Abstract
OBJECTIVE
To characterize the associations between diabetes mellitus (DM) and lower urinary tract symptoms (LUTS). This study focuses on the relationships between specific diabetic characteristics (eg, severity, biomarkers) and the prevalence of LUTS.
MATERIALS AND METHODS
The 2005-2008 cycles of the National Health and Nutrition Examination Survey were queried for men who completed both a DM and a kidney/prostate questionnaire. Men with LUTS were defined as those experiencing at least 1 out of 3 of the following: nocturia, hesitancy, or incomplete emptying. Men with DM were defined as having been diagnosed by a physician and being actively treated. Multivariate logistic regression with sample weighting was performed to assess effects of biomarker levels (HgbA1c, fasting glucose), medication use, and surrogates of disease progression on the presence of LUTS.
RESULTS
Of the 2127 male participants, those with DM (n = 405) were more likely to experience at least 1 urinary symptom (adjusted odds ratio 1.63, P <.0001). Men under the age of 70 with longstanding (>5 years) DM were more likely to report LUTS than those with a shorter duration of the disease (<5 years). Diabetes-specific biomarkers (HgbA1c, fasting glucose) were not predictors of LUTS in men with DM.
CONCLUSION
DM was confirmed to be strongly associated with patient-reported LUTS in men. Younger men and those with longer-standing disease appear to be most susceptible. In actively treated patients with DM, DM biomarkers were not helpful in predicting individual LUTS. Instead, biomarkers that indirectly reflect DM disease progression were most useful. UROLOGY 105: 141–148, 2017. Published by Elsevier Inc.
Lower urinary tract symptoms (LUTS) and diabetes mellitus (DM) are commonly reported conditions that can both negatively affect quality of life.1,2 Often present together, it is not surprising that the association between the 2 conditions, as well as the associations between DM and other commonly reported urologic conditions, has been well-established.3-6 However, despite the clinical relationship noted between these disease states, little is known about how DM management and DM progression can mitigate the development of LUTS and LUTS severity.7,8
Studies in type I DM (DM1) populations suggest that tighter glucose control might prevent LUTS development. However, this may not be the case in type 2 DM (DM2) population.9-11 Lack of a clear understanding of the pathophysiology linking the 2 conditions in male patients, hypothesized to involve both static and dynamic components, currently limits clinicians’ ability to adequately treat and prevent DM-associated LUTS.7 Thus, a better understanding of how these conditions relate to one another in the general population, including improving our ability to predict which patients with DM will develop LUTS, is desired.
The National Health and Nutrition Examination Survey (NHANES) has previously been used to analyze the relationship between LUTS and other disease processes such as depression and constipation.12,13 Rohrmann et al used NHANES III to become one of the first to demonstrate the strong correlation between LUTS and metabolic syndrome (including DM2) in men over the age of 60.14 In this study, we propose to expand on the use of this dataset to determine how both clinical indicators and serum biomarkers can be used as a means to predict the presence of LUTS in the DM population, hypothesizing that indicators of improved DM control will be associated with fewer LUTS.
MATERIALS AND METHODS
Study Population
After obtaining institutional review board exemption, we combined and analyzed the 2005-2006 and 2007-2008 NHANES datasets. The NHANES sampling methodology has been described elsewhere.15 In this study, we limited our cohort to men over the age of 40 who completed 3 different questionnaires related to diabetes, kidney/urologic conditions, and prostate conditions (Supplementary Fig. S1).
Assessment of LUTS
The NHANES questionnaires included the following 3 questions that we used to assess LUTS: (1) “Do you usually have trouble starting to urinate (pass water)?” (urinary hesitancy); (2) “After urinating (passing water), does your bladder feel empty?” (incomplete emptying); and (3) “During the past 30 days, how many times per night did you most typically get up to urinate, from the time you went to bed at night until the time you got up in the morning?” (nocturia). Patients were considered to have nocturia only if they woke up 2 times or more per night.16 The primary outcome of this study was the presence of any of the above LUTS. The secondary outcomes included the presence of any of the individual LUTS.
