SUMMARY
The morphological events forming the body’s musculature are sensitive to genetic and environmental perturbations with high incidence of congenital myopathies, muscular dystrophies and degenerations. Pattern formation generates branching series of states in the genetic regulatory network. Different states of the network specify pre-myogenic progenitor cells in the head and trunk. These progenitors reveal their myogenic nature by the subsequent onset of expression of the master switch gene MyoD and/or Myf5. Once initiated, the myogenic progression that ultimately forms mature muscle appears to be quite similar in head and trunk skeletal muscle. Several genes that are essential in specifying pre-myogenic progenitors in the trunk are known. Pax3, Lbx1, and a number of other homeobox transcription factors are essential in specifying pre-myogenic progenitors in the dermomyotome, from which the epaxial and hypaxial myoblasts, which express Myogenic Regulatory Factors (MRFs), emerge. The proteins involved in specifying pre-myogenic progenitors in the head are just beginning to be discovered and appear to be distinct from those in the trunk. The homeobox gene Pitx2, the T-box gene Tbx1, and the bHLH genes Tcf21 and Msc encode transcription factors that play roles in specifying progenitor cells that will give rise to branchiomeric muscles of the head. Pitx2 is expressed well before the onset of myogenic progression in the 1st branchial arch mesodermal core and is essential for the formation of 1st branchial arch derived muscle groups. Anterior-posterior patterning events that occur during gastrulation appear to initiate the Pitx2 expression domain in the cephalic and branchial arch mesoderm. Pitx2 therefore contributes to the establishment of network states, or kernels, that specify pre-myogenic progenitors for extraocular and mastication muscles. A detailed understanding of the molecular mechanisms that regulate head muscle specification and formation provides the foundation for understanding congenital myopathies. Current technology and mouse model systems help to elucidate the molecular basis on etiology and repair of muscular degenerative diseases.
Keywords: embryonic developemnt, muscle, transctiption factor, homeobox, branchial arch, somites
Trunk Myogenesis
Skeletal muscles of the trunk derive from paraxial somitic mesoderm, whereas skeletal muscles of the head derive mostly from the pre-chordal and non-somitic paraxial head mesoderm. Somites are epithelial structures derived from the paraxial mesoderm that receive signals from the local environment and differentiate into the dorsal dermomyotome and the ventral sclerotome (Aoyama and Asamoto, 1988; Denetclaw et al., 1997; Cheng et al., 2004). The dermomyotome appears to be further subdivided by partially overlapping expression patterns of homeodomain transcription factors. The dorsomedial dermomyotome gives rise to the epaxial, or primaxial, muscles, which include the deep muscles of the back. The ventrolateral dermomyotome gives rise to the hypaxial, or abaxial, muscles, which include the appendicular and abdominal body wall muscles (Christ and Ordahl, 1995; Burke and Nowicki, 2003).
Both appendicular and abdominal muscles are derived from molecularly specified muscle progenitor cells in the ventrolateral dermomyotome that are distinct from those in the myotome. Specified appendicular pre-myogenic progenitors delaminate from the ventrolateral dermomyotome of limb level somites, migrate into the lateral plate mesoderm-derived limb mesenchyme as it is being patterned, and re-aggregate in specific locations to form the muscle anlagen that presage the distinct muscles observed in adults. During this process they turn on MyoD and Myf5 to initiate the myogenic progression. Hypoglossal muscles of the tongue and the diaphragm muscles also come from this source of migratory progenitors. At abdominal levels the specified pre-myogenic progenitors proliferate without delaminating and thereby cause the dermomyotome to expand ventrally into the lateral plate mesoderm-derived somatopleure. The onset of the myogenic progression occurs during this expansion. The somatopleure and splanchnopleure are derivatives of the lateral plate mesoderm, which has been demonstrated to provide inductive cues for hypaxial muscle specification in chick embryos (Dietrich et al., 1998; Dietrich et al., 1999; Alvares et al., 2003; Mootoosamy and Dietrich, 2002).
The events that form the epaxial, or primaxial, musculature are less well characterized, but involve molecular specification in the myotome and dorsomedial dermomyotome prior to migration and growth (Cheng et al., 2004). A first wave of cells from the dorsomedial lip of the dermomyotome forms the early myotome, which consists of post-mitotic cells and which is the first structure of the body to express myogenin (Pownall et al., 1996). Cells from the dermomyotome continue to colonize the myotome in a second wave. These cells proliferate in the myotome, begin to express MyoD or Myf5, and steadily produce postmitotic muscle cells (Kahane et al., 1998a, b). As the myotome matures, it gives rise to deep muscles of the back. The intercostal and suspension muscles, which connect the limbs to the body (rhomboids, trapezii, pectorals, latissimus dorsii), are thought to be derived from a mixture of primaxial and abaxial precursors (Burke and Nowicki, 2003).
Head Myogenesis
Head muscles fall into the axial, laryngoglossal, branchial, and extraocular muscle (EOM) categories (Noden and Francis-Wesr, 2006). The axial muscles derive from the pre-otic somites, are located in the neck, and move the head. The laryngoglossal muscles derive from the pre-otic somites and branchial arch (BA) mesoderm and contribute to the movement of the muscles of larynx, mouth and tongue. The branchial muscles attach to the mandible, maxilla and pharynx. The 1st BA gives rise to mandibular adductors, intermandibular muscles, suprahyoid muscles, and at least two EOMs. The 2nd BA gives rise to mandibular depressors, stapedial muscle and facial expression muscles. The 3rd BA gives rise to the constrictor and stylopharyngeal muscles (Noden and Trainor, 2005). The EOMs derive from the prechordal and 1st BA mesoderm and move the eye.
Myogenic Progression
Networks of transcription factors are required for molecular specification and myogenic progression in both trunk and head muscle development. The MRFs include MyoD, Myf5, Myogenin and MRF4/myf6/herculin, are bHLH transcription factors, and control myogenic progression. MRFs activate transcription by heterodimerizing with ubiquitously expressed E-proteins to bind a consensus DNA motif (E box) (Lassar et al., 1991). MyoD and Myf5 have distinct regulatory functions in differentiation and maintenance of muscle progenitor lineages (Emerson et al., 1990). MyoD expression in the more lateral region of the myotome is controlled by signals from the dorsal ectoderm and can promote myoblast differentiation by activating Myogenin and Myocyte Enchancer Factor 2 (MEF2). Myogenin and MRF4 control the terminal differentiation of myoblasts into myotubes (Edmonson and Olson, 1989; Wright et al., 1989) and have cooperative functions with MyoD and Myf5 for the activation of contractile proteins (Charbonnier et al., 2002). MyoD and MEF2 act cooperatively to regulate the expression of skeletal muscle genes. Both these factors are associated with histone acetyltransferases (HATs) and histone deacetylases (HDACs) to control the activation and the repression of the muscle differentiation program respectively. Signaling systems that regulate the growth and differentiation of muscle cells act by regulating the intracellular localization and associations of these chromatin-remodeling enzymes with myogenic transcription factors (McKinsey et al., 2001). The acetyltransferase p300 activity is required for the activation of, and cooperates with MyoD and Myf5, to arrest the cell cycle and to mediate activation of Myogenin and MRF4 (Roth et al., 2003; Puri et al., 1997; Eckner et al., 1996). MyoD and Myf5 specify myogenic identity of uncommitted somitic mesoderm cells. Myogenin and MRF4 in conjunction with MEF2 proteins, allow myoblasts to exit cell cycle and to differentiate into polynucleated myocytes and mature microfibers (Buckingham, 2001). This process requires both repression of genes associated with proliferation and activation of muscle specific genes.
