Abstract
One-hundred four persons aged ≥ 18 years (62 males and 42 females) who were admitted for traumatic brain injury (TBI) underwent brain computed tomography (CT) scan and assay of serum cortisol, insulin-like growth factor 1 (IGF-1), thyrotropin (TSH) and free thyroxine (FT4). The main purpose was to assess any gender difference and the rate of empty sella (ES).
Women were more likely to have empty sella (19/42 [45.2%] vs 19/62 [30.6%], P = 0.15, OR = 1.9), which was more frequently total ES or TES (16/19 [84.2%] vs 3/19 [15.8%], P = 0.0025, OR = 11.6). Neuroradiology was normal in the remaining 65 patients. Patients with TES were approximately 20–30 years older than both patients with partial ES (PES) and normal sella, but only the comparison with normal sella was significant (P = 0.001 all patients, P = 0.005 males). Presumed deficiency of IGF-1, cortisol or TSH occurred in 33 persons (31.7%; 20 Males [32.2%], 13 Females [30.9%]), 14 (13.5%; 10 M [16.2%], 4F [9.5%]) or 8 (7.7%; 1 M [1.7%], 7F [16.7%]), with only TSH deficiency having significant intergender difference (P = 0.007). The highest or lowest rates of IGF-1 deficiency occurred in men with PES (41.7%) or men with TES (14.3%), of cortisol deficiency in men with PES (33.3%) or women with PES (zero), and TSH deficiency in women with TES (18.7%) or both men and women with PES (zero) and men with normal sella (zero). Within ES, males with no deficiency were older compared to males with at least one hormone deficiency (75.7 ± 17.4 vs 55.6 ± 18.9, P = 0.022); in turn, the former males were also older compared with normal sella males having no hormone deficiency (54.1 ± 25.2, P = 0.023).
In conclusion, ES is detectable in almost 40% of persons who undergo CT within 24 h from TBI. A number of intergender differences concerning ES and the hormones evaluated are apparent.
Keywords: Traumatic brain injury, Post-traumatic hypopituitarism, Empty sella, Computed tomography
Introduction
Several retrospective studies, prospective studies and reviews on traumatic brain injury (TBI)-induced hypopituitarism [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11] are available in the literature.
The generality of studies on TBI have focused on the endocrine side, especially comparing the evolution over time of hormone changes, with minimal or absent attention to the neuroradiology of the hypothalamus-pituitary region in the early phase of TBI as well as to possible gender differences. Particularly, a few studies focused on post-traumatic empty sella that is the herniation of the subarachnoid space within the sella turcica, resulting in flattening of the pituitary [4], [12].
A Czech prospective study evaluated 89 TBI patients (women, n = 23) aged 18–65 years (mean 36 years) with a Glasgow Coma Scale (GCS) score ranging from 3 to 14 (median score 7). Patients underwent hormone evaluation at the time of injury and at 3, 6, and 12 months post-injury. Magnetic resonance imaging (MRI), which was also performed at 12 months post-injury, demonstrated an empty sella syndrome more frequently in patients with some hormone deficiency compared with those without endocrine dysfunction [12]. One German study examined the independent association of gender and age with injury severity (measured by the GCS), clinical course, pituitary dysfunction and outcome after TBI as measured by the Glasgow Outcome Scale. Age, but not gender, influenced the GCS. Logistic regression revealed an effect only of age and the initial injury severity on the Glasgow Outcome Scale. Gender affected instead the rate of pituitary insufficiency [13]. A Dutch group studied 630 women (53 with TBI) and 533 men (63 with TBI) [14]. Linear regression analysis (adjusted for age, body mass index, chronic diseases, smoking, alcohol use, and gender) was performed to examine the association between TBI and serum anterior pituitary hormone levels and bone mineral density measured by dual-energy x-ray absorptiometry and quantitative ultrasound. Serum follicle stimulating hormone (FSH) was significantly higher in males who had had head trauma compared with those who had not had it. In a study on 51 patients (mean age 36.1 years; 46 men) who were evaluated at least one year after TBI, pituitary hormone deficits were reported to be particularly common and capable of adversely affecting activities of daily living reducing quality of life. There was no gender difference in the rate of growth hormone (GH) deficiency [15]. In an American study, 18 patients with TBI and 16 subjects with subarachnoid hemorrhage underwent pituitary hormone evaluations 5–12 months following TBI [16]. In this series of subjects, the type of event (traumatic or hemorrhagic) and gender did not influence the prevalence of hypopituitarism. In addition, TSH and GH deficiencies were associated with reduced performance-function [16].
We have realized that, in the context of limitations in the requests for biochemical and instrumental investigations imposed by the Italian Ministry of Health to ensure savings for the national health system, the overwhelming majority of patients admitted to the Emergency Units for head trauma are unwilling to monitor hormone levels over time to detect post-head trauma hypopituitarism (Pht-Hypo). However, when we probed our patients after discharge from the Emergency Unit of University Hospital of Messina and informed them of the risk of Pht-Hypo occurring even many years after head trauma, there was a high rate of willingness to pay out of their pocket for hormone assays, if neuroradiology evaluation in the Emergency Unit showed abnormalities in the hypothalamic-pituitary region. The existence of any such abnormality would have increased the risk of hypopituitarism and made endocrine screening cost-effective.
With this in mind, we started recommending to our neuroradiologists not to miss the study of the sellar region during the brain imaging by CT, brain CT being a routine procedure for patients admitted at our Emergency Unit for head trauma. Based on very pilot initial observations that showed an unsuspected high proportion of empty sella in men, we elected to perform the prospective study reported here.
The aims of this prospective study were (i) to assess the frequency of empty sella in the acute phase of trauma by minimizing enrollment of patients with other causes of empty sella; (ii) to detect possible gender differences in empty sella frequency; (iii) to correlate empty sella with baseline endocrine evaluation.
Materials and methods
Patients
During the 12 months from January 01 to December 31, 2016, we enrolled 104 consecutive patients aged 18 or more years.
Each patient or, when unconscious, one close relative (or the living parents if the son/daughter was not independent yet) had to sign an informed consent.
The following precautions were taken in order to minimize the possibility that radiological alterations of the sellar region pre-existed.
Based on information obtained by the patients and/or their relatives, exclusion criteria were: (i) having been previously admitted to any Emergency Unit for head trauma; (ii) having recollection of head trauma (such as fall, road accident, sports-related) not followed by admission to the hospital; (iii) planning to perform neuroradiology investigation for symptoms such as headache, visual impairment, dizziness; (iv) having endocrine disorders of the hypothalamus-pituitary region (including having undergone either neurosurgery or brain radiotherapy); (v) having other brain disorders (such as cerebrovascular disease, dementia).
