Abstract
Background
For hepatocellular carcinoma (HCC), liver resection is a classical curative modality, despite its technical complexity. The incidence of HCC in the oldest old people (aged ≥ 85 years) is rising along with the global increase in life expectancy. Currently, no report has addressed liver resection for HCC in this aged population.
Patients and methods
We conducted a retrospective review of 1889 patients receiving curative liver resection for newly diagnosed HCC from 1992 to 2016. At the time of operation, 1858 of them were aged < 85 years (group A), and 31 were aged ≥ 85 years (group B). Another 18 oldest old patients, whose HCC was considered resectable but were not operated on due to the patient’s refusal, served as the control group (group C). The clinicopathological characteristics and early and long-term outcomes were compared between groups A and B. All associated co-morbidities of the patients were well-treated before liver resection. The overall survival (OS) rates were also compared between groups B and C.
Result
Group B had a significantly higher incidence of associated co-morbidities and hepatitis C infection. Postoperative complication rates and 90-day mortality rates after liver resection did not differ between groups A and B (p = 0.834 and p = 1.000, respectively), though group B had a longer postoperative stay (p = 0.001). In groups A and B, the 5-year disease-free survival rates were 29.7% and 22.6% (p = 0.163), respectively, and their overall survival rates were 43.5% and 35.5% (p = 0.086). The overall survival rate of group B was significantly different from group C (35.5% vs. 0%, p = 0.001).
Conclusion
Despite a longer postoperative recovery period, liver resection for HCC in the oldest old patients may be justified if co-morbidities are well controlled.
Keywords: Hepatocellular carcinoma, Oldest old, Liver resection
Background
Hepatocellular carcinoma (HCC) is a high lethal malignancy with increasing prevalence. As an endemic area of hepatitis B virus, hepatocellular carcinoma (HCC) has ranked as the first or second commonest cause of cancer death each year over the last 20 years [1]. During the past three decades, many modalities have been developed for treating HCC [1, 2]. Among them, liver resection, liver transplantation, and radiofrequency ablation (RFA) are generally agreed curative modalities for HCC [1–3]. Many non-operative modalities, such as trans-arterial chemo-embolization (TACE), trans-arterial radio-embolization (TARE), or target therapies have also been developed for HCC recently [2–4]. Although liver resection is the most classical modality, with a mortality rate of < 3%, it remains a complex procedure with high postoperative complication rates (20–40%). Liver resection would be better performed at high-volume centers with well-experienced surgical teams [1, 5]. Selection for the appropriate modality at the right time for the appropriate patient groups may improve the survival of HCC.
On the other hand, human life expectancy is increasingly lengthened due to improved medication and health care. The definition of “elderly people” four decades ago was those aged “65 years old” [6]. The new definition of elderly has been extended to people > 80 years old [7]. Older patients usually have a higher incidence of the associated co-morbidities, which are often serious [8–10]. Elderly people can be roughly subdivided into three groups, viz., “young old,” over 65 but under 75 years; “intermediate old,” over 75 but under 85 years; and “the oldest old,” over 85 years [7].
In 1990, Fortner et al. first demonstrated that despite higher risks of liver resection in elderly patients (age ≥ 65 years), liver resection remains possible after careful patient selection [11]. Thereafter, a number of other investigators reported the feasibility of liver resections for HCC in old patients [12–14]. Their definition of old age was from 65 to 70 [12] and even 80 [13, 14]. Liver resection for HCC in the oldest old patients has not been addressed so far. The main age of HCC incidence is 50 to 70 years. On the other hand, non-operative modalities, as described above, also develop. Although these treatments do not cure the HCC, they do prolong the survival times with acceptable life quality [3]. Thus, the actual benefits of liver resection for HCC in oldest old patients remain to be determined.
The life expectancy of the Taiwanese population has increased rapidly during the last 20 years. As a tertiary referral center, we sometimes encounter oldest old HCC patients. This age is over the average life expectancy worldwide and in Taiwan [15], so we have been hesitant about aggressive surgical intervention for this particular population. Some oldest old HCC patients are in relatively good general condition to undergo the operation, but their tolerance for surgical complications and benefit from operation, such as median survival time, are still unclear. To elucidate the benefits of liver resection for HCC in these particular patients, we conducted a retrospective review of prospectively collected data of liver resection for HCC over the past 25 years.
