Abstract
Purpose
We describe haploidentical hematopoietic cell transplantation (HCT) with high-dose post-transplant cyclophosphamide (PTCy) in a boy with x-linked chronic granulomatous disease (CGD).
Methods
A persistent and life-threatening fungal infection was the indication for HSCT. Non-myeloablative conditioning with PTCy (50 mg/kg days 3 and 4) was used in the absence of fully matched donors.
Results
Engraftment occurred on day 24. The patient experienced Grade 2 graft-versus-host disease of the skin and gastrointestinal tract and CMV infection, both of which were controlled. Chimerism was 100 % at days 30 and 6 months. Cessation of antifungal therapy was consistent with cure of the infection.
Conclusions
Haploidentical HCT with high-dose PTCy for CGD is feasible and succeeded even in the context of active infection.
Keywords: Chronic granulomatous disease, haploidentical, hematopoietic cell transplantation, scedosporium apiospermum, post-transplant cyclophosphamide, graft versus host disease
Introduction
Haploidentical hematopoietic cell transplantation (HCT) has been an acceptable procedure for many years in severe combined immunodeficiency [1, 2]. Ex-vivo T-cell depletion reduces the alloreactivity of donor cells in a recipient whose ability to generate alloreactivity is limited, and this approach has been used in transplantation for chronic granulomatous disease (CGD) as well [3]. Recently, high-dose post-transplant cyclophosphamide has been used to allow haplo-identical transplantation for pre-malignant and malignant hematological conditions, with acceptable rates of engraftment and graft-versus-host disease (GVHD) [4].
We report, to our knowledge, the first successful haploidentical transplantation using post-transplant high dose cyclophosphamide (PTCy) in a 12 year old boy with CGD and an ongoing pericardial infection with Scedosporium apiospermum.
Methods
Case
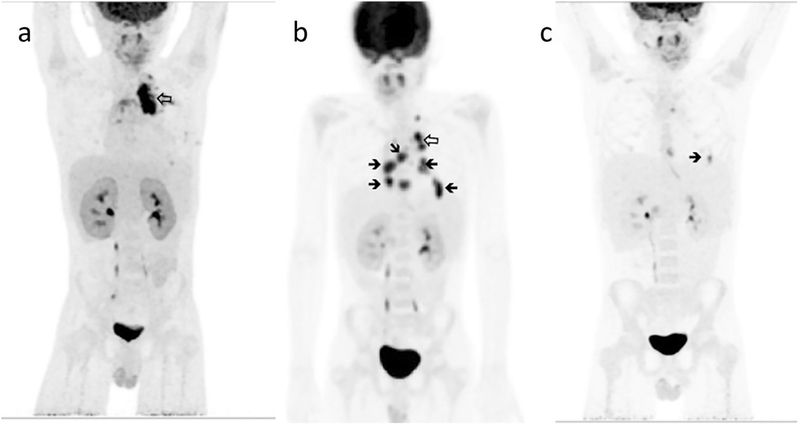
The patient’s X-linked CGD (mutation CYBB c.217 C > T; Arg73 stop; DHR stimulation index 1.4 consistent with quartile 1 superoxide production) presented in infancy with fungal lymphadenitis and retinitis. Thereafter he suffered from inflammatory bowel disease, with intermittent abdominal pain and bloody stools. Ten months prior to transplant, he presented to his local hospital with new fever after 4 months of a dry cough. A left upper lobe lung mass was seen on chest CTand a diagnostic specimen was obtained by video-assisted thoracoscopic surgery. Histological examination of the tissue demonstrated hyphal elements and culture grew Scedosporium apiospermum. He was referred to the National Institutes of Health (NIH) for further management. During the course of treatment he developed a moderate pericardial effusion that responded to a short course of oral corticosteroid. With resolution of signs and symptoms of active disease, he made a transition to maintenance antifungal therapy and returned home. Two months later fever recrudesced and radiologic evaluation (echocardiography/cardiac magnetic resonance imaging and positron-emission tomography [PET]) demonstrated multiple pericardial masses contiguous to the site of resected disease but also throughout the pericardial space that were associated with early tamponade. Intensification of antifungal treatment in addition to antibacterial therapy and granulocyte transfusions (twice weekly for 5 weeks) resulted in marked, but not complete, resolution as assessed by echocardiography and PET-CT 9 months after the initial diagnosis. (Fig. 1).
