Abstract
Aim:
Delirium is common and dangerous among elderly inpatients; yet, it is underdiagnosed and thus undertreated. This study aimed to test the diagnostic characteristics of a noninvasive point-of-care device with two-channel (bispectral) electroencephalography (EEG) for the screening of delirium in the hospital.
Methods:
Patients admitted to the University of Iowa Hospitals and Clinics were assessed for the presence of delirium with a clinical assessment, the Confusion Assessment Method for Intensive Care Unit and Delirium Rating Scale. Subsequently, we obtained a 10-min bispectral EEG (BSEEG) recording from a hand-held electroencephalogram device during hospitalization. We performed power spectral density analysis to differentiate between those patients with and without delirium.
Results:
Initially 45 subjects were used as a test dataset to establish a cut-off. The BSEEG index was determined to be a significant indicator of delirium, with sensitivity 80% and specificity 87.7%. An additional independent validation dataset with 24 patients confirmed the validity of the approach, with a sensitivity of 83.3% and specificity of 83.3%.
Conclusion:
In this pilot study, the BSEEG method was able to distinguish delirious patients from non-delirious patients. Our data showed the feasibility of this technology for mass screening of delirium in the hospital.
Keywords: delirium, electroencephalogram, point of care, power spectral analysis
DELIRIUM IN HOSPITALIZED elderly patients is dangerous, common, and expensive. It is also seriously underdiagnosed and, thus, undertreated.1–3 Delirium is an acute confusional state characterized by inattention, impaired cognition, psychomotor disturbances, and a waxing and waning course. It is common in hospitalized older adults, affecting 20% to 50% of patients on general medicine floors, 15% to 53% of postoperative surgical patients, and 70% to 87% of patients in intensive care units.2,4 This translates into 11.8 million patients over the age of 65 who are hospitalized annually in the USA with a minimum 15% to 20% chance of developing delirium (2–3 million cases a year).
Delirium is a strong predictor of poor patient outcomes. Patients with delirium have: increased rates of mortality; more complications, such as falls and aspiration pneumonia; longer lengths of hospital stays; persistent cognitive and functional declines; and more frequent institutionalization after discharge.1–3 The health and cost consequences of undetected delirium cannot be overstated. In one study, it was shown that incident delirium in hospitals increased length of stay by 7.8 days.5 In addition, 75% of patients with delirium are discharged into an institutional setting and have over twice the mortality rate.6 If undetected, delirium adds over $60 000 in health-care costs per delirium patient per year, costing the health-care system over $150 billion annually in the USA alone.3,7
Studies show that 30% to 40% of cases can be prevented by using low-tech, high-touch, and cost-saving interventions;3,8,9 thus, early identification and diagnosis of delirium is the key for better outcomes. Over the decades since 1990, when the Confusion Assessment Method (CAM) was originally developed as a tool for delirium screening, efforts to identify better methods of screening for delirium largely focused on the development of simpler and shorter questionnaire-style instruments.10–14 Although, these instruments have been shown to be sensitive and specific in research settings, the frequency with which these tools must be administered and their subjective nature make them impractical for use in real-world hospital workflows. These tools have been reported to be ineffective and have poor sensitivity (38% to 47%) in busy hospital environments, such as those in an intensive care unit (ICU).15,16
Electroencephalography (EEG) is highly useful in detecting delirium.17,18 However, it is not practical or timely for screening the high volume of elderly patients admitted. A typical traditional EEG machine is very expensive and not portable. It requires an experienced technician to correctly place 20 EEG leads upon a patient’s head. It also requires a specialized neurologist who can interpret the data and write a report, which delays treatment by at least 1 day – a significant delay in treating delirium patients.
EEG signals characteristic of delirium are called ‘diffuse slow wave’. The term ‘diffuse’ indicates that across all 20 leads, the brain wave signals are almost the same, showing low frequency (‘slowing’). In fact, slow EEG brainwave signals have been known to be associated with delirium since the 1940s.19 This fact allows for great simplification in lead placement on the subject’s head. Placing only two channels (i.e., bispectral EEG [BSEEG]) on the head will allow for even non-experts to apply the device. This meets a critical need in that specialized neurologists and technicians are not required to perform BSEEG, and it permits mass screening to occur.
