Abstract
Herbarium voucher specimens are used primarily for taxonomic confirmation. However, they also afford a record of the metabolic profile of a plant, potentially at the time it was collected, or at the very least, at the time of analysis. Even with the enhanced sensitivity of modern analytical techniques, analysis of the metabolites of a herbarium voucher requires removal and consumption of at least part of an entire specimen. We present herein a non-destructive method to analyze the metabolites of herbarium voucher specimens with the droplet-liquid microjunction-surface sampling probe (droplet probe) coupled to ultra-performance liquid chromatography and highresolution mass spectrometry. As proof of concept, a herbarium voucher specimen of Garcinia mangostana (mangosteen) was utilized due to the well-characterized xanthones biosynthesized by this plant, which are of interest as potential anticancer agents. Also, the juice of the fruits of this plant is used widely in the United States and in other countries as a botanical dietary supplement. Metabolite profiles of the sampled surfaces were compared to a subset of xanthone standards. Using this innovative method on the herbarium voucher specimen, we were able to readily identify cytotoxic prenylated xanthones while maintaining the integrity of the entire specimen.
Graphical Abstract
1. Introduction
Herbarium voucher specimens have been integral to science as far back as 1556, when the Italian botanist, Luca Ghini, first produced them from dried plants, employing sheets of heavy paper bound together and stored vertically, as formal and permanent documentation (Stearn, 1971). Subsequently, Carl Linnaeus in 1735 laid the foundation for taxonomic identification of plants via herbarium vouchers (Müller-Wille, 2006). In addition to being a permanent record of a plant, these specimens present a unique opportunity to study plants and other preserved species over time. They are a snapshot that researchers can examine, providing opportunities to look at the phenotype, genotype, and chemotype during a specific window of time (Culley, 2013; Willis et al., 2017), albeit with the recognition that some degradation or other changes may occur to the voucher during storage. Herbarium specimens provide a glimpse into the past that researchers of the present (and perhaps future) are capable of studying with modern and next-generation techniques, documenting and investigating changes over time (Drabkova, 2014; Jiang et al., 2005). However, to do this, some techniques require the destruction of a portion of the sample for DNA and/or metabolite analysis (Willis et al., 2017). While valuable, the sampling process is permanent, visible, and invasive, forever altering the integrity and scientific value of the voucher.
Over the past three years, our team has been utilizing the droplet-liquid microjunctionsurface sampling probe (droplet probe) as a tool to examine the chemistry of fungal cultures in situ. Essentially, this sampling system dispenses a droplet of approximately 4 μl on the surface of a sample, enabling a microextraction, which can then be analyzed by UPLC-HRMS (Paguigan et al., 2016; Sica et al., 2015; Sica et al., 2016a; Sica et al., 2017). In addition to a suite of applications on fungal cultures, we have also shown its potential in studying various plant parts in situ, including fruits, seeds, stems, and even rarely studied flower petals (Sica et al., 2016b). In the current study, we turned our attention to the use of droplet probe for the examination of herbarium vouchers. In principle, they should behave in a manner similar to plant tissues, which have been sampled with this technique previously (Sica et al., 2016b). However, due to the delicate, and at times precious, nature of these specimens, we wanted to ensure that the specimen’s appearance would not be altered by the analysis, as the physical characteristics of these specimens are critical for taxonomic studies.
Garcinia mangostana L. (Clusiaceae), commonly known as mangosteen, was chosen because this plant is known for its numerous biological applications, including as an antimicrobial, anti-inflammatory, and antioxidant, among many other purposes (Benatrehina et al., 2018; Chen et al., 2008; Chin and Kinghorn, 2008; Chin et al., 2008; Williams et al., 1995). Mangosteen has been used traditionally to treat disorders ranging from skin infection to dysentery to abdominal pains (Ibrahim et al., 2016). Xanthones are the major metabolites that have been isolated and investigated from this plant. Modern studies have shown antibacterial, antifungal, antihistamine, anti-HIV, antineoplastic, aromatase inhibitory, cancer chemopreventive, and cytotoxic activities from this class of compounds (Balunas et al., 2008; Chairungsrilerd et al., 1996; Chen et al., 1996; Chitchumroonchokchai et al., 2013; Chomnawang et al., 2007; Chomnawang et al., 2009; Gopalakrishnan et al., 1997; Han et al., 2009; Ibrahim et al., 2016; Jung et al., 2006; Matsumoto et al., 2003; Phongpaichit et al., 2006; Suksamrarn et al., 2003; Tousian Shandiz et al., 2017).
