Highlights
-
•
The individual components of the biodiesel had a lower toxicity threshold than in the complex mixture.
-
•
The B20 sample proved to be the most toxic for the red algae P. purpureum.
-
•
The B100 sample showed the highest level of toxicity for the microalgae A. ussuriensis, C. muelleri and H. akashiwo.
-
•
The sample of petroleum diesel B0 showed less toxicity compared to B20 and B100.
Keywords: Biodiesel, Ecotoxicology, Microalgae, Waste cooking oil biodiesel, Biodiesel blends, Aquatic pollution
Abstract
The world biodiesel production is increasing at a rapid rate. Despite its perceived safety for the environment, more detailed toxicity studies are mandatory, especially in the field of aquatic toxicology. While considerable attention has been paid to biodiesel combustion emissions, the toxicity of biodiesel in the aquatic environment has been poorly understood. In our study, we used an algae culture growth-inhibition test (OECD 201) for the comparison of the toxicity of B100 (pure biodiesel), produced by methanol transesterification of waste cooking oil (yellow grease), B0 (petroleum diesel fuel) and B20 (diesel-biodiesel blended of 20% biodiesel and 80% petroleum diesel fuel by volume). Two marine diatoms Attheya ussuriensis and Chaetoceros muelleri, the red algae Porphyridium purpureum and Raphidophyte Heterosigma akashiwo were employed as the aquatic test organisms. A sample of biodiesel from waste cooking oil without dilution with petroleum diesel (B100) showed the highest level of toxicity for the microalgae A. ussuriensis, C. muelleri and H. akashiwo, compared to hexane, methanol, petroleum diesel (B0) and diluted sample (B20). The acute EC50 in the growth-inhibition test (96 h exposure) of B100 for the four species was in the range of 3.75–23.95 g/L whereas the chronic toxicity EC50 (7d exposure) was in the range of 0.42–16.09 g/L.
1. Introduction
Biodiesel is a fuel composed of fatty acid alkyl esters made by transesterification of fatty acids (triglycerides) from plant oils or animal fats. Biodiesel was proposed as a “green” alternative for fossil fuel and with the intention of causing lower impact on human health and the environment by reduction of combustion derived hydrocarbons, carbon monoxide, polycyclic aromatic hydrocarbons (PAHs), and particulate matter (PM) [1]. However, concrete evidence which supports the reduced toxicity of biodiesel emissions is lacking. It is also apparent that biodiesel toxicity depends on the chemical composition and properties of the biodiesel made from diverse feedstocks which in turn can vary considerably. The impact of biodiesel on living systems is under intensive investigation using a variety of bioassays [2].
Residues of oils after cooking and from food waste, cleaning residues and animal fat wastes are a cheap source for biodiesel production and are widely used on an industrial scale. Used oils contain a high concentration of free fatty acids (FFA). According to the FFA content, used cooking oils are divided into two groups: yellow fat (FFA < 15%) and brown fat (FFA > 15% and water). Yellow fats are a common source of biodiesel and can be used after filtration and cleaning [[3], [4], [5]]. The world population growth has led to an increase in the volume of food industry wastes and by-products. The use of these wastes for biodiesel production has been encouraged as an effective utilization of an otherwise wasted resource. Thus, biofuels from waste edible oils, which can differ in the initial composition of fatty acids and, consequently, in properties and levels of toxicity often enter the market. The composition of the common fatty acids used in the production of biodiesel is described in a number of works [[6], [7], [8], [9], [10]].
Much of the work on the toxicity of biofuels has focused on comparison of hazardous properties of diesel engine emissions with biodiesel alternatives [[11], [12], [13]]. However, only limited information is available on the aquatic toxicity of biodiesel or other biofuels. Petroleum-related pollutants are a worldwide threat to aquatic organisms and humans [[14], [15], [16], [17]], so the ecotoxicity of biodiesel biofuel needs to be examined in terms of its impact on such species.
Comparisons of published studies of biodiesel water toxicity can be problematic because procedures differ for preparation of oil-in-water dispersions (OWDs) or water-accommodated fractions (WAFs). The use of biodiesels from different feedstocks as well as the employment of different test organisms must be considered. Hence, there is a lack of uniformity in the conclusions reached; some studies suggest that the toxicity of petroleum diesel exceeds that of biodiesel with differences of up to 1000 fold [[18], [19], [20]]. Therefore, clarification of the risks of biodiesel to aquatic species must be made in view of the growing popularity and environmental exposure of these fuels.
In our study, we used an algae culture growth-inhibition test for evaluation of the toxicity of a petroleum diesel (B100) and a B20 blend (20% biodiesel and 80% petroleum diesel) of biodiesel from waste cooking oil. The use of a phytoplankton for the assessment of marine pollution is well documented [21,22]. These algae are one of the primary organisms in the food chain, playing a key role in toxin biomagnification to higher predators, such as zooplankton, fish and ultimately humans [23].
2. Material and methods
2.1. Chemicals
Fuel samples were obtained from the Primorsky Krai (Russian Federation) market as B100 (pure biodiesel), produced by methanol transesterification of waste cooking oil (yellow grease), B0 (diesel fuel) and B20 (diesel-biodiesel blend of 20% biodiesel and 80% diesel fuel by volume). Hexane and methanol were used for comparison control. Potassium bichromate (K2Cr2O7) (source) was used as the positive control.
Propidium Iodide (PI), from Molecular Probes (Eugene, Oregon, USA), was used to measure cell death (or Growth-inhibition/vitality).
