Abstract
Purpose
The purpose of this study was to evaluate engraftment and adverse events with a conditioning and prophylactic regimen intended to achieve high rates of engraftment with minimal graft-versus-host disease (GVHD) in allogeneic transplantation for chronic granulomatous disease in a single center.
Methods
Forty patients, 37 male, with chronic granulomatous disease were transplanted. Transplant products were matched sibling peripheral blood stem cells (PBSCs) in four and matched unrelated donor (MUD) bone marrow in three, and one patient received mismatched unrelated PBSCs. Thirty-two patients received MUD PBSCs. All patients received a conditioning regimen of busulfan/alemtuzumab (with low-dose total body irradiation for MUD recipients) with sirolimus graft-versus-host disease prophylaxis.
Results
Engraftment occured in 38/40 recipients (95%). Acute or chronic GVHD occurred in 18 (45%) and 5 (12.5%), respectively, with 6 episodes of grades III–IVand/or steroid refractory GVHD. Overall survival was 33/40 (82.5%) and event-free survival was 30/40 (80%). Successful engraftment was associated with myeloid and NK cell, but not CD3+ chimerism. Myeloid engraftment was greater than 70% in 30/32 recipients at mean follow-up of 3.4 years. Evidence of persistent immunodeficiency was not seen in successful transplants. Attempts to rescue failed or poorly functioning grafts were associated with unacceptable morbidity and mortality.
Conclusions
A reduced-intensity allogeneic transplant protocol based on alemtuzumab and busulfan with sirolimus GVHD prophylaxis produced high rates of successful engraftment and minimal regimen-related toxicity. Prolonged clinical follow-up has confirmed its efficacy in ameliorating CGD-related disease. Outcomes were not acceptable with donor cell infusion rescue of cause with poor graft function.
Keywords: Chronic granulomatous disease, hematopoietic stem cell transplantation, reduced-intensity conditioning
Introduction
Chronic granulomatous disease of childhood is the most common primary immunodeficiency disorder specifically impairing the function of phagocytic cells, with approximately 1/200,000 live births affected in the USA. The defect in the myeloid lineage cells has made hematopoietic stem cell transplantation (HSCT) a curative option that is recommended therapy by some authorities [1]. The special considerations related to CGD and HSCT relate to the need for successful myeloid engraftment in a population with a substantial pretransplant burden of infectious and inflammatory disease. Transplant outcomes have been addressed in numerous studies, often with small numbers and/or with substantial variation in conditioning regimens [2–6]. HSCT provides better survival and clinical outcomes than conventional treatment, but rejection with the need for subsequent retransplantation has been needed [1, 7]. The heterogeneity of conditioning regimens makes it difficult to ascribe outcomes to individual components of the transplant procedure.
This point is illustrated in an initial report from European centers, in which T cell replete grafts and myeloablative (majority) conditioning led to a survival of 85.2% with grades 3–4 graft-versus-host disease (GVHD) in 4 of 27 recipients, with 4 deaths (all in patients with pretransplant refractory fungal infections) [8]. A subsequent report details results of 56 patients from 16 centers in 10 countries with reduced-intensity conditioning consisting of fludarabine/busulfan, with anti-thymocyte globulin (ATG) or alemtuzumab added for matched unrelated donor (MUD) transplants. This resulted in an event-free survival of 91% at 2 years and overall survival of 96%. There were three graft failures. GVHD grades 2–4 occurred in 11% and grades 3–4 in 4% (all MUD). Two deaths occurred, both in cases of steroid-refractory GVHD [9].
The US National Institutes of Health (NIH) reported results using matched sibling donors in 10 patients, with cyclophosphamide, fludarabine, and ATG conditioning, in addition to requiring a CD34+ cell dose of 5 × 106/kg (mean dose achieved 7.9 × 106) with depletion and then enrichment of the graft with CD3+ cells to achieve a final dose of 1 × 105/kg in all recipients. Of 10 patients, one highly alloimmunized recipient never engrafted but did have autologous recovery, and a second patient rejected after 8 months. Donor lymphocyte infusions were used in all but one patient. GVHD grades 2–4 occurred in three, with one death from grade 4 gastrointestinal (GI) and skin GVHD [10].
Here, we report our results with a regimen utilizing alemtuzumab and weight-based busulfan, with low-dose total body irradiation (TBI) for MUD recipients, and sirolimus GVHD prophylaxis. The regimen is designed to achieve maximum engraftment rates with minimal regimen-related toxicity (RRT) and low rates of GVHD grades 2–4.
Methods
Study Design and Procedures
We conducted a study to determine the efficacy and safety of non-myeloablative allogeneic HSCT for patients with CGD. The study was approved by the institutional review board of the National Institute of Allergy and Infectious Diseases and was independently monitored for safety and data accuracy. Written informed consent and assent were obtained for all patients and donors in accordance with the Declaration of Helsinki.
Patients between the ages of 2 and 65 years were eligible if they met the following criteria: confirmed CGD by dihydrorhodamine (DHR) 123 assay and confirmatory molecular testing, with sufficient complications from the CGD to warrant the risk of transplantation.
A 6/6 matched related donor (MRD), or 9–10/10 MUD donor was required. For unrelated donors, the donor is high-resolution matched (4 digits) at HLA-A, HLA-B, HLA-C, DRB1, and DQB1. For matched siblings, the donor and recipient are low-resolution HLA typed at class I (HLA-A, HLA-B, and HLA-C), and high-resolution confirmatory typing is performed for class II (DRB1 and DQB1). For peripheral blood stem cell collection at the NIH Clinical Center, matched related and unrelated donors received 5–6 days of granulocyte colony-stimulating factor (10 μg/kg/day), followed by apheresis on day 5 with the goal of collecting at least 5 × 106 CD34+ cells/kg of the recipient’s body weight. Recipients were evaluated for HLA antibodies and the presence of McLeod syndrome.