Patients and methods
Patient data
We undertook a retrospective review and analysis of the clinicopathological data of 1910 consecutive patients who had undergone liver resections for newly diagnosed HCC during the period from January 1992 to December 2016. Patients whose HCC resection needed cardiopulmonary bypass for tumor thrombus extending to the right atrium (n = 11) [16] and patients who received non-curative liver resection (n = 10, defined as gross residual tumor after operation [8]) were excluded.
Among all enrolled patients, 1858 of them were aged < 85 years at the time of operation (group A, median 62, range 18–84). The age of the remaining 31 patients was ≥ 85 years (group B, median 87.5 years, range 85–95). We then compared groups A and B regarding patients’ background features, tumor characteristics, and the early and long-term postoperative outcomes.
During the same study period, 18 oldest old patients were judged as resectable for HCC but were treated by non-operative modalities instead due to the refusal of operation by the patients or their families (group C). Their treatment modalities were RFA in 4, TACE in 9, oral sorafenib in 3, and conservative treatment in 2. TACE or RFA was performed by a senior radiologist (JIH).
Since July 2012, laparoscopic hepatectomy was performed on one patient in group B and 51 in group A. Tumors of these patients met the patient selection criteria of the Louisville statements of laparoscopic hepatectomy [17].
Preoperative assessments
As we have previously reported [8], HCC patients underwent measurement of conventional hemogram and liver function tests: serum α-fetoprotein (AFP), hepatitis B surface antigen (HBsAg), and anti-hepatitis C antibody (anti-HCV); indo-cyanine green (ICG) clearance test; gastroduodenal endoscopy; abdominal computed tomography (CT); and/or magnetic resonance imaging (MRI). The resectability and the extent of the liver to be resected were based on the tumor extension and modified Makuuchi criteria [18].
Patients whose co-morbidities should be well treated and controlled and who fell into American Society of Anesthesiology (ASA) class one or two were considered operable. After 2001, forced expiratory volume at 1 s (FEV 1) ≥ 75% and left ventricular ejection fraction (LVEF) in echocardiogram ≥ 50% were added to the patient selection criteria in patients older than 65 years. The treatment strategies, management plans, and tentative operative procedures of each patient were agreed upon before operation in conferences attended jointly by surgeons, gastroenterologists, anesthesiologists, radiologists, and co-morbidity-related physicians. The associated co-morbidities were the following: cardiopulmonary diseases, including hypertension, heart failure, cardiomyopathies, valvular heart disease, pericardial disease, syncope, aortic aneurysms, coronary arterial disease, and arrhythmia; lung malignancies, including chronic obstructive pulmonary disease, bronchiectasis, obstructive sleep apnea, interstitial lung disease, and pulmonary hypertension; neuromuscular disorders, including seizure, myopathies, stroke, dementia, and Parkinson’s disease; gastrointestinal diseases, including peptic ulcer disease, malignancies other than HCC, inflammatory bowel disease, gastrointestinal bleeding, biliary tract diseases, esophageal and gastric disorders; endocrine and metabolic diseases, including breast disease, thyroid disease, adrenal disorders, pituitary disorders, diabetes mellitus, and dyslipidemia; urologic and nephrologic diseases, including end-stage renal disease, urologic and genital malignancies, lithiasis, renal failure and glomerular disease; hematologic diseases, including anemia, platelet disorders, coagulopathies, leukemias, lymphomas and myeloproliferative disease; and rheumatologic diseases, including systemic lupus erythematosus, vasculitis, amyloidosis, various arthritis, and other autoimmune diseases. For patients with end-stage renal disease, peri-operative heparin-free hemodialysis was carried out [19]. For patients with hypersplenic thrombocytopenia (platelet count ≤ 80,000/mm3), concomitant splenectomy was carried out [20]. For patients with severe gastroesophageal varices (F3 or presence of red-colored sign), we performed pre-operative endoscopic ligation and/or sclerotherapy [21]. In addition to post-operative pain control, epidural catheter insertion for analgesic injection was performed routinely after 2005.
Intraoperative assessments
Liver parenchyma was transected using a Kelly crushing method under intermittent hepatic inflow blood occlusion (Pringle’s maneuver). During liver parenchymal transection, a low central venous pressure (CVP) policy (CVP < 5 cm H2O) was implemented by a senior anesthesiologist (CHS), who also oversaw the restrictive blood transfusion policy.