The concept of bispectral brain wave monitoring is not unique to us. In the area of anesthesiology, for the last two decades new devices utilizing EEG signals obtained from a few leads attached to a patient’s forehead to monitor depth of anesthesia have been gaining popularity – to the point where it is almost standard care for anesthetized surgical patients.20–23 For a psychiatric treatment, called ‘electroconvulsive therapy’ (ECT), the device includes an EEG function from a few leads to monitor seizure activities. Thus, although not currently used in screening for delirium, obtaining EEG signals from limited leads is an established technology.
The usefulness of an EEG using fewer electrodes in delirium research has only recently been systematically demonstrated. One study confirmed the excellent sensitivity and specificity of a limited number of EEG leads rather than the traditional 20 leads for confirming delirium.24 However, the study did not show the importance of integrating:
a device with simplified lead placement suitable for frontline staff application;
a device–user interface that incorporates automated signal analysis, eliminating the need for expert interpretation; and
a focus on early identification of delirium rather than merely confirming the presence of delirium.
The potential benefits of BSEEG have led us to pursue development of a portable, point-of-care technology suitable for frontline staff to use for early detection of delirium and prediction of poor outcomes. Specifically, we aimed to determine whether power spectral density analysis from limited forehead EEG leads can differentiate confirmed cases of delirium among hospitalized patients.
METHODS
Participants
Study subjects were recruited from patients who were admitted to the University of Iowa Hospitals and Clinics between January 2016 and March 2017. We recruited patients both with and without delirium from the general medicine floor as well as the medical ICU to compare features of brain wave signals obtained using a simplified EEG device. This study was approved by the University of Iowa Institutional Review Board.
Clinical assessment
We assessed baseline cognitive function using the Montreal Cognitive Assessment25 to evaluate subjects’ capacity to consent, and obtained consent from subjects or their legally authorized representative, as appropriate. For baseline dementia, we reviewed hospital records for past diagnosis of dementia. Then we screened for the presence of delirium by administering the CAM-ICU.10 For the validation dataset, we also assessed the level of potential delirium severity with the Delirium Rating Scale – Revised-98 (DRS-R-98)26 and used the Delirium Observation Screening Scale (DOSS),27 which was scored by nursing staff at every shift, and recorded subjects’ DOSS values of the most recent shift from the time of our assessment. We repeated CAM-ICU, DRS-R-98, and DOSS every time we obtained EEG recordings. We used all of these screening instruments to capture any possible incident of delirium, and a final decision of delirium category was made by detailed chart review by trained psychiatrists (G. S. or A. C.).
EEG device
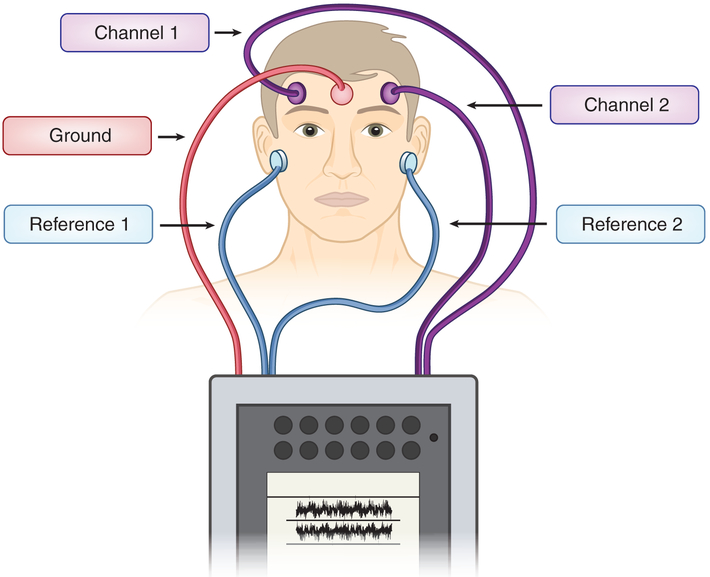
Following those evaluations, we placed EEG leads on each patient’s right and left side of the forehead with one ground on the center of the forehead, with references on each earlobe to obtain two-channel signals (Fig. 1), which were recorded for 10 min (Fig. 2). For data capture, we used a commercially available, handheld EEG device (CMS2100, Contec, Qinhuangdao, China). We used five electrodes placed on the forehead and earlobes bilaterally to obtain signals from two channels. For disposable electrodes, we used alligator clips with disposable electrode patches (Item #602924, Alligator Clip Lead; Item #388007, Nutab Disposable Electrodes, Rochester Electro-Medical, Lutz, FL, USA).