Conditioning Regimen
Conditioning consisted of alemtuzumab 0.03 mg/kg on day −8, 0.1 mg/kg day −7, 0.3 mg/kg days −6 to −4, and intravenous once-daily busulfan 5 mg/kg days −3 and −2. A series of busulfan levels were obtained at the end of the first infusion, but doses were not adjusted on this basis. MUD recipients received 300 cGy total body irradiation (TBI) on day −1. Cells were infused on day 0. Sirolimus (5 mg every 4 h for 3 doses and then 5 mg daily in adults, 3 mg/m2 on the same schedule for pediatric patients) was begun on day −1 and adjusted to maintain trough levels between 10 and 20 ng/ml.
Supportive Care
We followed standard guidelines for supportive care established at the NIH Clinical Center for patients undergoing allogeneic HSCT. Mucositis was graded according to the NCI Common Terminology Criteria for Adverse Events Grading Scale.
T, B, and NK Cells
CD14+ monocytes, CD3+/CD56+ NK cells, CD19+ B lymphocytes, and CD3+ T lymphocytes were quantified by flow cytometry pretransplantation and at designated intervals post-transplantation.
Analysis of Chimerism
Engraftment of donor cells was assessed using polymorphisms in regions known to contain short tandem repeats. Peripheral blood CD14+, CD3+/CD56+, CD19+, and CD3+ cells were selected using flow cytometry at the designated time points, and chimerism was assessed on these subpopulations. In addition, CD14+/CD15+ myeloid cells and CD3+ T lymphocytes were selected using immunobeads, and chime-rism was assessed on the selected cells. The lower limit of sensitivity for this method is 1–3% of donor-type polymorphic markers in the mixture; these sensitivities are on the basis of studies using mixtures of known proportions of allogeneic DNA samples.
Statistical Analysis
Overall survival and event-free analyses were conducted. The event-free survival is a composite outcome, i.e., either rejection, graft function poor enough to necessitate a donor cell infusion or new transplant, or death. The log-rank statistic was used to test the association between clinical variables and survival. S-Plus program was used in the statistical analyses. S-PLUS is a commercial implementation of the S programming language sold by TIBCO Software Inc., Palo Alto, CA.
Results
From 2007 to 2015, 40 patients were transplanted, of whom 37 were male. Transplant characteristics are shown in Table 1. Ages ranged from 4 to 32 years, mean 16 (IQR 8–23), with 23 (58%) children <18 years of age. All patients had confirmed CGD, which was X-linked (XL, CYBB, gp91phox) in 34, autosomal recessive (AR) p22 (CYBA p22phox) in 2, AR p47 (NCF1 p47phox) in2, and AR p40 (NCF4, p40phox) in 2. The distribution of NADPH-oxidase activity by DHR 123 assay in transplanted patients as a cohort of our institutional database is shown in Fig. 1. Two patients received second transplants, one of whom received a second transplant from the same donor.
Table 1.
Patient characteristics
| Age/sex | CGD type | Clinical disease | Disease at transplant | Busulfana | Donor/Product | TNC(109) | CD34+ (106)/kg | CD3+(107)/kg | Outcome/years | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 M | XL | Infection | 2249.50 | MUD PBSC | 9.87 | 8 | 25.5 | Rejection | |
| 2 | 4 M | XL | Mix | 2702.00 | MUD PBSC | 27.70 | 10 | 29.8 | Rejection/death day 508 | |
| 3 | 4 M | XL | Infection | 2336.00 | MUDBM | 14.90 | 5.16 | 14.9 | Cure/5 | |
| 4 | 5 M | XL | Mix | 2163.00 | MUDBM | 13.60 | 9.1 | 9.16 | Death day 296 | |
| 5 | 6 M | XL | Mix | A. nidulans pneumonia spinal osteomyelitis | 3747.00 | Msib PBSC | 13.60 | 7.36 | 14.3 | Cure/5.5 |
| 6 | 6 M | XL | Mix | 2656.00 | MUD PBSC | 23.30 | 9.43 | 8.43 | Cure/5 | |
| 7 | 6 M | XL | Mix | Fungal brain abscess | 4221.00 | MUD PBSC | 10.00 | 7.9 | 9.38 | Rejection/death day 397 |
| 8 | 8 M | XL | Inflammation | Pulmonary infiltrates | 2896.00 | MUD PBSC | 26.50 | 9.88 | 16.6 | Cure/5.1 |
| 9 | 8 M | XL | Mix | 4294.00 | Msib PBSC | 11.60 | 5.63 | 18.1 | Cure/3 | |
| 10 | 8 M | XL | Mix | 5242.00 | MUD PBSC | 65.90 | 9.41 | 41.1 | Cure/4.1 | |
| 11 | 8 M | XL | Mix | 2417.00 | MUD PBSC | 27.30 | 8.03 | 23.7 | Cure/4 | |
| 12 | 8 M | p40 | Inflammation | 7158.00 | MUD PBSC | 21.90 | 10.4 | 20.1 | Cure/5.1 | |
| 13 | 9 M | XL | Infection | Pulmonary infiltrate | 3662.00 | MUD PBSC | 20.00 | 9.54 | 28.8 | Cure/2.6 |
| 14 | 11 M | XL | Mix MacLeod syndrome | 3554.00 | MUD PBSC | 28.30 | 8 | 18.4 | Cure/3.5 | |
| 15 | 11 M | XL | Mix | 2333.90 | MUD PBSC | 13.50 | 8 | 20.3 | Cure/2.9 | |
| 16 | 11 M | XL | Infection | 3561.00 | MUD PBSC | 36.40 | 8 | 46.8 | Cure/4 | |
| 17 | 11 M | XL | Infection | 2854.00 | MUD PBSC | 34.50 | 8 | 30.5 | Cure/3.1 | |
| 18 | 13 F | AR p22 | Inflammation | 5406.00 | MUD PBSC | 10.30 | 2.1 | 5.08 | Cure/2.0 | |
| 19 | 13 M | XL | Mix | 2500.00 | MUD PBSC | 61.40 | 8.81 | 40.9 | Cure/5 | |
| 20 | 15 M | XL | Infection | 3399.00 | MUD PBSC | 40.70 | 6.14 | 33.1 | Cure/2 | |
| 21 | 17 M | AR p47 | Mix | 3470.00 | MUD PBSC | 73.70 | 6.67 | 45.1 | Death day 663 | |
| 22 | 17 F | AR p47 | Mix | 10,795.00 | MUD PBSC | 24.20 | 7.02 | 13.4 | Cure/6.5 | |
