Abstract
Background
Second allogeneic hematopoietic stem cell transplant (HCT) remains as an option for disease relapse after initial HCT.
Methods
We analyzed retrospectively the outcomes of 65 consecutive patients who underwent a second HCT for disease relapse at the University of Chicago. Univariate and multivariate analysis were conducted, and a scoring system was generated to select the patients who would benefit second HCT.
Results
All except four patients received T cell depleted (TCD) first HCT. The majority of patients had AML (n = 47) and high risk MDS (n = 5). The median age at second HCT was 45 years (11–73). 13 patients (20%) achieved CR before second HCT. 98% (n = 64) and 72% (n = 47) patients achieved neutrophil and platelet engraftment at a median interval of 10 and 18 days, respectively, following the second HCT. With a median follow up of 23 (5.5–140) months for survivors after second HCT, the estimated 2 years PFS was 17.5% and the 2 years OS was 22.6%. The day 100 cumulative incidence of non-relapse mortality rate was 23.6%, and the cumulative incidence of aGVHD and cGVHD were 16.9% and 7.7% respectively at 1 year after second HCT. In univariate analysis, patients with remission duration after first HCT of > 12 months and those in CR before second HCT had significantly better PFS and OS. A scoring system using disease status before second HCT (CR = 0 vs. non-CR = 1), and remission duration after first HCT (< 6 = 2, 6–12 = 1 and > 12 months = 0) was generated as an approach to classify patients into different risk categories in the purpose to provide guidance to the transplant physician to inform the outcomes to potential patients undergoing 2nd HCT. A score of < 2 (n = 26) identified a group with PFS and OS of 31.6% and 36.2% at 2 years after second HCT.
Conclusion
In conclusion, second HCT is a viable option for disease relapse after TCD HCT for patients entering second HCT in remission and/or remission duration > 12 months after first HCT with acceptable rates of GVHD and donor engraftment.
Keywords: Second allogeneic bone marrow transplantation, T cell depletion, Non-relapse mortality
Introduction
HCT is the only curative treatment for many high-risk hematologic diseases, including AML, and MDS. The numbers of HCT are steadily increasing worldwide, with improving outcomes as judged by lower treatment-related mortality. However, relapse after HCT remains the major cause for treatment failure. In most situations, treatment options at relapse after HCT are very limited with unacceptably high rates of disease relapse. The treatment options for disease relapse after HCT include withdrawal of immune suppression, chemotherapy, second allogeneic transplant, cytokine and adoptive cell therapy and donor lymphocyte infusion [1].
Second HCT has been demonstrated repeatedly to provide benefit, albeit in a small subset of patients [2–5], with larger more recent retrospective studies confirming this observation [6–8]. In addition, a recent retrospective registry study performed by the Acute Leukemia Working Party of EBMT reported the outcomes of adults with relapsed AML after RIC HCT [9]. Long-term survival was only achieved in patients who achieved CR by cyto-reductive therapy, followed either by donor lymphocyte infusion or second HCT as consolidation [9].
T-cell depletion is an approach that enhances procedure tolerability by reducing acute and chronic GVHD related morbidity and mortality. We have employed in vivo T-cell depletion with alemtuzumab for over a decade at the University of Chicago and confirmed lower rates of acute and chronic GVHD with similar overall survival to T cell repleted HCT [10]. Although relapse remains problematic after transplant [11], particularly for those with active disease at time of HCT, there has been concern that T-cell depletion might further increase the relapse rate. Though registry studies suggest an increased rate of disease recurrence after T-depleted transplantation [12], our own comparative analysis of alemtuzumab vs non-alemtuzumab based conditioning did not show an increased relapse incidence [10]. Neither did another study comparing CD34 selection with conventional GVHD prophylaxis [13, 14].
In this study, we analyzed outcomes in patients, many of whom received an initial T cell-depleted transplant, who then received second HCT at our center for relapsed disease. It is a retrospective single center analysis.
