Abstract
Amyloid beta (Aβ) and tau pathology have been described in the brains of captive aged great apes, but the natural progression of these age-related pathologies from wild great apes, including the gorilla, is unknown. In our previous study of western lowland gorillas (Gorilla gorilla gorilla) who were housed in American Zoos and Aquariums (AZA)-accredited facilities, we found an age-related increase in Aβ-positive plaques and vasculature, tau-positive astrocytes, oligodendrocyte coiled bodies, and neuritic clusters in the neocortex as well as hippocampus in older animals. Here, we demonstrate that aged wild mountain gorillas (Gorilla beringei beringei), who spent their entire lives in their natural habitat, also display an age-related increase in APP/Aβ-immunoreactive blood vessels and plaques, but very limited tau pathology, in the frontal cortex. These results indicate that Aβ and tau lesions are age-related events that occur in the brain of gorillas living in captivity and in the wild.
Keywords: aging, amyloid, cerebral cortex, wild great apes, tau, pathology
INTRODUCTION
While neurofibrillary tangles (NFTs) are pathological features exclusive to human neurodegenerative conditions (Bouras et al., 1994; Braak and Braak, 1991; Jackson and Lowe, 1996), beta amyloid (Aβ) deposits (neuritic and diffuse plaques) and Aβ angiopathy have been reported in several nonhuman primate species. The majority of these studies have been carried out in captive rhesus monkeys (Gearing et al., 1996; Martin et al., 1991; Mufson et al., 1994; Norvin et al., 2015; Poduri et al., 1994), long-tailed macaques (Kimura et al., 2003), Caribbean vervets (Lemere et al., 2004), squirrel monkeys (Walker et al., 1987), and cotton-top tamarins (Lemere et al., 2008), and a few in great apes (Edler et al., 2015; Gearing et al., 1997, 1996, 1994; Kimura et al., 2001; Perez et al., 2013; Rosen et al., 2008). Great apes, which include common chimpanzees, bonobos, gorillas, and orang-utans, are of significant interest in aging and AD research because they are the closest living relatives of humans and therefore could provide clues to the evolutionary changes underlying the onset of AD pathology.
Several reports demonstrate that aged chimpanzees (Gearing et al., 1996, 1994) orang-utans (Gearing et al., 1997), and gorillas (Kimura et al., 2001; Perez et al., 2013), display diffuse plaques and vascular amyloid, whereas AD-like NFTs have been observed only in the aged chimpanzee brain (Edler et al., 2015; Rosen et al., 2008). However, each of these investigations evaluated brain tissue obtained from great apes that lived in captivity their entire life or for a significant time. To our knowledge, there are no reports of AD-like pathology in the brain of wild-living apes, or any other primate species for that matter. Several studies suggest that environmental conditions and experiences influence brain function and the risk of developing AD-related pathology (Hu et al., 2010; Lazarov et al., 2005; Nichol et al., 2007; Snowdon, 2003; Stern et al., 1994). In this regard, a previous study reported that aged rhesus monkeys with early rearing in smaller cages exhibited extensive Aβ plaque deposition in the neocortex compared to aged monkeys reared in standard-sized cages. By contrast, non-aged monkeys raised in standard-sized cages exhibited only rare amyloid plaques, suggesting that life experience may constitute a risk factor for Aβ plaque deposition later in life (Merrill et al., 2011). Therefore, it is important to determine whether environmental factors influence the development of AD-like pathology in great apes. In our previous study of western lowland gorillas (Gorilla gorilla gorilla) housed in US zoos we demonstrated the presence of Aβ plaques, Aβ vascular deposition, and tau-like lesions in the neocortex and hippocampus in old male and female individuals (Perez et al., 2013). However, whether gorillas who lived their entire life in their natural habitat develop AD-like pathology is unknown. Therefore, we used immunohistochemical and histofluorescence techniques to reveal the presence of Aβ and tau pathology during aging in the frontal cortex of the wild mountain gorilla (Gorilla beringei beringei).
Mountain gorillas differ from western lowland gorillas in a number of features, including a greater reliance on a folivorous diet and more rapid growth and development (McFarlin et al., 2013). In addition, they are among the closest extant relatives of modern humans, as demonstrated by the recent sequencing of the gorilla genome (Scally et al., 2012). Mountain gorillas are found in the high elevations of the Virunga Mountains in the northwest border of Rwanda, Democratic Republic of Congo, and Uganda (Doran et al., 2002). The maximum lifespan of wild gorillas range from 35 to 40 years (Bronikowski et al., 2011), whereas captive great apes have exceeded 50 years (Perez et al., 2013).
Subjects
The study sample included a total of 10 adult (>10 years) wild mountain gorillas (G. b. beringei), with 7 females (16 to 42 years) and 3 males (>20 to 35 years), from the Virunga volcanic mountains in Rwanda. Although in this study the average age was 31.4 ± 8.6 years, the exact date of birth and age of some of the gorillas were not known but were estimated based on veterinarian records and dental examination. The estimated age of the female gorillas Um and Ki was >35 and >40 years old, respectively and the estimated age of the males Se (silver back) and Sh was >20 and >31 years old, respectively (see Table 1). All individuals died in the field, and brains (females 416 ± 13 g, males 461 ± 102 g) were collected and fixed within 48 hours in 4% paraformaldehyde and maintained in the same fixative for an average of 49 weeks (range 4 to 103 weeks). Afterwards, brains were stored in phosphate-buffered saline containing 0.1% sodium azide at 4°C. Right hemisphere blocks containing frontal cortex (Brodmann areas 6, 8, 9, 44, 45 and 46), anterior cingulate areas (Brodmann areas 32, 24, and 25), orbitofrontal cortex (Brodmann areas 11 and 47) and anterior insula were cryoprotected in a graded series of sucrose solutions at 4°C and cut in the coronal plane at 40 μm on a freezing sliding microtome. Sections were stored in a solution containing 30% glycerin-30% ethylene glycol, in 0.1 M phosphate buffer at −20°C until processing for immunohistochemistry.
