Abstract
Introduction:
The corneal epithelium is maintained by limbal stem cells (LSCs) that reside in the basal epithelial layer of the tissue surrounding the cornea termed the limbus. Loss of LSCs results in limbal stem cell deficiency (LSCD) that can cause severe visual impairment. Patients with partial LSCD may respond to conservative therapies designed to rehabilitate the remaining LSCs. However, if these conservative approaches fail or, if complete loss of LSCs occurs, transplantation of LSCs or their alternatives is the only option. While a number of clinical studies utilizing diverse surgical and cell culture techniques have shown favorable results, a universal cure for LSCD is still not available. Knowledge of the potential risks and benefits of current approaches, and development of new technologies, is essential for further improvement of LSCD therapies.
Areas covered:
This review focuses on cell-based LSCD treatment approaches ranging from current available clinical therapies to preclinical studies of novel promising applications.
Expert opinion:
Improved understanding of LSC identity and development of LSC expansion methods will influence the evolution of successful LSCD therapies. Ultimately, future controlled clinical studies enabling direct comparison of the diverse employed approaches will help to identify the most effective treatment strategies.
Keywords: Limbal Stem Cells, Limbal Stem Cell Deficiency, ABCB5, CLET, SLET, SEAM
1. Introduction
The cornea is located at the most anterior part of the eye and represents the first ocular structure crossed by the light on the way to the retina. Corneal transparency, which is essential for visual acuity, depends on the structural integrity of its three layers: the epithelium, the stroma and the endothelium. The corneal epithelium, the most superficial corneal layer, functions as an antimicrobial and permeability barrier and possesses high regenerative capacity. Histologically, it can be described as a non-keratinized, stratified squamous epithelium, which is comprised of several distinct cell populations. Rapid regeneration of the corneal epithelium is maintained by limbal stem cells (LSCs) residing in the conjunctional zone between the cornea and conjunctiva called the limbus [1]. In 1983, Thoft et al. proposed the X, Y, Z hypothesis of corneal epithelial regeneration, which posits that during corneal homeostasis LSCs generate transient amplifying cells (TACs) that migrate centripetally and anteriorly to become differentiated corneal epithelial cells [2, 3]. LSC loss or dysfunction results in conjunctival epithelial ingrowth, neovascularization of corneal stroma and corneal opacification [4, 5], a disease termed limbal stem cell deficiency (LSCD). LSCD can be caused by either genetic mutations in syndromes such as aniridia, multiple endocrine deficiency, dyskeratosis congenita or ectrodactyly-ectodermal dysplasia-clefting syndrome, or by acquired conditions, e.g. Stevens-Johnson syndrome, ocular cicatricial pemphigoid, chemical or thermal burns, contact lens over-wear, limbal tumors, corneal infections, and iatrogenic causes [6, 7, 8, 9, 10, 11, 12, 13]. LSCD can manifest itself either unilaterally, as a result of a localized injury, or bilaterally, as observed in patients with genetic diseases or acquired systemic conditions. While unilateral LSCD can be treated with autologous LSC transplantation, treatment of bilateral LSCD, where no autologous LSC source exists, remains highly challenging. In less severe cases, in which the remaining LSCs can be rehabilitated in the affected eyes, both unilateral and bilateral LSCD can be treated with conservative therapeutic approaches employing, for example: autologous serum drops, therapeutic scleral lenses, eye lubrication, corneal scraping or amniotic membrane transplantation [14]. In cases of bilateral LSCD, when resident LSCs are no longer available, transplantation of allogeneic LSCs or alternative autologous cell sources represent the only potentially therapeutic option for regeneration of the corneal epithelium.
Molecular and functional characterization of LSCs and their use for LSCD therapy represent highly investigated areas in ophthalmological research. This is evidenced by the rapidly increasing numbers of manuscripts published over the last two decades with the keyword “limbal stem cell” searchable in PubMed (Figure 1). The quest for a bona fide LSC marker has lead to the discovery of a number of molecules that can potentially be utilized as either positive selection markers such as p63 [15], Lgr5 [16], Tcf4[17], CD157 [18], CD71low/Integrin α6high [19], TrkA [20], N-Cadherin [21], ABCG2 [22, 23], Cytokeratin 15[24] and ABCB5[25], or as negative selection markers, e.g. ALDHdim [26], RHAMMbright [26] and Connexin-43 [27]. Here we will review the history of cell-based therapies for LSCD, as well as discuss novel strategies for identification of the most efficient approaches to corneal restoration utilizing LSCs or alternative cell-based therapeutic strategies.
Figure 1. Acceleration of scientific progress in the field of LSC biology.
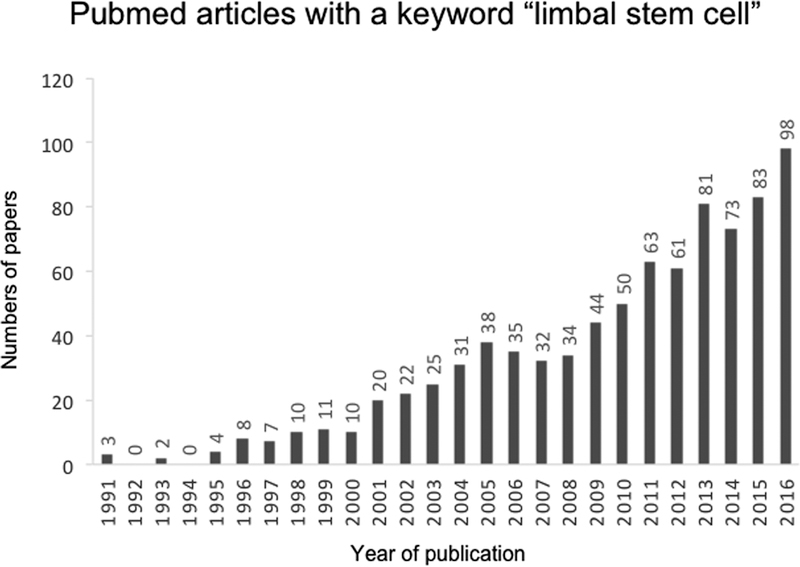
The bar graph represents the number of papers with the keyword “limbal stem cell” published in PubMed from 1991–2016.
2. Development of LSCD therapies: historical overview
The history of modern cell-based LSCD therapies started with conjunctival transplantation in 1977 and keratoepithelioplasty in 1984 performed by Thoft [28, 29] (Figure 2). While these techniques were successful in achieving early corneal re-epithelialization, long-term restoration could not be fully accomplished. Subsequent to the establishment of the LSC concept in 1986 [1], the first large series of conjunctival limbal autograft (CLAU) transplantation for unilateral LSCD was performed by Kenyon et al. in 1989 [30]. However, due to the extent of the biopsy necessary to generate CLAU, this procedure frequently lead to LSC depletion in healthy donor eyes and was also not applicable to patients with bilateral LSCD, who had no remaining LSCs. In order to address this problem, keratolimbal allografts (KLAL) and conjunctival limbal allografts (CLAL) were developed for allogeneic transplantation [31, 32, 33, 34]. KLAL and CLAL use the similar technique as CLAU but the grafts come from either cadaveric or living donor corneas. These approaches also require extensive systemic immunosuppression in order to overcome allograft rejection.
Figure 2. Historical overview of LSCD therapies.