Specification of appendicular pre-myogenic progenitors
Although myogenic progression is similar in trunk and head muscle groups, specification of the myogenic lineage appears to be distinct (Mootoosamy and Dietrich, 2002). Pax3 and Lbx1 have generally been placed prior to myogenic progression in the embryonic limb because they are expressed earlier and their mutations lead to a loss of migratory precursors before MRFs are normally expressed (Goulding et al., 1994; Bober et al., 1994; Mennerich et al., 1998; Schäfer and Braun, 1999; Gross et al., 2000). Forced expression of Lbx1 and Pax3 increases both proliferation and differentiation in muscle cell cultures, explants, and limb buds (Mennerich and Braun, 2001; Maroto et al., 1997, Epstein et al., 1995). Ablation of Pax3 (Bober et al., 1994; Goulding et al., 1994; Williams and Ordahl, 1994) or Lbx1 (Mennerich et al., 1998, Dietrich et al., 1999; Gross et al, 2000) leads to the loss of limb muscle precursors and limb muscle. In Pax3 mutants, Splotch (Sp, Spd), migrating pre-myogenic progenitor cells were not observed between the epithelial somite and the limb bud at early stages and all hypaxial muscles are missing at late stages (Franz et al., 1993: Goulding et al., 1994). Specification of pre-myogenic limb muscle progenitors appears to be linked to expression of Lbx1 and c-met within the ventrolateral Pax3 expression domain of dermomyotomes at limb levels (Dietrich et al., 1998; Gross et al., 2000). Lbx1 expression in mice begins in the dermomyotome lips at E9.25 at forelimb levels, and is required for lateral migration (Gross et al., 2000). These cells also express Msx1, but not MyoD or Myf5, at the earliest phase of migration (Houzelstein et al., 1999). Numerous Lbx1+/Pax3+ cells persist in all limb muscle anlagen until at least E12.5. In the period between E11 and E12.5 the muscle masses enlarge, split and ultimately become the muscle anlagen, which resemble the adult muscles in shape and position with respect to bone anlagen. Thus, the Pax3/Lbx1, MyoD and myogenin proteins mark phases prior to and during myogenic progression rather than discrete anlagen in the limb bud. In addition to Pax3, several other homeobox genes including Msx1, Pax7 and Lbx1 can activate myoblastic differentiation by regulating the MRFs (Epstein et al., 1995: Schafer and Braun, 1999).
Head muscle anlagen do not follow the same specification cues as somite-derived muscles (Mootoosamy and Dietrich, 2002). Four transcription factors are currently invoked to explain specification of the head muscles. Initiation of branchiomeric myogenesis in the 1st BA requires by the homeobox transcription factor Pitx2. Pitx2 mutant mice fail to develop EOM and mastication muscles (MAM). Pitx2 is required for the expression of the pre-myogenic specification markers Tbx1, Tcf21 and Msc in the 1st but not 2nd BA (Shih et al., 2007b). Initiation of the branchiomeric myogenesis in the 2nd BA is regulated by Tbx1, which regulates Myf5 and MyoD and maintains Fgf10 expression. The absence of Tbx1 results in sporadic branchiomeric myogenesis (Kelly et al., 2004). Both bHLH repressors Tcf21 and Msc are also required for activation of Myf5 in the head muscles. Double Tcf21 and Msc mutants (Tcf21−/−/Msc−/−) fail to develop MAM (Lu et al., 2002). The phenotype observed in the head musculature in the Tcf21−/−/Msc−/− mice resembles the MyoD−/−/Myf5−/− phenotype in all skeletal muscles (Rudnicki et al., 1993).
During development, a variety of inductive cues initiate transitions between the network states that define cell types. It appears that the distinct network states that define pre-myogenic progenitors in the head and trunk are set up by different inductive cues, as one would expect. Signals from the neural tube and notochord, including Wnt and Shh can activate Myf5 and MyoD in the epaxial myotome (Munsterberg et al., 1995; Tajbakhsh et al., 1998; Gustafsson et al., 2002). Signals from the lateral plate, BMP, can delay the activation of MRFs in the hypaxial lineages (Pourquie et al., 1996). However, head myogenesis is blocked by these signals. Formation of head muscle is inhibited in a trunk context and formation of trunk muscles is inhibited in a head context. Myogenesis can by induced in the head by the BMP inhibitors Noggin and gremlin, and the Wnt inhibitor Frzb (Tirosh-Finkel et al., 2006).
Pitx2 and Myogenesis
Pitx2 is a bicoid–related homeobox gene that is asymmetrically expressed in the lateral plate mesoderm and is regulated by the Nodal/lefty pathway (Gage and Camper 1997; Campione et al., 1999; Piedra et al., 1998; Logan et al., 1998;Yoshioka et al., 1998; Meno et al., 1998; Ryan et al., 1998). Pitx2 mutations (4q25–q26) cause Axenfeld-Rieger (AR, RIEG1) syndrome, an autosomal-dominant disorder, characterized by ocular abnormalities, dental hypoplasia, craniofacial dysmorphism, umbilical and heart defects (Semina et al., 1996) and myopathy (Summit et al, 1971). Ablation of all three Pitx2 isoforms (Pitx2abc−/−) (Gage et al., 1999; Kitamura et al., 1999; Lin et al., 1999; Lu et al., 1999) causes lethality in mouse at E10.5-E14.5 with axial malformations, open body wall, laterality and heart defects, and arrest of organ development. Pitx2 appears to be a downstream target of general growth factor signaling pathways that mediate cell-type specific control of proliferation. The Wnt1/Dvl2/ß-catenin/Pitx2 pathway controls proliferation of myoblasts by regulating the expression of critical G1 cell cycle control genes. Growth factor-dependent signaling results in release of Pitx2-associated co-repressors and mediates recruitment of specific co-activator complexes (Kioussi et al., 2002; Baek et al, 2002). Three independent events underlie Pitx2-specific activation of myoblast proliferation, Wnt-dependent activation of Pitx2, Wnt and growth factor-dependent relief of Pitx2 repression activity and serial recruitment of series of specific coactivator complexes that act in a promoter manner. Pitx2 is a positive regulator of FGF8 and a repressor of BMP4 signaling during mandibular organogenesis (Liu et al., 2003).