Table 1.
Gorilla demographics and cortical Alzheimer’s-like pathology
| ID | Age | Sex | Brain | Time in | Aβ-ir vascular | Aβ-ir plaques | Tau-ir | Tau-ir |
|---|---|---|---|---|---|---|---|---|
| Weight (g) | Fixative (w) |
neurites | cells | |||||
| Sa | 16 | F | 402 | 10 | -- | -- | + | -- |
| ‘In | 24 | F | 412 | 38 | -- | -- | + | -- |
| Kw | 33 | F | 423 | 53 | + | + | + | -- |
| Um | >35* | F | 433 | 4 | ++ | ++ | + | -- |
| Pu | 38 | F | 400 | 68 | ++ | -- | + | -- |
| Ki | >40* | F | 432 | 92 | + | + | + | + |
| Cy | 42 | F | 410 | 60 | ++ | ++ | + | + |
| Se | >20* | M | 345 | 53 | + | + | -- | -- |
| Sh | >31* | M | 538 | 6 | + | + | + | -- |
| Ti | 35 | M | 500 | 103 | + | + | -- | -- |
, estimated age; +: minor; ++: extensive; --: absent
(w): weeks
Immunohistochemistry and histofluorescence
Free-floating sections containing frontal cortical areas were singly stained with antibodies against APP/Aβ, Aβ, Aβ42, Aβ40, Tau (Alz50 and AT8), and the endothelial marker CD31 (see Table 2 for details). The molecular nature of the immunogens recognized by these antibodies and their specificity has been reported previously (Perez et al., 2013). Sections were washed in Tris-buffered saline (TBS) and incubated in 0.1 M sodium metaperiodate (Sigma, St. Louis, MO) to inactivate endogenous peroxidase. Tissue was then permeabilized in TBS containing 0.25% Triton-X (Thermofisher, Waltham, MA) and blocked in the same solution containing 3% goat serum for 1 h. Sections were incubated with the antibodies at appropriate dilutions (Table 2) overnight at room temperature in 0.25% Triton X-100, and 1% goat serum solution. The next day, and after several washes in TBS containing 1% goat serum, tissue was incubated with the appropriate secondary antibodies (Table 2) at a 1:200 dilution for 1 h (Vector Laboratories, Burlingame, CA). Following washes in TBS, sections were incubated in Vectastain ABC kit (Vector Laboratories) for 1 h, and developed in acetate-imidazole buffer containing 0.05% 3,3’-diaminobenzidine tetrahydrochochloride (DAB, Sigma) with or without nickel intensification (1% nickel sulfate). The reaction was terminated in acetate-imidazole buffer, the tissue was mounted on slides, dehydrated in alcohols, cleared in xylenes, and cover-slipped with DPX (Biochemica Fluka, Buchs, Switzerland). Select sections were also singly stained with anti-Aβ40, anti-Aβ42, and anti-Aβ antibodies (see Table 2) using the protocol described above. A pan-Aβ antibody, MOAB-2-immunostaining, was performed on sections mounted on charged slides that were pretreated for antigen-retrieval with 88% formic acid for 6 min. Selected sections were Nissl counterstained with cresyl violet. In addition, as a positive control for each antibody, we stained cortical sections from a 55 year-old female western lowland gorilla known to have amyloid plaque and tau-pathology from our previous study (Perez et al., 2013) and from the brain of a patient who died with AD. Cortical nomenclature was based upon the cytoarchitecture of the human brain (Brodmann, 1909; Simić and Hof, 2015).
Table 2.
Summary of antibodies
| Antigen | Primary antibodies | Dilution | Company: Catalog # | Secondary antibodies Company: Catalog # |
|---|---|---|---|---|
| APP/Aβ | mouse monoclonal to residues1–16 of Aβ (“DAEFRHDSGYEVHHQK”) (6E10) | 1:1000 | Covance: SIG-39320 | Biotinylated Goat anti-mouse IgG Vector: Ba-9200 |
| Aβ | mouse monoclonal to Aβ (MOAB-2) | 1:600 | Gift from M. J. LaDu | |
| Aβ40 | rabbit polyclonal to C terminus of human Aβ40 (“LMVGGVV”) | 1:100 | Millipore: AB5074P | Biotinylated goat anti-rabbit IgG Vector: Ba-1000 |
| Aβ42 | rabbit polyclonal to C terminus of human Aβ A4 protein | 1:100 | Invitrogen: 700254 | |
| CD31 | rabbit polyclonal to C terminus of mouse CD31 | 1:500 | Abcam: ab28364 | |
| Tau (Alz50) | mouse monoclonal to tau residues 5–15 (“QEFEVMEDHA”), 312–322 (“GSTENLKHQPGG”) | 1:1000 | Gift from P. Davies | Biotinylated Goat anti-mouse IgM Vector: Ba-2020 |
| Tau (AT8) | mouse monoclonal to tau phosphoSerine 202 and phosphoThreonine 205 | 1:800 | Therno Fisher: MN1020 | Biotinylated Goat anti-mouse IgG Vector: Ba-9200 |
| SMI-34 | mouse monoclonal to phosphorylated 200 kDa and 160 kDa neurofilaments | 1:800 | Abcam: 24571 |
Some sections also were double-immunolabeled for CD31 and APP/Aβ as well as for SMI-34 and Aβ42. After finishing the standard protocol for CD31 or Aβ42 using nickel intensification, sections were washed with TBS and incubated with antibody 6E10 or SMI-34 for 24 h in a medium containing TBS, 0.25% TritonX-100, and 1% goat normal serum. The next day, sections were incubated with biotinylated goat anti-mouse (Vector Laboratories) and visualized with DAB (brown) without nickel intensification. This dual staining resulted in a two-color reaction product: CD31immunoreactive (-ir) endothelial cells and Aβ42 positive plaques appeared dark blue/black whereas SMI-34 profiles, APP/Aβ-positive plaques and blood vessels were brown (Perez et al., 2012, 2011).
