Abstract
Locked nucleic acids (LNAs) are conformationally restricted RNA nucleotides. Their increased thermal stability and selectivity toward their complements make them well-suited for diagnostic and therapeutic applications. Although the structural and thermodynamic properties of LNA—LNA, LNA— RNA, and LNA—DNA hybridizations are known, the kinetic effects of incorporating LNA nucleotides into DNA strand displacement systems are not. Here, we thoroughly studied the strand displacement kinetics as a function of the number and position of LNA nucleotides in DNA oligonucleotides. When compared to that of an all-DNA control, with an identical sequence, the leakage rate constant was reduced more than 50-fold, to an undetectable level, and the invasion rate was preserved for a hybrid DNA/LNA system. The total performance enhancement ratio also increased more than 70-fold when calculating the ratio of the invading rate to the leakage rate constants for a hybrid system. The rational substitution of LNA nucleotides for DNA nucleotides preserves sequence space while improving the signal-to-noise ratio of strand displacement systems. Hybrid DNA/LNA systems offer great potential for high-performance chemical reaction networks that include catalyzed hairpin assemblies, hairpin chain reactions, motors, walkers, and seesaw gates.
Graphical abstract

1. INTRODUCTION
The themodynamics1–7 and kinetics8–12 of Watson—Crick hybridization and strand displacement are well known for DNA and RNA oligonucleotides. As an alternative to naturally occurring nucleic acids, locked nucleic acids (LNAs) are conformationally restricted RNA nucleotides, where the 2′ oxygen in the ribose bonds to the 4′ carbon.13–16 This covalent bond constrains the sugar in the N-type (C3′-endo) con-formation, which in turn preorganizes the phosphate backbone, promotes base stacking, and forces the double helix into its A-form.16–24 These attributes increase the LNA’s thermal stability on and selectivity toward its Watson—Crick complements: including LNA, RNA, and DNA. Naturally occurring nucleotides that neighbor LNA nucleotides also adopt the N-type conformation.23,25 When a hybrid DNA/LNA strand binds to an all-DNA oligonucleotide, the structure reflects the number of LNA nucleotides incorporated into the duplex. For example, the A- to B-form ratio increases as the number of LNA nucleotides increases.23
The stability of LNA-containing duplexes can be considered in terms of the Gibbs free energy, which accounts for the entropic and enthalpic contributions of including conformationally restricted nucleotides into a strand. The positive entropic change is from LNA preorganizing the phosphate backbone.26 In comparison, the more negative enthalpic change is from an increase in base stacking from LNA when compared to that in naturally occurring nucleotides such as RNA and DNA.26 Thermodynamic parameters have been reported for DNA duplexes with single LNA substitutions.27–30 The results indicate that LNA pyrimidines are more stable than LNA purines and that the overall duplex stability is highly dependent on the DNA nucleotides that neighbor the LNA nucleotides.27 As a consequence, the melting temperature of a DNA complex ranges from +1 to +8 °C for every LNA nucleotide added.28,29 In addition, the thermal stability of a DNA duplex saturates as the number of LNA nucleotides approaches ∼50% of the total content.15,30 For example, the melting temperature increased on average 5.3 °C per LNA for a 9-nucleotide DNA duplex that had three randomly distributed LNAs on one of its strands. In comparison, the melting temperature increased on average 4.5 °C per LNA when an equivalent duplex was fully saturated with LNA on one of its strands.19
In addition to thermodynamic parameters, kinetic parameters have been measured when incorporating LNA nucleotides into DNA systems.31 The results indicate that the LNA—DNA base pairs have an increased binding affinity when compared to that of the DNA—DNA base pairs because they have a slower dissociation rate rather than a faster hybridization rate.31,32 In spite of these structural, thermodynamic, hybridization, and dissociation attributes, the kinetics of incorporating LNA nucleotides into DNA strand displacement systems8,33–39 has not been explored. Furthermore, leakage suppression and total system performance (i.e., the signal-to-noise ratio)40–43 have not been studied in DNA reactions that include LNA nucleotides.
Presented here for the first time, LNA nucleotides were substituted for DNA nucleotides in a strand displacement system (Figure 1). Independent of the number or position of the substitutions, the oligonucleotide sequence was fixed. In our model system, the invader (i) hybridizes with the substrate complex at toehold domain 5 and displaces the signal strand(s), creating a waste complex. The signal strand then reacts with the reporter complex and releases a dye strand (d). Using a fluorometer, the intrinsic leakage rates were measured between zero toehold invaders and substrates. In contrast, invasion rates were measured between 6 nt toehold invaders and identical substrates. For both experiments, all-DNA oligonucleotide invader and substrate controls were compared to hybrid DNA/ LNA oligonucleotide invader and substrate variants, with identical sequences. Leakage was minimized by site-specifically incorporating LNA nucleotides into DNA substrates. Equally as important, the elevated invasion rates were maintained by incorporating LNA nucleotides into the invader strand. Experimental methods, results, and discussion for how to optimize the kinetic performance of a DNA stand displacement system by site-specifically substituting LNA for DNA nucleotides into the system are described below.
Figure 1.
