Abstract
Purpose:
Ibrutinib, a first-in-class, once-daily, oral inhibitor of Bruton’s tyrosine kinase, promotes apoptosis, and inhibits B-cell proliferation, adhesion, and migration. Ibrutinib has demonstrated single-agent efficacy and acceptable tolerability at doses of 420 and 840 mg in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) who were treatment naïve (TN) or had relapsed/refractory (R/R) CLL after ≥1 prior therapy in a phase 1b/2 study (PCYC-1102). Subsequently, the ibrutinib 420 mg dose was approved in CLL.
Experimental Design:
We report data with 44 months of follow-up on 94 patients with TN and R/R CLL/SLL receiving ibrutinib 420 mg once-daily in PCYC-1102 and the long-term extension study PCYC-1103.
Results:
Ninety-four CLL/SLL patients (27 TN, 67 R/R) were treated with ibrutinib (420 mg/day). Patients with R/R disease had received a median of 4 prior therapies (range, 1–12). Responses were rapid and durable and median duration of response was not reached. Best overall response was 91% (85% TN [complete response (CR) 26%] and 94% R/R [9% CR]). Median progression-free survival (PFS) was not reached in either group. The 30-month PFS rate was 96% and 76% for TN and R/R patients, respectively. Ibrutinib was well tolerated with extended follow-up; rates of grade ≥3 cytopenias and fatigue, as well as discontinuations due to toxicities decreased over time.
Conclusions:
Single-agent ibrutinib at 420 mg once-daily resulted in durable responses and was well tolerated with up to 44 months follow-up in patients with TN and R/R CLL/SLL. Presently, 66% of patients continue on ibrutinib.
Keywords: ibrutinib, CLL, durable response
Introduction
Bruton’s tyrosine kinase (BTK) plays an important role in B-cell receptor (BCR) signaling in normal and malignant B cells (1). Ibrutinib is a first-in-class, once-daily, oral covalent inhibitor of BTK (2). Preclinical data showed promising activity with ibrutinib via its ability to promote apoptosis (3), inhibit B-cell survival and proliferation (4), and inhibit B-cell adhesion and migration (5). In vivo analysis of cells of patients with chronic lymphocytic leukemia (CLL) showed that ibrutinib once-daily caused sustained inhibition of BTK and inhibition of BCR signaling in both circulating and tumor cells within the tissue microenvironment (6). Preclinical results translated well to the clinical setting with substantial activity observed across several B-cell histologies (7).
Results from a phase 1b/2 study (PCYC-1102) of ibrutinib 420 mg or 840 mg once-daily in 31 patients with treatment-naïve (TN),(8) symptomatic CLL or small lymphocytic lymphoma (SLL) aged ≥ 65 years and 85 patients with ≥1 prior therapy (R/R),(9) showed high response rates, durable remissions, and a satisfactory toxicity profile. After pharmacodynamic assessment indicated that ≥95% BTK occupancy and rapid absorption and elimination of ibrutinib were observed at both dose levels, the 840 mg dosing cohort was closed before full accrual of TN patients. Overall response rate (ORR) was similar in the 420 and 840 mg dose cohorts (71% PR+CR, with an additional 20% and 15% achieving partial response with lymphocytosis [PR-L], respectively)(9). )Clinical efficacy of oral ibrutinib 420 mg once daily has also been demonstrated in the phase 3 RESONATE™ study (PCYC-1112). This analysis of 391 patients showed that ibrutinib 420 mg resulted in significant reduction in the rate of progression or death compared with ofatumumab in patients with R/R CLL (10). This encouraging activity in patients with deletion 17p (del17p) CLL led to full approval of the 420 mg once-daily, oral dose of single-agent ibrutinib by the US Food and Drug Administration for treatment of patients with CLL who have received ≥1 prior therapy, and for patients with del17p CLL including as first-line therapy (11). More recently, 3-year follow-up data from PCYC-1102 and the long-term extension study PCYC-1103 in 132 patients (R/R CLL/SLL [n=85], TN [n=31], and CLL ≥2 prior therapies food effect [n=16]) showed that extended treatment with ibrutinib improved quality of response over time, durable remissions, and acceptable toxicity (12). We report safety and efficacy data, with continued follow-up (up to 44 months), for patients in the phase 1b/2 study (PCYC-1102) and extension study (PCYC-1103) who received ibrutinib 420 mg once-daily until progression.