Assessment of Diabetes, Diabetes Severity, and Diabetes Biomarkers
We defined the diagnosis of DM as answering “yes” to the following question: “Other than during pregnancy, have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?” Notably, the NHANES questions do not allow for researchers to distinguish between DM1 and DM2.
We assessed DM management and individual DM severity by analyzing each participant’s reported use of oral hypoglycemic medication, use of insulin, and presence of diabetic retinopathy. We used the “time from DM diagnosis” as a way to assess the cumulative effect that DM presence has on the development of urinary symptoms. Finally, to determine the effect that lifestyle and compliance with (presumed) DM treatment recommendations has on LUTS development, we analyzed if patient reports of “not controlling weight,” “not increasing physical activity,” “not reducing fat/calories in diet,” “seeing a physician infrequently,” “not seeing a diabetes specialist,” “not regularly checking A1C,” “checking blood sugar infrequently,” and “not checking feet for sores” increased the likelihood of individual LUTS reporting.
In the men who underwent serum analysis as part of their NHANES participation, we were able to assess if common biomarkers often associated with DM disease progression and DM severity, including HgbA1c levels, random plasma glucose, fasting plasma glucose, and fasting insulin levels, were associated with LUTS. In addition, various other biomarkers related to DM and chronic disease states were analyzed, including measurements for dyslipidemia (total cholesterol, low-density lipoprotein, high-density lipoprotein, triglycerides), renal insufficiency (serum creatinine, urine protein-to-creatinine ratio [UPC], hemoglobin), and systemic inflammation (WBC, C-reactive protein [CRP]).
Statistical Analysis
All statistics were completed using appropriate sample weights for the combined 2005-2008 NHANES cycle. Specific subsampling weights, including patients undergoing testing in the Mobile Examination Center and those on fasting protocols, were used where indicated. We first used descriptive statistics to determine the prevalence of LUTS (voiding symptoms and nocturia) in men with and without diabetes. Second, we conducted a stratified analysis of LUTS prevalence by age (groups of 10 years). We further used these age categories to assess LUTS in those with and without diabetes and then those with and without long-standing diabetes (>5 years since age of diagnosis) using the Cochran-Armitage test for trend. Third, 2 multivariable logistic regression models were constructed controlling for previously described risk factors, including age, body mass index (BMI), smoking status, alcohol use, and prostate-specific antigen (PSA), to determine (1) biomarkers that were independently associated with LUTS and (2) clinical management factors that predicted LUTS in men with diabetes. Finally, a stratified analysis was performed comparing the number of reported LUTS with 3 different biomarkers: HgbA1c, fasting glucose, and PSA. Statistical analyses were completed using SAS 9.3 (SAS Institute Inc., Cary, NC) with statistical significance set at P <.05.
RESULTS
LUTS Prevalence
Of 2127 men who met study criteria, 405 (19.0%) reported to have been diagnosed with diabetes by a physician and were actively being treated. The presence of DM was an independent predictor of reporting any LUTS (adjusted odds ratio [OR] 1.63, 95% confidence interval 1.27-2.08). The overall prevalence of any LUTS in the DM population was 52.7% compared with 36.5% in the entire study population (P <.0001). Individual urinary symptoms were also higher in patients with diabetes, with 41.4% (vs 23.4%, P = <.0001) reporting clinically significant nocturia, 11.7% (vs 8.9%, P = <.0001) reporting incomplete emptying, and 10.4% (vs 8.1%; P = <.0001) reporting issues with hesitancy.
