Abstract
Objectives
The ability to efficiently and accurately predict future risk of primary total hip and knee replacement (THR/TKR) in earlier stages of osteoarthritis (OA) has potentially important applications. We aimed to develop and validate two models to estimate an individual’s risk of primary THR and TKR in patients newly presenting to primary care.
Methods
We identified two cohorts of patients aged ≥40 years newly consulting hip pain/OA and knee pain/OA in the Clinical Practice Research Datalink. Candidate predictors were identified by systematic review, novel hypothesis-free ‘Record-Wide Association Study’ with replication, and panel consensus. Cox proportional hazards models accounting for competing risk of death were applied to derive risk algorithms for THR and TKR. Internal–external cross-validation (IECV) was then applied over geographical regions to validate two models.
Results
45 predictors for THR and 53 for TKR were identified, reviewed and selected by the panel. 301 052 and 416 030 patients newly consulting between 1992 and 2015 were identified in the hip and knee cohorts, respectively (median follow-up 6 years). The resultant model C-statistics is 0.73 (0.72, 0.73) and 0.79 (0.78, 0.79) for THR (with 20 predictors) and TKR model (with 24 predictors), respectively. The IECV C-statistics ranged between 0.70–0.74 (THR model) and 0.76–0.82 (TKR model); the IECV calibration slope ranged between 0.93–1.07 (THR model) and 0.92–1.12 (TKR model).
Conclusions
Two prediction models with good discrimination and calibration that estimate individuals’ risk of THR and TKR have been developed and validated in large-scale, nationally representative data, and are readily automated in electronic patient records.
Keywords: osteoarthritis, total hip replacement, total knee replacement, primary care, electronic health record, record-wide association study, risk prediction model
Key messages.
What is already known about this subject?
The majority of primary total hip replacement (THR) and primary total knee replacement (TKR) were performed for patients with end-stage osteoarthritis in the UK, but clinical risk predictions of THR and TKR in patients who newly presenting hip pain/osteoarthritis and knee pain/osteoarthritis at the primary care settings have not been developed.
What does this study add?
A novel approach for predictor selection has been developed. Two risk prediction models based on clinical variables that are available in the UK primary care electronic health record have been developed and validated. These algorithms can be used to inform clinical decision making, for example targeting intensive non-surgical management at patients identified at high risk of future primary THR or TKR.
How might this impact on clinical practice?
These algorithms can be used to inform clinical decision making, for example targeting intensive non-surgical management at patients identified at high risk of future primary THR or TKR.
Introduction
Osteoarthritis (OA) is the most common form of arthritis and a leading cause of disability in populations worldwide.1 Although characterised as a slowly progressive condition, recent studies have highlighted substantial heterogeneity between groups of patients in the course of symptoms,2–4 function5 and structural disease.6 Healthcare costs attributed to OA, driven largely by primary and revision arthroplasty,7 appear concentrated in a minority of patients.8 The development and application of prognostic models capable of identifying patients with OA at high risk of future progression is now recognised as a priority internationally and by patients, carers and health and social care professionals.9 Such models could have important clinical and research applications: better targeting of intensive non-surgical care; selection of patients for active monitoring; timely assessment and discussion of appropriateness for referral and recruitment of ‘high-risk’ patients as part of efficient clinical trial design evaluating new secondary prevention treatments.
Models that rely on pooling data from existing clinical trials and bespoke cohorts may offer the prospect of carefully measured, highly relevant predictors and outcomes, but are limited by the availability of large, long-term studies with sufficient harmonised data. Furthermore, due to the high prevalence of OA and to time and cost constraints, models that require the collection of biomarkers, imaging or lengthy patient-reported instruments are unlikely to be implemented at scale in routine primary care, irrespective of their informativeness in research settings. An alternative approach, and the one chosen in our study, is to investigate whether data already routinely available in large, representative primary electronic healthcare databases could provide accurate predictions which are feasible for implementing in routine primary care. This approach has been used to derive and validate risk algorithms for condition-specific outcomes in other chronic non-communicable diseases and for complex events such as hospital admissions.10–18
We sought to develop and validate multivariable prediction models, based exclusively on information routinely recorded within the primary care electronic health record, to estimate the risk of primary total hip replacement (THR) and total knee replacement (TKR) in patients newly presenting with hip pain/OA and knee pain/OA in UK primary care. To achieve this, we included a novel approach to identify candidate prognostic factors recorded in the primary care patient record.
Methods
Data source and study population
We used data from the Clinical Practice Research Datalink (CPRD) covering a representative sample of 7% of the UK general population.19 The definition20 and the selection of population were presented in online supplementary technical appendix, figures S1 and S2.
annrheumdis-2018-213894supp001.docx (2.9MB, docx)
Defining THR/TKR
Primary THR and TKR were identified within CPRD using the Read code list developed and applied in CPRD by Culliford and colleagues21 and validated by Hawley et al.22 23 Details of outcome definition was presented in online supplementary technical appendix.
Candidate predictors
Candidate predictors were identified from three sources: (i) a systematic review of previously published studies (further details available in online supplementary technical appendix); (ii) potentially relevant general predictors used within 12 QResearch risk algorithms and shown to be feasibly obtained from UK primary care (eg, sociodemographic, lifestyle related, comorbidities)10–18 24–26 (iii) a hypothesis-free record-wide association study (ReWAS) of all third-level Read morbidity and process of care codes and for prescribed medicine, third-level sections within the British National Formulary which had been recorded in ≥1% of cases in the 3 years prior to date of arthroplasty. There were 6109 third-level Read morbidity and process of care codes and 325 prescribed medications assessed. The ReWAS case-control analysis was conducted in CPRD, with replication of ‘hits’ in a separate UK regional primary care Electronic Health Record (EHR) dataset—Consultations in Primary Care Archive (further details available in online supplementary technical appendix). Morbidities, processes of care and prescribed medications that were statistically significantly associated with THR or TKR in the screen were taken forward (online supplementary figures S3 and S5 for TKR; online supplementary figures S4 and S6 for THR). The candidate predictors were assessed for clinical relevance by a review panel including seven members (six clinicians and one lay member), and the predictors agreed as relevant by ≥4 members were included in the modelling stage (online supplementary table S1). This process identified 29 candidate predictors for THR and 34 for TKR. These were extracted from records in the 3 years prior to index consultation.
Statistical analysis for model derivation
Primary THR and TKR occurring since the patients’ index consultation for hip pain/OA and knee pain/OA in primary care were treated as time-to-event outcomes in the THR and TKR models, respectively. Statistical method of predictor selection was presented in online supplementary technical appendix.
