Through conduct of a prospective longitudinal cohort, we identify factors that promote ADHD medication continuity from a variety of domains.
Abstract
OBJECTIVES:
To identify predictors of attention-deficit/hyperactivity disorder (ADHD) medication continuity, hypothesizing greater continuity among children with (1) greater child acceptance of treatment, (2) parent belief in longer time course for ADHD, (3) positive differential between parent-perceived need for and concerns about medication, and (4) greater parent-perceived alliance with their child’s doctor.
METHODS:
We conducted a prospective longitudinal cohort of 89 children aged 6 to 10 years old newly treated for ADHD by 1 of 44 pediatricians in 11 practices. Parents completed validated surveys on their beliefs about ADHD and medicine. We audited charts and obtained pharmacy dispensing records. In our analyses, we examined the relationship between predictor variables (eg, sociodemographic and clinical characteristics, quality of care, and belief measures) and short-term (first 90 days after starting medication) and long-term (91–450 days) medication continuity as defined by the number of days covered with medication.
RESULTS:
Children had a median of 81% of days covered over 0 to 90 days and 54% of days covered over 91 to 450 days after starting medicine. In the first 90 days, medication coverage related to child age, satisfaction with information about medicine, medication titration, symptom reduction, parent beliefs about control over symptoms, uncertainty about treating with medicine, and working alliance. Long-term medication continuity related to child acceptance of treatment and differential between parent-perceived need for and concerns about medication at 3 months, not baseline factors.
CONCLUSIONS:
Adherence is a process that can change over time in response to experiences with treatment. Interventions are needed to promote productive interactions between pediatricians and families in support of continuity.
What’s Known on This Subject:
Children treated for attention-deficit/hyperactivity disorder (ADHD) often stop taking medicine or periodically stop and restart. Given the impairments experienced by children with ADHD, discontinuity of treatment is a major public health concern. Factors that promote medication continuity have not been fully elucidated.
What This Study Adds:
We identified factors that promote continuity from a variety of domains including child and clinical characteristics, quality of ADHD care, and beliefs that influence family decision-making. Medication continuity is impacted by several potentially modifiable factors.
Pharmacological treatment, usually with stimulants, is one of the most frequently used evidence-based interventions for children with attention-deficit/hyperactivity disorder (ADHD).1,2 However, most children treated for ADHD discontinue treatment either by stopping altogether or periodically stopping and restarting medicine.3,4 Discontinuity of treatment prevents children from realizing the full therapeutic benefits.5 Given the impairing academic, social, and family difficulties experienced by children with ADHD,6 medication discontinuity is a major public health concern. Several researchers have demonstrated that specific child characteristics (eg, age, race),3,7–12 clinical characteristics (eg, amount of symptom reduction),3,8,11,13 and quality of ADHD care (eg, titration)3,9,14 influence medication continuity. However, qualitative research suggests that parent and child acceptance of treatment, parental beliefs about ADHD, medications, and the relationship with their child’s doctor all impact whether families continue treatment.15–21 Indeed, familial beliefs about illness (eg, expected time course, etc), treatment (eg, perceived need, concerns), and working alliance between patient and physician have been shown to be better predictors of medication continuity than demographic or clinical factors across a range of other conditions (eg, asthma, diabetes, depression, etc).22–26 To date, how these decision-making factors influence medication continuity in children receiving ADHD pharmacological treatment has not been examined in any study. Such research is essential to guide the development of tailored interventions to improve continuity. In this study, we examine a broad range of factors as they relate to ADHD medication continuity in community pediatric settings, including demographic and clinical characteristics, specific aspects of ADHD care, working alliance, and familial beliefs about ADHD and ADHD medicines. On the basis of qualitative research involving parents of children with ADHD,15–21 we hypothesized that the following factors would predict greater medication continuity: (1) greater child acceptance of treatment, (2) parent belief in longer time course for ADHD, (3) positive differential between parent-perceived need for and concerns about ADHD medication, and (4) greater parent-perceived alliance with their child’s doctor.
Methods
Study Design, Setting, and Participants
We conducted a prospective longitudinal cohort study with patients recruited from practices in the Cincinnati and northern Kentucky region from March 2010 to September 2013. Eligible families were English-speaking and had a child aged 6 to 10 years old who was ADHD medication naïve and being assessed for ADHD with or without co-occurring diagnoses. Children were retained in the cohort if they were prescribed an ADHD medication within 3 months of their assessment. A member of the office staff at each practice served as a research liaison to identify potentially eligible subjects at the time ADHD assessment was initiated and request the parent’s permission for research staff to contact them with more information about the study.
Procedures
After consenting to participate, the parent or guardian who self-identified as the child’s primary caregiver (hereafter referred to as “parent”) completed the assessment battery (see Supplemental Table 4 for psychometric properties of measures) after the visit with their child’s doctor to discuss assessment results and develop a treatment plan (hereafter referred to as “baseline”). Parents repeated the assessment battery 3 months later. At 18 months, we conducted a chart audit and collected pharmacy dispensing records. The institutional review board approved this study.
