Abstract
Recombinant adeno-associated virus (rAAV) has been developed as a successful vector for both basic research and human gene therapy. However, neutralizing antibodies (NAbs) against AAV capsids can abolish AAV infectivity on target cells, reducing the transduction efficacy. Absence of AAV NAb has become a prerequisite qualification for patients enrolled in gene therapy trials. Nevertheless, accurate assessment of AAV NAb has remained a challenging task. Here we developed a rapid assay based on the observations that AAV NAb inhibits rAAV binding to the host cell surface and NAb titers are negatively related to the amount of AAV genomes binding to the target cells. By quantifying the AAV genome on the target cells in the presence of anti-sera, AAV NAb titers can be accurately determined. The titer determined by this assay correlates well with the classical transduction-based assays. A major advantage of this method is that it can be carried out with a 30-min binding assay without the lengthy wait for a transduction outcome. This assay is independent of transduction performance of AAV serotype in the target cells. Therefore, the AAV cell-binding assay for NAb determination offers an alternative method for in vivo NAb assay.
Keywords: AAV, neutralizing antibody assay, gene therapy, vector
Introduction
Adeno-associated virus (AAV) is a successful vector for human gene therapy with an excellent safety profile and broad tissue tropisms.1 Over the decades, hundreds of AAV serotypes have been identified and developed as gene delivery tools.2 Nevertheless, host immunity against AAV capsids remains a major obstacle to broad and effective application of AAV vectors in human gene therapy.3 Although AAV has not been associated with any known human diseases, pre-existing AAV-neutralizing antibodies (NAbs) are widespread in the human population.4 Between 50%–90% of the human population has been exposed to AAV infection, of which around 50% of cases lead to the development of NAbs against AAV capsids.5, 6, 7, 8 In addition, there is a high degree of capsid amino acid sequence homology among AAV serotypes. Cross-reactivity of NAbs against various AAV serotypes is another issue that limits the usefulness of AAV vectors.9 It has been reported that a NAb titer as low as 1:5 in the blood can fully prevent AAV transduction in vivo.10 Accordingly, pre-screening for the presence of NAbs against specific AAV serotypes is a critical procedure in both animal experiments and clinical trials.
Efforts have been made to develop accurate assays for assessing the AAV NAb titers. ELISA-based capture assays are designed to measure the amount of immunoglobulin G (IgG) that can bind to AAV capsids.11 Although the assay itself is easy to set up and the amount of IgG with affinity to AAV capsids can be measured accordingly, this assay does not necessarily solely represent the neutralizing activity in the sample, since non-NAbs are equally measured and reported. Cellular transduction-based in vitro assays have also been developed and widely adopted for assessing anti-AAV NAb.12, 13, 14 These assays utilize AAV vector carrying a reporter gene. The titer of NAbs is determined by monitoring the reduction of the reporter gene expression in transducing cells in the presence of the testing sera. While this assay is straightforward to perform, it is highly reliant on efficient transduction. Most serotypes transduce tissue culture poorly. This drastically lowers detection sensitivity and in some cases is not possible even with high MOI. In vivo NAb assays can accurately capture the inhibitors in the testing sera that affect AAV transduction.6 However, in vivo assays are time consuming and more costly than in vitro methods.
Here we report a new in vitro NAb assay based on AAV-target cell binding. Through quantifying the genome copy number of rAAV bound to the cell surface in the presence of testing sera, the NAb titers can be determined. Since this assay does not rely on sensitive reporter expression, it can be applied to all AAV serotypes regardless of serotype performance in transducing the targeting cells. Therefore, this assay provides a fast, sensitive, and cost-effective way to determine NAb titers.