We formed the risk (cumulative incidence) equations for predicting an individual’s 10-year probability of primary THR and TKR since the index consultation for hip pain/OA and knee pain/OA, by using the developed model’s baseline cumulative incidence function (CIF) at 10 years, along with the estimated regression coefficients (β) and the individual’s predictor values (X) using the following equation27:
Validation of prediction models
We assessed the model discrimination using Harrell’s C-statistic and the model calibration using calibration slope (details in online supplementary technical appendix)28–30 over the 10 years of follow-up.
We assessed the apparent performance of the models; that is, the observed performance in exactly the same data used to develop the model. However, we also used an internal–external cross-validation (IECV) approach (online supplementary technical appendix) to evaluate the two derived prediction models over 13 geographical regions in the UK (presented in table 1).31–33
Table 1.
Characteristics of study populations for the primary total knee replacement (TKR) model and primary total hip replacement (THR) models
| Predictor | THR model | TKR model |
| N=301 052 | N=416 030 | |
| Outcome, n (%) | 15 509 (5.15) | 18 289 (4.40) |
| Median follow-up duration (range), years | 6.27 (2.00 to 24.49) | 6.21 (2.00 to 24.56) |
| Gender (female) | 191 288 (63.54) | 238 549 (57.34) |
| Ethnicity | ||
| White | 92 269 (30.65) | 125 982 (30.28) |
| Other ethnicity group | 3621 (1.20) | 6001 (1.44) |
| Not recorded | 205 162 (68.15) | 284 047 (68.28) |
| Region | ||
| North East | 6552 (2.18) | 8647 (2.08) |
| North West | 38 618 (12.83) | 51 331 (12.34) |
| Yorkshire and the Humber | 13 140 (4.36) | 17 282 (4.15) |
| East Midlands | 12 779 (4.24) | 16 670 (4.00) |
| West Midlands | 29 273 (9.72) | 40 383 (9.71) |
| East of England | 26 194 (8.70) | 36 488 (8.77) |
| South West | 24 885 (8.27) | 34 216 (8.22) |
| South Central | 32 048 (10.65) | 46 736 (11.32) |
| London | 23 541 (7.82) | 35 197 (8.46) |
| South East Coast | 28 760 (9.55) | 41 608 (10.00) |
| Northern Ireland | 11 445 (3.80) | 14 708 (3.54) |
| Scotland | 25 672 (8.53) | 34 547 (8.30) |
| Wales | 28 145 (9.35) | 38 217 (9.18) |
| Family history of arthritis/ osteoarthritis (OA) | 1862 (0.62) | 2135 (0.51) |
| Smoking status | ||
| Light smoker | 6845 (2.27) | 8502 (2.04) |
| Moderate/heavy smoker | 34 896 (11.59) | 48 398 (11.63) |
| Drinking status | ||
| Ex-drinker | 11 069 (3.68) | 13 924 (3.35) |
| Light drinker | 212 058 (70.44) | 294 273 (70.73) |
| Moderate drinker | 4172 (1.39) | 6523 (1.57) |
| Heavy drinker | 1945 (0.65) | 3177 (0.76) |
| Physical activity | ||
| Taking light physical activity | 26 855 (8.92) | 44 876 (10.53) |
| Taking moderate physical activity | 17 526 (5.82) | 29 665 (7.13) |
| Taking heavy physical activity | 1984 (0.66) | 3229 (0.78) |
| Having diet consultation | 110 780 (36.80) | 173 969 (41.82) |
| Asthma | – | 59 326 (14.26) |
| COPD | – | 20 114 (4.83) |
| Chronic liver disease | 6962 (2.31) | 10 798 (2.60) |
| Diabetes mellitus | 42 352 (14.07) | 54 339 (13.06) |
| Malabsorption | 1488 (0.49) | 2091 (0.50) |
| Inflammatory bowel disease | 16 863 (5.60) | 26 722 (6.42) |
| Dementia | 1265 (0.42) | 2710 (0.65) |
| Cerebral palsy | 153 (0.05) | 205 (0.05) |
| Multiple sclerosis | 942 (0.31) | 1280 (0.31) |
| Cerebrovascular disease | 11 819 (3.93) | 16 150 (3.88) |
| Mental disorder | ||
| Anxiety | 38 133 (12.67) | 56 163 (13.50) |
| Depression | 48 558 (16.13) | 36 034 (8.66) |
| Rheumatoid arthritis | 5031 (1.67) | 10 200 (2.45) |
| Systemic lupus erythematosus | 673 (0.22) | 865 (0.21) |
| Falls | 29 780 (9.89) | 49 859 (11.98) |
| Previous hip injury for THR model/previous knee injury for TKR model | 7115 (2.36) | 26 735 (6.43) |
| Osteoporosis | 12 953 (4.30) | 19 481 (4.68) |
| Knee effusion | – | 6243 (1.50) |
| Diabetic foot | 13 915 (4.62) | – |
| Bleed | 44 812 (14.89) | 70 543 (16.96) |
| Scoliosis/kyphosis | 2340 (0.78) | 3292 (0.79) |
| Development dysplasia of the hip | 67 (0.02) | 76 (0.02) |
| Chondrocalcinosis | 781 (0.26) | 1588 (0.38) |
| Recorded diagnosis of joint-specific OA | ||
| Hip OA for TKR model/knee OA for THR model | 272 (0.09) | 26 640 (6.40) |
| Hand OA | 9897 (3.29) | 12 419 (2.99) |
| Generalised OA | 7733 (2.57) | 10 715 (2.58) |
| Other joint OA | 12 380 (4.11) | 151 282 (3.64) |
| Recorded diagnosis of non-specific OA | 123 810 (41.13) | 147 103 (35.36) |
| Low back pain | 150 759 (50.08) | 218 702 (52.57) |
| Hypertension | 101 999 (33.88) | 149 307 (35.89) |
| Atrial fibrillation | 9842 (3.27) | 16 515 (3.97) |
| Congestive cardiac failure | 6397 (2.12) | 9661 (2.32) |
| Venous thromboembolism | 8040 (2.67) | 12 245 (2.94) |
| Valvular heart disease | 4157 (1.38) | 6823 (1.64) |
| Joint injection | – | 42 434 (10.20) |
| Knee arthroscopy | – | 8310 (2.00) |
| ACL reconstruction | – | 430 (0.10) |
| Phenytoin | 819 (0.27) | 1248 (0.30) |
| Physiotherapy | 26 972 (8.96) | 41 547 (9.99) |
| Corticosteroids | – | 62 127 (14.93) |
| Glucocosteroids | 32 203 (10.70) | 51 645 (12.41) |
| Antidepressant | 109 062 (36.23) | 159 899 (38.43) |
| Analgesics | ||
| Weak combination opioids | 192 249 (63.86) | 268 421 (64.52) |
| Moderate combination opioids | 3231 (1.07) | 4837 (1.16) |
| Strong/very strong combination opioids | 3034 (1.01) | 6359 (1.53) |
| Hormone treatment | 82 221 (27.31) | 107 321 (25.78) |
| Bisphosphonates | 15 249 (5.07) | 24 147 (5.80) |
| Topical NSAIDS | ||
| NSAIDS | 68 002 (22.59) | 112 705 (27.09) |
| Other | 3627 (1.20) | 7422 (1.78) |
| Drugs for rheumatoid disease and gout | ||
| NSAIDS | 183 107 (60.82) | 268 850 (64.62) |
| COX2 | 20 161 (6.70) | 26 748 (6.43) |
| Prostaglandins and oxytocics | 10 359 (3.44) | 15 837 (3.80) |
| Rheumatoid factor test | 876 (0.29) | 1124 (0.27) |
| Age, mean±SD, years | 62.98±12.17 | 60.71±12.39 |
| Body mass index, mean±SD, kg/m2 | 27.70±5.44 | 28.06±5.62 |
| Charlson comorbidity index, median (nterquartile) | 1 (0 to 2) | 1 (0 to 2) |
| Number of consultations, median (interquartile) | 56 (0 to 111) | 65 (32 to 113) |
| Number of referrals, median (interquartile) | 0 (0 to 1) | 1 (0 to 1) |
| Polypharmacy, median (interquartile) | 7 (5 to 9) | 6 (0 to 8) |
| Missing information: body mass index | 16 226 (5.39) | 23 555 (5.66) |
ACL, anterior cruciate ligament; COPD, chronic obstructive pulmonary disease; COX2, cyclooxygenase-2; NSAIDS, non-steroidal anti-inflammatory drugs.