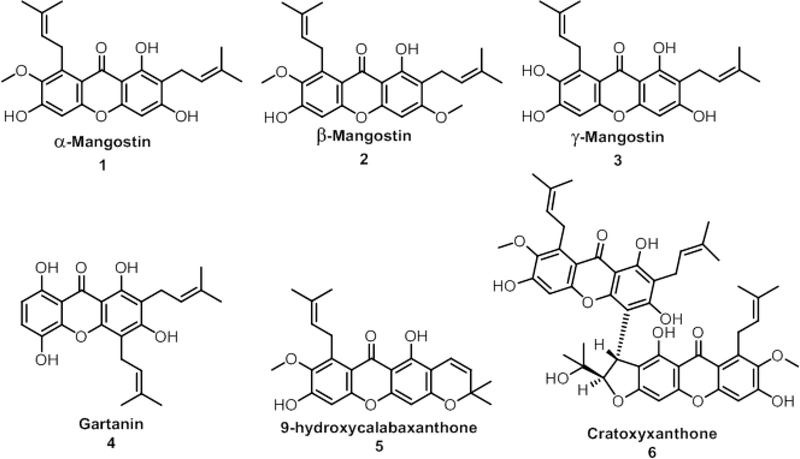
In this study, we investigated six xanthones of mangosteen: α-mangostin (1), β-mangostin (2), γ-mangostin (3), gartanin (4), 9-hydroxycalabaxanthone (5), and cratoxyxanthone (6) (Fig. 1) (Ji et al., 2007; Walker, 2007). Many of these compounds are found in one or more of the pericarp, stem, and whole fruit of G. mangostana (Obolskiy et al., 2009). Using a herbarium voucher specimen of G. mangostana, our goal was to extract chemical knowledge via the droplet probe technique without marring the physical appearance of the voucher.
Fig. 1.
Six prenylated xanthones previously isolated from Garcinia mangostana fruit or stem bark (Han et al., 2009; Jung et al., 2006). These were used as reference standards when analyzing the droplet probe data from the herbarium voucher.
2. Results and Discussion
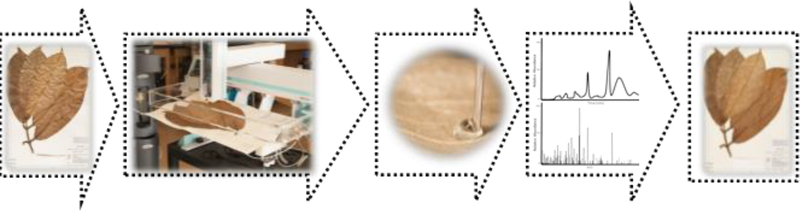
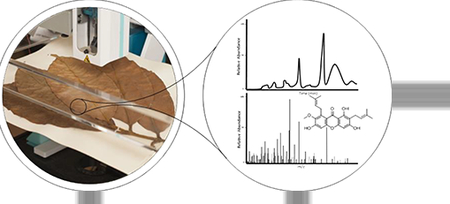
The main objective of this project was to identify prenylated xanthones from the herbarium voucher specimen of Garcinia mangostana, as compared to isolated reference standards, without damaging the voucher. The sampling of the herbarium voucher specimen was performed using the droplet probe technique, in which a droplet of approximately 4 μl of 50:50 DMSO:H2O was dispersed onto the surface of the sample. Metabolites from the herbarium voucher specimen were dissolved and analyzed using the droplet probe system (Fig. 2).
Fig. 2.
General procedure for detecting and analyzing prenylated xanthones from the Garcinia mangostana herbarium specimen.
