Abstract
OBJECTIVES:
The incidence of staphylococcal scalded skin syndrome (SSSS) is rising, but current practice variation in diagnostic test use is not well described. Our aim was to describe the variation in diagnostic test use in children hospitalized with SSSS and to determine associations with patient outcomes.
METHODS:
We performed a retrospective (2011–2016) cohort study of children aged 0 to 18 years from 35 children’s hospitals in the Pediatric Health Information System database. Tests included blood culture, complete blood count, erythrocyte sedimentation rate, C-reactive protein level, serum chemistries, and group A streptococcal testing. K-means clustering was used to stratify hospitals into groups of high (cluster 1) and low (cluster 2) test use. Associations between clusters and patient outcomes (length of stay, cost, readmissions, and emergency department revisits) were assessed with generalized linear mixed-effects modeling.
RESULTS:
We included 1259 hospitalized children with SSSS; 84% were ≤4 years old. Substantial interhospital variation was seen in diagnostic testing. Blood culture was the most commonly obtained test (range 62%–100%), with the most variation seen in inflammatory markers (14%–100%). Between hospital clusters 1 and 2, respectively, there was no significant difference in adjusted length of stay (2.6 vs 2.5 days; P = .235), cost ($4752 vs $4453; P = .591), same-cause 7-day readmission rate (0.8% vs 0.4%; P = .349), or emergency department revisit rates (0.1% vs 0.6%; P = .148).
CONCLUSIONS:
For children hospitalized with SSSS, lower use of diagnostic tests was not associated with changes in outcomes. Hospitals with high diagnostic test use may be able to reduce testing without adversely affecting patient outcomes.
Staphylococcal scalded skin syndrome (SSSS) is a toxin-mediated exfoliative dermatitis that primarily affects children. Authors of multiple studies have reported that the incidence of SSSS is rising.1–6 Although affected children are known to have a broad range of clinical severity,7–9 many require hospitalization.10 Hospitalizations for SSSS increased by 50% from 2009 to 2012, reaching an estimated 900 hospitalizations in the United States and increasing as a proportion of overall pediatric hospitalizations.11 Local fluctuations in the incidence of SSSS secondary to outbreaks from clustering within communities may contribute to the rising incidence of hospitalizations.12–14
Currently, there are no comprehensive evidence-based clinical practice guidelines to assist clinicians with the diagnostic evaluation and management of children with SSSS. Although some experts suggest diagnostic testing only in cases of expanding skin desquamation or nonresponse to antibiotic therapy, others recommend the universal use of diagnostic tests, including nasal and surface cultures.7,10,15 Recommendations for adjunct testing, such as inflammatory markers, are even less clear, but some experts recommend their use in severe cases.7 Bacterial culture is the most commonly recommended diagnostic test, yet within the published literature there is a lack of consensus regarding the optimal site to culture (eg, skin, nares, blood, or pharynx).1,4,9,16–19 Although skin biopsy can accurately differentiate SSSS from other desquamating skin diseases,20,21 its use is limited by the invasive nature of the procedure.15
Given conflicting expert recommendations and lack of strong evidence supporting standardization of practice for children with SSSS, we hypothesized that there is substantial variation in diagnostic test use. Variation in diagnostic testing and associated costs across hospitals has not been well described in the literature. Similarly, it is unclear whether a lower rate of test use is associated with differences in patient outcomes. With increasing attention on diagnostic test stewardship,22–26 understanding the role of diagnostic testing in SSSS is needed to promote more judicious use and help decrease unnecessary health care costs. Our aim with this study is to describe the variation in diagnostic testing across multiple US children’s hospitals and to determine if patterns in diagnostic testing are associated with differences in hospital length of stay (LOS), cost, readmission rates, and emergency department (ED) revisits.
Methods
We performed a multicenter, retrospective cohort study using data from 35 free-standing tertiary care US children’s hospitals located across 24 states within the Pediatric Health Information System (PHIS) database. The PHIS database is maintained by the Children’s Hospital Association in Lenexa, Kansas, and contains deidentified patient data, including demographics, diagnoses (with International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] and International Classification of Diseases, 10th Revision, Clinical Modification [ICD-10-CM]), procedures, and daily billing information. Participating hospitals and the Children’s Hospital Association ensure the quality and reliability of the data.27 The local institutional review board determined that this study did not constitute human subjects research because the information was deidentified.
Study Population
We included children aged 0 to 18 years who were hospitalized from July 2011 to June 2016 with a primary or secondary diagnosis of SSSS (ICD-9-CM: 695.81; ICD-10-CM: L00). We excluded children who were transferred from another hospital because patient data from the index hospital are not accessible in the PHIS database. We excluded children with complex chronic medical conditions as defined by Feudtner et al28 because these children may warrant a different diagnostic approach compared with the general population. Additionally, to minimize diagnostic ambiguity, we excluded children if they were coded for an alternative dermatologic diagnosis as a primary or secondary diagnosis (eg, scarlet fever or Stevens-Johnson Syndrome) (Supplemental Table 3). Finally, we excluded hospitals with <10 children with SSSS during the study period because of concern that individual patient cases may dramatically skew aggregate data for a hospital because of low patient volumes.