Fig. 1.
FDG-F18 PET scans. A 2 months after the onset of febrile illness. b 4 months later, at recrudescence of a febrile illness with pericardial disease. c 4 months later, after clinically successful therapy prior to haplo-identical HCT. Hollow arrows indicate the primary site of disease in the left upper lung, solid arrows the active lesions in the pericardial space
He had no full sibling or matched unrelated donor in the NMDP registry. His father was a haploidentical donor and the patient (weight 35 kg) underwent HCT with peripheral blood stem cells on 11/07/2014 with fludarabine days −6 through −2, (30 mg/m2 × 5), intravenous busulfan (102 mg/day intravenously in a single dose over 2 h on days −4 and −3, increased to 161 mg day −2 to achieve a cumulative AUC of ~9024 micromol*min, cumulative dose 10.4 mg/kg), cyclophosphamide 14.5 mg/kg × 2, days −6 and −5, and total body irradiation (200 cGy) day −1 conditioning with post-transplant cyclophosphamide (50 mg/kg days 3 and 4) and sirolimus GVHD prophylaxis. Antifungal therapy was continued through the transplant and granulocyte transfusions were given during neutropenia.
Results
The transplant course was complicated by febrile neutropenia with candidemia and polymicrobial bacteremia and eventually rash and diarrhea. Biopsy of the colon on day 21 was consistent with GVHD and cytomegalovirus (CMV) colitis. CMV viremia was noted the same day as the endoscopy. Stable neutrophil engraftment occurred on day 24. The bacteremia and candidemia were successfully treated. Foscarnet controlled the CMV reactivation and specific anti-CMV therapy was discontinued within 5 weeks. Grade 2 GVHD was successfully treated with a short course of tapering corticosteroid and tacrolimus in addition to continuation of protocol sirolimus. Post-transplant radiology/echocardiography was consistent with resolution of the Scedosporium infection. The patient is now well at 9 months post transplant with no evidence of GVHD.
Discussion
Hematopoietic cell transplantation is a potentially curative therapy for congenital immunodeficiencies, including CGD [5]. Acceptable rates of engraftment without transplant-related mortality or GVHD are the goals of transplantation. Results achieved with matched related sibling donors have justified the extension to alternative donors, especially matched unrelated donors. However, this still leaves a proportion of patients without a suitable donor based on classical matching criteria [6].
This problem has been addressed in other immunodeficiencies and hematological malignancies by extending the donor pool to include haploidentical siblings or parents [7]. Post-transplant cyclophosphamide has been used as single agent GVHD prophylaxis in other contexts, but the bidirectional adverse outcomes related to the haplo-mismatch are both attenuated by either T-cell depletion strategies or post-transplant high dose cyclophosphamide, as in our case [8, 9]. This has led to acceptable levels of engraftment and low levels of Grade III/IV GVHD [10]. Elevated risks of relapse of malignant disease are not considerations in congenital immunodeficiency, making this a particularly attractive option.
Haploidentical transplantation in CGD has been successful utilizing ex-vivo T-cell depletion with anti-CD3 antibodies. That case resulted in rapid engraftment and no GVHD but had prolonged red cell aplasia, presumably secondary to blood group incompatibility [3]. Other recent approaches have included the depletion of naïve T-cells with anti-CD45RA beads, but clinical reports are only now appearing [11, 12]. The relative successes of these approaches would require formal study. T-cell depletion will carry the standard risk of increased susceptibility to infectious complications, which is relevant in the transplant course of our case and in other congenital immunodeficiencies such as DOCK8, in which chronic pretransplant viral infections are a major component of the process [13]. Whether that risk would be balanced by successful transplantation and the control of viral reactivation should be assessed.