Thioflavine S histofluorescence was used to examine the fibrillary nature of the Aβ deposits in the vasculature and plaques as described previously (Perez et al., 2013). Bright field and fluorescence images were acquired using a Nikon Eclipse 80i microscope and a Zeiss LSM 710 confocal, respectively. APP/Aβ-ir profiles were counted using a 10x objective in a field size of 1.0 mm2.
RESULTS
Aβ deposition increases with age in the frontal cortex
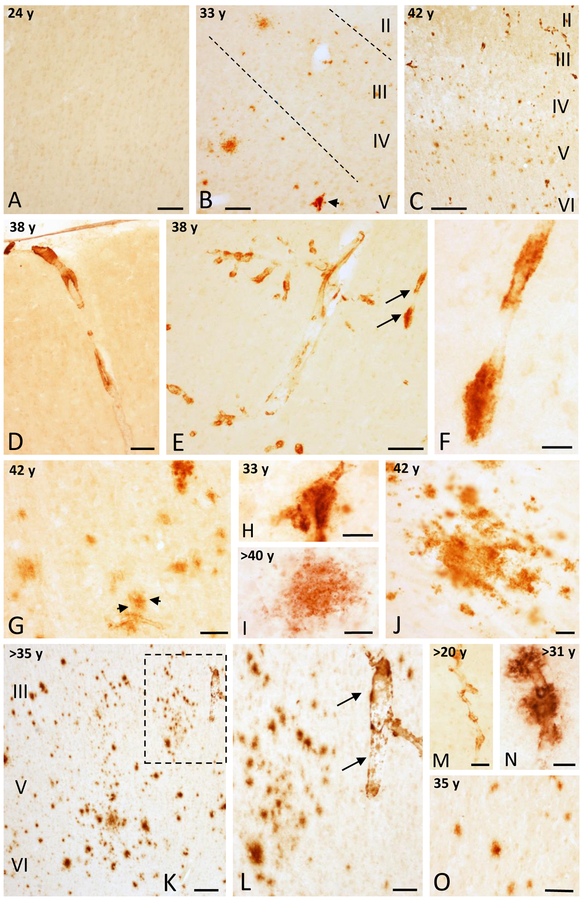
Bright field examination of immunostained wild mountain gorilla frontal cortex sections revealed the presence the APP/Aβ-positive blood vessels and plaques in subjects older than 25 years (Table 1 and Fig. 1A–C). No APP/Aβ-ir) profiles were found in young female mountain gorillas (16 and 24 year-old females) (Table 1 and Fig. 1A). By contrast, only few APP/Aβ-ir profiles were seen in a male gorilla older than 20 years (Table 1 and Fig. 1M). In general, vascular APP/Aβ-ir deposits were numerous between 30 to 40 years of age (Fig. 1C-F, M, N), and were found in meningeal arteries, arterioles, and capillaries, and as perivascular accumulations mainly in cortical layers I to IV (Fig. 1C–H). While APP/Aβ-ir plaques were seen either in association with blood vessels (Fig. 1H), as isolated profiles, or in clusters primarily in older ages (Fig. 1I, J).
Figure 1.
Photomicrographs showing the absence of APP/Aβ-ir profiles in area 9 in a 24 year-old female gorilla (A) and the presence of some APP/Aβ-ir profiles in a 33 (B) and many more in a 42 (C) year-old female gorilla. Images showing variation in APP/Aβ-ir blood vessels (D-F) in the area 9 in a 38 year-old female gorilla. F. High-magnification photomicrograph of APP/Aβ-positive blood vessel (arrows) shown in panel E. G and H. Aβ positive plaque (black arrows) associated to a blood vessel in a 42 year-old gorilla (G), and blood vessel with Aβ accumulations from panel B (arrow) in a 33 year-old female gorilla (H). I and J. High-magnification photomicrographs showing a diffuse APP/Aβ-ir plaques in the frontal cortex of a >40 (I) and a 42 (J) year-old female gorilla. K. Photomicrograph illustrating numerous APP/Aβ-positive plaques in the posterior orbital cortex (area 44) in a >35 year-old female gorilla. L. High-magnification photomicrograph of the boxed area in K showing APP/Aβ-positive plaques and an adjacent blood vessel (arrows). M and N. APP/Aβ-positive parenchymal blood vessels in a >20 and >31 year-old male gorillas, respectively. Note the Aβ accumulations in the blood vessel in >31 year-old male gorilla. O. APP/Aβ-positive plaques in a 35 year-old male gorilla. Abbreviations: I, II, III, IV, V and VI, cortical layers. Scale bars = 200 μm in K; 100 μm in B-E and O; 75 μm in L; 50 μm in A, G, and M; 25 μm in F, H-J, and N.