Postoperation assessment
Resected specimens were examined by a senior pathologist (YGJ), who determined the following: tumor capsular formation, resection margin width, tumor number, micro- or macro-vascular invasion, Ishak score, cirrhosis severity [22], and tumor differentiation (using Edmondson and Steiner grading). The AJCC Cancer staging system (8th edition) was applied after pathological examinations. Early postoperative complications, such as bile leakage, ascites, and liver failure, were recorded as defined according to the international consensus [23–25]. The severity of complications [26] was determined using the Clavien-Dindo classification. Any death that had occurred within 90 days after the operation was considered operative mortality.
Patients who survived from hepatectomy were followed up at the outpatient clinic during the first 2 years at intervals of 2 to 3 months, and thereafter at intervals of 4–6 months. Serum AFP, liver function, and imaging (abdominal ultrasonography, CT, or MRI) were checked. Recurrent HCCs were treated by re-hepatectomy, RFA, TACE, oral sorafenib, or other conservative treatments, as appropriate.
Statistical analysis
Continuous variables are expressed as median (range) and were compared using Mann-Whitney U test. Frequencies were compared using Fisher’s exact test or Pearson’s χ2 test. All patients were followed up till July 2018. The disease-free survival rates and overall survival rates were characterized by the Kaplan-Meier life-table method and compared using the log-rank test. p values < 0.05 were considered statistically significant.
Results
Patient background characteristics
Table 1 shows the clinicopathological characteristics of patients with resected HCC (groups A and B) and clinical features of the oldest old HCC patients without surgery (group C). Groups B and C had significantly higher incidences of associated co-morbidities and anti-HCV positivity compared with group A. Clinicopathological characteristics did not significantly differ between groups B and C.
Table 1.
Clinicopathological features in the three groups
| Clinical characteristics | Group A (n = 1858) | Group B (n = 31) | Group C (n = 18) | p value | |
|---|---|---|---|---|---|
| A vs. B | B vs. C | ||||
| Sex (M:F) | 1421:437 | 25: 6 | 11:7 | 0.741 | 0.102 |
| Age (years) | 63 (18–84) | 86 (85–95) | 87 (85–92) | < 0.001 | 0.914 |
| Serum hepatitis states | 0.024 | 0.894 | |||
| B + C+ | 108 (5.8%) | 1 (3.2%) | 0 | ||
| B + C− | 873 (47.0%) | 11 (35.5%) | 5 (27.8%) | ||
| B − C+ | 590 (31.8%) | 13 (41.9%) | 10 (55.6%) | ||
| B − C− | 287 (15.4%) | 6 (19.4%) | 3 (16.7%) | ||
| Serum AFP (ng/ml) | 30.9 (0.63–3,395,610) | 28.3 (1.29–15,321) | 34.6 (12.0–127,911) | 0.814 | 0.758 |
| ICG 15 (%) | 13.5 (1.38–59.65) | 15.3 (6.60–29.53) | 14.2 (5.4–38.0) | 0.814 | 0.758 |
| Child-Pugh grade | |||||
| A:B:C | 1612:205:41 | 27:3:1 | 16:2:0 | ||
| Need for splenectomy | 141 (7.5%) | 1 (3.2%) | 1 (5.3%) | 0.190 | 0.711 |
| Associated with EGV | 800 (16.0%) | 4 (12.9%) | 2 (11.1%) | 0.584 | 0.899 |
| Associated with comorbidities | 454 (24.3%) | 29 (93.8%) | 16 (88.9%) | < 0.001 | 0.916 |
| Cardiopulmonary | 201 | 20 | 12 | ||
| Neurologic | 10 | 2 | 0 | ||
| Hepatico-gastroenterologic | 66 | 4 | 0 | ||
| Endocrine and metabolic | 125 | 6 | 6 | ||
| Genitoenphrologic | 69 | 3 | 1 | ||
| Hematologic | 72 | 0 | 0 | ||
| Rheumatologic | 19 | 3 | 0 | ||
| Others | 41 | 2 | 2 | ||
Hepatitis states: B + C+, positive for HBsAg and anti-HCV; B + C−, positive for HBsAg, negative for anti-HCV; B− C+, negative for HBsAg and positive for anti-HCV; B− C−, negative for HBsAg and anti-HCV; AFP α-fetoprotein, ICG 15 indocyanine-green 15-min retention test, EGV esophagogastric varice
Operation result and early outcome
Group B had a significantly longer postoperative hospital stay compared with group A [18.0 (8–46) days vs. 10.0 (7–81) days, p = 0.001]. No significant differences were found in intra- or early post-operative results between the two operated groups (Table 2). The pathologic features between groups B and A have no significant difference (Table 3).