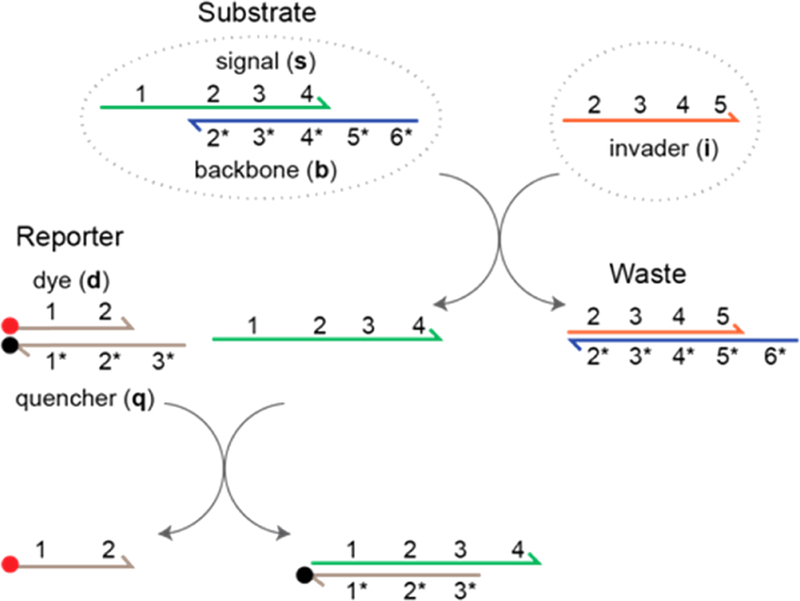
Schematic of a nucleic acid-based, toehold-mediated strand displacement system. Functional domains are represented by numbers, and complementary domains are denoted by numbers with asterisks. The substrate complex includes signal (s) and backbone (b) strands, whereas the reporter complex includes dye (d) and quencher (q) strands. Various LNA substitutions have been made to the signal, backbone, and invader (i) sequences in this study. During invasion, the invader hybridizes with the backbone at domain 5* and displaces the signal strand via three-way branch migration. The invader and backbone form a waste complex. The free signal strand then reacts with the reporter complex and releases a dye strand, which is monitored by a fluorometer.
2. RESULTS AND DISCUSSION
DNA nucleotides were site-specifically substituted by LNA nucleotides in the backbone (b), signal (s), and the backbone and signal of the original sb0 substrate (Figure 2A,B). LNA substitutions were made in the invaders (i) with 0 and 6 nucleotide (nt) toeholds (Figure 2C). All sequences are listed in Section S1 of the Supporting Information. Experimentally, leakage reactions were measured between invaders with 0 nt toeholds and the substrates, whereas invasion reactions were measured between invaders with 6 nt toeholds and the same substrates. The rate constants were extracted by fitting the data using a second-order reaction model (Section S2). The reporter kinetics and control experiments are shown in Sections S3 and S4, respectively.
Figure 2.

(A) Original sequence-level substrate (sb0). The black and red letters, respectively, denote DNA and LNA nucleotides. (B) Hybrid DNA/ LNA substrates. Substrates b1–b6 selectively substituted LNA for DNA nucleotides on the backbone strand. Substrates s1–s4 selectively substituted LNA for DNA nucleotides on the signal strand. Substrates sb1–sb8 selectively substituted LNA for DNA nucleotides on both the signal and backbone strands. (C) Zero nucleotide (nt) invaders (i1–i3) were used for the leakage reactions and 6 nt toehold invaders (i4–i6) were used for the invasion reactions.
2.1. Leakage Reactions between a DNA Invader and Hybrid DNA/LNA Substrates.
The leakage rate constants between the 0 nt toehold invader, i1, and the substrates (Figure 2B) are shown in Figure 3A. Select fluorescence traces for low leakage systems are shown in Figure 3B. For substrates with LNA substitutions on the backbone (b1, b2, b3, b4, b5, and b6), there is no enthalpic change in the base pairing during the leakage reaction, in which the DNA invader replaces the signal strand of the substrate. In general, the more the LNAs incorporated into the backbone, the greater the leakage suppression. For example, when 15 LNA nucleotides substituted for DNA nucleotides in the b6 backbone, leakage was suppressed by a factor of 7. In addition, substrates b1 and b3, with two LNAs near the terminal ends of the duplex, had a more pronounced effect on leakage reduction than that of substrate b2 with two LNAs in the center of the complex. This is likely attributed to the stronger DNA— LNA base pairing, which reduces the fraying frequency and hence reduces the probability for nucleation to occur between the substrates and the 0 nt toehold invader.
Figure 3.

Effects of LNA substitutions on strand displacement kinetics. (A) Leakage rate constants for multiple substrates that were exposed to DNA invader i1 with a 0 nt toehold. (B) Leakage kinetics for select substrates (20 nM) that were exposed to DNA invader i1 (2 μM). The black line is the original substrate (sb0), and the red line is the background reaction when the reporter complex (40 nM) was mixed with DNA invader i1 (2 μM) without the substrate. (C) Invasion rate constants for multiple substrates that were exposed to DNA invader i4 with a 6 nt toehold. (D) The performance enhancement ratio of each substrate was calculated by taking the ratio of the rate constants in (C) and (A). The error bars represent the standard deviation from three reactions with different invader concentrations.
For substrates s2, s3, and s4, with LNA substitutions on the signal strand, the leakage rate constants are smaller than those for substrates b2, b3, and b4 with the same number and position of LNAs on the backbone. The reason is that there is an enthalpic penalty in the leakage reaction, where the LNA—DNA base pairs in the substrates are replaced by the DNA—DNA base pairs in the waste complex.19 During branch migration, this enthalpic penalty renders a bias for the signal to hybridize to the backbone rather than being replaced by the invader strand.44 The data also shows that the leakage performances of substrates s1 and s3 are dramatically different even though they contain the same number and identity of LNAs.