2.2. Microalgal cultures
Four species of seawater microalgae isolated from the Peter the Great Gulf (Sea of Japan, the Primorsky Krai, Far-Eastern Russia) were used as the test organisms. The test species included two marine diatoms Attheya ussuriensisand Chaetoceros muelleri, a red algae Porphyridium purpureum and Raphidophyte Heterosigma akashiwo and were cultured with Guillard’s f/2 medium [24]. Microalgal culture medium was provided by National Scientific Center of Marine Biology, Far Eastern Branch of the Russian Academy of Sciences (NSCMB FEB RAS).
Culturing and toxicity test conditions were according to OECD 201 standard and are presented in Table 1.
Table 1.
Culturing and toxicity test conditions.
| Parameters | Culture conditions |
|---|---|
| Temperature | 20 ± 2 °C |
| pH | 8.0 ± 0.2 |
| Salinity | 33 ± 1‰ |
| Light intensity | 300 μmol∙m−2∙s-1, Cool White Fluorescent |
| Light cycle | 12:12 h light:dark |
| Test type | Flow cytometry |
| Test duration | 24 h, 96 h, 7d |
| Test chamber | 24-well plate |
| Age of test organism | 14-20 d, exponential growth phase |
| Initial bioassay cell density | 1-5 × 103 cells mL−1 |
| Control/diluent water | 0.22 μm filtered seawater |
| Test endpoints | Growth-inhibition/vitality (Propidium Iodide) |
The suitability of the each microalgae species as a test organism for the assay was carried out with the toxicant potassium bichromate. The results of microalgae sensitivity testing are presented in Table 2. Exposure of cells was carried out in 24-well plates. Each assay was run at concentrations of K2Cr2O7 of 1, 2, 4, 8, 16, 32 mg/L. For each concentration and control group (without toxicant), the experiment was conducted in 4 replications. The volume of microalgae aliquots in each replication was 1.5 mL. The number of living cells was determined using a CytoFLEX flow cytometer (Beckman Coulter, USA) after 96 h (acute toxicity) and after 7 days (chronic toxicity). The calculation of EC50 (growth inhibition) was performed using GraphPad Prism 7.04.
Table 2.
Preliminary testing of microalgae cultures sensitivity, mg L−1.
| Species | 96-h LC50 | 7-d LC50 |
|---|---|---|
| Attheya ussuriensis | >33.13 | 7.91 (7.00–8.96) |
| Porphyridium purpureum | 1.18 (0.57–1.80) | 0.15 (5,02e-005– 0,45) |
| Chaetoceros muelleri | >42.36 | 29.66 (24.00–38.20) |
| Heterosigma akashiwo | 13.88 (12.10–15.62) | 8.94 (7.89–10.17) |
Thus, the species sensitivity trend from more sensitive to less sensitive was as follows:
P. purpureum>H. akashiwo>A. ussuriensis>C. muelleri
2.3. Liquid chromatography-mass spectrometry (LC–MS) analysis
Diesel fuel (B0), blended sample (B20), and straight biodiesel (B100) were analyzed by liquid chromatography–mass spectrometry (LC–MS).
2.4. Flow cytometry analysis
The algae-bioassay (24 h, 96 h and 7 days) was carried out to test the toxicity of the fuel samples. Twenty-four-well plates were used for cultivation of the microalgal species with the biofuel at concentrations of 1, 5, 10, 20, 40, 80, 120 g/L. As comparison controls, the same bioassays with hexane and methanol at concentrations of 5, 10, 20, 40, 80 g/L were carried out. Wells with only f/2 medium were the control group. For each concentration and control group there were four replications. The volume of microalgae aliquots in each replication was 1,5 mL.
The accurate counting of algal cells was provided at 24 h, 96 h and 7 days by flow cytometer CytoFLEX (Beckman Coulter, USA) with the software package CytExpertv.2.0. Each sample was measured with a flow rate 40 μL/min during 75 s. Cells viability was determined by staining with Propidium Iodide (PI) according to standard protocol [25].
The parameters for determination of the target group of cells were made by the determination of cells having autofluorescence for chlorophyll a (light source - laser 488 nm, emission filter - PC5.5, 690 nm) in the FSC/PC5.5 dot diagram and elimination of dead cells from this range. The cells were considered dead if they had fluorescence in PI (light source - laser 488 nm, emission filter - ECD, 610 nm) in a FSC/ECD dot diagram.
2.5. Statistical analysis
Statistical analysis was carried out using GraphPad Prism 7.04 (GraphPad Software, USA).
3. Results and discussion
The results of the LC–MS analysis for the biodiesel (B100), blended sample (B20) and diesel fuel (B0) are presented in Table 3.
Table 3.
LC–MS analysis of fuel samples.