Patients and methods
Sixty-five consecutive patients who underwent second HCT for disease relapse at University of Chicago were retrospectively analyzed. This study included all the patients who received second HCT at our center without selection, and these patients who were able to move to second HCT could be well-selected since second HCT was only one of the options to treat disease relapse after HCT. Patient characteristics were listed in Table 1. All except 4 patients received T cell depleted (TCD) HCT as first HCT. The majority of patients (71%) received RIC during first HCT. As a strategy to prevent further relapse, the majority of patients (68%, n = 44) received myeloablative conditioning regimens for their second transplant. 18 patients received Fludarabine (Flu)-Busulfan (Bu)-alemtuzumab (campath), 7 patients received TBI-based regimens and two patients received Bu/cyclophosphamide (CTX). Twenty-one patients received reduced intensity conditioning including 5 patients received Flu-Mel-alemtuzumab, 7 patients received Clofarabine (Clo)-Mel-Campath and 7 patients received Flu-Mel-ATG. Tacrolimus and MMF were mainly used as GVHD prophylaxis. This study included a heterogeneous patient population, while the majority of the patients had AML/MDS and underwent RIC conditioning for second HCT.
Table 1.
Characteristics of the patients
| Variable | Total |
|---|---|
| Total patients | 65 |
| Median Age (range) | 45 (11–73) |
| Male/female | 48/17 |
| Diagnosis | |
| AML | 47 |
| MDS | 5 |
| Lymphoma | 6 |
| CML | 2 |
| Other leukemia | 5 |
| Disease status before second HCT | |
| Remission | 13 |
| Not in remission | 52 |
| Donor status | |
| MRD | 33 |
| MUD | 21 |
| Haplo-cord | 11 |
| Conditioning regimen for first HCT | |
| Myeloablative | 19 |
| RIC | 46 |
| Conditioning regimen for second HCT | |
| Myeloablative | 44 |
| Flu-Bu-Alemtuzumab | 18 |
| TBI-based | 7 |
| Bu/Cy | 7 |
| RIC | 21 |
| Flu-mel-Alemtuzumab | 5 |
| Clo-mel-Alemtuzumab | 7 |
| Flu-mel-ATG | 7 |
MRD matched related donor, MUD matched unrelated donor, MDS myelodysplastic syndrome
Endpoints
The primary endpoints of the retrospective analysis were OS, NRM, and progression-free survival (PFS). The analysis included OS, PFS, relapse, and NRM.
Statistical analysis
Patient and transplantation characteristics were analyzed using descriptive statistics. NRM estimates were calculated using cumulative incidence with relapse as the competing event. The estimated relapse was calculated using cumulative incidence with NRM as competing event. The estimate of aGVHD/cGVHD of second HCT was calculated using cumulative curves in the competing risk model. The estimate of time to engraftment of ANC and PLT was calculated using life tables. Successful engraftment was set as the positive event. The probabilities of OS and PFS were analyzed using Kaplan–Meier plotting and its univariate analysis. PFS was defined as time to relapse or death from any cause. OS and PFS were calculated from the date of second transplant. The p value was set at < .05 for statistical significance. In terms of multivariate analysis, Cox regression model was applied in which the OS and PFS was dependent variable respectively. Statistical analyses were performed with SPSS software.
Results
Patient characteristics
A total of 65 consecutive patients were included (Table 1). Forty-eight patients were male and 17 were female. The median age at second transplantation was 45 years (range 11 to 73 years). Underlying diagnoses were as follows: 47 patients were diagnosed with AML (72%) and 5 (8%) patients were diagnosed with MDS. Remission duration after first HCT was calculated from the date of first HCT to the date of disease relapse after first HCT. All the 65 patients had disease relapse after first HCT. All the relapsed patients after first HCT received disease control treatment prior to second HCT, but 52 (80%) patients still had active disease while 13 (20%) patients were in complete remission (CR) after treatment prior to second HCT. The donor for the second HCT was MRD in 33 patients, MUD in 21 patients, and haplo-cord in 11 patients. Forty-four patients (68%) received myeloablative conditioning (MAC), and 21 (32%) received a reduced intensity condition HCT. Most of the patients (73.8%, 48/65) still received T cell depleted HCT either with Campath for matched donor or ATG for haplo-cord donor for the second HCT. Further information of patient characteristics can be found in Table 1. More than half (58%) patient had duration of remission after first HCT that persisted for greater than 6 months prior to second HCT (Table 2), 75% patients were able to move to second HCT within 6 month after disease relapse from the first HCT. In these selected patients for the second HCT, it was not surprised that only six patients developed Grade II aGVHD, and only 2 patients had mild cGVHD after first HCT and before second HCT.