| 23 | 17 M | XL | Inflammation | 4408.00 | MUD PBSC | 40.60 | 6.64 | 39.5 | Cure/5 | |
| 24 | 18 M | XL | Mix | Pyrenochaeta liver/lung abscess | 3414.00 | Mismatch (5/6) UD | 44.70 | 5.34 | 34.9 | Death day 90 |
| 25 | 19 M | XL | Mix | 3748.00 | MUD PBSC | 44.70 | 5.34 | 34.9 | Rejection/death day 400 | |
| 26 | 19 M | p40 het | Inflammation | 6782.00 | Msib PBSC | 27.10 | 3.47 | Cure/2.0 | ||
| 27 | 20 M | XL | Infection | A. nidulans pneumonia spinal osteomyelitis | 3219.00 | MUD PBSC | 97.60 | 10 | 34.7 | Cure/2.0 |
| 28 | 21 M | XL | Mix Macleod syndrome | Fungal meningitis encephalitis | 4651.00 | MUD PBSC | 119.00 | 6.26 | 42.4 | Cure/5 |
| 29 | 22 M | XL | Mix | Nocardia pneumonia | 3051.00 | Msib PBSC | 14.20 | 2.75 | 5.84 | Cure/1.5 |
| 30 | 23 M | XL | Mix | 7712.00 | Msib PBSC | 13.30 | 2 | 5.77 | Cure/2.0 | |
| 31 | 23 M | XL | Infection | 3590.00 | MUD PBSC | 98.50 | 9.04 | 48.6 | Rejection | |
| 32 | 24 M | XI | Infection | 6182.00 | MUDBM | 16.30 | 1.9 | 3 | Cure/2.0 | |
| 33 | 25 M | XL | Infection | 3446.00 | MUD PBSC | 56.50 | 7.02 | 19.3 | Cure/5.7 | |
| 34 | 26 M | XI | Mix | 4418.00 | MUD PBSC | 44.90 | 7.98 | 30 | Cure/2.0 | |
| 35 | 26 M | XL | Mix | Fungal pneumonia | 2249.50 | MUD PBSC | 52.00 | 8.05 | 38.8 | Cure/1 |
| 36 | 29 M | XL | Mix | Nocardia pneumonia | 2703.00 | MUD PBSC | 74.50 | 8.83 | 37.5 | Cure/1.4 |
| 37 | 29 F | AR p22 | Mix | Pneumonia | 6373.00 | Msib PBSC | 13.60 | 1.56 | 7.38 | Cure/5.2 |
| 38 | 31 M | XL | Infection | Fungal osteomyelitis | 4265.00 | MUD PBSC | 43.10 | 1.32 | 16.4 | Cure/3.2 |
| 39 | 32 M | XL | Infection | Chronic kidney disease | 3758.00 | MUDBM | 14.90 | 2.86 | 3.33 | Death day 92 |
| 40 | 32 M | XL | Mix | 6000.00 | MUD PBSC | 51.60 | 6.94 | 32 | Rejection |
min*microm/L
Fig. 1.
Distribution of PMA-stimulated production from neutrophils isolated from CGD patients within NIH cohort
Matched sibling peripheral blood stem cells (PBSC) were used in four cases, matched unrelated bone marrow in three, and one patient received mismatched (9/10, mismatch at HLA-A) unrelated PBSCs. No non-sibling 6/6 related donors were used. The remaining 32 products were PBSCs from 10/10 HLA-matched unrelated donors. The median total nucleated cell dose (TNC) was 27.5 × 109 (IQR 14.4–50.0), CD34+ stem cell dose was 7.63 × 106/kg (IQR 5.34–8.83) and the CD3+ cell dose 2.37 × 108/kg (one missing data point) (IQR 1.34–3.49).
The busulfan median AUC was 3575.5 min*microm/L (IQR 2740.75–4592.75) or 14,677.4 ng/ml*h (IQR 11250.78–18,853.23 ng/ml*h). Our institution does not have onsite therapeutic drug monitoring for busulfan, and thus, no adjustment was made to the weight-based dose.
Conditioning was generally well tolerated, with only two patients experiencing grade 3 mucositis, one episode of posterior reversible encephalopathy syndrome, and no other organ dysfunction suggestive of sinusoidal obstruction syndrome or interstitial pneumonitis. The administration of alemtuzumab was almost always associated with fever and rigors, and these responded rapidly to antipyretics and meperidine, with the exception of a single case in which fevers persisted through and after the alemtuzumab, heralding a recrudescent Burkholderia cepacia complex bacteremia prior to neutropenia.
The indication for transplantation in five patients was inflammatory disease, predominantly inflammatory bowel disease or GI obstruction/stricture, or urinary tract obstruction. Twelve patients had predominantly infectious complications, such as lymphadenitis, pneumonia, osteomyelitis, and liver abscess. A mixed picture with infections and inflammatory disease was the indication for transplantation in 23 patients.
Major infections active at transplant included two pneumonias without microbiologic diagnosis, Aspergillus nidulans pneumonia and spinal osteomyelitis, Scedosporium apiospermum pneumonia and spinal osteomyelitis, liver abscess from Pyrenochaeta romeroi with extension through the diaphragm and involving the spine, Nocardia pneumonia, two cases of Aspergillus pneumonia with spread to the central nervous system, and fungal pneumonia with two species of Phellinus. Of nine patients with active infection, seven received granulocyte transfusions, which began at the onset of neutropenia and continued until neutrophil engraftment. One additional patient received granulocyte transfusion for B. cepacia complex sepsis that began during conditioning, prior to neutropenia. With the exception of this last patient, persistence of their major infection despite prolonged conventional therapy was an indication for proceeding with transplant.
Median days of neutropenia were 13.5 (IQR 10.5–17.5), days to engraftment 19.5 (IQR 20–25.5). Primary failure to engraft neutrophils occurred in two cases. Platelets were never lower than 20 × 109/ml in nine cases and never lower than 50 × 109/ml in one. In 12 cases, platelet transfusions obscure the period to platelet engraftment >20 × 109/ml. Days to platelet count >50 × 109/ml were 27 (IQR 16–28.5). Two patients never had platelet engraftment. Two patients with the McLeod blood phenotype successfully engrafted.