Patient Covariates
Patient variables included age, sex, ethnicity and/or race, payer type, level of care, and illness severity. Age was divided into the following categories: 0 to 59 days, 2 to 11 months, 1 to 4 years (preschool-aged), 5 to 10 years (school-aged), and 11 to 18 years (adolescent). Infants from 0 to 59 days of age were divided into a separate age group than older infants because they may warrant a different diagnostic approach. Racial and ethnic data were classified as white (non-Hispanic), African American (non-Hispanic), Hispanic, or other. Payer types were categorized as government (such as Medicaid), private, or other. Illness severity was assessed by using the All Patient Refined Diagnosis Related Group (3M, Maplewood, MN) severity levels.29 Level of care was classified as either intensive care or acute care. We characterized hospitals by US region, assigned as Northeast, Midwest, South, and West.
Diagnostic Test Use
We used PHIS billing codes to determine the diagnostic tests obtained in the study population. We assessed the general use of bacterial cultures, including wound cultures, cultures with an unspecified site, and cultures that were specific for Staphylococcus aureus or methicillin-resistant Staphylococcus aureus (MRSA). Tests chosen for further analysis were the most common distinct serum and microbiologic swab tests in the patient cohort and included the following: complete blood count (CBC), C-reactive protein (CRP) level or erythrocyte sedimentation rate (ESR), blood chemistry profile, blood culture, and group A streptococcal testing. A blood chemistry profile included the following: an 8- or 14-test chemistry profile, an electrolyte profile, a kidney profile, other multitest chemistry profile, or if all of the following individual tests were ordered: sodium + potassium + chloride + bicarbonate + blood urea nitrogen + creatinine + glucose. Group A streptococcal testing included the following: β-hemolytic streptococcal antigen detection, antistreptolysin O titer, streptococcal screen culture, other specified Streptococcus, or streptococcal antibody screen. We defined hospital-level diagnostic test use as the percentage of the patient cohort at each hospital that had each test ordered at least once during the admission.
Outcome Measures
Outcome measures included hospital-level LOS in days and all-cause and same-cause 7- and 14-day readmission and ED revisit rates. Readmissions and revisits were considered same-cause if a diagnosis for SSSS (ICD-9-CM: 695.81; ICD-10-CM: L00) was recorded for the encounter. Costs were estimated from charges by using hospital- and year-specific cost-to-charge ratios. Secondary outcomes included the following: frequency of diagnostic coding for bacteremia or sepsis30 (Supplemental Table 4), escalation of care to the ICU after hospital day 1, and mortality occurring during the admission.
Statistical Analysis
Summary statistics were calculated to assess patient characteristics, disposition, and LOS. Patient variables were compared across hospital groups by using χ2 statistics. Each hospital was assigned to 1 of 2 clusters on the basis of similar diagnostic testing patterns across 5 different diagnostic tests by using k-means clustering. Two clusters were confirmed through examination of the Scree plot. Hospitals were grouped into 2 clusters, named cluster 1 (high test use) and cluster 2 (low test use), so that each hospital was part of the cluster with the closest mean testing pattern in aggregate for each of the 5 diagnostic tests. This approach has been used with other diagnoses as a framework to evaluate hospital-level patterns in testing and their association with patient outcomes.31
Generalized linear mixed-effects models were used with random hospital intercepts (to account for patient grouping within a hospital) to model outcomes between the 2 hospital clusters. Models were adjusted for race, illness severity, payer, and region. Because of the nonnormal behavior of cost and LOS data, an exponential distribution was used for the models. All analyses were performed with SAS 9.4 (SAS Institute, Inc, Cary, NC), and P values of <.05 were considered statistically significant.