Formation of the Extraocular Muscles (EOM)
Pre-myogenic markers are distinct not only between trunk and head muscles but also amongst head muscles. EOM include the levator palpebrae superioris, which elevates the upper lid, and six muscles capable of rotating the eye in all directions. These include the four recti, ventral, medial, lateral and dorsal, and two obliqui, superior and inferior. The levator palpebrae superioris, obliquus inferior, and recti superior, inferior and medialis are innervated by the occulomotor nerve, the obliquus superior by the trochlear, and the rectus lateralis by the abducent nerve. All EOM work together for the continuous movement of the eye that is essential for vision. The lateral rectus and the dorsal oblique derive from the unsegmented paraxial head mesoderm, while the remaining recti and obliqui derive from the prechordal head mesoderm. EOM are characterized molecular profiles that are distinct from other head muscle groups (Porter et al., 2001; Mootoosamy and Dietrich, 2002).
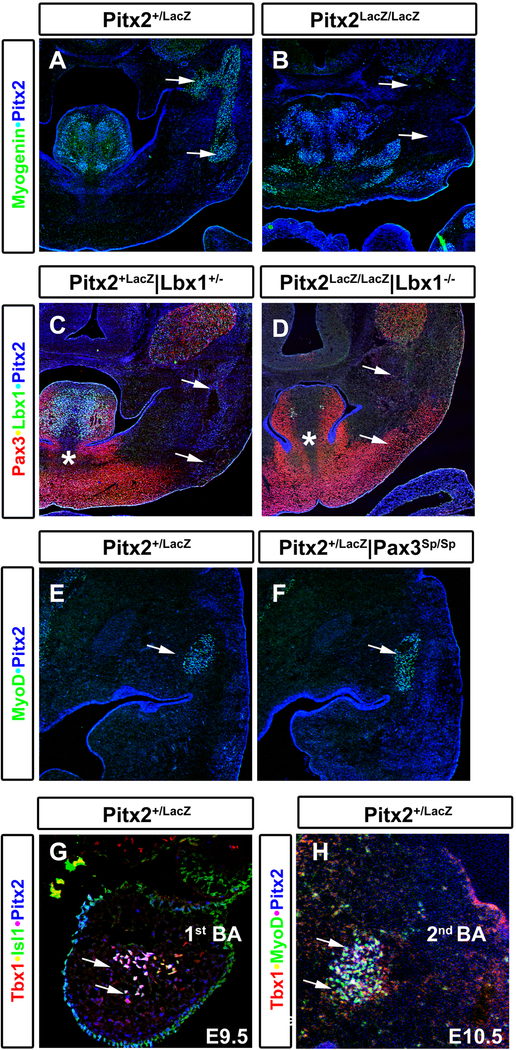
Pitx2abc−/− mice characterized by loss of EOMs, agenesis of corneal endothelium and stoma (Kitamura et al., 1999; Gage et al., 1999; Lu et al., 1999). Pitx2 is expressed strongly at E8.25 in the cephalic mesenchyme (Shih et al, 2007a; Fig. 3), which is the primordium for the cephalic and 1st BA mesoderm. Cephalic mesoderm gives rise to EOM anlagen. At later developmental stages, Pitx2 is expressed both in the mesoderm and neural crest cell populations that surround the eye, (Gage et al., 2005). Later still, it marks all EOM anlagen (Kitamura et al., 1999; Gage et al., 2005). Pitx2 is co-expressed with the myogenic bHLH marker myogenin (Fig. 1A) in newly formed EOM in Pitx2LacZ/+ E12.5 mouse embryos. Myogenin+/Pitx2+ EOM cells are not observed in Pitx2LacZ/LacZ mutants (Fig. 1B), which do not develop EOM at later stages. The homeodomain proteins that contribute to specification of pre-myogenic progenitors of trunk hypaxial muscles, Pax3 and Lbx1, were not observed in head mesoderm and derived muscles (Fig. 1C) and, as expected, Pax3 mutant mice, Pax3Sp/Sp, appear to have normal EOM development (Fig. 1D). However, Pax3Sp/Sp are characterized by absence of all hypaxial muscles (Tremblay et al., 1998) and Lbx1 mutants are characterized by loss of limb muscles (Gross et al., 2000).
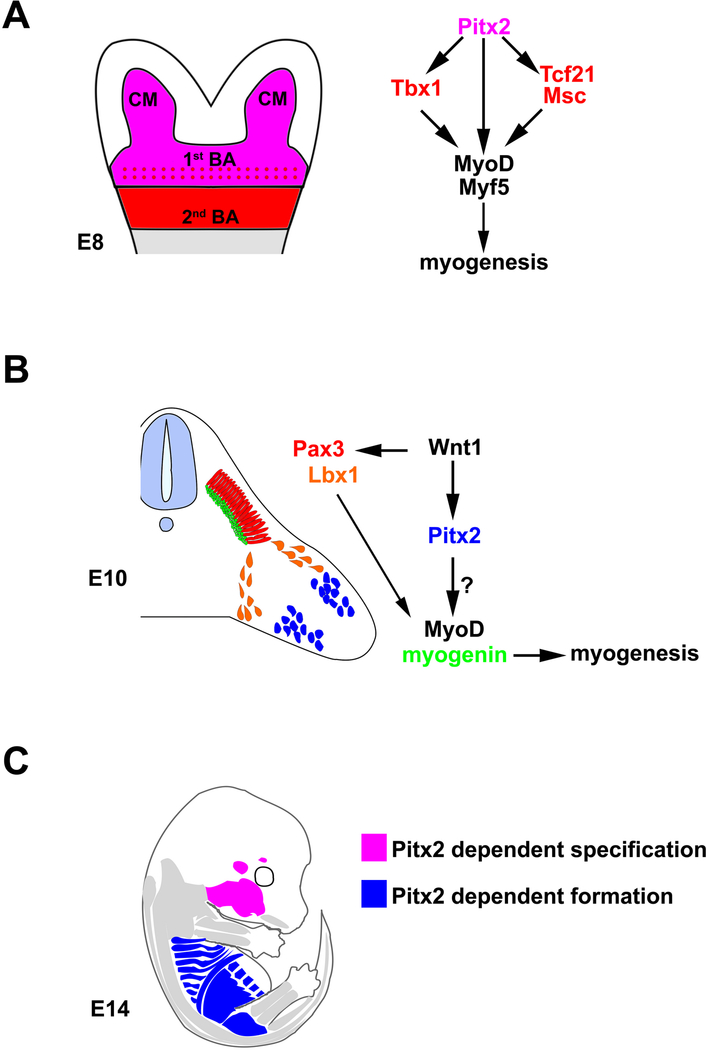
Figure 3. (A) Pitx2 specifies cephalic and 1st BA derived muscles.