To quantify the effects of secondary structure on the leakage rates of substrates s1 and s3, the probability that a base was unpaired at equilibrium was calculated for substrate sb0 using NUPACK (Section S4).12 According to our analysis, base availability was higher for the right side versus the left side of the sb0 duplex, indicating that the right side of the substrate is more susceptible to leakage because of fraying and hence favorable nucleation between the substrate and its zero nt toehold invader. As a consequence, site-specific substitution of LNAs for DNA nucleotides has a greater leakage suppression effect near the right side (domain 4) versus that on the left side (domain 2) of the sb0 substrate. By extension, substrate s3 has a greater leakage suppression than that from substrate s1. In support of this claim, the experimental results in Section S4 show that leakage is faster from the right side versus that from the left side of the substrate when the i1 invader was separately truncated by two nucleotides at its 5′ and then 3′ end.
Leakage suppression was maximized for substrates sb1, sb2, sb3, sb4, sb5, sb6, sb7, and sb8 with LNA nucleotides on both their signal and backbone strands. When compared to the original substrate (sb0), the leakage reduction for sb8 was ∼50- fold. For substrates sb4—sb8, the leakage shown in Figure 3B was saturated regardless of the number and location of the LNA substitution. This leakage could be attributed to the background cooperative leakage, which was discussed in Section S4. Thus, the leakage rate constant between zero toehold DNA invaders and LNA-modified substrates sb4—sb8 should be much less than 0.03 M−1 s−1, which could not be detected. This significant performance increase is because the LNA—LNA base pairs are more thermomechanically stable than the LNA—DNA or DNA—DNA base pairs.19 In addition, the energy penalty between the LNA—LNA and DNA—LNA base pairs during branch migration likely contributes to the slower leakage rates. For example, there is a stronger bias to form LNA—LNA base pairs between the signal and the backbone than that for DNA—LNA base pairs between the invader and the backbone. Surprisingly, substrate sb2 with two LNA—LNA base pairs near the duplex center has a similar leakage performance as that of substrate sb4 with two LNA—LNA base pairs near the terminal ends of the duplex. Although LNAs near the terminal ends of the substrate may reduce the fraying frequency and hence lower the probability of invader nucleation, they may not adequately transform the substrate from the B-form to the more stable A-form conformation. On the contrary, LNAs in the central region of the substrate not only change the structural conformation from the B-form to the more stable A-form but also impose a higher-energy barrier for branch migration to proceed.25,44 This might explain why substrate sb2, with only two LNA—LNA base pairs in the center of its duplex, reduced the leakage rate from 1.48 to 0.065 M−1 s−1.
Overall, substrates with LNA nucleotides show significant leakage suppression. The leakage rate constant was reduced from 1.48 M−1 s−1 to undetectable level. The LNA—DNA and LNA— LNA base pairs make the substrates less vulnerable to react with the zero toehold invader probably because of their increased thermomechanical stability. LNA nucleotides near the terminal end of the substrates are more likely to reduce fraying, and LNAs in the central region impose a higher-energy barrier during branch migration.
2.2. Invasion Reactions between a DNA Invader and Hybrid DNA/LNA Substrates.
The invading rate constants between the 6 nt toehold invader, i4, and the substrates (Figure 2B) are shown in Figure 3C. The invading rate constants for substrates b1–b6 are equivalent to those for the original substrate, sb0, because (1) the 6 nt toehold is well-established before the branch migration process proceeds and (2) the enthalpy change during branch migration is net neutral for substrates with and without LNA nucleotides. More specifically, stable toeholds provide a forward bias for the invader strand to displace the signal strand even though higher-energy barriers are confronted during the LNA—DNA versus DNA—DNA base pairing. In addition, LNA substitutions at the terminal ends of the duplex for substrates b1, s1, and sb1 do not affect the invading rates because terminal base pairs spontaneously dissociate during branch migration.45
With the exception of s1, substrates s2, s3, and s4, with LNA substitutions on the signal strand, reduce the invasion rates because the LNA—DNA base pairs are replaced by DNA—DNA during branch migration. This energy penalty minimizes the forward bias of the random walk process. The forward bias decreases as the number of LNA nucleotides increases on the signal strand. With the exception of sb1, it also decreases for substrates with LNAs on both the signal and backbone. As a consequence, substrates sb2–sb8 exhibit substantially lower invasion rates as the number of LNA—LNA base pairs increases. For example, the invading rate constant for substrate sb8 is reduced by ∼3 orders of magnitude when compared to that for sb0. To further understand the kinetics of strand invasion, the invasion rate constants were measured as a function of the toehold length and are shown in Section S6.
During strand invasion, the LNA—LNA base pairs in the substrate behave as barriers to strand invasion. The probability to overcome the barrier is dependent on both the position and number of LNA—LNA base pairs in the substrate. For example, as equivalent LNA—LNA base pairs move closer to the toehold, the i4 invasion rate generally shrunk, as reflected by sb1–sb3 and sb5–sb7. In addition, the invasion rate constants for sb1 and sb5 remained elevated, regardless of the invader used, because the last few bases at the terminus of branch migration spontaneously melt off.45 Substrate sb3 with two LNA—LNA bases close to the right terminal end had a faster invasion rate than that of sb2 with two LNA—LNA bases in the central region. This may be because the LNA substitutions that are close to the terminal ends of the duplex are not sufficient to induce structural changes to the duplex.
Overall, LNA substitutions on the substrate affect the invasion kinetics in the following ways. LNAs at the beginning of branch migration of the substrate impose a higher penalty for initiating branch migration.44 LNAs in the center of branch migration of the substrate slow down the rate of branch migration through a relatively large sawtooth amplitude associated with each step of branch migration.44 LNAs at the end of branch migration of the substrate do not affect the strand displacement kinetics.