Results
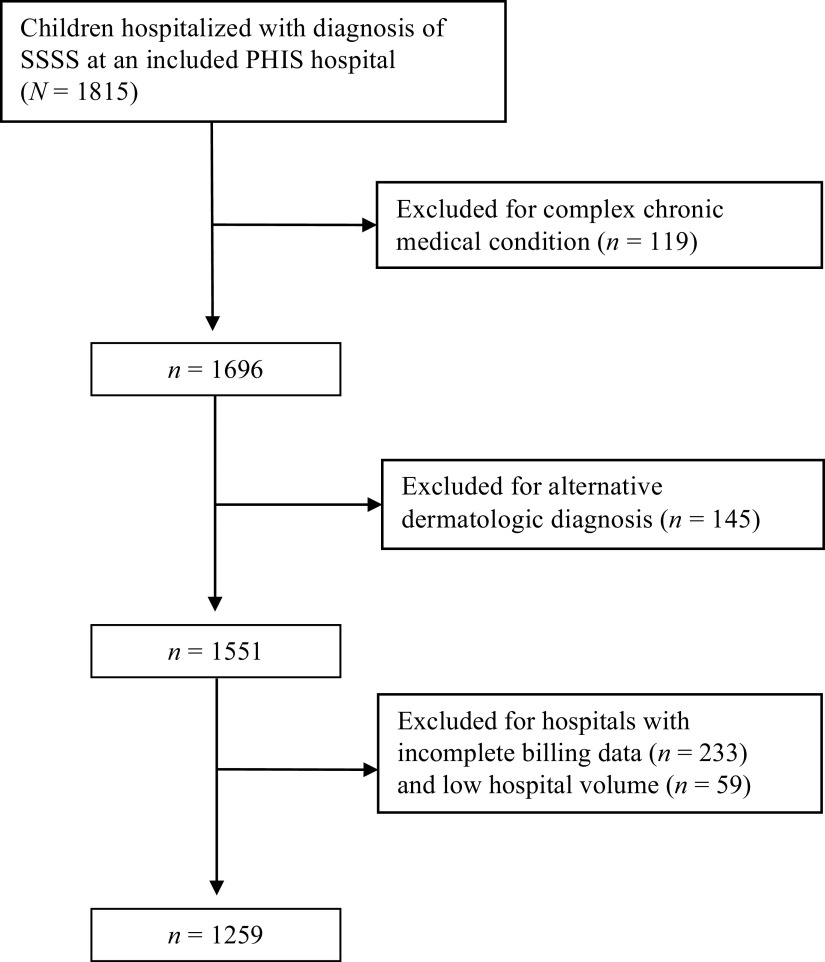
Overall, 1815 children with SSSS were admitted to a study hospital from 2011 to 2016. In total, 6.6% of hospitalized children were excluded because of a complex chronic condition, and an additional 8.6% of those remaining were excluded because of an alternative dermatologic diagnosis (Fig 1). The overall population is described in Table 1 (N = 1259). The majority of children (84%) were ≤4 years of age, 52.7% were boys, and 5% were admitted directly to an ICU. In the 33 hospitals with continuous patient data during the study period, patient cases increased from 2011 to 2016, with an increased incidence during summer and early fall of each year (Supplemental Fig 4).
FIGURE 1.
Flowchart of study population.
TABLE 1.
Comparison of Characteristics of Children With SSSS Across Hospital Clusters
| Characteristics | Overall | Hospital Cluster 1 | Hospital Cluster 2 | P |
|---|---|---|---|---|
| Patient total, n (%) | 1259 | 838 (67) | 421 (33) | |
| Level of care, n (%) | .703 | |||
| Intensive | 64 (5) | 44 (5) | 20 (5) | |
| Acute | 1195 (95) | 794 (95) | 401 (95) | |
| Age group, n (%) | .280 | |||
| 0–59 d | 168 (13) | 108 (13) | 60 (14) | |
| 2–11 mo | 231 (18) | 163 (20) | 68 (16) | |
| 1–4 y | 658 (52) | 428 (51) | 230 (55) | |
| 5–10 y | 180 (14) | 121 (14) | 59 (14) | |
| 11–18 y | 22 (2) | 18 (2) | 4 (1) | |
| Sex, n (%) | ||||
| Male | 664 (53) | 450 (54) | 214 (51) | .336 |
| Race and/or ethnicity, n (%) | <.001 | |||
| White | 634 (50) | 392 (47) | 242 (58) | |
| African American | 280 (22) | 189 (23) | 91 (22) | |
| Hispanic | 210 (17) | 178 (21) | 32 (8) | |
| Other | 135 (11) | 79 (9) | 56 (13) | |
| Payer, n (%) | <.001 | |||
| Government | 704 (56) | 496 (59) | 208 (49) | |
| Private | 518 (41) | 330 (39) | 188 (45) | |
| Other | 37 (3) | 12 (1) | 25 (6) | |
| US region, n (%) | <.001 | |||
| Midwest | 263 (21) | 115 (14) | 148 (35) | |
| Northeast | 166 (13) | 15 (2) | 151 (36) | |
| South | 631 (50) | 542 (65) | 89 (21) | |
| West | 199 (16) | 166 (20) | 33 (8) | |
| Illness severity,a n (%) | .146 | |||
| Low | 777 (62) | 529 (63) | 248 (59) | |
| High | 482 (38) | 309 (37) | 173 (41) |
—, not applicable.
Illness severity defined by All Patient Refined Diagnosis Related Group codes.