Scattered APP/Aβ-ir blood vessels and plaques were observed in anterior cingulate and orbitofrontal cortices in a 33 year-old female mountain gorilla (Fig. 1B). By contrast in a 38 year-old female we observed extensive APP/Aβ positive vasculature in layers I to IV (Fig. 1D–E), with very limited plaque pathology. In older female gorillas (~40 and 42 years old) many more APP/Aβ-positive plaques were seen throughout all cortical layers in the frontal cortical areas (Fig. 1C, G), including layers V and VI (Fig. 1C, G). For example, the oldest 42 year-old female gorilla the cingulate cortex showed a 2-fold increase in APP/Aβ-ir plaque density per 1 mm2 cortical area compared to a young 33 year-old female. The area size of cortical APP/Aβ-ir profiles range from 130 to 2,700μm2. Notably, a female, of an estimated age of at least 35 years, displayed a wide distribution of APP/Aβ-ir plaques and blood vessels within the posterior orbitofrontal cortex and anterior insula, which was comparable to the 42 year-old female gorilla (Fig. 1K, L). All three male mountain gorillas (>20 years, >31 years, and 35 years), displayed APP/Aβ-ir accumulations in blood vessels and plaques in the cortex (Fig. 1M–O). However, the two younger male subjects showed only sparse APP/Aβ-positive blood vessels and few plaques. By contrast the oldest male gorilla, showed a more widespread APP/Aβ-ir plaque and vascular pathology throughout the cortical areas (Fig. 1O).
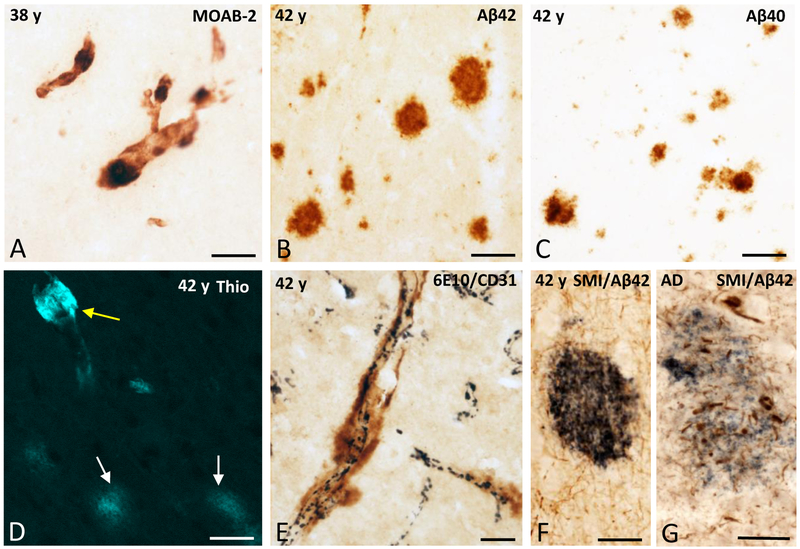
To examine Aβ species and the fibrillar nature of the amyloid deposits further, neocortical sections adjacent to those containing extensive APP/Aβ-positive plaques and vasculature were immunolabeled with antibodies that detected Aβ (MOAB-2) and the Aβ40 and Aβ42 species, as well as with thioflavine S. The morphology and topographic distribution of MOAB-2-labeled plaques and vessels in the 38 and 42 years old female gorillas were comparable to those stained by 6E10 (APP/Aβ), indicating that the main amyloidogenic component in gorilla plaques is Aβ (Fig. 2A). Plaques and blood vessels were positive for Aβ40 and Aβ42 at the ages examined (Fig. 2B, C). Blood vessels showed stronger thioflavine S fluorescence compared to plaques, which showed weak staining (Fig. 2D). Cortical sections double-labeled for APP/Aβ and the endothelial marker CD31 revealed APP/Aβ-ir plaque deposits in close apposition to CD31-positive blood vessels (Fig. 2E). Double immunostaining for phosphorylated neurofilaments using SMI-34 and Aβ42 did not reveal dystrophic neurites associated with Aβ42-ir plaques (Fig. 2F) in the gorilla cortex compared to the numerous SMI-34-ir neurites seen within the Aβ42-ir plaques in the AD cortex (Fig. 2G).
Figure 2.
A. Photomicrograph showing blood vessels stained with the pan-Aβ antibody MOAB-2 in the frontal cortex of a 38 year-old female gorilla. B and C. Aβ42 and Aβ40 plaques in the cingulate cortex (area 24) in a 42 year-old female gorilla, respectively. D. Thioflavine S (Thio) positive blood vessel in the area 24 of a 42 year-old female gorilla. Note the stronger thioflavine S fluorescence in the blood vessel (yellow arrow) compared to the plaques (white arrows). E. Dual immunolabeling showing APP/Aβ-positive accumulations (brown) in close apposition to the CD31-ir blood vessels (dark blue) in the frontal cortex of a 42 year-old female gorilla. F and G. Dual immunolabeling showing the absence of dystrophic SMI-34-ir neurites (brown) within the surrounding of a Aβ−42 positive plaque (dark blue) in the frontal cortex of a 42 year-old female gorilla (F), while numerous swollen and dystrophic SMI-34-ir neurites (brown) are seen within the Aβ42 positive plaque in the cortex of an AD case (G). Scale bars = 100 μm in A; 50 μm in B and C; 25 μm in D, E, F, G.