Imaging
A non-enhanced CT of the brain was performed using SOMATOM® Definition AS 64 (Siemens).
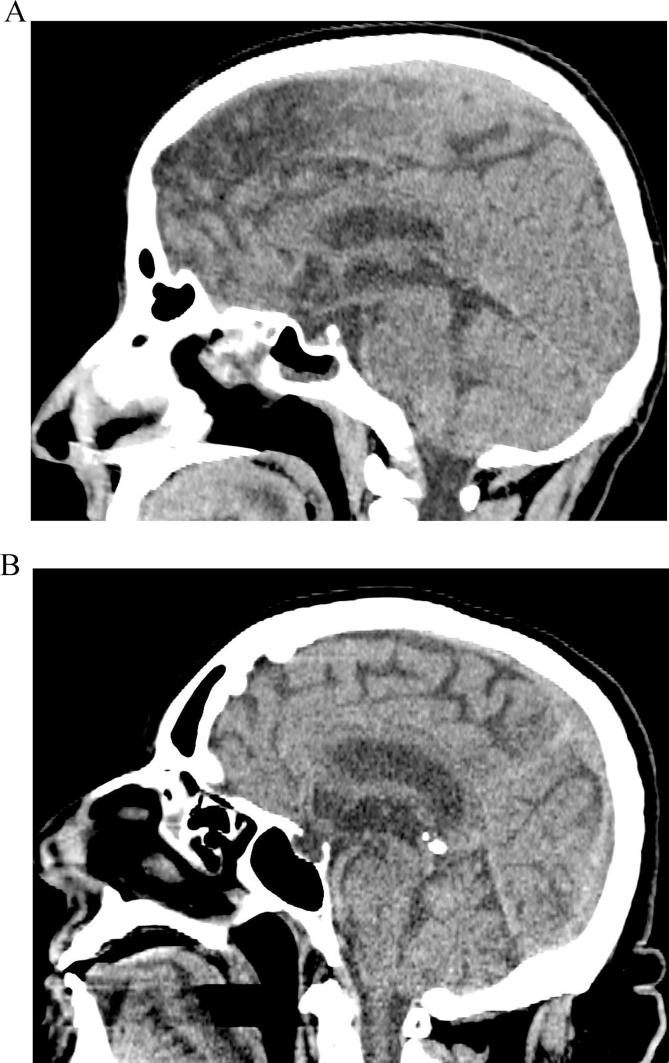
One coauthor (SLV) performed the CT scans. Partial empty sella (Fig. 1A) was considered when pituitary thickness was ≥3 mm and ≤50% of the sella was filled with cerebrospinal fluid, while total empty sella (Fig. 1B) was considered when pituitary thickness was <3 mm and >50% of the sella was filled with cerebrospinal fluid [17].
Fig. 1.
A) Partial empty sella; B) Total empty sella (Brain CT scan, sagittal plane).
Hormonal assessment
Endocrine investigations consisted in measurement of the following hormones: morning and afternoon serum cortisol (reference range: 6.7–22.6 µg/dl and <10 µg/dl, respectively), insulin-like growth factor-1 (IGF-1) (age-dependent reference range from 163 to 424 ng/ml for 1–20 years through 55–166 ng/ml for >80 years), TSH (reference range: 0.3–4.2 mU/L) and free thyroxine (FT4) (reference range: 0.61–1.12 ng/dl). For diagnosis of GH deficiency we relied only on IGF-1, since basal circulating GH measurement is not helpful to diagnose GH deficiency [7]. In case of low serum TSH, subclinical hyperthyroidism was excluded because of normal or low normal FT4 levels.
For the purposes of this study, all serum samples were stored at −20 °C until assay and a given hormone of all patients was measured in a single run to avoid inter-runs differences. Analytes were measured by these commercial immunoassay kits: Access-cortisol (Beckman Coulter, Inc, Brea, CA, USA), Access-Hypersensitive TSH (Beckman Coulter, Inc), Access-FT4 (Beckman Coulter, Inc), and Immulite 2000 IGF-1 (Siemens Medical Solutions-Diagnostics-USA, Malvern, PA, USA; formerly Diagnostic Products Corporation, Los Angeles, CA). The TSH kit is a third generation assay with a functional sensitivity of 0.01–0.02 mIU/L. Because (i) data are reported in an aggregate modality, and (ii) our study was based on neither dynamic tests nor invasive procedures but merely on a few baseline measurements on blood that had been drawn for routine blood chemistry, no Ethics Committee approval was needed.
Statistics
Data are reported as mean ± SD, median and range. Differences between means were analyzed by the ANOVA test. If data had non-gaussian distribution, the ANOVA test was performed after log10 transformation. Differences between proportions were analyzed by the χ2 test or Fisher’s exact test, as appropriate. The P value was set at ≤0.05 to indicate significant difference, and comprised between 0.05 and 0.10 to indicate borderline, trendwise difference.
Results
Age at trauma and neuroradiology of sella turcica
The average age at trauma (which coincides with age at our observation) was in the seventh decade, males being approximately 10 years younger than females (P = 0.012). The mean brain injury was mild, since GCS averaged 15 in either gender (Table 1).
Table 1.
Age at trauma and Glasgow Coma Scale of the 104 patients.*
| Mean ± SD, range [median] |
Age at trauma | Glasgow Coma Scale |
|---|---|---|
| All (n = 104) | 61.8 ± 26.9 [69.5; 18–93] |
14.6 ± 0.73 [15; 12–15] |
| Males (n = 62) | 57.4 ± 22.2 [62.1; 18–92] |
14.8 ± 0.46 [15; 12–15] |
| Females (n = 42) | 68.1 ± 17.6 [72.5; 22–93] |
14.3 ± 0.92 [15; 12–15] |
| Statistics, males vs females | P = 0.012 | P > 0.10 |
Statistically significant values are shown in bold.
Data are reported as mean ± SD [median; range].
Overall, empty sella was detected in over one-third of the 104 patients, and in females insignificantly more frequently than in males (45.2% vs 30.6%, P = 0.13). Interestingly, total empty sella was 3-fold more frequent in females than in males (38.1% vs 11.3%; P = 0.0012), while the opposite was true for partial empty sella (19.3% vs 7.1%, P = 0.096). Thus, total empty sella accounted for 84.2% of all cases of empty sella in females, compared with 36.8% in males (P = 0.0069) (Table 2). Only one patient (a 48-yr-old man) had other sella abnormalities. This consisted of increased volume of sella turcica, with the upper boundary of pituitary being mildly convex (Table 2). Further to trauma, this finding is consistent with pituitary tumor.
Table 2.