Hospital Clustering
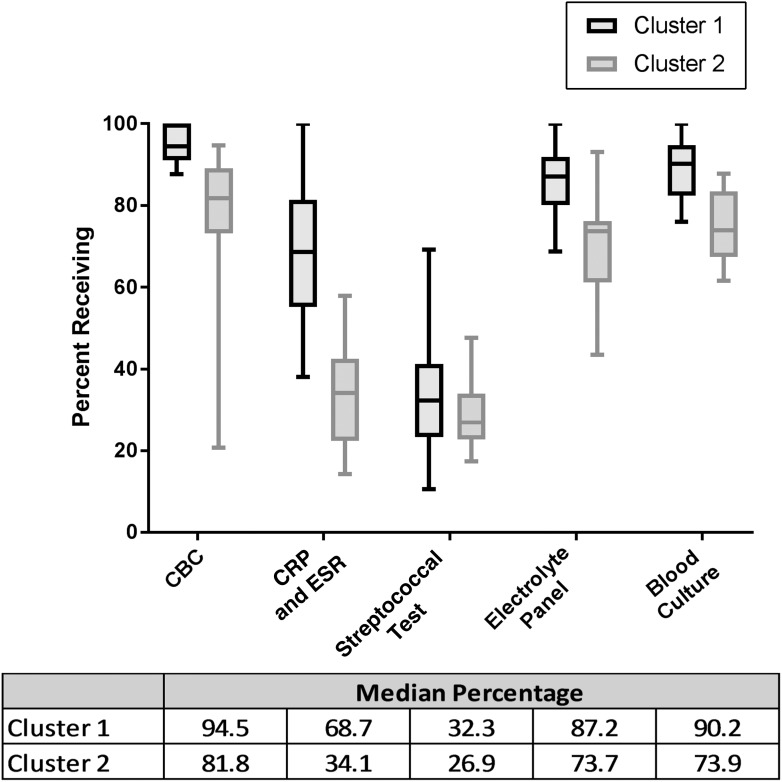
Cluster 1 contained 22 hospitals, whereas cluster 2 contained 13 hospitals. More children received each of the selected diagnostic tests at cluster 1 hospitals than at cluster 2 hospitals, although variation in some tests was greater than in others. In addition, differences in median testing rates between the clusters also varied by test type (Fig 2). Patient case mix between hospital clusters is shown in Table 1. Significantly more children with Hispanic ethnicity and public insurance were in cluster 1, and a larger percentage of hospitals were in the Southern United States. There were no significant differences in patient age, sex, illness severity, or ICU admission between the 2 cluster groups.
FIGURE 2.
Comparison of distribution of diagnostic testing for hospital clusters 1 and 2. Boxes extend to the 25th and 75th percentiles. Lines indicate median testing rates. Whiskers represent 1.5 × IQR from the edge of the box.
Variation in Diagnostic Test Use
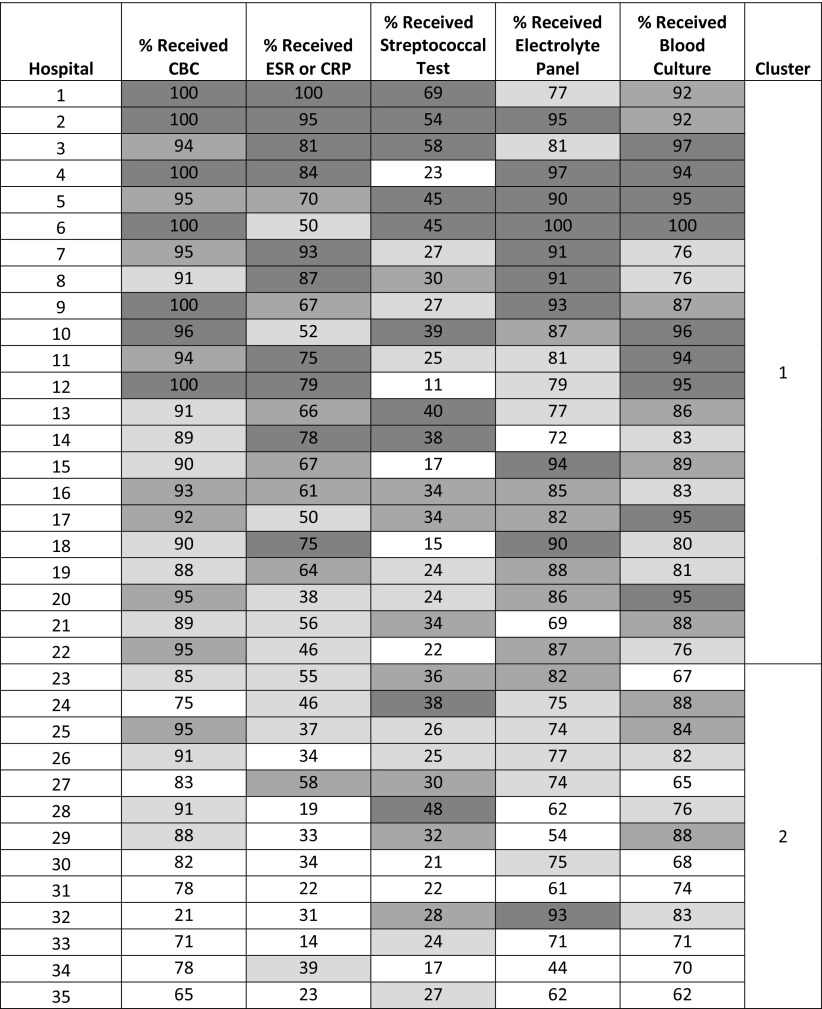
Substantial interhospital variation was seen in diagnostic test use (Fig 3). Blood cultures were obtained most frequently at individual hospitals, with use ranging from 62% to 100%, whereas group A streptococcal testing was obtained the least, with use ranging from 11% to 69%. Overall, 88% and 31% of children received blood cultures and streptococcal testing, respectively. The use of ESR or CRP revealed the greatest range across hospitals, from 14% to 100%. Overall, wound cultures were obtained in 28% of hospitalized children, 7% of children received a specific S aureus or MRSA culture (multiple sites), and 32% of children had bacterial cultures obtained but from an unspecified site of collection. Finally, skin biopsy was rarely performed (1.7% of children).