Clinical Characteristics
Parents completed measures to characterize their own characteristics and their child’s clinical symptoms and response to treatment. Parents completed the Rapid Estimate of Adult Literacy in Medicine-Short Form, a validated 7-item scale,27 and the Subjective Numeracy Scale, a validated 8-item scale.28,29 Parents reported on their own psychological distress using the K6 scale, a validated 6-item screen that is highly correlated with diagnostic interviews for serious mental illness.30 Parents reported child quality of life on the Pediatric Quality of Life Inventory version 4.0 generic core scale (PedsQL).31 We used T-scores from parent report on the Behavioral Assessment System for Children, Second Edition (BASC2)32 to characterize child externalizing symptoms including aggression and conduct problems. Parents reported child ADHD symptoms and related impairment using the Vanderbilt ADHD Parent Rating Scale (VAPRS).33 From this, we calculated the ADHD symptom severity and average impairment score at baseline and reduction in ADHD symptoms from baseline to 3 months. Parents completed the 13-item Pittsburgh Side Effects Rating Scale.34 From this, we calculated the number of side effects attributable to medication by subtracting the number of moderate or severe side effects reported at baseline from those reported at 3 months. We collected this measure at baseline because some children experience these symptoms (ie, headache, stomach aches, etc) before starting medicine. Our rationale is that most side effects will be apparent in the first 3 months of trying medication.
Chart Audit
After 18 months of participation, 1 of 2 research assistants extracted the following information from each patient chart (ie, problem and medication lists, visit progress notes, contact notes, scanned documents, etc) for any ADHD care provided during the study: (1) information on prescriptions written (ie, date, medication, dosage, amount dispensed), (2) dates of all ADHD-related treatment visits and contacts (ie, phone, e-mail correspondence), (3) dates of collection and scores for all parent- and teacher-completed ADHD rating scales, and (4) any co-occurring diagnoses. Interrater reliability for chart reviews was calculated by using a random sampling of 10% of charts, with intraclass correlations (ICCs) for continuous data and κ values for dichotomous data averaging 0.945.
Measures of ADHD Care Quality
We derived a number of clinical care delivery variables from chart audit data. We defined presence of a medication titration as having an adjustment (ie, dosage change, medication switch, or addition or removal of a medicine) in the first 3 months. We defined presence of a low complexity dosing regimen as child initially prescribed only an extended-release medication. We defined presence of initiation phase monitoring as having a visit with their doctor within 30 days of the initial prescription. We characterized treatment monitoring by the number of physician-parent contacts (ie, visits, phone calls, or e-mails to discuss the child’s response to ADHD treatment, excluding parent contacts with office staff solely to request a refill) and the number of behavior rating scales collected from a parent or teacher. At baseline, parents completed the Parent’s Perception of Primary Care (P3C) related to their child’s doctor’s practice35 and the Satisfaction with Information about Medicine Scale related to the information received about their child’s initial ADHD prescription.36
Measures of Parent and Child Beliefs
Parents and children completed a variety of validated measures to characterize their beliefs about ADHD, treatment of ADHD, and ADHD care. Children completed 3 items about their acceptance of ADHD medicine.37 Responses to each item were dichotomized as positive acceptance or neutral versus negative. Parents completed the 10-item Brief Illness Perceptions Questionnaire (BIPQ), which assesses the parents’ representation of ADHD (eg, expected time course, consequences, etc).38–41 Parents completed the Decisional Conflict Scale to characterize parent comfort with the initial treatment plan.42 Parents completed the Beliefs about Medicines Questionnaire (BMQ),43,44 which has subscales to assess beliefs about the overuse of medicine by doctors and the intrinsic harmfulness of medicine (assessed at baseline) as well as beliefs about the necessity of medicine and concerns about potential adverse effects (assessed at 3 months). A needs and concerns differential score is calculated by subtracting the subscale scores. Parents also completed the Working Alliance Inventory to assess agreement on the goals and tasks of treatment and the extent to which there was a strong personal bond with their child’s physician.24,45
Outcome Measures
On the basis of chart audit of prescriptions written verified by pharmacy dispensing records, we calculated short-term (first 90 days after initiating medication) and long-term (91–450 days postinitiation) medication continuity as defined by the number of days covered with medication (see Supplemental Information for details). We examined these periods separately because qualitative research suggests that factors influencing short-term and long-term adherence may differ.46 We chose medication obtained for the child because it provides an objective, unobtrusive, reliable measure that is a well-accepted proxy for medication consumption47,48 that has been reported in past ADHD studies.14,49–51 We double-coded the pharmacy data from a random sample of 21 subjects (23% of sample) to examine intrarater reliability. The intraclass correlation coefficient for number of days covered with medicine was 0.998.
Statistical Analyses
We calculated descriptive statistics for all variables. To reduce issues of multicollinearity in multivariable models and aid in data reduction, we created factor scores for the 10-item BIPQ. We used exploratory factor analysis with varimax rotation to determine the number of factors needed and the pattern of factor loadings. We retained 3 factors on the basis of eigenvalue >1. We used the regression method to create factor scores52 for each of the 3 factors and named each on the basis of the items included.
Out of the 30 predictors of interest, 10 had missing values (see Table 1). Missing values were imputed by using the nonparametric random forest method.53 In this approach, we used a random forest trained on the observed values of the data matrix to predict the missing values, with the ability to impute continuous and categorical data.
TABLE 1.