Neuroradiology of sella turcica and its relationship with age in patients stratified by gender.
| Empty sella |
|||||
|---|---|---|---|---|---|
| Normal | Partial | Total | Partial + Total | Other abnorm. | |
| All (n = 104) | 65 (62.5%) | 15 (14.4%) | 23 (22.1%) | 38 (36.5%) | 1 (1.0%) |
| Males (n = 62) | 42 (67.8%) | 12 (19.3%) [12/19 = 63.2%] |
7 (11.3%) [7/19 = 36.8%] |
19 (30.6%) | 1 (1.6%) |
| Females (n = 42) | 23 (54.8%) | 3 (7.1%) [3/19 = 15.8%] |
16 (38.1%) [16/19 = 84.2%] |
19 (45.2 %) |
0 |
| Statistics |
χ2 = 1.80 P = 0.18 OR = 1.7 (0.8–3.9 |
19.3 vs 7.1% P = 0.096 OR = 3.1 (0.8–11.8) |
11.3 vs 38.1% χ2 = 10.4 P = 0.0012 OR = 0.21 (0.07–0.56) |
χ2 = 2.3 P = 0.13 OR = 1.9 (0. 9–4.2) |
N/A |
| Statistics | 84.2 vs 15.8% 36.8 vs 63.2% P = 0.0069 OR = 9.1, 95% CI 1.9–45.9 |
N/A | |||
| Age, years | |||||
| All (n = 104) | 58.4 ± 23.0 [64;18–93] |
55.8 ± 17.9 [56; 33–84] |
75.3 ± 18.8 [73; 41–91] |
67.6 ± 17.4 [71;33–91] |
48 |
| Males (n = 62) | 54.2 ± 23.6 [57;18–92] |
56.2 ± 20.1 [52.5; 33–84] |
80.3 ± 9.6 [86;67–91] |
65.1 ± 20.5 [71;33–91] |
48 |
| Females (n = 42) | 66.5 ± 20.3 [73; 22–93] |
54.0 ± 3.5 [56] 50–56 |
73.1 ± 12.9 [73] 41–90 |
70.1 ± 13.8 [72] 41–90 |
|
| Statistics (Age) | |||||
| Males vs Females |
P = 0.035 (Normal) |
||||
| Normal vs Total empty sella |
P = 0.0011 (All) |
P = 0.005 (Males) |
|||
| Normal vs Partial + Total empty sella |
P = 0.035 (All) |
P = 0.08 (Males) |
|||
| Partial vs Total empty sella |
P = 0.0003 (All), P = 0.009 (Males) | ||||
Other abnormalities consists of increased volume of sella turcica, with the upper boundary of pituitary being mildly convex.
When χ2 is not given, difference was analyzed by the Fisher’s exact test. Values for odds ratio (OR) and, in parentheses, for 95% confidence interval are given. Borderline significant differences (P between 0.10 and 0.05) are typed bold-face italics, while statistically significant differences (P < 0.05) are typed bold-face.
In the comparisons concerning age, not to overload the Table, only borderline significant or statistically significant differences are given. Age comparisons involving females with partial empty sella were not done because of the small size of this group (n = 3).
Omitting from analysis the women with partial empty sella because of their small number (n = 3), patients with partial empty sella and normal sella had similar age. In contrast, patients with total empty sella were approximately 20–30 years older than both patients with partial empty sella and normal sella, but only the comparison with normal sella was statistically significant (P = 0.001 all patients, P = 0.005 males) (Table 2).
When patients are subdivided in age subgroups, it is evident that empty sella is always observed after 30 years (Table 3). After 60 years of age, empty sella is always of the total type in males and most frequently of the total type in females.
Table 3.
Distribution of empty sella in age subgroups.
| Males (n = 62) |
Females (n = 42)* |
|||||||
|---|---|---|---|---|---|---|---|---|
| Empty sella |
Empty sella |
|||||||
| No (n = 42) |
Yes (n = 19) |
No* (n = 23) |
Yes (n = 19) |
|||||
| Age, years |
Partial empty sella (n = 12) |
Total empty sella (n = 7) |
Part + Tot empty sella (n = 19) |
Partial empty sella (n = 3) |
Total empty sella (n = 16) |
Part + Tot empty sella (n = 19) |
||
| ≤30 | 11 | 0 | 0 | 0/11 | 1 | 0 | 0 | 0/1 |
| 31–40 | 4 | 4 | 0 | 4/8 (50%) | 3 | 0 | 0 | 0/3 |
| 41–50 | 4 | 2 | 0 | 2/7 (28.6%) | 1 | 1 | 1 | 2/3 (66.7%) |
| 51–60 | 3 | 1 | 0 | 1/4 (25%) | 3 | 2 | 1 | 3/6 (50%) |
| 61–70 | 4 | 0 | 1 | 1/5 (20%) | 1 | 0 | 3 | 3/4 (75%) |
| 71–80 | 12 | 3 | 2 | 5/17 (29.4%) | 6 | 0 | 6 | 6/15 (40%) |
| ≥81 | 4 | 2 | 4 | 6/10 (60%) | 5 | 0 | 5 | 5/10 (50%) |
Statistics of the intergender comparison within patients with empty sella. Partial empty sella, df = 4, χ2 = 6.67, P = 0.15; Total empty sella, df = 4, χ2 = 1.88, P = 0.76; Partial + Total empty sella, df = 5, χ2 = 6.18, P = 0.29.
One man (aged 48 years) had increased volume of sella turcica, with the upper boundary of pituitary being mildly convex.
In females empty sella is observed after 40 years, with partial empty sella being restricted to the age band 41–60 years. In contrast, partial empty sella has no clear age prevalence in males (Table 3).
Hormone levels and correlation with neuroradiology
Hormone deficiency of at least one hormone (as defined under Materials and Methods) and its relationship with sellar neuroradiology are summarized in Table 4, Table 5, while Table 6 gives details for hormone status in patients with empty sella.
Table 4.