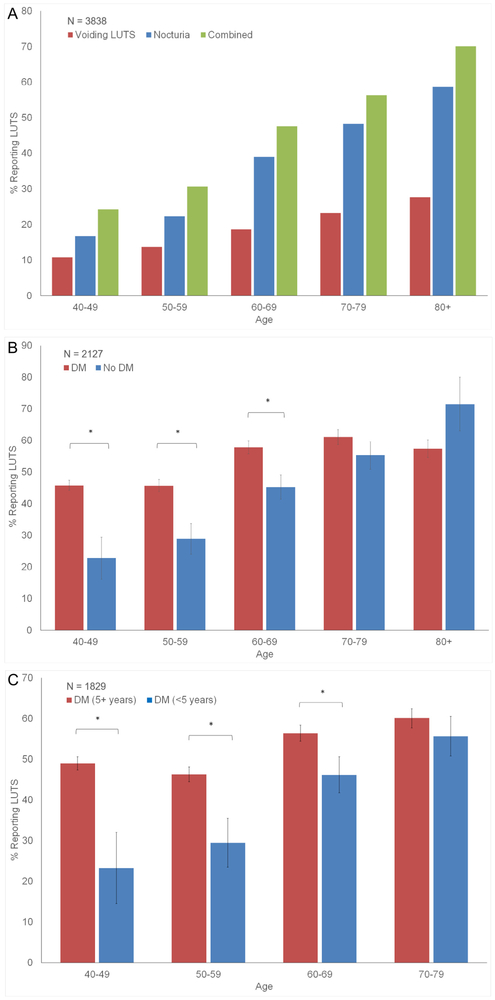
In the general population, the prevalence of both voiding LUTS and nocturia increased with age (Fig. 1A) but was higher in the DM populations (Fig. 1B). One of the largest discrepancies between the DM and non-DM populations was noted in the youngest age group, where 45.8% of the DM population reported LUTS vs 22.8% of the non-DM population (P = .0003). Controlling for age and the presence of DM, the length of time patients with DM had been diagnosed with the disease (here, dichotomized into <5 years and >5 years) was strongly associated with an increase in LUTS reporting (Fig. 1C).
Figure 1.
(A-C) Stratified analysis of LUTS as a function of age, DM, and length of DM. DM, diabetes mellitus; LUTS, lower urinary tract symptoms. (Color version available online.)
Biomarkers and LUTS
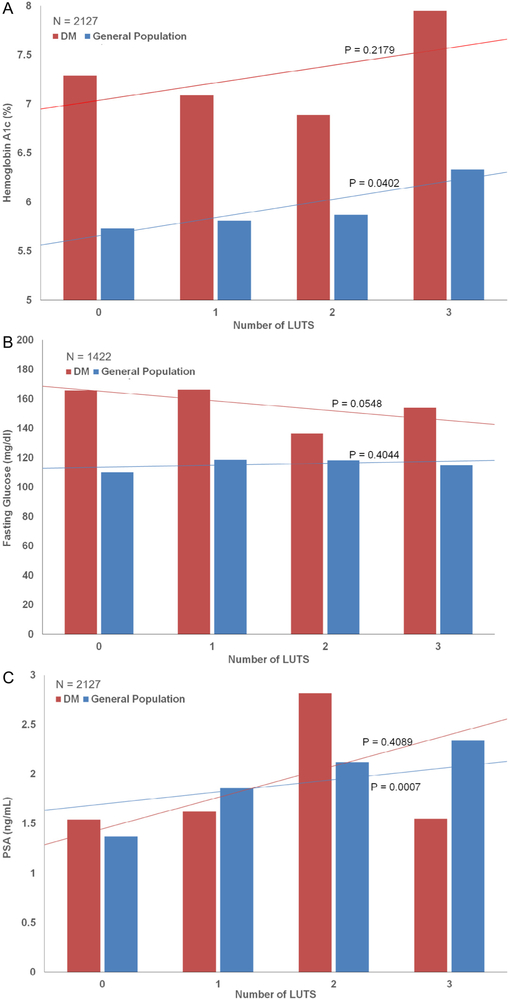
Overall HgbA1c levels were higher in the DM population as compared with the general population (7.22% vs 5.71%, P <.0001). However, when HgbA1c levels were stratified by the total number of reported symptoms, only in the non-DM population did increasing HgbA1c predict for more LUTS (Fig. 2A).
Figure 2.
(A-C) Hemoglobin A1c/fasting glucose/PSA vs LUTS in men with and without diabetes. LUTS, lower urinary tract symptoms; PSA, prostate-specific antigen. (Color version available online.)