Figure 1.
Location of five electrodes’ placement. One lead is placed on the right and left sides of the forehead, respectively, to obtain two channels. One lead is placed on each earlobe as references. A fifth lead is placed on the center of the forehead as ground.
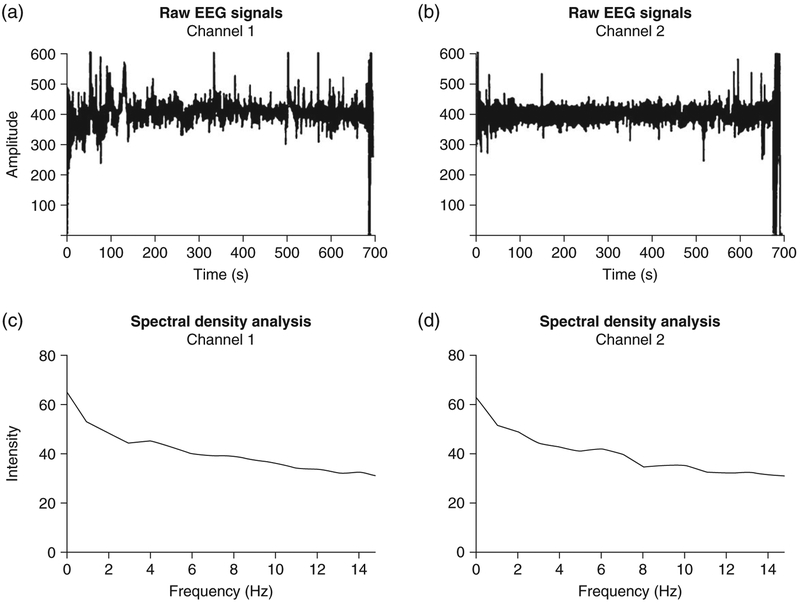
Figure 2.
(a,b) Raw electroencephalography (EEG) signals over 10 min of recording. (c,d) Power spectral density analysis from the corresponding signal.
EEG measurements
We asked patients to close their eyes and relax their jaws, then to sit still during recording as much as they could. We repeated this process twice a day during their hospitalization, for up to 7 days for most cases, and we terminated testing if no change in mental status was observed after a week. If we observed mental status changes, we continued beyond 1 week to monitor EEG changes over time. Data was transferred to a secure server for subsequent signal processing.
EEG data quality
For subgroups of the study subjects, we compared the quality of the brain wave signal from our limited-lead EEG device with the brain wave signal obtained from a traditional 20-lead EEG machine from the same patients at the same time. The equivalent quality was confirmed by a single team member who is an EEG expert (T. Y.). Based on the EEG data obtained through this initial study, we established that these EEG were fully functional with respect to their ability to measure brain waves derived from two EEG channels attached to patients’ foreheads.
EEG signal processing and analysis
Device EEG data were exported in European Data Format for further analysis. Signal windowing was then performed, whereby each channel of data was extracted and each channel was subsequently divided into 4-s windows. Next, we performed window filtering, with each window of data being interrogated for excessive noise, and those windows with interference being removed from further analysis. Each remaining window was then processed for extraction of the following signal features: power spectral density (PSD) obtained via fast Fourier transformation of remaining windows (Fig. 2), interquartile range of raw signal amplitude, and root mean square of raw signal amplitude. These features were then aggregated as the medians of all remaining windows. We used PSD ratio (PSDR) to obtain BSEEG score for further analysis.