Table 2.
Intra- and early-postoperative result in hepatectomy for HCC
| Group A (n = 1858) | Group B (n = 31) | p | |
|---|---|---|---|
| Operative time (hour) | 4.6 (3.2–14.9) | 4.0 (0.8–10) | 0.282 |
| Liver ischemic time (min) | 30.0 (14.8–204) | 28.8 (17.1–94.3) | 0.282 |
| Liver transection area (cm2) | 36.8 (2.4–164.0) | 42.6 (16.0–120.0) | 0.564 |
| Operative bleeding (ml) | 520 (20–10,024) | 600 (50–1900) | 0.395 |
| Need for blood transfusion | 329 (17.7) | 6 (19.4%) | 0.994 |
| Postoperative stay (day) | 18.0 (5–46) | 10 (7–81) | 0.001 |
| Postoperative complications | 361 (19.4%) | 7 (22.6%) | 0.834 |
| 90-day mortality | 17 (0.91%) | 0 | 1.000 |
| Clavian-Dindo grade | |||
| Grade III | 196 | 3 | |
| Grade IV | 62 | 1 | |
| Grade V | 16 | 0 | |
| Post-operation pneumonia | 2 | 0 | |
| Pleural effusion | 31 | 0 | |
| Ascites | 38 | 0 | |
| Bile leakage | 101 | 1 | |
| Liver failure | 16 | 0 | |
| Delay bowel movement | 13 | 3 | |
| Wound infection | 27 | 1 | |
Table 3.
Pathologic characteristics of resected specimen
| Pathological features | Group A (n = 1858) | Group B (n = 31) | p |
|---|---|---|---|
| Tumor size (cm) | 6.5 ± 5.1 | 5.6 ± 5.7 | |
| Histological characteristics | 1414 (75.7%) | 22 (74.0%) | 0.691 |
| Tumor number ≥ 2 | 352 (18.9%) | 4 (12.9%) | 0.540 |
| Microvascular | |||
| Invasion | 787 (42.1%) | 12 (38.7%) | 0.848 |
| Satellite nodule | 608 (32.5%) | 9 (29.0%) | 0.831 |
| Capsule formation | 961 (51.3%) | 21 (67.7%) | 0.103 |
| Resection margin (mm) | 3.0 (0–19.0) | 1.0 (0–65) | 0.961 |
| Tumor differentiation | |||
| Well differentiation | 308 (16.6%) | 3 (9.7%) | 0.546 |
| Moderately differentiation | 450 (24.2%) | 9 (29.0%) | |
| Poorly differentiation | 1100 (59.2%) | 19 (61.3%) | |
| AJCC-TNM stage | 0.936 | ||
| I | 739 (39.8%) | 12 (40.0%) | |
| II | 556 (29.9%) | 10 (33.3%) | |
| III | 548 (29.5%) | 9 (29.4%) | |
| IV | 15 (0.8%) | 0 (0%) | |
Long-term result
The difference in DFS between groups B and A was not significant (p = 0.163) (Fig. 1). The OS of Group B was slightly but not significantly lower than that of group A (p = 0.086). On the other hand, group C had very poor outcomes compared to group B (Fig. 2).
Fig. 1.

Disease-free survival rates (DFS) in groups A and B, p =0.163
Fig. 2.
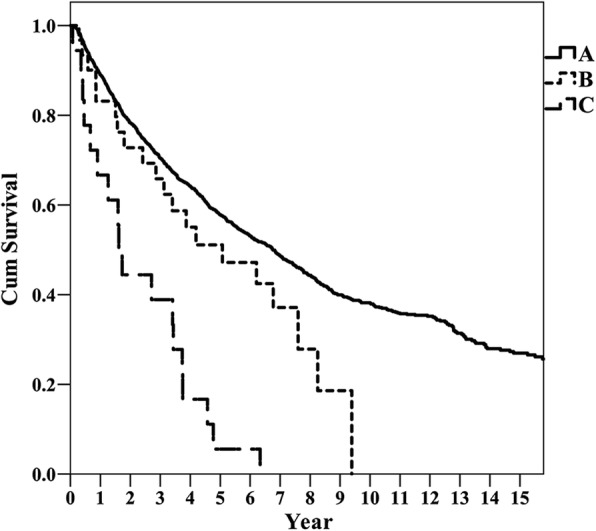
Overall survival curves in groups A, B, and C. p value for A vs. B, 0.080; B vs. C, < 0.001
A total of 1098 patients in group A died during the course of this study. Among them, deaths of 105 patients were not due to HCC, and the remaining 993 patient deaths were HCC-related. In contrast, only 20 patients in group B died, 6 of them due to other diseases (p < 0.001). The median survival times of group A, B, and C were 6.9, 5.4, and 1.9 years, respectively.