Pitx2 is strongly expressed in head and 1st BA mesoderm, in the pre-myogenic progenitors before muscle specification at E8. Pitx2 controls the transcription factors Tbx1, Tcf21, and Msc, which specify the pre-myoblast precursors and activate myogenic progression in head mesoderm. Loss of functional Pitx2 results in downregulation of these transcription factors in the 1st BA mesoderm and failure of muscle specification. Consequently, un-specified pre-myoblast precursors undergo into apoptosis, which leads to the loss of most, if not all, 1st BA muscles. (B) Pitx2 regulates somatic-derived muscles. Pitx2 is expressed in somites at E10, after the muscle precursor specification and the onset of Pax3, indicating it is not required for muscle specification. Although the formation of limb muscles seems to be unaffected in Pitx2 mutant mice, Affymetrix microarray studies show that Pitx2 represses expression of Pax3 and Lbx1 and activates expression of Tbx1, Pitx1, Six2, and Sox6 in pre-myogenic progenitors in the developing limbs. Consequently, myogenic progression is locally altered in Pitx2 mutants. It suggested that Pitx2 mediates a potential pathway that is parallel with classic myogenic progression pathway in controlling myogenesis transition. The majority of intercostal, diaphragm, and abdominal muscles are remarkably deformed suggesting that Pitx2 palys an important role in forming these muscles. However, it is unclear whether Pitx2 manipulates muscle formation by controlling myogenesis in this area. Because Pitx2 mutant mice also display severe body wall and body axial turning phenotypes, the deformities of body wall muscles could also due to a secondary effect of global patterning defect. (C) Pitx2-modulated head and trunk muscles. Pitx2 has distinct functions in different muscle groups related to their embryonic origin. Not affected muscles in Pitx2 null mutant mice, Pitx2 independent, are indicated in grey. Affected muscles in Pitx2 null mutant mice are indicated in magenta and blue. The Pitx2-dependent specification muscles, exraocular and mastication muscles are indicated in magenta. The Pitx2-dependent formation muscles, intercostal, diaphragm and abdominal muscles are indicated in blue. (BA) branchial arch, (CM) cephalic mesoderm
Figure 1. Loss of Extraocular Muscles in Pitx2 Mouse Mutants.
Triple and double labeling immunohistochemistry visualized by confocal miscroscopy on cross sections of E12.5 Pitx2LacZ/+, Pitx2LacZ/Lacz and Pax3Sp/Sp mutant mice. (A) Extraocular muscles are positive for Pitx2 and Myogenin in Pitx2 heterozygote mice Pitx2LacZ/+. (B) The same cells are absent in the Pitx2 mutant mice Pitx2LacZ/Lacz. (C) Pitx2 is expressed in extraocular muscles but neither Lbx1 nor Pax3. (D) Pax3 does not affect the formation of extraocular muscles. The Pax3 mutant mice Pax3Sp/Sp exhibit normal extraocular muscles. The MyoD+(green) /Pitx2+(ß-galactosidase, blue) cells are present in the mutant mice. Arrows indicate the same muscle in all sections.
The two obliqui, superior and inferior are more sensitive to Pitx2 signals than the rectii. Microarray analysis form Pitx2abc mutants and wild type EOM indicated the Pitx2 acts upstream of the muscle-specific transcription factor genes Myf5, Myog, Myod1, Smyd1, Msc, and Csrp3 (Diehl et al., 2006). The forkhead transcription factor Foxc1 is also expressed in the cranial mesoderm and neural crest cells (Gage et al., 2005). Pitx2 expression in the periocular mesoderm precedes Foxc1, which begins to be expressed at E9.5 (Kume et al., 1998). Foxc1 mutant mice are characterized with a disorganized cornea (Kume et al., 1998; Smith et al., 2000). Foxc1 lies in the 6p25 forkhead cluster and is associated with ocular anterior segment developmental defects (Lehmann et al., 2000). Thus, Pitx2 marks the EOM lineage and is essential for its specification and maintenance by controlling the myogenic gene network in the periocular mesenchyme.
Formation of the Mastication Muscles (MAM)
Some EOM and the MAM that connect maxilla and mandible to other regions of the skull are derived from the 1st BA. Mandibular muscles or jaw closure muscles became specialized later in evolution and operate in capturing and manipulating food. They include the masseter, temporalis, lateral and medial pterygoid in mammals. MAMs derive from the first branchial arch mesoderm and are innervated by the trigeminal ganglion.
Distinct transcription factors have been identified as specific regulators of head but not trunk myogenesis. The bHLH repressor Msc and Tcf21 are transiently expressed in migratory paraxial mesodermal cells of 1st and 2nd BA. Double mouse mutants, Tcf21−/−/Msc−/− are characterized by absence and severe reduction of early Myf5 and MyoD expression in the 1st BA, respectively. They fail to develop MAM (Lu et al., 2002). The homeobox transcription factor Tbx1 is expressed in the premyogenic mesoderm of the first and 2nd BA, before the onset of MRF expression (Kelly et al., 2004); Fig. 3). Tbx1−/− mice characterized by loss of the Myf5+/MyoD+ cells but not Tcf21+ cells, suggesting that Tbx1 affects the maturation of the BA myoblasts and formation of some head muscles by activating MRFs (Kelly et al., 2004). Tbx1 is activated by retinoic acid and Shh signals via Fox transcription factor, and regulates Fgf10 (Garg et al., 2001).
Pitx2 is expressed in the muscle anlagen in all stages of myogenic progression (Shih et al., 2007a). Pitx2 can regulate cell proliferation in myoblast cells lines by controlling the cell cycle machinery (Kioussi et al., 2002) and modulating MRFs expression cell autonomously (Diehl et al., 2006; von Scheven et al., 2006). Pitx2 is expressed in the Myogenin+ cells in the jaw (Fig. 2A, arrows) and when Pitx2 is mutated these muscles are missing (Fig. 2B, arrows). These Pitx2+/Myogenin+ jaw muscles do not express Pax3 nor Lbx1 and as expected Pax3 and Lbx1 do not contribute to their formation (Fig. 2C, D, arrows). Pitx2 is expressed prior the onset of cephalic myogenesis but after the onset of somitic myogenesis (Shih et al., 2007b). The BA mesoderm gives rise to the non-somite-derived branchiomeric muscles. Pitx2 is expressed in the 1st BA mesoderm (Pitx2+/Tbx1+) at E9.5 before the onset of MyoD (Fig. 2G). At E10.5 Pitx2 is co-expressed with Tbx1 and MyoD (Pitx2+/Tbx1+/MyoD+) in the 1st and 2nd BA (Fig. 2H). Pitx2 specifies the 1st BA prior to the specification of muscle anlagen from the mesodermal core by activating Tbx1, Tcf21 and Msc (Shih et al., 2007b). Loss of Pitx2 results to loss of 1st BA and deformation of the 2nd BA muscle anlagen, which results to loss of MAM (Dong et al., 2006; Shih et al., 2007b; Fig. 2A, B). Both Pax3 and Lbx1 do not contribute to the formation of head muscles. The Pitx2+/MyoD+ MAM are present in the Pax3Sp/Sp (Fig. 2F) and Lbx1 (Brohmann et al., 2000; Gross et al., 2000; and data not shown) mutant mice. However, both genes contribute to the formation of tongue muscles (Fig. 2C, D; Franz et al., 1993; Bladt et al., 1995; Tajbakhsh et al., 1997). Pitx2 and Tbx1 are molecular partners in different developmental fields including cranial, limb and heart muscle lineages and they are in the same genetic pathway during cardiac development (Nowotschin et al., 2006). Pitx2 is not only required but is also sufficient to activate Tbx1 in the 1st BA (Shih et al., 2007b).