Starting with conjunctival transplantation in 1977, several clinical applications have been developed up to now. Additional corneal epithelial cell alternatives are being tested in preclinical studies.
In an attempt to overcome the disadvantages of CLAU such as the potentially damaging large excision of donor corneas, Pellegrini et al. developed in 1997 the autologous cultivated limbal epithelial transplantation (CLET) approach, which utilized cells harvested from a small biopsy specimen recovered from the healthy contralateral limbus [35]. This technique was based on a novel corneal epithelial cell culture method, which allowed ex vivo generation of corneal epithelial sheets suitable for transplantation [36]. Compared to CLAU, CLET resulted in more efficient corneal epithelialization and reduced ocular surface inflammation and scarring [37]. Allogeneic CLET performed for patients with bilateral LSCD [38] was also more advantageous than a living donor KLAL or CLAL because it required a less expansive excision of the healthy donor cornea.
For a number of years, CLET has been the treatment of choice for LSCD patients, however its more universal use was limited by several logistical hurdles such as the need for on site clinical-grade laboratory support for ex vivo graft cultivation, a requirement for two stepwise surgical procedures, i.e. one for limbal excision and another for transplantation, as well as a prolonged, up to two-weeks cell sheet preparation period and the associated high costs. As an alternative approach to CLET, in 2012, Sangwan et al. reported simple limbal epithelial transplantation (SLET) that combines the benefits of CLAU and CLET [39]. Unlike CLET, SLET is a single-step procedure, which utilizes only minimal autologous donor tissue for transplantation onto the affected eye and does not require clinical-grade laboratory support.
In cases of bilateral LSCD, allo-transplantation is frequently complicated by rejection, adverse events associated with immunosuppression and/or potential disease transmission from the donor [14]. Another major concern is the significant shortage of donor corneas in some countries [40]. To overcome these barriers, in 2003, Nakamura et al. developed a method of autologous cultivated oral mucosal epithelial transplantation (COMET) for restoration of corneal epithelium, which resulted in cornea restoration in vivo in a preclinical rabbit LSCD model [41]. In 2004, Nishida et al. reported the first successful application of this technique to human patients [42]. In addition to COMET, Nishida et al. also developed a temperature-responsive harvesting system that allowed transplantation of a carrier-free cell sheet [42]. A further approach developed by Ricardo et al. in 2013 involved transplantation of autologous conjunctival epithelial cells cultivated ex vivo (EVCAU) [43]. This technique utilizes conjunctival epithelial cells, which share some characteristics of corneal epithelium, for the treatment of bilateral LSCD.
In addition to the above-mentioned clinical studies, alternative therapeutic approaches to LSCD are currently being tested in preclinical models. For example, corneal epithelial-like cells could be induced from embryonic stem cells (ESCs) [44] and from induced pluripotent stem cells (iPSCs) [45, 46]. Most recently in 2016, Hayashi et al. developed a self-formed ectodermal autonomous multi-zone (SEAM) of ocular cells using human iPSCs [47, 48] and showed that corneal epithelial stem/progenitor cells could be successfully isolated from the SEAM. Direct reprogramming of other cell types such as bone marrow mesenchymal stem cells (BM-MSCs), hair follicle stem cells (HFSCs), skin epithelial stem cells, fibroblasts and oral mucosal epithelial cells into corneal epithelial-like cells [49, 50, 51, 52, 53, 54, 55, 56, 57], or use of dental pulp stem cell sheets [58] and nasal mucosal epithelial cell sheets [59] might represent additional novel options for treatment of LSCD in the future.
Cell-free devices such as the Boston type I keratoprostheses are also available, but are associated with significant side-effects and complications [60, 61].
3. Cell-based LSCD therapies in clinical trials
Numerous clinical studies examined the use of cell-based approaches to the treatment of LSCD (Table 1). First, Kenyon et al. reported using CLAU in a series of 21 patients with unilateral disease. In this procedure, two grafts obtained from the limbus and the adjacent conjunctiva of the contralateral uninjured eye were transplanted onto the recipient eye [30]. Stable epithelial adhesion without recurrent erosion or persistent epithelial defect was observed in 20 cases (95.2%) and improved visual acuity was recorded in 17 cases (81.0%). As expected for autologous transplantation, no immune rejection was observed. Next, Holland et al. reported using KLAL in a series of 31 patients with bilateral LSCD caused by aniridia [62]. In this procedure, three keratolimbal crescents prepared from cadaveric limbal tissues were transplanted onto the recipient eye after removal of abnormal fibrovascular pannus and epithelium from the affected cornea. This resulted in regeneration of a normal ocular surface in 23 patients (74.2%) and improvement in visual acuity in 27 patients (87.1%). Despite the need for systemic immunosuppression, no adverse reactions were reported in this study. Notably, the success of this procedure was not universal. For example, Shimazaki et al. reported that the postoperative corneal epithelialization stability of KLAL was significantly worse than that of CLAU [63].
Table 1.
Representative clinical studies using LSCs or their alternatives to treat LSCD.
| Procedures | Year | Authors | Type of graft |
Number of patients | Success rate | Improved visual acuity | Follow up month Mean/Median [range] |
Reference |
|---|---|---|---|---|---|---|---|---|
| CLAU | 1989 | Kenyon et al. | Autograft | 21 | 95% | 81% | Median 24 [6–45] |
[30] |
| KLAL | 2003 | Holland et al. | Allograft | 31 | 74.2% | 87.1% | Mean 35.7 [12–117] |
[62] |
| CLET (3T3-J2, fibrin) | 2010 | Rama et al. | Autograft | 107 | 68.2% | 56.1% | Mean 35 [12–120] |
[64] |
| CLET (xeno-free, HAM) | 2011 | Sangwan et al. | Autograft | 200 | 71% | 60.5% | Mean 36 [12–91] |
[65] |
| SLET | 2016 | Basu et al. | Autograft | 125 | 76% | 75.2% | Median 18 [12–48] |
[68] |
| COMET | 2011 | Satake et al. | Autograft | 40 | 57.5% | 59% | Mean 25.5 [6–54.9] |
[71] |
| EVCAU | 2013 | Ricardo et al. | Autograft | 12 | 83.3% | 75% | Mean 18.5 [15–26] |
[43] |
The development of CLET lead to the emergence of multiple clinical studies employing diverse culture protocols and surgical approaches. In 2010, Rama et al. reported results of the first long-term CLET study of patients with unilateral LSCD [64]. In this study, autologous LSCs isolated from limbal biopsies up to 2 mm2 in size obtained from the uninjured contralateral eye were cultivated on fibrin and clinical-grade-certified sub-lethally irradiated 3T3-J2 feeder cells. The cultured epithelial sheets were then placed on the prepared corneal wound beds of recipient eyes after removal of fibrovascular corneal pannus. Of the 107 corneas treated in the course of this study, 73 transplants (68.2%) were considered successful and 60 transplants (56.1%) resulted in improved visual acuity. Other clinical trials employed xeno-free culture conditions and, in some cases, used human amniotic membrane (HAM) instead of fibrin. For example, Sangwan et al. reported 200 cases with CLET using a xeno-free explant culture system and HAM as a substrate [65]. A completely epithelialized, avascular and clinically stable corneal surface was reported in 142 cases (71%) and improvement in visual acuity was seen in 121 cases (60.5%). Subsequently, Haagdorens et al. reviewed the clinical outcomes of CLET of 1029 cases of autografts and 135 cases of allografts that utilized diverse cell expansion and surgical protocols [14]. The overall success rate was estimated to be 70% and improved visual acuity was obtained in 55% of the grafted eyes. Zhao et al. performed a meta-analysis of a different CLET series, which employed HAM for cell expansion [66]. In 572 cases examined, the success rate was 67%, and the two-line improvement in visual acuity was 62% with no significant difference observed between autologous and allogeneic transplants (odds ration (OR) 1.35 and 1.53, respectively). Similar conclusions were drawn by Holland et al. who examined the long-term outcome of another CLET series, which showed an overall success rate of 72% in 720 cases and two-line improvement in visual acuity in 63% of 539 cases [67].
