Abstract
Salmonella osteomyelitis is known to occur in immunocompromised and sickle cell disease patients. It rarely occurs in other hosts. We present a case of chronic femoral osteomyelitis due to S. enterica serovar Typhi seen in a Maryland resident. Potential risk factors included traveling to an endemic area as well as a newly diagnosed sickle cell trait and thalassemia trait. It is postulated that less severe hemoglobinopathies may also contribute to an elevated risk of Salmonella osteomyelitis.
Keywords: Salmonella, Typhoid fever, Osteomyelitis, Sickle cell, Thalassemia, Hemoglobinopathy
Case report
A 26-year-old man without any past medical history presented with a sudden onset of severe right leg pain and six days of subjective fever. Though he was an avid soccer player, the patient denied any antecedent trauma and was able to bear weight on both legs despite the pain. He denied rash, nausea, vomiting, diarrhea, cough, hemoptysis, shortness of breath, and night sweats. He had a single, long-term female sexual partner and used barrier contraceptives intermittently. Having denied any known sexually transmitted infections, his medical history was pertinent only for treated latent tuberculosis prior to his emigration from Ghana 15 years before. Since then the patient had traveled back to Ghana one year and Mexico five months prior. He did not recall any gastrointestinal illness during travel or upon return. Vaccination history was not known. Family history included multiple first-degree relatives who died from sickle cell disease prior to his birth.
Upon presentation, the patient was febrile with maximal temperature 40.6 °C, heart rate 95, respiratory rate 20 bpm, and blood pressure 132/82 mmHg. Physical examination was significant for marked tenderness on deep palpation over the right middle to distal anterior thigh. There was no outward signs of infection such as joint swelling, erythema, warmth, open wounds, induration, rash or decreased range of motion of the hips or knees. The patient was mildly leukopenic (WBC 4.5 K/mcl, normal 4.8–10.9), anemic (hemoglobin 11.9 g/dL, normal 13.5–17.5; hematocrit 34.3%, normal 41.0–53.0), with a normal platelet count. MCV was 77.6 fL/cell. He had elevated transaminase levels (AST 76 u/L and ALT 124 u/L), and mildly elevated C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) of 13.40 mg/L and 58 mm/hour, respectively. An X-ray of the right lower extremity demonstrated a lucent lesion of the central mid to distal medullary space of the femur (Fig. 1), suspicious for an infarct or chronic osteomyelitis. MRI revealed a 24 cm cystic lesion centered in the diaphysis spanning down to the distal metaphysis, with internal complex fluid levels and thickened cortex surrounding the collection (Fig. 2), concerning for chronic osteomyelitis with abscess formation.
Fig. 1.
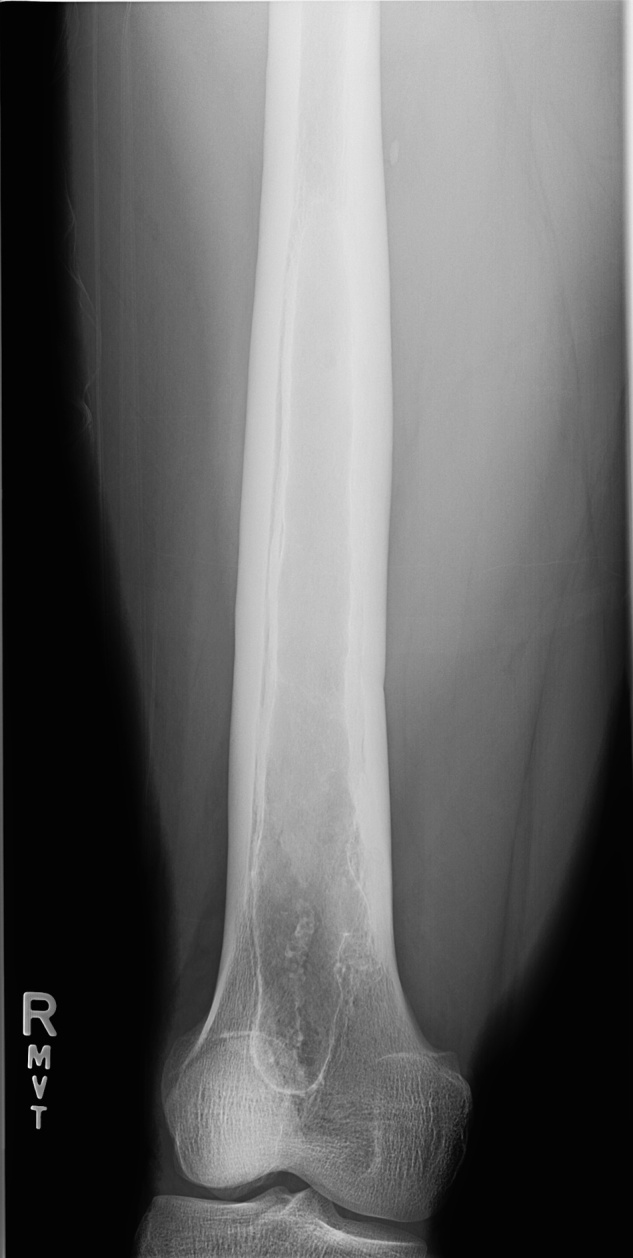
Plain radiograph showing lucent lesion of the central mid-to-distal right femoral medullary space.