Multiple imputation using chained equations was applied to handle missing values, and the imputation model included all candidate predictors and outcome (online supplementary technical appendix).34
Based on the 15 509 THRs and a total of 73 predictor parameters and 18,289 TKRs and 79 predictor parameters, we had an effective sample size of 212 events per predictor parameter for the THR derivation cohort and 232 events per predictor parameter for the TKR derivation cohort, above the minimum requirement suggested by Peduzzi.35
In a sensitivity analysis, we assessed the models’ performances when including patients with a THR and TKR within the first 2 years after the index consultation (an exclusion criteria for the main analysis). In the other sensitivity analysis, we derived model coefficients by applying final predictors into patients with a THR and TKR within the first 2 years after the index consultation (an exclusion criteria for the main analysis).
We used Stata MP V.15.1 version for all statistical analyses. This study was conducted and reported in line with the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) guidelines (online supplementary file 2).30
annrheumdis-2018-213894supp002.docx (213KB, docx)
Results
Study population
Table 1 summarises the baseline characteristics of the study populations, showing broadly similar characteristics between THR and TKR cohorts.
Model development
Of 45 candidate categorical predictors of primary THR, 26 were excluded due to multivariable −1%≤PAR≤1% (online supplementary table S2). Of the remaining 19 categorical predictors and 6 continuous predictors considered for inclusion in the multivariable prediction model, 14 categorical predictors and 6 continuous predictors were retained after backward elimination (table 2). Previous hip injury recorded within 3 years prior to index consultation was a strong predictor of increased risk of future primary THR (adjusted subdistribution HR 1.54, 95% CI 1.40 to 1.69). Age at index consultation and body mass index (BMI) showed non-linear adjusted associations with THR, peaking at 75 years and 47 kg/m2 respectively (online supplementary figure S7).
Table 2.
Adjusted subdistribution hazard ratios and final model coefficients
| Predictor | Subdistribution hazard ratio (95 CI) | Beta coefficient |
| Final model for primary total hip replacement | ||
| Gender: women vs men | 1.00 (0.96 to 1.04) | 0.002179 |
| Smoking status | ||
| Non-smoker/not recorded/ex-smoker | reference | |
| Light smoker | 0.64 (0.54 to 0.75) | −0.446637 |
| Moderate/heavy smoker | 0.75 (0.70 to 0.80) | −0.283425 |
| Drinking status | ||
| Non-drinker/not recorded | reference | |
| Ex-drinker | 1.01 (0.91 to 1.11) | 0.008542 |
| Light drinker | 1.18 (1.13 to 1.24) | 0.167673 |
| Moderate drinker | 1.36 (1.18 to 1.56) | 0.304068 |
| Heavy drinker | 1.06 (0.83, 1.36) | 0.062211 |
| Diabetes mellitus: yes vs no | 0.86 (0.81 to 0.91) | −0.154314 |
| Mental disorders: yes vs no | ||
| No/not recorded | reference | |
| Anxiety | 0.85 (0.80 to 0.90) | −0.162867 |
| Depression | 0.85 (0.80 to 0.90) | −0.164488 |
| Falls | 0.85 (0.80, 0.91) | −0.157293 |
| Previous hip injury: yes vs no | 1.54 (1.40 to 1.69) | 0.432446 |
| Recorded diagnosis of joint-specific osteoarthritis (OA) | ||
| No/not recorded | reference | |
| Knee OA | 1.02 (0.64 to 1.61) | 0.015210 |
| Hand OA | 0.21 (0.18 to 0.24) | −1.582914 |
| Generalised OA | 0.29 (0.25 to 0.33) | −1.242895 |
| Other joint OA | 0.23 (0.20 to 0.26) | −1.473753 |
| Recorded diagnosis of non-specific OA: yes vs no | 0.27 (0.26 to 0.28) | −1.312229 |
| Analgesics | ||
| No prescription | reference | |
| Weak combination opioids | 0.93 (0.89 to 0.97) | −0.072075 |
| Moderate combination opioids | 1.00 (0.85 to 1.19) | 0.002597 |
| Strong/very strong combination opioids | 1.06 (0.87 to 1.29) | 0.056441 |
| Antidepressant: yes vs no | 0.96 (0.92 to 1.01) | −0.036091 |
| Topical NSAIDS | ||
| No prescription | reference | |
| NSAIDS | 0.77 (0.73 to 0.80) | −0.266746 |
| Other | 0.78 (0.65 to 0.94) | −0.247566 |
| NSAIDS/COX2 | ||
| No prescription | reference | |
| NSAIDS | 1.06 (1.02 to 1.10) | 0.056447 |
| COX2 | 1.18 (1.10 to 1.26) | 0.164306 |
| Hormone treatment: yes vs no | 0.96 (0.92 to 1.01) | −0.039146 |
| (Age/10)^3 | – | 0.056927 |
| (Age/10)^3*ln(age/10) | – | −0.024913 |
| (Body mass index (BMI)/10)^2 | – | 0.137871 |
| (BMI/10)^3 | – | −0.024987 |
| ((Charlson comorbidity index+1)/10)^−2 | – | 0.001583 |
| ((Charlson comorbidity index+1)/10)^2 | – | −1.318626 |
| ((Number of referrals+1)/10)^−2 | – | −0.015522 |