Hormone deficiencies according to neurology.
| Normal (n = 65) | Empty sella (n = 38) |
All (n = 104)* | ||||
|---|---|---|---|---|---|---|
|
(42 M, 23F) |
Partial (12 M, 3F) |
Total (7 M, 16F) |
Partial + Total (19 M, 19F) |
(62 M, 42F) |
||
| Cortisol deficiency (<7 µg/dl) | ||||||
| All | 7 (10.8%) | 4 (26.7%) | 3 (13.0%) | 7 (18.4%) | 14 (13.5%) | |
| Males | 5 (11.9%) | 4 (33.3%) | 1 (14.3%) | 5 (26.3%) | 10 (16.2%) | |
| Females | 2 (8.7%) | 0 | 2 (12.5%) | 2 (10.5%) | 4 (9.5%) | |
| Cortisol deficiency (≤11 µg/dl) | ||||||
| All | 15 (23.1%) | 6 (40.0%) | 4 (17.4%) | 10 (26.3%) | 25 (24.0%) | |
| Males | 10 (23. 8%) | 5 (41.7%) | 1 (14.3%) | 6 (31.6%) | 16 (25.8%) | |
| Females | 5 (21.7%) | 1 (33.3%) | 3 (18.7%) | 4 (21.0%) | 9 (21.4%) | |
| TSH deficiency | ||||||
| All | 4 (6.1%) | 0 | 4 (10.5%) | 4 (10.5%) | 8 (7.7%) | |
| Males | 0 | 0 | 1 (14.3%) | 1 (5.3%) | 1 (1.6%) | |
| Females | 4 (17.4%) | 0 | 3 (18.7%) | 3 (15. 8%) | 7 (16.7%) | |
| IGF-I deficiency | ||||||
| All | 19 (29.2%) | 6 (40.0%) | 7 (18.4%) | 13 (34.2%) | 33 (31.7%) | |
| Males | 13 (30.9%) | 5 (41.7%) | 1 (14.3%) | 6 (31.6%) | 20 (32.2%) | |
| Females | 6 (26.1%) | 1 (33.3%) | 6 (37.5%) | 7 (36. 8%) | 13 (30.9%) | |
One man (aged 48 years) had increased volume of sella turcica, with the upper boundary of pituitary being mildly convex.
Table 5.
Combinations of hormone deficiencies according to neuroradiology.
| Normal (n = 65) | Empty sella (n = 38) |
All (n = 104)* | ||||
|---|---|---|---|---|---|---|
|
(42 M, 23F) |
Partial (12 M, 3F) |
Total (7 M, 16F) |
Partial + Total (19 M, 19F) |
(62 M, 42F) |
||
| Deficiencies (cortisol def. at <7 µg/dl) | ||||||
| Any one | 21 (32.3%) | 10 (76. 9%) | 3 (13.0%) | 13 (34.2%) | 35 (33.6%) | |
| Any two | 3 (4.6%) | 0 | 4 (17.4%) | 4 (10.5%) | 7 (6.7%) | |
| All three | 1 (1.5%) | 0 | 1 (4.3%) | 1 (2. 6%) | 2 (1.9%) | |
| Males | , any one | 14 (33.3%) | 9 (75.0%) | 0 | 9 (47.4%) | 24 (38.7%) |
| , any two | 2 (4.8%) | 0 | 0 | 0 | 2 (3.2%) | |
| , any three | 0 | 0 | 1 (14.3%) | 1 (5.3%) | 1 (1.6%) | |
| Females | , any one | 7 (30.4%) | 1 (33.3%) | 3 (18.7%) | 4 (21.0%) | 11 (26.2%) |
| , any two | 1 (4.3%) | 0 | 4 (25%) | 4 (21.0%) | 5 (11.9%) | |
| , any three | 1 (4.3%) | 0 | 0 | 0 | 1 (2.4%) | |
| Deficiencies (cortisol def. at ≤11 µg/dl) | ||||||
| Any one | 24 (36.9%) | 12 (80.0%) | 4 (17.4%) | 16 (42.1%) | 41 (39.4%) | |
| Any two | 7 (10.8%) | 1 (6.7%) | 4 (17.4%) | 5 (13.2%) | 12 (11.5%) | |
| All three | 1 (1.5%) | 0 | 1 (4.3%) | 1 (2.6%) | 2 (1.9%) | |
| Males | , any one | 18 (42.8%) | 10 (83.3%) | 0 | 10 (52.6%) | 29 (46.8%) |
| , any two | 3 (7.1%) | 1 (8.3%) | 0 | 1 (5.3%) | 4 (6.4%) | |
| , any three | 0 | 0 | 1 (14.3%) | 1 (5.3%) | 1 (1.6%) | |
| Females | , any one | 6 (26.1%) | 2 (66.7%) | 4 (25.0%) | 6 (31.6%) | 12 (28.6%) |
| , any two | 4 (17.4%) | 0 | 4 (25.0%) | 4 (21.0%) | 8 (19.0%) | |
| , any three | 1 (4.3%) | 0 | 0 | 0 | 1 (2.4%) | |
One man (aged 48 years) had increased volume of sella turcica, with the upper boundary of pituitary being mildly convex.
Table 6.
Hormonal levels according to neuroradiology and gender.*
| Males |
Females |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| no. | yrs | ES | Cortis (µg/dl) |
TSH (mU/L) |
IGF-1 (ng/ml) |
no. | yrs | ES | Cortis (µg/dl) |
TSH (mU/L) |
IGF-1 (ng/ml) |
|
| 1 | 9 | 33 | PES | 12.9 | 1.64 | 84 | 101 | 50 | PES | 27.2 | 11.6 | 81 |
| 2 | 78 | 34 | PES | 18.8 | 1.38 | 205 | 43 | 56 | PES | 21.1 | 1.04 | 128 |
| 3 | 42 | 35 | PES | 8.2 | 1.62 | 75 | 72 | 56 | PES | 9.6 | 2.71 | 99 |
| 4 | 82 | 40 | PES | 18.1 | 0.47 | 90 | 79 | 41 | TES | 25.7 | 0.02 | 72 |
| 5 | 75 | 42 | PES | 5.8 | 1.64 | 130 | 99 | 58 | TES | 5.9 | 1.13 | 89 |
| 6 | 31 | 45 | PES | 20.8 | 1.0 | 71.3 | 58 | 62 | TES | 25.5 | 0.71 | 154 |
| 7 | 104 | 60 | PES | 6.4 | 1.39 | 125 | 29 | 65 | TES | 22.3 | 0.06 | 29 |
| 8 | 74 | 71 | PES | 15.3 | 0.41 | 52 | 12 | 68 | TES | 15.2 | 0.64 | 93 |
| 9 | 95 | 71 | PES | 13.4 | 1.62 | 138 | 80 | 71 | TES | 12.7 | 1.37 | 110 |
| 10 | 97 | 79 | PES | 1.9 | 0.48 | 150 | 93 | 72 | TES | 4.8 | 0.02 | 87 |
| 11 | 81 | 81 | PES | 21.9 | 1.0 | 103 | 62 | 73 | TES | 17.3 | 0.54 | 206 |
| 12 | 34 | 84 | PES | 5.3 | 0.62 | 78 | 89 | 73 | TES | 9.2 | 0.73 | 122 |
| 13 | 28 | 67 | TES | 6.3 | 0.29 | 55 | 38 | 75 | TES | 21.3 | 1.37 | 61 |
| 14 | 36 | 71 | TES | 21.5 | 1.52 | 91 | 55 | 79 | TES | 22.6 | 1.0 | 24 |
| 15 | 13 | 73 | TES | 13.9 | 0.8 | 158 | 98 | 81 | TES | 24.5 | 0.64 | 114 |
| 16 | 5 | 86 | TES | 13.5 | 0.41 | 60 | 10 | 85 | TES | 47.0 | 1.13 | 67 |
| 17 | 91 | 86 | TES | 28.9 | 1.52 | 99 | 54 | 88 | TES | 31.9 | 2.25 | 105 |
| 18 | 23 | 88 | TES | 13.6 | 0.47 | 76 | 41 | 89 | TES | 23.3 | 0.66 | 50 |
| 19 | 103 | 91 | TES | 18.0 | 2.72 | 73 | 69 | 90 | TES | 20.1 | 0.73 | 72 |
| PES + TES (n = 19) |
13.9 ± 7.0 [13.6] 1.9–28.9 |
1.05 ± 0.53 [1.0] 0.29–2.72 |
100.7 ± 40.4 [90] 52–205 |
PES + TES (n = 19) |