In 2016, Basu et al. reported the outcome of SLET in 125 patients with unilateral LSCD caused by chemical or thermal burns [68]. In this procedure, a 1-clock hour limbal biopsy sample was obtained from an uninjured donor eye and cut into small pieces. The dissected limbal tissues were transplanted onto a HAM in the recipient eye and fixed with fibrin glue. A completely epithelialized avascular corneal surface was observed in 95 cases (76%) and improvement of visual acuity was seen in 94 cases (75.2%). Intriguingly, SLET also had better success rates compared to CLET in children (SLET: 71% vs. CLET: 37%) [68, 69]. Recently, the outcomes of SLET were evaluated further in a multicenter study [70], which reported clinical success in 57 out of 68 cases (83.8%) and improvement of visual acuity in 44 eyes (64.7%).
Several clinical trials have investigated the efficiency of other cell types. In 2011, Satake et al. reported using COMET in forty patients with LSCD [71]. The grafts were generated from 8-mm diameter biopsies excised from autologous buccal mucosa. Resected tissues were dissociated into single cell suspensions and seeded onto HAM- or fibrin-coated culture plates inserted into plates containing mitomycin C-treated 3T3 cells. Five to six layers of cultivated oral mucosal epithelial sheets were transplanted onto the corneal surface after excision of invaded fibrovascular tissues. A clear corneal appearance with no epithelial defect, minimal fibrovascular tissue invasion and anatomical reconstruction of the ocular surface was obtained in 23 cases (57.5%), while improved visual acuity was observed in 59% of the treated eyes. Ricardo et al. performed transplantation of autologous conjunctival epithelial cells cultivated ex vivo (EVCAU) on 12 eyes with LSCD [43]. Cells were obtained from a 6 mm2 superior forniceal conjunctival biopsy and cultured on denuded HAM. Subsequently, EVCAU were transplanted on the affected eye after removal of fibrovascular pannus and conjunctival tissue ingrowth. Clinical improvement was observed in 10 cases (83.3%) and improved visual acuity was achieved in 9 cases (75%).
While multiple clinical trials show that the majority of the currently approved procedures can improve vision, there appears to be no significant difference in their success rates. For this reason, there is no standard treatment approach that is currently universally accepted. The choice of a particular treatment protocol might also be influenced by the risk of complications as detailed in the section below.
4. Clinical complications of the current LSCD therapies
The common postoperative complications of current therapies include recurrent or persistent epithelial defects, conjunctivalization, subconjunctival hemorrhage, corneal thinning/melting/perforation, infectious keratitis and inflammation. Among infectious etiologies, the most common are bacterial infections caused by methicillin-resistant Staphylococcus aureus, Streptococcus pneumonia, and fungal infections [72, 73]. In addition, some studies also reported cases of herpetic keratitis [64, 71, 74]. In case of CLAU and CLAL, biopsy-related epithelial abnormalities ranging from minor transient irritation to infection and destruction of the donor corneal epithelium have been observed [32, 75]. Miri et al. conducted a retrospective study of the long-term changes and safety implications for donor eyes [76] showing implications of the biopsy site location for successful post-procedure recovery. In particular, the study revealed that when limbal biopsy was performed at 2 clock-hours of the superior and inferior limbus with 3×3 mm of adjacent conjunctiva it did not precipitate any long-term complications in the healthy eye. Even though 4 of the 50 donor eyes examined developed minor complications such as filamentary keratitis and subconjunctival hemorrhage, all complications were resolved without any lasting consequences or visible effects [76]. In another study, Busin et al. reported that LSCs repopulated the donor area within one year [77]. In cases of KLAL, recipient eyes tended to suffer more frequently from glaucoma and needed repeated surgeries compared to the eyes transplanted with CLAU [63]. One case of ocular surface squamous neoplasia occurred in a patient treated with CLAL [78].
Compared to autologous transplants, KLAL, CLAL and allogeneic CLET carry increased risk of transplant rejection [79]. In addition, systemic immunosuppression with agents such as cyclosporine A, tacrolimus, mycophenolate and steroids was in some cases complicated by anemia, hyperglycemia, infection, and renal and liver function abnormalities [80, 81]. Among the agents utilized for systemic immunosuppression, prednisone and tacrolimus were responsible for the majority of adverse effects associated with systemic immunosuppression [80].
For COMET, one of the major concerns is the development of superficial corneal neovascularization under the emerging epithelial sheet [42, 82]. This could be related to diminished secretion of anti-angiogenic molecules such as thrombospondin-1 (TSP-1) and soluble vascular endothelial growth factor receptor-1 (soluble VEGFR-1) [83, 84]. This suggests that use of anti-angiogenic therapies immediately after transplantation might reduce COMET-associated neovascularization [83]. The use of animal products such as 3T3 cells and fetal bovine serum for cell culture methods employed for CLET, COMET and EVCAU [35, 42, 43, 64, 71] can be associated with increased risk of transmission of zoonotic infection [85]. To address this concern, several novel xeno-free cell culture methods have recently been developed [65, 86].
Regarding treatment costs, Sangwan et al. compared the cost of CLAU, CLET and SLET in their clinical reports [39, 87]. Based on their analyses, CLET costs approximately 12,000 Euros per patient, and is approximately 8 times more expensive than CLAU and SLET. In addition, severe adverse events and prolonged hospitalizations can increase the costs for any of the procedures. Further research on the relative cost effectiveness of these procedures might influence the eventual selection of treatment procedures with similar clinical outcomes.
5. Development of future LSCD therapies
To overcome the worldwide shortages of donor corneas for the treatment of bilateral LSCD, alternative sources for autologous corneal epithelial derivation have been investigated. Transplantation of oral mucosal epithelium and conjunctival epithelium has already been successfully applied in the clinic with favorable outcomes [42, 43, 71]. In addition, other alternatives have recently been developed in the laboratory [44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59]. These include (i) derivation of corneal epithelial cells from pluripotent cells, (ii) derivation of corneal epithelial cells from differentiated cells by direct reprogramming, and (iii) using surface ectoderm-derived cells.