| ((Number of referrals+1)/10)^−2*ln((number of referrals+1)/10) | – | −0.005468 |
| ((Number of consultations+1)/1000)^−0.5 | – | −0.171643 |
| ((Number of consultations+1)/1000)^−0.5*ln((number of consultations+1)/1000) | – | −0.017409 |
| ((Number of BNF chapters+1)/10)^−2 | – | 0.110533 |
| ((Number of BNF chapters+1)/10)^−2*ln((number of BNF chapters+1)/10) | – | 0.046402 |
| Final model for primary total knee replacement | ||
| Gender: women vs men | 0.87 (0.85 to 0.90) | −0.136204 |
| Ethnicity | ||
| White | reference | – |
| Other ethnicity group | 0.90 (0.75 to 1.08) | −0.105427 |
| Not recorded | 1.04 (1.01 to 1.08) | 0.042038 |
| Smoking status | ||
| Non-smoker/not recorded/ex-smoker | reference | – |
| Light smoker | 0.75 (0.64 to 0.89) | −0.281159 |
| Moderate/heavy smoker | 0.75 (0.71 to 0.80) | −0.281584 |
| Drinking status | ||
| Non-drinker/not recorded | reference | – |
| Ex-drinker | 1.13 (1.09 to 1.50) | 0.126359 |
| Light drinker | 1.21 (1.07 to 1.38) | 0.192886 |
| Moderate drinker/heavy drinker | 1.34 (1.19 to 1.50) | 0.289711 |
| Asthma, yes vs no | 1.07 (1.03 to 1.12) | 0.070898 |
| COPD, yes vs no | 0.71 (0.66 to 0.77) | −0.341224 |
| Diabetes mellitus: yes vs no | 0.88 (0.84 to 0.93) | −0.126142 |
| Mental disorders: yes vs no | ||
| Anxiety | 0.76 (0.73 to 0.80) | −0.268390 |
| Depression | 0.85 (0.81, 0.89) | −0.164173 |
| Previous knee injury: yes vs no | 1.29 (1.24 to 1.35) | 0.256978 |
| Recorded diagnosis of joint-specific OA | ||
| No/not recorded | reference | – |
| Hip OA | 0.59 (0.55 to 0.63) | −0.528500 |
| Hand OA | 0.61 (0.56 to 0.68) | −0.486247 |
| Generalised OA/ | 0.76 (0.69 to 0.83) | −0.278839 |
| Other joint OA | 0.74 (0.69 to 0.80) | −0.296179 |
| Recorded diagnosis of non-specific OA: yes vs no | 1.17 (1.12 to 1.22) | 0.159204 |
| Low back pain: yes vs no | 0.87 (0.84 to 0.90) | −0.142815 |
| Hypertension: yes vs no | 0.96 (0.93 to 0.99) | −0.043010 |
| Joint injection: yes vs no | 1.66 (1.60 to 1.72) | 0.504619 |
| Knee arthroscopy: yes vs no | 14.47 (13.95 to 15.02) | 2.672150 |
| Antidepressant: yes vs no | 0.95 (0.91 to 0.98) | −0.054225 |
| Analgesics | ||
| No prescription | reference | – |
| Weak combination opioids | 1.33 (1.27 to 1.39) | 0.286170 |
| Moderate combination opioids | 1.37 (1.22 to 1.54) | 0.318097 |
| Strong/very strong combination opioids | 1.59 (1.45 to 1.75) | 0.465081 |
| Topical NSAIDS | ||
| No prescription | reference | – |
| NSAIDS | 0.93 (0.90 to 0.96) | −0.074381 |
| Other | 1.17 (1.08 to 1.28) | 0.160424 |
| NSAIDS/COX2 | ||
| No prescription | reference | – |
| NSAIDS | 1.41 (1.35 to 1.48) | 0.343065 |
| COX2 | 1.27 (1.19 to 1.36) | 0.238566 |
| (Age/10)^3 | – | 0.029250 |
| (Age/10)^3*ln(age/10) | – | −0.013246 |
| (BMI/10)^2 | – | 0.280906 |
| (BMI/10)^3 | – | −0.047226 |
| ((Charlson comorbidity index+1)/10)^−2 | – | 0.002381 |
| ((Charlson comorbidity index+1)/10)^2 | – | −1.442397 |
| ((Number of referrals+1)/10)^−2 | – | −0.010564 |
| ((Number of referrals+1)/10)^−2*ln((number of referrals+1)/10) | – | −0.002929 |
| ((Number of consultations+1)/1000)^−0.5 | – | −0.542288 |
| ((Number of consultations+1)/1000)^−0.5*ln((number of consultations+1)/1000) | – | −0.073453 |
BNF, British National Formulary; COPD, chronic obstructive pulmonary disease; COX2, cyclooxygenase-2; NSAIDS, steroidal anti-inflammatory drugs.
Of 53 candidate categorical predictors of primary TKR, 20 were excluded due to multivariable −1%≤PAR≤1% (online supplementary table S3). Of the remaining 33 categorical predictors and 6 continuous predictors entered into the multivariable prediction model, 19 categorical predictors and 5 continuous predictors were retained after backward elimination (table 2). Oral NSAID and opioid analgesic prescriptions, intra-articular injections and previous arthroscopic knee surgery in the 3 years prior to index consultation were strong predictors of increased risk of future primary TKR. Age and BMI showed non-linear adjusted associations, peaking at 70 years and 40 kg/m2 respectively (online supplementary figure S8).
Apparent predictive performance of the models
Our final THR prediction model was able to discriminate between patients with and without a primary THR with a C-statistic of 0.73 (95% CI 0.72 to 0.73); our final TKR prediction model was also able to discriminate between patients with and without TKR with a C-statistic of 0.79 (0.78 to 0.79) over the 10-year follow-up period. The calibration slope was 1.00 (0.98 to 1.02) and 1.00 (0.99 to 1.01) for THR and THR, respectively, as we would expect.