Overall fasting glucose levels were higher in the DM population as compared with the general population (161.2 mg/dL vs 112.7 mg/dL, P <.0001). In the DM population, lower fasting glucose levels were associated with increased LUTS (Fig. 2B), although a similar association was not noted in the non-DM population.
PSA levels were statistically similar between the general population and the population with diabetes (1.50 ng/ mL vs 1.66 ng/mL, P = .2758). Figure 2C demonstrates that increasing PSA levels were associated with more LUTS in the general population (P = .0007) but not in the population with diabetes (P = .4089).
Multivariable Analysis
Biomarkers.
We next assessed the associations between clinical characteristics, diabetes, biomarkers, and LUTS. Univariate analysis of biomarkers between individuals with and without DM was first assessed (Supplementary Table S1). Multivariable analysis, controlling for age, BMI, smoking status, alcohol use, and PSA was performed (Table 1). In the model, only PSA was found to be a significant predictor of voiding LUTS. Nocturia was predicted by UPC (OR 2.74,1.28-5.85), hemoglobin (OR 0.81, 0.68-0.98), bicarbonate (OR 1.16, 1.03-1.31), and C-reactive protein levels (OR 1.95, 1.09-3.47). Traditional biomarkers used for monitoring DM, including HgbA1c, fasting glucose, and fasting insulin, were not predictive of either voiding LUTS or nocturia in the DM population.
Table 1.
Multivariate logistic analysis of biomarkers associated with self-reported LUTS in men with diabetes
| Voiding LUTS N = 271 |
Nocturia N = 270 |
|||
|---|---|---|---|---|
| Model | Adjusted Odds Ratio* | 95% CI | Adjusted Odds Ratio* | 95% CI |
| Age | 1.00 | 0.95-1.05 | 1.02 | 0.99-1.04 |
| BMI | 1.02 | 0.97-1.07 | 1.01 | 0.95-1.07 |
| Current/former smoker | 2.05 | 0.70-6.06 | 1.04 | 0.42-2.57 |
| Current/former alcoholic | 1.11 | 0.52-2.38 | 1.06 | 0.47-2.42 |
| PSA | 1.21 | 1.10-1.33 | 0.95 | 0.86-1.05 |
| Biomarker | Adjusted Odds Ratio* | 95% CI | Adjusted Odds Ratio* | 95% CI |
|---|---|---|---|---|
| Random plasma glucose (mg/dL) | 0.82 | 0.28-2.41 | 1.61 | 0.60-4.37 |
| Fasting plasma glucose† (mg/dL) | 1.00 | 0.99-1.00 | 1.00 | 1.00-1.01 |
| Fasting insulin† (pU/mL) | 0.96 | 0.92-1.00 | 0.99 | 0.94-1.04 |
| Hemoglobin A1c (%) | 1.03 | 0.87-1.23 | 0.96 | 0.82-1.13 |
| Serum creatinine (mg/dL) | 1.29 | 0.75-2.21 | 1.21 | 0.65-2.27 |
| Urine protein/creatinine | 1.24 | 0.86-1.79 | 2.74 | 1.28-5.85 |
| BUN (mg/dL) | 0.98 | 0.94-1.03 | 1.03 | 1.00-1.07 |
| Hemoglobin (g/dL) | 0.93 | 0.75-1.16 | 0.81 | 0.68-0.98 |
| Bicarbonate (mmol/L) | 0.94 | 0.85-1.04 | 1.16 | 1.03-1.31 |
| Total cholesterol (mg/dL) | 0.99 | 0.98-1.00 | 1.00 | 1.00-1.01 |
| LDL† (mg/dL) | 0.99 | 0.97-1.00 | 1.01 | 0.99-1.02 |
| HDL† (mg/dL) | 1.01 | 0.97-1.05 | 0.99 | 0.95-1.03 |
| Triglycerides† (mmol/L) | 1.00 | 0.99-1.00 | 1.00 | 1.00-1.01 |
| WBC (1000 cells/uL) | 0.90 | 0.73-1.11 | 0.86 | 0.68-1.09 |
| C-reactive protein (mg/dL) | 1.19 | 0.75-1.87 | 1.95 | 1.09-3.47 |
BMI, body mass index; BUN, blood urea nitrogen; CI, confidence interval; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LUTS, lower urinary tract symptoms; PSA, prostate-specific antigen.