To guide strand invasion design, the performance enhance-ment ratio is defined as the ratio of the invading rate constant for the 6 nt toehold invader and the leakage rate constant for the 0 nt toehold invader (performance enhancement ratio = kinvading/ kleak). The performance enhancement ratios of hybrid DNA/ LNA substrates are shown in Figure 3D. The performance enhancement ratio of substrate sb0 is normalized to 1 unit, and the higher ratios reflect a better performance. For example, the performance enhancement ratio of substrate sb5 is 18 times better than that of the original sb0 substrate.
2.3. Leakage Reactions between Hybrid DNA/LNA Invaders and Hybrid DNA/LNA Substrates.
The leakage kinetics between 0 nt toehold invaders, with LNA substitutions, and select substrates (Figure 2B) is summarized in Figure 4. Invaders with LNA substitutions have a faster leakage kinetics than that of the DNA invader for three potential reasons. First, invader i2 with LNA substitutions could increase the probability to form stable nuclei with substrate backbones. Second, the high affinity of the LNA—DNA and LNA—LNA base pairs may increase the thermodynamic driving force for strand invasion to proceed. Third, invaders with LNA substitutions likely lower the kinetic barrier and promote the forward bias during strand invasion.
Figure 4.

Leakage rate constants for select substrates and zero toehold invaders with and without LNA substitutions. The black and red letters, respectively, denote DNA and LNA nucleotides. The error bars are the standard deviation from three reactions with different invader concentrations.
In addition to the presence of LNAs in invaders i2 and i3, substrate sb0 leakage is highly dependent on LNA location. For example, invader i2 increased the leakage rate by a factor of 10, whereas invader i3 increased it only by a factor of 2.7. In short, LNAs near the terminus of invader i2 stabilize the nuclei between the invader and the backbone strands, which most likely increases the probability for branch migration to proceed to completion. In contrast, the central location of the LNAs in invader i3 is expected to support a similar nucleation behavior as that supported by invader i1 without any LNA. However, invader i3 has a higher probability of completing branch migration than that of i1 because its LNA—DNA base pairs encourage the forward bias to displace the signal strand. Therefore, even though invaders i2 and i3 have the same number and identity of LNA substitutions, i2 exhibits much faster reaction rates than those of i3 when invading the original substrate (sb0).
Invaders with LNA substitutions have faster leakage rates than those of the all-DNA invader, independent of the substrates tested. Compared with sb0, sb6 includes 15 LNAs on its backbone, most of which are at the terminal ends of domains 2* and 4*. When exposed to invaders with LNA substitutions, the leakage rate was ∼10 times faster for i2 than i3. Briefly, invader i2 has a faster leakage rate because it stabilizes the nucleation event by replacing two DNA— LNA base pairs in the substrate with two LNA—LNA base pairs in the waste complex. In contrast, substrates s2 and s4, with LNA substitutions on the signal strand, consistently have greater leakage rates than those of substrates sb2 and sb4, which have LNAs on their signal and backbone strands. Regardless of the invaders chosen, substrates sb2 and sb4 exhibit a greater leakage suppression than that from substrates s2 and s4, with LNA substitutions only on their signal strand.
2.4. Invasion Reactions between Hybrid DNA—LNA Invaders and Hybrid DNA—LNA Substrates.
When using the 0 nt toehold invader, i1, the sb substrates with LNA on both the signal and backbone strands yielded the greatest leakage suppression (Figure 3A). In comparison, the invasion rates decreased for all sb substrates, excluding sb1, that were invaded by i4, a DNA invader with a 6 nt toehold (Figure 5A). To improve the invasion performance of the sb substrates, LNA substitutions were site-specifically included into invaders i5 and i6, both of which had 6 nt toeholds. Regardless of the strand displacement systems in Figure 5, toehold hybridization was identical between all substrates and all invaders. As a consequence, nucleation between the invader strands and the substrates was assumed to be identical. In many cases, the invasion rates for i5 and i6 had comparable invasion performance to that of DNA invader i4 on the original substrate (sb0). Primary examples include the invasion of substrates sb1, sb2, sb5, and sb6 with either invader i5 or i6.
Figure 5.

(A) Invasion rate constants for substrates with LNA nucleotides on both signal and backbone strands that were exposed to pure DNA and hybrid DNA/LNA invaders with 6 nt toeholds. (B) The performance enhancement ratio of each substrate was calculated by taking the ratio of the invasion and leak rate constants. The error bars represent the standard deviation from three reactions with different invader concentrations.
Between substrates sb1 and sb8, the invasion rates were elevated for i5. During the invasion reaction, but after the invader toehold bound to the substrate, two LNA substitutions on domain 4 of invader i5 formed a stable LNA—LNA or LNA—DNA base pair with the backbone. The increased stability of these base-pairs renders a forward bias during branch migration, which caused invader i5 to have an increased probability to the branch point return to the toehold binding domain. In comparison, invader i6, with two LNA substitutions on domain 3, shows far slower invasion rates for substrates sb3, sb4, sb7, and sb8. The LNA—LNA base pairs that are on the substrate near the onset of branch migration are expected to render a higher-energy barrier after i6 binds to the toehold. Thus, the signal strand has a stronger bias to hybridize to the backbone strand, which encourages the invader to stay on the toehold domain. Thus, LNA substitutions on the invader, that are further away from the toehold domain, do not improve the invading rate constants.