Patients and Methods
The phase 1b/2 study (PCYC-1102) enrolled 132 patients with TN or R/R CLL/SLL to receive either oral ibrutinib 420 mg or 840 mg once-daily on a continuous schedule until progressive disease (PD) or intolerable toxicity. Full details of the study methodology were described previously (8,9). This analysis describes the efficacy and safety of ibrutinib in patients treated with the approved 420 mg daily-dose for treatment of CLL.
All patients were diagnosed with CLL or SLL. The TN group included patients with previously untreated CLL/SLL aged ≥65 years; the R/R group included patients with ≥2 prior therapies including a purine analog, and patients with high-risk CLL/SLL (progression of disease within 24 months of chemoimmunotherapy or failure to respond). Patients completing a minimum of 6 treatment cycles with no evidence of PD were enrolled in the long-term extension study (PCYC-1103). Responses are per investigator assessment, based on International Workshop on CLL (iwCLL) 2008 criteria with marrow aspiration required to confirm CR. PR in the setting of persistent lymphocytosis was characterized as PR-L and included in the reported response rate. In PCYC-1102, all-grade adverse events (AEs) were collected. In PCYC-1103, only grade ≥3 AEs, serious AEs, and AEs leading to discontinuation or dose modification were captured. All AEs were reported as maximum grade per patient. Patients provided written informed consent, and studies were conducted in accordance with the principles of the Declaration of Helsinki. The independent review board of each participating institution reviewed study protocols before implementation. Both studies are registered at clinicaltrials.gov (PCYC-1102: NCT01105247; PCYC-1103: NCT01109069).
Results
Patient characteristics
Of the 132 CLL/SLL patients, 94 were treated with ibrutinib 420 mg per day, including 27 TN patients aged ≥65 years, and 67 R/R patients. The median age was 71 years (range, 65–84) in the TN cohort and 66 years (range, 37–82) in the R/R cohort. Baseline patient and disease characteristics are summarized in Table 1. Previously treated patients received a median of 4 prior therapies (range, 1–12), predominantly of chemotherapy (100%) and anti-CD20 antibody (99%).
Table 1.
Patient and disease characteristics and comorbidity at baseline
| Characteristic | TN ≥65 years 420 (n = 27), % |
R/R 420 (n = 67), % |
|---|---|---|
| Median age, years (range) | 71 (65–84) | 66 (37–82) |
| ≥70 years | 74% | 36% |
| ECOG PS | ||
| 0 | 78% | 40% |
| 1 | 22% | 57% |
| 2 | 0% | 3% |
| Rai stage | ||
| 0-II | 41% | 45% |
| III-IV | 56% | 51% |
| Unknown | 4% | 4% |
| Bulky nodes | ||
| >5 cm | 11% | 52% |
| >10 cm | 0% | 9% |
| β2-microglobulin level >3 mg/L | 30% | 42% |
| FISH cytogenetic abnormalities | ||
| Del17p | 7% | 34% |
| Del11q | 0% | 33% |
| Any cytopenia | 67% | 54% |
| Median ANC, 109/L (range) | 4 (0–19) | 2.5 (0–14) |
| ≤1.5 × 109/L | 4% | 27% |
| Median hemoglobin, g/L (range) | 122 (77–157) | 118 (66–176) |
| ≤110 g/L | 37% | 33% |
| Median platelets, 109/L (range) | 113 (32–217) | 107 (29–310) |
| ≤100 × 109/L | 41% | 39% |
| Median CrCl rate, mL/min (range) | 67 (31–114) | 81 (36–213) |
| ≤60 mL/min | 30% | 19% |
| Median prior therapies, n (range) | 4 (1–12) | |
| 1–2 | -- | 31% |
| 3 | 13% | |
| ≥4 | 55% | |
| Type of prior systemic therapy | -- | |
| Chemotherapy | 100% | |
| Nucleoside analog | 94% | |
| Alkylator (including bendamustine) | 90% | |
| Any anti-CD20–based therapy | 99% | |
| Anti-CD20–based chemoimmunotherapy | 97% | |
| Anti-CD52–based therapy (alemtuzumab) | 24% | |
| Idelalisib | 6% |
ANC, absolute neutrophil count; CrCl, creatinine clearance; ECOG PS, Eastern Cooperative Oncology Group performance status; FISH, fluorescence in situ hybridization; R/R, relapsed/refractory; TN, treatment-naïve.