ESCs are pluripotent cells that can give rise to derivatives of all three germ layers, i.e. endoderm, mesoderm and ectoderm [88, 89]. Ahmad et al. reported successful induction of corneal epithelial-like cells from ESCs cultured on collagen IV in medium conditioned by limbal fibroblasts [44]. However, future clinical use of ESCs for treatment of LSCD might be complicated by ethical concerns, immunogenicity, and uncontrolled growth resulting in increased tumorigenicity [90]. Using a similar approach, Shalom-Feuerstein et al. reported the first induction of corneal epithelial-like cells from human iPSCs [46]. Using this technique, they revealed that successful adoption of the corneal phenotype was critically dependent on induction of PAX6 expression, which was regulated by microRNAs miR-450b-5p and miR-184. In 2016, Hayashi et al. described generation of SEAM of ocular cells from iPSCs [47, 48]. When cultured in the presence of rho kinase inhibitor and keratinocyte growth factor (KGF) [91], corneal epithelial stem and progenitor cells isolated from SEAM formed an epithelial cell sheet, which successfully recovered corneal function in an experimentally induced rabbit model of LSCD. These discoveries highlight the unique potential of iPSC-derived corneal epithelium for the treatment of bilateral LSCD. However, the future widespread application of this technology could be hampered by prohibitively high costs, significant length of time required for iPSCs generation, and concerns for tumorigenicity of iPSC- derived cells [92]. Creation of HLA-typed iPSC banks might help to overcome the problem of high expense and long iPSC generation time [93], while direct reprogramming of differentiated cells instead of iPSCs might reduce the risk of tumorigenicity [94].
Several cell types have been tested as a potential source for corneal epithelial derivation via direct reprogramming [49, 50, 51, 52, 53, 54, 55, 56, 57]. BM-MSCs and HFSCs could be induced to differentiate into corneal epithelial-like cells when placed in the LSC niche environment in vitro [49, 50, 54, 55]. Additionally, BM-MSCs, adipose tissue-derived MSCs and dental pulp stem cells could be induced to differentiate into corneal epithelial cells in vivo when directly transplanted into animals with experimentally induced LSCD [56, 57, 95]. Alternatively, epidermal skin cells, fibroblasts and oral mucosal epithelial cells transfected with PAX6 exhibited a corneal epithelial phenotype [51, 52, 53]. Similar to oral mucosal epithelial cells and conjunctival epithelial cells, other surface ectoderm-derived cells such as nasal mucosal epithelial cells also have a potential to work as a substitute for functional ocular surface epithelial cells [59]. Use of this cell population carries the additional advantage of concurrent derivation of functional goblet cells, which stabilize the ocular surface.
Of the future therapies, in addition to use of MSCs for treatment of bilateral LSCD patients [50, 54, 55, 56, 57], transplantation of differentiated iPSCs is also highly promising. Even though some iPSC-derived tissues were reported to be immunogenic [96, 97], creation of iPSC banks for HLA-matched transplantation might help to overcome major allogeneic barriers for the majority of bilateral LSCD patients. For example, Taylor et al. reported that the top 50 highest ranked homozygous HLA types can provide a zero HLA mismatch for 79% of potential UK recipients [93]. Since iPSC generation has a potential for mutagenicity, comprehensive genetic analyses would be required before use of these cell populations in clinical practice.
Recently, our laboratories demonstrated that ATP-binding cassette (ABC) superfamily member ABCB5 identifies LSCs with the ability to restore and maintain the corneal epithelium upon transplantation in preclinical models of LSCD [25]. Specifically, our studies showed that prospectively isolated human or murine ABCB5-positive LSC, but not ABCB5-negative limbal epithelial cells, possessed the capacity to fully restore the corneal epithelium upon grafting to LSC-deficient mice in xenogeneic or syngeneic transplantation models [25]. Thus, we posit that prospective isolation and purification of human LSC through use of the cell surface marker ABCB5 might have the potential to further improve therapeutic outcomes. In addition, based on our findings of ABCB5 expression by dermal stem cell (DSC) subpopulations [98, 99] and prior studies demonstrating corneal differentiation capacity of other skin populations [49, 52], we hypothesize that ABCB5-positive DSCs might also possess an ability to restore LSCD. Our most recent studies demonstrating the capability of ABCB5-positive DSCs to induce allograft tolerance to HLA mismatched transplants highlight the potential of this cell sub-population as an attractive cell source for allogeneic transplantation in patients with bilateral LSCD [100].
In addition to cellular transplantation, using biologically active molecules capable of mobilizing functional residual LSCs in the setting of partial LSCD is another attractive option for LSCD therapy. Recently, Yeh et al. reported that pigment epithelial-derived factor (PEDF) peptide administered locally in the form of an eye ointment induced limbal regeneration both structurally and functionally [101]. PEDF peptide induces the proliferation of LSCs remaining in the eyes in LSCD, thus replenishing the LSC population. This in situ treatment does not require donor cell transplantation and is therefore free from the potential disadvantages of cell therapies discussed earlier.
As described here, an increasing number of novel techniques using diverse cell sources are currently being investigated for the treatment of LSCD. Future clinical trials will help to determine the applicability of these approaches to patient care.
6. Conclusion
Our improved understanding of LSC identity and the development of LSC expansion methods, as well as recent discoveries of alternative techniques that can be employed for corneal restoration, will influence the evolution of clinical approaches to LSCD therapy. Up to now, a number of clinical studies have already been performed using various surgical and culture methods. While most studies have shown favorable results, reliable and universal cures are currently not yet available, highlighting the need for further progress. Knowing the potential risks and benefits of the current approaches, and developing new technologies, is hereby essential for further improvement of LSCD therapies.
7. Expert Opinion
Current tissue sources for the treatment of LSCD can be categorized into three groups; i) autologous limbal epithelium, ii) allogeneic limbal epithelium, and iii) autologous limbal epithelium alternatives. Unilateral LSCD is treated with autologous limbal epithelium. Bilateral LSCD can be treated with allogeneic limbal epithelium, but the requirement of immunosuppression and a shortage of donor corneas are the main disadvantages. Bilateral LSCD treatments using autologous corneal epithelium alternatives such as oral mucosal epithelium and conjunctival epithelium are ways to solve those problems. All methods have both advantages and disadvantages, but clinical studies show certain degrees of success in all methods. Care should be taken when comparing the reported success rates and improved visual acuity rates among these procedures because of the diversity of diseases treated, variations in treatment protocols, use of non-standardized outcome measures, and differences in the length of follow-up times. It is too early to determine which procedure is to be preferred in specific conditions. More clinical studies using rigorously controlled comparisons of these procedures are required.
Additionally, most surgical techniques require special skills and instruments including pre-operative preparation and post-operative clinical care. Availability of transplant tissues is also a critical issue. One of the potential options for making transplantation of cultured epithelial cells more accessible is to enable sharing of a single cell-processing center (CPC) by multiple hospitals [102]. For example, cells obtained from a particular patient are transported to a CPC for processing and expansion, and then the cultured cell sheet is sent back to the original hospital, where transplantation is performed.
Article Highlights Box.
Limbal stem cell deficiency (LSCD) resulting from diverse genetic or acquired conditions is a major cause of corneal blindness.
Unilateral LSCD can be treated with autologous limbal stem cell (LSC)-containing grafts using various techniques.
Currently, treatment of patients with bilateral LSCD relies of allogeneic donor cell grafts requiring immunosuppression.
Alternative therapeutic strategies utilizing stem cells derived from other tissues are currently being tested in preclinical studies.
ABCB5-positive LSC represent a novel molecularly defined stem cell population with promising therapeutic potential.
