To the Editor:
Biallelic mutations in the dedicator of cytokinesis 8 (DOCK8) genecause autosomal-recessivehyper-IgEsyndrome,acombined immunodeficiency characterized by sinopulmonary infections, skin and systemic viral infections, eczema, and food allergy. DOCK8 deficiency can lead to early death from infection and malignancy.1,2 The disease is curable by hematopoietic cell transplantation (HCT).3-5 Thus, it is important to ascertain the diagnosis of DOCK8 deficiency to institute early treatment.
The vast majority of DOCK8-deficient patients lack DOCK8 expression and many have deletions in the DOCK8 gene.1,2 Confirmation of the diagnosis has relied on immunoblotting of blood cell lysates and/or gene sequencing, techniques that are not routinely available. We present here a flow cytometry assay that could facilitate the diagnosis of DOCK8 deficiency, detection of carrier status, and investigation of lineage-specific DOCK8 expression following HCT.
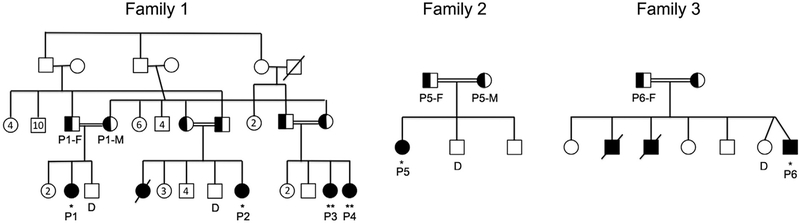
The pedigrees of patients studied are shown in Fig E1 and their mutations in Table E1 (see Online Repository at www.jacionline..org/). All studies were obtained after informed consent and approval of the Boston Children’s Hospital Institutional Review Board. Intracellular staining for DOCK8 was performed on mononuclear cells from peripheral blood or bone marrow (BM) using the CytoFix/CytoPerm kit (BD Biosciences, San Jose, Calif), mouse monoclonal anti-DOCK8 (clone G-2, Santa Cruz Biotechnology, Dallas, Tex, raised against amino acids 119–277), mouse IgG1 isotype control (Biolegend,SanDiego,Calif), and fluorescein isothiocyanate–conjugated rat anti-mouse IgG1 (Biolegend). Expression was calculated as the difference in mean fluorescence intensity (ΔMFI) between cells stained with anti-DOCK8 antibody and isotype control; the results were analyzed as a percentage of ΔMFI of healthy control assayed on the same day or in aggregate compared with theaverage of allhealthy controls (see the Methods section in this article’s Online Repository at www.jacionline.org).
FIG E1.
Pedigrees of families studied. Family 1 is an extended family from Kuwait with 4 affected children. Family 2 is from Syria with 1 affected child. Family 3 is from Saudi Arabia with 2 previously affected children who died and 1 affected child. Where indicated, patients were studied after undergoing matched related (*) or after closely matched unrelated donor HCT (**).
Table E1.
Characteristics of DOCK8-deficient patients studied
| Patient | Mutation | Age at transplant (y) |
Conditioning | Donor | Status and donor chimerism |
|---|---|---|---|---|---|
| P1 | c.[1-?_5281?del]1[1-?_5281?del] (includes deletion of exons 1 and 5) |
7 | Busulfan, fludarabine | Matched sibling | Well, full |
| P2 | c.[1-?_5281?del]1[1-?_5281?del] (includes deletion of exons 1 and 5) |
5 | Busulfan, fludarabine | Matched sibling | Well, full |
| P3 | c.[1-?_5281?del]1[1-?_5281?del] (includes deletion of exons 1 and 5) |
3 | Busulfan, fludarabine, ATG | 10/10 unrelated donor | Well, full |
| P4 | c.[1-?_5281?del]1[1-?_5281?del] (includes deletion of exons 1 and 5) |
2 | Busulfan, fludarabine, ATG | 9/10 HLA-A mismatched unrelated donor | Well, mixed at 4.5 mo CD3 94% CD19 51% CD15 49% |
| P5 | c.[1-?_1561?del]1[1-?_1561?del] (includes deletion of exons 1 and 2) |
10 | Busulfan, cyclophosphamide | Matched sibling | Well, full |
| P6 | Homozygous point mutation in splice site IVS4411G>A resulting in exon 44 skipping |
3 | Busulfan, cyclophosphamide | Matched sibling | Well, full |
ATG, Antithymocyte globulin.