Results
rAAV Particles and Host Cell Binding Positively Correlates to AAV Transduction Efficacy
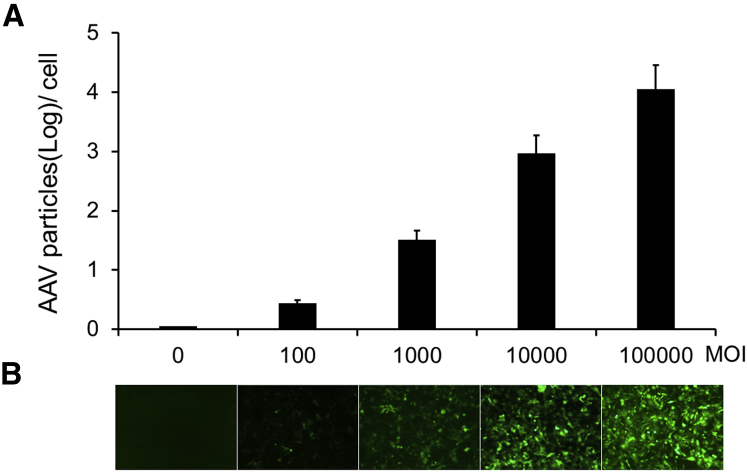
The typical in vitro AAV NAb assay is based on the reporter gene expression carried in AAV vectors, which can only be observed upon successful transduction. Since AAV-host cell binding is the first step leading to a cascade of events that result in reporter gene expression, we investigated the relationship between the initial AAV-host binding and the final reporter gene expression. To measure the AAV-host cell binding, self-complementary AAV vectors with an EGFP reporter gene (scAAV2-EGFP) were incubated with GM16095 cells at varying MOI (0, 100, 1,000, and 10,000), at 4°C for 30 min to allow rAAV binding to the cell surface without entering the cells. After repeated washing steps with cold PBS to get rid of unbound rAAV, the cells were collected, and rAAV bound to the cell surface was quantified with qRT-PCR (Figure 1A). The results showed that AAV binding to the cells is proportional to the MOI of vectors applied to the cells, which is similar to the increased transduction with the increased MOI.
Figure 1.
Correlation of AAV2 Vector-Host Cell Binding to GFP Reporter Gene Expression Level
(A) Measurement of AAV2 genomes binding to the host cells at different MOIs. scAAV2-EGFP at the MOIs indicated on the x axis were incubated with GM16095 cells at 4°C for 30 min, and then the vector genome copy numbers were determined by qRT-PCR, which is shown on the y axis. Data are expressed as log per cell means ± SD. (B) The GFP expression at 48 hr post-infection was observed by fluorescence microscopy. A typical view image is shown for each MOI.
To confirm that the cell surface-binding ability directly correlates to transducing efficiency, the GFP-positive cells corresponding to the above infection were observed at 48 hr post-infection (Figure 1B). The rAAV MOIs, AAV-host cell binding, and positive GFP expression cells correlated very well. Of interest, at a low MOI of 100, the AAV binding to the cells was distinctly different from the control of mock infection or binding at an MOI of 0 (p = 0.0013); in contrast, there were no differences in transduction observed at MOIs 0 and 100. This result suggests that an NAb assay based on AAV-host cell binding could be more sensitive than the classic reporter gene assays.
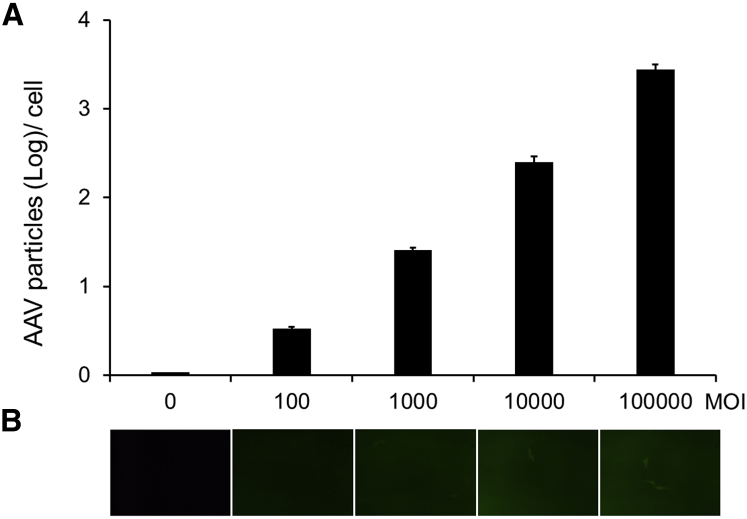
Many AAV serotypes transduce in tissue culture poorly despite their superior performance in vivo. It is especially difficult to get an accurate NAb readout when reporter gene expression is low. Here we examined the binding properties of AAV8, whose transduction efficiency is much worse than AAV2 in tissue-cultured cells. The numbers of AAV8 genomes that were binding to the target cells determined by qRT-PCR were positively correlated to MOI (Figure 2A); in contrast, there was no obvious transduction observed in the traditional transducing reporter gene assay (Figure 2B). These results suggest that assays based on the cell surface-binding property may overcome the limitations of rAAVs with low or no transducing efficiency in vitro.