Predictor Variables
| No. (%) or Mean (SD), Total N = 89a | |
|---|---|
| Child and parent demographics | |
| Child age (y)3,7–9,11–13,54 | 8.3 (1.4) |
| Child white and/or non-Hispanic3,7,8,11 | 63 (71%) |
| Public insurance8,10,11,54 | 34 (38%) |
| Clinical characteristics | |
| Parent with severe psychological distress present54 (baseline)b | 9 (10%) |
| PedsQL total score (baseline) [range 0–100, higher = better quality]6 | 67.3 (12.4) |
| BASC2 externalizing symptom T-score (baseline)3,8,11,13 | 68.0 (13.7) |
| VAPRS total symptom score (baseline) [range 0–54, higher = more symptoms]13 | 36.5 (9.5) [n = 88] |
| VAPRS impairment score (baseline) [range 0–5, higher = more impairment]8 | 3.1 (0.6) [n = 84] |
| Reduction in ADHD symptoms from baseline to 3 mo [range 0–54]17 | 15.2 (12.8) [n = 84] |
| No. moderate or severe side effects attributable to medication at 3 mo [range = 0–13, with higher numbers indicating more side effects]15,16,55–57 | 0.1 (1.8) [n = 85] |
| Quality of care | |
| P3C summary score (baseline) [range 0–100, higher = better quality]15 | 86.6 (10.7) [n = 79] |
| Satisfaction with information about Medicine Scale total score (baseline) [range 0–14, higher = more satisfied]15 | 12.3 (2.4) |
| Presence of a low-complexity dosing regimen3,9 | 62 (70%) |
| Presence of a visit in first 30 d of treatment14 | 30 (34%) |
| Presence of a medication titration in first 3 mo14 | 61 (69%) |
| No. physician-parent contacts (0–90 d of treatment)14 | 3.0 (2.6) |
| No. physician-parent contacts (91–450 d of treatment)14 | 3.9 (3.1) |
| No. physician-collected behavior rating scales (0–90 d of treatment)1 | 1.7 (1.9) |
| No. physician-collected behavior rating scales (91–450 d of treatment)1 | 1.8 (2.2) |
| Child acceptance of medication | |
| How do you feel about the medicine? [child responded = “I don’t like taking medicine,” 3 mo]15,16 | 16 (22%) [n = 72] |
| Does the medicine help you? [child responded = no, 3 mo]15,16 | 6 (8%) [n = 72] |
| Do you want to take the medicine again? [child responded = no, 3 mo]15,16 | 15 (21%) [n = 73] |
| Parent beliefs about ADHD, medicine, and their child’s doctor | |
| BIPQ factor 1: impact of ADHD on life (baseline) [range 0–10, higher = stronger belief]15–21 | 7.2 (1.5)c |
| How much do you think your child’s ADHD affects his or her life? | |
| How much does your child’s ADHD affect your life? | |
| How much does your child experience symptoms from his or her ADHD? | |
| How concerned are you about your child’s ADHD? | |
| How much does your child’s ADHD affect you emotionally? | |
| BIPQ factor 2: amount of control over ADHD (baseline) [range 0–10, higher = stronger belief]15–21 | 3.3 (2.1)c |
| How much control do you feel your child has over his or her ADHD? | |
| How much control do you feel you have over your child’s ADHD? | |
| BIPQ factor 3: understanding ADHD and course (baseline) [range 0–10, higher = stronger belief]15–21 | 7.1 (1.5)c |
| How long do you think your child’s ADHD will continue? | |
| How much do you think your child’s treatment can help his or her ADHD? | |
| How well do you feel you understand your child’s ADHD? | |
| Decisional Conflict Scale total score (baseline) [range 0–100, higher = more conflict]15–21 | 20.5 (15.3) [n = 88] |
| BMQ overuse subscale score (baseline) [range 1–5, higher = stronger belief]15–21 | 2.9 (0.8) |
| BMQ harm subscale score (baseline) [range 1–5, higher = stronger belief]15–21 | 2.2 (0.7) |
| BMQ needs and concerns differential score (3 mo) [range −4 to 4, >1 favors needs]15–21 | 1.0 (1.1) [n = 76] |
| Working Alliance Inventory (baseline) [range 12–60, higher = greater alliance]15–21 | 50.7 (4.9) |
Those variables with missing data have the correct N in brackets.
K6 scale produces a total score with range from 0 (no distress) to 24 (maximal distress); scores of 13 or higher are suggestive of serious mental illness.30
Average score of factor loading items, mean (SD).
We centered all predictor and outcome variables to account for clustering at the practice level.58 This approach was justified because preliminary analyses revealed significant variation at the practice level for both short- and long-term medication continuity, (ICC = 0.14 [95% confidence interval 0.02–0.44] and 0.17 [95% confidence interval 0.04–0.43], respectively) but not at the physician level (ICC = 0 and 0, respectively). Data from all subjects are included in all analyses. Linear regression analysis was used to evaluate the univariate association between each predictor (see Table 1 for predictors) and short- and long-term medication continuity separately. For multivariable models, we used least absolute shrinkage and selection operator (lasso) regression analysis59,60 to identify the most important subset of predictors of short- and long-term medication continuity separately. The lasso approach is well-suited for high-dimensional data in which the number of predictors may be large relative to the sample size and when predictors may be correlated. We report the standardized coefficients for these variables as well as the variance in medication continuity explained by these variables. To examine temporal (ie, summer) influences on short-term medication continuity, we conducted sensitivity analyses that excluded children who started medicine in any month in which the mean number of days covered for the 3-month interval was >1 SD below the grand mean. Please see Supplemental Information for additional details on the analytic approach.