Values in bold are statistically significant (P < 0.05).
Adjusted for age, BMI, smoking habits, alcohol habits, and PSA.
Calculated with fasting subsample.
Lifestyle and Diabetes Management.
Table 2 depicts a multivariable model, controlling for age, BMI, smoking status, and alcohol use, which aims to identify lifestyle factors within the DM population that predict LUTS. Analyzing voiding LUTS revealed the only predictive management/lifestyle factor to be “never checking blood sugar” (OR 4.28, 1.39-13.20). Analyzing nocturia revealed the only predictive management/lifestyle factor to be patient-reported “diabetic retinopathy” (OR 1.84, 1.07-3.16). The current DM therapy as reflected by usage of insulin (voiding LUTS: OR 0.92, 0.48-1.78; nocturia: OR 0.61, 0.36-1.05) or oral hypoglycemics (voiding LUTS: OR 1.12, 0.53-2.38; nocturia: OR 0.98, 0.54-1.80) did not predict LUTS.
Table 2.
Multivariate logistic analysis of patient-reported diabetic control factors on the presence of LUTS
| Voiding LUTS N = 314 |
Nocturia N = 499 |
|||
|---|---|---|---|---|
| Question Response | Adjusted Odds Ratio* | 95% CI | Adjusted Odds Ratio* | 95% CI |
| Overall health management | ||||
| Told to control weight | 0.67 | 0.29-1.56 | 0.62 | 0.38-1.02 |
| Told to increase physical activity | 1.24 | 0.64-2.39 | 0.83 | 0.52-1.31 |
| Told to reduce calories/fat | 0.69 | 0.30-1.60 | 0.96 | 0.57-1.64 |
| Not controlling weight | 2.13 | 0.88-5.15 | 1.23 | 0.71-2.13 |
| Not increasing physical activity | 1.01 | 0.56-1.84 | 1.04 | 0.59-1.83 |
| Not reducing calories/fat | 1.67 | 0.67-4.13 | 1.35 | 0.78-2.32 |
| Diabetes medical management | ||||
| Taking insulin | 0.92 | 0.48-1.78 | 0.61 | 0.36-1.05 |
| Taking oral hypoglycemic | 1.12 | 0.53-2.38 | 0.98 | 0.54-1.80 |
| Diabetes self-management | ||||
| Never seen a diabetes specialist | 1.74 | 0.84-3.61 | 1.58 | 0.91-2.66 |
| Single diabetes doctor | 0.68 | 0.28-1.67 | 0.95 | 0.58-1.54 |
| More than 10 health-care appointments | 1.14 | 0.41-3.16 | 1.57 | 0.61-4.04 |
| Never check blood sugar | 4.28 | 1.39-13.20 | 1.61 | 0.80-3.20 |
| Infrequent vs daily checks | 1.56 | 0.75-3.24 | 1.26 | 0.70-2.29 |
| Do not know what HgbA1c is | 1.33 | 0.66-2.65 | 1.68 | 0.93-3.02 |
| No HgbA1c checks | 1.57 | 0.70-3.49 | 1.92 | 0.73-5.04 |
| No self-foot checks | 1.42 | 0.60-3.36 | 1.46 | 0.89-2.39 |
| End-organ damage | ||||
| Diabetic retinopathy | 1.57 | 0.53-4.67 | 1.84 | 1.07-3.16 |
Values in bold are statistically significant (P< 0.05).
Adjusted for age, BMI, smoking habits, and alcohol habits.