FIGURE 3.
Heat map of hospital-specific use of diagnostic testing. Numbers indicate the percentage of children at each hospital that received each test. Hospitals are sorted by highest to lowest mean testing rate across the 5 tests with the cluster number indicated. Shading corresponds to use quartile, with darker shading indicating higher use.
Patient Outcomes and Association With Diagnostic Test Use
For the entire patient cohort, the median LOS was 3 days (interquartile range [IQR]: 2–3); 23% of children required 4 or more days of admission. Forty-two children (2.4%) were also coded for the diagnosis of bacteremia or sepsis. No children in our cohort required escalation of care to the ICU after admission, and none died.
In cluster 1 hospitals, the unadjusted median LOS was 3 days (IQR: 2–3) versus 2 days (IQR: 2–3) in cluster 2 hospitals. After adjustment, there was no difference in median LOS or ED revisit rates at 7 or 14 days between cluster 1 and cluster 2 hospitals (Table 2). The 7-day all-cause hospital readmission rate was lower in cluster 2 hospitals, but there was no significant difference in same-cause 7- and 14-day readmission rates. Among children who experienced an ED revisit or readmission, none had a diagnosis code of bacteremia or sepsis recorded for the encounter. There was no significant difference in adjusted cost between cluster 1 and cluster 2 hospitals.
TABLE 2.
Adjusted Patient Outcomes Between Hospital Clusters
| Outcomea | Hospital Cluster 1 | Hospital Cluster 2 | P |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| LOS, d | 2.6 (2.4, 2.7) | 2.5 (2.3, 2.6) | .235 |
| Cost, US dollars | 4752 (4009, 5495) | 4453 (3617, 5288) | .591 |
| All cause | % (IQR) | % (IQR) | |
| 7-d readmission | 1.3 (0.5, 2.1) | 0.3 (0, 0.9) | .049 |
| 14-d readmission | 1.5 (0.6, 2.3) | 0.7 (0, 1.4) | .138 |
| 7-d ED revisit | 0.9 (0.3, 1.5) | 1.4 (0.4, 2.5) | .436 |
| 14-d ED revisit | 1.6 (0.7, 2.4) | 2 (0.3, 3.7) | .436 |
| Same cause | % (IQR) | % (IQR) | |
| 7-d readmission | 0.8 (0.1, 1.4) | 0.4 (0, 1) | .349 |
| 14-d readmission | 0.8 (0.1, 1.4) | 0.4 (0, 1) | .621 |
| 7-d ED revisit | 0.1 (0, 0.4) | 0.6 (0, 1.3) | .148 |
| 14-d ED revisit | 0.1 (0, 0.4) | 0.6 (0, 1.3) | .148 |
Adjustments made for severity, race, payer, and census region.
Discussion
We performed a multicenter, retrospective study of hospitalized children with SSSS, including assessment of hospital-level variation in diagnostic testing and the association with patient outcomes. We found that significant variation exists in diagnostic testing among children hospitalized with SSSS. Lower rates of test use were not associated with significant differences in hospital-level LOS, readmissions, ED revisits, or escalations of care. Our findings reveal that some patient outcomes might not be adversely affected with an overall reduction in diagnostic testing.