Acknowledgments
Funding:
This work was supported by a National Institutes of Health (NIH)/National Eye Institute (NEI) grant RO1EY025794–01A1 grant to NY Frank, BR Ksander and MH Frank, and a by NIH/NEI Schepens Core Grant P30EY003790 to BR Ksander. It was also supported by Veterans Affairs (VA) Rehabilitation Research and Development (RR&D) Merit Review Award 1I01RX000989 and a Harvard Stem Cell Institute (HSCI) seed grant award to NY Frank. Support was also received via the Kanae Foundation for the Promotion of Medical Science (Tokyo, Japan), Alcon Japan Ltd. (Tokyo, Japan) and Japan Eye Bank Association (Tokyo, Japan) to Y Sasamoto.
Footnotes
Declaration of Interest:
M.H. Frank, B.R. Ksander and N.Y. Frank are inventors or co-inventors of US and international patents assigned to Brigham and Women’s Hospital, and/or Boston Children’s Hospital, and/or Massachsetts Eye and Ear Infirmary, and/or VA Boston Healthcare System, Boston, MA, licensed to Ticeba GmbH (Heidelberg, Germany) and Rheacell GmbH & Co. KG (Heidelberg, Germany). M.H. Frank. serves as a scientific advisor to Ticeba GmbH and Rheacell GmbH & Co. KG. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
References
- 1.Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol 1986. July;103(1):49–62.*This manuscript described for the first time the concept of limbal stem cells (LSCs) and their location.
- 2.Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci 1983. October;24(10):1442–3. [PubMed] [Google Scholar]
- 3.Yoon JJ, Ismail S, Sherwin T. Limbal stem cells: Central concepts of corneal epithelial homeostasis. World J Stem Cells 2014. September 26;6(4):391–403. doi: 10.4252/wjsc.v6.i4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen JJ, Tseng SC. Corneal epithelial wound healing in partial limbal deficiency. Invest Ophthalmol Vis Sci 1990. July;31(7):1301–14. [PubMed] [Google Scholar]
- 5.Tseng SC. Concept and application of limbal stem cells. Eye (Lond) 1989;3 ( Pt 2):141–57. doi: 10.1038/eye.1989.22. [DOI] [PubMed] [Google Scholar]
- 6.Aslan D, Akata RF. Dyskeratosis congenita and limbal stem cell deficiency. Exp Eye Res 2010. March;90(3):472–3. doi: S0014–4835(09)00355–8 [pii] 10.1016/j.exer.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Baylis O, Figueiredo F, Henein C, et al. 13 years of cultured limbal epithelial cell therapy: a review of the outcomes. J Cell Biochem 2011. April;112(4):993–1002. doi: 10.1002/jcb.23028. [DOI] [PubMed] [Google Scholar]
- 8.Bobba S, Di Girolamo N, Mills R, et al. Nature and incidence of severe limbal stem cell deficiency in Australia and New Zealand. Clin Exp Ophthalmol 2017. March;45(2):174–181. doi: 10.1111/ceo.12813. [DOI] [PubMed] [Google Scholar]
- 9.Chan CC, Holland EJ. Severe limbal stem cell deficiency from contact lens wear: patient clinical features. Am J Ophthalmol 2013. March;155(3):544–549 e2. doi: S0002–9394(12)00643–5 [pii] 10.1016/j.ajo.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Di Iorio E, Kaye SB, Ponzin D, et al. Limbal stem cell deficiency and ocular phenotype in ectrodactyly-ectodermal dysplasia-clefting syndrome caused by p63 mutations. Ophthalmology 2012. January;119(1):74–83. doi: S0161–6420(11)00623–3 [pii] 10.1016/j.ophtha.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 11.Lim P, Fuchsluger TA, Jurkunas UV. Limbal stem cell deficiency and corneal neovascularization. Semin Ophthalmol 2009. May-Jun;24(3):139–48. doi: 911119158 [pii] 10.1080/08820530902801478. [DOI] [PubMed] [Google Scholar]
- 12.Mohammadpour M, Javadi MA. Keratitis associated with multiple endocrine deficiency. Cornea 2006. January;25(1):112–4. doi: 00003226-200601000-00020 [pii]. [DOI] [PubMed] [Google Scholar]
- 13.Nishida K, Kinoshita S, Ohashi Y, et al. Ocular surface abnormalities in aniridia. Am J Ophthalmol 1995. September;120(3):368–75. [DOI] [PubMed] [Google Scholar]
- 14.Haagdorens M, Van Acker SI, Van Gerwen V, et al. Limbal Stem Cell Deficiency: Current Treatment Options and Emerging Therapies. Stem Cells Int 2016;2016:9798374. doi: 10.1155/2016/9798374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pellegrini G, Dellambra E, Golisano O, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A 2001. March 13;98(6):3156–61. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brzeszczynska J, Ramaesh K, Dhillon B, et al. Molecular profile of organ culture-stored corneal epithelium: LGR5 is a potential new phenotypic marker of residual human corneal limbal epithelial stem cells. Int J Mol Med 2012. May;29(5):871–6. doi: 10.3892/ijmm.2012.904. [DOI] [PubMed] [Google Scholar]
- 17.Lu R, Qu Y, Ge J, et al. Transcription factor TCF4 maintains the properties of human corneal epithelial stem cells. Stem Cells 2012. April;30(4):753–61. doi: 10.1002/stem.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horenstein AL, Sizzano F, Lusso R, et al. CD38 and CD157 ectoenzymes mark cell subsets in the human corneal limbus. Mol Med 2009. Mar-Apr;15(3–4):76–84. doi: 10.2119/molmed.2008.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi R, Yamato M, Saito T, et al. Enrichment of corneal epithelial stem/progenitor cells using cell surface markers, integrin alpha6 and CD71. Biochem Biophys Res Commun 2008. March 07;367(2):256–63. doi: 10.1016/j.bbrc.2007.12.077. [DOI] [PubMed] [Google Scholar]
- 20.Qi H, Li DQ, Shine HD, et al. Nerve growth factor and its receptor TrkA serve as potential markers for human corneal epithelial progenitor cells. Exp Eye Res 2008. January;86(1):34–40. doi: 10.1016/j.exer.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashi R, Yamato M, Sugiyama H, et al. N-Cadherin is expressed by putative stem/progenitor cells and melanocytes in the human limbal epithelial stem cell niche. Stem Cells 2007. February;25(2):289–96. doi: 10.1634/stemcells.2006-0167. [DOI] [PubMed] [Google Scholar]
- 22.Budak MT, Alpdogan OS, Zhou M, et al. Ocular surface epithelia contain ABCG2-dependent side population cells exhibiting features associated with stem cells. J Cell Sci 2005. April 15;118(Pt 8):1715–24. doi: 10.1242/jcs.02279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Paiva CS, Chen Z, Corrales RM, et al. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells 2005;23(1):63–73. doi: 23/1/63 [pii] 10.1634/stemcells.2004-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshida S, Shimmura S, Kawakita T, et al. Cytokeratin 15 can be used to identify the limbal phenotype in normal and diseased ocular surfaces. Invest Ophthalmol Vis Sci 2006. November;47(11):4780–6. doi: 10.1167/iovs.06-0574. [DOI] [PubMed] [Google Scholar]
- 25.Ksander BR, Kolovou PE, Wilson BJ, et al. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature 2014. July 17;511(7509):353–7. doi: nature13426 [pii] 10.1038/nature13426.*This paper described a novel limbal stem cell population capable of long-term cornea restoration upon transplantation that can be prospectively isolated from mixed cell suspensions based on the expression of a cell membrane protein ABCB5.