20.4 ± 9.9 [21.8] 4.8–47.0 | 0.93 ± 0.70 [0.73] 0.02–2.71 |
92.8 ± 42.8 [89] 24–206 |
|||||
| PES (n = 7) |
12.4 ± 6.8 [13.1] 1.9–21.9 | 1.02 ± 0.53 [1.0] 0.41–1.64 |
108.4 ± 42.9 [96.5] 52–205 |
PES (n = 3) |
19.3 ± 8.9 [21.1] 9.6–27.2 |
1.04, 2.71 | 102.7 ± 23.7 [99] 81–128 |
|||||
| TES (n = 12) |
16.5 ± 7.2 [13.9] 6.3–28.9 |
0.96 ± 0.61 [0.80] 0.29–2.72 |
87.4 ± 34.8 [76] 55–158 |
TES (n = 16) |
20.6 ± 10.3 [21.8] 4.8–47 |
0.81 ± 0.57 [0.72] 0.02–2.25 |
90.9 ± 45.8 [88] 24–206 |
|||||
| Normal (n = 42) |
14.5 ± 5.5 [13.9] 3.1–27.3** |
1.63 ± 0.96 [1.39] 0.48–5.06 |
134.5 ± 85.2 [104] 41–354 |
Normal (n = 23) |
18.8 ± 8.6 [19.6] 5.8–34.6** |
1.39 ± 1.02 [0.96] 0.005–3.45 |
101.0 ± 50.7 [88] 27–233 |
|||||
| Normal vs PES + TES | P = 0.86 | P = 0.019 | P = 0.27 | Normal vs PES + TES | P = 0.64 | P = 0.20 | P = 0.79 | |||||
For females, means ± SD, median and ranges were calculated by omitting case no. 1, who is suspicious for subclinical primary hypothyroidism.
Abbreviations: ES = empty sella; PES = partial empty sella; TES = total empty sella; Cortis = cortisol. There was neither borderline nor statistical significant difference when comparing cortisol, TSH or IGF-1 in the PES group with the corresponding analyte in the TES group. Cortisol, TSH or IGF-1 are typed bold face when subnormal.
In the intergender comparison, significant was only one difference in the normal group and regarded cortisol (14.5 ± 5.5 vs 18.8 ± 8.6 µg/dl, P = 0.017 by ANOVA).
The only patient, a 48-yr-old man, with increased volume of sella turcica had a single deficiency (IGF-1, 24 ng/ml [data not shown]; reference range for age 46–50: 94–252 ng/ml). IGF-1 deficiency prevailed over the other two deficiencies, since it was observed in one-third of patients regardless of gender (Table 4). This frequency was similar in patients with partial empty sella and patients with normal sella. However, within empty sella, IGF-1 deficiency prevailed in patients with partial empty sella compared to those with total empty sella (40% vs 18%, P = 0.044), the difference being accounted for by males (41.7% PES vs 14.3% TES, P = 0.33) (Table 4). TSH deficiency was the rarest (7.7% of the 104 patients), particularly in males compared to females (1.6% vs 16.7%, P = 0.007; OR = 0.02 [0.01–0.7]); this sex-dimorphism was observed both in the normal group and empty sella group (Table 4). Of note, there was no instance of TSH deficiency in the partial empty sella group. Depending on threshold used, cortisol deficiency occurred in approximately one-fourth or one-eighth of the 104 patients. Using the more stringent threshold of serum cortisol <7.0 µg/dl, cortisol deficiency was slightly more frequent in males than in females, particularly in the empty sella group (26.3% vs 10.5%). The highest frequency was observed in males with partial empty sella (33.3%, but 41.7% using the threshold of serum cortisol ≤11.0 µg/dl) (Table 4).
Consistently, occurrence of any one deficiency was the most frequent pattern, while occurrence of all three deficiencies was the rarest (Table 5). Within the empty sella group, two or all three deficiencies were observed in the total empty sella group solely, using the threshold of serum cortisol at <7.0 µg/dl, or most frequently, using the threshold of serum cortisol at ≤11.0 µg/dl (Table 5). Using the threshold of serum cortisol at <7.0 µg/dl, two or three deficiencies were observed more frequently in the empty sella group compared to the normal sella group, and this difference was accounted for by females. Small numbers did not permit to reach statistical significance (Table 5).
Within the empty sella group, low serum cortisol ranged 1.9–6.4 or to 8.2 µg/dl (depending on threshold) in males, and 4.8–5.9 or to 9.6 µg/dl in females (Table 6). Within the normal sella group, low serum cortisol ranged 3.1–6.5 or to 10.7 µg/dl (depending on threshold) in males, and 5.8–6.6 or to 9.6 µg/dl (depending on threshold) in females (data not shown). However, cortisol levels did not change significantly according to neuroradiology. In the intergender comparison, statistically significant was only one difference and solely in the normal group, viz. serum cortisol levels were lower in males compared to females (14.5 ± 5.5 [median 13.9] vs 18.8 ± 8.6 [median 19.6] µg/dl, P = 0.017).
In all patients serum FT4 was within the reference range (see 2.3 Hormonal assessment). Within the empty sella group (Table 6), TSH deficiency was marked in all three women (0.02–0.06 mU/L), as compared with the minimal fall of serum TSH in the single male patient with such deficiency (0.285 mU/L). In the other patients with TSH deficiency (4 women with normal sella), TSH ranged 0.05–0.28 mU/L (data not shown). In males, but not in females, serum TSH was significantly lower in patients with empty sella compared to those with normal sella (1.05 ± 0.53 [median 1.0] vs. 1.63 ± 0.96 mU/L [median 1.39], P = 0.019) (Table 6).
