Results from a QI collaborative indicated that family-driven goal setting enhances outcomes in the standardization of management of constipation and insomnia in children with ASD.
Abstract
OBJECTIVES:
Constipation and insomnia are not consistently identified and treated in children with autism spectrum disorder (ASD) despite their high prevalence and deleterious impact in this population. To standardize care, a constipation practice pathway and an insomnia practice pathway were previously developed by Autism Treatment Network clinicians. Our objective was to implement and refine these practice pathways in clinical settings.
METHODS:
Eleven Autism Treatment Network sites participated in a Learning Collaborative (ie, multidisciplinary quality improvement team) and chose to implement either the constipation or insomnia practice pathway in the clinical setting. Families set intervention goals (eg, increase stool frequency, decrease nighttime awakenings) before treatment. Each site began implementation with 1 patient and then increased implementation by factors of 5. Before each increase, the Learning Collaborative evaluated progress and refined the practice pathways. Process improvement was measured primarily by duration until goal attainment and by percentage of families who meet their goals.
RESULTS:
Across sites, 82 children with ASD and constipation and 101 children with ASD and insomnia were managed. Difficulties with intervention adherence and communication between providers and families were reported and were subsequently improved with parallel refinements to both practice pathways. The most notable modification was incorporating a goal-setting session in which families generated their own intervention goals (ie, family-driven goals). In this quality improvement initiative, 75% of families met at least 1 constipation or insomnia goal, with the median time to improvement being 6 weeks.
CONCLUSIONS:
By integrating a family-centered approach into the standardization of care, constipation and insomnia practice pathways may improve engagement, adherence, and management of medical conditions in children with ASD.
Constipation (ie, infrequent and painful and/or difficult defecation) and insomnia (ie, difficulty with sleep onset, sleep maintenance, and/or waking too early) are common medical conditions in children with autism spectrum disorder (ASD), affecting ∼9% to 34% and 50% to 80% of individuals in this population, respectively.1–9 Medical conditions such as constipation and insomnia are often cited as negatively impacting global health and quality of life in this population.10–12 In studies of children with ASD, researchers have identified associations between constipation and increased maladaptive behaviors (eg, irritability, social withdrawal, stereotypy, and hyperactivity), anxiety, somatic complaints, and externalizing problems.2,13 Children with ASD and insomnia have greater internalizing and externalizing behaviors, fewer well-developed adaptive skills, fewer socialization skills, and more restricted or repetitive behaviors.14–16
Management of medical conditions in children with ASD results in subsequent improvements in daytime behaviors and family functioning.17 However, medical conditions are not consistently and effectively identified and treated in medical practices that provide care for children with ASD. In a clinical sample of 1518 children with ASD, 71% met the criteria for clinically significant sleep problems although only 30% had been given a sleep disorder diagnosis in the clinic.18 Barriers to assessment and treatment may include communication deficits, restricted feeding regimens, medication effects, sensory symptoms, and psychiatric disorders. To address such challenges, routine screening, reduced threshold for referral to specialists, and recognition of alternate symptom presentations have been recommended.19 Previous literature indicates that treatment-response monitoring and patient follow-up from providers is critical to successful management of medical conditions in this population.20 At this time, no standardized, evidence-based approach to assessment and treatment has been published.
To standardize care, the Gastroenterology Committee and the Sleep Committee of the Autism Treatment Network (ATN) developed 2 clinical practice pathways on the basis of empirical literature and expert consensus to assess and manage constipation and insomnia in children with ASD.20,21 Within the framework of flowcharts, both practice pathways incorporate routine screenings, behavioral- and educational-based interventions when possible, medication or referral to specialists when needed, and follow-up and reevaluation (both flowcharts are available in the respective references). Piloting of the practice pathways indicated promising outcomes.20,21 A quality improvement (QI) model was hypothesized to aid in the implementation and refinement of the practice pathways and enhance outcomes. To date, no QI program had ever been applied to the management of insomnia or constipation in individuals with ASD.
In association with the National Institute for Children’s Health Quality, a QI program was initiated to implement and refine the practice pathways. The current QI program was based on the Breakthrough Series model, which incorporates partnership across sites (ie, Learning Collaborative) to improve health care.22 The Learning Collaborative used data-driven, peer-learning methods to identify areas for improvement and refine intervention processes as they were moved from small-scale to clinic-wide implementation. Our primary aim is to present the lessons learned by the Learning Collaborative and any process improvements made to the practice pathways. Our secondary aim is to report rates of improvement associated with the insomnia and constipation practice pathways in a sample of children with ASD.
Methods
Setting and Program Design
The Learning Collaborative was established by a partnership between the National Institute for Children’s Health Quality, the Autism Speaks Autism Treatment Network (AS ATN), and the Autism Intervention Research Network on Physical Health (AIR-P). The AS ATN aims to improve medical care for children and adolescents with ASD by providing comprehensive, coordinated, family-centered care. Building on the infrastructure of the AS ATN, the AIR-P provides support for research, QI activities, health care guideline and toolkit development, and dissemination of findings. The Nationwide Children’s Hospital Institutional Review Board exempted the current initiative from review because of its status as a QI project.