Figure 2. Loss of Masticatory Muscles in Pitx2 Mutants.
Triple and double labeling immunohistochemistry visualized by confocal miscroscopy on frontal head sections of E12.5 Pitx2 heterozygote Pitx2LacZ/+, Pitx2 mutant Pitx2LacZ/Lacz, Lbx1 mutant Lbx1−/− and Pax3 mutant Pax3Sp/Sp mice. (A) Pitx2 positive (ß-Gal) and Myogenin positive cells are present in mastication (arrows) and tongue muscles in the Pitx2 heterozygote mice Pitx2LacZ/+. (B) the same cells are absent in the mastication muscles of the maxilla region in the Pitx2 mutant Pitx2LacZ/LacZ mouse. Additionally, some of the tongue muscles are distorted. (C) Lbx1 and Pax3 are not expressed in the Pitx2+(ß-Gal) mastication muscles but are co-expressed in some of the tongue muscles. (D) Loss of the mastication muscles in the double mutant mice for Pitx2 and Lbx1, Pitx2LacZ/Lacz/Lbx1−/− mice is due to Pitx2 function. However, the loss of some tongue muscles is due to Lbx1 function (asterisk). (E, F) The Pitx2 positive and MyoD positive (Pitx2+/MyoD+) mastication muscles are present in the Pax3 mutant Pax3Sp/Sp mice. (G) Pitx2 is co-expressed with mesodermal markers Tbx1 and Islet1 in the 1st BA at E9.5. (H) Pitx2 is co-expressed with the muscle markers Tbx1 and MyoD in the 2nd BA at E10.5. V: trigeminal ganglion; tg: tongue.
Different pre-myoblastic regions of the embryo require different combinations of transcription factors to activate either Myf5 or MyoD and initiate myogenic progression. Myogenic progression appears to be a common process, or plug-in, that cells can activate if the pattern formation process creates appropriate network states in them. Various distinct network states have the ability to activate either MyoD or Myf 5 and thereby initiate the plug-in called the myogenic progression. Myf5, the first myogenetic marker, employs different enhancer elements for head and trunk myogenesis (Summerbell et al., 2000; Hadchouel et al., 2000). Pitx2 specifies the specifies pre-myoblast mesoderm and initiates myogenic progression in the cephalic mesoderm and 1st BA mesoderm by activating the pre-myoblast specification markers, Tbx1, Tcf21 and Msc and contributes to the earliest specification of jaw and development of EOM and MAM (Fig. 3, Table 1).
Table 1:
Transcription Factors in Muscle Lineages
| Transcription Factor | Family | Expression Pattern | Function | Reference |
|---|---|---|---|---|
|
Pitx2 Otlx2, Brx1, Ptx2 |
HD | muscle anlagen 1st and 2nd BA muscles (E8) body and limb muscles (E10) |
specification of EOM specification of MAM |
Kitamura et al., 1999 Dong et al., 2006 Gage et al., 2005 Shih et al., 2007a, b |
|
Tbx1 T-box protein 1 |
T-box | 1st and 2nd BA muscles (E9) limb muscles (E10) |
formation of 1st and 2nd BA muscles | Kelly et al., 2004 |
|
Tcf21 capsulin, Pod-1 |
bHLH | 1st and 2nd BA muscles (E9) | formation 1st BA muscles | Lu et al., 2002 |
|
Msc MyoR, musculin |
bHLH | 1st and 2nd BA muscles (E9) limb muscles (E10) |
formation 1st BA muscles | Lu et al., 2002 |
|
En2 engrailed 2 |
HD | 1st BA muscles (E9.5) | ND |
Joyner and Martin, 1987 Degenhardt et al., 2001 Degenhardt et al., 2002 |
|
Pax3 paired box gene 3 |
HD | somitic muscles | specification of somitic muscles delamination of limb muscle progenitors |
Franz et al., 1993 Goulding et al., 1994 Bober et al., 1994 Williams and Ordahl, 1994 Bladt et al., 1995 Tajbakhsh et al., 1997 |
|
Lbx1 ladybird homeobox homolog 1 |
HD | migratory limb muscle progenitors hypoglossal muscles |
delamination of limb muscle progenitors |
Mennerich et al., 1998 Dietrich et al., 1999 Gross et al., 2000 |
|
Myf5 Myogenic factor 5 |
bHLH | muscle anlagen | initiation of myogenic progression |
Emerson et al., 1990 Rudnick et al., 1993 |
|
MyoD myogenic differentiation 1 |
bHLH | muscle anlagen | initiation of myogenic progression |
Emerson et al., 1990 Rudnick et al., 1993 |
|
Myog myogenin |
bHLH | muscle anlagen | post-mitotic muscle differentiation |
Edmonson and Olson, 1989 Wright et al., 1989 Emerson et al., 1990 |
HD: Homeodomain, bHLH: Basic helix-loop-helix, BA: branchial arch, EOM: extraocular muscles, MAM: mastication muscles, ND: not determined
Conclusions and Perspectives
Mammalian skeletal muscles derive from mesoderm segments flanking the embryonic midline. Trunk muscles derive from the paraxial somitic mesoderm and serve in locomotion. Head muscles derive from the pre-chordal and paraxial head mesoderm (referred as cranial mesoderm) and control mastication, breathing, eye movement and facial expression. In both trunk and head, myogenic progression is the same and followed by the expression of MRFs. Proliferating myoblasts express Myf5 and MyoD. Later myogenic differentiation is marked by the expression of myogenin, cell exit the cell cycle and terminally differentiate to contractile muscles. Trunk muscles require the expression of paired homeobox gene Pax3 for activation of myogenesis. Limb muscles require both Pax3 and Lbx1. However, the specification of trunk and head muscles is significantly different. The proteins involved in specifying pre-myogenic progenitors in the head are just beginning to be discovered. Head muscles are more diverse. Pitx2 specifies and marks the EOM and the 1st BA derived MAM and is expressed after the onset of myogenic commitment in the trunk. Tbx1 is expressed in the premyogenic precursors in both 1st and 2nd BA and required for the development of some head muscles and similarly to Pitx2, in the myogenic trunk precursors. The availability of new gene-driven lineage-tracing mouse systems allow us to better understand the developmental program of muscle at the genetic, cellular and molecular levels to serve the need for skeletal muscle regeneration research. A more detailed picture of how muscle cell lineages diverge and assemble to form complex systems, such the skeletal muscle groups is now available and we expect that the lineage roadmaps and gene functions elucidated for mouse muscle development will be closely relevant to human muscle development and regeneration. They will provide the basic scientific knowledge to understand congenital myopathies at the cellular and molecular level, which ultimately will lead to treatment and cure.