Results
Participants
We approached pediatricians in a practice-based research network comprising 30 practices. Forty-four pediatricians in 11 practices agreed to participate. Research liaisons approached 265 parents and 209 agreed to be contacted with information about the study. Of these, 35 were unable to be contacted by research staff, 20 were found to be ineligible, 20 declined participation, and 131 enrolled in the study. Of these, 89 children met the final eligibility criteria of being prescribed ADHD medication within 3 months of assessment for ADHD. Pediatricians were predominantly white (84%), women (61%), with a median age (interquartile range) of 48 (44–56) years, and saw a median (interquartile range) number of ADHD patients per week of 5 (3–10). The mean (SD) number of children enrolled per pediatrician was 2.4 (1.6) (range 1–6]). Children were predominantly boys (68%) (see Table 1 for additional characteristics). A minority of children had a co-occurring mental health diagnosis (7 out of 89 = 7.9%) (eg, adjustment disorder, depression, anxiety, obsessive-compulsive disorder), learning disorder (4 out of 89 = 4.5%), or speech disorder (8 out of 89 = 9.0%) documented in their chart. Parents were primarily women (94%), married (60%), and had a mean (SD) age of 35.9 (7.6) years. The vast majority had graduated high school (60%) or college (29%), had a high literacy level (87% greater than or equal to ninth grade), and had average to high numeracy (77%).
Predictors
We provide descriptive statistics for all predictors in Table 1. At baseline, children had ADHD symptoms and functional impairment. On average, medication was associated with ADHD symptom reductions. There was wide variation on ADHD quality of care measures.
Outcome Measures
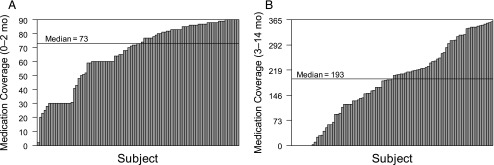
We summarize medications prescribed in Table 2. Variability in short- and long-term medication continuity is depicted in Fig 1 A and B. In the first 90 days after initiating medication, children had a median of 81% (73 out of 90) of days covered with medicine. Children who started medication in May (n = 10) had a mean (SD) number of days covered = 40.7 (18.3). This was the only month in which the mean was below the SD for the grand mean = 66.2 (22.3). Between 91 and 450 days postinitiation, children had a median of 54% (193 out of 360) of days covered with medicine.
TABLE 2.
Medication Summary
| No. (%) | |
|---|---|
| Initial prescription | |
| Extended release stimulant alone | 80 (90) |
| Immediate release stimulant alone | 6 (7%) |
| Extended release nonstimulant alone | 3 (3%) |
| >1 category of ADHD medication | 0 |
| Receipt of first prescription at baseline visit | 63 (71) |
| Medication changes | Mean (SD) |
| No. drug changes per child | 1.0 (1.4) |
| No. dosage changes per child | 2.2 (2.0) |
| No. times a medication was added per child | 0.3 (0.8) |
| No. times a medication was removed per child | 0.3 (0.8) |
| Final daily dosage in MPH-equivalent milligramsa | 26.5 (12.0) |
MPH, methylphenidate.
For children prescribed stimulant medication, the daily dosage for the final prescription written was calculated in MPH-equivalent milligrams by converting other stimulant medication by using the following conversions (mixed salt amphetamines dose or dexmethylphenidate dose × 2; lisdexamfetamine dimesylate × 0.8).
FIGURE 1.
Days covered with medication for (A) short-term medication continuity and (B) long-term medication continuity. Each bar represents an individual subject’s days of coverage.
Predicting Short-term Medication Continuity
The most important predictors of increased medication continuity during the first 90 days of treatment were younger child age, greater satisfaction with information received about medicine, presence of a medication titration in first 3 months, greater reduction in ADHD symptoms from baseline to 3 months, stronger parent beliefs about control over ADHD symptoms, lower decisional conflict scale total score, and lower working alliance (see Table 3). These variables accounted for 32% of the variance in short-term medication continuity. Of note, working alliance was not related to the outcome in unadjusted analyses, but exerted enhancer effects61 on other predictor variables retained in the multivariable lasso model (see Supplemental Information for details). Sensitivity analyses excluded the 10 children who started medicine in May. This lasso model retained the same variables with the exception of decisional conflict, which was no longer important.
TABLE 3.