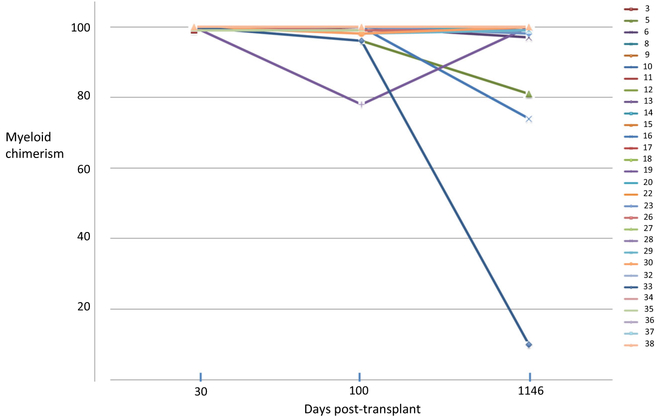
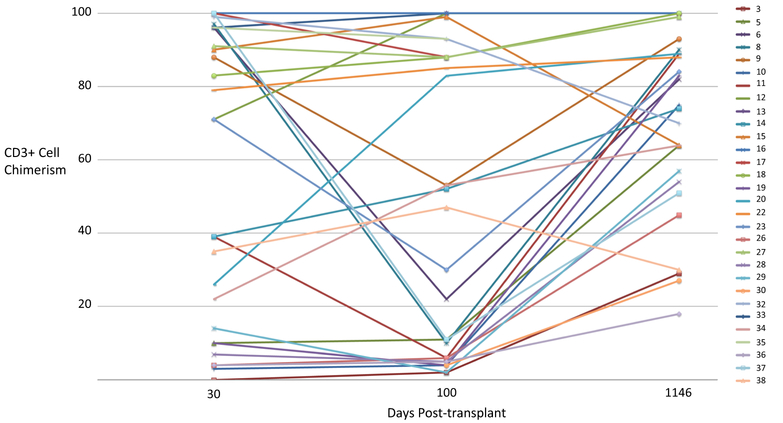
Overall survival was 82.5% (33/40) and event-free survival 80% (30/40) at a median follow-up of 3.4 years (Fig. 2). Overall survival at 2 and 3 years are the same, 81.3% (95% CI 69.7%, 94.9%) and event-free survival at 2 and 3 years are the same, 74.8% (95% CI 62.5%, 89.6%). For associations with overall and event-free survival, the univariate analysis is shown in Table 2. Multivariate analysis did not show greater ability to predict events than univariate analysis. Graft chimerism for myeloid engraftment at day 30 was 98–100% in all successful engraftments and is shown in Fig. 3 for 30 recipients with persistent graft function through day 1146. CD19+ and NK cell engraftments in assessable cases (too few cells in 23 cases) were 88% (IQR 56–98.5) and 97% (IQR 88–99), respectively. Day 100 myeloid and NK chimerisms were associated with overall survival (P = 0.033 and 0.024, respectively). For overall survival, both day 30 myeloid and NK chimerisms (P = 0.034 and P = 0.023, respectively) and day 100 myeloid chimerisms (P < 0.001 and P = 0.008, respectively) were predictive. Mean CD3+ cell chimerism was 71% (IQR 10–96) at day 30, in 35 evaluable cases. Day 30 CD3+ cell counts were median 69 × 109/ml (IQR 24–157.5) in 30 evaluable cases. Day 100 myeloid chimerism in 37 cases was median 100% donor (IQR 98–100), CD3+ cell chimerism 33.5% (IQR 5–89), NK cell % 98.5 (IQR 89–100), and CD19+ cell median 93% (IQR 86–100). Mixed CD3+ cell chimerisms are shown in Fig. 4 for 30 recipients with persistent graft function through day 1146. Day 100 CD3+ cell counts were median 76.5 × 109/ml (IQR 35.25–220) in 33 evaluable cases.
Fig. 2.
Event-free survival and overall survival
Table 2.
Overall and event-free survival, univariate analysis
| Overall survival | Event-free survival | |
|---|---|---|
| Age | 0.634 | 0.847 |
| Sex | 0.413 | 0.333 |
| Clinical diseasea | 0.128 | 0.400 |
| Busulfan AUC (min*microm/L) | 0.136 | 0.254 |
| Total nucleated cell dose | 0.630 | 0.844 |
| CD34+ cell dose | 0.985 | 0.669 |
| CD3+ cell dose | 0.934 | 0.476 |
| Day 30 myeloid chimerism | 0.245 | 0.034 |
| Day 30 CD3+ chimerism | 0.764 | 0.548 |
| Day 30 NK chimerism | 0.593 | 0.023 |
| Day 100 myeloid chimerism | 0.033 | <0.0001 |
| Day 100 CD3+ chimerism | 0.902 | 0.325 |
| Day 100 NK chimerism | 0.024 | 0.008 |
Clinical disease includes the categories infectious, inflammatory, or mixed
Fig. 3.
Myeloid chimerism of 30 patients with engraftment through day 1146. Data sample numbers are consistent with patient designations in Table 1
Fig. 4.
CD3+ cell chimerism of 30 patients with engraftment through day 1146. Data sample numbers are consistent with patient designations in Table 1
Viral reactivation occurred in 26 cases (CMV 12, EBV 18, CMV/EBV 4, EBV/BK 2). One child had CMV viremia prior to conditioning. Only one case of EBV-associated post-transplant lymphoproliferative disease (PTLD) occurred and was managed successfully with rituximab. Thirteen patients had bacteremias, four of which were prior to neutropenia.