Patient disposition and safety
The median time on treatment was 30 months (range, 1–44) for TN and 22 months (range, 0–45) for R/R patients. Twenty-two (81%) TN and 40 (60%) R/R patients remain on ibrutinib treatment in the extension study. The primary reasons for discontinuing therapy included PD in 12 (13%) of 94 patients (1 [4%] of 27 TN patients, 11 [16%] of 67 R/R patients) and AEs in 12 (13%) patients (3 [11%] TN, 9 [13%] R/R); of those, 3 (13%) patients had transformation events. Of patients discontinuing due to PD, 5 occurred in year 1 (1 TN, 4 R/R), 4 in year 2 (R/R), and 3 in ≥ year 3 (R/R). For 12 patients discontinuing due to AEs, 7 discontinued in year 1, 3 in year 2, and 2 beyond year 3 (Supplementary Table S1). Four AEs leading to treatment discontinuation were considered related to ibrutinib treatment (1 each grade 3 subdural hematoma and influenza in 2 R/R patients, and 1 each grade 3 pruritic rash and fatigue in 2 TN patients). See Supplementary Table S2 for a further summary of AEs.
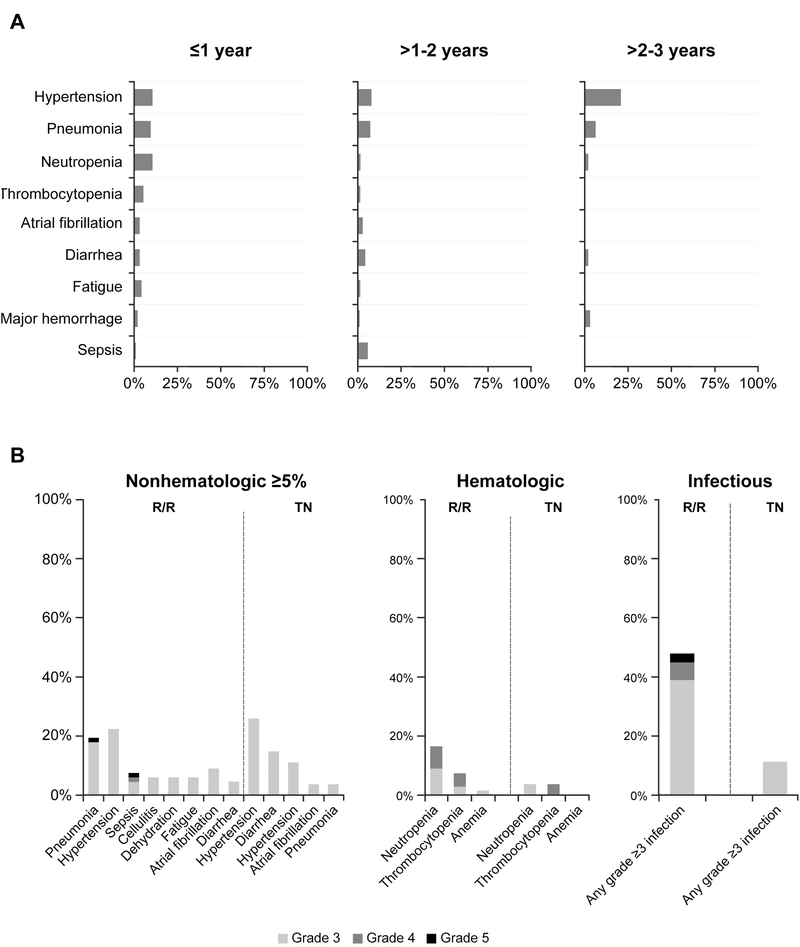
The most common grade ≥3 AEs observed were hypertension (26% TN, 22% R/R) and pneumonia (4% TN, 19% R/R). Grade ≥3 cytopenias were less common in TN than R/R patients (4% vs. 16% for neutropenia, 4% vs. 7% thrombocytopenia, and 0 vs. 1% anemia, respectively). A similar pattern was seen with grade ≥3 infections (11% TN, 48% R/R). Major hemorrhage events were observed in 5% of patients over a median 25 months of treatment. The frequency of grade ≥3 AEs occurring during years ≤1, >1–2, and >2–3 on therapy generally decreased with time including for infection (27%, 25%, and 16%), neutropenia (11%, 1%, 2%), thrombocytopenia (5%, 1%, 0%), atrial fibrillation (3%, 3%, 0%), diarrhea (3%, 4%, 2%), pneumonia (10%, 6%, 6%), and fatigue (4%, 1%, 0%, respectively). The frequency of hypertension appeared higher during the later parts of exposure: 11% during year ≤1 and 20% during year >2–3 (Figure 1A).