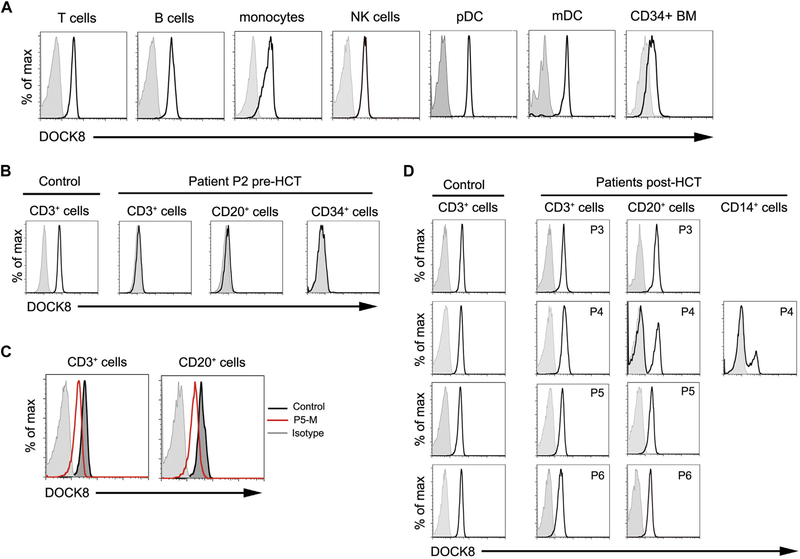
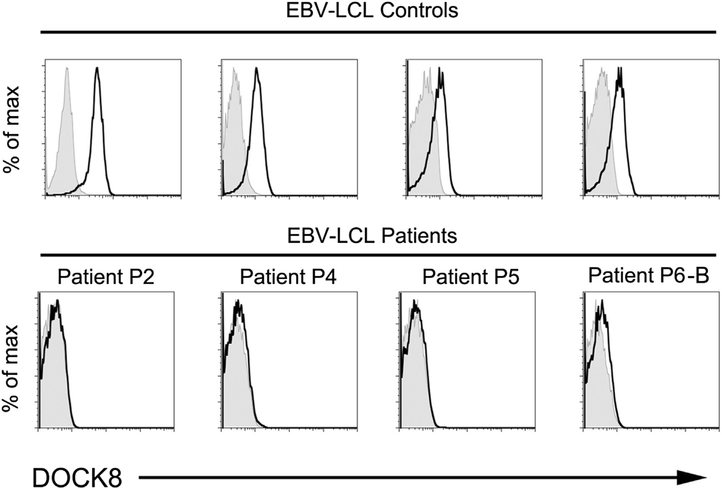
Expression of DOCK8 could be detected by flow cytometry in subsetsof normal peripheral bloodand in CD34+BM cells (Fig1, A). DOCK8 could not be detected by flow cytometry in T cells,B cells, and CD34+ BM cells from 3 patients (Fig 1, B and data not shown). Because proteins in shipped blood samples may degrade, we also examined EBV-transformed lymphoblastoid cell lines (EBV-LCL). Flow cytometry readily detected DOCK8 in EBV-LCL derived from 4 healthy controls but not in EBV-LCL derived from patients P2, P4, P5, or the brother of P6 (see Fig E2 in this article’s Online Repository at www.jacionline.org).
FIG 1.
DOCK8 expression by flow cytometry. Shaded histograms indicate isotype control. A, Control cells. B, Cells from control and patient P2. C, Lymphocytes from mother of P5 (red) and control (dark gray). D, Lineage-specific expression in control and in 4 patients at 7 months (P3), 5 months (P4), 25 months (P5), and 36 months (P6) after HCT. NK, Natural killer.
FIG E2.
Analysis of DOCK8 expression in EBV-LCL from 4 healthy controls (top row) and 4 patients (bottom row).