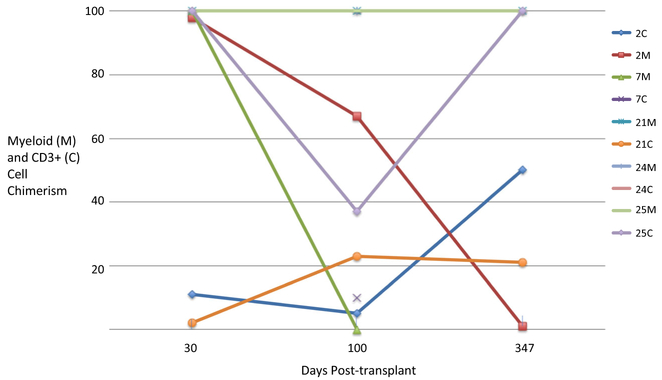
Outcomes for recipients with the composite endpoint of rejection/poor graft function/death are shown in Table 3. Six deaths were associated with grades III–IV or steroid-refractory GVHD. One of these deaths occurred in a primary graft failure patient, and in five of these cases (three after day 100), donor cell infusions were given to boost poor graft function and/or declining myeloid chimerism, or in one case, refractory Evans syndrome with mixed B cell chimerism. One patient died of cerebral Aspergillus infection as a complication of the treatment of severe skin GVHD. One death occurred after aplasia developed after a busulfan-conditioned transplant for late rejection of the first graft. The relation of myeloid and CD3+ cell chimerisms to outcomes in five patients who received donor cell infusions is shown in Fig. 5.
Table 3.
Engraftment, rejection, and death summary outcomes
| Age sex | Engraftment (day) | Rejection (day) | Death (day) | GVHD | Myeloid chimerism prior/post intervention | CD3+ chimerism prior/post intervention | Intervention (day) | Post-transplant infections | Complications and cause of death |
|---|---|---|---|---|---|---|---|---|---|
| 4 M | 15 | 73 | K. pneumoniae bacteremia | Survived | |||||
| 32 M | No | 92 | SR | 0/92 | 0/62 | DCI | K. pneumoniae bacteremia | Multiorgan failure/ K. pneumoniae | |
| D 58 | bacteremia/sepsis | ||||||||
| 17 M | 20 | 230 | 663 | SR | 6/39 | 99/99 | 2nd TX | A. terreus pneumonia | Evans syndrome myositis, multiorgan |
| D 616 | failure | ||||||||
| 6 M | 26 | 75 | 397 | SR | 0/13 | 0/91 | 2nd Tx | S. maltophilia bacteremia | Respiratory/renal failure |
| D 134 | |||||||||
| 18 M | 21 | 90 | Skin, liver | 100/100 | 100/100 | DCI | KPC K. pneumoniae bacteremia | DAH/VOD | |
| D 82 | K. pneumoniae sepsis | ||||||||
| 32 M | No | 35 | No | K. pneumoniae bacteremia/fungal pneumonia | Bleeding at ostomy survived | ||||
| 4 M | 21 | 264 | 508 | Grades 3–4 | 50/55 | 1/0 | 2nd Tx | HHV6 encephalitis | Respiratory, renal, liver failure |
| D 314 | |||||||||
| 5 M | 28 | 296 | Skin | CNS aspergillosis | CNS aspergillosis | ||||
| 23 M | 39 | 161 | K. pneumoniae bacteremia | Survived | |||||
| 19 M | 20 | 53 | 400 | Skin/GI | 37/100 | 100/100 | DCI | KPC K. pneumoniae bacteremia | Respiratory, renal failure |
| D 98 |
DCI unmanipulated donor cell infusion, TX same donor cell infusion with conditioning, GI gastrointestinal, SR steroid refractory, KPC Klebsiella pneumoniae carbapenemase-producing, CNS central nervous system, DAH diffuse alveolar hemorrhage, VOD veno-occlusive disease of the liver
Fig. 5.
Myeloid (M) and CD3+ (C) cell chimerism of five patients with adverse outcomes related to donor cell infusions. Patient 2 had a second transplant which failed. Patient 7 had insufficient cells for analysis at day 30 and died prior to further analysis after day 100. Patient 21 had Evans syndrome with full myeloid chimerism at all points. Patient 24 received a donor cell infusion for persistent cytopenias in the context of ongoing infection but was fully engrafted in both compartments throughout. Patient 25 received a donor cell infusion after day 100 for cytopenias in the context of ongoing infection. Data sample numbers are consistent with patient designations in Table 1
Two patients had primary graft failure. Cell doses were 2.86 and 6.94 × 106 CD34+ cells/kg. Both had received intensive transfusion, including granulocytes, and one was demonstrably broadly alloimmunized. This latter patient received an infusion of the stored autologous product which we keep to avoid prolonged aplasia, as he had both hemorrhage (at an ostomy site) and a new infiltrate before recovery, which then occurred at day 51 (neutrophils) and day 73 (platelets). The second patient with primary graft failure died of renal failure and the complications of GVHD after a donor cell infusion. Two patients died after infusion of donor cells without conditioning, one in the context of steroid-refractory GVHD and one as a consequence of GVHD after the infusion to boost poor graft function for declining chimerism. Four patients died after retransplantation (with conditioning). One death was due to central nervous system aspergillosis that developed during corticosteroid therapy for GVHD.
We utilized granulocyte transfusions to support nine patients with active infections (all fungal with the exception of one Nocardia pneumonia) during transplant-induced neutropenia. None had progression of their infection during transplant. Two patients had undiagnosed pulmonary infiltrates during transplantation and an additional patient, who would develop fatal dissemination of Aspergillus disease to the brain, probably developed invasive pulmonary aspergillosis during engraftment, but no confirmatory diagnosis was made.
Fifty-six diagnostic procedures to evaluate tissue for GVHD were performed in 26 patients. Twenty-one of these biopsies (37.5%) were not consistent with GVHD. There were 18 episodes of acute GVHD (skin 13, GI tract 10, liver 3). As noted above, in six cases, death was attributed to complications of grades 3–4 or steroid-refractory GVHD. All other cases responded to either topical therapy or a short course of 1 mg/kg of prednisone daily. Five cases of chronic GVHD involved the skin, oral cavity, and eyes. All chronic GVHD was limited in extent, mild in severity, and resolved at last follow-up with one exception, continued use of topical therapy for mild ocular disease. All patients successfully stopped sirolimus.