Figure 2.
Sensitivity Assessment of AAV8 Vector-Host Cell Binding Compared to Reporter Gene Expression
(A) AAV8 genomes binding to the host cells at different MOIs. scAAV8-EGFP vectors at the MOIs indicated on the x axis were incubated with GM16095 cells at 4°C for 30 min, and then the vector genome copy numbers were measured by qRT-PCR, which is shown on the y axis. Data are expressed as log per cell means ± SD. (B) The GFP expression at 48 hr post-infection was observed by fluorescence microscopy. A typical view image is shown for each MOI.
Capsid-Host Cell Binding as an Assay to Determine NAb Titer
Based on the above observations, we designed a new method to determine the NAb titer based on AAV and host cell binding. The detailed procedures are outlined in Figure 3A. Briefly, serially diluted testing sera were incubated with rAAV (MOI: 1,000) for 1 hr at 37°C. Cells cultured overnight on 96-well plates were cooled down at 4°C for 5 min and incubated with the rAAV-sera mixture for 30 min. After thoroughly washing with cold PBS to remove any non-specifically bound rAAV, rAAV bound to the cell surface was recovered, as described in the Materials and Methods. Finally, the genome numbers of rAAV were determined by qRT-PCR. Theoretically, the presence of NAb in the sera inactivates rAAV, thus reducing rAAV binding to the cell surface. Therefore, by detecting the numbers of rAAV bound to the cell surface, we would be able to infer the NAb titers of a test sera.
Figure 3.
AAV-Binding Assay Procedure and NAb Calculation Demonstration
(A) Flowchart of AAV-neutralizing antibody determination using an AAV-binding assay. (B and C) An example of NB50 calculation. ssAAV2-EGFP (B) or ssAAV8-EGFP (C) and varying dilutions of neutralizing antibodies were incubated at 37°C for 1 hr. The resulting complex of AAV vector and neutralizing antibodies (NAbs) was then applied to GM16095 cells and incubated at 4°C for 30 min. The genome copy number of AAV bound to the cell was determined by qRT-PCR. The NB50 values were then calculated, as represented by the antibody dilutions needed to neutralize 50% of the input vector, which was 1:453 for AAV2 and 1:114 for AAV8. NC, negative control with no antibody added. Data are expressed as mean percent neutralized vector ± SD.
To test this hypothesis, we first used well-characterized anti-AAV2 and anti-AAV8 antibodies. rAAV2 and rAAV8 (MOI: 1,000) were pre-incubated with the anti-AAV2 or anti-AAV8 antibodies, respectively, at dilutions indicated in Figures 3B and 3C. Following the increase of antibodies from 1:10,000 to 1:10, the cell surface-binding genome numbers of rAAV2 decreased (Figure 3B). Similarly, increasing the concentrations of anti-AAV8 antibody (1:1,000 to 1:10) decreased the genome numbers of cell-bound rAAV8 (Figure 3C). Based on these data and curve fitting, the titers neutralizing 50% binding (NB50) were determined to be 1:453 and 1:114 for anti-AAV2 and anti-AAV8 antibodies, respectively. These results demonstrate that NAb titers in sera can be quantitatively determined by examining the reduction of the cell surface-bound rAAV genome numbers.