| № | Compound | B0 Diesel |
B20 Blended sample |
B100 Biodiesel |
|---|---|---|---|---|
| 1 | Methanol | >5% | 15.0% | |
| 2 | Ethanol | >5% | 8.5% | 3.8% |
| 3 | 2-Pentene | 0.3% | – | – |
| 4 | Hexane | >15% | ||
| 5 | Hexane,3-methyl | – | – | 18.4% |
| 6 | Heptane | >70% | 68.1% | |
| 7 | Benzene,1,3-dimethyl | 0.1% | ||
| 8 | Decane | 0.1% | 0.1% | – |
| 9 | Undecane | 0.1% | 0.1% | – |
| 10 | Octanoicacid, methyl ester | 0.1% | ||
| 11 | Undecane, 2,6-dimethyl | 0.1% | – | – |
| 12 | Dodecane, 4,6-dimethyl | 0.1% | – | – |
| 13 | Tetradecane | 0.1% | 0.1% | – |
| 14 | Pentadecane | 0.1% | 0.1% | – |
| 15 | Hexadecane | 0.1% | 0.05% | – |
| 16 | Heptadecane | 0.1% | 0.1% | – |
| 17 | Metyltetradecanoate | – | 0.1% | |
| 18 | 9-Hexadecanoic acid, methyl ester | – | 0.1% | |
| 19 | Hexadecanoicacid, methyl ester | 1.0% | 12.1% | |
| 20 | 9,12-Octadecanoic acid (z,z)-, methyl ester | – | 0.1% | |
| 21 | Heptadecanoicacid, methyl ester | – | 0.1% | |
| 22 | 9-Octadecanoic acid, methyl ester | – | 2.2% | – |
| 23 | 9,12-Octadecanoic acid (z,z)-, methyl ester and 10,13-Octadecanoic acid, methyl ester | 3.7% | 15.6% | |
| 24 | 11-Octadecanoic acid, methyl ester | 0.1% | – | |
| 25 | 8-Octadecanoic acid, methyl ester | – | 30.0% | |
| 26 | 16-Octadecanoic acid, methyl ester and 9-Octadecanoic acid, methyl ester | – | 12.5% | |
| 27 | 9,12,15-Octadecatrienoic acid, methyl ester and 7,10,13-Octadecatrienoic acid, methyl ester | – | 0.5% | |
| 28 | Octadecanoicacid, methyl ester | 0.4% | 4.7% | |
| 29 | 9,11-Octadecanoic acid, methyl ester | – | 0.1% | |
| 30 | 8,11-Octadecanoic acid, methyl ester and 9,12-Octadecanoic acid, methyl ester | – | 0.2% | |
| 31 | 9,12,15-Octadecatrienoic acid, methyl ester | – | 0.1% | |
| 32 | 1,5,9,11-Tridecatetraene, 12-methyl | – | 0.1% | |
| 33 | 11-Eicosenoic acid, methyl ester | – | 0.3% | |
| 34 | Eicosanoicacid, methyl ester | – | 0.5% | |
| 35 | Docosanoicacid, methyl ester | – | 0.5% | |
| 36 | Tetracosanoicacid, methyl ester | – | 0.1% |
The diesel fuel B0 consisted of alkanes (mainly n-heptane and n-hexane) plus methanol and ethanol (with latter two alcohols representing more than 10%) as additives. An insignificant content of unsaturated hydrocarbons was observed, including 0.1% of several aromatic compounds.
The blended sample B20 also consisted of alkanes (predominantly n-heptane), alcohols (about 23.5%) and a small amount of methyl esters of fatty acids.
The main components of the biodiesel sample B100 were methyl esters of fatty acids (77.6%). The predominant components were methyl esters of hexadecanoic acid (C16: 0) and methyl esters of saturated and unsaturated octadecanoic acids (C18: 0, C18: 1, C18: 2, C18: 3). In addition, BA100 contained ethanol (3.8%) and a significant amount of volatile organic compounds, such as 3-methylhexane (18.4%).
The toxicological properties of organic substances with different molecular structures often differ significantly. The assessment of individual hydrocarbons toxicity is based on the testing of model substances and the prediction of hazardous properties for substances having a similar structure; quantitative structure-activity relationship [26]. Hydrocarbons are divided into categories of substances with common properties by the number of carbon atoms, the content of common composite elements, the content of aromatic compounds, and the presence of components with unusual toxicity [4]. Consequently, the potential toxicity of individual hydrocarbons might be evaluating by using read-across hydrocarbons in general or to similar model substances for which the required data are available. To avoid underestimating the hazardous properties of a substance, the concept of a "reasonable worst case" is usually developed.
The toxic control and scientifically based regulation of petroleum or oil-based substances is a formidable challenge. Such chemicals of multi-constituent nature are defined as UVCB (Unknown or Variable Composition, Complex Reaction Products and Biological Materials) substances. Category read-across approaches for UVCBs may not always be sufficient. Thus, the concept of a potential hazardous chemical classification should be based on an integrative framework consisting of in vitro testing and high-throughput genomics data analyses for read-across assessment [27,28].
The only aliphatic hydrocarbon having an unusual toxicity is n-hexane. N-hexane is noted as an exception due to its toxicological properties that differ from the other aliphatic hydrocarbons with a similar structure or the same number of carbon atoms, including n-pentane, n-heptane, other hexane and heptane isomers, and cyclohexane. The unusual toxic properties of n-hexane are caused by the formation of a specific intermediate metabolite 2,5-hexanedione which, on the grounds of its structure, may interfere with the formation of microtubules and spindle fibers [29,30], leading to inhibition of meiosis [31].
Thus, in the existing literature, n-hexane and solvents containing n-hexane at levels > 5% are included in a separate category C6 (hexanes). C7-C9 (aliphatics) are classified separately. Among the tested samples n-heptane (B0- 70%, B20 - 68%) and 3-methylhexane (B100 - 18.4%) can be grouped into the category C7-C9.
According to McKee et al., [26] and the HPV documentation for this category of solvents [32], the C7–C9 aliphatic hydrocarbons are not genotoxic. The biodegradation of isoheptane in water was 51.3% after 28 days, and 60.2% after 60 days [33].