Table 2.
Information of patients before the second HCT
| Characteristics | No (%) |
|---|---|
| Duration of remission after first HCT (months) | |
| < 6 | 27 (42%) |
| ≥ 6–12 | 15 (23%) |
| ≥ 12 | 23 (35%) |
| Time from relapse to second HCT (months) | |
| < 6 | 49 (75%) |
| ≥ 6–12 | 12 (18%) |
| ≥ 12 | 4 (6%) |
Hematopoietic recovery after second HCT
Following the second HCT, 64 patients (98%) achieved engraftment of neutrophil and 47 patients (72%) achieved engraftment of platelet with median times of 10 days (8–55 days) and 18 days (8–146 days) respectively (Table 3). The cumulative incidence of day 28 neutrophil and platelet engraftment was 98% and 73% respectively (Table 3). Donor engraftment analysis were routinely done in all the patients, and most of patients had full donor chimerism at day 30 after second HCT, but the data was not captured in this analysis as part of hematopoietic recovery after second HCT.
Table 3.
Transplant outcomes after second HCT
| Characteristics | No (%) |
|---|---|
| Neutrophil recovery | |
| Neutrophil engraftment by day 28 | 98% |
| Median (day) | 10 (8–55) |
| Platelet recovery | |
| Platelet engraftment by day 28 | 73% |
| Median (day) | 18 (8–146) |
| NRM | |
| Day 100 | 23.6% |
| 1 year | 36.9% |
| aGVDH Grade II–IV at 1 year | 16.9% |
| cGVHD at 1 year | 7.7% |
| Relapse at 1 years | 33.8% |
| 1 year PFS | 29.3% |
| 1 year OS | 33.3% |
| Death | 47 |
| Relapse | 22 (47%) |
| Infection | 11 (23%) |
| GVHD | 6 (13%) |
| Other | 8 (17%) |
Survival, relapse, NRM, and GVHD after second HCT
With a median follow-up of surviving patients of 695 days (range 166 to 4191 days), PFS and OS were 29.3% and 33.3% at 1 year respectively (Fig. 1), and both curves stayed flat after 2 years follow-up, indicating that very few patients were at risk of disease relapse more than 2 years after second HCT. The cumulative incidence of relapse was 36.9% at 1 year. The cumulative incidence of non-relapse mortality (NRM) was 23.6% at day 100, and 33.8% at 1 year after the second HCT (Table 3). The cumulative incidence of acute GVHD was 16.9%, and of chronic GVHD was 7.7% at 1 year post-transplant (Table 3). There were 47 deaths at the end of follow-up. The most common cause of death was disease relapse in 22 patients (47%), followed by infection in 11 patients and GVHD in 6 patients (Table 3).
Fig. 1.
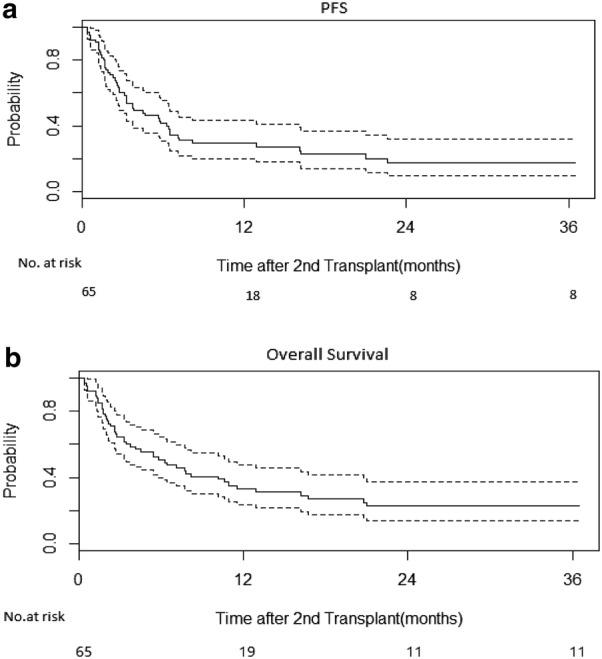
Long term survival could be achieved in patients after second HCT. The estimated 2 years PFS and OS was 17.5% and 22.6% respectively
Univariate analysis was utilized as an approach in identifying the factors affecting the PFS and OS after second HCT. Factors evaluated included pre-transplant patient characteristics, conditioning regimen, donor options etc. In our analysis with limited power due to small patient number, there was no association of PFS and OS with donor type(MRD vs MUD vs Haplo-cord),performance status (≥ 90 vs < 90), HCT-CI(≥ 3 vs < 3), conditioning intensity (Myeloablative vs RIC), development of GVHD after second HCT or not, patient age (≤ 60 vs. > 60), or use of alemtuzumab as part of conditioning regimen for the second HCT (data not shown).