The strategy we adopted is modeled on that pioneered at the Johns Hopkins Hospital and modified in transplantation for other congenital immunodeficiencies at our institution. The fundamental regimen is based on low dose irradiation, which is effective with fludarabine at the doses we use, and low dose cyclophosphamide induction, which improves donor chimerism [14, 15]. Our institutional adaptation has been to add busulfan in order to facilitate myeloid engraftment in patients with congenital immunodeficiencies, such as DOCK8 and GATA2 deficiencies, and is consistent with other protocols for transplantation in chronic granulomatous disease [5, 16]. Treosulfan has been used in Europe but is not commercially available in the United States. Targeted dose busulfan has been used widely, but presents the challenge of real-time busulfan levels, which we do not have. Thus, our approach has been to estimate a dose and make modifications as possible if we receive a busulfan level from day one early enough to allow subsequent changes. With the goal of a higher cell dose, we have used peripheral blood stem cells and have targeted higher numbers (11 × 106 CD34 cells in this case).
We have used sirolimus as GVHD prophylaxis in matched-related and unrelated transplantion for CGD. Sirolimus is a tolerizing agent (as opposed to calcineurin inhibitors such as tacrolimus or cyclosporine). It is generally well tolerated and we have chosen it to further decrease the degree of conditioning required for engraftment. This is similar to the NHLBI Sickle Cell protocol, which is also using single agent sirolimus [17, 18].
We developed a protocol for haploidentical transplantation in the context of need for the patient reported here. As noted, we considered T-cell depletion strategies, but at our first review the ex-vivo depletion product was not yet available. Transplantation in the context of ongoing/refractory infection is a concept in evolution [19]. Infection in transplantation for other congenital immunodeficiencies has been associated with poorer outcomes [20]. However, our experience in this and other cases supports the idea that transplantation can be implemented successfully by decreasing the burden of disease before conditioning and using aggressive diagnostic, prophylactic and therapeutic maneuvers during neutropenia. It is possible that at some point the need for “control” of infection, however that might be measured, would be overstated and transplantation with restoration of effective immunity would trump other therapies. Transplantation has defined mortality. It is more difficult to sum the adverse events and consequences of the natural course of CGD. Both infections, chronic inflammatory complications, and the toxicities and burdens of treating these are substantial and sometimes overwhelming. Our priority is thus to improve the outcomes of transplantation in order to better weigh its place in the management of this congenital immunodeficiency.
The use of PET scans for the assessment of invasive fungal disease in CGD has been described and was important in staging this patient’s disease prior to transplantation [21, 22]. Management of Scedosporium apiospermum infection, a left-upper lobe pulmonary mass and pericarditis in this case, is poorly understood [23, 24]. We had anti-fungal susceptibility tests from the patient’s original isolate, but neither these nor synergy tests seemed to explain his course. The patient’s disease relapsed on secondary prophylaxis after an initial response, with extension of disease to the pericardial space that threatened tamponade. Granulocyte transfusions with a change in antifungals were used to control the alarming recrudescence of disease in the pericardium. The collection of granulocytes is dependent on the availability of immediate donation (cells are not stored and donors are selected by CMV status when possible, but CMV seronegative eligible donors are rare). There is no established protocol to prevent alloimmunization, although rituximab has been used [25]. Rituximab may have prolonged effects, and so we chose sirolimus, with which we have experience (unpublished observation, E. Kang) during the course of granulocyte transfusions, to decrease the risk of alloimmunization [26–29]. Repeat anti-HLA antibody testing after the therapeutic course of granulocytes revealed only weak and narrow reactivity, none relevant to the haploidentical donor, and we did not test for anti-neutrophil antibodies. We were able to repeat the granulocyte transfusions three times during the transplant, with sufficient durability of the improvement in counts to presume that no anti-neutrophil immunity had occurred.