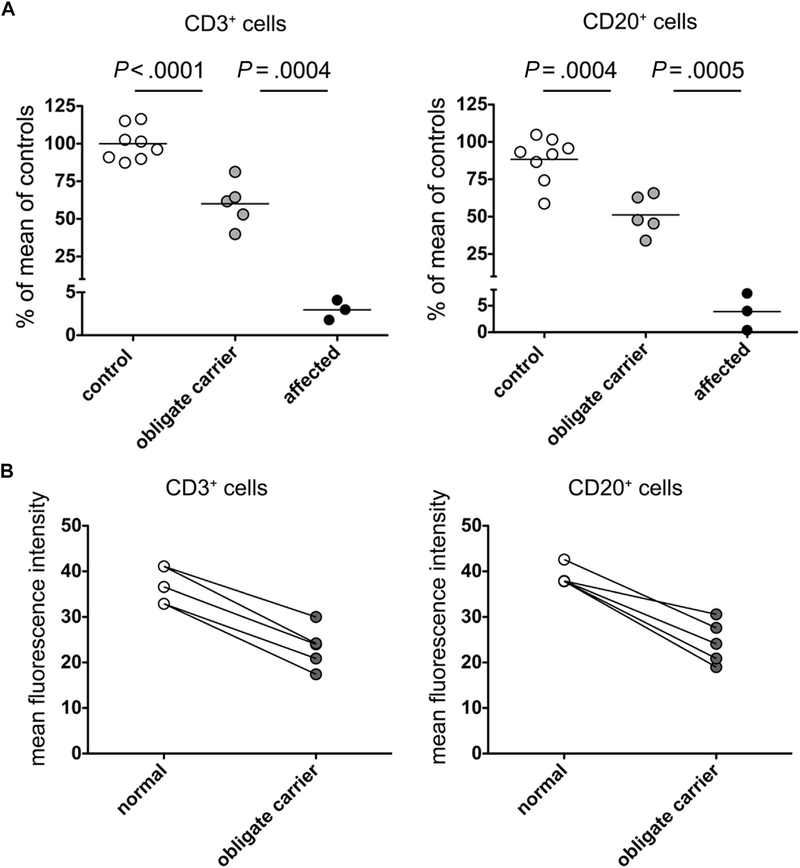
We investigated whether flow cytometry could detect a difference in DOCK8 expression between obligate carriers and healthy controls. DOCK8 expression in obligate carriers was intermediate between patients and healthy controls (Fig 1, C). When calculated as a percentage of DOCK8 expression of the control assayed on the same day, the mean DOCK8 expression of 5 obligate carriers was approximately half that in controls in T cells (57.7%; range, 44.4% to 69.9%) and B cells (52.9%; range, 36.4% to 65.8%). For comparison, the mean DOCK8 expression of 3 patients was 2.2% in T cells (range, 1.9% to 2.6%) and 2.5% in B cells (range, 0.4% to 4.7%). When analyzed in aggregate, the difference in ΔMFI of all controls compared with all carriers was highly statistically significant. While there was overlap in the range of controls and carriers in B cells, in paired analysis the MFI of carriers was always lower than that of the control assayed on the same day (see Fig E3 and Tables E2 and E3 in this article’s Online Repository at www.jacionline.org). Given the overlap between carriers and normal individuals, this assay cannot be used alone to diagnose carrier status and must be confirmed by appropriate genetic testing. Missense mutations or in-frame small deletions that do not affect protein expression may not be detected; however, such cases have not been reported and represent a minority of patients (T. A. Chatila; unpublished data, 2014).
FIG E3.
Analysis of DOCK8 expression in patients and carriers. A, Quantitative expression in T and B lymphocytes in 3 patients (P1, P2, and P4) and in 5 obligate carriers (P1-F, P1-M, P5-F, P5-M, and P6-F). Bars indicate the mean and P values unpaired t tests. B, The mean fluorescence intensity of DOCK8 expression in CD3+ (left panel) and CD20+ cells (right panel) in samples from healthy controls and obligate carriers is shown in paired fashion.
Table E2.
Range of expression of DOCK8 in healthy individuals, carriers, and affected patients shown as percent of mean ΔMFI
| Group | T cell % of mean MFI of controls |
B cell % of mean MFI of controls |
|---|---|---|
| Healthy | 87.4–116.4 | 66.4–118.7 |
| Carrier | 39.9–81.3 | 38.5–74.4 |
| Affected | 1.8–4.1 | 0.5–8.7 |
Table E3.