Figure 1. Grade ≥3 adverse events.
(A) Grade ≥3 adverse events by time to event onset. Listed adverse events include those that occurred in ≥5% of patients in all-treated population; denominator for each term and time period can vary based on those at risk. (B) Frequency of grade ≥3 adverse events by treatment-naïve (TN) or relapsed/refractory (R/R) status.
Generally, TN patients experienced fewer grade ≥3 toxicities, particularly infectious and hematologic toxicities, compared with R/R patients (Figure 1B). Grade ≥3 drug-related AEs were reported in 6 (22%) TN patients and 25 (37%) R/R patients. Grade ≥3 drug-related serious AEs were reported in 1 (4%) TN patient and 8 (12%) R/R patients. Dose reduction due to an AE occurred in 8 patients (1 TN, 7 R/R), with 7 patients having a dose reduction in year 1, and 1 patient (R/R) with a dose reduction in year 3. The most common AE leading to dose reduction was nausea.
Response
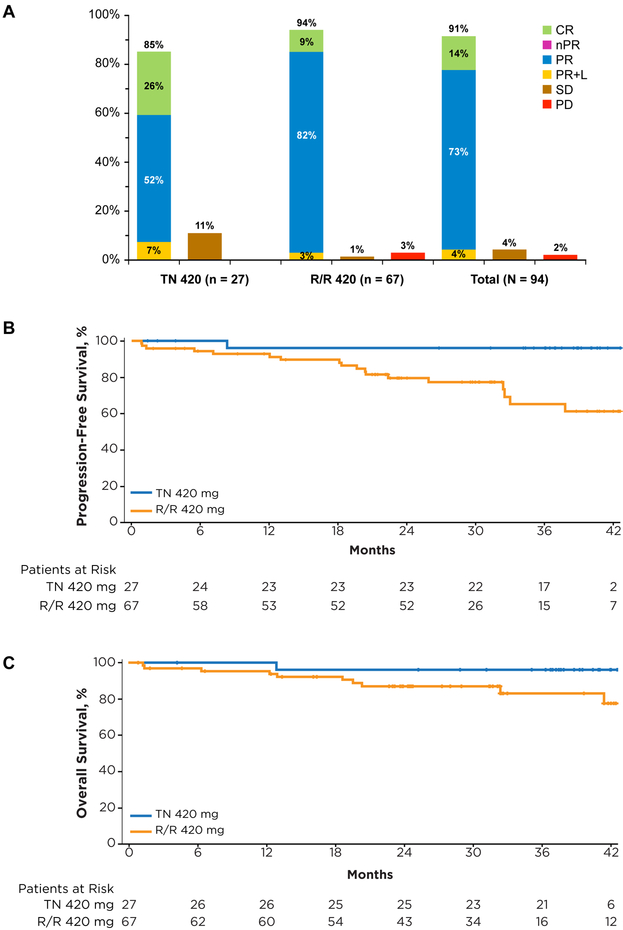
Patients with TN CLL had an ORR of 85%, with 26% complete response (CR), 52% partial response (PR), and 7% PR-L. For R/R patients, an ORR of 94% was observed, with 9% CR, 82% PR, and 3% PR-L. The overall best response to ibrutinib is summarized in Figure 2A. Among patients achieving PR or better, median time to initial response (PR-L or better) was 2 months, with median time to best response of 7 months, and median time to CR of 17 months (Supplementary Table S3). With longer-term follow-up, analysis of cumulative best response over time showed that 49 of 53 (92%) patients with PR-L converted to a deeper response (CR or PR) with longer duration of therapy (Supplementary Table S4). Median duration of response was not reached in both TN and R/R patients.