Assessment of NAb against AAV2 in Human Serum Samples
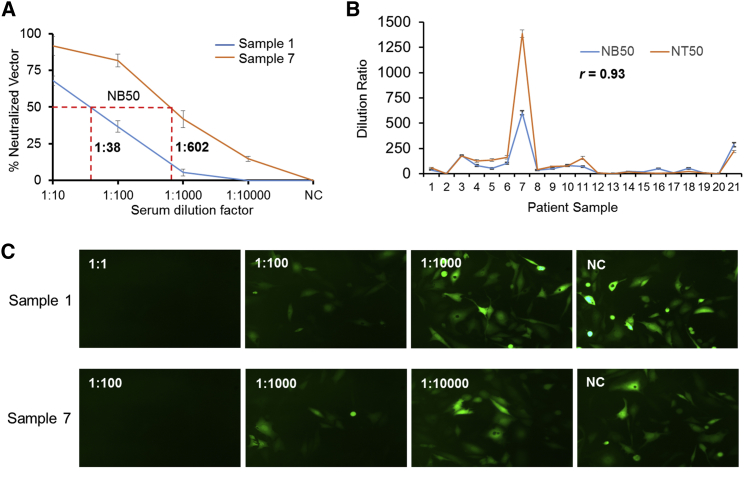
To further validate the cell-binding assays, we evaluated serum samples from a total of 21 patients for the presence of AAV NAb. Two typical cases, serum samples 1 and 7, are illustrated in Figure 4A, which shows NB50 values of 1:38 and 1:602, respectively. Using this approach, the NB50 values of all samples were determined and compared with NT50 values determined by the classical transducing assays (Figure 4B; Table S1). Statistical analysis showed an excellent correlation between NB50 values determined by our new method and NT50 values determined by the classical transducing assays (r = 0.93). A set of representative in vitro-transducing assays for serum samples 1 and 7 is shown in Figure 4C. Similarly, the NB50 values against AAV8 of these serum samples were determined and are presented as supplemental data (Figure S1; Table S1). These results demonstrate that this new assay based on capsid-cell binding for NAb titer in vitro is validated and feasible.
Figure 4.
Determination of Neutralizing Titer in Two Typical Human Serum Samples for AAV2 Serotype
(A) The neutralizing activity of human serum against AAV2 vectors. Neutralizing titers (NTs) were calculated as the serum dilution required to neutralize 50% of the input vector quantified by genome copy numbers obtained from qRT-PCR assay. Graphic determination for patients 1 and 7 yielded NTs against AAV2 of 1:38 and 1:602, respectively. NC, negative control with no serum added. Data are expressed as mean percent neutralized vector ± SD. (B) Correlation between NB50 and NT50 values. 21 patient samples were analyzed to determine the r value, which was 0.93. Data are expressed as mean dilution ratio ± SD. (C) Observation of GFP gene expression through fluorescence microscopy following the transduction-based assay of NAb against AAV2. GM16095 cells were incubated with the mixture of ssAAV2-EGFP (MOI: 1,000) and several dilutions of human serum from patients 1 and 7, as described in the Materials and Methods.
Discussion
AAV-based gene therapy for genetic diseases has yielded promising outcomes in clinical trials. However, the presence of NAbs against AAV capsids in the general population calls for an effective assay to determine NAb titer in the patients before they are involved in the clinical trials.
One potential caveat of the in vivo transduction inhibition assay is that transduction involves complicated cellular processes, including cell surface binding, cell entrance, trafficking to the nucleus, and other intracellular events that are not influenced by NAbs. In addition, it should be noted that in vivo transduction inhibition assays do not take into consideration the potential antibody-dependent cellular phagocytosis that may block vector neutralization,15 the possible change in tissue targeting of AAV, or the presence of NAb in the testing animals, all of which may adversely affect the reliability and validity of assay results.16
Nevertheless, in vitro assays can offer a fast and economical way for initial screening. A reporter gene-based assay, initially presented as early as 1998, relies on the reduction of transduction in the presence of NAb inhibition.17 Although this is a straightforward and convenient assay, it requires efficient transduction of host tissue cultures. However, many new AAV serotypes, including AAV1, AAV8, and AAV9, do not transduce tissue culture cells well even at a high MOI, despite their superior performance in their target tissues in vivo. The high level of in vitro transduction observed with AAV2 combined with the use of sensitive luminescence reporter genes permits the use of an MOI as low as 100.18 On the other hand, the low levels of in vitro transduction efficiency observed in some of the newer AAV serotypes, such as AAV8, demand the use of an MOI as high as 10,000.9, 18, 19, 20 Tailoring the minimum numbers of AAV particles per cell for each AAV serotype may optimize assay sensitivity for NAb titer determination, but this can be costly and time consuming. It also compromises the comparison of NAb titers between different AAV serotypes. Therefore, a new in vitro assay that does not rely on the transduction of the reporter gene is highly desirable.