Internal–external cross-validation
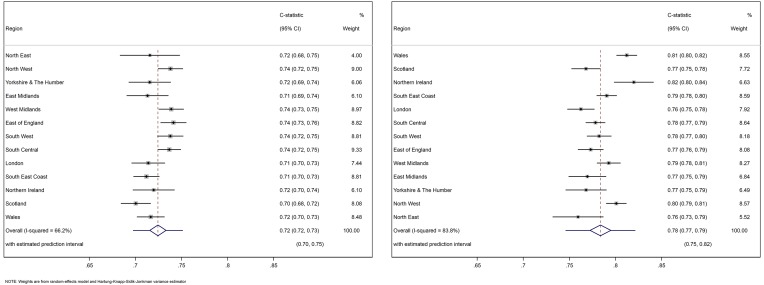
The internal-external cross-validation revealed that the C-statistic was similar in each of the 13 geographical regions, ranging from 0.70 (0.68 to 0.72) to 0.74 (0.73 to 0.75) for the THR model (figure 1 left panel) and between 0.76 (0.73 to 0.79) and 0.82 (0.80 to 0.84) for the TKR model (figure 1 right panel). After meta-analysis, the summary C-statistic was 0.72 (0.72 to 0.73) for the THR model and 0.78 (0.77 to 0.80) for the TKR model. Based on the 95% prediction intervals, if the models were applied in a new (but similar) setting, we would expect the C-statistic to be between 0.70 and 0.75 for the THR model and between 0.75 and 0.82 for the TKR model.
Figure 1.
C-statistics in 13 validation cohorts and the overall estimation across validation cohorts. The left panel is for THR model and the right is for TKR model.
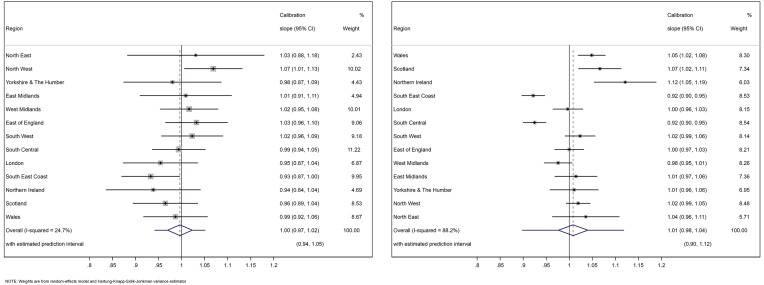
The calibration slope was also similar across the 13 regions, ranging between 0.93 (0.87 to 1.00) and 1.07 (1.01 to 1.13) for THR model (figure 2 left panel) and between 0.92 (0.90 to 0.95) and 1.12 (1.05 to 1.19) for TKR model (figure 2 right panel). After meta-analysis, the summary calibration slope was 1.00 (0.97 to 1.02) for the THR model and 1.01 (0.98 to 1.04) for the TKR model. If the models were applied in a new (but similar) setting, we would expect the calibration slope to be between 0.94 and 1.05 for the THR model and between 0.98 and 1.12 for the TKR model.
Figure 2.
Calibration slope in 13 validation cohorts and the overall estimation across validation cohorts. The left panel is for THR model and the right is for TKR model.
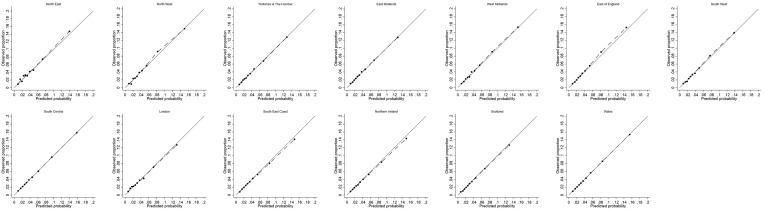
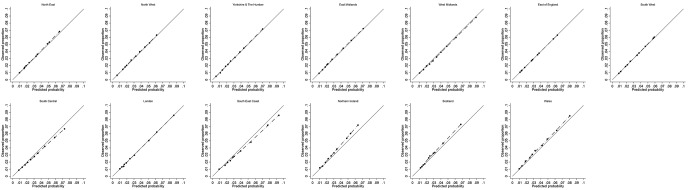
The calibration plot in each validation cohort was presented for one imputed dataset, but comparable to the calibration plots in the other imputations (figure 3 for THR and in figure 4 for TKR). Good agreement between observed and predicted risks was observed in each geographical region cohort for THR. For those at highest risk of TKR (>10th decile), the observed risk was slightly higher than the predicted risk from the model in Northern Ireland, Scotland and Wales. We hypothesised that this slight miscalibration could result from systematic differences between these devolved nations and the English regions in the entry year or follow-up duration. However, on inspection, this was not the case (online supplementary figures S9 and S10).
Figure 3.
Assessment of calibration for model predicting 10-year risk of primary total hip replacement in validation cohorts.
Figure 4.
Assessment of calibration for model predicting 10-year risk of primary total knee replacement in validation cohorts.
The sensitivity analysis including patients with early outcomes (THR/TKR within 2 years of index consultation) gave similar levels of discrimination and calibration (online supplementary figure S11 and table S4). Similar model coefficients were derived by applying final predictors into patients with early outcomes (THR/TKR within 2 years of index consultation) (online supplementary table S5).
Clinical examples
Online supplementary tables S6 and S7 give clinical examples of the application of THR and TKR risk prediction models to predict 10-year risk of THR and TKR, with examples chosen to illustrate ‘low’ and ‘high’ risk.
Discussion
We have developed and validated two algorithms to predict the absolute risk of future primary THR and TKR in patients who newly present to UK general practice with hip pain/osteoarthritis and knee pain/osteoarthritis. Internal–external cross-validation showed consistently good calibration when models are applied to the different geographical regions, and the models have good discrimination with C-statistics of greater than 0.70 for both models. To our knowledge, these are the first such risk prediction tools, for primary THR and TKR in osteoarthritis developed in large-scale cohort data.
Strengths and limitations of study
Our study was based on a large, representative and contemporary UK population with data obtained from a validated research database.19 Risk prediction tools relying on routinely collected primary care data are more readily implementable in primary care practice10, and this was an important motivation for our study design. Potential limitations include missing predictor data and known predictors that are not measured or recorded in the primary care EHR. Around 5% of cohort participants did not have a recorded value for BMI in the 3 years prior to index pain/osteoarthritis consultation, but we found little difference in findings between complete dataset and multiple imputed datasets. We assumed no consultation record of a morbidity or prescription meant, there had been no such event within primary care. Although it is a fairly standard approach to use the most recent record for time-varying exposures that are likely to be generally stable, this approach might still be conservative (ie, underestimate the exposure–outcome association) to the extent that it misclassifies the exposure level relevant to the outcome (eg, lifetime cumulative exposure to smoking).
Primary THR and TKR are complex, multiply determined outcomes and can be considered as a composite measure of osteoarthritis progression, since these procedures are indicated for a combination of pain, functional disability, impact on quality of life, radiological changes and failed conservative treatment.36 Joint replacement is an important outcome of osteoarthritis, and this is reflected in its role when judging the validity of imaging-related primary endpoints for clinical trials of structure-modifying drugs.37 However, it is important to recognise that a proportion of individuals with progressive OA may not be offered, or accept, TKR/THR. The receipt of TKR/THR can also reflect extraneous factors such as patient age, sex, ethnicity, willingness to undergo surgery, comorbidity, patients’ needs, patients’ coping skills, physician effect and prevailing supply-side factors.38 We found adjusted rates of THR and TKR were lower given the following patient characteristics at or before index hip/knee consultation: age over 80–85 years, non-white ethnicity,39 higher levels of comorbidity (including diagnosed mental health disorder, generalised OA and low back pain) and very high levels of obesity. Osteoarthritis progression in the context of these factors will be underestimated by risk algorithms based on the outcome of receipt of primary joint replacement.