- 26.Ahmad S, Kolli S, Li DQ, et al. A putative role for RHAMM/HMMR as a negative marker of stem cell-containing population of human limbal epithelial cells. Stem Cells 2008. June;26(6):1609–19. doi: 10.1634/stemcells.2007-0782. [DOI] [PubMed] [Google Scholar]
- 27.Chen Z, Evans WH, Pflugfelder SC, et al. Gap junction protein connexin 43 serves as a negative marker for a stem cell-containing population of human limbal epithelial cells. Stem Cells 2006. May;24(5):1265–73. doi: 10.1634/stemcells.2005-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thoft RA. Conjunctival transplantation. Arch Ophthalmol 1977. August;95(8):1425–7. [DOI] [PubMed] [Google Scholar]
- 29.Thoft RA. Keratoepithelioplasty. Am J Ophthalmol 1984. January;97(1):1–6. [DOI] [PubMed] [Google Scholar]
- 30.Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology 1989. May;96(5):709–22; discussion 722–3. doi: S0161–6420(89)32833–8 [pii].**This is the first report of limbal tissue transplantation, which lead to subsequent development of several methods for treatment of corneal blindness due to the limbal stem cell deficiency (LSCD).
- 31.Kenyon KR, Rapoza PA. Limbal allograft transplantation for ocular surface disorders. Ophthalmology 1995;102(suppl):101–102.7831023 [Google Scholar]
- 32.Tan DT, Ficker LA, Buckley RJ. Limbal transplantation. Ophthalmology 1996. January;103(1):29–36. doi: S0161–6420(96)30737–9 [pii]. [DOI] [PubMed] [Google Scholar]
- 33.Tsai RJ, Tseng SC. Human allograft limbal transplantation for corneal surface reconstruction. Cornea 1994. September;13(5):389–400. [DOI] [PubMed] [Google Scholar]
- 34.Holland EJ. Epithelial transplantation for the management of severe ocular surface disease. Trans Am Ophthalmol Soc 1996;94:677–743. [PMC free article] [PubMed] [Google Scholar]
- 35.Pellegrini G, Traverso CE, Franzi AT, et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997. April 05;349(9057):990–3. doi: S0140–6736(96)11188–0 [pii] 10.1016/S0140-6736(96)11188-0.*This is the first report of autologous cultivated limbal epithelial transplantation (CLET) utilizing a novel cell culture method minimizing the size of the incision of the contralateral donor eye. This report lead to rapid development of limbal stem cells (LSCs) expansion methods.
- 36.Lindberg K, Brown ME, Chaves HV, et al. In vitro propagation of human ocular surface epithelial cells for transplantation. Invest Ophthalmol Vis Sci 1993. August;34(9):2672–9. [PubMed] [Google Scholar]
- 37.Ang LP, Sotozono C, Koizumi N, et al. A comparison between cultivated and conventional limbal stem cell transplantation for Stevens-Johnson syndrome. Am J Ophthalmol 2007. January;143(1):178–80. doi: S0002-9394(06)00903-2 [pii] 10.1016/j.ajo.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 38.Schwab IR, Reyes M, Isseroff RR. Successful transplantation of bioengineered tissue replacements in patients with ocular surface disease. Cornea 2000. July;19(4):421–6. [DOI] [PubMed] [Google Scholar]
- 39.Sangwan VS, Basu S, MacNeil S, et al. Simple limbal epithelial transplantation (SLET): a novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br J Ophthalmol 2012. July;96(7):931–4. doi: bjophthalmol-2011-301164 [pii] 10.1136/bjophthalmol-2011-301164. [DOI] [PubMed] [Google Scholar]
- 40.Gain P, Jullienne R, He Z, et al. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol 2016. February;134(2):167–73. doi: 2474372 [pii] 10.1001/jamaophthalmol.2015.4776. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura T, Endo K, Cooper LJ, et al. The successful culture and autologous transplantation of rabbit oral mucosal epithelial cells on amniotic membrane. Invest Ophthalmol Vis Sci 2003. January;44(1):106–16. [DOI] [PubMed] [Google Scholar]
- 42.Nishida K, Yamato M, Hayashida Y, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med 2004. September 16;351(12):1187–96. doi: 10.1056/NEJMoa040455351/12/1187[pii].*Autologous oral mucosal epithelial cells were used as an alternative cell source for the treatment of patients with bilateral limbal stem cell deficiency (LSCD). This study provided a first example of using autologous cell source for patients with bilateral LSCD when limbal tissue was no longer available.
- 43.Ricardo JR, Cristovam PC, Filho PA, et al. Transplantation of conjunctival epithelial cells cultivated ex vivo in patients with total limbal stem cell deficiency. Cornea 2013. March;32(3):221–8. doi: 10.1097/ICO.0b013e31825034be. [DOI] [PubMed] [Google Scholar]
- 44.Ahmad S, Stewart R, Yung S, et al. Differentiation of human embryonic stem cells into corneal epithelial-like cells by in vitro replication of the corneal epithelial stem cell niche. Stem Cells 2007. May;25(5):1145–55. doi: 2006-0516 [pii] 10.1634/stemcells.2006-0516. [DOI] [PubMed] [Google Scholar]
- 45.Hayashi R, Ishikawa Y, Ito M, et al. Generation of corneal epithelial cells from induced pluripotent stem cells derived from human dermal fibroblast and corneal limbal epithelium. PLoS One 2012;7(9):e45435. doi: 10.1371/journal.pone.0045435PONE-D-12-19308[pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shalom-Feuerstein R, Serror L, De La Forest Divonne S, et al. Pluripotent stem cell model reveals essential roles for miR-450b-5p and miR-184 in embryonic corneal lineage specification. Stem Cells 2012. May;30(5):898–909. doi: 10.1002/stem.1068. [DOI] [PubMed] [Google Scholar]
- 47.Hayashi R, Ishikawa Y, Katori R, et al. Coordinated generation of multiple ocular-like cell lineages and fabrication of functional corneal epithelial cell sheets from human iPS cells. Nat Protoc 2017. April;12(4):683–696. doi: nprot.2017.007 [pii] 10.1038/nprot.2017.007. [DOI] [PubMed] [Google Scholar]
- 48.Hayashi R, Ishikawa Y, Sasamoto Y, et al. Co-ordinated ocular development from human iPS cells and recovery of corneal function. Nature 2016. March 17;531(7594):376–80. doi: nature17000 [pii] 10.1038/nature17000.*The paper describes the generation from human induced pluripotent cells (iPSCs) of a self-formed ectodermal autonomous multi-zone (SEAM) mimicing whole-eye developmenst. Development of the entire eye was reproduced in a dish from human induced pluripotent cells (iPSCs). Corneal epithelial stem/progenitor cells isolated from the ocular surface ectodermal zone of the SEAM can restore limbal stem cell deficiency (LSCD).