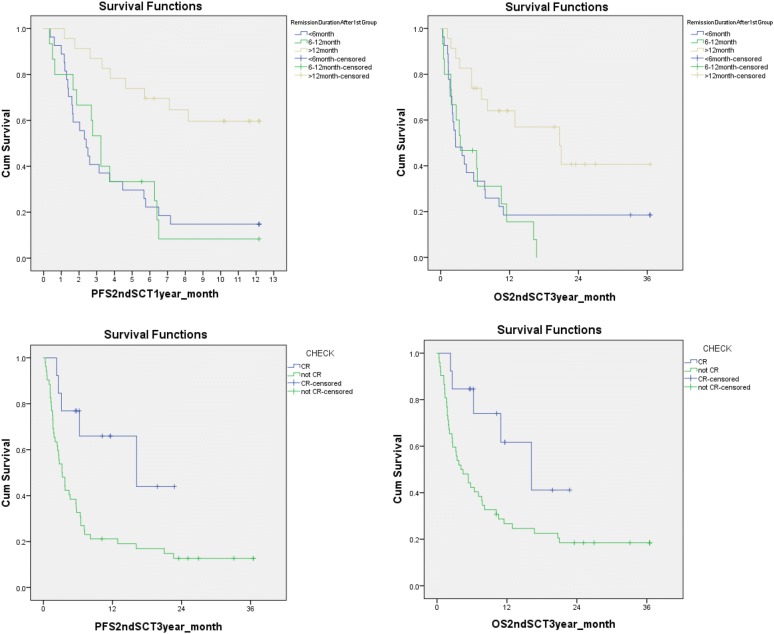
In contrast, univariate analysis demonstrated that patients with remission duration after first HCT of > 12 months or being in CR prior to the second HCT was associated significantly with better PFS and OS respectively. The difference of PFS and OS stratified by these two variables was very striking (Fig. 2).
Fig. 2.
Long duration of remission after first HCT and disease remission prior to second HCT predicted better survival significantly after second HCT. Patients whose remission duration > 12 months after first HCT had significant better outcome (top panels), as well as the patients who entered complete remission prior to second HCT (bottom panels)
In addition, Cox regression model was applied for multivariate analysis in which the OS and PFS was dependent variable respectively. According to the univariate analysis, remission duration after first HCT and being in CR prior to the second HCT or not was input in the model. In the PFS model, remission duration and being in CR prior to the second HCT or not were risk factors. The patients whose remission duration after first HCT < 12 months had high risk of relapse or death than those whose duration > 12 months, with a hazard ratio (HR) of 1.54. Patients who were not in CR prior to second HCT had high risk of relapse or death than those who were in CR, with a HR of 2.48. In the OS model, only being in CR prior to the second HCT or not had been kept in the Cox equation. Patients who were not in CR prior to second HCT had high risk of death than those in CR, with a HR of 31.80.