Since NAb can bind to AAV and interfere with AAV’s attachment to the target cells, the property of AAV binding to target cells can be utilized for the development of NAb assays, avoids many complications of steps prior to reporter gene expression, and increases the NAb assay sensitivity. In the present study, we offer a more sensitive, efficient assay based on rAAV binding to the cell surface. The measurement is independent of AAV serotypes and reliable even for AAV serotypes like AAV8 with a very low in vitro transduction efficiency. Further, even though we used GM16095 cells, researchers can use different cell types with different receptors to optimize for different serotypes. For a standardized comparison of NAb titers among all AAV serotypes, all relevant test samples must have the same MOI input. Our results showed that, for both AAV2 and AAV8, the numbers of AAV bound to the cell surface is positively correlated with a wide range of MOIs (0–10,000). Particularly, whereas transduction inhibition assays work with a starting dilution of 1:2–1:20, the new method reported here begins at a higher starting dilution of 1:10 and runs over a wide range to 1:100,000, demonstrating the sensitivity of this method. This wide range makes it applicable to different AAV serotypes.
As demonstrated in our studies, an MOI as low as 100 generated cell-bound genome numbers that can be determined by qRT-PCR, making this method compatible with both low and high MOIs. However, to avoid variations with low MOI, we used an MOI of 1,000 in our subsequent screening of serum samples and based on our results. The benefits of a higher MOI help with potential issues that may arise from the concern of qRT-PCR sensitivity, because the copy numbers of vector genomes detected fall into the linear range of the standard curve. The standard curve for the NAb assay was established using an MOI of 1,000, which mitigates issues that are not well defined in cell-vector binding, such as non-specific binding to the wells of tissue culture plates. For these reasons, we recommend an MOI of 1,000 for most applications. For use with more sensitive systems, like digital droplet PCR (ddPCR), a lower MOI may be used.
Furthermore, we applied this new method to test 21 clinical samples, and we compared with the classic transducing assays. The results showed an excellent correlation between NB50 values determined by this new method and NT50 values determined by the classical transducing assays (r = 0.93). According to the transducing assays, 59% of these samples were positive against AAV2, whereas the cell-binding assays revealed that 68% of sample sera were positive against AAV2. In addition, NT50 values against AAV8 were undetectable because of low transduction efficiency; however, two positive samples against AAV8 vectors were able to be captured by our new method. In conclusion, a cell-binding-based method for the determination of AAV NAb is a rapid, efficient, and sensitive method, although there still exists some variation when compared with the classic transduction-based assay. Therefore, this new potential method needs to be standardized using more clinical samples.
Materials and Methods
Cell Lines
HEK293 cells and GM16095 cells (a human fibroblast cell line purchased from the Coriell Institute, Camden, NJ) were cultured in DMEM supplemented with 10% fetal bovine serum, 100 μg/mL penicillin, and 100 units/mL streptomycin (Invitrogen, Carlsbad, CA). All cells were maintained in a humidified 37°C incubator with 5% CO2.
rAAV Production and Purification
AAV vectors were generated using the triple plasmid co-transfection method, as described previously.21, 22 Briefly, pAAV-Rep-Cap (serotypes 2 and 8), pAd helper, and the transgene plasmids pdsAAV-CB-EGFP were co-transfected into HEK293 cells and cultured in roller bottles at a ratio of 1:1:1. The vectors from transfected cells and medium were harvested 72 hr post-transfection, purified by two rounds of cesium chloride (CsCl) gradient ultracentrifugation, and extensively dialyzed against PBS containing 5% D-sorbitol. Viral genomes (vg) were quantified by real-time PCR and vector titers are expressed as vg/mL. Serum samples and NAb standards were as follows: human serum samples (n = 21) were obtained from the New Bolton Center (University of Pennsylvania, Philadelphia, PA); serum samples were heat inactivated at 56°C for 30 min and archived at −80°C for later NAb analysis; and anti-AAV2 antibody and anti-AAV8 antibody were gifts from James Wilson (University of Pennsylvania, Philadelphia, PA) for use as positive controls.
In Vitro rAAV-Binding Assays
The in vitro rAAV-binding assay procedures are detailed as follows:
-
1.
Seed GM16095 cells into a 96-well plate at 1 × 105 cells/mL and a volume of 100 μL/well. Incubate the plate overnight at 37°C and 5% CO2.
-
2.
Dilute the test sera at ratios of 1:10, 1:100, 1: 1,000, and 1:10,000 using DMEM. Mix 100 μL of each test serum dilution with AAV2 vectors and AAV8 vectors, separately, each at an MOI of 1,000. Incubate the mixtures for 1 hr at 37°C to allow any neutralizing activity to occur.
-
3.