The impact of CMV serostatus in granulocyte donors on subsequent viremia or disease in transplant recipients of granulocyte transfusions may be attenuated in the context of surveillance for disease and preemptive therapy [30]. The granulocyte transfusions did infect our patient with CMV, which recrudesced with his GI GVHD. There were other consequences of the treatment of the pre-transplant infectious disease that had to be taken into consideration. Although not quantifiable, the renal effects of several weeks of amphotericin may have intensified the electrolyte abnormalities seen with foscarnet treatment for CMV disease. In addition, the best evidence in this case supported some better efficacy for high dose voriconazole (used with omeprazole to boost levels), but that therapy had already caused painful periostitis [31]. That led us to bridge the period from control of the pericarditis until transplant with posaconazole, and his infection had broken through secondary prophylaxis with that drug.
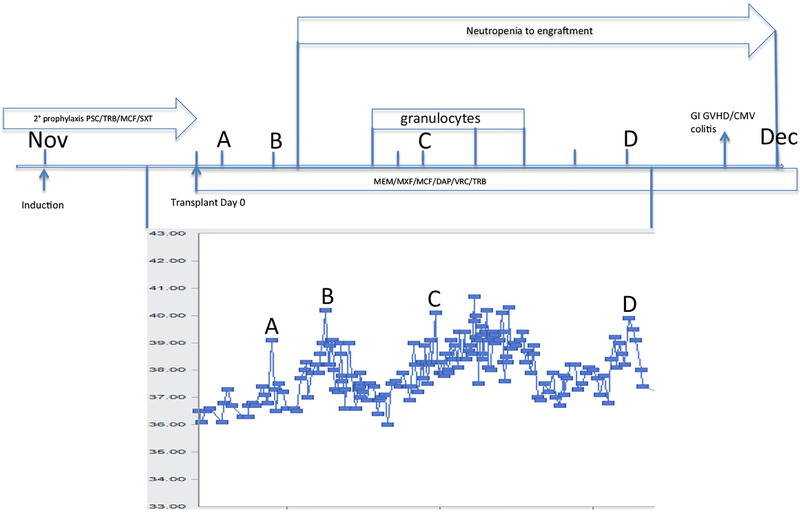
The transplant course itself was somewhat stormy. Exuberant fever as reported elsewhere occurred at infusion and persisted until the treatment for GVHD became effective [32]. We were aware that fevers can occur either with cell infusion or with the post-transplant cyclophosphamide (C. Kanakry, personal communication), but separating fevers and symptoms due to the transplant from persistent or new infections (bacteremia and candidemia, probably both catheter-associated during neutropenia) was challenging. (Fig. 2) The Grade 2 GVHD was managed successfully over the course of weeks rather than months with a combination of sirolimus, low dose tacrolimus and tapering doses of corticosteroids. It is equally remarkable that the CMV infection was only moderately difficult to manage, as the patient was a CMV positive recipient from a CMV negative donor [33]. Viremia was quickly suppressed and the major complication was managing electrolyte abnormalities caused by foscarnet. There was evidence of anti-CMV T-cell reconstitution (reactivity to CMVpp65 and CMVIE1) by 6 weeks after the onset of disease. (personal communication, C. Bollard). Therapy with siRNA against IE1 compared to that against the viral polymer-ase or kinase has potent activity in vitro in suppressing viral protein production, replication and the number of cells infected [34]. Our data by which to quantitate the components of the patient’s reconstitution are otherwise limited, with one immunophenotype at day 38 post-transplant with a CD8 count of 309 cells/μl and the above specific CMV reactivity at day 61 post-transplant. We would interpret these as fairly early and vigorous reconstitution, and this brings into question what role T-cell depletion, ex- or in vivo, would have had. We cannot comment specifically on the role of planned immunosuppression with post-transplant sirolimus alone, as the patient’s GVHD required the addition of corticosteroids and tacrolimus. Both the GVHD and CMV infection course might speak to an early, successful common mechanism of CD4+ Foxp3+ T regulatory and antiviral cell donor engraftment [35]. Alternatively, the survival of recipient T memory cells after PTCy has been demonstrated [36]. Chimerism studies demonstrated 100 % donor engraftment in the CD3 and myeloid lineages from day 30 through 6 months post-transplant.
Fig. 2.