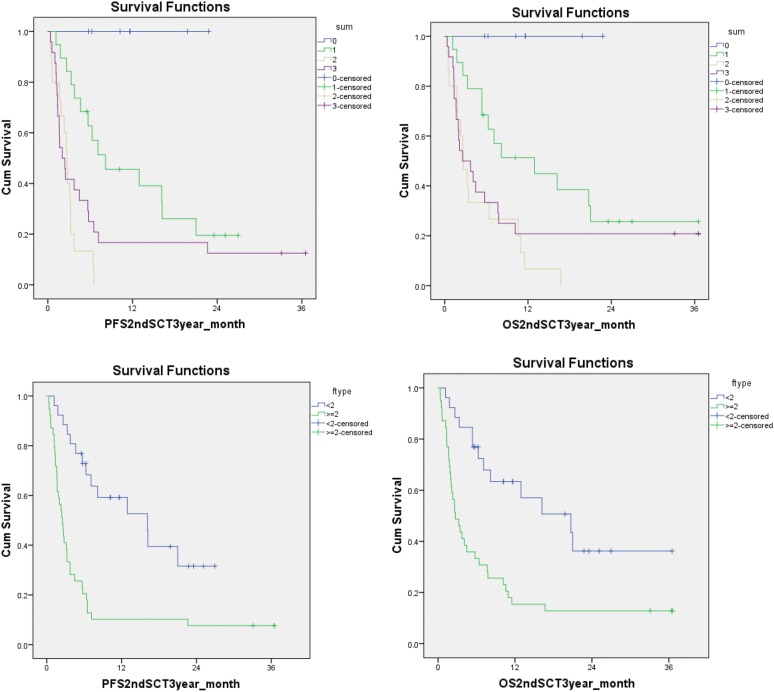
In the purpose to provide guidance to the transplant physician to inform the outcomes to the potential patients undergoing 2nd HCT, we built a scoring system using the two variables which were statistically significant in univariable analysis: remission status prior to second HCT and remission duration after first HCT (Table 4); The scoring system could separate the PFS and OS effectively (Fig. 3, top panel); Summarizing the use of this scoring system, patients who scored < 2 demonstrated statistically significant better 2 years PFS of 31.6% and OS of 36.2% comparing to the patients who scored ≥ 2 at 7.7% and 12.8% as 2 years PFS and OS respectively (Fig. 3, low panel). Thus, patients who score < 2 on the scoring system might be considered for second HCT given the enhanced possibility for long-term survival. In order to make this score system valid to apply to other transplant centers, it will need to be validated using much larger patient population, which is not possible at this time. This scoring system has been routinely used at our transplant center to guide the decision to move forward to second HCT, and to inform the potential patients the outcome after second HCT to obtain informed consent for second HCT.
Table 4.
A scoring system using the remission status prior to second HCT, and remission duration after first HCT
| Score | CR or not Prior to second HCT |
Remission duration after first HCT (months) |
|---|---|---|
| 0 | CR | > 12 |
| 1 | Not CR | 6–12 |
| 2 | < 6 |
Fig. 3.
A scoring system using disease status before second HCT and remission duration after first HCT can predict survival outcome after second HCT. Disease status before second HCT (0: CR vs. 1: non-CR) and remission duration after first HCT (0: > 12 months; 1: 6 to 12 months; 2: < 6 months) could be used to predict the outcomes after second HCT. The outcomes of the patients with a score < 2 were significant better than those who ≥ 2, which selected out the patients who could benefit most from second HCT
Discussion
Relapse remains the leading cause of treatment failure in hematologic malignancies after allogeneic stem cell transplant. Treatment options for disease relapse after HCT remains limited, and long term survival even with new agents is disappointing. Although there is significant mortality, second HCT has been demonstrated to provide enhanced long term survival in well-selected patients [6–8, 15]. Our center has been using in vivo T cell depletion with alemtuzumab as part of the conditioning regimen. Our single center retrospective study presented the analysis of the second HCT outcomes for disease relapse after T cell depleted HCT in patient with hematologic malignancies. As we are aware, it is the largest retrospective study focusing T cell depleted HCT to determine the outcomes after second HCT.
Limited by relatively small patient numbers, a heterogeneous population with different disease diagnosis, disease and donor status and conditioning regimens, our results showed that donor type (MRD vs. MUD vs. haplo-cord), conditioning intensity (RIC vs. myeloablative), and patient age had no influence on PFS and OS. We could not exclude that these variables might have effect in a much large analysis. While our analysis could not confirm the detrimental effects on survival from T-cell depletion with alemtuzumab, avoidance of T cell depletion using alemtuzumab especially in patients receiving second matched related donor HCT had been recommended in our center in the recent years of clinical practice. In the univariate analysis, patients with remission duration after first HCT (> 12 months) or CR status before second HCT had significantly better PFS and OS. These observations suggested that the remission duration and CR status may serve as positive predictive prognostic factors for survival after second HCT at least in our patient population undergoing T cell depleted HCT. The scoring system we devised using disease status before second HCT (CR vs. non-CR), and remission duration after first HCT (< 6, 6–12 and ≥ 12 months) (Table 4) could be utilized to identify patients who might benefit second HCT effectively (Fig. 3). Patients scored < 2 on the scoring system had enhanced 2 years PFS and OS, and second HCT might be considered in these well selected population.