Range of expression of DOCK8 in healthy individuals, carriers, and affected patients shown as raw MFI
| Group | T cell average MFI (range) |
B cell average MFI (range) |
|---|---|---|
| Healthy | 35.8 (32.1–41.1) | 37.0 (26.4–42.6) |
| Carrier | 23.3 (17.4–30.0) | 24.4 (19.0–30.6) |
| Affected | 7.4 (5.17–11.8) | 6.1 (3.55–8.01) |
The immunological manifestations of DOCK8 deficiency are corrected by allogeneic HCT.3−5 We examined DOCK8 expression in the blood of 4 of 6 patients after HCT (Fig 1, D; Table E1). DOCK8 was detected in T and B cells from patients P3, P5, and P6, who achieved 100% chimerism. Despite all patients receiving myeloablative conditioning, patient P4 had mixed chimerism and showed much higher expression of DOCK8 in T cells (97.8%) than in B cells (30.4%) and monocytes (21.5%) (Fig 1, D). Our finding suggests that DOCK8-expressing cells may have a competitive advantage over DOCK8-deficient cells in the T-cell lineage, as has been reported in murine mixed BM chimeras,6,7 but not in the myeloid or B-cell lineages. Two patients with DOCK8 deficiency undergoing HCT with reduced or no conditioning similarly had mixed chimerism, with engraftment limited to the lymphoid or T lineage and absent in the myeloid lineage.3,5
Assessment of chimerism in different hematopoietic lineages after HCT may predict the likely clinical outcome. High-level Donor chimerism in theTlineage is likely to correlate with freedom from viral infection and possibly virus-associated malignancy. B-cell and natural killer cell functions are impaired in DOCK8 deficiency8,9; however, the level of mixed chimerism in these lineages sufficient to control other manifestations of the disease is yet to be determined. Monitoring mixed chimerism in patients after reduced intensity or minimal conditioning approaches and correlation with clinical symptoms will be key to answering questions regarding the optimal approach and expected efficacy of HCT.
In conclusion, we describe a simple and robust flow cytometry assay for DOCK8 protein expression that can be completed in hours and uses commercially available reagents and standard techniques with sufficient dynamic range to diagnose DOCK8 deficiencyin affected patients and detect potential carrier status in family members. The assay promises to have high clinical applicability in the diagnosis and post-transplant monitoring of current and future corrective therapies in DOCK8 deficiency.
Acknowledgments
The work was supported by a Translational Investigator Service Award (to S.-Y.P.) and the National Institutes of Health (grant nos. 5R01AI065617 and 1R21AI087627 to T.A.C. and grant no. 1R01AI100315 to R.S.G.).
Footnotes
Disclosure of potential conflict of interest: S.-Y. Pai has received salary support from a Translational Investigator Service Award, via Boston Children’s Hospital, her place of employment; she and her institution have grants pending from the National Institute of Allergy and Infectious Diseases. H. de Boer, T. Chatila, and R. S. Geha’s institutions have received funding from the National Institutes of Health. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Zhang Q, Davis J, Lamborn I, Freeman A, Jing H, Favreau A, et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med 2009;361: 2046–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engelhardt KR, McGhee S, Winkler S, Sassi A, Woellner C, Lopez-Herrera G, et al. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J Allergy Clin Immunol 2009;124:1289–302.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bittner TC, Pannicke U, Renner ED, Notheis G, Hoffmann F, Belohradsky BH, et al. Successful long-term correction of autosomal recessive hyper-IgE syndrome due to DOCK8 deficiency by hematopoietic stem cell transplantation. Klin Padiatr 2010;222:351–5. [DOI] [PubMed] [Google Scholar]
- 4.Gatz SA, Benninghoff U, Schütz C, Schulz A, Hönig M, Pannicke U, et al. Curative treatment of autosomal-recessive hyper-IgE syndrome by hematopoietic cell transplantation. Bone Marrow Transplant 2010;46:552–6. [DOI] [PubMed] [Google Scholar]
- 5.Al-Mousa H, Hawwari A, Alsum Z. In DOCK8 deficiency donor cell engraftment post-genoidentical hematopoietic stem cell transplantation is possible without conditioning. J Allergy Clin Immunol 2013;131:1244–5. [DOI] [PubMed] [Google Scholar]
- 6.Randall KL, Lambe T, Johnson A, Treanor B, Kucharska E, Domaschenz H, et al. Dock8 mutations cripple B cell immunological synapses, germinal centers and long-lived antibody production. Nat Immunol 2009;10:1283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Randall KL, Chan SS-Y, Ma CS, Fung I, Mei Y, Yabas M, et al. DOCK8 deficiency impairs CD8 T cell survival and function in humans and mice. J Exp Med 2011; 208:2305–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jabara HH, McDonald DR, Janssen E, Massaad MJ, Ramesh N, Borzutzky A, et al. DOCK8 functions as an adaptor that links TLR-MyD88 signaling to B cell activation. Nat Immunol 2012;13:612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizesko MC, Banerjee PP, Monaco-Shawver L, Mace EM, Bernal WE, Sawalle-Belohradsky J, et al. Defective actin accumulation impairs human natural killer cell function in patients with dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol 2013;131:840–8. [DOI] [PMC free article] [PubMed] [Google Scholar]