Tau-like pathology in the frontal cortex
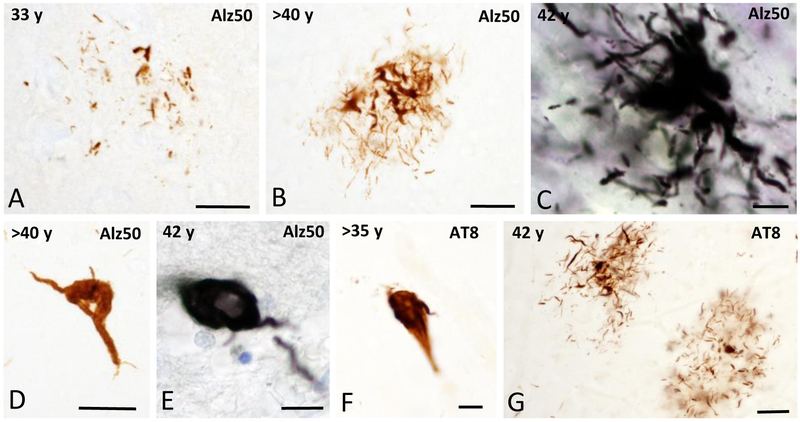
To examine the presence of alterations in tau protein, frontal cortex sections were stained with antibodies against Alz50 a tau conformational epitope, and AT8 a phosphorylation tau marker. In most of the wild mountain gorillas examined, with the exception of two males (>20 and 35 years old only a few scattered tau immunoreactive profiles (neuritic threads and glial cells) were seen in the frontal cortex (Table 1). Clusters of Alz50-ir neuritic threads were found in all female gorillas (Fig. 3A–C), while an occasional Alz50-ir profile resembling a glial cell was observed in the oldest gorilla (Fig. 3D, E). Similarly, AT8 positive profiles were found in the frontal cortical areas examined (Fig. 3F, G). Tau positive cortical profiles did not display an apparent laminar or regional distribution pattern. Interestingly, no flame-shaped Alz50 or AT8-ir neurons were found in the gorilla frontal cortex as are seen in AD.
Figure 3.
A-C. Photomicrographs showing Alz50-ir clusters of neurites in the frontal cortex in female gorillas across ages. D and E. Cortical Alz50-ir profiles resembling glial cells in a >40 and 42 year-old female gorillas, respectively. F and G. Cortical AT8-ir gial cell profile and clusters of neurites in a >35 and 42 year-old female gorillas, respectively. Tissues in C and E were counterstained with cresyl violet. Scale bars = 25 μm in A, B and G; 10 μm in C, E, and F.
DISCUSSION
Although amyloid plaque, vascular, and tau pathology similar to those typically found in the AD brain have been described in the neocortex and hippocampus of captive western lowland gorillas (Kimura et al., 2001; Perez et al., 2013), virtually nothing is known about these changes in wild-living gorillas. Results from captive western lowland gorillas and wild mountain gorillas might be expected to vary due to species-specific differences in genetics and biology, as well as differences in environmental stressors, activity level, and diet. In particular, the wild mountain gorillas would have had ample opportunities for aerobic exercise and a low-fat diet of mostly leaves, bark, and pith (Doran and McNeilage, 2001; Robbins, 2007; Watts, 1996). These factors could interact with AD-like pathology progression. Potential stressors in the wild that would not be experienced in the stable environment of a zoo enclosure, however, include the risk of social upheaval and infanticide when a resident male is overtaken. We had the unique opportunity to examine AD-like pathology in the frontal cortex of wild mountain gorillas during aging, but not in the hippocampus due to limited tissue availability in the individuals examined. Similar to our previous findings in captive western lowland gorillas (Perez et al., 2013), APP/Aβ-positive blood vessels and plaques first appear in the cortex of wild mountain gorillas between 25 to 30 years of age, but not in younger subjects (16 and 24 years old). At younger ages, cortical vascular Aβ deposits were more common than plaques and occurred in meningeal and arterial vasculature, as well as in the form of perivascular accumulations, as we previously described in captive western lowland gorillas (Perez et al., 2013). Conversely, the oldest 42 year-old female mountain gorilla, displayed more widespread frontal cortical plaque pathology compared to younger female gorillas. Similar to western lowland gorillas (Perez et al., 2013), vascular and plaque deposits were positive for Aβ40 and Aβ42 in the wild mountain gorilla. However, in the present study due to the limited amount of sections and other technical limitations we could not assess which of the two Aβ species was more abundant in the wild mountain gorilla or whether both coexisted in the same Aβ profiles. However, based on our previous findings in western lowland gorilla (Perez et al., 2013) and in other great apes (Gearing et al., 1996, 1997), where short and less fibrillogenic Aβ40 was more abundant, we speculate that Aβ40 would be the major form in the wild mountain gorilla cortex. Thioflavine S histochemisty revealed strong vascular Aβ staining compared to weaker plaque fluorescence, supporting the fibrillar nature of the Aβ in the blood vessels, but to a lesser extent in plaques. In addition, we did not observe cortical SMI-34 positive dystrophic neurites surrounding Aβ plaques in the wild mountain gorilla. These results are similar to our previous report in the captive western lowland gorilla (Perez et al., 2013), and other great apes (Gearing et al., 1997, 1996) lending support the concept that aged gorillas displayed mainly diffuse plaques but not neuritic plaques (Kimura et al., 2001; Perez et al., 2013). Interestingly, neuritic plaques, rather than diffuse plaques, correlate with cognitive decline in normal aging and AD (Arriaga et al., 1992; Giannakopoulos et al., 2003; Nagy et al., 1995). Whether plaque pathology is associated with cognitive decline in gorillas living in the wild or in captivity remains unknown. Our data indicate that Aβ deposits in the microvasculature and diffuse plaques are common features in the cerebral cortex, occur at the same chronological ages in both the natural habitat and captivity independent of environmental conditions.