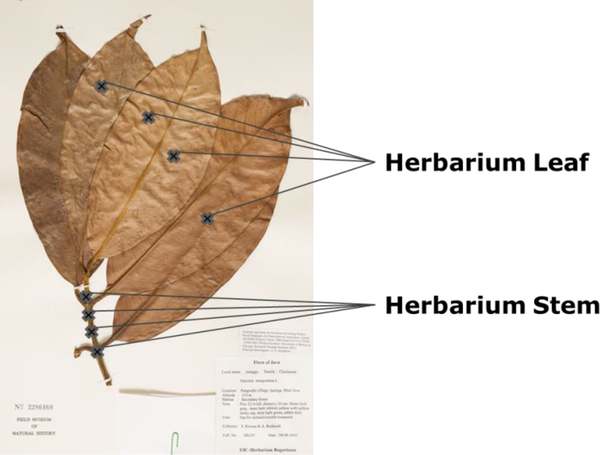
The herbarium voucher was sampled at four sites of both the leaf and the stem (Fig. 3). Essentially, the droplet probe performs a microextraction from the surface of the voucher, and the concentrated droplet is then analyzed via UPLC-HRMS. This permitted a comparison of chromatographic and spectrometric data to that of the reference standards (Figs. 1 and S1), while keeping the whole sample intact and undamaged in appearance for taxonomic posterity.
Fig. 3.
Garcinia mangostana leaf herbarium specimen (No. 2286460) from The Field Museum of Natural History in Chicago, IL. Sites that were sampled by the droplet probe are marked with crosses on the leaf and stem of the voucher. A droplet containing approximately 4 μl of 50:50 DMSO:H2O with an internal standard was dispensed onto the surface at each of these sights before UPLC-HRMS analysis.
A herbarium specimen is important for future taxonomic studies. To demonstrate this innocuous property of this technique, photographic close ups of the voucher, both before and after droplet probe analysis are included (Fig. 4). Chemical sampling of the G. mangostana voucher via droplet probe was non-destructive, and at least at the optical level, deterioration of the specimen was not observed. This was important since a non-destructive chemical analysis of a voucher has not yet been reported, although recently there has been a non-destructive analysis of DNA from herbarium specimens (Shepherd, 2017).
Fig. 4.
Garcinia mangostana herbarium voucher specimen before and after sampling by droplet probe. The photos were taken within three hours of each other and show the same area with a zoom in before and after sampling.
Previous methods from our laboratory have utilized water and methanol as the solvent combination for droplet microextractions (Paguigan et al., 2016; Sica et al., 2015; Sica et al., 2016a; Sica et al., 2016b), but the xanthones were found to be semi-soluble in methanol and not at all soluble in water. The droplet probe technique relies on droplets that contain water, which maintains surface tension when making contact with the sample. Thus, DMSO was included in the microextraction solvent because of its improved solubility with the xanthones.
In preliminary studies, a total of five microextractions per droplet were used to sample the herbarium voucher specimen; however, the xanthones were not well detected. In order to concentrate the microextraction further, the number of microextractions was increased by five until α-mangostin (1), β-mangostin (2), and γ-mangostin (3) could be detected consistently on the herbarium voucher specimen. In total, 15 microextractions per droplet were performed, which was three times more than required previously with fungal cultures (Sica et al., 2015).
The ability to retain a droplet on the tip of the syringe and concentrate the microextractions via repeated sampling is a benefit of the droplet probe system. However, in doing so, some amount of the solvent may be retained on the sample, and this could result in inconsistencies and variability in sampling. Therefore, we sought an internal standard that could be used to normalize the data, and to solve this challenge Alizarin Red S (0.0001 mg/mL) was included in the sampling solvent.
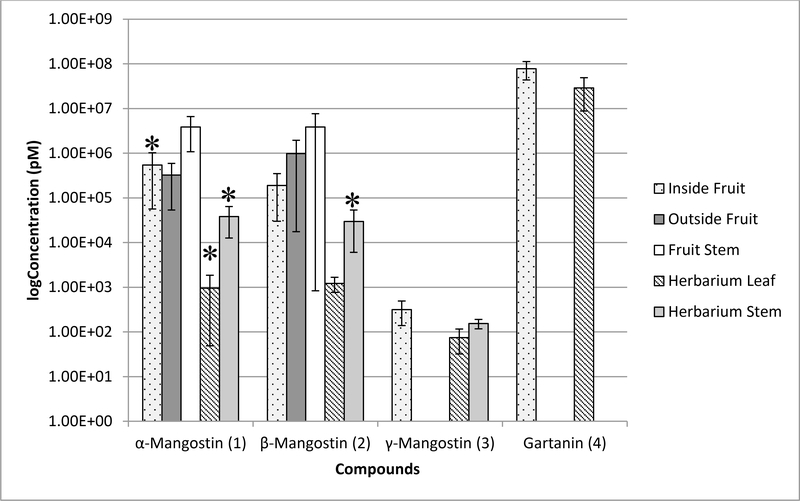
The six xanthones were analyzed from each site on the herbarium voucher specimen (Fig. 4), and the average of the leaf and stem sites respectively were averaged together (Fig. 5). Of the prenylated xanthones, α-mangostin (1) and β-mangostin (2) were detected in the highest concentrations of all the compounds across all sites, whereas 9-hydroxycalabaxanthone (5) and cratoxyxanthone (6) were not detectable at all. The herbarium voucher leaves were observed to have less of each of the compounds, if present at all, compared to the sites on the herbarium voucher stem. It is unknown whether these observations were due to the amount of those compounds in the leaves at the time of collection, or if they degraded upon processing and storage as a voucher; there is literature precedent for the latter with benzofuran ketones (Lee et al., 2015; Lee et al., 2017). Irrespective, it is a fact that at the time of analysis, the herbarium voucher leaves had a smaller amount of those compounds.