Pre-chill the cell culture in the 96-well plate at 4°C for 5 min.
-
4.
Remove the old medium and wash wells with cold PBS. Add 100 μL/well test serum-AAV vector mixture with 3 replicates for each mixture. Incubate the cell culture plate at 4°C for 30 min to allow the binding of AAV vectors to cell surfaces (but not vector entry).
-
5.
Wash with cold PBS twice to remove any AAV vectors not bound to cell surfaces (as a result of being bound to NAbs instead).
-
6.
Add 100 μL lysis buffer containing proteinase K (40 μg/mL) into each well to allow lysis of cells, and then incubate the cell lysates at 56°C for 60 min to allow lysis of vector capsids for the release of viral genomes.
-
7.
Inactivate proteinase K by heating at 95°C for 10 min, then keep the samples at 4°C.
-
8.
Determine viral genome copy numbers via real-time qPCR assay.
-
9.
Calculate the anti-AAV-NAb titer by finding the serum dilution ratio that causes 50% inhibition of vector binding to cells (NB50).
In Vitro rAAV Reporter Gene Expression Assays
The in vitro rAAV reporter gene expression assay procedures are detailed as follows:
-
1.
Seed GM16095 cells onto a 96-well plate at 1 × 105 cells/mL and a volume of 100 μL/well. Incubate the plate overnight at 37°C and 5% CO2.
-
2.
Dilute the test sera at ratios of 1:10, 1:100, 1: 1,000, and 1: 10,000 using DMEM. Mix 100 μL of each test serum dilution with AAV2 vectors and AAV8 vectors, separately, each at an MOI of 1,000. Incubate the mixtures for 1 hr at 37°C to allow any neutralizing activity to occur.
-
3.
Replace the old medium with 100 μL/well of the test serum-AAV vector mixture with 3 replicates for each mixture. Incubate the cell culture plate at 37°C for 24 hr to allow AAV vector binding and entry into cells.
-
4.
Use a fluorescence microscope to observe EGFP expression at 24 hr post-infection. Examine 5 fields of vision, and identify GFP-positive cells out of a total count of 200 cells selected at random in each.
-
5.
Calculate the anti-AAV-NAb titer by finding the serum dilution ratio that causes 50% inhibition of transduction efficacy (NT50).
Calculation of the Anti-AAV-NAb Titer
The neutralizing titer of the sample is determined as the first dilution at which 50% of the input vector (MOI: 1,000) experiences inhibition by NAbs. For example, if 50% inhibition is observed at a 1:10 dilution of the sample, the titer is reported as 1:10. For the cell-binding assay, NB50 values were derived from qRT-PCR results as follows: add 100 μL lysis buffer containing proteinase K (40 μg/mL) into each well to allow lysis of cells, and then incubate the cell lysates at 56°C for 60 min to allow lysis of vector capsids for the release of viral genomes; inactivate proteinase K by heating at 95°C for 10 min, then keep the samples at 4°C; and determine viral genome copy numbers via real-time qPCR assay. All qRT-PCR analyses were performed using the Fast SYBR Green Master Mix, which has been previously described,20, 23 using the following primers: EGFP forward primer, 5′-TGACCCTGAAGTTCATCTGC-3′; and EGFP reverse primer, 5′-GAAGTCGTGCTGCTTCATGT-3′. The genome copy number of vectors found in each sample was determined by comparison with a standard curve made from serial dilutions of plasmid containing the corresponding AAV genomes.
Cycle threshold (Ct) values obtained from the qRT-PCR assay represent the logarithm of viral genome copy numbers and were converted into the latter. Ct values of known plasmid copy number dilutions (107, 106, 105, 104, and 103) were used to plot a standard curve and yield an equation describing the relationship between Ct values and the log of genome copy numbers, which were used then to calculate the corresponding log of genome copy numbers. The log was removed by raising 10 to the power of these derived values, and the final viral genome copy number per milliliter was obtained by multiplying by the total dilution factor applied to the original sample.
These viral genome copy numbers represent AAV vectors that remained bound to the cell surface following exposure to NAbs and washes with cold PBS. To determine the number of AAV vectors that were otherwise unable to bind to cells due to contact with NAbs, cell-bound AAV vector numbers were subtracted from the total number of input vectors, as defined by the genome copy number calculated for the negative control serum without the presence of any antibodies. The percent inhibition value for each serum dilution ratio was then obtained through dividing the number of neutralized vectors by the total input number of vectors and multiplying by 100. A line of best fit that gave the highest R2 value was then plotted from these values, and its equation was then used to calculate the serum dilution ratio that yields a 50% inhibition, designated as the NB50 value.