A strength of our study was the comprehensive identification of candidate predictors from a variety of sources including a novel hypothesis-free ‘ReWAS’ study with replication in an independent primary care EHR dataset. This latter technique yielded a small number of candidate predictors not previously reported (eg, arthroscopy). ReWAS also confirmed known prognostic factors or suggested prognostic factors that were most likely proxy markers for known predictors not obtainable from the EHR (eg, analgesic prescriptions as a proxy for pain severity). All such ‘hits’ had to be judged clinically relevant by review panel of clinicians and lay member in order to be included in the modelling stage. Unsurprisingly, many candidate predictors of future primary THR or TKR previously identified in the literature were not routinely available within the primary care record. These included multi-item patient-reported measures of pain severity,40 41 structural disease markers from plain X-rays or MRI40 42 and measures of occupational and leisure time physical activity.43–46 It is not known if their inclusion would significantly improve model performance.
Comparison with other studies
The majority of relevant previous studies have focused on one or more potential causal exposures for future joint replacement. We identified only three small studies that had previously derived and reported a multivariable prediction model for total hip or knee replacement based mainly on patient-reported and imaging variables.40 41 47 Although the overall performance of the prognostic model is of primary importance, the direction and magnitude of association between some of the included predictors and outcome deserves comment. It must be recognised though that these associations are not intended to be, and cannot be interpreted as, valid estimates of causal effect (total, direct or indirect) on primary hip/knee replacement: they are chosen for their informativeness in predicting primary THR/TKR. They may or may not be causal or reversible; all associations were conditioned on having an index consultation for hip or knee osteoarthritis/pain; each coefficient was adjusted for all covariates in the model, but the minimally sufficient set of covariates needed to adjust for confounding would likely differ for each (the ‘table 2 fallacy’48). With these concerns in mind, we note that adjusted rates of THR and TKR were lower among moderate/heavy current smokers49 and higher among those with a previous injury.50 Prior arthroscopic knee surgery was strongly associated with future TKR, an association which may include a very small direct causal effect51 but which otherwise we interpret as reflecting a mixture of disease severity, risk of future progression and willingness to undergo a surgical procedure for the knee.
Implications
Our newly developed risk algorithms could have important applications in clinical practice by helping direct annual monitoring, intensive non-surgical care and timely assessment and discussion of the need for surgical referral to those most at risk of progression. The algorithms can specifically identify the individuals who, in the context of current healthcare policies and resources, are at higher risk of future joint replacement, and therefore can be targeted for individual care ranging from earlier surgery to non-invasive care that might postpone the need for surgery. The hypothetical higher risk individual illustrated in online supplementary table S6 and S7 might, for instance, be targeted for a programme of more intensive multimodal therapy including graded supervised exercise and supported weight loss. The algorithm also uses future joint replacement as a proxy for future progression of osteoarthritis, and therefore potentially attempting to identify individuals more broadly who can be targeted for more intensive monitoring and interventions that might prevent such future progressions and severity regardless of whether they would actually have had a joint replacement. For each of these clinical activities, a more targeted approach based on risk of progression may help. Monitoring of patients with osteoarthritis for progression of symptoms and impact is regarded as an important aspect of quality of care,52 but current The National Institute for Health and Care Excellence guidance would result in this being applied to a very large number of patients.53 Consideration for joint replacement should only be made after proper conservative care.3 While consistent evidence supports the effectiveness for knee OA of supervised, individually tailored exercise programmes progressed over several visits,6 many patients do not receive this52 partly due to limited physiotherapy resource8 and lack of referral.7 For many patients, joint replacement will still be the most cost-effective intervention and an earlier recognition of patients’ risk of future joint replacement may facilitate more timely assessment and discussion of appropriateness for referral. However, we caution against over-reliance on these risk algorithms, particularly for individuals whose characteristics mean that they will not be candidates for surgery despite experiencing progressive disease, and against their crude application to ration what are highly cost-effective procedures of primary THR and TKR for osteoarthritis.
Conclusions
We have developed and validated two new risk prediction equations to quantify the absolute risks of primary THR and TKR in patients’ newly presenting with hip pain/OA or knee pain/OA in the primary care setting. The models have the advantage of being based on information routinely available in UK primary care EHR, making them potentially implementable for automatic risk calculation in electronic medical record software. They can be used to identify patients at high risk of end-stage OA for further assessments and intensive non-surgical intervention. The algorithms are readily amenable to further external validation in many developed countries that have routine records available for research. Further research is warranted to evaluate the clinical outcomes and cost effectiveness of using these risk equations in primary care.
Acknowledgments
The authors would like thank Professor Peter Croft and Professor Elaine Hay for insightful comments on the draft manuscript. This study is based in part on data from the Clinical Practice Research Datalink obtained under license from the UK Medicines and Healthcare Products Regulatory Agency. The interpretation and conclusions contained in this study are those of the authors alone.
Footnotes
Handling editor: Prof Josef S Smolen
Contributors: GMP, KPJ, and DY designed the study. KS and RR designed the validation approach. KS supervised the data analysis. CW as the PPI member attended each stage of the study (study design, reviewed the research protocol and predictors, review and revised the manuscript). DY performed the analysis and drafted the manuscript. JB, JE, KPJ, and GMP defined code lists for predictors and entry criteria, and outcome. JB, JE, CM, VT, VU, DPA, and CW supplemented predictors, reviewed and selected predictors. All authors interpreted the findings. All authors contributed to revision of the paper and have approved the final version. All authors were involved in the interpretation of the data, contributed towards critical revision of the manuscript, and approved the final draft.
Funding: This study was funded by NIHR School for Primary Care Research Funding Round 9 (Project No: 258) and by Public Health England. CDM is funded by the NIHR Collaborations for Leadership in Applied Health Research and Care West Midlands, the NIHR School for Primary Care Research and a NIHR Research Professorship in General Practice (NIHR-RP-2014-04-026). JE is a NIHR Academic Clinical Lecturer. The views expressed in this paper are those of the author(s) and not necessarily those of the NHS, the NIHR, Public Health England, or the Department of Health. This research is funded by the National Institute for Health Research School for Primary Care Research (NIHR SPCR).
Competing interests: None declared
Patient consent: Not required.