The toxicity of the fatty acid methyl esters (FAMEs) is still poorly understood. According to the OECD standard, FAMEs are not dangerous substances. All FAMEs are readily biodegrated in water, soil and sediment; within 10 days, 62% of FAMEs are biodegrated. However, the data on the water toxicity of biodiesel widely vary. The existing data on the water toxicity of the main components of the test samples on the most common small planktonic crustacean Daphnia magna and on the green algae Raphidocelis subcapitata (formerly known as Pseudokirchnerella subcapitata or Selenastrum capricornutum) are presented in Table 4.
Table 4.
EC50, aquatic toxicity of the main components of tested samples, mg L−1.
| Species/Guideline | Test duration | Toxicant |
||||
|---|---|---|---|---|---|---|
| Methanol | Ethanol | n-hexane | C7-C9n-alkanes, isoalkanes | Fatty acids, C10-18 and C12-22-unsatd. alkyl esters | ||
|
Daphnia magna OECD 202 |
24-h | 20 803 [34] |
13 715 [34] |
1 000 [35] |
10 [36] |
n/a |
| 48-h | > 10 000 [37] |
> 10 000 [37] |
31.9 [35] |
3.8 [38] |
2 504 [39] |
|
| 96-h | 18 260 [40] |
n/a | n/a | n/a | n/a | |
|
Raphidocelis subcapitata OECD 201 |
24-h | n/a | n/a | n/a | n/a | n/a |
| 72-h | n/a | 12 900 [38] |
55 [35] |
10 – 30 [38] |
73 729 [39] |
|
| 96-h | 22 000 [41] | 10 000 [41] |
n/a | 13 [38] |
>1 000 [39] |
|
In our work n-hexane and methanol were used as comparison controls. For all studied substances the concentrations were determined, at which the cell growth rate was reduced by 50% (EC50) compared to the control. The EC50 values (with 95% confidence intervals) based on flow cytometry analysis and determined by GraphPad Prism 7.04 are presented in Table 5.
Table 5.
EC50 values for microalgae species exposed to tested toxicants (g L−1).
| Toxin/Exposure | 24-h EC50 | 96-h EC50 | 7-d EC50 |
|---|---|---|---|
| Attheya ussuriensis | |||
| Hexane | – | >52.92 | >57.37 |
| Methanol | – | 77.56 (55.99–132.2) | 40.29 (30–57.48) |
| B0 | >120 | 29.84 (25.19–35.14) | 12.82 (11.39–14.40) |
| B20 | >40 | 10.41 (9.40–11.44) | 0.92 (0.76–1.07) |
| B100 | 19.69 (15.88–24.22) | 9.83 (8.67–11.09) | 0.72 (0.42–1.02) |
| Porphyridium purpureum | |||
| Hexane | – | 10.54 (<19.23) | 41.71 (<64.78) |
| Methanol | – | 13.17 (11.26–15.50) | 16.53 (12.17–21.21) |
| B0 | 35.05 (24.44–50.95) | 20.44 (17.08–23.95) | 12.33 (7.99–16.09) |
| B20 | 2.81 (1.44–4.52) | 8.25 (7.51–9.05) | 5.53 (5.16–5.98) |
| B100 | 7.72 (4.66–18.82) | 9.89 (8.45–11.4) | 6.59 (6.24–6.94) |
| Chaetoceros muelleri | |||
| Hexane | – | 52.70 (46.91–58.09) | 42.79 (42.58–43.00) |
| Methanol | – | >156.2 | >108.1 |
| B0 | >120 | 51.81 (48.27–54.31) | >40 |
| B20 | 10.89 (9.99–11.84) | 6.50 (6.04–6.99) | – |
| B100 | 6.46 (5.98–7.02) | 2.53 (2.19–2.93) | – |
| Heterosigma akashiwo | |||
| Hexane | – | 30.41 (29.89–31.46) | 27.24 (22.07–32.86) |
| Methanol | – | 32.16 (31.26–33.05) | 30.65 (26.28–33.38) |
| B0 | >118.8 | 10.05 (9.01–11.14) | 5.73 (4.75–6.63) |
| B20 | 13.74 (12.55–14.99) | 17.51 (15.46–19.74) | ˜2.40 |
| B100 | 6.24 (5.67–6.84) | ˜3.75 | ˜2.24 |
According to the results of the experiments for microalgae A. ussuriensis, C. muelleri and H. akashiwo, the maximum level of toxicity was shown by a sample of pure biodiesel B100 and the diesel-biodiesel blend B20 sample for the red alga P. purpureum.
P. purpureum was the most sensitive of the tested species for potassium bichromate.
n-Hexane and methanol proved to be the most stable for B100 and B20. In addition, P. purpureum and H. akashiwo showed the ability to adapt to the presence of the tested pollutants. So, for P. purpureum, the EC50 increased for hexane and methanol following 7 days of exposure (chronic toxicity) compared to 96 h (acute toxicity), while for B20 and B100, an increase in the EC50 was observed from 24 h to 96 h. On the 7th day of the experiment the EC50 decreased. H. akashiwo during the experiment adapts only to B20, showing an increase in the EC50 from 24 h to 96 h and a sharp decrease in the EC50 by the 7th day. For the diatoms of C. muelleri and A. ussuriensis, the level of toxicity increased in direct proportion to the increase of cells exposure time. C. muelleri and H. akashiwo demonstrated the highest acute toxic effect for B100. The highest chronic toxicity was shown by A. ussuriensis for B20 and B100.