Transplant course. Temperature curve in degrees Celsius. A) transplant day 1 B) transplant day 3, post-transplant cyclophosphamide C) bacteremia/fungemia D) onset of graft-versus-host disease/CMV colitis. PSC, posaconazole; TRB, terbinafine; MCF, micafungin; SXT, sulfamethoxazoletrimethoprim; MEM, meropenem; MXF, moxifloxacin; DAP, daptomycin; VRC, voriconazole
Conclusion
We have used haploidentical transplantation with post-transplant high dose cyclophosphamide in a patient with chronic granulomatous disease with refractory infection as the indication for transplantation. Further experience will be necessary to firmly establish this modality of transplant in this context, but its success in this most challenging case provides sufficient grounds for exploring this as an acceptable transplant procedure for patients with no suitable matched sibling or unrelated donor.
Acknowledgments
We would like to acknowledge the expert advice provided by Dr. Catherine Bollard of Children’s National Medical Center and Drs. Dennis Hickstein and Christopher Kanakry of the Experimental Transplantation and Immunology Branch of the National Cancer Institute.
Funding This project has been funded in whole or in part with federal funds from the following components of the National Institutes of Health (NIH): National Cancer Institute, NIH, under Contract No. HHSN261200800001E; National Institute of Allergy and Infectious Disease under Intramural Project #1-ZAI-AI000989. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research; and [in part] by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases.
Footnotes
Compliance with Ethical Standards All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Human and Animal Rights This article does not contain any studies with animals performed by any of the authors.
Informed Consent Informed consent was obtained from all individual participants included in the study under IRB approved NIH Protocol #s 94-I-0073, 05-I-0213 and/or 15-I-0007.
References
- 1.Friedrich W, Goldmann SF, Ebell W, Blutters-Sawatzki R, Gaedicke G, Raghavachar A, et al. Severe combined immunodeficiency: treatment by bone marrow transplantation in 15 infants using HLA-haploidentical donors. Eur J Pediatr. 1985;144(2): 125–30. [DOI] [PubMed] [Google Scholar]
- 2.Buckley RH, Schiff SE, Schiff RI, Markert L, Williams LW, Roberts JL, et al. Hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 1999;340(7):508–16. [DOI] [PubMed] [Google Scholar]
- 3.Hoenig M, Niehues T, Siepermann K, Jacobsen EM, Schutz C, Furlan I, et al. Successful HLA haploidentical hematopoietic SCT in chronic granulomatous disease. Bone Marrow Transplant. 2014;49(10):1337–8. [DOI] [PubMed] [Google Scholar]
- 4.Kanakry CG, Tsai HL, Bolanos-Meade J, Smith BD, Gojo I, Kanakry JA, et al. Single-agent GVHD prophylaxis with posttrans-plantation cyclophosphamide after myeloablative, HLA-matched BMT for AML, ALL, and MDS. Blood. 2014;124(25):3817–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gungor T, Teira P, Slatter M, Stussi G, Stepensky P, Moshous D, et al. Reduced-intensity conditioning and HLA-matched haemopoietic stem-cell transplantation in patients with chronic granulomatous disease: a prospective multicentre study. Lancet. 2014;383(9915):436–48. [DOI] [PubMed] [Google Scholar]
- 6.Kang EM, Marciano BE, DeRavin S, Zarember KA, Holland SM, Malech HL. Chronic granulomatous disease: overview and hematopoietic stem cell transplantation. J Allergy Clin Immunol. 2011;127(6):1319–26 quiz 27–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuchs EJ. Haploidentical transplantation for hematologic malignancies: where do we stand? Hematol Am Soc Hematol Educ Program 2012;2012:230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertaina A, Merli P, Rutella S, Pagliara D, Bernardo ME, Masetti R, et al. HLA-haploidentical stem cell transplantation after removal of alphabeta + T and B cells in children with nonmalignant disorders. Blood. 2014;124(5):822–6. [DOI] [PubMed] [Google Scholar]