DISCUSSION
In this analysis of 2005-2008 NHANES data, we confirm the previously established relationship between the presence of DM and the patient-reported LUTS.4,17 Furthermore, in the population with diabetes, we identified several predictors of LUTS, including length of time with the disease (>5 years), clinical markers of DM-related end-organ damage (eg, retinopathy), and serum biomarkers associated with renal insufficiency (eg, UPC) and systemic inflammation (eg, CRP). When controlling for age, DM presence was found to be most predictive of LUTS in the younger populations. However, the findings did not support the study’s original hypothesis, as we found serum biomarkers associated with DM control and progression, including HgbA1c and fasting glucose, were not associated with the presence of LUTS in the DM population.
Biomarkers and LUTS
CRP was found to be predictive of LUTS (nocturia only) in the DM cohort—a finding similar to Chung et al who noted elevated CRP levels in 1301 men with DM and nocturia,18 perhaps suggesting systemic endothelial dysfunction or intraprostatic inflammation as possible contributors to DM-related LUTS.19,20 Importantly, it must be noted that CRP levels can be falsely elevated in patients with renal failure and indeed, when both CRP and UPC are forced into the model (results not shown), CRP loses its ability to predict for LUTS. Furthermore, because CRP was only predictive of nocturia, CRP may be more predictive of renal-concentrating ability than of endothelial function in the DM cohort.21
Elevations in the UPC and decreases in hemoglobin were both predictive of nocturia in the DM cohort, which may be a function of impaired renal-concentrating ability seen with renal insufficiency.22 Without a voiding diary, it is difficult to determine cause and effect, although this association may suggest that aggressive behavioral intervention (through safe water restriction and timely diuretic use) and patient education might help to mitigate this problem in patients with diabetes.23
PSA was the only biomarker found to be independently associated with voiding LUTS and likely related to prostate size.24 Importantly, whereas baseline PSA levels were similar between the DM and non-DM populations, PSA levels were linearly associated with LUTS only in the general population. This suggests that in populations with diabetes, factors other than prostate size may be more responsible for reporting LUTS than in populations without diabetes.
We did not find biomarkers most commonly associated with DM, including HgbA1c, fasting glucose, and fasting insulin, to be predictive of LUTS in the DM population, as has been suggested in other cross-sectional studies.10,25 However, HgbA1c levels were predictive of LUTS in the general population—a population that presumably has not been influenced by medical management of serum glucose. It is unclear what this means clinically, as simply asking our patients with DM-related LUTS to improve their DM control may not immediately improve LUTS. Rather, the effects of DM on LUTS development are likely a cumulative process, as suggested by the study findings that longer time of disease exposure, evidence of renal insufficiency, and diabetic retinopathy (the latter two being indicators of disease progression), are strong predictors of LUTS.
DM Control and LUTS
Patient answers to questions about their current DM management and DM control were largely unhelpful in predicting the presence of LUTS. This again may suggest that a cross-sectional look at any individual patient with DM may not be enough to predict urinary sequelae. Interestingly, the only DM management finding that predicted LUTS was patient-reported “I never check my blood glucose.” This might suggest that a patient not willing, or unable, to achieve tighter blood glucose control may be at a higher long-term risk of LUTS development.26
Surprisingly, the impact that intensive glycemic control has on the presence and development of LUTS and other urologic sequelae remains largely unknown. Van Den Eeden et al demonstrated that in men with DM1, 10 years of intensive vs conventional glycemic control (with median difference of 2.0% HgbA1c) had little to no effect on the development of storage or voiding LUTS.9 Although a similar, prospective, longitudinal study has not been performed in the DM2 population, the findings from our study suggest that a similar outcome might be obtained were one to be performed. However, as our younger men with DM appeared to be most susceptible to LUTS relative to non- DM peers, early and aggressive intervention may be most warranted here to prevent the apparent cumulative effects of elevated glucose.27,28
Limitations
There are limitations that deserve mentioning. First, the NHANES contains only cross-sectional data which do not allow us to establish a cause and effect relationship between our biomarker and LUTS findings, although the ability to stratify by both age and time with disease strongly suggests that DM-related LUTS is a cumulative process. Second, cross-sectional HgbA1c and fasting glucose generally reflects only a 3-month time period, and because treatment compliance is known to vary widely, the crosssectional value obtained for a specific patient may not accurately reflect their entire disease course. Third, our outcome of interest (LUTS) was confined to assessing only 3 symptoms, leaving us with the inability to analyze other important storage (urgency, frequency) and obstructive (weak stream, straining) symptoms typical of LUTS workup. Therefore, we are forced to make the assumption that all variations of LUTS appear with equal frequency in the DM population. Finally, the NHANES does not specifically differentiate between men with DM1 and men with DM2. Although the nature of the questionnaire administered and the characteristics of the population suggest that the majority of men have DM2, we cannot verify this fact with the publically available data.