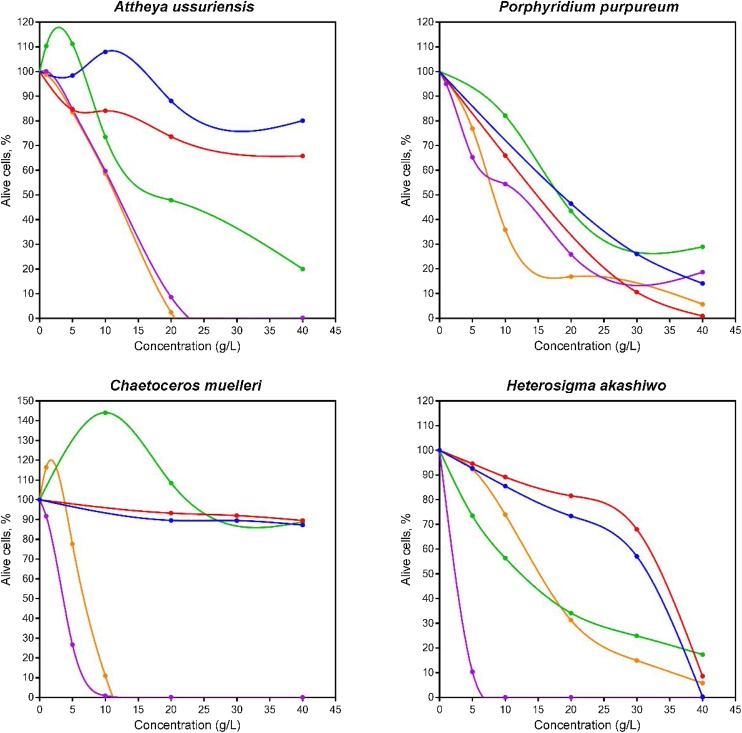
Comparison of the mortality rates of the four microalgae species under 96 h exposure to toxicants is shown in Fig. 1. The stimulating effect of low concentrations of n-hexane, B0 and B20 on the diatoms C. muelleri and A. ussuriensis is probably caused by a response to the presence of the petroleum hydrocarbons, using them as an additional source of carbon. The stimulation of microalgae growth due to low concentrations of the low molecular weight hydrocarbons is consistent with existing studies [42].
Fig. 1.
Mortality rates (mean) of four microalgae species in acute toxicity tests (96 h) of Hexane (blue), Methanol (red), Diesel fuel B0 (green), waste cooking oil biodiesel B100 (purple), diesel-biodiesel blend B20 (orange).
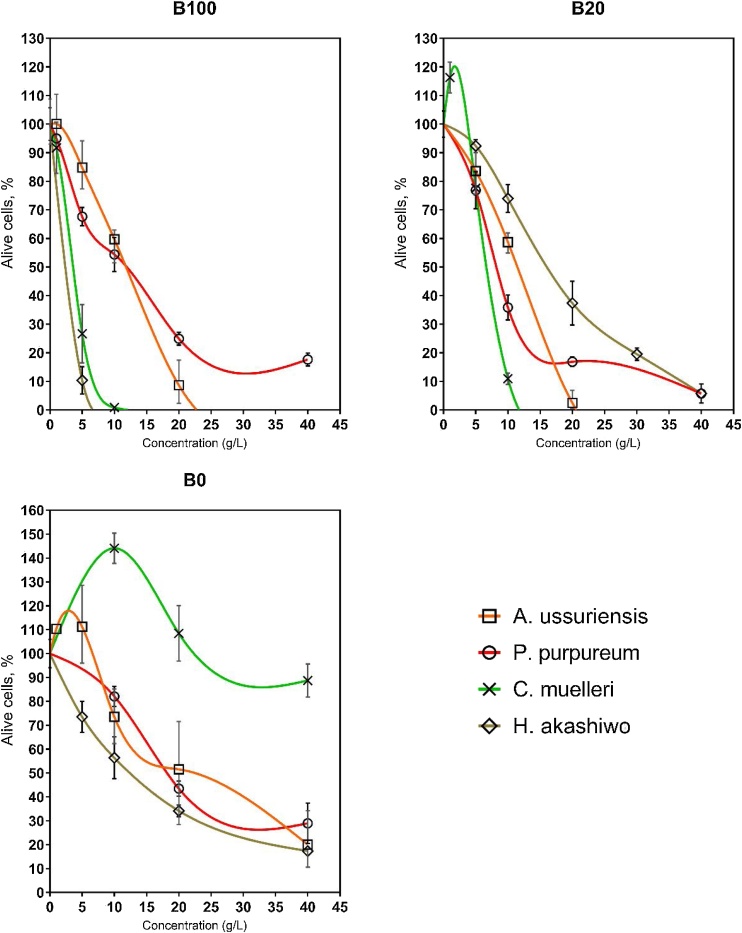
Many authors have reported biodiesel as a biodegradable and non-toxic fuel [5,43,44], but the sensitivity of these distinct species to different toxicants can widely vary, as indicated in the Fig. 2. In particular, the sensitivity and stability of the algae to specific pollutants can be different for different species [45] and for more reliable bio-testing it is preferable to use several species. It has been reported that biodiesel from used cooking oils can pose a significant danger to aquatic organisms [18].
Fig. 2.
The sensitivity of the four microalgae species to B100 and B20.
The sensitivity trend for potassium bichromate, hexane, methanol and diesel fuel B0 for the four microalgae was the same. However, the B100 biodiesel was more toxic for H. akashiwo and C. muelleri and was the least toxic for P. purpureum. The species sensitivity trend for B100 (biodiesel) was: H. akashiwo > C. muelleri > A. ussuriensis > P. purpureum, which is quite different compared to preliminary testing of microalgae sensitivity by potassium bichromate.