Myeloid engraftment of 97% or greater was present in 27 and greater than 70% in 30/32 recipients evaluable at the latest follow-up. Only one recipient had a myeloid chimerism <50% at the latest follow-up, and this patient still had resolution of his susceptibility to frequent/severe infections. After transplant, no further infectious episodes suggestive of immunodeficiency occurred after successful myeloid engraftment, and the manifestations of inflammatory disease, principally obstructive and inflammatory gastrointestinal disease, were cured, although protein-losing enteropathy did persist in one case.
Discussion
This non-myeloablative conditioning regimen, with weight-based busulfan, alemtuzumab, low-dose TBI, and sirolimus GVHD prophylaxis, provided excellent results, with survival of 82.5% in a population of patients with a heavy burden of disease from CGD and risk assessed by residual oxidase production. Surviving patients with engraftment have remission of their CGD-related diseases, whether inflammatory or infectious, at a mean follow-up of 3.4 (IQR 2–5) years. The decision in transplanting patients is confounded by the need for a potentially life-saving intervention in a patient least likely to tolerate the complications of transplantation. Several patients in this protocol were transplanted in the context of aggressive and medically uncontrollable infection. Residual oxygen intermediate production, as assessed by DHR 123 assay, Fig. 1, demonstrates an important discrepancy between predicted illness/mortality and objective data combined with a patient’s or provider’s subjective opinion that the risk of HSCT is proportional to the impact of the disease. For instance, two outliers with normal ROI production were patients with inflammatory bowel disease, and the lack of correlation with ROI production and gastrointestinal disease has been shown [11].
The risk of conditioning with busulfan and alemtuzumab was associated with manageable toxicities, with frequent grade 1–2 mucositis and reactions to alemtuzumab that required only symptomatic treatment. There was no indication of severe end-organ busulfan toxicity (neurologic, pulmonary or sinusoidal obstruction syndrome) even though we made no modifications to the weight-based dose, nor was there a statistical association with engraftment by busulfan level. Data on dose adjustments and therapeutic drug monitoring (TDM) are conflicting, and our result is not in accordance with literature that suggest that TDM is mandatory [12, 13]. In a recent non-comparative multicenter European trial, treosulfan was well tolerated as an alternative to busulfan. However, although mucositis was acknowledged as one of the commonly reported toxicities of both agents, it is absent from the assessed treosulfan toxicities, and thus, comparison for that factor is not possible [14]. Long-term toxicities with low-dose TBI remain to be determined, but the methodologic challenges in attributing long-term events to TBI when the toxicities of multiple components of the conditioning are similar is an unsolved dilemma [15].
Ten patients had unacceptable outcomes, with two primary graft failures, one of whom survived, one secondary failure with survival after autologous recovery, and seven deaths, of which six were subsequent to donor cell infusions that led to severe or steroid-refractory GVHD and its attendant complications. The statistical interpretation that relates chimerism to outcome in this context is clouded by the disproportionate effect of unmanipulated donor cell infusions or retransplantation, so that adverse outcomes are associated with our response to chime-risms rather than to an objective measure of chimerism itself. This response was often driven by the presence of comorbidi-ties which had already made successful transplantation the only viable option for some of our patients. For instance, the patient with a Pyrenochaeta (fungal) lung abscess with extension across the diaphragm received a 5/6 matched transplant which we would have not used in any other circumstance. The development of GVHD predictably led to the patient’s demise. Rather than excuse adverse outcomes as related to the severity of illness present at transplantation, we think it is critical to review the events that led to deaths, since we had no deaths associated with rejection alone. Risks for graft failure (heavy alloimmunization) were identifiable in two cases, although only two cases of primary failure occurred [16]. There is an important component of infectious mortality during the course of patients with failed engraftment or GVHD. These patients had a significantly higher (P = 0.002) incidence of K. pneumoniae bacteremia (5/10), although not of bacteremia overall. This obscures the effect of multi-drug resistant KPC (K. pneumoniae carbapenemase)-producing K. pneumoniae bacteremia in two cases in which this infection, virtually untreatable at the time of these events, contributed directly to the death of the patient. We changed our management of failed transplantation in the course of this protocol, as deaths occurred in patients who rejected and then developed eventually fatal GVHD/end-organ failure after donor cell infusions or retransplantation. Second transplantation for graft failure has been successful in patients with non-malignant diseases, but no CGD patients are included in the largest series, which reported only 20% survival in malignant diseases [17]. We no longer consider donor cell infusions for declining chimerism. Two failures to engraft in adults with a history of prior granulocyte transfusions and alloimmunization suggest that the current immuno-suppression and cell dose are not sufficient to overcome that barrier. Both patients survived, one with autologous recovery and one with receipt of a banked autologous rescue product, which we obtain prior to transplantation whenever possible.
The results of transplantation for CGD vary depending on the population and conditioning. Gungor et al. reported the multicenter European experience in 56 patients with reduced-intensity conditioning based on busulfan, fludarabine, ATG/thymoglobulin, or alemtuzumab. Graft failure occurred in 5% with stable chimerism in 52 (93%) survivors [9]. A small series (3 patients) utilizing this regimen led to graft loss in all patients, which the authors associated with multiple factors, among them differences in busulfan exposure [18]. Martinez et al. reported the results of myeloablative conditioning, based on busulfan, cyclophosphamide and cytarabine (MRD) or fludarabine (MUD), with alemtuzumab added in all cases. All 11 patients reached >95% chimerism before day 100, but thereafter, two had declining chimerism which stabilized at a mean of 70%. Grade 1 acute GVHD occurred in 4/11 [6]. The results of a subsequent international multi-center trial using regimens most similar to the reduced-intensity conditioning of Gungor et al. are less favorable than those of Gungor and Martinez, as noted above, with overall survival of 91.4%, with 12% graft failure and 12% grades 3–4 GVHD, but the variability in conditioning, other than the use of treosulfan as noted above, is substantial [14].
We chose to use alemtuzumab based on its success in other non-malignant diseases and a favorable toxicity profile, which is needed in transplanting patient with active disease and organ dysfunction. The majority of patients had successful and sustained engraftment with some mild acute GVHD. We believe we have provided data on the evaluation of our patients for tissue evidence of GVHD that is often lacking in the transplant literature. The diagnosis of GVHD remains clinical, and so, this report from a single center with extensive pathologic data contributing to the diagnosis may obviate some of the observer bias that might lead to unrealistically low levels of GVHD in some reports. Only two cases of grades 3–4 GVHD occurred after first transplant; all others followed donor cell infusions with or without conditioning.