Discussion
In Taiwan, the average life expectancy had exceeded 80.0 years by 2014 (mean 80.0 years; male 76.8 years; female 83.4 years in 2016). People aged ≥ 65 years constitute 13.3% of the population, and the country’s aging index (the number of people ≥ 60 years old per 100 people < 15 years old) was 100.18 in 2016 [15]. This means increasing population aging is impending, mirroring the global trend. The remaining life expectancy of 65-, 75-, and 85-year-olds was 19.9, 12.6, and 6.8 years, respectively [15].
The elderly population has a higher risk of HCC formation [27]. In our daily clinical practice, increasingly more elderly subjects have been diagnosed with HCC. Our previous study explored the potential benefits of hepatectomy in octogenarians [8]. Since then, a number of studies have reported on the feasibility of liver resection to treat HCC in old patients. Uwatoko et al. [28] reported successful resection of HCC in two patients aged > 90 years, but they did not present long-term outcomes. To our best knowledge, ours is the first study regarding the outcomes of hepatectomy in oldest old patients.
This study is a single-institution observation of HCC resection with a relatively large sample observed over a relatively long period of time. The liver resection strategy and surgical procedure were homogenous across patients. Surgical indications, operation methods, and follow-up policies were all similar during the study period. Furthermore, an anesthesiologist, radiologist, gastroenterologist, and co-morbidities-related physician jointly discussed the treatment options preoperatively. Our average case intake was approximately 75 cases/year in volume, which is considered high for HCC resection in hospitals [5, 29].
During the study period, we have improved on the surgical equipment and techniques, concepts, and medication prescriptions [30] to treat early surgical complications. The positive trend of long-term outcomes of our liver resections for HCC has noticeably risen with time.
Our group B and C patients had higher incidences of co-morbidity and hepatitis C infection. These results are consistent with previous studies [27, 31]. Furthermore, poor organ function and liver function in the elderly have been reported in the early literature [32]. Yamada et al. [33] showed that old patients (> 80 years) had lower serum albumin than younger patients. Okinaga et al. [29], on the other hand, reported that the number of patients with multiple co-morbidities dropped in patients aged > 80 years compared with younger patients (70–80 years).
In a previous study, we used the ASA score as the sole criterion for patient selection. One 84-year-old patient expired due to acute myocardial infarction 3 weeks after the HCC resection. Therefore, in the present study, we added more criteria for patient selection: FEV1 ≥ 75% and LVEF ≥ 50% for those aged ≥ 65 years. No postoperative deaths had occurred under the screening after 2004 with these new criteria.
Age is a risk factor of post-liver resection pulmonary complication. Kim et al. [34] reported that older patients (> 70 years of age) had higher incidences of postoperative pneumonia. Here, we found no severe pulmonary complication in the older group. Our pre-operative cardiopulmonary function test, used as a patient selection criterion, and respiratory training with incentive spirometer and postoperative pain control were arranged to exclude the possibility of pulmonary complications. In addition, we noted that older patients required longer periods for postoperative recovery without increasing the complication rate. We believe that this finding was related to the longer recovery of postoperative ileus in the older group [8].
Nozawa et al. [9] also reported a higher incidence of postoperation cardiovascular complication and delirium in super-elderly patients (aged > 80 years). Apart from this, no difference in liver-associated postoperative complications was found between the older and younger patients [29]. We used a pre-operative echocardiogram and LVEF as additional criteria for patient selection. Postoperative ileus, delirium, cardiovascular disease, and pulmonary complications should contraindicate liver resection surgery in patients aged ≥ 70 years [35]. Our multi-departmental consensus conference on the treatment modalities for each HCC patient helped to support the feasibility and safety of hepatectomy for elderly patients, including those ≥ 85 years.