Ethics approval: This project was approved by the Independent Scientific Advisory Committee (reference number: 15_211) for the CPRD data.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. GBD 2015 DALYs and HALE Collaborators Global, regional, and nationaldisability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE),1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1603–58. 10.1016/S0140-6736(16)31460-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Verkleij SP, Hoekstra T, Rozendaal RM, et al. Defining discriminative paintrajectories in hip osteoarthritis over a 2-year time period. Ann Rheum Dis 2012;71:1517–23. 10.1136/annrheumdis-2011-200687 [DOI] [PubMed] [Google Scholar]
- 3. Collins JE, Katz JN, Dervan EE, et al. Trajectories and risk profiles of painin persons with radiographic, symptomatic knee osteoarthritis: data from the osteoarthritis initiative.OsteoarthritisCartilage 2014;22:622–30. 10.1016/j.joca.2014.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nicholls E, Thomas E, vander Windt DA,et al. Pain trajectorygroups in persons with, or at high risk of, knee osteoarthritis: findings from the Knee Clinical AssessmentStudy and the Osteoarthritis Initiative. Osteoarthritis Cartilage 2014;22:2041–50. 10.1016/j.joca.2014.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holla JF, vander Leeden M,Heymans MW, et al. Three trajectories of activitylimitations in early symptomatic knee osteoarthritis: a 5-year follow-up study. Ann Rheum Dis 2014;73:1369–75. 10.1136/annrheumdis-2012-202984 [DOI] [PubMed] [Google Scholar]
- 6. Bartlett SJ, Ling SM, Mayo NE, et al. Identifying common trajectories ofjoint space narrowing over two years in knee osteoarthritis. Arthritis Care Res 2011;63:1722–8. 10.1002/acr.20614 [DOI] [PubMed] [Google Scholar]
- 7. Losina E, Paltiel AD, Weinstein AM, et al. Lifetime medical costs of kneeosteoarthritis management in the United States: impact of extending indications for total knee arthroplasty.ArthritisCare Res 2015;67:203–15. 10.1002/acr.22412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lo T,Parkinson L, Cunich M, et al. A six-year trend of the healthcare costof arthritis in a population-based cohort of older women. International Journal for Population Data Science 2017;1:147 10.23889/ijpds.v1i1.166 [DOI] [Google Scholar]
- 9. Arden N, Richette P, Cooper C, et al. Can we identify patients with high risk ofosteoarthritis progression who will respond to treatment? a focus on biomarkers and frailty.DrugsAging 2015;32:525–35. 10.1007/s40266-015-0276-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hippisley-Cox J, Coupland C, Vinogradova Y, et al. Derivation and validation of QRISK, anew cardiovascular disease risk score for the United Kingdom: prospective open cohort study. BMJ 2007;335:136 10.1136/bmj.39261.471806.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hippisley-Cox J, Coupland C. Predicting risk of osteoporotic fracture in men and women inEngland and Wales: prospective derivation and validation of QFractureScores. BMJ 2009;339:b4229 10.1136/bmj.b4229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hippisley-Cox J, Coupland C. Derivation and validation of updated QFracture algorithm topredict risk of osteoporotic fracture in primary care in the United Kingdom: prospective open cohort study.BMJ 2012;344:e3427 10.1136/bmj.e3427 [DOI] [PubMed] [Google Scholar]
- 13. Hippisley-Cox J, Coupland C. Predicting risk of upper gastrointestinal bleed and intracranialbleed with anticoagulants: cohort study to derive and validate the QBleed scores. BMJ 2014;349:g4606 10.1136/bmj.g4606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hippisley-Cox J, Coupland C. Development and validation of risk prediction equations toestimate future risk of blindness and lower limb amputation in patients with diabetes: cohort study.BMJ 2015;351:h5441 10.1136/bmj.h5441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hippisley-Cox J, Coupland C, Brindle P. Derivation and validation of QStroke score for predicting risk ofischaemic stroke in primary care and comparison with other risk scores: a prospective open cohort study.BMJ 2013;346:f2573 10.1136/bmj.f2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hippisley-Cox J, Coupland C. Development and validation of risk prediction algorithm(QThrombosis) to estimate future risk of venous thromboembolism: prospective cohort study. BMJ 2011;343:d4656 10.1136/bmj.d4656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hippisley-Cox J, Coupland C. Predicting the risk of chronic Kidney Disease in men and women inEngland and Wales: prospective derivation and external validation of the QKidney Scores. BMC Fam Pract 2010;11:4911–229649. 10.1186/1471-2296-11-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hippisley-Cox J, Coupland C. Predicting risk of emergency admission to hospital using primarycare data: derivation and validation of QAdmissions score. BMJ Open 2013;3:e003482-2013-003482 10.1136/bmjopen-2013-003482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herrett E, Gallagher AM, Bhaskaran K, et al. Data Resource Profile: ClinicalPractice Research Datalink (CPRD). Int J Epidemiol 2015;44:827–36. 10.1093/ije/dyv098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jordan KP, Jöud A, Bergknut C, et al. International comparisons of theconsultation prevalence of musculoskeletal conditions using population-based healthcare data from Englandand Sweden. AnnRheum Dis 2014;73:212–8. 10.1136/annrheumdis-2012-202634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Culliford D, Maskell J, Judge A, et al. Future projections of total hip andknee arthroplasty in the UK: results from the UK Clinical Practice Research Datalink. OsteoarthritisCartilage 2015;23:594–600. 10.1016/j.joca.2014.12.022 [DOI] [PubMed] [Google Scholar]
- 22. Hawley S, Delmestri A, Judge A. Total Hip and Knee Replacement Among Incident Osteoarthritis andRheumatoid Arthritis Patients Within the UK Clinical Practice Research Datalink (CPRD) Compared to HospitalEpisode Statistics (HES): A Validation Study. Pharmacoepidemiol drug safety 2016;25:251. [Google Scholar]
- 23. Törner A, Dickman P, Duberg AS, et al. A method to visualize and adjust forselection bias in prevalent cohort studies. Am J Epidemiol 2011;174:969–76. 10.1093/aje/kwr211 [DOI] [PubMed] [Google Scholar]
- 24. Collins GS, Altman DG. Identifying patients with undetected colorectal cancer: an independent validation of QCancer (Colorectal). Br J Cancer 2012;107:260–5. 10.1038/bjc.2012.266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Collins GS, Altman DG. Identifying women with undetected ovarian cancer: independent andexternal validation of QCancer(®) (Ovarian) prediction model. Eur J Cancer Care 2013;22:423–9. 10.1111/ecc.12015 [DOI] [PubMed] [Google Scholar]