The toxicity level of diesel-biodiesel blend B20 is mostly similar to B100. A notable difference, however, is observed for H. akashiwo, where the microalgae cells showed a significantly greater resistance to B20 compared to pure biodiesel B100.
It is noteworthy that for all the algae, the toxicity level of samples containing biodiesel from waste cooking oil (B20 and B100) was higher than it was for hexane, methanol or diesel fuel. The results of our studies showed that the sample B20 containing 68.1% n-heptane, 15% methanol and 8.5% ethanol was significantly more toxic for all species than pure hexane, pure methanol and the sample B0 containing about 70% n-heptane, but with a fraction of methanol and ethanol (˜5%).
Several groups have reported [46,47] that when biodiesel enters the aquatic environment, the biodegradation of fuel gradually increases the concentration of methanol that is formed as a result of hydrolysis that converted the transesterification reaction. Thus, it is most likely that the high toxicity of B20 is associated with an increased content of alcohol.
Quality biodiesel consists mainly of fatty acid methyl esters, the low toxicity and biodegradability of which determines their safety o for the environment. The low toxicity of FAMEs has been shown in several studies [48,49]. However, based on our results, sample B100 consisting of 77.6% of FAMEs exhibited the greatest aquatic toxicity among all the samples tested. This was probably caused by the presence of the aliphatic iso-paraffinic constituent 3-methylhexane (18.4%) included in C7-C9 category of petroleum pollutants. These paraffins have a high aquatic ecotoxicity potential, possibility due to the increasing concentration of methanol following FAME hydrolysis.
4. Conclusions
The properties of biodiesel as a non-toxic and environmentally friendly alternative fuel may differ depending on the feedstock, the method of production and the chemical composition of the final product. Yassine, M. H., et al [50] remarked that the process of transesterification of biodiesel could be a critical factor in determining the aquatic toxicity of the fuel. At the same time, the combination of individual components with relatively low toxicity, can cause a synergistic effect and have unpredictable level of toxicity [[51], [52], [53], [54]]. Judging from the literature and our results, the individual components of the biodiesel (n-hexane, n-pentane, methanol, FAMEs) had a lower toxicity threshold than in the complex mixture.
The B100 sample showed the highest level of toxicity for the microalgae A. ussuriensis, C. muelleri and H. akashiwo in comparison with hexane, methanol, B0 and B20. The acute EC50 in the growth-inhibition test (96 h exposure) of B100 for the four species of microalgae was in the range 3.75–23.95 g/L, while the chronic toxicity EC50 (7 d exposure) ranged from 0.42 to 16.09 g/L.
B20 proved to be the most toxic for the red algae P. purpureum. The acute C50 of the diesel-biodiesel blend B20 for the four microalgae species was in the range 6.04–19.74 g/L; the chronic toxicity EC50 was from 0.76 to 5.98 g/L.
A sample of petroleum diesel B0 showed less toxicity compared to B20 and B100. The diatom microalga C. muelleri proved to be much resistant to B0. The presence of B0 in concentrations up to 20 g/L caused an increase in the rate of cell growth. The acute EC50 of B0 for the four microalgae species was in the range 9.01–54.31 g/L; the chronic toxicity EC50 was in the range of 4.75–40 g/L.
The findings of this study provide an important foundation for further investigation into the effects of such products on coastal aquatic ecosystems.
Competing interests
AT is the Editor-in-Chief for the journal, but had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article.
Transparency document
Acknowledgements
The work was funded by the Russian Science Foundation (No. 15-14-20032-P).
The authors would like to thank the staff team of FEFU CCU (Center of Collective Use) ‘Interdepartmental center for analytical control of the environment’ and the Laboratory of Toxicology, University of Crete, Greece, for their dedicated involvement in this study.