Regarding the long-term survival after HCC resection, most studies reported no difference in OS or DFS between older and younger patients [9, 32, 34, 36–38] (Fig. 2). Here, we also found no significant differences between the older and younger group, though in the older group, we found a tendency of shorter OS. Furthermore, we also observed that the operated oldest old patients had better prognosis than the non-operated control group. According to these results, we believe that hepatectomy for HCC has long-term benefits, consistent with previous studies.
For late deaths in our oldest old operated patients, we observed fewer HCC-related deaths compared with the younger patients. Nozawa et al. [9] also reported that older patients who underwent hepatectomy for HCC had fewer cancer-related deaths than younger patients. On the other hand, we found that all of non-operated oldest old patients succumbed to HCC. Toro et al. [3] assessed life quality within 2 years after hepatectomy for HCC, finding that it was better than those who underwent RFA, TACE, or no treatment. Therefore, regarding HCC treatment and quality of life, liver resection is mandatory in well-selected oldest old patients.
The current study showed that median survival time after liver resection for HCC in group B (5.4 years) was approximately 1.4 years lower than the life expectancy of the general oldest old population (6.8 years) in Taiwan. In contrast, the median survival time of group C was 1.9 years (4.9 years below the life expectancy). We concluded that liver resection for HCC in oldest old patients might let these patients be able to achieve their natural life expectancy without viable HCC, a lethal malignancy.
There are some limitations to this study. First, this is a cohort study, and many perioperative assessments, postoperative care schedule, and strategies of treatments were not fully consistent during the whole period of study. Second, our strategy could have changed with some biases and with time. Finally, we provided no randomized comparisons of other medical non-operative modalities (e.g., RFA, TACE, or TARE) due to the small sample size.
Despite these limitations, we can still conclude that liver resection for HCC may be justified in highly selected oldest old patients with well-controlled health conditions.
Conclusion
Despite a longer postoperative recovery period, liver resection for HCC in the oldest old patients may be justified if co-morbidities are well controlled.
Acknowledgements
The authors express their thanks to Mr. JP Chen, a member of the biostatistical team of Taichung Veterans General Hospital, for his statistical assistance.
Funding
No funding was received.
Availability of data and materials
Please contact the corresponding author with request for data.
Authors’ contributions
FHW, CHS, SCL, JIH, WSC, HZY, YY, SBC, YLL, FKP, and CCW analyzed and interpreted the patient data regarding the hepatocellular carcinoma. YGJ performed the histological examination of the liver. FHW and CCW were major contributors in writing the manuscript. All authors approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Institutional Review Boards of Taichung Veterans General Hospital, Taichung (CE18170A). The research was conducted in accordance with the principals of the 1964 Declaration of Helsinki and its later amendments.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Feng-Hsu Wu, Email: b101091110@tmu.edu.tw.
Shao-Ciao Luo, Email: ytppytpp@hotmail.com.
Jen-I Hwang, Email: jihwang@vghtc.gov.tw.
Wen-Shan Chao, Email: nini8327@yahoo.com.tw.
Hong-Zen Yeh, Email: yhz@vghtc.gov.tw.
Yee-Gee Jan, Email: Janyejan@vghtc.gov.tw.
Yun Yen, Email: yen@tmu.edu.tw.
Shao-Bin Cheng, Email: sbc@vghtc.gov.tw.
Cheng-Chung Wu, Phone: +886-4-23592525-82564, Email: hegsvghtc@gmail.com.
Yi-Ling Lin, Email: elaine@vghtc.gov.tw.
Fang-Ku P’eng, Email: elaine39_22@yahoo.com.tw.