- 49.Blazejewska EA, Schlotzer-Schrehardt U, Zenkel M, et al. Corneal limbal microenvironment can induce transdifferentiation of hair follicle stem cells into corneal epithelial-like cells. Stem Cells 2009. March;27(3):642–52. doi: stemcells.2008–0721 [pii] 10.1634/stemcells.2008-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gu S, Xing C, Han J, et al. Differentiation of rabbit bone marrow mesenchymal stem cells into corneal epithelial cells in vivo and ex vivo. Mol Vis 2009;15:99–107. doi: 10 [pii] [PMC free article] [PubMed] [Google Scholar]
- 51.Kitazawa K, Hikichi T, Nakamura T, et al. OVOL2 Maintains the Transcriptional Program of Human Corneal Epithelium by Suppressing Epithelial-to-Mesenchymal Transition. Cell Rep 2016. May 10;15(6):1359–68. doi: S2211–1247(16)30433–8 [pii] 10.1016/j.celrep.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 52.Ouyang H, Xue Y, Lin Y, et al. WNT7A and PAX6 define corneal epithelium homeostasis and pathogenesis. Nature 2014. July 17;511(7509):358–61. doi: nature13465 [pii] 10.1038/nature13465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sasamoto Y, Hayashi R, Park SJ, et al. PAX6 Isoforms, along with Reprogramming Factors, Differentially Regulate the Induction of Cornea-specific Genes. Sci Rep 2016. February 22;6:20807. doi: srep20807 [pii] 10.1038/srep20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang TS, Cai L, Ji WY, et al. Reconstruction of the corneal epithelium with induced marrow mesenchymal stem cells in rats. Mol Vis 2010. July 14;16:1304–16. doi: 144 [pii]. [PMC free article] [PubMed] [Google Scholar]
- 55.Rohaina CM, Then KY, Ng AM, et al. Reconstruction of limbal stem cell deficient corneal surface with induced human bone marrow mesenchymal stem cells on amniotic membrane. Transl Res 2014. March;163(3):200–10. doi: 10.1016/j.trsl.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 56.Reinshagen H, Auw-Haedrich C, Sorg RV, et al. Corneal surface reconstruction using adult mesenchymal stem cells in experimental limbal stem cell deficiency in rabbits. Acta Ophthalmol 2011. December;89(8):741–8. doi: 10.1111/j.1755-3768.2009.01812.x. [DOI] [PubMed] [Google Scholar]
- 57.Galindo S, Herreras JM, Lopez-Paniagua M, et al. Therapeutic Effect of Human Adipose Tissue-Derived Mesenchymal Stem Cells in Experimental Corneal Failure Due to Limbal Stem Cell Niche Damage. Stem Cells 2017. October;35(10):2160–2174. doi: 10.1002/stem.2672. [DOI] [PubMed] [Google Scholar]
- 58.Gomes JA, Geraldes Monteiro B, Melo GB, et al. Corneal reconstruction with tissue-engineered cell sheets composed of human immature dental pulp stem cells. Invest Ophthalmol Vis Sci 2010. March;51(3):1408–14. doi: iovs.09–4029 [pii] 10.1167/iovs.09-4029. [DOI] [PubMed] [Google Scholar]
- 59.Kobayashi M, Nakamura T, Yasuda M, et al. Ocular surface reconstruction with a tissue-engineered nasal mucosal epithelial cell sheet for the treatment of severe ocular surface diseases. Stem Cells Transl Med 2015. January;4(1):99–109. doi: sctm.2014–0169 [pii] 10.5966/sctm.2014-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aravena C, Bozkurt TK, Yu F, et al. Long-Term Outcomes of the Boston Type I Keratoprosthesis in the Management of Corneal Limbal Stem Cell Deficiency. Cornea 2016. September;35(9):1156–64. doi: 10.1097/ICO.0000000000000933. [DOI] [PubMed] [Google Scholar]
- 61.Sejpal K, Yu F, Aldave AJ. The Boston keratoprosthesis in the management of corneal limbal stem cell deficiency. Cornea 2011. November;30(11):1187–94. doi: 10.1097/ICO.0b013e3182114467. [DOI] [PubMed] [Google Scholar]
- 62.Holland EJ, Djalilian AR, Schwartz GS. Management of aniridic keratopathy with keratolimbal allograft: a limbal stem cell transplantation technique. Ophthalmology 2003. January;110(1):125–30. doi: S0161-6420(02)01451-3 [pii]. [DOI] [PubMed] [Google Scholar]
- 63.Shimazaki J, Shimmura S, Tsubota K. Donor source affects the outcome of ocular surface reconstruction in chemical or thermal burns of the cornea. Ophthalmology 2004. January;111(1):38–44. doi: 10.1016/j.ophtha.2003.02.003S0161-6420(03)01024-8[pii]. [DOI] [PubMed] [Google Scholar]
- 64.Rama P, Matuska S, Paganoni G, et al. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med 2010. July 08;363(2):147–55. doi: NEJMoa0905955 [pii] 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 65.Sangwan VS, Basu S, Vemuganti GK, et al. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: a 10-year study. Br J Ophthalmol 2011. November;95(11):1525–9. doi: bjophthalmol-2011–300352 [pii] 10.1136/bjophthalmol-2011-300352. [DOI] [PubMed] [Google Scholar]
- 66.Zhao Y, Ma L. Systematic review and meta-analysis on transplantation of ex vivo cultivated limbal epithelial stem cell on amniotic membrane in limbal stem cell deficiency. Cornea 2015. May;34(5):592–600. doi: 10.1097/ICO.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 67.Holland EJ. Management of Limbal Stem Cell Deficiency: A Historical Perspective, Past, Present, and Future. Cornea 2015. October;34 Suppl 10:S9–15. doi: 10.1097/ICO.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 68.Basu S, Sureka SP, Shanbhag SS, et al. Simple Limbal Epithelial Transplantation: Long-Term Clinical Outcomes in 125 Cases of Unilateral Chronic Ocular Surface Burns. Ophthalmology 2016. May;123(5):1000–10. doi: S0161–6420(16)00003–8 [pii] 10.1016/j.ophtha.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 69.Sejpal K, Ali MH, Maddileti S, et al. Cultivated limbal epithelial transplantation in children with ocular surface burns. JAMA Ophthalmol 2013. June;131(6):731–6. doi: 1675579 [pii] 10.1001/jamaophthalmol.2013.2308. [DOI] [PubMed] [Google Scholar]
- 70.Vazirani J, Ali MH, Sharma N, et al. Autologous simple limbal epithelial transplantation for unilateral limbal stem cell deficiency: multicentre results. Br J Ophthalmol 2016. October;100(10):1416–20. doi: 10.1136/bjophthalmol-2015-307348. [DOI] [PubMed] [Google Scholar]
- 71.Satake Y, Higa K, Tsubota K, et al. Long-term outcome of cultivated oral mucosal epithelial sheet transplantation in treatment of total limbal stem cell deficiency. Ophthalmology 2011. August;118(8):1524–30. doi: S0161–6420(11)00072–8 [pii] 10.1016/j.ophtha.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 72.Basu S, Ali H, Sangwan VS. Clinical outcomes of repeat autologous cultivated limbal epithelial transplantation for ocular surface burns. Am J Ophthalmol 2012. April;153(4):643–50, 650 e1–2. doi: S0002–9394(11)00732-X [pii] 10.1016/j.ajo.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 73.Shimazaki J, Aiba M, Goto E, et al. Transplantation of human limbal epithelium cultivated on amniotic membrane for the treatment of severe ocular surface disorders. Ophthalmology 2002. July;109(7):1285–90. doi: S0161–6420(02)01089–8 [pii]. [DOI] [PubMed] [Google Scholar]