Figure 2. Best response by investigator assessment and survival with ibrutinib over time.
(A) Best response (investigator-assessed) to ibrutinib therapy. (B) Progression-free and (C) overall survival in patients with TN and R/R CLL.
Survival
The estimated 30-month PFS rate was 96% (95% CI, 74%−99%) in TN and 76% (95% CI, 63%−85%) in R/R patients. Median PFS was not reached for either group (Figure 2B). The PFS with ibrutinib varied by cytogenetic abnormality (Supplementary Table S5; Supplementary Figure 1A); among the previously treated population, patients with del17p (n=23) had a 30-month estimated PFS rate of 60% (95% CI, 34%−78%), less than the 82% (95% CI, 55%−94%) observed for patients with del11q (n=18), and the 85% (95% CI, 60%−95%) observed when neither of these aberrations were present (n=23).
The estimated 30-month overall survival (OS) rate was 96% (95% CI, 76%−99%) in TN patients and 87% (95% CI, 76%−93%) in R/R patients (Figure 2C). As with PFS, OS also varied by interphase cytogenetic abnormality; among the previously treated population, patients with del17p CLL had a 30-month estimated OS rate of 81% (95% CI, 58%−93%), less than the 88% (95% CI, 61%−97%) observed for patients with del11q, and the 90% (95% CI, 66%−98%) 30-month OS rate observed in patients lacking both of these aberrations (Supplementary Table S5; Supplementary Figure 1B).
Changes in cytopenias
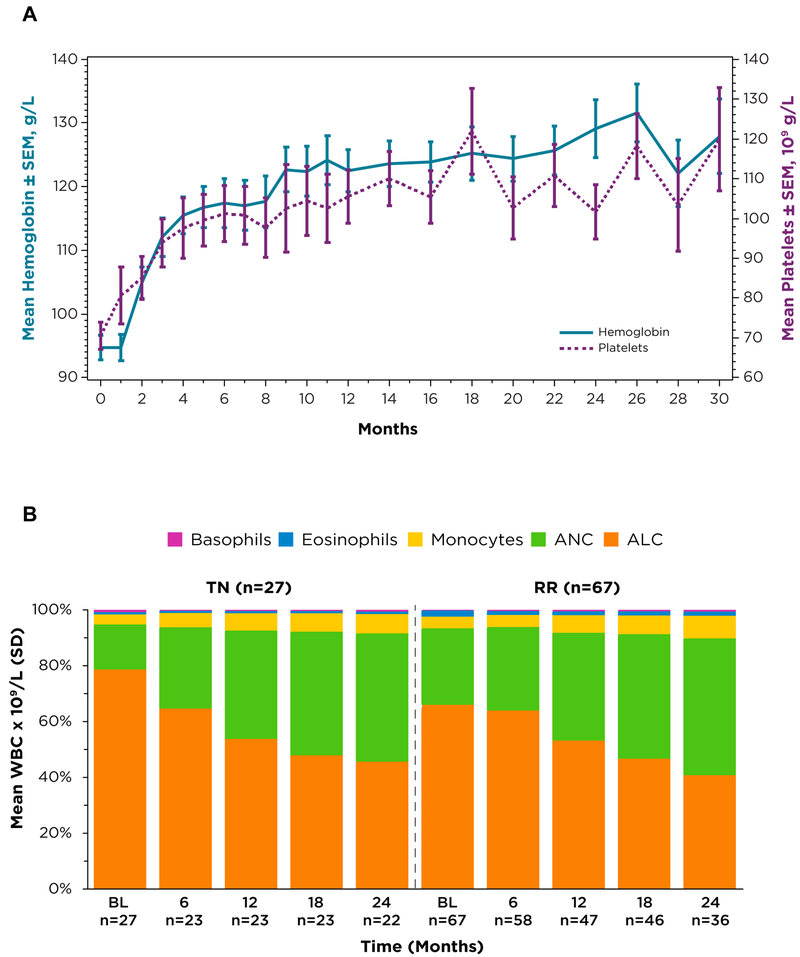
Median baseline hemoglobin (Hgb) level was 122 g/L (range, 77–157) for TN patients, with 37% of TN patients having anemia at baseline (Hgb ≤110 g/L). For patients with R/R disease, median Hgb was 118 g/L (range, 66–176) with 33% of patients with R/R CLL having anemia at baseline (Hgb ≤110 g/L). Median baseline platelet counts were 113 × 109/L (range, 32–217) for TN patients with 41% of TN patients having thrombocytopenia at baseline (platelets ≤100 × 109/L). For patients with R/R disease, median platelet count was 107 × 109/L (range, 29–310), with 39% of patients with R/R CLL having thrombocytopenia (platelets ≤100 × 109/L) at baseline. Among all treated patients with baseline anemia or thrombocytopenia, hemoglobin levels and platelet counts increased over time (Figure 3A).
Figure 3. Change in blood cell counts over time.