- 9.Kanakry CG, O’Donnell PV, Furlong T, de Lima MJ, Wei W, Medeot M, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol: Off J Am Soc Clin Oncol. 2014;32(31):3497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCurdy SR, Kanakry JA. Showel MM. Bolanos-Meade J, Rosner GL, et al. Risk-stratified outcomes of nonmyeloablative, HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide Blood: Tsai HL; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teschner D, Distler E, Wehler D, Frey M, Marandiuc D, Langeveld K, et al. Depletion of naive T cells using clinical grade magnetic CD45RA beads: a new approach for GVHD prophylaxis. Bone Marrow Transplant. 2014;49(1):138–44. [DOI] [PubMed] [Google Scholar]
- 12.Triplett BM, Shook DR, Eldridge P, Li Y, Kang G, Dallas M, et al. Rapid memory T-cell reconstitution recapitulating CD45RA-depleted haploidentical transplant graft content in patients with hematologic malignancies. Bone Marrow Transplant. 2015;50(7): 968–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuellar-Rodriguez J, Freeman AF, Grossman J, Su H, Parta M, Murdock H, et al. Matched related and unrelated donor hematopoietic stem cell transplantation for DOCK8 deficiency. Biol Blood Marrow Transplant. 2015;21(6):1037–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Donnell PV, Luznik L, Jones RJ, Vogelsang GB, Leffell MS, Phelps M, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttrans-plantation cyclophosphamide. Biol Blood Marrow Transplant. 2002;8(7):377–86. [DOI] [PubMed] [Google Scholar]
- 15.Luznik L, Jalla S, Engstrom LW, Iannone R, Fuchs EJ. Durable engraftment of major histocompatibility complex-incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood. 2001;98(12):3456–64. [DOI] [PubMed] [Google Scholar]
- 16.Grossman J, Cuellar-Rodriguez J, Gea-Banacloche J, Zerbe C, Calvo K, Hughes T, et al. Nonmyeloablative allogeneic hematopoietic stem cell transplantation for GATA2 deficiency. Biol Blood Marrow Transplant. 2014;20(12):1940–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng Y, Collins SL, Lutz MA, Allen AN, Kole TP, Zarek PE, et al. A role for mammalian target of rapamycin in regulating T cell activation versus anergy. J Immunol. 2007;178(4):2163–70. [DOI] [PubMed] [Google Scholar]
- 18.Powell JD, Fitzhugh C, Kang EM, Hsieh M, Schwartz RH, Tisdale JF. Low-dose radiation plus rapamycin promotes long-term bone marrow chimerism. Transplantation. 2005;80(11):1541–5. [DOI] [PubMed] [Google Scholar]
- 19.Ozsahin H, von Planta M, Muller I, Steinert HC, Nadal D, Lauener R, et al. Successful treatment of invasive aspergillosis in chronic granulomatous disease by bone marrow transplantation, granulocyte colony-stimulating factor-mobilized granulocytes, and liposomal amphotericin-B. Blood. 1998;92(8):2719–2724. [PubMed] [Google Scholar]
- 20.Pai SY, Logan BR, Griffith LM, Buckley RH, Parrott RE, Dvorak CC, et al. Transplantation outcomes for severe combined immuno-deficiency, 2000–2009. N Engl J Med. 2014;371(5):434–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theobald I, Fischbach R, Hulskamp G, Franzius C, Frosch M, Roth J, et al. [Pulmonary aspergillosis as initial manifestation of septic granulomatosis (chronic granulomatous disease, CGD) in a premature monozygotic female twin and FDG-PET diagnosis of spread of the disease]. Radiologe. 2002;42(1):42–5. [DOI] [PubMed] [Google Scholar]