Overall, LNA substitutions on the invader affect the invasion kinetics in the following ways. LNAs on the invader bias the random walk forward at the onset of branch migration and hence increase the invasion rate constant. As the LNA substitutions move away from the toehold domain of the invader, the invasion performance gradually decreases.
The performance enhancement ratios of strand displacement systems between substrates and invaders, with and without LNA substitutions, are shown in Figure 5B. The performance enhancement ratio of substrate sb0 was normalized to 1 unit, and higher ratios reflect increased performance. The perform- ance enhancement ratios of all of the LNA-substituted substrates were improved. The performance enhancement of substrate sb6 and invader i5 showed a 70-fold improvement compared to the performance of the original substrate sb0 and invader i4.
2.5. Optimization of the Performance Enhancement Ratio of Hybrid DNA/LNA Systems.
To explore effective practices for incorporating LNA substitutions into DNA strand displacement systems, four original systems were investigated in Figure 6. Identical to substrate sb0 in Figure 3, the control system (sy1) was entirely made from DNA oligonucleotides. For each system, the leakage rate and invasion rate constants were measured and then compared to the DNA control. For all four systems, the performance enhancement ratio was calculated from the ratio of the invasion rate to the leakage rate constants. The performance enhancement ratio of sy1 was normalized to 1 unit and showed the largest leakage rate (Figure 6B). In comparison, the invasion rate constants were equivalent for all four systems (Figure 6C). The total system performance enhancement ratio improved from twofold to ninefold (Figure 6D).
Figure 6.
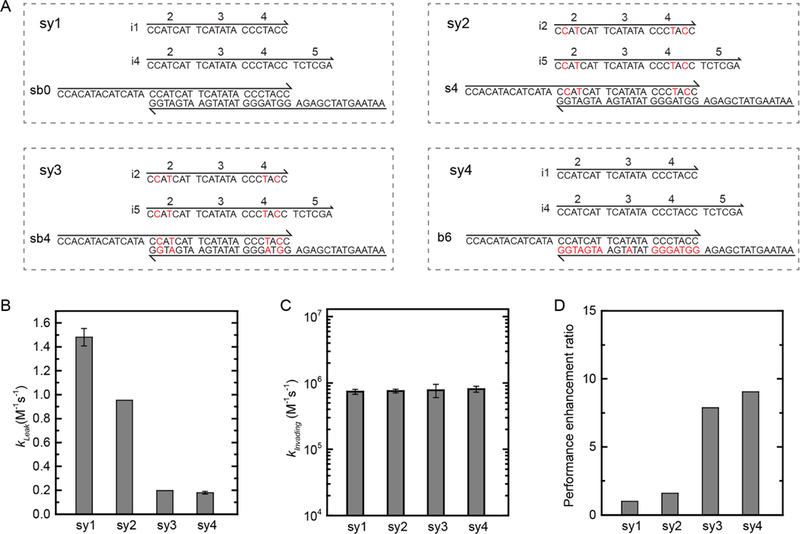
Optimized hybrid DNA/LNA systems. (A) Strands and sequences for four unique systems. The black and red letters, respectively, denote DNA and LNA. Each system consists of a zero toehold invader, a 6 nt toehold invader, and a substrate. For example, “sy1” denotes system 1, in which i1 is the zero toehold invader, i4 is the 6 nt toehold invader, and sb0 is the original substrate. (B) Leakage rate constants of each system. (C) Invasion rate constants of each system. (D) The performance enhancement ratio of each system was calculated by taking the ratio of the rate constants in (C) and (B). The error bars represent the standard deviation from three reactions with different invader concentrations.
In sy1, sy2, and sy3, domains 2 through 4 have identical nucleic acid composition. In sy2 and sy3, LNA substitutions were introduced into the reactants: 0 nt toehold invaders, 6 nt toehold invaders, and the substrates. Aside from the 6 nt toehold region (domain 5), there is not an enthalpic change in the leakage and invasion reactions. Although the thermodynamic driving force was equal for sy1, sy2, and sy3, the performance enhancement ratios of sy2 and sy3 were greater than that of sy1 by a factor of 1.6 and 8, respectively. The increased performance is attributed to an increase in the number of LNA nucleotides in the backbone of sy3. If the thermodynamic driving force is constant, the more the LNA nucleotides introduced into the backbone of the substrate, the better the performance. As a consequence, it is projected that an all-LNA system would outperform an all-DNA system.
That being said, caution is required when incorporating LNA nucleotides into DNA strand displacement systems because of their strong binding affinity to complementary LNA or DNA nucleotides, especially for single strands.19 To minimize secondary structures in single-stranded reactants, LNA nucleotides can be limited to the duplex backbone. In sy4, the single- stranded reactants are DNA oligonucleotides and the substrate complex has LNA nucleotides in its backbone. The performance enhancement ratio of sy4 is improved by a factor of 9 when compared to that of the all-DNA sy1. Unique hybrid DNA/LNA systems are demonstrated in Section S7 with performance improvement as high as 50-fold.