CONCLUSION
DM is an independent risk factor for the development of LUTS in men. Younger men with DM appear to be at higher risk for developing symptoms relative to their peers, and the duration of the disease likely matters. The role of glycemic control remains controversial as neither HgbA1c nor fasting glucose appears to correlate directly with LUTS, implying that at least short-term DM control may not reverse the presence of LUTS. However, markers of end-organ damage (retinopathy, vasculopathy, and nephropathy) appear to be most predictive of LUTS, suggesting that improving long-term control of DM may prevent DM-related urinary symptoms. Further prospective work is needed to identify at-risk men with diabetes who may benefit from intervention or preventative strategies to reduce urologic morbidity.
Supplementary Material
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
Supplementary Data
Supplementary data associated with this article can be found, in the online version, athttp://dx.doi.org/10.1016/j.urology.2017.03.040.
References
- 1.Huang ES, Brown SE, Ewigman BG, Foley EC, Meltzer DO. Patient perceptions of quality of life with diabetes-related complications and treatments. Diabetes Care. 2007;30:2478–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kupelian V, Wei JT, O’Leary MP, et al. Prevalence of lower urinary tract symptoms and effect on quality of life in a racially and ethni-cally diverse random sample: the Boston Area Community Health (BACH) Survey. Arch Intern Med. 2006;166:2381–2387. [DOI] [PubMed] [Google Scholar]
- 3.Sarma AV, Burke JP, Jacobson DJ, et al. Associations between diabetes and clinical markers of benign prostatic hyperplasia among community-dwelling Black and White men. Diabetes Care. 2008;31:476–482. [DOI] [PubMed] [Google Scholar]
- 4.Van Den Eeden SK, Ferrara A, Shan J, et al. Impact of type 2 diabetes on lower urinary tract symptoms in men: a cohort study. BMC Urol. 2013;13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pashootan P, Ploussard G, Cocaul A, de Gouvello A, Desgrandchamps F. Association between metabolic syndrome and severity of lower urinary tract symptoms (LUTS): an observational study in a 4666 European men cohort. BJU Int. 2015;116:124–130. [DOI] [PubMed] [Google Scholar]
- 6.Sarma AV, Kanaya AM, Nyberg LM, et al. Urinary incontinence among women with type 1 diabetes—how common is it? J Urol. 2009;181:1224–1230. discussion 1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarma AV, Parsons JK, McVary K, Wei JT. Diabetes and benign prostatic hyperplasia/lower urinary tract symptoms—what do we know? J Urol. 2009;182(6 suppl):S32–S37. [DOI] [PubMed] [Google Scholar]
- 8.Sasaki K, Yoshimura N, Chancellor MB. Implications of diabetes mellitus in urology. Urol Clin North Am. 2003;30:1–12. [DOI] [PubMed] [Google Scholar]
- 9.Van Den Eeden SK, Sarma AV, Rutledge BN, et al. Effect of intensive glycemic control and diabetes complications on lower urinary tract symptoms in men with type 1 diabetes: Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes Care. 2009;32:664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiu AF, Huang MH, Wang CC, Kuo HC. Higher glycosylated hemoglobin levels increase the risk of overactive bladder syndrome in patients with type 2 diabetes mellitus. Int J Urol. 2012;19:995–1001. [DOI] [PubMed] [Google Scholar]
- 11.Liu RT, Chung MS, Lee WC, et al. Prevalence of overactive bladder and associated risk factors in 1359 patients with type 2 diabetes. Urology. 2011;78:1040–1045. [DOI] [PubMed] [Google Scholar]