Fig. 2.
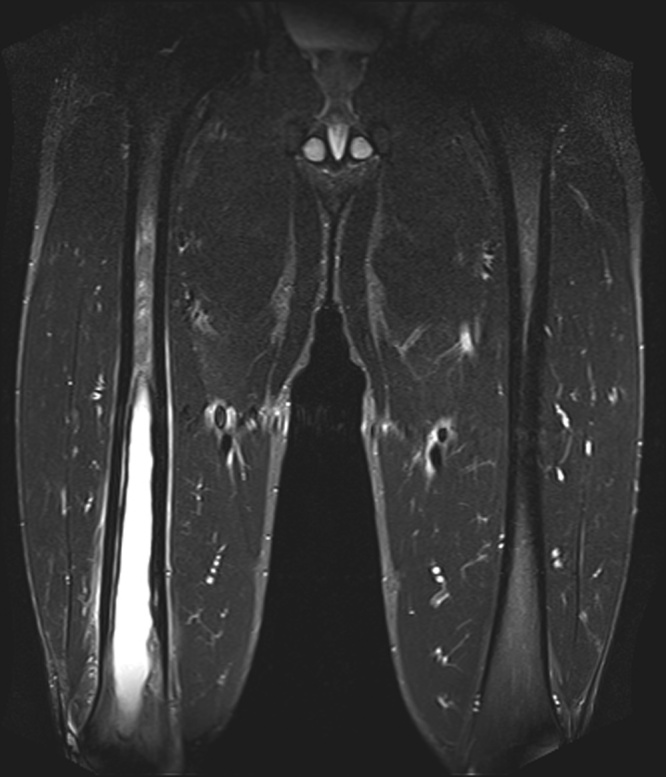
MRI (Short-TI Inversion Recovery, STIR) without contrast showing a 24-cm cystic lesion of the right femur with surrounding bony sclerosis and periosteal edema consistent with atypical infection.
Aspiration of the femur on hospital day 2 revealed serosanguinous fluid, with Gram-negative rods visualized on Gram stain. The patient underwent an open biopsy and debridement on day 4; intraoperative findings included a relative decrease of marrow contents and a cystic cavity without purulence. Pathology showed acute on chronic osteomyelitis. Aspirate and open bone cultures grew Salmonella enterica, serovar Typhi, susceptible to levofloxacin, ceftriaxone, trimethoprim/sulfamethoxazole, and ampicillin/sulbactam. Blood cultures remained negative, as were AFB stains of the fluid, bone cultures, N. gonorrhea urine NAAT and HIV Ag/Ab.
Based on country of origin, family history of sickle cell disease, and Salmonella osteomyelitis, hemoglobin (Hb) electrophoresis was pursued. Results showed 62.8% HbA, 33.2% HbS, 3.7% HbA2 and 0.3% HbF, consistent with both sickle cell trait and likely thalassemia trait (due to HbS level <35%). Abdominal ultrasound showed normal splenic echotexture and dimensions (11 × 5 x 5.5 cm).
The patient received one week of ceftriaxone intravenously while inpatient, followed by ciprofloxacin 750 mg orally twice daily for a planned total 6-week course for Salmonella typhi chronic osteomyelitis. On day 10, CRP had decreased to 5.7 mg/L but ESR had increased to 102 mm/hr. Duration of antimicrobial course was extended to allow for follow up CT imaging, but this was not completed. Patient received approximately 8 weeks of antimicrobials in total. At last known follow up, patient had achieved clinical resolution of all prior symptoms. Given extent of involvement seen on initial imaging and surgery, further surgical intervention was suggested, but not pursued by patient due to lack of health insurance. Radiograph did confirm a healing bone lesion.
Discussion
We describe here a case of femoral Salmonella osteomyelitis in an otherwise healthy young adult with exposure to two endemic areas, who presented with high fever and severe leg pain. The patient was found to be a carrier of sickle cell trait and thalassemia trait.