References
- 1.Murugesan A. Bio-diesel as an alternative fuel for diesel engines- a review. Renewable Sustainable Energy Rev. 2009;13(3):653–662. [Google Scholar]
- 2.Eck-Varanka B. Eco- and genotoxicity profiling of a rapeseed biodiesel using a battery of bioassays. Ecotoxicol. Environ. Saf. 2018;151:164–171. doi: 10.1016/j.ecoenv.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 3.Canakci M., Van Gerpen J. Biodiesel production from oils and fats with high free fatty acids. Trans. ASAE. 2001;44:1429–1436. [Google Scholar]
- 4.Clark C.R. A GHS-consistent approach to health hazard classification of petroleum substances, a class of UVCB substances. Regul. Toxicol. Pharmacol. 2013;67:409–420. doi: 10.1016/j.yrtph.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 5.Leung D.Y.C. Degradation of biodiesel under different storage conditions. Bioresour. Technol. 2006;97:250–256. doi: 10.1016/j.biortech.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Atabani A.E. Non-edible vegetable oils: a critical evaluation of oil extraction, fatty acid compositions, biodiesel production, characteristics, engine performance and emissions production. Renewable Sustainable Energy Rev. 2013;18:211–245. [Google Scholar]
- 7.Hoekman S.K. Review of biodiesel composition, properties, and specifications. Renewable Sustainable Energy Rev. 2012;16:143–169. [Google Scholar]
- 8.Lin L. Opportunities and challenges for biodiesel fuel. Appl. Energy. 2011;88:1020–1031. [Google Scholar]
- 9.Misra R.D., Murthy M.S. Straight vegetable oils usage in a compression ignition engine-A review. Renewable Sustainable Energy Rev. 2010;14:3005–3013. [Google Scholar]
- 10.Singh S.P., Singh D. Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: a review. Renewable Sustainable Energy Rev. 2010;14:200–216. [Google Scholar]
- 11.Chang Y.-C. Effects of waste cooking oil-based biodiesel on the toxic organic pollutant emissions from a diesel engine. Appl. Energy. 2014;113:631–638. [Google Scholar]
- 12.Kooter I.M. Toxicological characterization of diesel engine emissions using biodiesel and a closed soot filter. Atmos. Environ. 2011;45:1574–1580. [Google Scholar]
- 13.Madden M.C. A paler shade of green? The toxicology of biodiesel emissions: recent findings from studies with this alternative fuel. Biochim. Et Biophys. Acta. 2016;1860:2856–2862. doi: 10.1016/j.bbagen.2016.05.035. [DOI] [PubMed] [Google Scholar]
- 14.Bellas J. Evaluation of artificially-weathered standard fuel oil toxicity by marine invertebrate embryogenesis bioassays. Chemosphere. 2013;90:1103–1108. doi: 10.1016/j.chemosphere.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Djomo J.E. Toxic effects of some major polyaromatic hydrocarbons found in crude oil and aquatic sediments on Scenedesmus subspicatus. Water Res. 2004;38:1817–1821. doi: 10.1016/j.watres.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 16.Santander-Avancena S.S. Acute Toxicity of water-accommodated fraction and chemically enhanced WAF of bunker C oil and dispersant to a microalga Tetraselmis tetrathele. Bull. Environ. Contam. Toxicol. 2016;96:31–35. doi: 10.1007/s00128-015-1696-0. [DOI] [PubMed] [Google Scholar]
- 17.Adekunle A.S. Determination of polycyclic aromatic hydrocarbon levels of groundwater in Ife north local government area of Osun State, Nigeria. Toxicol. Rep. 2017 doi: 10.1016/j.toxrep.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan N. A comparison of acute toxicity of biodiesel, biodiesel blends, and diesel on aquatic organisms. J. Air Waste Manage. Assoc. 2007;57:286–296. doi: 10.1080/10473289.2007.10465333. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y.Y. Biological toxicities of emissions from an unmodified engine fueled with diesel and biodiesel blend. J. Environ. Sci. and Health Part A. 2008;43:1735–1743. doi: 10.1080/10934520802330438. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y.Y. Carbonyl compounds and toxicity assessments of emissions from a diesel engine running on biodiesels. J. Air Waste Manage. Assoc. 2009;59:163–171. doi: 10.3155/1047-3289.59.2.163. [DOI] [PubMed] [Google Scholar]
- 21.Franklin N.M. Development of an improved rapid enzyme inhibition bioassay with marine and freshwater microalgae using flow cytometry. Arch. Environ. Contam. Toxicol. 2001;40:469–480. doi: 10.1007/s002440010199. [DOI] [PubMed] [Google Scholar]
- 22.Metting F.B. Biodiversity and application of microalgae. J. Ind. Microbiol. Biotechnol. 1996;17:477–489. [Google Scholar]
- 23.Desai S.R. Evaluation of genotoxic responses of Chaetoceros tenuissimus and Skeletonema costatum to water accommodated fraction of petroleum hydrocarbons as biomarker of exposure (vol 44, pg 7, 2007) Water Res. 2011;45 doi: 10.1016/j.watres.2009.12.048. 1906-1906. [DOI] [PubMed] [Google Scholar]
- 24.Guillard R.R., Ryther J.H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can. J. Microbiol. 1962;8:229–239. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- 25.Ostrander G.K. CRC Press; 2005. Techniques in Aquatic Toxicology. [Google Scholar]
- 26.McKee R.H. Characterization of the toxicological hazards of hydrocarbon solvents. Crit. Rev. Toxicol. 2015;45:273–365. doi: 10.3109/10408444.2015.1016216. [DOI] [PubMed] [Google Scholar]
- 27.Grimm F.A. A chemical-biological similarity-based grouping of complex substances as a prototype approach for evaluating chemical alternatives. Green Chem. 2016;18:4407–4419. doi: 10.1039/c6gc01147k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsitou P. Toxicogenomics in vitro as an alternative tool for safety evaluation of petroleum substances and PAHs with regard to prenatal developmental toxicity. Toxicol. Vitr. 2015;29:299–307. doi: 10.1016/j.tiv.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Frontali N. Experimental neurotoxicity and urinary metabolites of the C5-C7 aliphatic hydrocarbons used as glue solvents in shoe manufacture. Clin. Toxicol. 1981;18:1357–1367. doi: 10.3109/15563658108990344. [DOI] [PubMed] [Google Scholar]
- 30.Ono Y. A comparative study on the toxicity of n-hexane and its isomers on the peripheral nerve. Int. Arch. Occup. Environ. Health. 1981;48:289–294. doi: 10.1007/BF00405616. [DOI] [PubMed] [Google Scholar]
- 31.Abou-Donia M.B. The relative neurotoxicities of n-hexane, methyl n-butyl ketone, 2,5-hexanediol, and 2,5-hexanedione following oral or intraperitoneal administration in hens. Toxicol. Appl. Pharmacol. 1982;62:369–389. doi: 10.1016/0041-008x(82)90139-9. [DOI] [PubMed] [Google Scholar]
- 32.OECD, SIDS Initial Assesment Profile. C7-C9 Aliphatic Hydrocarbon Solvents Category. In: US/ICCA, (Ed.), Vol. SIAM 30, 2010.