RESULTS
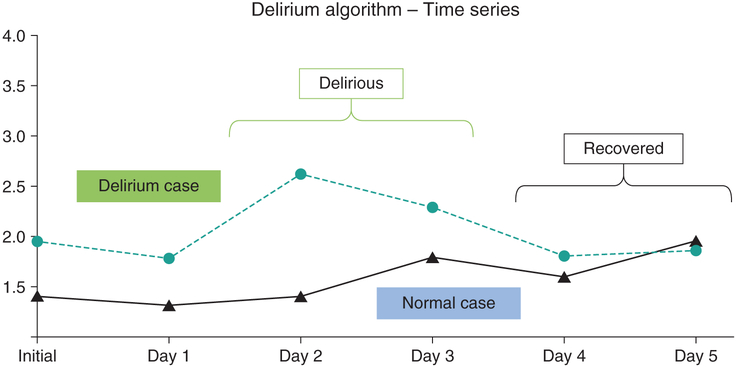
We first conducted preliminary analyses of the data from a limited number of cases involving patients with and without delirium. Our initial PSDR analysis showed that our EEG device clearly differentiated between patients with and without delirium. The PSDR analysis also detected delirium and the absence of delirium in the same patient at different times (Fig. 3).
Figure 3.
Preliminary data comparing power spectral density analysis between delirious case (blue) and normal control (black) over 5 days. Although this was analyzed by a simple algorithm, the data clearly distinguish the two cases, as well as the delirious and the recovered state from the same individual.
Analysis of the training dataset
We then extended the sample size during an ongoing recruitment effort and 184 EEG recordings were obtained from 45 patients. For this test dataset group, the average age was 74.4 years with an SD of 12.7 years, including 15 male and 30 female subjects. Forty-four of the 45 were Caucasian. A comparison of characteristics between the delirium group and the control group revealed no statistically significant differences other than in regards to the CAM-ICU results (Table 1). Our initial analysis from test datasets showed that this method differentiated the two groups of subjects (Fig. 4). With a PSDR threshold of 1.44 (positive ≥ 1.44; negative < 1.44), the performance metrics were as follows: accuracy, 87.5%; sensitivity, 80.0%; and specificity, 87.7%. Our preliminary analysis with the present algorithm was successful in differentiating delirium with a receiver–operator curve (ROC) of 0.70 with the test dataset.
Table 1.
Comparisons of study subject characteristics between delirium cases and controls from test dataset subjects and validation dataset subjects
| Classification | Test subjects |
Validation subjects |
||||
|---|---|---|---|---|---|---|
| Delirious | Control | Delirious | Control | |||
| n | 4 | 41 | 12 | 12 | ||
| P | P | |||||
| Mean age – years | 63.3 | 75.5 | NS | 74.1 | 77.7 | NS |
| SD | 20.8 | 10.5 | 7.6 | 7.7 | ||
| Female sex (n) | 2 | 28 | NS | 5 | 3 | NS |
| % | 50% | 68.3% | 42% | 25% | ||
| Race | ||||||
| White (n) | 4 | 40 | NS | 12 | 12 | NS |
| % | 100% | 97.5% | 100% | 100% | ||
| Other (n) | 0 | 1 | NS | 0 | 0 | NS |
| % | 0% | 2.5% | 0% | 0% | ||
| Admission to ICU (n) | 1 | 4 | NS | 0 | 1 | NS |
| % | 25% | 9.8% | 0% | 8.3% | ||
| Dementia (n) | 2 | 7 | NS | 7 | 6 | NS |
| % | 50% | 17% | 58% | 50% | ||
| CAM-ICU positive (n) | 3 | 3 | <0.001 | 8 | 0 | 0.0013 |
| % | 75% | 7.3% | 66.6% | 0% | ||
| Mean DRS-R-98 score | NA | NA | 19.0 | 6.9 | <0.001 | |
| Mean DOSS score | NA | NA | 6.0 | 0.33 | <0.001 | |
| Mean MoCA score | 8.5 | 20.6 | 0.067 | 15.3 | 21.7 | 0.072 |
CAM-ICU, Confusion Assessment Method – Intensive Care Unit; DOSS, Delirium Observation Screening Scale; DRS-R-98, Delirium Rating Scale – Revised-98; ICU, intensive care unit; MoCA, Montreal Cognitive Assessment; NA, not applicable; NS, not significant.