References
- 1.Wu CC. Progress of liver resection for hepatocellular carcinoma in Taiwan. Jpn J ClinOncol. 2017;47(5):375–380. doi: 10.1093/jjco/hyx007. [DOI] [PubMed] [Google Scholar]
- 2.Cheng AL, Amarapurkar D, Chao Y, et al. Re-evaluating transarterial chemoembolization for the treatment of hepatocellular carcinoma: consensus recommendations and review by an International Expert Panel. Liver Int. 2014;34(2):174–183. doi: 10.1111/liv.12314. [DOI] [PubMed] [Google Scholar]
- 3.Toro A, Pulvirenti E, Palermo F, Di Carlo I. Health-related quality of life in patients with hepatocellular carcinoma after hepatic resection, transcatheter arterial chemoembolization, radiofrequency ablation or no treatment. SurgOncol. 2012;21(1):e23–e30. doi: 10.1016/j.suronc.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 5.Yasunaga H, Horiguchi H, Matsuda S, et al. Relationship between hospital volume and operative mortality for liver resection: data from the Japanese Diagnosis Procedure Combination database. Hepatol Res. 2012;42(11):1073–1080. doi: 10.1111/j.1872-034X.2012.01022.x. [DOI] [PubMed] [Google Scholar]
- 6.Kowal P, Dowd JE. Definition of an older person. Proposed working definition of an older person in Africa for the MDS Project. World Health Organization, Geneva, doi. 2001;10 (2.1):5188.9286.
- 7.Campion EW. The oldest old. N Engl J Med. 1994;330(25):1819–1820. doi: 10.1056/NEJM199406233302509. [DOI] [PubMed] [Google Scholar]
- 8.Wu CC, Chen JT, Ho WL, et al. Liver resection for hepatocellular carcinoma in octogenarians. Surgery. 1999;125(3):332–338. doi: 10.1016/S0039-6060(99)70245-X. [DOI] [PubMed] [Google Scholar]
- 9.Nozawa A, Kubo S, Takemura S, et al. Hepatic resection for hepatocellular carcinoma in super-elderly patients aged 80 years and older in the first decade of the 21st century. Surg Today. 2015;45(7):851–857. doi: 10.1007/s00595-014-0994-1. [DOI] [PubMed] [Google Scholar]
- 10.Inoue Y, Tanaka R, Fujii K, et al. Surgical outcome and hepatic regeneration after hepatic resection for hepatocellular carcinoma in elderly patients. Dig Surg. 2018. p. 1–13. [Epub ahead of print] [DOI] [PubMed]
- 11.Fortner JG, Lincer RM. Hepatic resection in the elderly. Ann Surg. 1990;211(2):141–145. doi: 10.1097/00000658-199002000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takenaka K, Shimada M, Higashi H, et al. Liver resection for hepatocellular carcinoma in the elderly. Arch Surg. 1994;129(8):846–850. doi: 10.1001/archsurg.1994.01420320072014. [DOI] [PubMed] [Google Scholar]
- 13.Shirabe K, Kajiyama K, Harimoto N, et al. Early outcome following hepatic resection in patients older than 80 years of age. World J Surg. 2009;33(9):1927–1932. doi: 10.1007/s00268-009-0122-3. [DOI] [PubMed] [Google Scholar]
- 14.Riffat F, Chu F, Morris DL. Liver resection in octogenarians. HPB (Oxford) 2006;8(3):206–210. doi: 10.1080/13651820500497173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ministry of the Interior T. Statistics of population in Taiwan. 2017; https://www.moi.gov.tw/chi/chi_site/stat/news_detail.aspx?sn=11735. Accessed 11 Mar 2017.
- 16.Wu CC, Hseih S, Ho WM, Tang JS, Liu TJ, P'Eng FK. Surgical treatment for recurrent hepatocellular carcinoma with tumor thrombi in right atrium: using cardiopulmonary bypass and deep hypothermic circulatory arrest. J SurgOncol. 2000;74(3):227–231. doi: 10.1002/1096-9098(200007)74:3<227::aid-jso15>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 17.Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: the Louisville Statement, 2008. Ann Surg. 2009;250(5):825–830. doi: 10.1097/SLA.0b013e3181b3b2d8. [DOI] [PubMed] [Google Scholar]
- 18.Makuuchi M, Kosuge T, Takayama T, et al. Surgery for small liver cancers. SeminSurgOncol. 1993;9(4):298–304. doi: 10.1002/ssu.2980090404. [DOI] [PubMed] [Google Scholar]
- 19.Cheng SB, Wu CC, Shu KH, et al. Liver resection for hepatocellular carcinoma in patients with end-stage renal failure. J SurgOncol. 2001;78(4):241–246. doi: 10.1002/jso.1160. [DOI] [PubMed] [Google Scholar]