Fig. 5.
Concentration of compounds 1–4 from Garcinia mangostana detected from a dried fruit hull compared to the herbarium specimen. The UPLC-HRMS data collected from four sites were sampled, normalized, and then averaged at each location (n=4). However, due to some variability in the measurements, the Grubbs Test was used to eliminate outliers, and in those cases, n=3. The bars with an asterisk are where n=4. Two of the compounds, 9hydroxycalabaxanthone (5) and cratoxyxanthone (6), were tested but not detected at all sites.
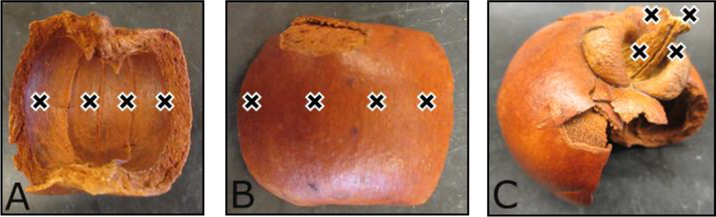
Furthermore, there was interest in comparing the xanthones of the herbarium voucher specimen to a dried fruit sample (Fig. 6) of the same species. Overall, the sampling of the stem was observed to contain higher concentrations of xanthones on both the dried fruit as well as the herbarium voucher specimen. However, gartanin (4) was not detected on the herbarium voucher specimen, but the metabolite was detected in the dried fruit sample, notably on the endocarp (Inside Fruit) and the pedicel (Fruit Stem) (Fig. 5). As with the herbarium voucher specimen, there was no detection of 9-hydroxycalabaxanthone (5) and cratoxyxanthone (6) on any of the dried fruit sites, despite the fact that the dried fruit sites showed an overall higher concentration of the xanthones compared to the herbarium voucher specimen. In a previous study from the stem bark of G mangostana, compounds 1 and 2 had been isolated in significantly greater quantities compared to compounds 4-6 (Han et al., 2009). This may explain the lower concentration or lack of detection in some cases for compounds 4-6.
Fig. 6.
Garcinia mangostana dried fruit specimen. The sampling locations are denoted with an X, and 15 microextractions per droplet were performed on the A) Inside Fruit, B) Outside Fruit, and C) Fruit Stem.
The data in Fig. 5 show that there was some variability in the measurements, especially for compounds of lower concentration, and there could be several reasons for this. The xanthones detected on the endocarp may have varied because that surface was more absorbent, leading to fluctuating droplet recovery of as low as 1% to as high as ~50% over the course of 15 microextractions per site. In contrast, the pericarp, leaf, and stems all provided hard and/or relatively flat surfaces that permitted facile and reproducible microextraction sampling. In addition, there may be inconsistency between the sites sampled with the droplet probe, simply due to inherent variability of secondary metabolite distribution in the plant (Sukatta et al., 2013). Since it was observed that the stems have a higher concentration of xanthones, it is possible that examining a spot closer to the stem of a leaf or fruit may be more concentrated in secondary metabolites. Moreover, the effects of collection, preparation, and storage time of herbarium voucher specimens are not known, which can be limiting when performing in situ analyses.