3. CONCLUSIONS
The kinetics of incorporating LNA nucleotides into a DNA strand displacement system has been studied. LNA substitutions affect the kinetics of strand displacement in three ways. First, LNAs in the substrates stabilize the duplex probably by reducing the fraying frequency at the terminus of the duplex regions, which lowers the probability of successful nucleation between the substrates and zero toehold invaders. Second, LNAs in the substrates induce B-form to A-form structural changes, which may hinder the branch migration process. Third, LNAs in the substrate or the invaders bias random walks during branch migration, which alters the probability of strand displacement to proceed. When incorporating LNA substitutions into a DNA strand displacement system, the leakage rate was reduced more than 50-fold and the invasion rate was maintained elevated. In comparison, kinetics for hybrid DNA/LNA systems, the performance enhancement ratio can be improved by a factor of 70, providing insights for how to design future high-performance chemical reaction networks made from DNA and LNA. By site- specifically substituting LNA nucleotides for DNA nucleotides, while maintaining the original sequence design, the performance of chemical reaction networks made from nucleic acids can be optimized. For example, LNAs can be strategically incorporated into different systems such as catalyzed hairpin assemblies,36,46 hairpin chain reactions,47,48 DNA walkers,49 and seesaw gate systems50,51 to minimize unwanted reactions and increase the rate of the desired reactions.
4. METHODS
4.1. Materials.
DNA and hybrid DNA/LNA oligonucleotides were synthesized with high-performance liquid chromatography purification by Integrated DNA Technologies (IDT) and Exqion, respectively. Reporter complexes were also labeled by IDT with 5′ TET fluorophores and 3′ Iowa Black FQ quenchers. Once received, the oligonucleotides were suspended in a 1× TE buffer (10 mM Tris—HCl, pH 8.0, 1 mM ethylenediaminete- traacetate (EDTA)). The stock concentrations were measured from their 260 nm absorbance using the extinction coefficients provided by IDT and Exqion. To minimize the loss from nonspecific binding, poly-T oligonucleotides were added to the dilute stock solutions (less than 1 μM) to reach a final poly-T concentration of 1 μM. Unless stated otherwise, chemicals and solvents were purchased from Sigma-Aldrich. The 10× TAE buffer (40 mM Tris, 40 mM acetate, 1 mM EDTA, pH 8.3–8.5) was purchased from Hoefer or Fisher Scientific. To reach a final concentration of 125 mM Mg2+, Mg(C2H3O2)2·4H2O was added to the 10× TAE buffer. The oligonucleotide components were diluted to 30 μM in a 1× TAE buffer with 12.5 mM Mg2+ and then annealed at 95 °C for 5 min using a Eppendorf Mastercycler Nexus Gradient Thermocycler. Once annealed, the samples were cooled from 95 °C to room temperature over ∼90 min to form the substrates and reporters used in the below listed experiments.
4.2. Purification.
Substrate and reporter complexes were purified by gel electrophoresis using a 10% polyacrylamide gel that was made from a 30% acrylamide bis solution in a 29:1 ratio. The native gels were run for 2 h at 16 °C using a 150 V bias and a VWR International chiller. To eliminate malformed complexes at room temperature, the substrates were stoichiometrically incubated with zero toehold DNA invaders at 15 μM for 5 h before loading the gel. The loading buffer was made from a 1:1 ratio of bromophenol blue dye and ficoll solution (Type 400, 20% water). The desired bands were cut from the gels and then eluted in a 1× TE/Mg2+ buffer for 2 days at 4 °C. The buffer included 1× TE with 12.5 mM MgCl2·6H2O (Acros Organics). Once purified, the substrate and reporter concentrations were quantified from their 260 nm absorbance.
4.3. Spectrofluorimetry.
Fluorescence spectrophotometers from Agilent and Varian Technologies (Cary Eclipses) were used to measure the reaction kinetics. The slit sizes were set to 2.5 nm for the excitation (510 nm) and 10 nm for the emission (538 nm) wavelengths. All experiments were performed at 25 °C, in 0.4 mL glass cuvettes made from Starna Cells, containing a 1× TE/Mg2+ buffer, with a total volume of 0.2 mL. The final fluorescence was normalized to 1 a.u. and corresponded to the lower concentration of the substrate or invader used in the experiments.
Supplementary Material
ACKNOWLEDGMENTS
This project was supported in part by the following: (1) the W.M. Keck Foundation, (2) NIH Grant No. K25GM093233 from the National Institute of General Medical Sciences, (3) NIH Grant No. P20GM103408 from the INBRE Program of the National Center for Research Resources, and (4) The Micron Foundation. Special thanks are extended to Dr. Peter Allen from the University of Idaho for his thoughtful review of this work.
Footnotes
Notes
The authors declare no competing financial interest.