Within the empty sella group, the range of low IGF-1 levels was 52–90 ng/ml in males, and 24–89 in females (Table 6). Within the normal sella group, the range of low IGF-1 levels was 40.7–109 ng/ml in males, and 26.9–101 in females (data not shown).
As shown in Table 7, there was a pattern in the empty sella group. Indeed, patients with normal hormone levels were older compared to those with at least one hormone deficiency, regardless of the threshold used for cortisol deficiency. The gap in age was striking in males, as those with normal hormone levels were approximately 20 years older than males with at least one deficiency (75.7 vs. 55.6 years); the equivalent gap in females was 2- to 3-fold lower or approximately 9–7 years, based on the threshold of cortisol used (73 or 74.9 vs. 66.1 years) (Table 7).
Table 7.
Age (years) of patients according to hormonal status and neuroradiology.
| Neuroradiology of sella turcica |
||||
|---|---|---|---|---|
| Normal | Empty | Statistics, Empty vs Normal |
||
| Normal (cortisol normal at ≥7.1 µg/dl) | ||||
| Males | 54.1 ± 25.2 [64], n = 26 | 75.7 ± 17.4 [81], n = 9 | P = 0.023 | |
| Females | 66.7 ± 19.6 [72.5], n = 14 | 73.0 ± 12.0 [73], n = 11 | P = 0.56 | |
| Statistics, M vs F | P = 0.14 | P = 0.40 | ||
| Normal (cortisol normal at ≥11.1 µg/dl) | ||||
| Males | 54.8 ± 25.8 [64], n = 22 | 75.7 ± 17.4 [81], n = 9 | P = 0.036 | |
| Females | 70.1 ± 15.3 [73], n = 13 | 74.9 ± 11.9 [73], n = 9 | P = 0.55 | |
| Statistics, M vs F | P = 0.11 | P = 0.56 | ||
| At least one deficiency (cortisol def. at <7 µg/dl) | ||||
| Males | 54.3 ± 21.7 [52.5], n = 16 | 55.6 ± 18.9 [52.5], n = 10 | P = 0.87 | |
| Females | 66.1 ± 22.6 [73], n = 9 | 66.1 ± 15.9 [68.5], n = 8 | P = 0.97 | |
| Statistics | , M vs F | P = 0.22 | P = 0.25 | |
| , vs Normal (M) | P = 0.86 | P = 0.022 | ||
| , vs Normal (F) | P = 0.93 | P = 0.41 | ||
| At least one deficiency (cortisol def. at ≤ 11 µg/dl) | ||||
| Males | 53.5 ± 21.7 [52.5], n = 20 | 55.6 ± 18.9 [52.5], n = 10 | P = 0.77 | |
| Females | 61.7 ± 21.5 [64], n = 10 | 65.8 ± 14.6 [68.5], n = 10 | P = 0. 85 | |
| Statistics | , M vs F | P = 0.36 | P = 0.23 | |
| , vs Normal (M) | P = 0.98 | P = 0.022 | ||
| , vs Normal (F) | P = 0.81 | P = 0.23 | ||
Statistically significant values are shown in bold.
Discussion
Secondary empty sella may be caused by pituitary adenomas undergoing spontaneous necrosis (ischemia or hemorrhage), by infective, autoimmune, and traumatic causes, or by radiotherapy, drugs, and surgery [17]. Studies with evaluation performed in the early phase of TBI are detailed in Table 8 [12], [18], [19], [20], [21], [22], [23], [24]. The frequency of empty sella depends on the setting. For instance, in the general population, it has been reported to range from 8 to 38% using MRI [17], [25]. Rates of GH/IGF-I deficiency, ACTH/cortisol deficiency or FT4 deficiency were 12.5%, 62.5% or 50% in 16 patients with empty sella on MRI. Concerning gonadotropin/testosterone deficiency, which we did not evaluate, its rate was 18.7% [26].
Table 8.
Studies with evaluation performed in the early phase of Traumatic Brain Injury.
| Ref | Patients | Methods | Pertinent results |
|---|---|---|---|
| 18 | 48 consecutive patients aged 15–70 years, with severe TBI, (GCS score ≤ 8), who were referred within 24 h after head trauma. | Blood sampled at day 1 and day 4 after TBI twice per day: in the morning (8–10 am) and in the evening (5–7 pm). Cortisol, GH, IGF-1, PRL, TSH, FT3, FT4, FSH, LH, testosterone, SHBG (only in men) were measured in the morning, whereas cortisol and GH were also measured in the evening. | Day 1- Low cortisol (<10 µg/dl) in 54.5% of patients sampled in the morning and in 52.3% of patients sampled in the evening. Very low cortisol (<3.6 µg/dl) in 18.6% and 15.9%, respectively. Low TSH (<0.27 mU/L) in 4.5%; low FT4 (<12 pmol/L or < 0.93 ng/dl) in 5.5%. Low age-related IGF-1 values in 30.2%. Day 4- Low cortisol in 70.5% of patients sampled in the morning and in 59.1% of patients sampled in the evening. Very low cortisol in 22.7% and 24.4%, respectively. Low TSH in 15.9% of patients; low FT4 27.3% of patients; Low age-related IGF-1 values in 2.3% of patients. |
| 19 | 50 consecutive patients admitted with severe or moderate TBI (initial GCS score 3–13) | Stimulation test for cortisol and GH, baseline thyroid function, PRL, IGF-1, gonadotrophins, testosterone or estradiol and glucagon evaluated at a median of 12 days (range 7–20) following TBI. | Low baseline cortisol (<50 nmol/L or 1.8 μg/dl) in 40% of patients; low cortisol peak after glucagon challenge (<450 nmol/L or 16.3 μg/dl) in 16% of patients. Low GH (peak after glucagon challenge <5 ng/ml) in 18% of patients. No statistical difference in plasma IGF-1 levels between the GH-sufficient and GH-deficient subjects. TSH deficiency (<0.5 mU/L) in 2% of patients. |
| 20 | 81 subjects with primary ES (70 females, 11 males; mean age 49.9 ± 14.5 years) | All patients with TES (n = 34) and PES (n = 47) underwent endocrinological evaluation. This consisted of measurement of fasting morning GH, IGF-1, FSH, LH, 17β estradiol (females), total testosterone (males), cortisol, ACTH, TSH, FT3 and FT4. |