Predictive Models
| Short-term Continuity (0–90 d) | Long-term Continuity (91–450 d) | |||
|---|---|---|---|---|
| Unadjusteda | Multivariable Lassob | Unadjusteda | Multivariable Lassob | |
| Child and parent demographics | ||||
| Child age, y | −0.19 | −0.18 | −0.19 | |
| Child white and/or non-Hispanic | 0.14 | −0.05 | ||
| Public insurance | −0.10 | −0.06 | ||
| Clinical characteristics | ||||
| Parent with severe psychological distress present | −0.07 | −0.22* | ||
| PedsQL (baseline) | −0.03 | 0.09 | ||
| BASC2 external symptom T-score (baseline) | 0.18 | 0.19 | ||
| VAPRS total symptom score (baseline) | 0.06 | 0.10 | ||
| VAPRS impairment score (baseline) | 0.13 | 0.01 | ||
| Reduction in ADHD symptoms from baseline to 3 mo | 0.18 | 0.13 | 0.13 | |
| No. moderate or severe medicine side effects at 3 mo | 0.09 | 0.11 | ||
| Quality of care | ||||
| P3C summary score (baseline) | 0.07 | 0.02 | ||
| Satisfaction with information about Medicine Scale total score (baseline) | 0.23* | 0.32 | 0.16 | |
| Presence of a low-complexity dosing regimen | −0.03 | −0.16 | ||
| Presence of a visit in first 30 d | 0.15 | 0.11 | ||
| Presence of a medication titration in first 3 mo | 0.24* | 0.29 | 0.05 | |
| No. physician-parent contacts during relevant time period (ie, 0–90 or 90–450 d) | 0.19 | 0.00 | ||
| No. physician-collected behavior rating scales during relevant time period (ie, 0–90 or 90–450 d) | 0.13 | −0.16 | ||
| Child acceptance of medication | ||||
| Child dislikes medicine (3 mo) | −0.03 | −0.30* | −0.25 | |
| Child doesn’t think medicine helps (3 mo) | −0.02 | −0.08 | ||
| Child doesn’t want to take medicine again (3 mo) | −0.02 | −0.22* | ||
| Parent beliefs | ||||
| BIPQ factor 1: impact of ADHD on lifec | 0.23* | 0.10 | ||
| BIPQ factor 2: amount of control over ADHDc | 0.18 | 0.26 | 0.10 | |
| BIPQ factor 3: understanding ADHD and coursec | 0.18 | 0.10 | ||
| Decisional conflict scale total score (baseline) | −0.28* | −0.22 | −0.26* | |
| BMQ overuse subscale score (baseline) | −0.12 | −0.27* | ||
| BMQ harm subscale score (baseline) | −0.13 | −0.21* | ||
| BMQ needs and concerns differential score (3 mo) | 0.20 | 0.37* | 0.33 | |
| Working alliance inventory (baseline) | −0.06 | −0.34 | 0.04 | |
Standardized regression coefficients.
Standardized partial regression coefficients of the variables retained in the final model were selected on the basis of the lowest residual sum of squares with model averaging. The retained variables predict medication continuity and the ones not retained do not predict medication continuity.
Predictors were entered in models as standardized factor scores estimated from regression method.52
P < .05.
Predicting Long-term Medication Continuity
Between 91 and 450 days postinitiation, the most important predictors of increased medication continuity were higher child’s acceptance of medication and higher needs or concerns differential score (see Table 3). These variables accounted for 20% of the variance in long-term medication continuity.
Discussion
With this study, we filled a sizable gap in the literature by prospectively assessing the impact of child and parent beliefs about ADHD and medication on treatment continuity using validated measures. Moving beyond past research that attributed variation in medication continuity to nonmodifiable participant characteristics is a necessary step to inform interventions. Characteristics such as younger child age, nonminority race and/or ethnicity, and absence of public insurance have predicted greater medication continuity in past studies.3,7–12,54 Consistent with this literature, younger child age predicted greater short-term medication continuity, but neither child race and/or ethnicity nor public insurance status was related to continuity in unadjusted or multivariate lasso models.
We identified 7 important predictors of short-term medication continuity. Our finding that stronger parent perceptions of the controllability of ADHD symptoms at baseline predicted continuity is similar to reports involving adults with a variety of chronic conditions.25 Consistent with past research, greater parent satisfaction with information about medicine36 and comfort with the treatment plan62 were important predictors. Consistent with our past research,14 the presence of a medication titration, which is recommended in ADHD clinical practice guidelines to maximize benefit and minimize side effects,1 was an important predictor of continuity. Improving the prevalence and timeliness of titration for children newly treated for ADHD holds promise to improve subsequent medication continuity.4,14,63 However, no algorithms or quality metrics exist to guide such efforts.1,64 Greater reduction in symptoms was also an important predictor, similar to past research.13 It is difficult to interpret the enhancer role that working alliance is playing. However, because this is the first report examining alliance in the context of pediatric pharmacotherapy, additional research is warranted.
We identified 2 important predictors of long-term medication continuity. As hypothesized, the differential between parent perception of need for and concerns about ADHD medication predicted greater long-term continuity. This finding is similar to a recent meta-analysis of 25 studies among adult patients23 and a study of parents of children with asthma22 in which significant correlations between the needs/concerns differential and adherence were reported. This measure includes items related to concerns about side effects and may have better captured the influence of side effects than counting the number of moderate or severe side effects reported by parents, which was unrelated to continuity in our study. It is possible that parent report of side effects underestimated the true impact if some parents based their side effect ratings at 3 months on child’s experiences after stopping the medicine that caused side effects. Indeed, researchers for several,15,16,55–57 but not all,65 retrospective studies have identified side effects as an influential factor. As hypothesized, child dislike for taking medicine predicted worse long-term medication continuity. This is akin to past studies revealing child oppositional symptoms being influential, presumably because of child refusal to take medicine.13 Parent belief in a longer time course for ADHD (as measured by BIPQ factor 3) did not impact medication continuity during the first 15 months of treatment. It is possible that parent beliefs about “outgrowing ADHD” exert an influence later.
It is noteworthy that the baseline factors that predicted getting off to a good start did not predict long-term continuity. Rather, child acceptance of medicine and parent perception of need for and concerns about ADHD medication became important factors. This is consistent with conceptualizing adherence as a process that can change over time in response to experiences with treatment.66 Pediatricians can help support families early in the treatment process by providing education about treatment options and engaging in shared decision-making to decrease decisional conflict.49 Moreover, pediatricians should titrate medicine to maximize symptom reduction and minimize side effects. Additional research is needed to determine whether monitoring child and parent beliefs about medicine over time with validated measures has clinical utility that would aid physicians to support medication continuity.