- 12.Breyer BN, Kenfield SA, Blaschko SD, Erickson BA. The association of lower urinary tract symptoms, depression and suicidal ideation: data from the 2005-2006 and 2007-2008 National Health and Nutrition Examination Survey. J Urol. 2014;191:1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thurmon KL, Breyer BN, Erickson BA. Association of bowel habits with lower urinary tract symptoms in men: findings from the 2005-2006 and 2007-2008 National Health and Nutrition Examination Survey. J Urol. 2013;189:1409–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rohrmann S, Smit E, Giovannucci E, Platz EA. Association between markers of the metabolic syndrome and lower urinary tract symptoms in the Third National Health and Nutrition Examination Survey (NHANES III). Int J Obes (Lond). 2005;29:310–316. [DOI] [PubMed] [Google Scholar]
- 15.Johnson CL, Dohrmann SM, Burt VL, Mohadjer LK. National Health and Nutrition Examination Survey: sample design, 2011-2014. Vital HealthStat 2. 2014;1–33. [PubMed] [Google Scholar]
- 16.Tikkinen KA, Johnson TM 2nd, Tammela TL, et al. Nocturia frequency, bother, and quality of life: how often is too often? A population-based study in Finland. Eur Urol. 2010;57:488–496. [DOI] [PubMed] [Google Scholar]
- 17.Wang CC, Chancellor MB, Lin JM, Hsieh JH, Yu HJ. Type 2 diabetes but not metabolic syndrome is associated with an increased risk of lower urinary tract symptoms and erectile dysfunction in men aged <45 years. BJU Int. 2010;105:1136–1140. [DOI] [PubMed] [Google Scholar]
- 18.Chung MS, Chuang YC, Lee JJ, Lee WC, Chancellor MB, Liu RT. Prevalence and associated risk factors of nocturia and subsequent mortality in 1,301 patients with type 2 diabetes. Int Urol Nephrol. 2014;46:1269–1275. [DOI] [PubMed] [Google Scholar]
- 19.Kupelian V, McVary KT, Barry MJ, et al. Association of C-reactive protein and lower urinary tract symptoms in men and women: results from Boston Area Community Health survey. Urology. 2009;73:950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rohrmann S, De Marzo AM, Smit E, Giovannucci E, Platz EA. Serum C-reactive protein concentration and lower urinary tract symptoms in older men in the Third National Health and Nutrition Examination Survey (NHANES III). Prostate. 2005;62:27–33. [DOI] [PubMed] [Google Scholar]
- 21.Ortega O, Rodriguez I, Gallar P, et al. Significance of high C-reactive protein levels in pre-dialysis patients. Nephrol Dial Transplant. 2002;17:1105–1109. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal R, Light RP, Bills JE, Hummel LA. Nocturia, nocturnal activity, and nondipping. Hypertension. 2009;54:646–651. [DOI] [PubMed] [Google Scholar]
- 23.Jin MH, Moon DG. Practical management of nocturia in urology. Indian J Urol. 2008;24:289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinsky PF, Kramer BS, Crawford ED, et al. Prostate volume and prostate-specific antigen levels in men enrolled in a large screening trial. Urology. 2006;68:352–356. [DOI] [PubMed] [Google Scholar]
- 25.Tai HC, Tai TY, Yang WS, Wang SW, Yu HJ. Associations between lower urinary tract dysfunction and glycemic control in women with type 2 diabetes: a cross-sectional study. J Diabetes Complications. 2016;30:415–419. [DOI] [PubMed] [Google Scholar]
- 26.Loveman E, Frampton GK, Clegg AJ. The clinical effectiveness of diabetes education models for Type 2 diabetes: a systematic review. Health Technol Assess. 2008;12:1–116, iii. [DOI] [PubMed] [Google Scholar]
- 27.Kim HJ. New horizons in geriatric urology. Korean J Urol. 2015;56:335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam DW, LeRoith D. The worldwide diabetes epidemic. Curr Opin Endocrinol Diabetes Obesity. 2012;19:93–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.