Our study is consistent with several recently published retrospective studies demonstrating that the duration of first HCT remission and diseases status prior to second allo-ST were important factors in determining the outcome after second HCT [6–8, 15]. Specifically, Ruutu et al. retrospectively analyzed the outcome of predictive factor after second HCT using data from EBMT; they demonstrated that an advanced stage of disease predicted poor outcome; a longer remission after the first transplantation was a favorable predictive factor for survival; and the patients with an interval of more than 1 year between the transplantations had a clearly better survival than those with a shorter interval [8]. Our data demonstrated that for patients who relapsed after T-cell depleted HCT, second HCT remains a viable option for selected patients. Similar to the observation by Ruutu et al. the best outcomes were achieved in patients who relapsed more than 12 months after the first HCT, and disease in remission prior to second HCT (Fig. 3) in our T cell depleted first HCT patients. Our results are also consistent with the observations of Schmid et al. in relapsed AML patients after RIC HCT. In their analysis, only patients who could enter CR after chemotherapy induction followed by cellular therapies, either donor lymphocyte infusion or second HCT, were long-term survivors [9].
In addition, according to the univariate analysis results, a scoring system using remission status and remission duration after first HCT was generated; and patients who scored < 2 on the scoring system demonstrated decent 2 years PFS of 31.6% and OS of 36.2% comparing to the patients who scored ≥ 2 at 7.7% and 12.8% of 2 years PFS and OS respectively. Thus, patients who score < 2 on the score system should be considered for second HCT for long-term survival.
Due to the very high 1 year NRM at 33.8% and high relapse rate at 36.9% at 1 year for the whole population, and 2 years PFS of only 7.7% for patients scored ≥ 2 in the scoring system, patients who scored ≥ 2 on the scoring system might not be offered second HCT, while clinical trial using targeted therapies or novel immunotherapies including antibody-based therapy or CAR-T cell therapy if available should be considered other than second HCT. The ongoing development of novel agents and treatment modalities may help to place more patients who relapsed after HCT into a further complete remission or decent disease control allowing a new bridge to second HCT.
Not surprisingly, disease relapse remains the major cause of mortality even after second HCT, and it contributed to 47% death (Table 3) after second HCT in our analysis. The strategies to prevent disease relapse after second HCT will not differ significantly from those available prior to the first HCT: (1) to achieve deeper minimal residual disease negative remission with novel agents since minimal residual disease prior to HCT predicts disease relapse [16–18]; (2) novel conditioning approaches to eliminate residual disease without increase of toxicities; (3) early intervention after HCT to prevent disease relapse, including maintenance treatment or prophylactic intervention, like prophylactic donor lymphocyte infusion or immunotherapy. In this regard, we do have ongoing clinical trial to incorporate Intensity Modulated Total Marrow Irradiation (IM-TMI) with fludarabine and melphalan as conditioning regimen for patients undergoing second HCT (NCT02333162) with the hope to eliminate residual disease prior to second HCT to prevent disease relapse after second HCT.
Our analysis had several limitations. The sample size precludes subset analysis or robust multivariate analysis. The predominance of T-cell depletion for first and second HCT may reduce generalizability. Finally, as with most series, patients undergoing second HCT were likely a selected subset of patients who relapsed.
Conclusion
In conclusion, second HCT is a viable option for disease relapse after T cell depleted HCT with acceptable GVHD and good engraftment for those entering transplant in remission with remission duration > 12 months after first HCT.
Authors’ contributions
All the authors made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data. All the authors had been involved in drafting the manuscript or revising it critically for important intellectual content; all the authors had given final approval of the version to be published. Each author had participated sufficiently in the work to take public responsibility for appropriate portions of the content; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank all the patients and their families who were included in this analysis. We would also like to thank all the nurses, clinic and research staffs who provided care and support to our patients and our research.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent to publish
Not applicable.
Ethics approval and consent to participate
This study conformed to the Declaration of Helsinki and was approved by institutional review boards. This data analysis was under the approved consents by BSD IRB at The University of Chicago Biological Sciences Division/University of Chicago Medical Center. These patients who underwent stem cell transplant all were consented for research data analysis. All the patients were consented to participate.