TES vs. PES:
|
| 21 | 58 children and adolescents (21 females, 37 males median age 11.3 years) evaluated after a TBI (GCS range 3–12) | Measurement of TSH, FT4, IGF-1, PRL, morning cortisol, FSH, LH, and testosterone (in boys) or estradiol (in girls) in the early post-traumatic period (2–14 days, T0), at 3 months (T3), 6 months (T6) and 12 months (T12) | At T0, 45% of patients had central hypothyroidism. At T3, 3% of patients showed combined pituitary hormone deficiency. At T6, only one patient (2%) was diagnosed with GH deficiency. At T12, two girls and one boy (5%) had GH deficiency. The boy had also central hypothyroidism. ES was detected in two boys with GH deficiency. |
| 12 | 89 patients (23 females, 66 males; mean age 36 years) evaluated after a TBI (GCS range 3–14) | TSH and FT4 in the acute stage of TBI; cortisol, TSH, FT4, IGF-1, and testosterone and SHBG (in men) at 3–6 months after TBI; morning cortisol, TSH, FT4 and stimulation tests (ACTH, arginine, glucagon) in case of clinical suspicion at one year after TBI | ES was more frequent in patients with major deficits compared to those without (78% vs. 20%) Acute phase: 53% of patients with ≥1 deficiency of one axis (37% GH axis, 10% ACTH axis, 3% TSH axis). At 3 and 6 months, recovery of normal function was documented in 55% and 37%, respectively. At 12 months 19 patients (21%) still had major hormonal deficiencies (63% GH axis, 0% TSH axis, 0% ACTH axis). |
| 22 | 56 consecutive patients (44 females, 12 males, aged 18–45 years) with diagnosis of head trauma | All patients were sampled for total and free T3, total and free T4, TSH, ACTH, cortisol, DHEA, DHEAS, GH, IGF-1, PRL, LH, FSH and testosterone (in males) or estradiol (females) in the early post-traumatic phase (0–10 days), at 6 and 12 months. Stimulation tests (ACTH test, insulin tolerance test), performed only in selected cases. | Hormonal dysfunction seen in 39 patients (70%) in the early phase. One, two and three axis dysfunction was reported in 26, 8 and 5 patients, respectively. Central hypothyroidism, hypocortisolism and GH deficiency was seen in 16 (29%), 7 (13%) and 11 (20%) patients, respectively. Pituitary deficiencies persisted in 7 (13%) and 8 (15%) patients at 6 and 12 months post TBI. |
| 23 | 63 patients (11 females, 52 males, 37.5 ± 17.0 years) with severe head injury (GCS < 8) | Measurement of serum TSH, FT4, ACTH, cortisol, GH, IGF-1, LH, FSH, and testosterone (in men) on admission, at the intensive care unit, and subsequently for 10 years. | Overall, hypopituitarism was diagnosed in 68% of patients, but in 38% in the early phase (<1 year post TBI). GH deficiency, central hypothyroidism and secondary adrenal failure were found in 51%, 22% and 9%, respectively. No correlation between hypopituitarism and clinical parameters on admission or at intensive care unit. |
| 24 | 163 patients admitted to neurorehabilitation, of whom 111 after severe TBI (28 females, 83 males) | Measurement of FSH, LH, testosterone (in men), estrogen (women), TSH, and ACTH stimulation test at admission and at 1-year follow-up | Central hypothyroidism diagnosed in 9% of patients. |
Abbreviations: ES = empty sella; GCS = Glasgow Coma Scale; TBI = traumatic brain injury; PES = partial empty sella; TES = total empty sella; SHBG = sex hormone-binding globulin; PRL = prolactin. GH = growth hormone; IGF-1 = insulin-like growth factor 1; TSH = thyrotropin; FT3 = free triiodothyronine; FT4 = free thyroxine; FSH = follicle stimulating hormone; LH = luteinizing hormone; ACTH = adrenocorticotropic hormone; DHEA = dehydroepiandrosterone; DHEAS = Dehydroepiandrosterone sulfate.
Only data on hypothalamus-pituitaryadrenal/thyroid axis and GH-IGF-1 axis are reported.
In patients with total and partial primary empty sella, these hormone abnormalities have been reported: 59% and 8% (GH/IGF-I deficiency), 47% and 4% (TSH/FT4 deficiency), 15% and 4% (ACTH/cortisol deficiency), 55.9% and 10.6%% (gonadotropin/testosterone or estrogen deficiency) [20].
The somatotrophic cells are supplied by the long portal vessels, located in the wings of the pituitary gland, and are exquisitely sensitive to damage. GH deficiency is thought to be the most common endocrine disturbance occurring after three months from TBI and is the one most likely to recover spontaneously [27].
There are limitations in our study. These include the relatively small cohort of patients evaluated and the lack of a comprehensive endocrine evaluation consisting of both baseline and dynamic tests. Exclusion criteria contributed to the relatively small size of our cohort, but, importantly, they minimized the possibility that empty sella antedated the occurrence of TBI. However, the aim of the study was not to perform an exhaustive endocrine evaluation of all axes.
Data presented in this study form an argument to identify patients discharged from the Emergency Unit who are worthy of being followed up because of post-traumatic empty sella, and therefore prone to develop hypopituitarism. Larger studies with a long follow-up are needed to confirm the gender differences we have described.