With the droplet probe, metabolites can be detected from herbarium vouchers via surface sampling. In addition, with this method we can discern metabolite concentrations that may vary across the sample, which yields physiological information without requiring multiple parts of the plant for extraction purposes. By maintaining the integrity of a herbarium voucher specimen with this technique, there is now the opportunity to safely apply chemotype analysis to such samples and yet preserve their appearance. This type of chemical analysis, where the integrity of the source material is preserved, may become increasingly valuable. Whether these samples are on species that are derived from threatened, endangered, and even extinct plants, or perhaps materials that illustrate pieces of history, chemical analyses need to be both effective and conserve these materials for future generations.
3. Experimental Section
3.1. General experimental procedures
The droplet−liquid microjunction-surface sampling probe was optimized for natural products studies via an earlier collaboration with Oak Ridge National Laboratory (Sica et al., 2015). Briefly, a CTC/LEAP HTC PAL autosampler (LEAP Technologies Inc., Pflugerville, TX) was converted into an automated droplet system via dropletProbe Premium, which was developed by Oak Ridge National Laboratory (Kertesz and Van Berkel, 2013, 2014; Kertesz et al., 2014; Kertesz et al., 2015; Ovchinnikova et al., 2011). Extractions were performed using Fisher Optima LC/MS grade solvents consisting of 50:50 DMSO:H2O to enhance the solubility of the prenylated xanthones of interest. For the purpose of investigating the xanthones present, each droplet contained 0.0001 mg/mL of Alizarin Red S (Sigma-Aldrich) as an internal standard. Approximately 4 μl of solvent was drawn into the syringe for each microextraction and dispensed onto the surface of the herbarium specimen at a rate of 2 μl/s. The syringe with the dispensed droplet paused over the surface for two seconds before being drawn back into the syringe. This process was repeated a total of 15 times for a single spot prior to injection into the UPLC−HRMS system. We suggest that users of this methodology notate where samples were acquired on the herbarium specimen, so as to inform future scientists. The droplet probe was coupled with a Waters Acquity ultraperformance liquid chromatography (UPLC) system (Waters Corp.) to a Thermo QExactive Plus MS (ThermoFisher).
The QExactive Plus collected data from 200 to 2000 m/z at a mass resolution of 70,000. The voltage for the positive ionization mode was set to 4.0 kV with a nitrogen sheath gas set to 50 arb and an auxiliary gas set to 15 arb. The S-Lens RF level was set to 50.0 and capillary temperature at 300°C. The flow rate of the UPLC was set to 0.3 mL/min using a BEH C18 (2.1 × 50 mm, 1.7 μm particle size) column. The column was equilibrated at 40 °C, and the sample manager was not used. The mobile phase consisted of Fisher Optima UPLC−HRMS grade CH3CN:H2O with 0.1% HCOOH. The initial gradient began at 75% CH3CN and increased linearly to 100% CH3CN over 2 min. For 1.5 minutes, the column was held at 100% CH3CN to clean the column before being re-equilibrated to the initial starting conditions. The PDA was set to acquire from 200 to 500 nm with a resolution of 4 nm.
3.2. Plant material
The herbarium voucher (No. 2286460) of Garcina mangostana L. (Clusiaceae) was provided by The Field Museum (Chicago, IL). The G. mangostana dried fruit, which included the pericarp, calyx, and pedicel, was provided by one of the authors (ADK).
3.3. Droplet Probe Analysis
The xanthone standards were isolated and characterized as detailed previously (Han et al., 2009; Jung et al., 2006). Calibration curves were recorded using a minimum of 12 different concentrations for compounds 1–6 (Figs. S2-S7). The UPLC-HRMS data for each compound, ranging from 0.00125–512 μM, were measured in triplicate. Calculations of concentrations were based on peak area ratios, and calibration curves were generated by weighted (1/X) linear regression. All data were processed using the Quan Browser module of Xcalibur version 2.2 (Thermo Fisher Scientific). The chromatographic peak areas were integrated using the Xcalibur Processing Setup version 2.2 (Thermo Fisher Scientific). ICIS peak detection was applied for a list of putatively annotated ions. The Grubbs’ test was performed for an n=4 number of observations after the average and standard deviation of each compound was determined at each site. The measurements that were considered outliers at a 5% significance level were excluded from our assessments (Grubbs, 1969).
4. Conclusion
With this method, secondary metabolites from a herbarium voucher specimen of Garcinia mangostana were compared and identified with droplet probe coupled to UPLC-HRMS. Importantly, chemical information was gleaned from this technique without the physical alteration of even a portion of the delicate sample. While this study focused on herbarium vouchers, we postulate that it may be applicable to other materials, such as macrofungi, rare/endangered species, or even historical samples and artifacts. This approach may pave the way for the in situ chemical analysis of delicate and/or priceless pieces from across time.