Funding
Dr. Hongtao Liu was supported by a Cancer Research Foundation Young Investigator award, and a K12 Paul Calabresi award. The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- AE
adverse event
- AML
acute myeloid leukemia
- ANC
absolute neutrophil count
- ECOG
Eastern Cooperative Oncology Group
- G-CSF
granulocyte-colony stimulating factor
- MDS
myelodysplastic syndromes
- NE
not estimable
- OS
overall survival
- RBC
red blood cells
- PFS
progression free survival
- MRD
matched related donor
- MUD
matched unrelated donor
- Haplo-Cord
haploidentical-cord
- HCT
allogeneic stem cell transplantation
- GVHD
graft versus host disease
- CR
complete remission
- HCT-CI
hematopoietic cell transplantation-comorbidity index
Contributor Information
Yun Fan, Email: fanyun2336@bjhmoh.cn.
Andrew S. Artz, Email: aartz@medicine.bsd.uchicago.edu
Koen van Besien, Email: kov9001@med.cornell.edu.
Wendy Stock, Email: wstock@medicine.bsd.uchicago.edu.
Richard A. Larson, Email: rlarson@medicine.bsd.uchicago.edu
Olatoyosi Odenike, Email: todenike@medicine.bsd.uchicago.edu.
Lucy A. Godley, Email: lgodley@medicine.bsd.uchicago.edu
Justin Kline, Email: jkline@medicine.bsd.uchicago.edu.
John M. Cunningham, Email: jcunning@peds.bsd.uchicago.edu
James L. LaBelle, Email: jlabelle@peds.bsd.uchicago.edu
Michael R. Bishop, Email: mbishop@medicine.bsd.uchicago.edu
Hongtao Liu, Email: hliu2@medicine.bsd.uchicago.edu.
References
- 1.Petrovic A, Hale G. Clinical options after failure of allogeneic hematopoietic stem cell transplantation in patients with hematologic malignancies. Expert Rev Clin Immunol. 2011;7(4):515–525. doi: 10.1586/eci.11.24. [DOI] [PubMed] [Google Scholar]
- 2.Radich JP, Sanders JE, Buckner CD, Martin PJ, Petersen FB, Bensinger W. Second allogeneic marrow transplantation for patients with recurrent leukemia after initial transplant with total-body irradiation-containing regimens. J Clin Oncol. 1993;11:304–313. doi: 10.1200/JCO.1993.11.2.304. [DOI] [PubMed] [Google Scholar]
- 3.Hosing C, Saliba RM, Shahjahan M, Estey EH, Couriel D, Giralt S. Disease burden may identify patients more likely to benefit from second allogeneic hematopoietic stem cell transplantation to treat relapsed acute myelogenous leukemia. Bone Marrow Transplant. 2005;36:157–162. doi: 10.1038/sj.bmt.1705011. [DOI] [PubMed] [Google Scholar]
- 4.Bosi A, Laszlo D, Labopin M, Reffeirs J, Michallet M, Gluckman E. Second allogeneic bone marrow transplantation in acute leukemia: results of a survey by the European cooperative group for blood and marrow transplantation. J Clin Oncol. 2001;19:3675–3684. doi: 10.1200/JCO.2001.19.16.3675. [DOI] [PubMed] [Google Scholar]
- 5.Eapen M, Giralt SA, Horowitz MM, Klein JP, Wagner JE, Zhang MJ. Second transplant for acute and chronic leukemia relapsing after first HLA-identical sibling transplant. Bone Marrow Transplant. 2004;34:721–727. doi: 10.1038/sj.bmt.1704645. [DOI] [PubMed] [Google Scholar]
- 6.Vrhovac R, Labopin M, Ciceri F, Finke J, Holler E, Tischer J, Lioure B, Gribben J, Kanz L, Blaise D, et al. Second reduced intensity conditioning allogeneic transplant as a rescue strategy for acute leukaemia patients who relapse after an initial RIC allogeneic transplantation: analysis of risk factors and treatment outcomes. Bone Marrow Transplant. 2016;51(2):186–193. doi: 10.1038/bmt.2015.221. [DOI] [PubMed] [Google Scholar]