GLOSSARY
- CEPHALIC MESODERM
Head mesenchymal cells located beneath the rostral neural plate, followed caudally by the notochord
- PARAXIAL MESODERM
Un-segmented embryonic tissue that forms in both sides of the notochord and gives rise to somites
- PRE-CHORDAL MESODERM
Embryonic tissue that is contiguous laterally with the paraxial mesoderm
- SOMITES
Metameric condensations of paraxial mesoderm that develop stepwise, are located in both sides of the neural tube and give rise to ventral mesenchymal SCLEROTOME and dorsal epithelial DERMOMYOTOME from which they delaminate to form the skeletal muscle of the MYOTOME
- BRANCHIOMERIC MESODERM
The mesodermal core of branchial arches derives from the paraxial mesoderm and anterior occipital mesoderm and gives rise to the branchiomeric skeletal muscles of head and neck
- EPAXIAL MUSCLES
Muscles of the back that develop from the dermomyotome
- HYPAXIAL MUSCLES
Majority of body and limb muscles that derive from the venro-lateral somites
- PRIMAXIAL MUSCLES
Somitic derived ribs and intercoastal muscles
- ABAXIAL MUSCLES
Somitic derived limb, abdominal and pectoral muscles
- APPENDICULAR MUSCLES
Control the movements of the upper and lower limbs and stabilize and control the movements of the pectoral and pelvic girdles
References
- Alvares LE, Schubert FR, Thorpe C, Mootoosamy RC, Cheng L, Parkyn G, Lumsden A, and Dietrich S. Intrinsic, Hox-dependent cues determine the fate of skeletal muscle precursors. Dev Cell. 2003; 5:379–90. [DOI] [PubMed] [Google Scholar]
- Aoyama H, Asamoto K. () Determination of somite cells: independence of cell differentiation and morphogenesis. Development 1988; 104:15–28. [DOI] [PubMed] [Google Scholar]
- Baek SH, Kioussi C, Briata P, Wang D, Nguyen HD, Ohgi KA, Glass CK, Wynshaw-Boris A, Rose DW and Rosenfeld MG Regulated subset of G1 growth-control genes in response to derepression by the Wnt pathway. Proc Natl Acad Sci U S A. 2003; 100: 3245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature 1995; 376:768–71. [DOI] [PubMed] [Google Scholar]
- Bober E, Franz T, Arnold HH, Gruss P, and Tremblay P. Pax-3 is required for the development of limb muscles: a possible role for the migration of dermomyotomal muscle progenitor cells. Development 1994; 120:603–12. [DOI] [PubMed] [Google Scholar]
- Brohmann H, Jagla K, Birchmeier C. The role of Lbx1 in migration of muscle precursor cells.Development 2000;127:437–45. [DOI] [PubMed] [Google Scholar]
- Buckingham M Skeletal muscle formation in vertebrates. Curr Opin Genet Dev 2001; 11: 440–8. [DOI] [PubMed] [Google Scholar]
- Burke AC, Nowicki JL. A new view of patterning domains in the vertebrate mesoderm. Dev Cell. 2003; 4:159–65. [DOI] [PubMed] [Google Scholar]
- Campione M, Steinbeisser H, Schweickert A, Deissler K, van Bebber F, Lowe LA, Nowotschin S, Viebahn C, Haffter P, Kuehn MR, Blum M. The homeobox gene Pitx2: mediator of asymmetric left-right signaling in vertebrate heart and gut looping. Development 1999; 126:1225–34. [DOI] [PubMed] [Google Scholar]
- Charbonnier F, Gaspera BD, Armand AS, Van der Laarse W., Launay T, Becker C, Gallien CL and Chanoine C. Two myogenin-related genes are differentially expressed in Xenopus laevis myogenesis and differ in their ability to transactivate muscle structural genes. J Biol Chem. 2002; 277: 1139–47. [DOI] [PubMed] [Google Scholar]
- Cheng L, Alvares LE, Ahmed MU, El-Hanfy AS, and Dietrich S. The epaxial-hypaxial subdivision of the avian somite. Dev Biol. 2004; 274:348–69. [DOI] [PubMed] [Google Scholar]
- Christ B, Ordahl, CP. Early stages of chick somite development. Anat Embryol (Berl). 1995)191: 381–96. [DOI] [PubMed] [Google Scholar]
- Degenhardt K, Sassoon DA. A role for Engrailed-2 in determination of skeletal muscle physiologic properties. Dev Biol. 2001; 231:175–89. [DOI] [PubMed] [Google Scholar]
- Degenhardt K, Rentschler S, Fishman G, Sassoon DA. Cellular and cis-regulation of En-2 expression in the mandibular arch.Mech Dev. 2002; 111:125–36 [DOI] [PubMed] [Google Scholar]
- Denetclaw WF Jr, Christ B, Ordahl CP. Location and growth of epaxial myotome precursor cells. Development 1997; 124:1601–10. [DOI] [PubMed] [Google Scholar]
- Diehl AG, Zareparsi S, Qian M, Khanna R, Angeles R, Gage PJ. Extraocular muscle morphogenesis and gene expression are regulated by Pitx2 gene dose. Invest Ophthalmol Vis Sci. 2006; 47:1785–93. [DOI] [PubMed] [Google Scholar]
- Dietrich S, Abou-Rebyeh F, Brohmann H, Bladt F, Sonnenberg-Riethmacher E, Yamaai T, Lumsden A, Brand-Saberi B, and Birchmeier C The role of SF/HGF and c-Met in the development of skeletal muscle. Development 1999; 126, 1621–1629. [DOI] [PubMed] [Google Scholar]
- Dietrich S, Schubert FR, Healy C, Sharpe PT, Lumsden A.. Specification of the hypaxial musculature. Development 1998; 125, 2235–2249. [DOI] [PubMed] [Google Scholar]
- Dong F, Sun X, Liu W, Ai D, Klysik E, Lu MF, Hadley J, Antoni L, Chen L, Baldini A, Francis-West P, Martin JF. Pitx2 promotes development of splanchnic mesoderm-derived branchiomeric muscle. Development 2006; 133:4891–9. [DOI] [PubMed] [Google Scholar]
- Eckner R, Yao TP, Oldread E,. Livingston DM. Interaction and functional collaboration of p300/CBP and bHLH proteins in muscle and B-cell differentiation. Genes Dev. 1996; 10: 2478–90. [DOI] [PubMed] [Google Scholar]
- Edmondson DG, Olson EN. A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev. 1989; 3: 628–40. [DOI] [PubMed] [Google Scholar]
- Emerson CP. Myogenesis and developmental control genes. Curr Opin Cell Biol. 1990; 2: 1065–75. [DOI] [PubMed] [Google Scholar]
- Epstein JA, Lam P, Jepea l., Maas RL Shapiro DN. Pax3 inhibits myogenic differentiation of cultured myoblast cells. J Biol Chem. 1995; 270: 11719–22. [DOI] [PubMed] [Google Scholar]