Supplementary Material
HIGHLIGHTS.
Non-damaging method to detect secondary metabolites on herbarium voucher specimen.
Secondary metabolites analyzed in situ via coupling droplet probe to UPLC-HRMS.
Analysis of xanthones from voucher and dried fruits of Garcinia mangostana.
Technique valuable for in situ chemical analysis of precious or delicate materials.
Acknowledgements
Diana Kao was supported by the National Center for Complementary and Integrated Health, NIH via grant F31 AT009264. Support also came from the National Cancer Institute, NIH via grant P01 CA125066. To the Botany Collections Manager, John G. Searle Herbarium of the Field Museum, we express our thanks for the loan of the Garcinia mangostana specimen and for the permission to submit the specimen for the present study. The images of the plant specimen were graciously photographed by Daniel Smith of UNCG. We thank, Drs. Vilmos Kertesz and Gary J. Van Berkel (Mass Spectrometry and Laser Spectroscopy Group, Chemical Sciences Division, Oak Ridge National Laboratory) for inspiration and guidance with the droplet probe. We also thank Tyler Graf and Dr. Daniel Todd of UNCG for helpful discussions pertaining to evaluation of metabolites. The mass spectrometry data were acquired in the Triad Mass Spectrometry Facility.
Footnotes
Appendix A. Supplementary Data
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balunas MJ, Su B, Brueggemeier RW, Kinghorn AD, 2008. Xanthones from the botanical dietary supplement mangosteen (Garcinia mangostana) with aromatase inhibitory activity. J. Nat. Prod 71, 1161–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benatrehina PA, Pan L, Naman CB, Li J, Kinghorn AD, 2018. Usage, biological activity, and safety of selected botanical dietary supplements consumed in the United States. J. Tradit. Complement. Med 8, 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chairungsrilerd N, Furukawa K, Ohta T, Nozoe S, Ohizumi Y, 1996. Histaminergic and serotonergic receptor blocking substances from the medicinal plant Garcinia mangostana. Planta Med. 62, 471–472. [DOI] [PubMed] [Google Scholar]
- Chen LG, Yang LL, Wang CC, 2008. Anti-inflammatory activity of mangostins from Garcinia mangostana. Food Chem. Toxicol 46, 688–693. [DOI] [PubMed] [Google Scholar]
- Chen SX, Wan M, Loh BN, 1996. Active constituents against HIV-1 protease from Garcinia mangostana. Planta Med. 62, 381–382. [DOI] [PubMed] [Google Scholar]
- Chin YW, Kinghorn AD, 2008. Structural characterization, biological effects, and synthetic studies on xanthones from mangosteen (Garcinia mangostana), a popular botanical dietary supplement. Mini Rev. Org. Chem 5, 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin YW, Jung HA, Chai H, Keller WJ, Kinghorn AD, 2008. Xanthones with quinone reductase-inducing activity from the fruits of Garcinia mangostana (Mangosteen). Phytochemistry 69, 754–758. [DOI] [PubMed] [Google Scholar]
- Chitchumroonchokchai C, Thomas-Ahner JM, Li J, Riedl KM, Nontakham J, Suksumrarn S, Clinton SK, Kinghorn AD, Failla ML, 2013. Anti-tumorigenicity of dietary α-mangostin in an HT-29 colon cell xenograft model and the tissue distribution of xanthones and their phase II metabolites. Mol. Nutr. Food Res 57, 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomnawang MT, Surassmo S, Nukoolkarn VS, Gritsanapan W, 2007. Effect of Garcinia mangostana on inflammation caused by Propionibacterium acnes. Fitoterapia 78, 401–408. [DOI] [PubMed] [Google Scholar]
- Chomnawang MT, Surassmo S, Wongsariya K, Bunyapraphatsara N, 2009. Antibacterial activity of Thai medicinal plants against methicillin-resistant Staphylococcus aureus. Fitoterapia 80, 102–104. [DOI] [PubMed] [Google Scholar]