(A) Mean hemoglobin levels and platelet counts over time among all patients receiving ibrutinib 420 mg/day with baseline anemia (defined as baseline hemoglobin ≤110 g/L) or thrombocytopenia (defined as baseline platelets ≤100 × 109/L). (B) Change in white blood cell differential and in absolute numbers of white blood cells over time.
Lymphocytosis
Transient lymphocytosis, an expected pharmacodynamic effect of ibrutinib, may result from impaired BCR- and chemokine-controlled retention of malignant cells in marrow and lymph nodes5. Lymphocytosis resolved in 100% of TN and 94% of R/R patients in a median time of 11.3 and 19 weeks respectfully. Analysis of the white blood cell differential showed that, over time, the proportion of lymphocytes decreased (Figure 3B). In contrast, there was a coincident increase in the proportion of neutrophils within the leukocyte differential over time (Figure 3B). Change in absolute numbers of white blood cells over time is tabulated below Figure 3B.
Discussion
Over 3 years of follow-up, patients with TN or R/R CLL/SLL receiving oral ibrutinib at the indicated dose of 420 mg once-daily experienced a high frequency of durable responses including CRs. Previously, patients treated with 840 and 420 mg doses were combined as efficacy was similar, and ≥95% BTK occupancy was achieved at both dose levels. With approval of the oral ibrutinib 420 mg once-daily dose in CLL, understanding the long-term safety and efficacy of this dose is clinically relevant. The current analysis provided up to 44 months of follow-up on older TN and R/R CLL/SLL patients receiving 420 mg single-agent ibrutinib once daily in the PCYC-1102 phase 1b/2 and PCYC-1103 long-term extension studies. This data is consistent with similar results from a previous report of this study that combined the dose groups (12). Most patients continue daily ibrutinib with notable improvement in the quality of response over time including conversion to PR or better in a majority of patients achieving PR-L initially. The ORR was 85% in TN patients (26% CR) and 94% in patients with R/R CLL (9% CR), with a median time to CR of 17 months. The higher CR rate in TN patients may result from either a larger proportion of patients continuing on therapy beyond the 1- and 2-year timepoint, or from these patients receiving ibrutinib earlier in their disease course. Median DOR was not reached in either group, with estimated 30-month progression-free rates of 95% (TN) and 79% (R/R).