This study’s findings must be interpreted in light of study limitations. Medication supply served as a proxy for consumption. Chart reviews may have underestimated the amount of care provided if ADHD care (ie, visits, phone calls, e-mails, behavioral rating scales, etc) was not documented . Our sample size was modest, had a fairly high literacy rate, and was limited geographically. Therefore, our results may not be generalizable to other populations. Pediatricians volunteered for our study and some may have endeavored to improve ADHD care quality. Although this likely provided the variability in ADHD care needed to detect associations with medication continuity, these pediatricians may not be representative of all pediatricians. In addition, our analyses were focused on children who started medication within 3 months of being assessed for ADHD. Additional research is needed to understand how continuity might differ among families who delay treatment initiation.
Conclusions
Medication continuity is impacted by several factors that are potentially modifiable. Interventions are needed to promote productive interactions between pediatricians and families in support of continuity.
Acknowledgments
We are grateful for the support of our practice-based research network, the Cincinnati Pediatric Research Group, and the pediatricians who made this study possible.
Glossary
- ADHD
attention-deficit/hyperactivity disorder
- BASC2
Behavioral Assessment System for Children, Second Edition
- BIPQ
Brief Illness Perceptions Questionnaire
- BMQ
Beliefs about Medicines Questionnaire
- ICC
intraclass correlation
- PedsQL
Pediatric Quality of Life Inventory version 4.0 generic core scale
- P3C
Parent’s Perception of Primary Care
- VAPRS
Vanderbilt ADHD Parent Rating Scale
Footnotes
Dr Brinkman conceptualized and designed the study, and drafted the initial manuscript; Dr Epstein conceptualized and designed the study, reviewed and revised the manuscript; Dr Sucharew carried out the analyses, reviewed and revised the manuscript; Ms Majcher designed the data collection instruments, and coordinated and supervised data collection, and critically reviewed the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Brinkman and Epstein were supported by grants K23MH083027 and K24MH064478 from the National Institute of Mental Health, respectively. Supported by an Institutional Clinical and Translational Science Award, National Institutes of Health and National Center for Research Resources grant UL1TR01425. The funders played no role in study design or conduct; data collection, management, analysis, or interpretation; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Wolraich M, Brown L, Brown RT, et al. ; Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management . ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The MTA Cooperative Group; Multimodal Treatment Study of Children with ADHD A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 1999;56(12):1073–1086 [DOI] [PubMed] [Google Scholar]
- 3.Marcus SC, Wan GJ, Kemner JE, Olfson M. Continuity of methylphenidate treatment for attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 2005;159(6):572–578 [DOI] [PubMed] [Google Scholar]
- 4.Perwien A, Hall J, Swensen A, Swindle R. Stimulant treatment patterns and compliance in children and adults with newly treated attention-deficit/hyperactivity disorder. J Manag Care Pharm. 2004;10(2):122–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MTA Cooperative Group National Institute of Mental Health Multimodal Treatment Study of ADHD follow-up: changes in effectiveness and growth after the end of treatment. Pediatrics. 2004;113(4):762–769 [DOI] [PubMed] [Google Scholar]
- 6.Klassen AF, Miller A, Fine S. Health-related quality of life in children and adolescents who have a diagnosis of attention-deficit/hyperactivity disorder. Pediatrics. 2004;114(5). Available at: www.pediatrics.org/cgi/content/full/114/5/e541 [DOI] [PubMed] [Google Scholar]
- 7.Cox ER, Motheral BR, Henderson RR, Mager D. Geographic variation in the prevalence of stimulant medication use among children 5 to 14 years old: results from a commercially insured US sample. Pediatrics. 2003;111(2):237–243 [DOI] [PubMed] [Google Scholar]
- 8.Leslie LK, Weckerly J, Landsverk J, Hough RL, Hurlburt MS, Wood PA. Racial/ethnic differences in the use of psychotropic medication in high-risk children and adolescents. J Am Acad Child Adolesc Psychiatry. 2003;42(12):1433–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez RJ, Crismon ML, Barner JC, Bettinger T, Wilson JP. Assessment of adherence measures with different stimulants among children and adolescents. Pharmacotherapy. 2005;25(7):909–917 [DOI] [PubMed] [Google Scholar]
- 10.Stevens J, Harman JS, Kelleher KJ. Race/ethnicity and insurance status as factors associated with ADHD treatment patterns. J Child Adolesc Psychopharmacol. 2005;15(1):88–96 [DOI] [PubMed] [Google Scholar]
- 11.Visser SN, Lesesne CA, Perou R. National estimates and factors associated with medication treatment for childhood attention-deficit/hyperactivity disorder. Pediatrics. 2007;119(suppl 1):S99–S106 [DOI] [PubMed] [Google Scholar]