Compared to the extent of Aβ-positive structures, there were very limited tau-ir profiles, in the neocortex in the wild mountain gorilla. Compared to the western lowland gorilla, in which older individuals (e.g. 49, 50 and 55 years old) were examined and displayed more extensive tau pathology (Perez et al., 2013), Alz50 and AT8-ir lesions were rarely found in the frontal cortex of the wild mountain gorilla. Very few cortical clusters of Alz50 and AT8-ir neuritic threads were present in the frontal cortex, and only scattered Alz50 and AT8-ir cells resembling glial cells were observed in the oldest wild mountain gorilla, while virtually no Alz50 or AT8-ir neurons and fibers were found across the ages examined. These discrepancies in cortical Alz50-positive profiles may due to species differences, or perhaps age, methodological factors, as well as environmental conditions related to captivity in the western lowland gorilla. In this context, it is possible that the shorter lifespan of mountain gorilla (43 years) in the wild (Bronikowski et al., 2011) underlies the differences in the extent of tau pathology described in the captive western lowland gorilla or in chimpanzees (Edler et al., 2015), which have longer lifespans in captivity (>50 years). In addition, the antigenicity of tau epitopes is dependent upon fixation and postmortem interval (Conti et al., 1988; Fox et al., 1985; Garver et al., 1994; Oh et al., 2010; Riederer, 1989; Riederer et al., 1993). Because the wild mountain gorilla brains were obtained from animals found dead in the wild with recovery intervals up to 48 hours and long fixation periods (up to 100 weeks), it is likely that these factors also affected the immunohistochemical detection of tau. In this regard, subject Se, with a relatively small brain weight (343 g) and subject Ti, with a prolonged fixation time (103 weeks), did not display cortical Alz50-ir profiles suggesting that both postmortem interval and the length of fixation play a role in the lower prevalence of tau-positive profiles in the wild mountain compared to western lowland gorillas who lived in zoos and whose brain could therefore be recovered and processed faster. Nonetheless, we found that the oldest wild mountain gorillas displayed more tau-positive lesions compared to the youngest, suggesting that tau pathology in gorillas (Perez et al., 2013), as in other great apes (Elder et al., 2015) is an age-related event in the wild and in captivity. Since we do not have behavioral data for the wild mountain gorilla, we are unable to determine whether the presence of tau in the frontal cortex is a normal aging or pathological response that affects function.
Our findings revealed that cortical Aβ and tau pathology occur in aged gorillas independent of environmental conditions. However, epidemiological and preclinical studies have demonstrated that environmental conditions influence risk of developing clinical (Snowdon, 2003; Stern et al., 1994) and pathological AD (Hu et al., 2010; Lazarov et al., 2005; Nichol et al., 2007). In fact, diet, infections, social environment and other husbandry factors influence ageing and associated pathologies in great apes and other non-human primates housed in zoos or in biomedical facilities (Finch and Austad, 2012). Specifically, a study in aged rhesus monkeys (Macaca mulatta) showed that individuals housed in small cages at early ages displayed significantly more plaque pathology than those housed their entire life in standard cages (Merrill et al., 2011). A 41 year-old female chimpanzee who suffered a stroke and had distribution of NFT-like pathology, which differed somewhat from that seen in human AD, was remarkably obese and had high cholesterol levels, both AD risk factors (Rosen et al., 2008). These observations suggest that husbandry might lower the threshold for Aβ pathology and tauopathy in captive nonhuman primates.
Our data reveal the presence of Aβ-positive microvasculature and diffuse plaques together with a very limited tau pathology in the frontal cortex in aged wild mountain gorillas, supporting previous findings from another gorilla species (Perez et al., 2013). However, a detailed neuropathological evaluation of the hippocampus, which is affected early in AD, is needed in both aged wild mountain and western lowland gorillas. Despite the close phylogenetic affinity between humans and gorillas, neither neuritic plaques nor frank NFTs, hallmarks of AD, were observed in the brain of wild or captive gorillas suggesting that NFT pathology differentiates these species during the aging process.
ACKNOWLEDGEMENTS
This work was supported in part by the Great Ape Aging Project and NIH grant AG014308 (to J.M.E.). We thank Cheryl D. Stimpson for expert technical assistance and Dr. Jordi Galbany for dental examination of the gorillas.
Footnotes
DISCLOSURE STATEMENT
The authors declare that they have no conflict of interest.