- 22.Gungor T, Engel-Bicik I, Eich G, Willi UV, Nadal D, Hossle JP, et al. Diagnostic and therapeutic impact of whole body positron emission tomography using fluorine-18-fluoro-2-deoxy-D-glucose in children with chronic granulomatous disease. Arch Dis Child. 2001;85(4):341–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jabado N, Casanova JL, Haddad E, Dulieu F, Fournet JC, Dupont B, et al. Invasive pulmonary infection due to scedosporium apiospermum in two children with chronic granulomatous disease. Clin Infect Dis: Off Publ Infect Dis Soc Am. 1998;27(6):1437–41. [DOI] [PubMed] [Google Scholar]
- 24.Troke P, Aguirrebengoa K, Arteaga C, Ellis D, Heath CH, Lutsar I, et al. Treatment of scedosporiosis with voriconazole: clinical experience with 107 patients. Antimicrob Agents Chemother. 2008;52(5):1743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shigemura T, Nakazawa Y, Yoshikawa K, Hirabayashi K, Saito S, Kobayashi N, et al. Successful cord blood transplantation after repeated transfusions of unmobilized neutrophils in addition to anti-fungal treatment in an infant with chronic granulomatous disease complicated by invasive pulmonary aspergillosis. Transfusion. 2014;54(3):516–21. [DOI] [PubMed] [Google Scholar]
- 26.Stroncek DF, Leonard K, Eiber G, Malech HL, Gallin JI, Leitman SF. Alloimmunization after granulocyte transfusions. Transfusion. 1996;36(11–12):1009–15. [DOI] [PubMed] [Google Scholar]
- 27.Heim KF, Fleisher TA, Stroncek DF, Holland SM, Gallin JI, Malech HL, et al. The relationship between alloimmunization and post-transfusion granulocyte survival: experience in a chronic granulomatous disease cohort. Transfusion. 2011;51(6):1154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anasetti C, Amos D, Beatty PG, Appelbaum FR, Bensinger W, Buckner CD, et al. Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukemia or lymphoma. N Engl J Med. 1989;320(4):197–204. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan B, Kopyltsova Y, Khokhar A, Lam F, Bonagura V. Rituximab and immune deficiency: case series and review of the literature. J Allergy Clin Immunol Pract. 2014;2(5):594–600. [DOI] [PubMed] [Google Scholar]
- 30.Vij R, DiPersio JF, Venkatraman P, Trinkaus K, Goodnough LT, Brown RA, et al. Donor CMV serostatus has no impact on CMV viremia or disease when prophylactic granulocyte transfusions are given following allogeneic peripheral blood stem cell transplantation. Blood. 2003;101(5):2067–9. [DOI] [PubMed] [Google Scholar]
- 31.Wang TF, Wang T, Altman R, Eshaghian P, Lynch JP 3rd, Ross DJ, et al. Periostitis secondary to prolonged voriconazole therapy in lung transplant recipients. Am J Transplant: Off J Am Soc Transplant Am Soc Transplant Surg. 2009;9(12):2845–50. [DOI] [PubMed] [Google Scholar]
- 32.O’Donnell P, Raj K, Pagliuca A. High fever occurring 4 to 5 days post-transplant of haploidentical bone marrow or peripheral blood stem cells after reduced-intensity conditioning associated with the use of post-transplant cyclophosphamide as prophylaxis for graft-versus-host disease. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2015;21(1):197–8. [DOI] [PubMed] [Google Scholar]
- 33.Thomas S, Herr W. Natural and adoptive T-cell immunity against herpes family viruses after allogeneic hematopoietic stem cell transplantation. Immunotherapy. 2011;3(6):771–88. [DOI] [PubMed] [Google Scholar]
- 34.Hamilton ST, Milbradt J, Marschall M, Rawlinson WD. Human cytomegalovirus replication is strictly inhibited by siRNAs targeting UL54, UL97 or UL122/123 gene transcripts. PLoS One. 2014;9(6):e97231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ganguly S, Ross DB, Panoskaltsis-Mortari A, Kanakry CG, Blazar BR, Levy RB, et al. Donor CD4+ Foxp3+ regulatory T cells are necessary for posttransplantation cyclophosphamide-mediated protection against GVHD in mice. Blood. 2014;124(13):2131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberto A, Castagna L, Zanon V, Bramanti S, Crocchiolo R. McLaren JE, et al. Blood: Role of naive-derived T memory stem cells in T cell reconstitution following allogeneic transplantation; 2015. [DOI] [PMC free article] [PubMed]