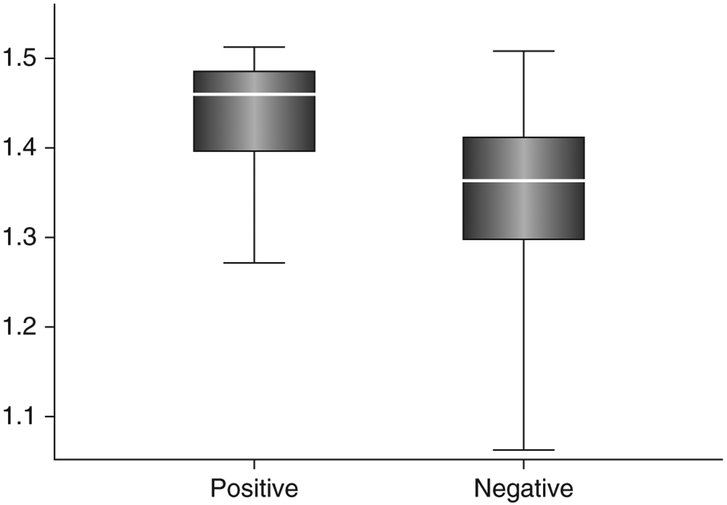
Figure 4.
Data comparing electroencephalography scores based on power spectral density analysis between delirium cases (positive) and normal controls (negative) from a total of 45 subjects. By selecting a cut-off score of 1.44, the two groups were distinguished with an accuracy of 87.5%.
Validation dataset analysis
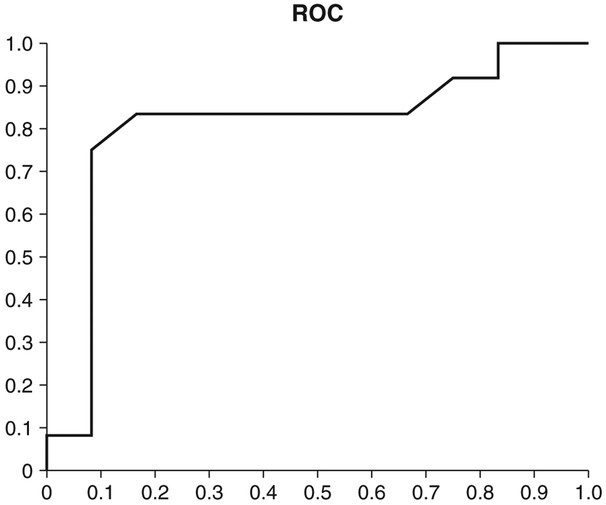
Next, based on the cut-off value of 1.44 from the preliminary results, we tested an independent new set of data consisting of 24 cases for validation. For this validation dataset group, the average age was 76.0 years with an SD of 7.9 years, including 13 male and 11 female subjects. A comparison of characteristics between the delirium group and the control group revealed no statistically significant difference except in regards to delirium screen items, such as CAM-ICU, DRS, and DOSS (Table 1). When we used maximum EEG score to differentiate positive cases versus negative cases, the performance metrics were as follows (Fig. 5): accuracy, 83.3%; sensitivity, 83.3%; and specificity, 83.3%. The area under the ROC was 0.81, and Fisher’s exact test two-tailed P-value was 0.0033.
Figure 5.
Receiver–operator curve (ROC) from the validation dataset. The area under the curve was 0.805.
DISCUSSION
Our results provide the first evidence of the utility of a simplified, portable, automated EEG with bispectral density analysis for delirium screening. Using such a strategy, our algorithm training data demonstrated the following performance characteristics: accuracy of 87.5%, sensitivity of 80.0%, and specificity of 87.7%. Performance on the validation dataset demonstrated accuracy of 83.3%, sensitivity of 83.3%, and specificity of 83.3%.