- 74.Baradaran-Rafii A, Eslani M, Djalillian AR. Complications of keratolimbal allograft surgery. Cornea 2013. May;32(5):561–6. doi: 10.1097/ICO.0b013e31826215eb. [DOI] [PubMed] [Google Scholar]
- 75.Jenkins C, Tuft S, Liu C, et al. Limbal transplantation in the management of chronic contact-lens-associated epitheliopathy. Eye (Lond) 1993;7 ( Pt 5):629–33. doi: 10.1038/eye.1993.145. [DOI] [PubMed] [Google Scholar]
- 76.Miri A, Said DG, Dua HS. Donor site complications in autolimbal and living-related allolimbal transplantation. Ophthalmology 2011. July;118(7):1265–71. doi: S0161-6420(10)01270-4 [pii] 10.1016/j.ophtha.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 77.Busin M, Breda C, Bertolin M, et al. Corneal Epithelial Stem Cells Repopulate the Donor Area within 1 Year from Limbus Removal for Limbal Autograft. Ophthalmology 2016. December;123(12):2481–2488. doi: S0161–6420(16)30931–9 [pii] 10.1016/j.ophtha.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 78.Shah KJ, Mogilishetty G, Holland EJ. Ocular Surface Squamous Neoplasia in a Living-Related Conjunctival Limbal Allograft. Cornea 2016. February;35(2):274–6. doi: 10.1097/ICO.0000000000000697. [DOI] [PubMed] [Google Scholar]
- 79.Satake Y, Dogru M, Yamaguchi T, et al. Immunological rejection following allogeneic cultivated limbal epithelial transplantation. JAMA Ophthalmol 2013. July;131(7):920–2. doi: 1675576 [pii] 10.1001/jamaophthalmol.2013.15. [DOI] [PubMed] [Google Scholar]
- 80.Krakauer M, Welder JD, Pandya HK, et al. Adverse effects of systemic immunosuppression in keratolimbal allograft. J Ophthalmol 2012;2012:576712. doi: 10.1155/2012/576712 PubMed PMID: 22523651; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tsubota K Ocular surface management in corneal transplantation, a review. Jpn J Ophthalmol 1999. Nov-Dec;43(6):502–8. doi: S0021515599001409 [pii]. [DOI] [PubMed] [Google Scholar]
- 82.Nakamura T, Inatomi T, Sotozono C, et al. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br J Ophthalmol 2004. October;88(10):1280–4. doi: 10.1136/bjo.2003.03849788/10/1280[pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kanayama S, Nishida K, Yamato M, et al. Analysis of soluble vascular endothelial growth factor receptor-1 secreted from cultured corneal and oral mucosal epithelial cell sheets in vitro. Br J Ophthalmol 2009. February;93(2):263–7. doi: 93/2/263 [pii] 10.1136/bjo.2008.141580. [DOI] [PubMed] [Google Scholar]
- 84.Sekiyama E, Nakamura T, Kawasaki S, et al. Different expression of angiogenesis-related factors between human cultivated corneal and oral epithelial sheets. Exp Eye Res 2006. October;83(4):741–6. doi: S0014–4835(06)00184–9 [pii] 10.1016/j.exer.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 85.Murphy FA. The public health risk of animal organ and tissue transplantation into humans. Science 1996. August 09;273(5276):746–7. [DOI] [PubMed] [Google Scholar]
- 86.Kolli S, Ahmad S, Mudhar HS, et al. Successful application of ex vivo expanded human autologous oral mucosal epithelium for the treatment of total bilateral limbal stem cell deficiency. Stem Cells 2014. August;32(8):2135–46. doi: 10.1002/stem.1694. [DOI] [PubMed] [Google Scholar]
- 87.Miri A, Al-Deiri B, Dua HS. Long-term outcomes of autolimbal and allolimbal transplants. Ophthalmology 2010. June;117(6):1207–13. doi: S0161–6420(09)01223–8 [pii] 10.1016/j.ophtha.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 88.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981. July 09;292(5819):154–6. PubMed PMID: 7242681; eng. [DOI] [PubMed] [Google Scholar]
- 89.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998. November 06;282(5391):1145–7. [DOI] [PubMed] [Google Scholar]
- 90.Grinnemo KH, Sylven C, Hovatta O, et al. Immunogenicity of human embryonic stem cells. Cell Tissue Res 2008. January;331(1):67–78. doi: 10.1007/s00441-007-0486-3. [DOI] [PubMed] [Google Scholar]
- 91.Miyashita H, Yokoo S, Yoshida S, et al. Long-term maintenance of limbal epithelial progenitor cells using rho kinase inhibitor and keratinocyte growth factor. Stem Cells Transl Med 2013. October;2(10):758–65. doi: sctm.2012–0156 [pii] 10.5966/sctm.2012-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miura K, Okada Y, Aoi T, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol 2009. August;27(8):743–5. doi: nbt.1554 [pii] 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- 93.Taylor CJ, Peacock S, Chaudhry AN, et al. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell 2012. August 03;11(2):147–52. doi: S1934–5909(12)00429–8 [pii] 10.1016/j.stem.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 94.Kelaini S, Cochrane A, Margariti A. Direct reprogramming of adult cells: avoiding the pluripotent state. Stem Cells Cloning 2014;7:19–29. doi: 10.2147/SCCAA.S38006sccaa-7-019[pii]. PubMed PMID: 24627642; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kerkis I, Kerkis A, Dozortsev D, et al. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs 2006;184(3–4):105–16. doi: 10.1159/000099617. [DOI] [PubMed] [Google Scholar]
- 96.Zhao T, Zhang ZN, Rong Z, et al. Immunogenicity of induced pluripotent stem cells. Nature 2011. May 13;474(7350):212–5. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 97.Guha P, Morgan JW, Mostoslavsky G, et al. Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell Stem Cell 2013. April 04;12(4):407–12. doi: 10.1016/j.stem.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 98.Frank NY, Pendse SS, Lapchak PH, et al. Regulation of progenitor cell fusion by ABCB5 P-glycoprotein, a novel human ATP-binding cassette transporter. J Biol Chem 2003. November 21;278(47):47156–65. doi: 10.1074/jbc.M308700200M308700200[pii]. [DOI] [PubMed] [Google Scholar]
- 99.Schatton T, Yang J, Kleffel S, et al. ABCB5 identifies immunoregulatory dermal cells. Cell Rep 2015. September 08;12(10):1564–74. doi: S2211–1247(15)00881–5 [pii] 10.1016/j.celrep.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Frank MH, Frank NY. Restoring the cornea from limbal stem cells. Regen Med 2015;10(1):1–4. doi: 10.2217/rme.14.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yeh SI, Ho TC, Chen SL, et al. Pigment epithelial-derived factor peptide regenerated limbus serves as regeneration source for limbal regeneration in rabbit limbal deficiency. Invest Ophthalmol Vis Sci 2016. May 01;57(6):2629–36. doi: 2522918 [pii] 10.1167/iovs.15-17171. [DOI] [PubMed] [Google Scholar]
- 102.Oie Y, Nishida K. Regenerative medicine for the cornea. Biomed Res Int 2013;2013:428247. doi: 10.1155/2013/428247. [DOI] [PMC free article] [PubMed] [Google Scholar]