References
- (1).Watson JD; Crick FHC Molecular structure of nucleic acids - a structure for deoxyribose nucleic acid. Nature 1953, 171, 737–738 [DOI] [PubMed] [Google Scholar]
- (2).Zuker M Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 2003, 31, 3406–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Dirks RM; Bois JS; Schaeffer JM; Winfree E; Pierce NA Thermodynamic analysis of interacting nucleic acid strands. SIAM Rev 2007, 49, 65–88. [Google Scholar]
- (4).SantaLucia J Jr.; Allawi HT; Seneviratne A Improved nearest- neighbor parameters for predicting DNA duplex stability. Biochemistry 1996, 35, 3555–3562. [DOI] [PubMed] [Google Scholar]
- (5).SantaLucia J Jr.; Hicks D The thermodynamics of DNA structural motifs. Annu. Rev. Biophys. Biomol. Struct 2004, 33, 415–440. [DOI] [PubMed] [Google Scholar]
- (6).Cooper A; Johnson CM; Lakey JH; Nöllmann M. Heat does not come in different colours: entropy—enthalpy compensation, free energy windows, quantum confinement, pressure perturbation calorimetry, solvation and the multiple causes of heat capacity effects in biomolecular interactions. Biophys. Chem 2001, 93, 215–230. [DOI] [PubMed] [Google Scholar]
- (7).Petruska J; Goodman MF Enthalpy-entropy compensation in DNA melting thermodynamics. J. Biol. Chem 1995, 270, 746–750. [DOI] [PubMed] [Google Scholar]
- (8).Zhang DY; Winfree E Control of DNA strand displacement kinetics using toehold exchange. J. Am. Chem. Soc 2009, 131, 17303–17314. [DOI] [PubMed] [Google Scholar]
- (9).Panyutin IG; Hsieh P The kinetics of spontaneous DNA branch migration. Proc. Natl. Acad. Sci. U.S.A 1994, 91, 2021–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Green C; Tibbetts C Reassociation rate limited displacement of DNA strands by branch migration. Nucleic Acids Res 1981, 9, 1905–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Morrison LE; Stols LM Sensitive fluorescence-based thermodynamic and kinetic measurements of DNA hybridization in solution. Biochemistry 1993, 32, 3095–3104. [DOI] [PubMed] [Google Scholar]
- (12).Olson X; Kotani S; Padilla JE; Hallstrom N; Goltry S; Lee J; Yurke B; Hughes WL; Graugnard E Availability: A Metric for nucleic acid strand displacement systems. ACS Synth. Biol 2017, 6, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Koshkin AA; Singh SK; Nielsen P; Rajwanshi VK; Kumar R; Meldgaard M; Olsen CE; Wengel J LNA (Locked Nucleic Acids): Synthesis of the adenine, cytosine, guanine, 5-methylcytosine, thymine and uracil bicyclonucleoside monomers, oligomerisation, and unprecedented nucleic acid recognition. Tetrahedron 1998, 54, 3607–3630. [Google Scholar]
- (14).Koshkin AA; Rajwanshi VK; Wengel J Novel convenient syntheses of LNA [2.2. 1] bicyclo nucleosides. Tetrahedron Lett 1998, 39, 4381–4384. [Google Scholar]
- (15).Obika S; Nanbu D; Hari Y; Morio K-I; In Y; Ishida T; Imanishi T Synthesis of 2′-O,4′-C-methyleneuridine and -cytidine. Novel bicyclic nucleosides having a fixed C3, -endo sugar puckering. Tetrahedron Lett 1997, 38, 8735–8738. [Google Scholar]
- (16).Kværnø L; Wengel J Investigation of restricted backbone conformations as an explanation for the exceptional thermal stabilities of duplexes involving LNA (Locked Nucleic Acid): synthesis and evaluation of abasic LNA. Chem. Commun 1999, 657–658.
- (17).Singh SK; Koshkin AA; Wengel J; Nielsen P LNA (locked nucleic acids): synthesis and high-affinity nucleic acid recognition. Chem. Commun 1998, 455–456.
- (18).Koshkin AA; Nielsen P; Meldgaard M; Rajwanshi VK; Singh SK; Wengel J LNA (locked nucleic acid): an RNA mimic forming exceedingly stable LNA: LNA duplexes. J. Am. Chem. Soc 1998, 120, 13252–13253. [Google Scholar]
- (19).Singh SK; Wengel J Universality of LNA-mediated highaffinity nucleic acid recognition. Chem. Commun 1998, 1247–1248.
- (20).Braasch DA; Corey DR Locked nucleic acid (LNA): finetuning the recognition of DNA and RNA. Chem. Biol 2001, 8, 1–7. [DOI] [PubMed] [Google Scholar]
- (21).Wengel J; Petersen M; Nielsen KE; Jensen GA; Håkansson AE; Kumar R; Sørensen MD; Rajwanshi VK; Bryld T; Jacobsen JP LNA (locked nucleic acid) and the diastereoisomeric α-L-LNA: conformational tuning and high-affinity recognition of DNA/RNA targets. Nucleosides, Nucleotides Nucleic Acids 2001, 20, 389–396. [DOI] [PubMed] [Google Scholar]
- (22).Petersen M; Bondensgaard K; Wengel J; Jacobsen JP Locked nucleic acid (LNA) recognition of RNA: NMR solution structures of LNA:RNA hybrids. J. Am. Chem. Soc 2002, 124, 5974–5982. [DOI] [PubMed] [Google Scholar]
- (23).Nielsen KE; Singh SK; Wengel J; Jacobsen JP Solution structure of an LNA hybridized to DNA: NMR study of the d(CTLGCTLTLCTLGC):d(GCAGAAGCAG) duplex containing four locked nucleotides. Bioconjugate Chem 2000, 11, 228–238. [DOI] [PubMed] [Google Scholar]
- (24).Nielsen KE; Rasmussen J; Kumar R; Wengel J; Jacobsen JP; Petersen M NMR Studies of fully modified locked nucleic Acid (LNA) hybrids: solution structure of an LNA:RNA hybrid and characterization of an LNA:DNA hybrid. Bioconjugate Chem 2004, 15, 449–457. [DOI] [PubMed] [Google Scholar]
- (25).Petersen M; Nielsen CB; Nielsen KE; Jensen GA; Bondensgaard K; Singh SK; Rajwanshi VK; Koshkin AA; Dahl BM; Wengel J; et al. The conformations of locked nucleic acids (LNA). J. Mol. Recognit 2000, 13, 44–53. [DOI] [PubMed] [Google Scholar]