Compared to traditional EEG, which requires >20 leads placed all over the head of patients by a trained EEG technician, our system requires only a few leads placed on the forehead, thus requiring minimal training. With only 10 min of EEG signal required and automated signal analysis, rapid screening can be achieved. This is a significant advantage compared to a traditional EEG reading by specialists, which introduces significant delays. This bispectral simplified, EEG (BSEEG) is also an improvement over screening methods currently used in practice, such as questionnaire-style methods, which are prone to subjective variation by examiners.
If our results are upheld with confirmatory studies, then BSEEG will lead to a drastic change in screening and prevention of complications from delirium. Such an approach will significantly improve the targeting of often-limited hospital resources for better patient outcomes with decreased hospital length of stay and associated financial costs.
Our study has several notable strengths. It is the first study showing the usefulness of a small, portable, bedside, point-of-care BSEEG device with simplified lead placement in differentiating delirium states. If this method becomes available for daily clinical practice, we envision it to be helpful for everyday practice to predict, screen, and monitor delirium for large volumes of patients in numerous settings, including inpatients, outpatients, emergency room, nursing homes, and potentially even at home, which would never be possible with a traditional EEG.
This method may have the potential to be used as a fifth vital sign, after heart rate, blood pressure, body temperature, and respiratory rate. Although we used a binary PSDR threshold to differentiate positive and negative, PSDR is a continuous scale, and thus, just like a blood pressure or heart rate, the EEG score can be used as a guideline to assess risk for urgency in terms of outcomes.
The authors acknowledge several limitations of the present study, including a relatively small sample size, which is the nature of such a proof-of-concept study. Nevertheless, with patients in this study, the technology appears to demonstrate evidence of reliability, and there is much room for the technology to mature to improve such an approach.
Although these preliminary data were established by using clinical data with categories of delirium and controls as the training set and validation set, respectively, it is vital that we test the algorithm among independent cohorts to further validate the algorithm and test for accuracy. Toward that aim, we are extending our current recruitment effort to a new group of subjects.
The goal of the next stage of this line of research will be to prospectively follow those subjects admitted to University of Iowa Hospitals and Clinics to identify the onset of delirium among them. Once we confirm the validity of this method in the prospective study, that result will support our hypothesis that BSEEG with the automated signal-processing algorithm can properly classify patients at risk for developing delirium. This would be important data because it would indicate that this technology can identify patients at risk for delirium; thus, it would provide the opportunity to prevent and address the potentially reversible causes of delirium ahead of time.
ACKNOWLEDGMENTS
This study was supported by the University of Iowa Research Foundation GAP funding award for Gen Shinozaki and John Cromwell. Gen Shinozaki has grant support from NSF1664364 and K23 MH107654.
Footnotes
DISCLOSURE STATEMENT
Gen Shinozaki and John Cromwell are co-founders of Predelix Medical LLC.