LITERATURE CITED
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 1992; 42:631–39. [DOI] [PubMed] [Google Scholar]
- Bouras C, Hof PR, Giannakopoulos P, Michel JP, Morrison JH. Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of elderly patients: a quantitative evaluation of a one-year autopsy population from a geriatric hospital. Cereb Cortex 1994; 4:138–50. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991; 82:239–59. [DOI] [PubMed] [Google Scholar]
- Brodmann K Vergleichende Lokalisationslehre der Grosshirnrinde. Leipzig: Johann Ambrosius Barth Verlag; 1909. [Google Scholar]
- Bronikowski AM, Altmann J, Brockman DK, Cords M, Fedigan LM, Pusey A, Stoinski T, Morris WF, Strier KB, Alberts SC. Aging in the natural world: comparative data reveal similar mortality patterns across primates. Science 2011; 331:1325–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti CJ, Larcher F, Chesner J, Aldaz CM. Polyacrylamide gel electrophoresis and immunoblotting of proteins extracted from paraffin-embedded tissue sections. J Histochem Cytochem 1988; 36:547–50. [DOI] [PubMed] [Google Scholar]
- Doran DM, McNeilage A, Greer D, Bocian C, Mehlman P, Shah N. Western lowland gorilla diet and resource availability: new evidence, cross-site comparisons, and reflections on indirect sampling methods. Am J Primatol 2002; 58:91–116. [DOI] [PubMed] [Google Scholar]
- Doran DM, McNeilage A. Subspecific variation in gorilla behavior: the influence of ecological and social factors In: Robbins MM, Sicotte P, Stewart KJ, editors. Mountain Gorillas: Three decades of research at Karisoke. Cambridge: Cambridge University Press; 2001. p. 123–149. [Google Scholar]
- Edler MK, Hof PR, Mufson EJ, Perez SE, Hopkins WD, Ely JJ, Erwin JM, Sherwood CC, Raghanti MA. Alzheimer’s disease pathology in aged chimpanzees Society for Neuroscience Abstract, Chicago, 2015. [Google Scholar]
- Finch CE, Austad SN. Primate aging in the mammalian scheme: the puzzle of extreme variation in brain aging. Age 2012; 34:1075–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CH, Johnson FB, Whiting J, Roller PP. Formaldehyde fixation. J Histochem Cytochem 1985; 33:845–53. [DOI] [PubMed] [Google Scholar]
- Garver TD, Harris KA, Lehman RA, Lee VM, Trojanowski JQ, Billingsley ML. Tau phosphorylation in human, primate, and rat brain: evidence that a pool of tau is highly phosphorylated in vivo and is rapidly dephosphorylated in vitro. J Neurochem 1994; 63:2279–87. [DOI] [PubMed] [Google Scholar]
- Gearing M, Rebeck GW, Hyman BT, Tigges J, Mirra SS. Neuropathology and apolipoprotein E profile of aged chimpanzees: implications for Alzheimer disease. Proc Natl Acad Sci USA 1994; 91:9382–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing M, Tigges J, Mori H, Mirra SS. Aβ40 is a major form of beta-amyloid in nonhuman primates. Neurobiol Aging 1996; 17:903–8. [DOI] [PubMed] [Google Scholar]
- Gearing M, Tigges J, Mori H, Mirra SS. β-Amyloid (Aβ) deposition in the brains of aged orangutans. Neurobiol Aging 1997; 18:139–46. [DOI] [PubMed] [Google Scholar]
- Giannakopoulos P, Herrmann FR, Bussière T, Bouras C, Kövari E, Perl DP, Morrison JH, Gold G, Hof PR. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology 2003; 60:1495–1500. [DOI] [PubMed] [Google Scholar]
- Hu YS, Xu P, Pigino G, Brady ST, Larson J, Lazarov O. Complex environment experience rescues impaired neurogenesis, enhances synaptic plasticity, and attenuates neuropathology in familial Alzheimer’s disease-linked APPswe/PS1ΔE9 mice. FASEB J 2010; 24:1667–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M, Lowe J. The new neuropathology of degenerative frontotemporal dementias. Acta Neuropathol 1996; 91:127–34. [DOI] [PubMed] [Google Scholar]
- Kimura N, Nakamura S, Goto N, Narushima E, Hara I, Shichiri S, Saitou K, Nose M, Hayashi T, Kawamura S, Yoshikawa Y. Senile plaques in an aged western lowland gorilla. Exp Anim 2001; 50:77–81. [DOI] [PubMed] [Google Scholar]
- Kimura N, Tanemura K, Nakamura S, Takashima A, Ono F, Sakakibara I, Ishii Y, Kyuwa S, Yoshikawa Y. Age-related changes of Alzheimer’s disease-associated proteins in cynomolgus monkey brains. Biochem Biophys Res Commun 2003; 310:303–11. [DOI] [PubMed] [Google Scholar]
- Lazarov O, Robinson J, Tang YP, Hairston IS, Korade-Mirnics Z, Lee VM, Hersh LB, Sapolsky RM, Mirnics K, Sisodia SS. Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell 2005; 120:701–13. [DOI] [PubMed] [Google Scholar]
- Lemere CA, Beierschmitt A, Iglesias M, Spooner ET, Bloom JK, Leverone JF, Zheng JB, Seabrook TJ, Louard D, Li D, Selkoe DJ, Palmour RM, Ervin FR. Alzheimer’s disease abeta vaccine reduces central nervous system Aβ levels in a non-human primate, the Caribbean vervet. Am J Pathol 2004; 165:283–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemere CA, Oh J, Stantish HA, Peng Y, Pepivani I, Fagan AM, Yamaguchi H, Westmoreland SV, Mansfield KG. Cerebral amyloid-beta protein accumulation with aging in cotton-top tamarins: A model of early Alzheimer’s disease? Rejuven Res 2008; 11:321–32. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Sisodia SS, Koo EH, Cork LC, Dellovade TL, Weidemann A, Beyreuther K, Masters C, Price DL. Amyloid precursor protein in aged nonhuman primates. Proc Natl Acad Sci USA 1991; 88:1461–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlin SC, Barks SK, Tocheri MW, Massey JS, Eriksen AB, Fawcett KA, Stoinski TS, Hof PR, Bromage TG, Mudakikwa A, Cranfield MR, Sherwood CC. Early brain growth cessation in wild Virunga mountain gorillas (Gorilla beringei beringei). Am J Primatol 2013; 75:450–63. [DOI] [PubMed] [Google Scholar]