Higher alemtuzumab levels have been associated with a lower risk of acute GVHD, but also more mixed chimerism and delayed lymphocyte recovery [19, 20]. We have not used therapeutic drug monitoring with alemtuzumab, and the analysis of our data may point in a different direction in seeking improvement in outcomes. Statistical significance for outcomes (event free and overall survival) in our data is heavily weighted toward myeloid and NK cell chimerisms, but not CD3+ cell chimerism. Our population does demonstrate persistent mixed CD3+ cell chimerism, but without evidence that this influences the success of myeloid engraftment. Long-term engraftment has been associated with CD3+, NK, and myeloid chimerisms, and these may support the importance of myeloid and NK cell chimerism as found in our study, although CD3+ chimerism has often been a predictive factor [21, 22]. NK cells might contribute to long-term engraftment via downregulation of CD8+ T cell proliferation [23]. They may also influence CD4+ T cell and donor-derived dendritic cell-mediated graft-versus host disease [24, 25].
The other component of immunomodulation in our protocol is sirolimus, an inhibitor of mTOR which has been used successfully as GVHD prophylaxis and for its immune-tolerizing activity [26, 27]. The latter may contribute to the fact that prolonged CD3+ cell cytopenias had no statistical effect on the success of engraftment. The combined use of alemtuzumab and sirolimus is derived from that used in transplantation for sickle cell disease, but CGD is a state of broad immune dysregulation rather than just cytopenias or single-cell line dysfunction, as seen in the substantial number of patients in our cohort who either had isolated inflammatory disease or non-infectious inflammatory disease in combination with infections.
This is the largest single-center report on HSCT for CGD. It thus has the advantage of consistency of approach. The data set has limitations that do not allow for characterization of some variables of interest. The population is overwhelmingly X-linked CGD, male, and the majority of transplant products were PBSC/MUD (30/37), and so the numbers in our cohort do not allow us to distinguish between success rates in these categories.
If there was a comparably large and lengthy experience with a myeloablative regimen as used by Martinez et al. incorporating alemtuzumab and GVHD prophylaxis based on a calcineurin inhibitor that achieved superior results, it would support the use of a more myeloablative regimen to improve engraftment, combined with additional measures for GVHD prophylaxis. Modification of the alemtuzumab regimen might influence the high rate of mixed chimerism in our study. However, the influence of mixed chimerism is not apparent in our statistical analysis that finds no association of outcome with CD3+ cell chimerism. T cell depletion was a component of our institution’s initial experience with HSCT for CGD, and we abandoned that strategy in favor of that used in the current report because of our dissatisfaction with our previous results [10]. PBSCs were the preferred product in this protocol because of the higher cell dose available to potentially aid in overcoming barriers to engraftment in this population. Whether strategies that employ newer technologies for T cell depletion and quantitated T cell add back would alter the utility of this strategy might be tested.
In summary, this non-myeloablative regimen for HSCT in CGD yields high rates of engraftment. This is attenuated by severe adverse consequences as a result of rescue therapies for failed engraftment. In order to improve these results, our preference in ongoing work is to target a higher cell dose to improve engraftment and to use post-transplant cyclophosphamide to ameliorate the risk of GVHD.
Acknowledgements
This project has been funded in whole or in part with federal funds from the following components of the National Institutes of Health (NIH): National Cancer Institute, NIH, under Contract No. HHSN261200800001E; National Institute of Allergy and Infectious Disease under Intramural Project No. 1-ZAI-AI000989. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research; and [in part] by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Compliance with Ethical Standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
This article does not contain any studies with animals performed by any of the authors.
Informed consent was obtained from all individual participants included in the study under IRB approved NIH Protocol No. 07-I-0075.
References
- 1.Ahlin A, Fugelang J, de Boer M, Ringden O, Fasth A, Winiarski J. Chronic granulomatous disease-haematopoietic stem cell transplantation versus conventional treatment. Acta Paediatr. 2013;102(11): 1087–94. [DOI] [PubMed] [Google Scholar]
- 2.Gozdzik J, Pituch-Noworolska A, Skoczen S, Czogala W, Wedrychowicz A, Baran J, et al. Allogeneic haematopoietic stem cell transplantation as therapy for chronic granulomatous disease—single centre experience. J Clin Immunol. 2011;31(3):332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tewari P, Martin PL, Mendizabal A, Parikh SH, Page KM, Driscoll TA, et al. Myeloablative transplantation using either cord blood or bone marrow leads to immune recovery, high long-term donor chimerism and excellent survival in chronic granulomatous disease. Biol Blood Marrow Transplant. 2012;18(9):1368–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soncini E, Slatter MA, Jones LB, Hughes S, Hodges S, Flood TJ, et al. Unrelated donor and HLA-identical sibling haematopoietic stem cell transplantation cure chronic granulomatous disease with good long-term outcome and growth. Br J Haematol. 2009;145(1): 73–83. [DOI] [PubMed] [Google Scholar]
- 5.Mehta B, Mahadeo K, Kapoor N, Abdel-Azim H. Low-dose total-body irradiation and alemtuzumab-based reduced-intensity conditioning regimen results in durable engraftment and correction of clinical disease among children with chronic granulomatous disease. Pediatr Transplant. 2015;19(4):408–12. [DOI] [PubMed] [Google Scholar]