- (26).Searle MS; Williams DH On the stability of nucleic acid structures in solution: enthalpy - entropy compensations, internal rotations and reversibility. Nucleic Acids Res 1993, 21, 2051–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).McTigue PM; Peterson RJ; Kahn JD Sequence-dependent thermodynamic parameters for locked nucleic acid (LNA)—DNA Duplex formation. Biochemistry 2004, 43, 5388–5405. [DOI] [PubMed] [Google Scholar]
- (28).Braasch DA; Liu Y; Corey DR Antisense inhibition of gene expression in cells by oligonucleotides incorporating locked nucleic acids: effect of mRNA target sequence and chimera design. Nucleic Acids Res 2002, 30, 5160–5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Kurreck J; Wyszko E; Gillen C; Erdmann VA Design of antisense oligonucleotides stabilized by locked nucleic acids. Nucleic Acids Res 2002, 30, 1911–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Kværnø L; Kumar R; Dahl BM; Olsen CE; Wengel J Synthesis of abasic locked nucleic acid and two seco-LNA derivatives and evaluation of their hybridization properties compared with their more flexible DNA counterparts. J. Org. Chem 2000, 65, 5167–5176. [DOI] [PubMed] [Google Scholar]
- (31).Christensen U; Jacobsen N; Rajwanshi VK; Wengel J; Koch T Stopped-flow kinetics of locked nucleic acid (LNA)-oligonucleotide duplex formation: studies of LNA-DNA and DNA-DNA interactions. Biochem. J 2001, 354, 481–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Arora A; Kaur H; Wengel J; Maiti S Effect of locked nucleic acid (LNA) modification on hybridization kinetics of DNA duplex. Nucleic Acids Symp. Ser 2008, 52, 417–418. [DOI] [PubMed] [Google Scholar]
- (33).Zhang DY; Turberfield AJ; Yurke B; Winfree E Engineering entropy-driven reactions and networks catalyzed by DNA. Science 2007, 318, 1121–1125. [DOI] [PubMed] [Google Scholar]
- (34).Zhang DY; Winfree E Robustness and modularity properties of a non-covalent DNA catalytic reaction. Nucleic Acids Res 2010, 38, 4182–4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Yin P; Yan H; Daniell XG; Turberfield AJ; Reif JH A unidirectional DNA walker that moves autonomously along a track. Angew. Chem., Int. Ed 2004, 43, 4906–4911. [DOI] [PubMed] [Google Scholar]
- (36).Yin P; Choi HM; Calvert CR; Pierce NA Programming biomolecular self-assembly pathways. Nature 2008, 451, 318–322. [DOI] [PubMed] [Google Scholar]
- (37).Yurke B; Turberfield AJ; Mills AP; Simmel FC; Neumann JL A DNA-fuelled molecular machine made of DNA. Nature 2000, 406, 605–608. [DOI] [PubMed] [Google Scholar]
- (38).Yurke B; Mills A Using DNA to power nanostructures. Genet. Program. Evolvable Mach 2003, 4, 111–122. [Google Scholar]
- (39).Allen PB; Arshad SA; Li B; Chen X; Ellington AD DNA circuits as amplifiers for the detection of nucleic acids on a paperfluidic platform. Lab Chip 2012, 12 (16), 2951–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Thachuk C; Winfree E; Soloveichik D DNA Computing and Molecular Programming; Phillips A, Yin P, Eds.; Springer International Publishing, 2015; Vol. 9211, pp 133–153. [Google Scholar]
- (41).Jiang YS; Bhadra S; Li B; Ellington AD Mismatches improve the performance of strand-displacement nucleic acid circuits. Angew. Chem 2014, 126, 1876–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Chen X; Briggs N; McLain JR; Ellington AD Stacking nonenzymatic circuits for high signal gain. Proc. Natl. Acad. Sci. U.S.A 2013, 110, 5386–5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Tomov TE; Tsukanov R; Liber M; Masoud R; Plavner N; Nir E Rational design of DNA motors: fuel optimization through single-molecule fluorescence. J. Am. Chem. Soc 2013, 135, 11935–11941. [DOI] [PubMed] [Google Scholar]
- (44).Srinivas N; Ouldridge TE; Sulc P; Schaeffer JM; Yurke B; Louis AA; Doye JPK; Winfree E On the biophysics and kinetics of toehold-mediated DNA strand displacement. Nucleic Acids Res 2013, 41, 10641–10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Machinek RR; Ouldridge TE; Haley NE; Bath J; Turberfield AJ Programmable energy landscapes for kinetic control of DNA strand displacement. Nat. Commun 2014, 5, No. 5324. [DOI] [PubMed] [Google Scholar]
- (46).Li B; Jiang Y; Chen X; Ellington AD Probing spatial organization of DNA strands using enzyme-free hairpin assembly circuits. J. Am. Chem. Soc 2012, 134, 13918–13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Dirks RM; Pierce NA Triggered amplification by hybridization chain reaction. Proc. Natl. Acad. Sci. U.S.A 2004, 101, 15275–15278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Huss D; Choi HMT; Readhead C; Fraser SE; Pierce NA; Lansford R Combinatorial analysis of mRNA expression patterns in mouse embryos using hybridization chain reaction. Cold Spring Harbor Protoc 2015, 2015, 259. [DOI] [PubMed] [Google Scholar]
- (49).Shin J-S; Pierce NA A synthetic DNA walker for molecular transport. J. Am. Chem. Soc 2004, 126, 10834–10835. [DOI] [PubMed] [Google Scholar]
- (50).Qian L; Winfree E Scaling up digital circuit computation with DNA strand displacement cascades. Science 2011, 332, 1196–1201. [DOI] [PubMed] [Google Scholar]
- (51).Qian L; Winfree E; Bruck J Neural network computation with DNA strand displacement cascades. Nature 2011, 475, 368–372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