Blood culture was the most common type of bacterial culture performed in our population, and hospital-level use varied from 62% to 100%. However, the use of blood culture for isolation of S aureus may be less helpful because bacteremia is thought to be uncommon in SSSS.15,21,32 Authors of 1 study found that 3.8% of children with SSSS had toxigenic S aureus isolated from the blood,1 and in our cohort, only 2.3% were coded for a diagnosis of sepsis or bacteremia. The association between the use of blood culture in SSSS and patient outcomes has not been described in the literature. In uncomplicated skin and soft tissue infections, blood culture yield is low, and its routine use is found to prolong LOS.33 Furthermore, routine blood culture is no longer recommended in uncomplicated cellulitis.34 Similarly, routine blood culture may be most indicated in severe cases of SSSS,7,15 but further large-scale research is needed to confirm in which clinical situations blood cultures are most useful.
In our cohort, hospital-level use of CBC, CRP, ESR, and electrolytes varied more widely than the use of blood culture. Despite being frequently used at some hospitals, the utility of these tests in pediatric SSSS has not been well studied. Authors of a study with a small sample size found that white blood cell count and levels of CRP were elevated in patients with SSSS compared with healthy controls. However, these tests had lesser accuracy to diagnose SSSS as compared with procalcitonin.35 In our cohort, we chose not to analyze procalcitonin because it was rarely ordered. In addition, electrolyte tests were performed in 80% of children in our cohort. Authors of other studies have demonstrated that electrolyte tests in hospitalized patients may be overused,23,36 but the appropriateness of these electrolyte tests in our cohort is unclear without the associated clinical context. The utility of using the above tests to diagnose and effectively manage SSSS remains uncertain. Additional studies are needed to determine if they lend any prognostic value for patient outcomes or severity of disease. However, as opposed to tests with less variation between hospitals, tests with more variation (such as CRP) may represent a greater opportunity to reduce testing in hospitals with high use.
Although our study was isolated to US children’s hospitals, patient outcomes were comparable with available data from other studies of SSSS. Overall median LOS was 3 days (adjusted median LOS of 2.5–2.6 days). In other studies, LOS ranged from 3 days6,16,37 to 5 to 8 days,38 and even longer in studies with primarily neonatal populations.13 Authors of a recent study found that being >2 years of age, being African American or multiracial, and having chronic conditions were associated with a longer LOS6; however, the authors of this study did not examine the impact of diagnostic testing. Also, we observed no mortalities in our cohort, which aligns with a recent US study in which a 0.3% mortality rate was reported.6 Our same-cause readmission rate of <1% is not surprising. Data on readmissions have not been well described, but recurrent SSSS is extremely rare, and most children have a good prognosis.10,39
We observed that variation in diagnostic test use was not strongly associated with patient outcomes in our cohort. Hospitals with lower rates of diagnostic test use did not experience significantly different LOS, cost, readmissions, or ED revisits. Interestingly, a lower rate of test use was not associated with lower costs. This may reflect the more sizable impact of costs related to LOS in relation to laboratory tests, which has been described in other studies.36,40 In the literature examining SSSS, the association between overall use of diagnostic tests and patient outcomes has not been specifically described. Further research focused on individual diagnostic test use and the results of those tests is needed to better evaluate the association between test use and patient outcomes. Researchers should also examine the population factors that may have contributed to differences in test use, such as government versus private payers and the socioeconomic status of patients. Researchers of future efforts could also focus on costs from the parental and societal perspectives for unnecessary test use. All of these factors could influence how hospitals might approach diagnostic test stewardship in children hospitalized with SSSS.
This study was subject to limitations inherent to the use of an administrative database. Identification of children with SSSS relied on recorded ICD-9-CM and ICD-10-CM diagnostic codes, which could result in patient misclassification. However, our cohort’s age demographic and higher incidence of SSSS in summer and early fall, which has been previously described with SSSS,1,4,6 suggest that children with SSSS were appropriately identified. Test results were not available, and therefore we cannot determine the clinical utility of performing the test. Culture results were not available to confirm a diagnosis of bacteremia or sepsis. Additionally, we were unable to identify the primary source of cultures with an unspecified site because of differences in hospital coding practices. For this reason, site-specific cultures were not quantified (such as nasopharyngeal cultures). Clinical details regarding progression of illness, such as the duration of fever or degree of skin desquamation, may have contributed to variability in diagnostic test use between hospitals and are not available in the PHIS database.7 Finally, because only PHIS hospitals were included, the description of practice and outcomes may not reflect all practice settings.
Conclusions
We found significant interhospital variation in diagnostic test use across hospitals with a similar case mix. Similar patient outcomes were present in our cohort between hospitals with high versus low rates of test use, suggesting that clinicians may be able to safely decrease diagnostic test use in SSSS. Further research with specific diagnostic test results may help guide the appropriate use of diagnostic testing and inform future development of clinical practice guidelines for children hospitalized with SSSS.