- 33.ECHA . Latvian Environment, Geology and Meteorology Centre; 2015. Substance Evaluation Conclusion Document. Evaluation Report for Isoheptane. [Google Scholar]
- 34.Lilius H. A comparison of the toxicity of 30 reference chemicals to Daphnia magna and Daphnia pulex. Environ. Toxicol. Chem. 1995;14:2085–2088. [Google Scholar]
- 35.European Chemicals Agency . 2018. N-hexane, 2018.https://echa.europa.eu/registration-dossier/-/registered-dossier/15741 (12 October 2018). Available from: [Google Scholar]
- 36.European Chemicals Agency . 2018. Hydrocarbons, C7-C9, n-alkanes, isoalkanes, cyclics, 2018.https://echa.europa.eu/registration-dossier/-/registered-dossier/14510 (14 October 2018). Available from: [Google Scholar]
- 37.Kühn R. Results of the harmful effects of selected water pollutants (anilines, phenols, aliphatic compounds) to Daphnia magna. Water Res. 1989;23:495–499. [Google Scholar]
- 38.US Environmental Protection Agency . US Environmental Protection Agency, Mid-Continent Ecology Division; Duluth, USA: 1996. ECOTOX Database Program, Version 1. [Google Scholar]
- 39.European Chemicals Agency . 2018. Fatty Acids, C10-18 and C12-22-unsatd., C14-18 and C16-18-unsatd. Alkyl Esters, 2018.https://echa.europa.eu/registration-dossier/-/registered-dossier/15446/11 (13 October 2018). Available from: [Google Scholar]
- 40.Dom N. Discrepancies in the acute versus chronic toxicity of compounds with a designated narcotic mechanism. Chemosphere. 2012;87:742–749. doi: 10.1016/j.chemosphere.2011.12.069. [DOI] [PubMed] [Google Scholar]
- 41.Cho C.-W. The ecotoxicity of ionic liquids and traditional organic solvents on microalga Selenastrum capricornutum. Ecotoxicol. Environ. Saf. 2008;71:166–171. doi: 10.1016/j.ecoenv.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Dunstan W.M. Stimulation and inhibition of phytoplankton growth by low molecular weight hydrocarbons. Mar. Biol. 1975;31:305–310. [Google Scholar]
- 43.Lapinskiene A. Eco-toxicological studies of diesel and biodiesel fuels in aerated soil. Environ. Pollut. 2006;142:432–437. doi: 10.1016/j.envpol.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 44.Zivkovic S., Veljkovic M. Environmental impacts the of production and use of biodiesel. Environ. Sci. Pollut. Res. - Int. 2018;25:191–199. doi: 10.1007/s11356-017-0649-z. [DOI] [PubMed] [Google Scholar]
- 45.Miazek K. Effect of organic solvents on microalgae growth, metabolism and industrial bioproduct extraction: a review. Int. J. Mol. Sci. 2017;18(7):1429. doi: 10.3390/ijms18071429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leite M. Toxicity of water-soluble fractions of biodiesel fuels derived from castor oil, palm oil, and waste cooking oil. Environ. Toxicol. Chem. 2011;30:893–897. doi: 10.1002/etc.444. [DOI] [PubMed] [Google Scholar]
- 47.Pereira S.A. Toxicity of biodiesel, diesel and biodiesel/diesel blends: comparative sub-lethal effects of water-soluble fractions to microalgae species. Bull. Environ. Contam. Toxicol. 2012;88:234–238. doi: 10.1007/s00128-011-0430-9. [DOI] [PubMed] [Google Scholar]
- 48.Littlefield-Wyer J.G. Application of biochemical fingerprinting and fatty acid methyl ester profiling to assess the effect of the pesticide Atradex on aquatic microbial communities. Environ. Pollut. 2008;153:393–400. doi: 10.1016/j.envpol.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 49.Synowiec A. Effect of fatty acid methyl esters on the herbicidal effect of essential oils on corn and weeds. Weed Technol. 2017;31:301–309. [Google Scholar]
- 50.Yassine M.H. Microtox aquatic toxicity of petrodiesel and biodiesel blends: the role of biodiesel’s autoxidation products. Environ. Toxicol. Chem. 2012;31(12):2757–2762. doi: 10.1002/etc.2001. [DOI] [PubMed] [Google Scholar]
- 51.Docea A.O. Study design for the determination of toxicity from long-term-low-dose exposure to complex mixtures of pesticides, food additives and lifestyle products. Toxicol. Lett. 2016;258S:S179. [Google Scholar]
- 52.Docea A.O. Six months exposure to a real life mixture of 13 chemicals’ below individual NOAELs induced non monotonic sex-dependent biochemical and redox status changes in rats. Food Chem. Toxicol. 2018 doi: 10.1016/j.fct.2018.03.052. doi:S0278-6915(18)30201-1. [DOI] [PubMed] [Google Scholar]
- 53.Tsatsakis A.M. New challenges in risk assessment of chemicals when simulating real exposure scenarios; simultaneous multi-chemicals’ low dose exposure. Food Chem. Toxicol. 2016;96:174–176. doi: 10.1016/j.fct.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 54.Tsatsakis A.M. Simulating real-life exposures to uncover possible risks to human health: a proposed consensus for a novel methodological approach. Hum. Exp. Toxicol. 2017;36(6):554–564. doi: 10.1177/0960327116681652. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.