- Culley TM, 2013. Why vouchers matter in botanical research. Appl. Plant Sc 1, apps.1300076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabkova LZ, 2014. DNA extraction from herbarium specimens. Methods Mol. Biol 1115, 69–84. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan G, Banumathi B, Suresh G, 1997. Evaluation of the antifungal activity of natural xanthones from Garcinia mangostana and their synthetic derivatives. J. Nat. Prod 60, 519–524. [DOI] [PubMed] [Google Scholar]
- Grubbs FE, 1969. Procedures for detecting outlying observations in samples. Technometrics 11, 1–21. [Google Scholar]
- Han AR, Kim JA, Lantvit DD, Kardono LB, Riswan S, Chai H, Carcache de Blanco EJ, Farnsworth NR, Swanson SM, Kinghorn AD, 2009. Cytotoxic xanthone constituents of the stem bark of Garcinia mangostana (mangosteen). J. Nat. Prod 72, 2028–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MY, Hashim NM, Mariod AA, Mohan S, Abdulla MA, Abdelwahab SI, Arbab IA, 2016. α-Mangostin from Garcinia mangostana Linn: An updated review of its pharmacological properties. Arab. J. Chem 9, 317–329. [Google Scholar]
- Ji X, Avula B, Khan IA, 2007. Quantitative and qualitative determination of six xanthones in Garcinia mangostana L. by LC-PDA and LC-ESI-MS. J. Pharm. Biomed. Anal 43, 1270–1276. [DOI] [PubMed] [Google Scholar]
- Jiang B, Yang H, Nuntanakorn P, Balick MJ, Kronenberg F, Kennelly EJ, 2005. The value of plant collections in ethnopharmacology: a case study of an 85-year-old black cohosh (Actaea racemosa L.) sample. J. Ethnopharmacol 96, 521–528. [DOI] [PubMed] [Google Scholar]
- Jung HA, Su BN, Keller WJ, Mehta RG, Kinghorn AD, 2006. Antioxidant xanthones from the pericarp of Garcinia mangostana (Mangosteen). J. Agric. Food Chem 54, 2077–2082. [DOI] [PubMed] [Google Scholar]
- Kertesz V, Van Berkel GJ, 2013. Automated liquid microjunction surface sampling-HPLCMS/MS analysis of drugs and metabolites in whole-body thin tissue sections. Bioanalysis 5, 819–826. [DOI] [PubMed] [Google Scholar]
- Kertesz V, Van Berkel GJ, 2014. Sampling reliability, spatial resolution, spatial precision, and extraction efficiency in droplet-based liquid microjunction surface sampling. Rapid Commun. Mass Spectrom 28, 1553–1560. [DOI] [PubMed] [Google Scholar]
- Kertesz V, Paranthaman N, Moench P, Catoire A, Flarakos J, Van Berkel GJ, 2014. Liquid microjunction surface sampling of acetaminophen, terfenadine and their metabolites in thin tissue sections. Bioanalysis 6, 2599–2606. [DOI] [PubMed] [Google Scholar]
- Kertesz V, Weiskittel TM, Van Berkel GJ, 2015. An enhanced droplet-based liquid microjunction surface sampling system coupled with HPLC-ESI-MS/MS for spatially resolved analysis. Anal. Bioanal. Chem 407, 2117–2125. [DOI] [PubMed] [Google Scholar]
- Lee ST, Cook D, Davis TZ, Gardner DR, Johnson RL, Stonecipher CA, 2015. A Survey of Tremetone, Dehydrotremetone, and Structurally Related Compounds in Isocoma spp. (Goldenbush) in the Southwestern United States. J. Agric. Food Chem 63, 872–879. [DOI] [PubMed] [Google Scholar]
- Lee ST, Davis TZ, Cook D, 2017. Evaluation of the Stability of Benzofuran Ketones in Rayless Goldenrod (Isocoma pluriflora) and White Snakeroot (Ageratina altissima) Under Different Storage Conditions. IJPPR 4, 36–42. [Google Scholar]
- Matsumoto K, Akao Y, Kobayashi E, Ohguchi K, Ito T, Tanaka T, Iinuma M, Nozawa Y, 2003. Induction of apoptosis by xanthones from mangosteen in human leukemia cell lines. J. Nat. Prod 66, 1124–1127. [DOI] [PubMed] [Google Scholar]
- Müller-Wille S, 2006. Linnaeus’ herbarium cabinet: a piece of furniture and its function. Endeavour 30, 60–64. [DOI] [PubMed] [Google Scholar]