Median PFS was also not reached for either group, with estimated 30-month PFS rates of 96% (TN) and 76% (R/R). Among the R/R population, the estimated 30-month PFS rate was lower in patients with del17p (60%) compared with patients with del11q (82%) and with neither cytogenetic abnormality (85%). The estimated 30-month OS rate was 81% in patients with del17p, 88% in patients with del11q, and 90% in patients with neither cytogenetic abnormality. Median PFS of 32 months observed in patients with R/R del17p CLL was a major advance, given that the R/R population was heavily pretreated with a median of 4 prior therapies, and that survival outcomes are typically poor. These patients usually have limited OS of 1 to 2 years (13), with inadequate outcomes such as median PFS ≤1 year even from first-line therapies (14,15). Median OS was not reached for any patients, including those with del17p or del11q CLL.
Ibrutinib was generally well tolerated allowing for extended dosing in most patients (81% of TN and 60% of R/R) with all but 3 (95%) continuing on 420 mg of ibrutinib daily. Adverse events leading to treatment discontinuation and the rate of grade ≥3 infections decreased over time. Grade ≥3 infectious AEs and severe hematologic toxicity were reported at a higher rate for patients who received prior therapy, suggesting that prior therapy and/or general disease state may have contributed to the occurrence of these events in this uncontrolled study.
Prolonged treatment with ibrutinib 420 mg once daily also resulted in early and sustained improvement in hemoglobin and platelet counts for patients with baseline anemia or thrombocytopenia. Incremental changes in the leukocyte differential were observed over time, with continual decrease in lymphocytes and increase in the proportion of neutrophils. These findings are consistent with general restoration of hematologic function with ibrutinib. Treatment-related lymphocytosis, a pharmacodynamic effect observed with ibrutinib as demonstrated in this study, typically occurred early in the course of treatment, was asymptomatic, associated with concurrent improvement in lymph node, spleen, and/or cytopenias, and resolved in the majority of patients with continued treatment (12). This effect, seen within the class of inhibitors of BCR signaling, led the iwCLL to clarify that the lymphocytosis seen with these agents in the context of improvement in other parameters should not be considered a sign of treatment failure (16). Results of a landmark analysis using data from PCYC-1102 showed no significant difference in PFS or OS between ibrutinib-treated patients who achieved PR-L versus patients who achieved traditional PR or CR, supporting the hypothesis that patients with persistent lymphocytosis achieve similar survival outcomes (17). In the present study, the majority of patients with PR-L converted to a deeper response (CR or PR) with continued ibrutinib treatment, supporting earlier findings that treatment-related lymphocytosis improves or resolves with time.
Notably, with up to 44 months of follow-up, 66% of patients (81% TN and 60% R/R) continue on single-agent ibrutinib 420 mg once daily in the PCYC-1103 extension study. The recently completed randomized RESONATE study (10) and initial RESONATE-2 study results (18) confirm the efficacy of ibrutinib versus controlled comparators in TN and R/R patients with CLL/SLL. Additional studies in these settings are evaluating efficacy of ibrutinib in combination with CD20 antibodies-or other targeted agents.
Supplementary Material
Translational Relevance.
Ibrutinib, a first-in-class, once-daily, oral, covalent inhibitor of BTK is approved at the 420 mg dose for treatment of patients with CLL/SLL, and for CLL/SLL with deletion 17p. Approval of ibrutinib in CLL was based on data from the PCYC-1102 study (420 mg/day). Initial reports of the PCYC-1102 study at 420 or 840 mg/day showed high response rates and durable remissions with approximately 21 months of follow-up. A recent 3-year follow-up showed improvement in both frequency and quality of responses as well as acceptable toxicity with continued ibrutinib (420 or 840 mg/day). We report safety and efficacy outcomes in the subset of patients from the PCYC-1102/1103 studies receiving the approved ibrutinib dose in CLL of 420 mg/day with up to 44 months of follow-up. Prolonged treatment with ibrutinib is well tolerated with durable responses, in patients with treatment-naïve and previously treated CLL.
Acknowledgments
Editorial assistance was provided by Susan O’Donnell, PharmD of Nexus GG Science LLC, and supported by Pharmacyclics LLC, an AbbVie Company.