- Merrill DA, Masliah E, Roberts JA, McKay H, Kordower JH, Mufson EJ, Tuszynski MH. Association of early experience with neurodegeneration in aged primates. Neurobiol Aging 2011; 32:151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Benzing WC, Cole GM, Wang H, Emerich DF, Sladek JR Jr, Morrison JH, Kordower JH. Apolipoprotein E-immunoreactivity in aged rhesus monkey cortex: colocalization with amyloid plaques. Neurobiol Aging 1994; 15:621–27. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Esiri MM, Jobst KA, Morris JH, King EM, McDonald B, Litchfield S, Smith A, Barnetson L, Smith AD. Relative roles of plaques and tangles in the dementia of Alzheimer’s disease: correlations using three sets of neuropathological criteria. Dementia 1995; 6:21–31. [DOI] [PubMed] [Google Scholar]
- Nichol KE, Parachikova AI, Cotman CW. Three weeks of running wheel exposure improves cognitive performance in the aged Tg2576 mouse. Behav Brain Res 2007; 184:124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norvin D, Kim G, Baker-Nigh A, Geula C. Accumulation and age-related evalution of amyloid-β within basal forebrain cholinergic neurons in the rhesus monkey. Neuroscience 2015; 298:102–11. [DOI] [PubMed] [Google Scholar]
- Oh KJ, Perez SE, Lagalwar S, Vana L, Binder L, Mufson EJ. Staging of Alzheimer’s pathology in triple transgenic mice: a light and electron microscopic analysis. Int J Alzheimer’s Dis 2010; July 15, doi: 10.4061/2010/780102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez SE, Getova DP, He B, Counts SE, Geula C, Desire L, Coutadeur S, Peillon H, Ginsberg SD, Mufson EJ. Rac1b increases with progressive tau pathology within cholinergic nucleus basalis neurons in Alzheimer’s disease. Am J Pathol 2012; 180:526–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez SE, He B, Muhammad N, Oh KJ, Fahnestock M, Ikonomovic MD, Mufson EJ. Cholinotrophic basal forebrain system alterations in 3xTg-AD transgenic mice. Neurobiol Dis 2011; 41:338–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez SE, Raghanti MA, Hof PR, Kramer L, Ikonomovic MD, Lacor PN, Erwin JM, Sherwood CC, Mufson EJ. Alzheimer’s disease pathology in the neocortex and hippocampus of the western lowland gorilla (Gorilla gorilla gorilla). J Comp Neurol 2013; 521:4318–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poduri A, Gearing M, Rebeck GW, Mirra SS, Tigges J, Hyman BT. Apolipoprotein E4 and beta amyloid in senile plaques and cerebral blood vessels of aged rhesus monkeys. Am J Pathol 1994; 144:1183–87. [PMC free article] [PubMed] [Google Scholar]
- Riederer BM, Porchet R, Marugg RA, Binder LI. Solubility of cytoskeletal proteins in immunohistochemistry and the influence of fixation. J. Histochem Cytochem 1993; 41:609–16. [DOI] [PubMed] [Google Scholar]
- Riederer BM. Antigen preservation tests for immunocytochemical detection of cytoskeletal proteins: influence of aldehyde fixatives. J Histochem Cytochem 1989; 37: 675–81. [DOI] [PubMed] [Google Scholar]
- Robbins MM. Gorillas: diversity in ecology and behavior In: Campbell CJ, Fuentes A, MacKinnon KC, Panger M, Bearder SK, editors. Primates in perspective. New York: Oxford University Press; 2007. p. 304–321. [Google Scholar]
- Rosen RF, Farberg AS, Gearing M, Dooyema J, Long PM, Anderson DC, Davis-Turak J, Coppola G, Geschwind DH, Pare JF, Duong TQ, Hopkins WD, Preuss TM, Walker LC. Tauopathy with paired helical filaments in an aged chimpanzee. J Comp Neurol 2008; 509:259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scally A, Dutheil JY, Hillier LW, Jordan GE, Goodhead I, Herrero J, Hobolth A, Lappalainen T, Mailund T, Marques-Bonet T, McCarthy S, Montgomery SH, Schwalie PC, Tang YA, Ward MC, Xue Y, Yngvadottir B, Alkan C, Andersen LN, Ayub Q, Ball EV, Beal K, Bradley BJ, Chen Y, Clee CM, Fitzgerald S, Graves TA, Gu Y, Heath P, Heger A, Karakoc E, Kolb-Kokocinski A, Laird GK, Lunter G, Meader S, Mort M, Mullikin JC, Munch K, O’Connor TD, Phillips AD, Prado-Martinez J, Rogers AS, Sajjadian S, Schmidt D, Shaw K, Simpson JT, Stenson PD, Turner DJ, Vigilant L, Vilella AJ, Whitener W, Zhu B, Cooper DN, de Jong P, Dermitzakis ET, Eichler EE, Flicek P, Goldman N, Mundy NI, Ning Z, Odom DT, Ponting CP, Quail MA, Ryder OA, Searle SM, Warren WC, Wilson RK, Schierup MH, Rogers J, Tyler-Smith C, Durbin R. Insights into hominid evolution from the gorilla genome sequence. Nature 2012; 483:169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simić G, Hof PR. In search of the definitive Brodmann’s map of cortical areas in human. J Comp Neurol 2015; 523:5–14. [DOI] [PubMed] [Google Scholar]
- Snowdon DA. Healthy aging and dementia: findings from the Nun Study. Ann Intern Med 2003; 139:450–54. [DOI] [PubMed] [Google Scholar]
- Stern RG, Mohs RC, Davidson M, Schmeidler J, Silverman J, Kramer-Ginsberg E, Searcey T, Bierer L, Davis KL. A longitudinal study of Alzheimer’s disease: measurement, rate, and predictors of cognitive deterioration. Am J Psychiatry 1994; 151:390–96. [DOI] [PubMed] [Google Scholar]
- Walker LC, Kitt CA, Schwam E, Buckwald B, Garcia F, Sepinwall J, Price DL. Senile plaques in aged squirrel monkeys. Neurobiol Aging 1987; 8:291–6. [DOI] [PubMed] [Google Scholar]
- Watts DP. Comparative socio-ecology of gorillas In: McGrew WC, Marchant LF, Nishida T, editors. Great ape societies. New York: Cambridge University Press; 1996. p. 16–28. [Google Scholar]