- 12.Cummings JR, Ji X, Allen L, Lally C, Druss BG. Racial and ethnic differences in ADHD treatment quality among Medicaid-enrolled youth. Pediatrics. 2017;139(6):e20162444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thiruchelvam D, Charach A, Schachar RJ. Moderators and mediators of long-term adherence to stimulant treatment in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2001;40(8):922–928 [DOI] [PubMed] [Google Scholar]
- 14.Brinkman WB, Baum R, Kelleher KJ, et al. Relationship between attention-deficit/hyperactivity disorder care and medication continuity. J Am Acad Child Adolesc Psychiatry. 2016;55(4):289–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brinkman WB, Sherman SN, Zmitrovich AR, et al. Parental angst making and revisiting decisions about treatment of attention-deficit/hyperactivity disorder. Pediatrics. 2009;124(2):580–589 [DOI] [PubMed] [Google Scholar]
- 16.Charach A, Skyba A, Cook L, Antle BJ. Using stimulant medication for children with ADHD: what do parents say? A brief report. J Can Acad Child Adolesc Psychiatry. 2006;15(2):75–83 [PMC free article] [PubMed] [Google Scholar]
- 17.Leslie LK, Plemmons D, Monn AR, Palinkas LA. Investigating ADHD treatment trajectories: listening to families’ stories about medication use. J Dev Behav Pediatr. 2007;28(3):179–188 [DOI] [PubMed] [Google Scholar]
- 18.Arcia E, Fernández MC, Jáquez M. Latina mothers’ stances on stimulant medication: complexity, conflict, and compromise. J Dev Behav Pediatr. 2004;25(5):311–317 [DOI] [PubMed] [Google Scholar]
- 19.dosReis S, Mychailyszyn MP, Myers M, Riley AW. Coming to terms with ADHD: how urban African-American families come to seek care for their children. Psychiatr Serv. 2007;58(5):636–641 [DOI] [PubMed] [Google Scholar]
- 20.Olaniyan O, dosReis S, Garriett V, et al. Community perspectives of childhood behavioral problems and ADHD among African American parents. Ambul Pediatr. 2007;7(3):226–231 [DOI] [PubMed] [Google Scholar]
- 21.Ahmed R, Borst J, Wei YC, Aslani P. Parents’ perspectives about factors influencing adherence to pharmacotherapy for ADHD. J Atten Disord. 2017;21(2):91–99 [DOI] [PubMed] [Google Scholar]
- 22.Conn KM, Halterman JS, Lynch K, Cabana MD. The impact of parents’ medication beliefs on asthma management. Pediatrics. 2007;120(3). Available at: www.pediatrics.org/cgi/content/full/120/3/e521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foot H, La Caze A, Gujral G, Cottrell N. The necessity-concerns framework predicts adherence to medication in multiple illness conditions: a meta-analysis. Patient Educ Couns. 2016;99(5):706–717 [DOI] [PubMed] [Google Scholar]
- 24.Fuertes JN, Mislowack A, Bennett J, et al. The physician-patient working alliance. Patient Educ Couns. 2007;66(1):29–36 [DOI] [PubMed] [Google Scholar]
- 25.Hagger MS, Orbell S. A meta-analytic review of the common-sense model of illness representations. Psychol Health. 2003;18(2):141–184 [Google Scholar]
- 26.Yoos HL, Kitzman H, Henderson C, et al. The impact of the parental illness representation on disease management in childhood asthma. Nurs Res. 2007;56(3):167–174 [DOI] [PubMed] [Google Scholar]
- 27.Arozullah AM, Yarnold PR, Bennett CL, et al. Development and validation of a short-form, rapid estimate of adult literacy in medicine. Med Care. 2007;45(11):1026–1033 [DOI] [PubMed] [Google Scholar]
- 28.Fagerlin A, Zikmund-Fisher BJ, Ubel PA, Jankovic A, Derry HA, Smith DM. Measuring numeracy without a math test: development of the Subjective Numeracy Scale. Med Decis Making. 2007;27(5):672–680 [DOI] [PubMed] [Google Scholar]
- 29.Zikmund-Fisher BJ, Smith DM, Ubel PA, Fagerlin A. Validation of the Subjective Numeracy Scale: effects of low numeracy on comprehension of risk communications and utility elicitations. Med Decis Making. 2007;27(5):663–671 [DOI] [PubMed] [Google Scholar]
- 30.Kessler RC, Barker PR, Colpe LJ, et al. Screening for serious mental illness in the general population. Arch Gen Psychiatry. 2003;60(2):184–189 [DOI] [PubMed] [Google Scholar]
- 31.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800–812 [DOI] [PubMed] [Google Scholar]
- 32.Reynolds CR, Kamphaus RW. Manual for the Behavior Assessment System for Children. 2nd ed. Circle Pines, MN: AGS Publishing; 2004 [Google Scholar]
- 33.Wolraich ML, Lambert W, Doffing MA, Bickman L, Simmons T, Worley K. Psychometric properties of the Vanderbilt ADHD diagnostic parent rating scale in a referred population. J Pediatr Psychol. 2003;28(8):559–567 [DOI] [PubMed] [Google Scholar]
- 34.Pelham WE Jr, Carlson C, Sams SE, Vallano G, Dixon MJ, Hoza B. Separate and combined effects of methylphenidate and behavior modification on boys with attention deficit-hyperactivity disorder in the classroom. J Consult Clin Psychol. 1993;61(3):506–515 [DOI] [PubMed] [Google Scholar]
- 35.Seid M, Varni JW, Bermudez LO, et al. Parents’ perceptions of primary care: measuring parents’ experiences of pediatric primary care quality. Pediatrics. 2001;108(2):264–270 [DOI] [PubMed] [Google Scholar]
- 36.Horne R, Hankins M, Jenkins R. The Satisfaction with Information about Medicines Scale (SIMS): a new measurement tool for audit and research. Qual Health Care. 2001;10(3):135–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bowen J, Fenton T, Rappaport L. Stimulant medication and attention deficit-hyperactivity disorder. The child’s perspective. Am J Dis Child. 1991;145(3):291–295 [DOI] [PubMed] [Google Scholar]