- 6.Martinez CA, Shah S, Shearer WT, Rosenblatt HM, Paul ME, Chinen J, et al. Excellent survival after sibling or unrelated donor stem cell transplantation for chronic granulomatous disease. J Allergy Clin Immunol. 2012;129(1):176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole T, Pearce MS, Cant AJ, Cale CM, Goldblatt D, Gennery AR. Clinical outcome in children with chronic granulomatous disease managed conservatively or with hematopoietic stem cell transplantation. J Allergy Clin Immunol. 2013;132(5):1150–5. [DOI] [PubMed] [Google Scholar]
- 8.Seger RA, Gungor T, Belohradsky BH, Blanche S, Bordigoni P, Di Bartolomeo P, et al. Treatment of chronic granulomatous disease with myeloablative conditioning and an unmodified hemopoietic allograft: a survey of the European experience, 1985–2000. Blood. 2002;100(13):4344–50. [DOI] [PubMed] [Google Scholar]
- 9.Gungor T, Teira P, Slatter M, Stussi G, Stepensky P, Moshous D, et al. Reduced-intensity conditioning and HLA-matched haemopoietic stem-cell transplantation in patients with chronic granulomatous disease: a prospective multicentre study. Lancet. 2014;383(9915):436–48. [DOI] [PubMed] [Google Scholar]
- 10.Horwitz ME, Barrett AJ, Brown MR, Carter CS, Childs R, Gallin JI, et al. Treatment of chronic granulomatous disease with nonmyeloablative conditioning and a T-cell-depleted hematopoietic allograft. N Engl J Med. 2001;344(12):881–8. [DOI] [PubMed] [Google Scholar]
- 11.Kuhns DB, Alvord WG, Heller T, Feld JJ, Pike KM, Marciano BE, et al. Residual NADPH oxidase and survival in chronic granulomatous disease. N Engl J Med. 2010;363(27):2600–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vassal G, Michel G, Esperou H, Gentet JC, Valteau-Couanet D, Doz F, et al. Prospective validation of a novel IV busulfan fixed dosing for paediatric patients to improve therapeutic AUC targeting without drug monitoring. Cancer Chemother Pharmacol. 2008;61(1):113–23. [DOI] [PubMed] [Google Scholar]
- 13.Malar R, Sjoo F, Rentsch K, Hassan M, Gungor T. Therapeutic drug monitoring is essential for intravenous busulfan therapy in pediatric hematopoietic stem cell recipients. Pediatr Transplant. 2011;15(6): 580–8. [DOI] [PubMed] [Google Scholar]
- 14.Morillo-Gutierrez B, Beier R, Rao K, Burroughs L, Schulz A, Ewins AM, et al. Treosulfan-based conditioning for allogeneic HSCT in children with chronic granulomatous disease: a multicenter experience. Blood. 2016;128(3):440–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatia S, Davies SM, Scott Baker K, Pulsipher MA, Hansen JA. NCI, NHLBI first international consensus conference on late effects after pediatric hematopoietic cell transplantation: etiology and pathogenesis of late effects after HCT performed in childhood—methodologic challenges. Biol Blood Marrow Transplant. 2011;17(10):1428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ottinger HD, Rebmann V, Pfeiffer KA, Beelen DW, Kremens B, Runde V, et al. Positive serum crossmatch as predictor for graft failure in HLA-mismatched allogeneic blood stem cell transplantation. Transplantation. 2002;73(8):1280–5. [DOI] [PubMed] [Google Scholar]
- 17.Remberger M, Mattsson J, Olsson R, Ringden O. Second allogeneic hematopoietic stem cell transplantation: a treatment for graft failure. Clin Transpl. 2011;25(1):E68–76. [DOI] [PubMed] [Google Scholar]
- 18.Oshrine B, Morsheimer M, Heimall J, Bunin N. Reduced-intensity conditioning for hematopoietic cell transplantation of chronic granulomatous disease. Pediatr Blood Cancer. 2015;62(2):359–61. [DOI] [PubMed] [Google Scholar]
- 19.Chakraverty R, Orti G, Roughton M, Shen J, Fielding A, Kottaridis P, et al. Impact of in vivo alemtuzumab dose before reduced intensity conditioning and HLA-identical sibling stem cell transplantation: pharmacokinetics, GVHD, and immune reconstitution. Blood. 2010;116(16):3080–8. [DOI] [PubMed] [Google Scholar]
- 20.Marsh RA, Kim MO, Liu C, Bellman D, Hart L, Grimley M, et al. An intermediate alemtuzumab schedule reduces the incidence of mixed chimerism following reduced-intensity conditioning hematopoietic cell transplantation for hemophagocytic lymphohistiocytosis. Biol Blood Marrow Transplant. 2013;19(11):1625–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ketterl TG, Flesher M, Shanley R, Miller W. Early CD3+/CD15+ peripheral blood leukocyte chimerism patterns correlate with long-term engraftment in non-malignant hematopoietic SCT. Bone Marrow Transplant. 2014;49(4):572–5. [DOI] [PubMed] [Google Scholar]
- 22.Breuer S, Preuner S, Fritsch G, Daxberger H, Koenig M, Poetschger U, et al. Early recipient chimerism testing in the T-and NK-cell lineages for risk assessment of graft rejection in pediatric patients undergoing allogeneic stem cell transplantation. Leukemia. 2012;26(3):509–19. [DOI] [PubMed] [Google Scholar]
- 23.Zecher D, Li Q, Oberbarnscheidt MH, Demetris AJ, Shlomchik WD, Rothstein DM, et al. NK cells delay allograft rejection in lymphopenic hosts by downregulating the homeostatic proliferation of CD8+ T cells. J Immunol. 2010;184(12):6649–57. [DOI] [PubMed] [Google Scholar]
- 24.Laffont S, Seillet C, Ortaldo J, Coudert JD, Guery JC. Natural killer cells recruited into lymph nodes inhibit alloreactive T-cell activation through perforin-mediated killing of donor allogeneic dendritic cells. Blood. 2008;112(3):661–71. [DOI] [PubMed] [Google Scholar]
- 25.Noval Rivas M, Hazzan M, Weatherly K, Gaudray F, Salmon I, Braun MY. NK cell regulation of CD4 T cell-mediated graft-versus-host disease. J Immunol. 2010;184(12):6790–8. [DOI] [PubMed] [Google Scholar]
- 26.Powell JD, Fitzhugh C, Kang EM, Hsieh M, Schwartz RH, Tisdale JF. Low-dose radiation plus rapamycin promotes long-term bone marrow chimerism. Transplantation. 2005;80(11):1541–5. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh MM, Kang EM, Fitzhugh CD, Link MB, Bolan CD, Kurlander R, et al. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N Engl J Med. 2009;361(24):2309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]