- Franz T, Kothary R, Surani MA, Halata Z, Grim M. The Splotch mutation interferes with muscle development in the limbs. Anat Embryol (Berl). 1993; 187: 153–60. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Camper SA. Pituitary homeobox 2, a novel member of the bicoid-related family of homeobox genes, is a potential regulator of anterior structure formation. Hum Mol Genet 1997; 6:457–64. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Suh H, Camper SA. Dosage requirement of Pitx2 for development of multiple organs. Development 1999; 126: 4643–51. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Rhoades W, Prucka SK, Hjalt T. Fate maps of neural crest and mesoderm in the mammalian eye. Invest Ophthalmol Vis Sci. 2005; 46:4200–8. [DOI] [PubMed] [Google Scholar]
- Garg V, Yamagishi C, Hu T, Kathiriya IS, Yamagishi H, Srivastava D. Tbx1, a DiGeorge syndrome candidate gene, is regulated by sonic hedgehog during pharyngeal arch development.Dev Biol. 2001;235(1):62–73. [DOI] [PubMed] [Google Scholar]
- Goulding M, Lumsden A, Paquette AJ. Regulation of Pax-3 expression in the dermomyotome and its role in muscle development. Development 1994; 120: 957–71. [DOI] [PubMed] [Google Scholar]
- Gross MK, Moran-Rivard L, Velasquez T, Nakatsu MN, Jagla K, Goulding M. Lbx1 is required for muscle precursor migration along a lateral pathway into the limb. Development 2000; 127, 413–424. [DOI] [PubMed] [Google Scholar]
- Gustafsson MK, Pan H, Pinney DF, Liu Y, Lewandowski A, Epstein DJ, Emerson CP Jr. Myf5 is a direct target of long-range Shh signaling and Gli regulation for muscle specification. Genes Dev. 2002; 16:114–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadchouel J, Tajbakhsh S, Primig M, Chang TH, Daubas P, Rocancourt D, Buckingham M. Modular long-range regulation of Myf5 reveals unexpected heterogeneity between skeletal muscles in the mouse embryo. Development 2000; 127:4455–67. [DOI] [PubMed] [Google Scholar]
- Houzelstein D, Auda-Boucher G, Cheraud Y, Rouaud T, Blanc I, Tajbakhsh S, Buckingham ME., Fontaine-Perus J, Robert B. The homeobox gene Msx1 is expressed in a subset of somites, and in muscle progenitor cells migrating into the forelimb. Development 1999; 126, 2689–2701. [DOI] [PubMed] [Google Scholar]
- Joyner AL, Martin GR. En-1 and En-2, two mouse genes with sequence homology to the Drosophila engrailed gene: expression during embryogenesis. Genes Dev. 1987; 1:29–38. [DOI] [PubMed] [Google Scholar]
- Kahane N, Cinnamon Y, Kalcheim C. The cellular mechanism by which the dermomyotome contributes to the second wave of myotome development. Development 1998; 125, 4259–4271. [DOI] [PubMed] [Google Scholar]
- Kahane N, Cinnamon Y, Kalcheim C. The origin and fate of pioneer myotomal cells in the avian embryo. Mech Dev 1998; 74, 59–73. [DOI] [PubMed] [Google Scholar]
- Kelly RG, Jerome-Majewska LA, Papaioannou VE. The del22q11.2 candidate gene Tbx1 regulates branchiomeric myogenesis. Hum Mol Genet. 2004;13:2829–40. [DOI] [PubMed] [Google Scholar]
- Kioussi C, Briata P, Baek SH, Rose DW, Hamblet NS, Herman T, Ohgi KA, Lin C, Gleiberman A, Wang J, Brault V, Ruiz-Lozano P, Nguyen H., Kemler R, Glass CK, Wynshaw-Boris A, Rosenfeld MG. Identification of a Wnt/Dvl/beta-Catenin --> Pitx2 pathway mediating cell-type-specific proliferation during development. Cell 2002; 111: 673–85. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Miura H, Miyagawa-Tomita S, Yanazawa M, Katoh-Fukui Y, Suzuki R, Ohuchi H, Suehiro A, Motegi Y, Nakahara Y, Kondo S, Yokoyama M. Mouse Pitx2 deficiency leads to anomalies of the ventral body wall, heart, extra- and periocular mesoderm and right pulmonary isomerism. Development 1999; 126: 5749–58. [DOI] [PubMed] [Google Scholar]
- Kume T, Deng KY, Winfrey V, Gould DB, Walter MA, Hogan BL. The forkhead/winged helix gene Mf1 is disrupted in the pleiotropic mouse mutation congenital hydrocephalus. Cell 1998; 93:985–996. [DOI] [PubMed] [Google Scholar]
- Lassar AB, Davis RL, Wright WE, Kadesch T, Murre C, Voronova A, Baltimore D, Weintraub H. Functional activity of myogenic HLH proteins requires hetero-oligomerization with E12/E47-like proteins in vivo. Cell. 1991; 66: 305–15. [DOI] [PubMed] [Google Scholar]
- Lehmann OJ, Ebenezer ND, Jordan T, Fox M, Ocaka L, Payne A, Leroy BP,Clark BJ, Hitchings RA, Povey S, Khaw PT, Bhattacharya SS. Chromosomal duplication involving the forkhead transcription factor gene FOXC1 causes iris hypoplasia and glaucoma. Am J Hum Genet. 2000; 67:1129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CR, Kiouss C, O’Connell S, Briata P, Szeto D, Liu F, Izpisua-Belmonte JC, Rosenfeld MG. Pitx2 regulates lung asymmetry, cardiac positioning and pituitary and tooth morphogenesis. Nature 1999; 401: 279–82. [DOI] [PubMed] [Google Scholar]
- Liu W, Selever J, Lu MF, Martin JF. Genetic dissection of Pitx2 in craniofacial development uncovers new functions in branchial arch morphogenesis, late aspects of tooth morphogenesis and cell migration. Development 2003; 130, 6375–6385. [DOI] [PubMed] [Google Scholar]
- Logan M, Pagan-Westphal SM, Smith DM, Paganessi L, Tabin CJ. The transcription factor Pitx2 mediates situs-specific morphogenesis in response to left-right asymmetric signals. Cell 1998; 94:307–17. [DOI] [PubMed] [Google Scholar]
- Lu MF, Pressman C, Dyer R, Johnson RL, Martin JF. Function of Rieger syndrome gene in left-right asymmetry and craniofacial development. Nature 1999; 401, 276–78. [DOI] [PubMed] [Google Scholar]
- Lu JR, Bassel-Duby R, Hawkins A, Chang P, Valdez R, Wu H, Gan L, Shelton JM, Richardson JA, Olson EN. Control of facial muscle development by MyoR and capsulin. Science 2002; 298:2378–81. [DOI] [PubMed] [Google Scholar]
- Maroto M, Reshef R, Munsterberg AE, Koester S, Goulding M, Lassar AB. Ectopic Pax-3 activates MyoD and Myf-5 expression in embryonic mesoderm and neural tissue. Cell 1997; 89: 139–48. [DOI] [PubMed] [Google Scholar]
- McKinsey TA, Zhang CL, Olson EN. Control of muscle development by dueling HATs and HDACs. Curr Opin Genet Dev 2001; 11:497–504 [DOI] [PubMed] [Google Scholar]
- Mennerich D, Schafer K, Brau T. Pax-3 is necessary but not sufficient for lbx1 expression in myogenic precursor cells of the limb. Mech Dev 1998; 73, 147–158. [DOI] [PubMed] [Google Scholar]