- 20.Wu CC, Cheng SB, Ho WM, et al. Appraisal of concomitant splenectomy in liver resection for hepatocellular carcinoma in cirrhotic patients with hypersplenic thrombocytopenia. Surgery. 2004;136(3):660–668. doi: 10.1016/j.surg.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Liu HT, Cheng SB, Wu CC, Yeh HZ, Chang CS, Wang J. Impact of severe oesophagogastricvarices on liver resection for hepatocellular carcinoma in cirrhotic patients. World J Surg. 2015;39(2):461–468. doi: 10.1007/s00268-014-2811-9. [DOI] [PubMed] [Google Scholar]
- 22.Knodell RG, Ishak KG, Black WC, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1(5):431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 23.Mullen JT, Ribero D, Reddy SK, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204(5):854–862. doi: 10.1016/j.jamcollsurg.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 24.Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149(5):680–688. doi: 10.1016/j.surg.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Ishizawa T, Hasegawa K, Kokudo N, et al. Risk factors and management of ascites after liver resection to treat hepatocellular carcinoma. Arch Surg. 2009;144(1):46–51. doi: 10.1001/archsurg.2008.511. [DOI] [PubMed] [Google Scholar]
- 26.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 27.Asahina Y, Tsuchiya K, Tamaki N, et al. Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infection. Hepatology. 2010;52(2):518–527. doi: 10.1002/hep.23691. [DOI] [PubMed] [Google Scholar]
- 28.Uwatoko S, Yamamoto K, Sasaki T, et al. Age is no longer a limit: two cases of hepatectomy in patients over 90 years old. Case Rep Gastroenterol. 2015;9(1):49–55. doi: 10.1159/000368115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okinaga H, Yasunaga H, Hasegawa K, Fushimi K, Kokudo N. Short-term outcomes following hepatectomy in elderly patients with hepatocellular carcinoma: an analysis of 10,805 septuagenarians and 2,381 Octo- and nonagenarians in Japan. Liver Cancer. 2018;7(1):55–64. doi: 10.1159/000484178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu CC, Ho WM, Cheng SB, et al. Perioperative parenteral tranexamic acid in liver tumor resection: a prospective randomized trial toward a “blood transfusion”-free hepatectomy. Ann Surg. 2006;243(2):173–180. doi: 10.1097/01.sla.0000197561.70972.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Portolani N, Baiocchi GL, Coniglio A, et al. Limited liver resection: a good indication for the treatment of hepatocellular carcinoma in elderly patients. Jpn J ClinOncol. 2011;41(12):1358–1365. doi: 10.1093/jjco/hyr154. [DOI] [PubMed] [Google Scholar]
- 32.Su CW, Lei HJ, Chau GY, et al. The effect of age on the long-term prognosis of patients with hepatocellular carcinoma after resection surgery: a propensity score matching analysis. Arch Surg. 2012;147(2):137–144. doi: 10.1001/archsurg.2011.288. [DOI] [PubMed] [Google Scholar]
- 33.Yamada S, Shimada M, Miyake H, et al. Outcome of hepatectomy in super-elderly patients with hepatocellular carcinoma. Hepatol Res. 2012;42(5):454–458. doi: 10.1111/j.1872-034X.2011.00952.x. [DOI] [PubMed] [Google Scholar]
- 34.Kim JM, Cho BI, Kwon CH, et al. Hepatectomy is a reasonable option for older patients with hepatocellular carcinoma. Am J Surg. 2015;209(2):391–397. doi: 10.1016/j.amjsurg.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Wei F. Does an extreme age (≥80 years) affect outcomes in patients after liver cancer surgery? A meta-analysis. ANZ J Surg. 2018. [Epub ahead of print] [DOI] [PubMed]
- 36.Motoyama H, Kobayashi A, Yokoyama T, et al. Impact of advanced age on the short- and long-term outcomes in patients undergoing hepatectomy for hepatocellular carcinoma: a single-center analysis over a 20-year period. Am J Surg. 2015;209(4):733–741. doi: 10.1016/j.amjsurg.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 37.Kaibori M, Yoshii K. Hasegawa K, et al. Ann Surg: Treatment optimization for hepatocellular carcinoma in elderly patients in a Japanese Nationwide Cohort; 2018. [DOI] [PubMed] [Google Scholar]
- 38.Cucchetti A, Sposito C, Pinna AD, et al. Effect of age on survival in patients undergoing resection of hepatocellular carcinoma. Br J Surg. 2016;103(2):e93–e99. doi: 10.1002/bjs.10056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact the corresponding author with request for data.