References
- 1.Benvenga S., Campenní A., Ruggeri R.M., Trimarchi F. Clinical review 113: hypopituitarism secondary to head trauma. J Clin Endocrinol Metab. 2000;85:1353–1361. doi: 10.1210/jcem.85.4.6506. [DOI] [PubMed] [Google Scholar]
- 2.Benvenga S. Brain injury and hypopituitarism: the historical background. Pituitary. 2005;8:193–195. doi: 10.1007/s11102-006-6040-6. [DOI] [PubMed] [Google Scholar]
- 3.Bondanelli M., Ambrosio M.R., Zatelli M.C., De Marinis L., degli Uberti E.C. Hypopituitarism after traumatic brain injury. Eur J Endocrinol. 2005;152:679–691. doi: 10.1530/eje.1.01895. [DOI] [PubMed] [Google Scholar]
- 4.Schneider H.J., Kreitschmann-Andermahr I., Ghigo E., Stalla G.K., Agha A. Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: a systematic review. JAMA. 2007;298:1429–1438. doi: 10.1001/jama.298.12.1429. [DOI] [PubMed] [Google Scholar]
- 5.Dusick J.R., Wang C., Cohan P., Swerdloff R., Kelly D.F. Pathophysiology of hypopituitarism in the setting of brain injury. Pituitary. 2012;15:2–9. doi: 10.1007/s11102-008-0130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karamouzis I., Pagano L., Prodam F., Mele C., Zavattaro M., Busti A. Clinical and diagnostic approach to patients with hypopituitarism due to traumatic brain injury (TBI), subarachnoid hemorrhage (SAH), and ischemic stroke (IS) Endocrine. 2016;52:441–450. doi: 10.1007/s12020-015-0796-2. [DOI] [PubMed] [Google Scholar]
- 7.Tanriverdi F., Schneider H.J., Aimaretti G., Masel B.E., Casanueva F.F., Kelestimur F. Pituitary dysfunction after traumatic brain injury: a clinical and pathophysiological approach. Endocr Rev. 2015;36:305–342. doi: 10.1210/er.2014-1065. [DOI] [PubMed] [Google Scholar]
- 8.Klose M., Feldt-Rasmussen U. Chronic endocrine consequences of traumatic brain injury - what is the evidence? Nat RevEndocrinol. 2018;14:57–62. doi: 10.1038/nrendo.2017.103. [DOI] [PubMed] [Google Scholar]
- 9.Leal-Cerro A., Rincón M.D. Domingo MP; Grupo de Trabajo de Disfunción Neuroendocrina y agresión Cerebral de la Sociedad Española de Endocrinología y Nutrición (SEEN) Neuroendocrine dysfunction and brain damage. A consensus statementEndocrinol Nutr. 2009;56:293–302. doi: 10.1016/S1575-0922(09)71944-8. [DOI] [PubMed] [Google Scholar]
- 10.Ghigo E., Masel B., Aimaretti G., Léon-Carrión J., Casanueva F.F., Dominguez-Morales M.R. Consensus guidelines on screening for hypopituitarism following traumatic brain injury. Brain Inj. 2005;19:711–724. doi: 10.1080/02699050400025315. [DOI] [PubMed] [Google Scholar]
- 11.Tan C.L., Alavi S.A., Baldeweg S.E., Belli A., Carson A., Feeney C. The screening and management of pituitary dysfunction following traumatic brain injury in adults: British Neurotrauma Group guidance. J Neurol Neurosurg Psychiatry. 2017;88:971–981. doi: 10.1136/jnnp-2016-315500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krahulik D., Zapletalova J., Frysak Z., Vaverka M. Dysfunction of hypothalamic-hypophysial axis after traumatic brain injury in adults. J Neurosurg. 2010;113:581–584. doi: 10.3171/2009.10.JNS09930. [DOI] [PubMed] [Google Scholar]
- 13.Renner C., Hummelsheim H., Kopczak A., Steube D., Schneider H.J., Schneider M. The influence of gender on the injury severity, course and outcome of traumatic brain injury. Brain Inj. 2012;26:1360–1371. doi: 10.3109/02699052.2012.667592. [DOI] [PubMed] [Google Scholar]
- 14.Rabelink N.M., Peeters G.M., van Schoor N.M., Drent M.L., Lips P. Self-reported loss of consciousness after head trauma does not predispose to hypopituitarism in an older population. J Head Trauma Rehabil. 2011;26:90–97. doi: 10.1097/HTR.0b013e31820367b3. [DOI] [PubMed] [Google Scholar]
- 15.Kozlowski Moreau O., Yollin E., Merlen E., Daveluy W., Rousseaux M. Lasting pituitary hormone deficiency after traumatic brain injury. J Neurotrauma. 2012;29:81–89. doi: 10.1089/neu.2011.2048. [DOI] [PubMed] [Google Scholar]
- 16.Srinivasan L., Roberts B., Bushnik T., Englander J., Spain D.A., Steinberg G.K. The impact of hypopituitarism on function and performance in subjects with recent history of traumatic brain injury and aneurysmal subarachnoid haemorrhage. Brain Inj. 2009;23:639–648. doi: 10.1080/02699050902970778. [DOI] [PubMed] [Google Scholar]
- 17.De Marinis L., Bonadonna S., Bianchi A., Maira G., Giustina A. Primary empty sella. J Clin Endocrinol Metab. 2005;90:5471–5477. doi: 10.1210/jc.2005-0288. [DOI] [PubMed] [Google Scholar]
- 18.Olivecrona Z., Dahlqvist P., Koskinen L.O. Acute neuro-endocrine profile and prediction of outcome after severe brain injury. Scand J Trauma Resusc Emerg Med. 2013;21:33. doi: 10.1186/1757-7241-21-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agha A., Rogers B., Mylotte D., Taleb F., Tormey W., Phillips J. Neuroendocrine dysfunction in the acute phase of traumatic brain injury. Clin Endocrinol (Oxf) 2004;60:584–591. doi: 10.1111/j.1365-2265.2004.02023.x. [DOI] [PubMed] [Google Scholar]
- 20.Zuhur S.S., Kuzu I., Ozturk F.Y., Uysal E., Altuntas Y. Anterior pituitary hormone deficiency in subjects with total and partial primary empty sella: do all cases need endocrinological evaluation? Turk Neurosurg. 2014;24:374–379. doi: 10.5137/1019-5149.JTN.8671-13.0. [DOI] [PubMed] [Google Scholar]
- 21.Krahulik D., Aleksijevic D., Smolka V., Klaskova E., Venhacova P., Vaverka M. Prospective study of hypothalamo-hypophyseal dysfunction in children and adolescents following traumatic brain injury. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2017;161:80–85. doi: 10.5507/bp.2016.047. [DOI] [PubMed] [Google Scholar]
- 22.Hari Kumar K.V., Swamy M.N., Khan M.A. Prevalence of hypothalamo pituitary dysfunction in patients of traumatic brain injury. Indian J Endocrinol Metab. 2016;20:772–778. doi: 10.4103/2230-8210.192917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nemes O., Kovacs N., Szujo S., Bodis B., Bajnok L., Buki A. Can early clinical parameters predict post-traumatic pituitary dysfunction in severe traumatic brain injury? Acta Neurochir (Wien) 2016;158:2347–2353. doi: 10.1007/s00701-016-2995-x. [DOI] [PubMed] [Google Scholar]
- 24.Marina D., Klose M., Nordenbo A., Liebach A., Feldt-Rasmussen U. Early endocrine alterations reflect prolonged stress and relate to 1-year functional outcome in patients with severe brain injury. Eur J Endocrinol. 2015;172:813–822. doi: 10.1530/EJE-14-1152. [DOI] [PubMed] [Google Scholar]
- 25.Foresti M., Guidali A., Susanna P. Primary empty sella. Incidence in 500 asymptomatic subjects examined with magnetic resonance. Radiol Med. 1991;81:803–807. [PubMed] [Google Scholar]
- 26.Rani P.R., Maheshwari R., Reddy T.S., Prasad N.R., Reddy P.A. Study of prevalence of endocrine abnormalities in primary empty sella. Indian J Endocrinol Metab. 2013;17:S125–S126. doi: 10.4103/2230-8210.119527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scranton R.A., Baskin D.S. Impaired pituitary axes following traumatic brain injury. J Clin Med. 2015;4:1463–1479. doi: 10.3390/jcm4071463. [DOI] [PMC free article] [PubMed] [Google Scholar]