REFERENCES
- 1.Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: Diagnosis, prevention and treatment. Nat. Rev. Neurol 2009; 5: 210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inouye SK. Delirium in older persons. N. Engl. J. Med. 2006; 354: 1157–1165. [DOI] [PubMed] [Google Scholar]
- 3.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet 2014; 383: 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pisani MA, McNicoll L, Inouye SK. Cognitive impairment in the intensive care unit. Clin. Chest Med 2003; 24: 727–737. [DOI] [PubMed] [Google Scholar]
- 5.McCusker J, Cole MG, Dendukuri N, Belzile E. Does delirium increase hospital stay? J. Am. Geriatr. Soc 2003; 51: 1539–1546. [DOI] [PubMed] [Google Scholar]
- 6.Boustani M, Baker MS, Campbell N et al. Impact and recognition of cognitive impairment among hospitalized elders. J. Hosp. Med 2010; 5: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch. Intern. Med 2008; 168: 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inouye SK, Bogardus ST Jr, Charpentier PA et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N. Engl. J. Med 1999; 340: 669–676. [DOI] [PubMed] [Google Scholar]
- 9.Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: A randomized trial. J. Am. Geriatr. Soc 2001; 49: 516–522. [DOI] [PubMed] [Google Scholar]
- 10.Ely EW, Inouye SK, Bernard GR et al. Delirium in mechanically ventilated patients: Validity and reliability of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). JAMA 2001; 286: 2703–2710. [DOI] [PubMed] [Google Scholar]
- 11.Inouye SK, Kosar CM, Tommet D et al. The CAM-S: Development and validation of a new scoring system for delirium severity in 2 cohorts. Ann. Intern. Med 2014; 160: 526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: The confusion assessment method. A new method for detection of delirium. Ann. Intern. Med 1990; 113: 941–948. [DOI] [PubMed] [Google Scholar]
- 13.Marcantonio ER, Ngo LH, O’Connor M et al. 3D-CAM: Derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: A cross-sectional diagnostic test study. Ann. Intern. Med 2014; 161: 554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuczmarska A, Ngo LH, Guess J et al. Detection of delirium in hospitalized older general medicine patients: A comparison of the 3D-CAM and CAM-ICU. J. Gen. Intern. Med 2016; 31: 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Eijk MM, van den Boogaard M, van Marum RJ et al. Routine use of the confusion assessment method for the intensive care unit: A multicenter study. Am. J. Respir. Crit. Care Med 2011; 184: 340–344. [DOI] [PubMed] [Google Scholar]
- 16.Nishimura K, Yokoyama K, Yamauchi N et al. Sensitivity and specificity of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) and the Intensive Care Delirium Screening Checklist (ICDSC) for detecting post-cardiac surgery delirium: A single-center study in Japan. Heart Lung 2016; 45: 15–20. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson S, Jerrier H. EEG in delirium. Semin. Clin. Neuropsychiatry 2000; 5: 86–92. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson SA, Leuchter AF, Walter DO. Conventional and quantitative EEG in the diagnosis of delirium among the elderly. J. Neurol. Neurosurg. Psychiatry 1993; 56: 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engel GL, Romano J. Delirium, a syndrome of cerebral insufficiency. J. Chronic Dis 1959; 9: 260–277. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Singh H, White PF. Electroencephalographic bispectral index correlates with intraoperative recall and depth of propofol-induced sedation. Anesth. Analg 1997; 84: 185–189. [DOI] [PubMed] [Google Scholar]
- 21.Doi M, Gajraj RJ, Mantzaridis H, Kenny GN. Effects of cardiopulmonary bypass and hypothermia on electroencephalographic variables. Anaesthesia 1997; 52: 1048–1055. [DOI] [PubMed] [Google Scholar]
- 22.Schmidlin D, Hager P, Schmid ER. Monitoring level of sedation with bispectral EEG analysis: Comparison between hypothermic and normothermic cardiopulmonary bypass. Br. J. Anaesth 2001; 86: 769–776. [DOI] [PubMed] [Google Scholar]
- 23.Powers KS, Nazarian EB, Tapyrik SA et al. Bispectral index as a guide for titration of propofol during procedural sedation among children. Pediatrics 2005; 115: 1666–1674. [DOI] [PubMed] [Google Scholar]
- 24.van der Kooi AW, Zaal IJ, Klijn FA et al. Delirium detection using EEG: What and how to measure. Chest 2015; 147: 94–101. [DOI] [PubMed] [Google Scholar]
- 25.Nasreddine ZS, Phillips NA, Bedirian V et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc 2005; 53: 695–699. [DOI] [PubMed] [Google Scholar]
- 26.Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the Delirium Rating Scale-Revised-98: Comparison with the Delirium Rating Scale and the Cognitive Test for Delirium. J. Neuropsychiatry Clin. Neurosci 2001; 13: 229–242. [DOI] [PubMed] [Google Scholar]
- 27.Schuurmans MJ, Shortridge-Baggett LM, Duursma SA. The Delirium Observation Screening Scale: A screening instrument for delirium. Res. Theory Nurs. Pract 2003; 17: 31–50. [DOI] [PubMed] [Google Scholar]