Salmonella enterica is clinically divided into typhoidal strains (serovar Typhi and Paratyphi) which cause typhoid or enteric fever, and nontyphoidal strains which most commonly cause gastroenteritis. Bacteremia is universal in enteric fever, but can also complicate nontyphoidal gastroenteritis, and rarely leads to osteomyelitis. Salmonella is the etiologic agent in less than 1% of osteomyelitis, but accounts for 5–64% of cases in patients with sickling hemoglobinopathies, with rates differing by region [1]. This and other manifestations of severe or disseminated disease are predominantly seen in patients with immunosuppression, biliary and urinary tract abnormalities, co-infections, and hemoglobinopathies, most notably sickle cell disease [[1], [2], [3], [4]]. In one series of Salmonella osteomyelitis, 60 patients were noted to have hemoglobinopathies; 2 had sickle-thalassemia and 1 had sickle cell trait [5]. Additional case reports of patients with Salmonella osteomyelitis with thalassemia [6,7], thalassemia trait [8,9], sickle-thalassemia [[10], [11], [12], [13]], or sickle cell trait [14] have been reported.
Salmonella osteomyelitis is a known complication in SCD, thought to be caused by a combination of factors. Sickling events may lead to infarct of both gut and bone, creating a permissive environment for both bacterial entry from the gut to the blood stream, followed by seeding of damaged bone by blood borne bacteria. Immune compromise due to dysfunction of liver and spleen and abnormal complement function may suppress clearance from the blood stream [14,15].
In contrast, patients with sickle cell trait or thalassemia trait are not thought to be routinely subject to sickling events that lead to this permissive environment for Salmonella. However, extensive research of military recruits during basic training demonstrates higher rates of sudden cardiac death in recruits with sickle cell trait, attributable to exertional heat illness (EHI) [16,17]. Intense sustained exercise leads to tissue hypoxia, acidosis, red blood cell dehydration, and elevated temperature, all of which may lead to vaso-occlusion in patients possessing Hb-S. Therefore patients with sickle cell trait, while often asymptomatic, may develop sub-clinical sickling events under conditions of intense exercise [18,19]. The patient presented here played recreational soccer, likely contributing to subclinical sickling events in his gut and bones, increasing the risk of development of Salmonella osteomyelitis.
An estimated 1–4% of patients with untreated enteric fever develop chronic carriage, defined as persistence of Salmonella in stool of urine for periods greater than one year, but one quarter of these patients do not report a history of enteric fever [20]. Approximately 470 people in the US annually are diagnosed with enteric fever (data from 2008 to 2012), 87% of whom were foreign borne US residents visiting friends and family in endemic areas. Approximately 8% of this group had received typhoid vaccination within 5 years [21]. Our patient emigrated from and traveled to Ghana, a country with an incidence of 100–1199.9 cases per 100,000 people years [22]. While the patient did not report recent gastrointestinal illness, one quarter infected with Salmonella enterica serovar Typhi do not. The patient or any contacts with similar travel history may have been had asymptomatic chronic carriage of the bacteria as well.
Conclusion
Although Salmonella osteomyelitis is rare in industrialized countries and in patients without known sickle cell disease, it remains an essential consideration in patients with who have traveled to endemic regions. Given possibility of sickling events in patients with milder and potentially undiagnosed hemoglobinopathies, patients presenting with Salmonella osteomyelitis may benefit from evaluation for hemoglobinopathy. This case also highlights the importance of vaccination in the travelers ‘visiting friends and family’.
Funding sources
None.
Conflicts of interest
The authors have no relevant conflicts of interest to report.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Authorship statement
All authors had a role in writing and revising this case report. All authors reviewed and approved final manuscript prior to submission. Individual CRediT roles are noted below.
Stephanie Stephanie:
Conceptualization of Case Report focus
Roles/Writing – original draft
Writing – review and editing
Sarah Schmalzle:
Conceptualization of Case Report focus
Writing – review and editing
Supervision
Contributor Information
Stephanie Stephanie, Email: StephaniePStephanie@berkeley.edu.
Sarah A. Schmalzle, Email: sschmalzle@ihv.umaryland.edu.