- Mennerich D, Braun T. Activation of myogenesis by the homeobox gene Lbx1 requires cell proliferation. EMBO J 2001; 20, 7174–7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meno C, Shimono A, Saijoh Y, Yashiro K, Mochida K, Ohishi S, Noji S, Kondoh H, Hamada H. lefty-1 is required for left-right determination as a regulator of lefty-2 and nodal. Cell 1998; 94:287–97. [DOI] [PubMed] [Google Scholar]
- Mootoosamy RC, Dietrich S. Distinct regulatory cascades for head and trunk myogenesis. Development 2002; 129:573–83. [DOI] [PubMed] [Google Scholar]
- Munsterberg AE, Kitajewski J, Bumcrot DA, McMahon AP, Lassar AB. Combinatorial signaling by Sonic hedgehog and Wnt family members induces myogenic bHLH gene expression in the somite. Genes Dev. 1995; 9: 2911–22. [DOI] [PubMed] [Google Scholar]
- Noden DM, Trainor PA. Relations and interactions between cranial mesoderm and neural crest populations. J Anat. 2005; 207:575–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noden DM, Francis-West P. The differentiation and morphogenesis of craniofacial muscles. Dev Dyn. 2006; 235:1194–218. [DOI] [PubMed] [Google Scholar]
- Nowotschin S, Liao J, Gage PJ, Epstein JA, Campione M, Morrow BE. Tbx1 affects asymmetric cardiac morphogenesis by regulating Pitx2 in the secondary heart field. Development 2006; 133:1565–73. [DOI] [PubMed] [Google Scholar]
- Piedra ME, Icardo JM, Albajar M, Rodriguez-Rey JC, Ros MAPitx2 participates in the late phase of the pathway controlling left-right asymmetry. Cell 1998; 94:319–24. [DOI] [PubMed] [Google Scholar]
- Pourquie O, Fan CM, Coltey M, Hirsinger E, Watanabe Y, Breant C, Francis-West P, Brickell P, Tessier-Lavigne M, Le Douarin NM. Lateral and axial signals involved in avian somite patterning: a role for BMP4. Cell 1996; 84:461–71. [DOI] [PubMed] [Google Scholar]
- Pownall ME, Gustafsson MK, and Emerson CP Jr Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos. Annu Rev Cell Dev Biol. 2002; 18:747–83. [DOI] [PubMed] [Google Scholar]
- Puri PL, Avantaggiati ML, Balsano C, Sang N, Graessmann A, Giordano A, Levrero M. p300 is required for MyoD-dependent cell cycle arrest and muscle-specific gene transcription. Embo J. 1997; 16: 369–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth JF, Shikama N, Henzen C, Desbaillets I, Lutz W, Marino S, Wittwer J, Schorle H, Gassmann M, Eckner R. Differential role of p300 and CBP acetyltransferase during myogenesis: p300 acts upstream of MyoD and Myf5. Embo J. 2003; 22: 5186–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicki M, Schnegelsber PN, Stead RH, Braun, Arnold HH, Jaenisch R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993; 75: 1351–9. [DOI] [PubMed] [Google Scholar]
- Ryan AK, Blumberg B, Rodriguez-Esteban C, Yonei-Tamura S, Tamura K, Tsukui T, de la Pena J, Sabbagh W, Greenwald J, Choe S, Norris DP, Robertson EJ, Evans RM, Rosenfeld MG, Izpisua Belmonte JC. Pitx2 determines left-right asymmetry of internal organs in vertebrates. Nature 1998; 394:545–51. [DOI] [PubMed] [Google Scholar]
- Schafer K, Braun T. Early specification of limb muscle precursor cells by the homeobox gene Lbx1h. Nat Genet 1999; 23, 213–216. [DOI] [PubMed] [Google Scholar]
- Semina EV, Reiter R, Leysens NJ, Alward WL, Small KW, Datson NA, Siegel-Bartelt J, Bierke-Nelson D, Bitoun P, Zabel BU, Carey JC, Murray JC. Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat Genet 1996;14:392–9. [DOI] [PubMed] [Google Scholar]
- Shih HP, Gross MK, Kioussi C. Expression pattern of the homeodomain transcription factor Pitx2 during muscle development. Gene Expr Patterns 2007a; 7:441–51. [DOI] [PubMed] [Google Scholar]
- Shih HP, Gross MK, Kioussi C. Cranial muscle defects of Pitx2 mutants result from specification defects in the first branchial arch. Proc Natl Acad Sci U S A. 2007b; 104:5907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS, Zabaleta A, Kume T, Savinova OV, Kidson SH, Martin JE, Nishimura DY, Alward WL, Hogan BL, John SW. Haploinsufficiency of the transcription factors FOXC1 and FOXC2 results in aberrant ocular development. Hum Mol Genet 2000; 9:1021–32. [DOI] [PubMed] [Google Scholar]
- Summerbell D, Ashby PR, Coutelle O, Cox D, Yee S, Rigby PW. The expression of Myf5 in the developing mouse embryo is controlled by discrete and dispersed enhancers specific for particular populations of skeletal muscle precursors. Development 2000; 127:3745–57. [DOI] [PubMed] [Google Scholar]
- Summitt RL, Hiatt RL, Duenas D, Johnson WW. Mesoectodermal dysplasia of the iris and cornea, mental retardation and myopathy: a sporadic case. Birth Defects Orig Artic Ser. 1971; 7, 129–135. [PubMed] [Google Scholar]
- Tajbakhsh., Rocancourt D, Cossu G, Buckingham M. Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell 1997; 89, 127–138. [DOI] [PubMed] [Google Scholar]
- Tirosh-Finkel L, Elhanany H, Rinon A, Tzahor E. Mesoderm progenitor cells of common origin contribute to the head musculature and the cardiac outflow tract. Development 2006; 133:1943–53. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Dietrich S, Mericskay M, Schubert F, Li Z, Paulin D. A crucial role for Pax3 in the development of the hypaxial musculature and the long-range migration of muscle precursors. Dev. Biol 1998; 203, 49–61 [DOI] [PubMed] [Google Scholar]
- Wright WE, Sassoon DA, Lin VK Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell 1989; 56, 607–617. [DOI] [PubMed] [Google Scholar]
- Yoshioka H, Meno C, Koshiba K, Sugihara M, Itoh H, Ishimaru Y, Inoue T, Ohuchi H, Semina EV, Murray JC, Hamada H, Noji S. Pitx2, a bicoid-type homeobox gene, is involved in a lefty-signaling pathway in determination of left-right asymmetry. Cell 1998; 94:299–305. [DOI] [PubMed] [Google Scholar]
- von Scheven G, Alvares LE, Mootoosamy RC, Dietrich S. Neural tube derived signals and Fgf8 act antagonistically to specify eye versus mandibular arch muscles. Development. 2006;133:2731–45. [DOI] [PubMed] [Google Scholar]