- Obolskiy D, Pischel I, Siriwatanametanon N, Heinrich M, 2009. Garcinia mangostana L.: a phytochemical and pharmacological review. Phytother. Res 23, 1047–1065. [DOI] [PubMed] [Google Scholar]
- Ovchinnikova OS, Kertesz V, Van Berkel GJ, 2011. Molecular surface sampling and chemical imaging using proximal probe thermal desorption/secondary ionization mass spectrometry. Anal. Chem 83, 598–603. [DOI] [PubMed] [Google Scholar]
- Paguigan ND, Raja HA, Day CS, Oberlies NH, 2016. Acetophenone derivatives from a freshwater fungal isolate of recently described Lindgomyces madisonensis (G416). Phytochemistry 126, 59–65. [DOI] [PubMed] [Google Scholar]
- Phongpaichit S, Rungjindamai N, Rukachaisirikul V, Sakayaroj J, 2006. Antimicrobial activity in cultures of endophytic fungi isolated from Garcinia species. FEMS Immunol. Med. Microbiol. 48, 367–372. [DOI] [PubMed] [Google Scholar]
- Shepherd LD, 2017. A non-destructive DNA sampling technique for herbarium specimens. PLoS ONE 12, e0183555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica VP, Raja HA, El-Elimat T, Kertesz V, Van Berkel GJ, Pearce CJ, Oberlies NH, 2015. Dereplicating and spatial mapping of secondary metabolites from fungal cultures in situ. J. Nat. Prod 78, 1926–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica VP, Figueroa M, Raja HA, El-Elimat T, Darveaux BA, Pearce CJ, Oberlies NH, 2016a. Optimizing production and evaluating biosynthesis in situ of a herbicidal compound, mevalocidin, from Coniolariella sp. J. Ind. Microbiol. Biotechnol 43, 11491157. [DOI] [PubMed] [Google Scholar]
- Sica VP, El-Elimat T, Oberlies NH, 2016b. In situ analysis of Asimina triloba (paw paw) plant tissues for acetogenins via the droplet-liquid microjunction-surface sampling probe coupled to UHPLC-PDA-HRMS/MS. Anal. Methods 8, 6143–6149. [Google Scholar]
- Sica VP, Rees ER, Raja HA, Rivera-Chavez J, Burdette JE, Pearce CJ, Oberlies NH, 2017. In situ mass spectrometry monitoring of fungal cultures led to the identification of four peptaibols with a rare threonine residue. Phytochemistry 143, 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearn WT, 1971. Sources of information about botanic gardens and herbaria. Biol. J. Linn. Soc. Lond 3, 225–233. [Google Scholar]
- Sukatta U, Takenaka M, Ono H, Okadome H, Sotome I, Nanayama K, Thanapase W, Isobe S, 2013. Distribution of major xanthones in the pericarp, aril, and yellow gum of mangosteen (Garcinia mangostana linn.) fruit and their contribution to antioxidative activity. Biosci. Biotechnol. Biochem 77, 984–987. [DOI] [PubMed] [Google Scholar]
- Suksamrarn S, Suwannapoch N, Phakhodee W, Thanuhiranlert J, Ratananukul P, Chimnoi N, Suksamrarn A, 2003. Antimycobacterial activity of prenylated xanthones from the fruits of Garcinia mangostana. Chem. Pharm. Bull 51, 857–859. [DOI] [PubMed] [Google Scholar]
- Tousian Shandiz H, Razavi BM, Hosseinzadeh H, 2017. Review of Garcinia mangostana and its xanthones in metabolic syndrome and related complications. Phytother. Res 31, 1173–1182. [DOI] [PubMed] [Google Scholar]
- Walker EB, 2007. HPLC analysis of selected xanthones in mangosteen fruit. J. Sep. Sci 30, 1229–1234. [DOI] [PubMed] [Google Scholar]
- Williams P, Ongsakul M, Proudfoot J, Croft K, Beilin L, 1995. Mangostin inhibits the oxidative modification of human low density lipoprotein. Free Radic. Res 23, 175–184. [DOI] [PubMed] [Google Scholar]
- Willis CG, Ellwood ER, Primack RB, Davis CC, Pearson KD, Gallinat AS, Yost JM, Nelson G, Mazer SJ, Rossington NL, Sparks TH, Soltis PS, 2017. Old plants, new tricks: Phenological research using herbarium specimens. Trends Ecol. Evolut. (Amst.) 32, 531–546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.