- 38.Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res. 2006;60(6):631–637 [DOI] [PubMed] [Google Scholar]
- 39.Weinman J, Petrie KJ, Moss-Morris R, Horne R. The illness perception questionnaire: a new method for assessing the cognitive representation of illness. Psychol Health. 1996;11(3):431–445 [Google Scholar]
- 40.Moss-Morris R, Weinman J, Petrie KJ, Horne R, Cameron LD, Buick D. The revised illness perception questionnaire (IPQ-R). Psychol Health. 2002;17(1):1–1628891328 [Google Scholar]
- 41.Horne R, Weinberg J. Self-regulation and self-management in asthma: exploring the role of illness perceptions and treatment beliefs in explaining non-adherence to preventer medication. Psychol Health. 2002;17(1):17–32 [Google Scholar]
- 42.O’Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15(1):25–30 [DOI] [PubMed] [Google Scholar]
- 43.Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;14(1):1–24 [Google Scholar]
- 44.Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47(6):555–567 [DOI] [PubMed] [Google Scholar]
- 45.Tracey TJ, Kokotovic AM. Factor structure of the working alliance inventory. Psychol Assess. 1989;1(3):207–210 [Google Scholar]
- 46.Ahmed R, McCaffery KJ, Aslani P. Factors influencing parental decision making about stimulant treatment for attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2013;23(3):163–178 [DOI] [PubMed] [Google Scholar]
- 47.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50(1):105–116 [DOI] [PubMed] [Google Scholar]
- 48.Lau HS, de Boer A, Beuning KS, Porsius A. Validation of pharmacy records in drug exposure assessment. J Clin Epidemiol. 1997;50(5):619–625 [DOI] [PubMed] [Google Scholar]
- 49.Brinkman WB, Hartl Majcher J, Poling LM, et al. Shared decision-making to improve attention-deficit hyperactivity disorder care. Patient Educ Couns. 2013;93(1):95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Epstein JN, Kelleher KJ, Baum R, et al. Impact of a web-portal intervention on community ADHD care and outcomes. Pediatrics. 2016;138(2):e20154240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Epstein JN, Kelleher KJ, Baum R, et al. Specific components of pediatricians’ medication-related care predict attention-deficit/hyperactivity disorder symptom improvement. J Am Acad Child Adolesc Psychiatry. 2017;56(6):483–490.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DiStefano C, Zhu M, Mindrila D. Understanding and using factor scores: considerations for the applied researcher. Pract Assess Res Eval. 2009;14(20):1–11 [Google Scholar]
- 53.Stekhoven DJ, Bühlmann P. MissForest–non-parametric missing value imputation for mixed-type data. Bioinformatics. 2012;28(1):112–118 [DOI] [PubMed] [Google Scholar]
- 54.Leslie LK, Aarons GA, Haine RA, Hough RL. Caregiver depression and medication use by youths with ADHD who receive services in the public sector. Psychiatr Serv. 2007;58(1):131–134 [DOI] [PubMed] [Google Scholar]
- 55.Bussing R, Zima BT, Mason D, Hou W, Garvan CW, Forness S. Use and persistence of pharmacotherapy for elementary school students with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2005;15(1):78–87 [DOI] [PubMed] [Google Scholar]
- 56.Hansen DL, Hansen EH. Caught in a balancing act: parents’ dilemmas regarding their ADHD child’s treatment with stimulant medication. Qual Health Res. 2006;16(9):1267–1285 [DOI] [PubMed] [Google Scholar]
- 57.Toomey SL, Sox CM, Rusinak D, Finkelstein JA. Why do children with ADHD discontinue their medication? Clin Pediatr (Phila). 2012;51(8):763–769 [DOI] [PubMed] [Google Scholar]
- 58.Feng Z, McLerran D, Grizzle J. A comparison of statistical methods for clustered data analysis with Gaussian error. Stat Med. 1996;15(16):1793–1806 [DOI] [PubMed] [Google Scholar]
- 59.Sauerbrei W, Schumacher M. A bootstrap resampling procedure for model building: application to the Cox regression model. Stat Med. 1992;11(16):2093–2109 [DOI] [PubMed] [Google Scholar]
- 60.Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Series B (Methodol). 1996;58(1):267–288 [Google Scholar]
- 61.Friedman L, Wall M. Graphical views of suppression and multicollinearity in multiple linear regression. Am Stat. 2005;59(2):127–136 [Google Scholar]
- 62.Sun Q . Predicting Downstream Effects of High Decisional Conflict: Meta-Analysis of the Decisional Conflict Scale [master’s thesis]. . Ottawa, Canada: University of Ottawa; 2005 [Google Scholar]
- 63.Epstein JN, Kelleher KJ, Baum R, et al. Variability in ADHD care in community-based pediatrics. Pediatrics. 2014;134(6):1136–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.National Committee for Quality Assurance HEDIS 2018 technical specifications for physician measurement. Available at: http://www.ncqa.org/Portals/0/HEDISQM/HEDIS2018/Summary of Changes for Physician Measurement 2018.pdf?ver=2017-12-15-070645-503. Accessed March 28, 2018
- 65.Firestone P. Factors associated with children’s adherence to stimulant medication. Am J Orthopsychiatry. 1982;52(3):447–457 [DOI] [PubMed] [Google Scholar]
- 66.Charach A, Fernandez R. Enhancing ADHD medication adherence: challenges and opportunities. Curr Psychiatry Rep. 2013;15(7):371. [DOI] [PMC free article] [PubMed] [Google Scholar]