Footnotes
FINANCIAL DISCLOSURE: Dr Aronson is supported by a Clinical and Translational Science Award (grant KL2 TR001862) from the National Center for Advancing Translational Science, a component of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The other authors have indicated they have no financial relationships relevant to this article to disclose. Funded by the National Institutes of Health (NIH).
FUNDING: No external funding.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
Dr Neubauer conceptualized and designed the study, performed data analysis, and drafted the initial manuscript; Dr Hall contributed to the conceptualization and design of the study, conducted the statistical analysis, and revised the manuscript; Drs Lopez and Wallace contributed to the conceptualization and design of the study and data analysis and critically reviewed and revised the manuscript; Drs Cruz, Queen, Foradori, Aronson, Markham, Nead, Hester, and McCulloh contributed to the design of the study and data analysis and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
References
- 1.Lamand V, Dauwalder O, Tristan A, et al. Epidemiological data of staphylococcal scalded skin syndrome in France from 1997 to 2007 and microbiological characteristics of Staphylococcus aureus associated strains. Clin Microbiol Infect. 2012;18(12):E514–E521 [DOI] [PubMed] [Google Scholar]
- 2.Hulten KG, Kok M, King K, Mason EO, Lamberth LB, Kaplan SL. Increasing numbers of staphylococcal scalded skin syndrome cases at Texas Children’s Hospital are caused by ST121. Open Forum Infect Dis. 2016;3(suppl 1):671. [DOI] [PubMed] [Google Scholar]
- 3.Faden H. Neonatal staphylococcal skin infections. Pediatr Infect Dis J. 2003;22(4):389. [DOI] [PubMed] [Google Scholar]
- 4.Li MY, Hua Y, Wei GH, Qiu L. Staphylococcal scalded skin syndrome in neonates: an 8-year retrospective study in a single institution. Pediatr Dermatol. 2014;31(1):43–47 [DOI] [PubMed] [Google Scholar]
- 5.Hayward A, Knott F, Petersen I, et al. Increasing hospitalizations and general practice prescriptions for community-onset staphylococcal disease, England. Emerg Infect Dis. 2008;14(5):720–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Staiman A, Hsu DY, Silverberg JI. Epidemiology of staphylococcal scalded skin syndrome in U.S. children. Br J Dermatol. 2018;178(3):704–708 [DOI] [PubMed] [Google Scholar]
- 7.Blyth M, Estela C, Young AE. Severe staphylococcal scalded skin syndrome in children. Burns. 2008;34(1):98–103 [DOI] [PubMed] [Google Scholar]
- 8.Hubiche T, Bes M, Roudiere L, Langlaude F, Etienne J, Del Giudice P. Mild staphylococcal scalded skin syndrome: an underdiagnosed clinical disorder. Br J Dermatol. 2012;166(1):213–215 [DOI] [PubMed] [Google Scholar]
- 9.Courjon J, Hubiche T, Phan A, et al. Skin findings of Staphylococcus aureus toxin-mediated infection in relation to toxin encoding genes. Pediatr Infect Dis J. 2013;32(7):727–730 [DOI] [PubMed] [Google Scholar]
- 10.Ladhani S, Joannou CL, Lochrie DP, Evans RW, Poston SM. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clin Microbiol Rev. 1999;12(2):224–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Healthcare Cost and Utilization Project.Overview of the kids’ inpatient database (KID). Available at: www.hcup-us.ahrq.gov/kidoverview.jsp. Accessed October 16, 2017
- 12.Doudoulakakis A, Spiliopoulou I, Spyridis N, et al. Emergence of a Staphylococcus aureus clone resistant to mupirocin and fusidic acid carrying exotoxin genes and causing mainly skin infections. J Clin Microbiol. 2017;55(8):2529–2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neylon O, O’Connell NH, Slevin B, et al. Neonatal staphylococcal scalded skin syndrome: clinical and outbreak containment review. Eur J Pediatr. 2010;169(12):1503–1509 [DOI] [PubMed] [Google Scholar]
- 14.Dancer SJ, Simmons NA, Poston SM, Noble WC. Outbreak of staphylococcal scalded skin syndrome among neonates. J Infect. 1988;16(1):87–103 [DOI] [PubMed] [Google Scholar]
- 15.Ladhani S, Joannou CL. Difficulties in diagnosis and management of the staphylococcal scalded skin syndrome. Pediatr Infect Dis J. 2000;19(9):819–821 [DOI] [PubMed] [Google Scholar]
- 16.Chi CY, Wang SM, Lin HC, Liu CC. A clinical and microbiological comparison of Staphylococcus aureus toxic shock and scalded skin syndromes in children. Clin Infect Dis. 2006;42(2):181–185 [DOI] [PubMed] [Google Scholar]