- 26. Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England andWales: population based cohort study using the QResearch database. BMJ 2010;340:c2197 10.1136/bmj.c2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analysesfor competing risk data. Stat Med 2017;36:4391–400. 10.1002/sim.7501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harrell FE, Califf RM, Pryor DB, et al. Evaluating the yield of medical tests.JAMA 1982;247:2543–6. 10.1001/jama.1982.03320430047030 [DOI] [PubMed] [Google Scholar]
- 29. Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of predictionmodels: a framework for traditional and novel measures. Epidemiology 2010;21:128–38. 10.1097/EDE.0b013e3181c30fb2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moons KG, Altman DG, Reitsma JB, et al. Transparent Reporting of amultivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration.AnnIntern Med 2015;162:W1–73. 10.7326/M14-0698 [DOI] [PubMed] [Google Scholar]
- 31. Royston P, Parmar MK, Sylvester R. Construction and validation of a prognostic model across severalstudies, with an application in superficial bladder cancer. Stat Med 2004;23:907–26. 10.1002/sim.1691 [DOI] [PubMed] [Google Scholar]
- 32. Debray TP, Moons KG, Ahmed I, et al. A framework for developing, implementing, and evaluating clinical prediction models in an individual participant data meta-analysis.StatMed 2013;32:3158–80. 10.1002/sim.5732 [DOI] [PubMed] [Google Scholar]
- 33. Riley RD, Ensor J, Snell KI, et al. External validation of clinicalprediction models using big datasets from e-health records or IPD meta-analysis: opportunities andchallenges. BMJ 2016;353:i3140 10.1136/bmj.i3140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidancefor practice. StatMed 2011;30:377–99. 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 35. Peduzzi P, Concato J, Feinstein AR, et al. Importance of events per independentvariable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates.JClin Epidemiol 1995;48:1503–10. 10.1016/0895-4356(95)00048-8 [DOI] [PubMed] [Google Scholar]
- 36. Gademan MGJ, Hofstede SN, VlietVlieland TPM,et al. Indicationcriteria for total hip or knee arthroplasty in osteoarthritis: a state-of-the-science overview.BMCMusculoskelet Disord 2016;17:463-016-1325-z 10.1186/s12891-016-1325-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reginster JY, Reiter-Niesert S, Bruyère O, et al. Recommendations for an update of the2010 European regulatory guideline on clinical investigation of medicinal products used in the treatment ofosteoarthritis and reflections about related clinically relevant outcomes: expert consensus statement.OsteoarthritisCartilage 2015;23:2086–93. 10.1016/j.joca.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 38. McAlindon TE, Driban JB, Henrotin Y, et al. OARSI Clinical Trials Recommendations:Design, conduct, and reporting of clinical trials for knee osteoarthritis. OsteoarthritisCartilage 2015;23:747–60. 10.1016/j.joca.2015.03.005 [DOI] [PubMed] [Google Scholar]
- 39. Smith MC, Ben-Shlomo Y, Dieppe P, et al. Rates of hip and knee joint replacementamongst different ethnic groups in England: an analysis of National Joint Registry data. OsteoarthritisCartilage 2017;25:448–54. 10.1016/j.joca.2016.12.030 [DOI] [PubMed] [Google Scholar]
- 40. Cicuttini FM, Jones G, Forbes A, et al. Rate of cartilage loss at two yearspredicts subsequent total knee arthroplasty: a prospective study. Ann Rheum Dis 2004;63:1124–7. 10.1136/ard.2004.021253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gossec L, Tubach F, Baron G, et al. Predictive factors of total hipreplacement due to primary osteoarthritis: a prospective 2 year study of 505 patients. Ann Rheum Dis 2005;64:1028–32. 10.1136/ard.2004.029546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bruyere O, Cooper C, Pavelka K, et al. Changes in structure and symptoms inknee osteoarthritis and prediction of future knee replacement over 8 years. Calcif Tissue Int 2013;93:502–7. 10.1007/s00223-013-9781-z [DOI] [PubMed] [Google Scholar]
- 43. Flugsrud GB, Nordsletten L, Espehaug B, et al. Risk factors for total hip replacementdue to primary osteoarthritis: a cohort study in 50,034 persons. Arthritis Rheum 2002;46:675–82. 10.1002/art.10115 [DOI] [PubMed] [Google Scholar]
- 44. Michaëlsson K, Byberg L, Ahlbom A, et al. Risk of severe knee and hiposteoarthritis in relation to level of physical exercise: a prospective cohort study of long-distanceskiers in Sweden. PLoSOne 2011;6:e18339 10.1371/journal.pone.0018339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ageberg E, Engström G, Gerhardssonde Verdier M,et al. Effect ofleisure time physical activity on severe knee or hip osteoarthritis leading to total joint replacement: apopulation-based prospective cohort study. BMC Musculoskelet Disord 2012;13:7313–247473. 10.1186/1471-2474-13-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Johnsen MB, Hellevik AI, Baste V,et al. Leisure timephysical activity and the risk of hip or knee replacement due to primary osteoarthritis: a population basedcohort study (The HUNT Study). BMC Musculoskelet Disord 2016;17:86-016-0937-7 10.1186/s12891-016-0937-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chan WP, Hsu SM, Huang GS, et al. Creation of a reflecting formula todetermine a patient's indication for undergoing total knee arthroplasty. J Orthop Sci 2010;15:44–50. 10.1007/s00776-009-1418-8 [DOI] [PubMed] [Google Scholar]
- 48. Westreich D, Greenland S. The table 2 fallacy: presenting and interpreting confounder andmodifier coefficients. Am J Epidemiol 2013;177:292–8. 10.1093/aje/kws412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Johnsen MB, Vie GÅ, Winsvold BS, et al. The causal role of smoking on the riskof hip or knee replacement due to primary osteoarthritis: a Mendelian randomisation analysis of the HUNTstudy. OsteoarthritisCartilage 2017;25:817–23. 10.1016/j.joca.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 50. Muthuri SG, McWilliams DF, Doherty M, et al. History of knee injuries and kneeosteoarthritis: a meta-analysis of observational studies. Osteoarthritis Cartilage 2011;19:1286–93. 10.1016/j.joca.2011.07.015 [DOI] [PubMed] [Google Scholar]
- 51. Brignardello-Petersen R, Guyatt GH, Buchbinder R, et al. Knee arthroscopy versus conservativemanagement in patients with degenerative knee disease: a systematic review. BMJ Open 2017;7:e016114-2017-016114 10.1136/bmjopen-2017-016114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Edwards JJ, Jordan KP, Peat G, et al. Quality of care for OA: the effect of apoint-of-care consultation recording template. Rheumatology 2015;54:844–53. 10.1093/rheumatology/keu411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. National Institute for Health andClinical Excellence (NICE) Osteoarthritis care and management in adults.UK: National Institute for Health and Clinical Excellence (NICE),2014. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2018-213894supp001.docx (2.9MB, docx)
annrheumdis-2018-213894supp002.docx (213KB, docx)