Footnotes
Financial support: Pharmacyclics, LLC, an AbbVie Company
Disclosure of Potential Conflicts of Interest: SEC has been engaged in a consulting or advisory role for Janssen and Pharmacyclics and received research funding from AbbVie and Pharmacyclics; RRF has received honoraria, has been engaged in a consulting or advisory role, and has been a member of a speakers’ bureau for Pharmacyclics; IWF received research funding from Pharmacyclics and Janssen; JB has been engaged in a consulting or advisory role for Janssen, Boehringer Ingelheim, and Portola, received research funding from Pharmacyclics and Gilead, and received travel or accommodation reimbursement from Roche and Janssen; KB received research funding from Celgene, Novartis, Janssen, Pharmacyclics, Seattle Genetics, Millennium, Gilead, MorphoSys, and Constellation Pharmaceuticals; JS received honoraria from and has been engaged in a consulting or advisory role with Pharmacyclics, received research funding from Pharmacyclics, Gilead, and Janssen, and is a member of speakers’ bureau for Gilead; JJ has been engaged in a consulting or advisory role with Pharmacyclics and received research funding from Pharmacyclics and AbbVie; WW received honoraria from and has been engaged in a consulting or advisory role for Sanofi, Genentech/Roche, Pharmacyclics, Celgene, Gilead, GSK/Novartis, Genzyme, Merck, AbbVie, and Emergent and received research funding from GSK/Novartis, AbbVie, Karyopharm, Genentech, Pharmacyclics, Acerta, Gilead, Janssen, Emergent, Juno, and Kite; AT, CZ, EB, and DFJ declare employment by and ownership interest in Pharmacyclics; SO has received honoraria and research funding from and has been engaged in a consulting or advisory role for Pharmacyclics. WZ, NAH, AJJ, and JCB declare no competing financial interests.
Previously presented at the 2015 AACR Annual Meeting.
References
- 1.Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A 2010;107:13075–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burger JA, Buggy JJ. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765). Leuk Lymphoma 2013;54:2385–91 [DOI] [PubMed] [Google Scholar]
- 3.Herman SE, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood 2011;117:6287–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ponader S, Chen SS, Buggy JJ, Balakrishnan K, Gandhi V, Wierda WG, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood 2012;119:1182–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Rooij MF, Kuil A, Geest CR, Eldering E, Chang BY, Buggy JJ, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood 2012;119:2590–4 [DOI] [PubMed] [Google Scholar]
- 6.Herman SE, Mustafa RZ, Gyamfi JA, Pittaluga S, Chang S, Chang B, et al. Ibrutinib inhibits BCR and NF-kappaB signaling and reduces tumor proliferation in tissue-resident cells of patients with CLL. Blood 2014;123:3286–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol 2013;31:88–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Brien S, Furman RR, Coutre SE, Sharman JP, Burger JA, Blum KA, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol 2014;15:48–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013;369:32–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 2014;371:213–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.IMBRUVICA® (ibrutinib) capsules [package insert]. Sunnyvale, CA: Pharmacyclics, Inc; 2015. [Google Scholar]
- 12.Byrd JC, Furman RR, Coutre SE, Burger JA, Blum KA, Coleman M, et al. Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood 2015;125:2497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stilgenbauer S, Zenz T. Understanding and managing ultra high-risk chronic lymphocytic leukemia. Hematology / the Education Program of the American Society of Hematology. American Society of Hematology. Education Program 2010;2010:481–8 [DOI] [PubMed] [Google Scholar]
- 14.Hillmen P, Skotnicki AB, Robak T, Jaksic B, Dmoszynska A, Wu J, et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol 2007;25:5616–23 [DOI] [PubMed] [Google Scholar]
- 15.Stilgenbauer S, Schnaiter A, Paschka P, Zenz T, Rossi M, Dohner K, et al. Gene mutations and treatment outcome in chronic lymphocytic leukemia: results from the CLL8 trial. Blood 2014;123:3247–54 [DOI] [PubMed] [Google Scholar]
- 16.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008;111:5446–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woyach JA, Smucker K, Smith LL, Lozanski A, Zhong Y, Ruppert AS, et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood 2014;123:1810–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N Engl J Med 2015;373:2425–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.