- 7.Orti G, Sanz J, Bermudez A, Caballero D, Martinez C, Sierra J, Cabrera Marin JR, Espigado I, Solano C, Ferra C, et al. Outcome of second allogeneic hematopoietic cell transplantation after relapse of myeloid malignancies following allogeneic hematopoietic cell transplantation: a retrospective cohort on behalf of the Grupo Espanol de Trasplante Hematopoyetico. Biol Blood Marrow Transplant. 2016;22(3):584–588. doi: 10.1016/j.bbmt.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Ruutu T, de Wreede LC, van Biezen A, Brand R, Mohty M, Dreger P, Duarte R, Peters C, Garderet L, Schonland S, et al. Second allogeneic transplantation for relapse of malignant disease: retrospective analysis of outcome and predictive factors by the EBMT. Bone Marrow Transplant. 2015;50(12):1542–1550. doi: 10.1038/bmt.2015.186. [DOI] [PubMed] [Google Scholar]
- 9.Schmid C, Labopin M, Nagler A, Niederwieser D, Castagna L, Tabrizi R. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood. 2012;119:1599–1606. doi: 10.1182/blood-2011-08-375840. [DOI] [PubMed] [Google Scholar]
- 10.van Besien K, Kunavakkam R, Rondon G, De Lima M, Artz A, Oran B, Giralt S. Fludarabine-melphalan conditioning for AML and MDS: alemtuzumab reduces acute and chronic GVHD without affecting long-term outcomes. Biol Blood Marrow Transplant. 2009;15(5):610–617. doi: 10.1016/j.bbmt.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van den Brink MR, Porter DL, Giralt S, Lu SX, Jenq RR, Hanash A, Bishop MR. Relapse after allogeneic hematopoietic cell therapy. Biol Blood Marrow Transplant. 2010;16(1 Suppl):S138–S145. doi: 10.1016/j.bbmt.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soiffer RJ, Lerademacher J, Ho V, Kan F, Artz A, Champlin RE, Devine S, Isola L, Lazarus HM, Marks DI, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117(25):6963–6970. doi: 10.1182/blood-2011-01-332007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barba P, Hilden P, Devlin SM, Maloy M, Dierov D, Nieves J, Garrett MD, Sogani J, Cho C, Barker JN, et al. Ex Vivo CD34+-selected T cell-depleted peripheral blood stem cell grafts for allogeneic hematopoietic stem cell transplantation in acute leukemia and myelodysplastic syndrome is associated with low incidence of acute and chronic graft-versus-host disease and high treatment response. Biol Blood Marrow Transplant. 2017;23(3):452–458. doi: 10.1016/j.bbmt.2016.12.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, Volin L, Ruutu T, Heim DA, Schwerdtfeger R, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10(9):855–864. doi: 10.1016/S1470-2045(09)70225-6. [DOI] [PubMed] [Google Scholar]
- 15.Duncan CN, Majhail NS, Brazauskas R, Wang Z, Cahn JY, Frangoul HA, Hayashi RJ, Hsu JW, Kamble RT, Kasow KA, et al. Long-term survival and late effects among one-year survivors of second allogeneic hematopoietic cell transplantation for relapsed acute leukemia and myelodysplastic syndromes. Biol Blood Marrow Transplant. 2015;21(1):151–158. doi: 10.1016/j.bbmt.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anthias C, Dignan FL, Morilla R, Morilla A, Ethell ME, Potter MN, Shaw BE. Pre-transplant MRD predicts outcome following reduced-intensity and myeloablative allogeneic hemopoietic SCT in AML. Bone Marrow Transplant. 2014;49(5):679–683. doi: 10.1038/bmt.2014.9. [DOI] [PubMed] [Google Scholar]
- 17.Appelbaum FR. Measurement of minimal residual disease before and after myeloablative hematopoietic cell transplantation for acute leukemia. Best Pract Res Clin Haematol. 2013;26(3):279–284. doi: 10.1016/j.beha.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Balsat M, Renneville A, Thomas X, de Botton S, Caillot D, Marceau A, Lemasle E, Marolleau JP, Nibourel O, Berthon C, et al. Postinduction minimal residual disease predicts outcome and benefit from allogeneic stem cell transplantation in acute myeloid leukemia with npm1 mutation: a study by the acute leukemia french association group. J Clin Oncol. 2017;35(2):185–193. doi: 10.1200/JCO.2016.67.1875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.