References
- 1.Thanni L.O. Bacterial osteomyelitis in major sickling heamoglobinopathies: geographic difference in pathogen prevalence. Afr Health Sci. 2006;6(4):236–239. doi: 10.5555/afhs.2006.6.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Workman M.R., Philpott-Howard J.N., Casewell M.W., Bellingham A.J. Salmonella bacteraemia in sickle cell disease at King’s College Hospital: 1976-1991. J Hosp Infect. 1994;27(3):195–199. doi: 10.1016/0195-6701(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 3.Gunn J.S., Marshall J.M., Baker S., Dongol S., Charles R.C., Ryan E.T. Salmonella chronic carriage: epidemiology, diagnosis and gallbladder persistence. Trends Microbio. 2014;22(22):648–655. doi: 10.1016/j.tim.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pham O.H., McSorley S.J. Protective host immune responses to Salmonella infection. Future Microbiol. 2015;10:101–110. doi: 10.2217/fmb.14.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen J.I., Bartlett J.A. Ralph Corey G. Extra-intestinal manifestations of Salmonella infections. Medicine (Baltimore) 1987;66(4):349–388. doi: 10.1097/00005792-198709000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Doctor P.N., Verma M., Varaiya A., Merchant R.H. Emphysematous osteomyelitis caused by Salmonella typhi in beta thalassemia major. J Postgrad Med. 2018 doi: 10.4103/jpgm.JPGM_689_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behr M.A., McDonald J. Salmonella neck abscess in a patient with beta-thalassemia major: case report and review. Clin Infect Dis. 1996;23:404–405. doi: 10.1093/clinids/23.2.404. [DOI] [PubMed] [Google Scholar]
- 8.Farrar H., Abbey A., Patel B., Nair R. Osteomyelitis, discitis, epidural and psoas abscess secondary to Salmonella enterica in a man with diabetes mellitus and newly diagnosed α-thalassaemia trait. BMJ Case Rep. 2015 doi: 10.1136/bcr-2014-207008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rayan F., Mukundan C., Shukla D.D. A case of relapsing Salmonella osteomyelitis in a thalassaemia trait patient. J Orthopaed Traumatol. 2009;10:31–33. doi: 10.1007/s10195-008-0033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarles H.E. Osteomyelitis complication a sickle-cell thalassemia syndrome. Tex Rep Biol Med. 1964;22:356–366. [PubMed] [Google Scholar]
- 11.Borsotti C., Dacatra U., Del Sasso L., Giancola R. Chir Ital. 1985;37(2):219–223. [PubMed] [Google Scholar]
- 12.Weiss H., Katz S. Salmonella paravertebral abscess and cervical osteomyelitis in sickle-thalassemia disease. South Med J. 1970;63(3):339–341. doi: 10.1097/00007611-197003000-00023. [DOI] [PubMed] [Google Scholar]
- 13.Nazarli A.G., Abrakhanova Kh N. Probl Gematol Pereliv Krovi. 1976;21(1):61–63. [PubMed] [Google Scholar]
- 14.Adeyokunnu A.A., Hendrickse R.G. Salmonella osteomyelitis in childhood. Arch Dis in Childhood. 1980;55:175–184. doi: 10.1136/adc.55.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anand A.J., Glatt A.E. Salmonella osteomyelitis and arthritis in sickle cell disease. Semin Arthritis Rheum. 1994;24(3):211–221. doi: 10.1016/0049-0172(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 16.Kark J.A., Posey D.M., Schumacher H.R., Ruehle C.J. Sickle cell trait as a risk factor for sudden death in physical training. N Engl J Med. 1987;317:781–787. doi: 10.1056/NEJM198709243171301. [DOI] [PubMed] [Google Scholar]
- 17.Smith L.E., Kark J.A., Gardner J.W., Ward F.T. Unrecognized exertional heat illness as a risk factor for exercise-related sudden cardiac death among young adults. J Cardiovasc Manag. 1997;29:447A–448A. [Google Scholar]
- 18.Loosemore M., Walsh S.B., Morris E., Stewart G., Porter J.B., Montgomery H. Sudden exertional death in sickle cell trait. Br J Sports Med. 2012;46:312–314. doi: 10.1136/bjsports-2011-090521. [DOI] [PubMed] [Google Scholar]
- 19.Kark J.A., Ward F.T. Exercise and hemoglobin S. Semin Hematol. 1994;31:181–225. [PubMed] [Google Scholar]
- 20.Parry C.M., Hien T.T., Dougan G., White N.J., Farrar J.J. Typhoid fever. New England J Med Surg Collat Branches Sci. 2002;347(22):1770–1782. doi: 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- 21.Date K.A., Newton A.E., Medalla F., Blackstock A., Richardson L., McCullough A. Changing patterns in enteric fever incidence and increasing antibiotic resistance of enteric fever isolates in the United States, 2008-2012. Clin Infect Dis. 2016;63(3):322–329. doi: 10.1093/cid/ciw232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radhakrishnan A., Als D., Mintz E.D., Crump J.A., Stanaway J., Breiman R.F. Introductory article on global burden and epidemiology of typhoid fever. Am J Trop Med Hyg. 2018;99(3S):4–9. doi: 10.4269/ajtmh.18-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]