For the transduction assay, NT50 values were derived from GFP-positive cell counts. Five fields were viewed under a fluorescence microscope to obtain the average number of GFP-positive cells observed out of a total of 200 cells. This indicates the number of vectors that escaped NAbs and subsequently entered and induced cells to express the GFP transgene. The number of vectors that were instead captured and neutralized by antibodies was inferred as the remainder when the number of GFP-positive cells was subtracted from the total number of cells present. This was divided by the total number of cells and multiplied by 100 to obtain percent inhibition values, which were then, as described above, accordingly manipulated to ultimately give the serum dilution ratio that produces a 50% inhibition, denoting the NT50 value. Serum samples were considered to have neutralizing activity if the 1:20 serum dilution inhibited vector transduction by at least 50%.
Note, the most suitable serum dilution ratios to use were determined by first setting a broad range, including 1:10, 1:100, 1:1,000, and 1:10,000. The cutoff point where inhibition levels no longer changed significantly with dilution ratio was identified as the sensitivity limit, and then it was used to further narrow down the dilution range until the best possible R2 value for the plotted curve was attained for each test serum sample analyzed.
Statistical Analysis
Both the two-tailed Student’s t test and one-way ANOVA with Bonferroni multiple comparisons following test were performed. The differences were considered significant when the p value was <0.05. The degree of relationship between results from two assays was compared by correlation analysis. The analysis was performed using SPSS version (v.)13.0 (SPSS, Chicago, IL).
Author Contributions
P.G., J.Z., M.C., J.H., H.C., J.A.F., N.S., Y.D., and W.X. contributed to the design and implementation of the research, to the analysis of the results, and to the writing of the manuscript.
Conflicts of Interest
The authors have no conflicts of interest.
Acknowledgments
This work was supported by grants from the NIH (reference HL114152 and HL130871), the National Natural Science Foundation of China (reference 81371672 and 81371669), Major Project of University and Industry Cooperation in Fujian Province of China (reference 2018Y4009), and Project of Science and Technology of Quanzhou (reference 2016N006). We highly appreciate the contributions of Dr. James Wilson (University of Pennsylvania).
Footnotes
Supplemental Information includes one figure and one table and can be found with this article online at https://doi.org/10.1016/j.omtm.2018.11.007.
Contributor Information
Yong Diao, Email: diaoyong@hqu.edu.cn.
Weidong Xiao, Email: wxiao@temple.edu.
Supplemental Information
References
- 1.Kotterman M.A., Schaffer D.V. Engineering adeno-associated viruses for clinical gene therapy. Nat. Rev. Genet. 2014;15:445–451. doi: 10.1038/nrg3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao G., Vandenberghe L.H., Alvira M.R., Lu Y., Calcedo R., Zhou X., Wilson J.M. Clades of Adeno-associated viruses are widely disseminated in human tissues. J. Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calcedo R., Wilson J.M. Humoral immune response to AAV. Front. Immunol. 2013;4:341. doi: 10.3389/fimmu.2013.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell P., Moscioni A.D., McCarter R.J., Wu D., Gao G., Hoang A., Sanmiguel J.C., Sun X., Wivel N.A., Raper S.E. Analysis of tumors arising in male B6C3F1 mice with and without AAV vector delivery to liver. Mol. Ther. 2006;14:34–44. doi: 10.1016/j.ymthe.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Xiao W., Chirmule N., Berta S.C., McCullough B., Gao G., Wilson J.M. Gene therapy vectors based on adeno-associated virus type 1. J. Virol. 1999;73:3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang M., Crosby A., Hastie E., Samulski J.J., McPhee S., Joshua G., Samulski R.J., Li C. Prediction of adeno-associated virus neutralizing antibody activity for clinical application. Gene Ther. 2015;22:984–992. doi: 10.1038/gt.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calcedo R., Morizono H., Wang L., McCarter R., He J., Jones D., Batshaw M.L., Wilson J.M. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin. Vaccine Immunol. 