- 17.Saida K, Kawasaki K, Hirabayashi K, et al. Exfoliative toxin A staphylococcal scalded skin syndrome in preterm infants. Eur J Pediatr. 2015;174(4):551–555 [DOI] [PubMed] [Google Scholar]
- 18.Yamasaki O, Yamaguchi T, Sugai M, et al. Clinical manifestations of staphylococcal scalded-skin syndrome depend on serotypes of exfoliative toxins. J Clin Microbiol. 2005;43(4):1890–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ladhani S. Recent developments in staphylococcal scalded skin syndrome. Clin Microbiol Infect. 2001;7(6):301–307 [DOI] [PubMed] [Google Scholar]
- 20.Elston DM, Stratman EJ, Miller SJ. Skin biopsy: biopsy issues in specific diseases. J Am Acad Dermatol. 2016;74(1):1–16; quiz 17–18 [DOI] [PubMed] [Google Scholar]
- 21.Handler MZ, Schwartz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J Eur Acad Dermatol Venereol. 2014;28(11):1418–1423 [DOI] [PubMed] [Google Scholar]
- 22.Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307(14):1513–1516 [DOI] [PubMed] [Google Scholar]
- 23.Zhi M, Ding EL, Theisen-Toupal J, Whelan J, Arnaout R. The landscape of inappropriate laboratory testing: a 15-year meta-analysis. PLoS One. 2013;8(11):e78962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lippi G, Bovo C, Ciaccio M. Inappropriateness in laboratory medicine: an elephant in the room? Ann Transl Med. 2017;5(4):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Messacar K, Parker SK, Todd JK, Dominguez SR. Implementation of rapid molecular infectious disease diagnostics: the role of diagnostic and antimicrobial stewardship. J Clin Microbiol. 2017;55(3):715–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan DJ, Malani P, Diekema DJ. Diagnostic stewardship-leveraging the laboratory to improve antimicrobial use. JAMA. 2017;318(7):607–608 [DOI] [PubMed] [Google Scholar]
- 27.Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048–2055 [DOI] [PubMed] [Google Scholar]
- 28.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sedman AB, Bahl V, Bunting E, et al. Clinical redesign using all patient refined diagnosis related groups. Pediatrics. 2004;114(4):965–969 [DOI] [PubMed] [Google Scholar]
- 30.Aronson PL, Thurm C, Alpern ER, et al. ; Febrile Young Infant Research Collaborative. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134(4):667–677 [DOI] [PubMed] [Google Scholar]
- 31.Thomson J, Hall M, Berry JG, et al. Diagnostic testing and hospital outcomes of children with neurologic impairment and bacterial pneumonia. J Pediatr. 2016;178:156–163.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berk DR, Bayliss SJ. MRSA, staphylococcal scalded skin syndrome, and other cutaneous bacterial emergencies. Pediatr Ann. 2010;39(10):627–633 [DOI] [PubMed] [Google Scholar]
- 33.Malone JR, Durica SR, Thompson DM, Bogie A, Naifeh M. Blood cultures in the evaluation of uncomplicated skin and soft tissue infections. Pediatrics. 2013;132(3):454–459 [DOI] [PubMed] [Google Scholar]
- 34.Stevens DL, Bisno AL, Chambers HF, et al. ; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America [published correction appears in Clin Infect Dis. 2015;60(9):1448]. Clin Infect Dis. 2014;59(2):e10–e52 [DOI] [PubMed] [Google Scholar]
- 35.Zeng M, Guo Z, Shen S, Liu S. Value of serum procalcitonin and interleukin-6 in patients with bullous impetigo and staphylococcal scalded skin syndrome. J Dermatol. 2014;41(11):1028–1029 [DOI] [PubMed] [Google Scholar]
- 36.Johnson DP, Lind C, Parker SE, et al. Toward high-value care: a quality improvement initiative to reduce unnecessary repeat complete blood counts and basic metabolic panels on a pediatric hospitalist service. Hosp Pediatr. 2016;6(1):1–8 [DOI] [PubMed] [Google Scholar]
- 37.Braunstein I, Wanat KA, Abuabara K, McGowan KL, Yan AC, Treat JR. Antibiotic sensitivity and resistance patterns in pediatric staphylococcal scalded skin syndrome. Pediatr Dermatol. 2014;31(3):305–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lipový B, Brychta P, Chaloupková Z, Suchánek I. Staphylococcal scalded skin syndrome in the Czech Republic: an epidemiological study. Burns. 2012;38(2):296–300 [DOI] [PubMed] [Google Scholar]
- 39.Davidson J, Polly S, Hayes PJ, Fisher KR, Talati AJ, Patel T. Recurrent staphylococcal scalded skin syndrome in an extremely low-birth-weight neonate. AJP Rep. 2017;7(2):e134–e137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rauh SS, Wadsworth EB, Weeks WB, Weinstein JN. The savings illusion–why clinical quality improvement fails to deliver bottom-line results. N Engl J Med. 2011;365(26):e48. [DOI] [PubMed] [Google Scholar]