2011;18:1586–1588. doi: 10.1128/CVI.05107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calcedo R., Vandenberghe L.H., Gao G., Lin J., Wilson J.M. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boutin S., Monteilhet V., Veron P., Leborgne C., Benveniste O., Montus M.F., Masurier C. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum. Gene Ther. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 10.Wang L., Calcedo R., Bell P., Lin J., Grant R.L., Siegel D.L., Wilson J.M. Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors. Hum. Gene Ther. 2011;22:1389–1401. doi: 10.1089/hum.2011.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blacklow N.R., Hoggan M.D., Rowe W.P. Serologic evidence for human infection with adenovirus-associated viruses. J. Natl. Cancer Inst. 1968;40:319–327. [PubMed] [Google Scholar]
- 12.Jungmann A., Müller O., Rapti K. In: Cell-Based Measurement of Neutralizing Antibodies Against Adeno-Associated Virus (AAV). Cardiac Gene Therapy: Methods in Molecular Biology. Ishikawa K., editor. Humana Press; 2017. pp. 109–126. [DOI] [PubMed] [Google Scholar]
- 13.Rincon M.Y., Prada C.E., Lopez M., Castillo V., Echeverria L.E., Serrano N. Determination of Anti-Adeno-Associated Viral Vector Neutralizing Antibodies in Patients With Heart Failure in the Cardiovascular Foundation of Colombia (ANVIAS): Study Protocol. JMIR Res. Protoc. 2016;5:e102. doi: 10.2196/resprot.5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meliani A., Leborgne C., Triffault S., Jeanson-Leh L., Veron P., Mingozzi F. Determination of anti-adeno-associated virus vector neutralizing antibody titer with an in vitro reporter system. Hum. Gene Ther. Methods. 2015;26:45–53. doi: 10.1089/hgtb.2015.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenberg A.H., Hudson L., Shen L., Roitt I.M. Antibody-dependent cell-mediated cytotoxicity due to a “null” lymphoid cell. Nat. New Biol. 1973;242:111–113. doi: 10.1038/newbio242111a0. [DOI] [PubMed] [Google Scholar]
- 16.Vandamme C., Adjali O., Mingozzi F. Unraveling the complex story of immune responses to AAV vectors trial after trial. Hum. Gene Ther. 2017;28:1061–1074. doi: 10.1089/hum.2017.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manning W.C., Zhou S., Bland M.P., Escobedo J.A., Dwarki V. Transient immunosuppression allows transgene expression following readministration of adeno-associated viral vectors. Hum. Gene Ther. 1998;9:477–485. doi: 10.1089/hum.1998.9.4-477. [DOI] [PubMed] [Google Scholar]
- 18.Halbert C.L., Miller A.D., McNamara S., Emerson J., Gibson R.L., Ramsey B., Aitken M.L. Prevalence of neutralizing antibodies against adeno-associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: Implications for gene therapy using AAV vectors. Hum. Gene Ther. 2006;17:440–447. doi: 10.1089/hum.2006.17.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rapti K., Louis-Jeune V., Kohlbrenner E., Ishikawa K., Ladage D., Zolotukhin S., Hajjar R.J., Weber T. Neutralizing antibodies against AAV serotypes 1, 2, 6, and 9 in sera of commonly used animal models. Mol. Ther. 2012;20:73–83. doi: 10.1038/mt.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauck B., Chen L., Xiao W. Generation and characterization of chimeric recombinant AAV vectors. Mol. Ther. 2003;7:419–425. doi: 10.1016/s1525-0016(03)00012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q., Firrman J., Wu Z., Pokiniewski K.A., Valencia C.A., Wang H., Wei H., Zhuang Z., Liu L., Wunder S.L. High-Density Recombinant Adeno-Associated Viral Particles are Competent Vectors for In Vivo Transduction. Hum. Gene Ther. 2016;27:971–981. doi: 10.1089/hum.2016.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Q., Dong B., Firrman J., Roberts S., Moore A.R., Cao W., Diao Y., Kapranov P., Xu R., Xiao W. Efficient production of dual recombinant adeno-associated viral vectors for factor VIII delivery. Hum. Gene Ther. Methods. 2014;25:261–268. doi: 10.1089/hgtb.2014.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao L., Liu Y., During M.J., Xiao W. High-titer, wild-type free recombinant adeno-associated virus vector production using intron-containing helper plasmids. J. Virol. 2000;74:11456–11463. doi: 10.1128/jvi.74.24.11456-11463.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.