Eleven North American AS ATN and AIR-P academic medical centers participated in the Learning Collaborative to implement and refine the ATN constipation and insomnia practice pathways20,21 in clinical settings. Each site elected to implement either the insomnia or the constipation practice pathway. There was no overlap between patients being treated for insomnia or patients being treated for constipation. The practice pathways were made available to each site in the form of provider checklists although the explicit process was customized by the individual site on the basis of the flow and information technology infrastructure of the clinic.
At each site, the Learning Collaborative met twice per month with the aim to evaluate and refine the practice pathways. Learning Collaborative meetings were facilitated by an experienced improvement advisor and included an autism clinic director, an autism medical specialist (eg, developmental behavioral pediatrician), at least 1 family advisor, and a data manager. During meetings, Learning Collaborative teams reviewed patient progress toward meeting intervention goals, identified areas for process improvement, and discussed strategies for improvements.
Enrollment Procedures
As part of standard AS ATN data collection procedures, all children had received a clinical diagnosis of ASD on the basis of diagnostic history and the Autism Diagnostic Observation Schedule.23 Children with ASD and insomnia or constipation concerns were identified through retrospective chart review, in-clinic request of services, and phone or e-mail communication from families. Families of children with ASD and insomnia or constipation were managed by the Learning Collaborative if they reported that addressing insomnia or constipation was a high priority, and they agreed to provide weekly progress reports. Baseline packets were sent to families, which included tools relevant to the intervention focus (insomnia or constipation), such as the AS ATN Sleep Toolkit or the Constipation Action Plan. Families also answered questions regarding symptoms, daily routine (eg, sleep diary), and medications. Baseline packets were reviewed by each site team to determine treatment strategies and a communication plan (method and frequency) with the Learning Collaborative. A variety of communication methods were used, including Research Electronic Data Capture, Google Drive, FluidSurveys, text messaging, phone calls, and electronic medical records.
Measures
Constipation and Insomnia Goals
After screening, Learning Collaborative teams helped families identify up to 4 intervention goals. Goals were initially chosen from a predefined list of constipation goals (eg, more frequent stooling, decreased pain with stooling, presence of soft stools, and decreased encopresis) and insomnia goals (eg, decreased time to sleep onset, decreased nighttime awakening, and decreased early morning awakening). Throughout intervention, families completed daily ratings of progress toward their insomnia or constipation goals. On a weekly basis, families communicated with the site Learning Collaborative team to review progress toward goals, discuss what worked and what was less useful, and plan next steps.
Insomnia Satisfaction Survey
Families at several sites with an insomnia focus were also asked to complete the Insomnia Satisfaction Survey. For this survey, our aim was to assess caregiver satisfaction with treatment as well as caregiver-reported insomnia outcomes. The Insomnia Satisfaction Survey was modeled from other patient satisfaction surveys (eg, the Clinician and Group Consumer Assessment of Healthcare Providers and Systems survey)24 and modified to fit the needs of the QI initiative. After testing, revisions were made to the survey on the basis of family feedback that the initial survey was too time intensive.
Analysis
The Learning Collaborative tested modifications to the practice pathways using serial plan-do-study-act (PDSA) cycles.25 Process improvement was measured by improvement rate (percentage of children with ASD who met 1 and ≥2 constipation- or insomnia-related goals) and duration to improvement (number of weeks from enrollment to goal completion). Modifications were initially made on a smaller sample of patients and then implemented on a larger scale if they proved successful. Implementation began with an N of 1 model and then increased by a factor of 5 (ie, from 1 family to 5, to 25, to 125, to all), with refinement occurring before each increase. Each clinic worked intensively with 1 family to optimize care. The Learning Collaborative identified areas for improvement and implemented changes in the practice pathways with the aim to provide positive outcomes for the family. Next, each clinic implemented the modified practice pathway to 5 families. As before, the intervention was refined to optimize outcomes for these families, implemented on a larger scale, and so on.
Results
Refinement of Practice Pathways
On the basis of feedback from PDSA cycles, modifications were implemented to the practice pathways to address provider- and family-reported challenges and improve intervention outcomes. At the first stages of implementation, sites reported mixed results with meeting treatment goals, particularly insomnia targets. The Learning Collaborative teams implemented several refinements to the practice pathways to increase treatment adherence. It was proposed that a collaborative goal-setting session may increase family engagement and treatment adherence. Compared to the original goal-setting process (ie, choosing goals from a predefined list), caregivers now collaborated with providers to develop family-driven goals. Family-driven goal setting occurred before intervention implementation. During goal setting, teams assessed parental readiness for change and worked collaboratively with families to increase engagement and motivation. Scripts were used by providers to ensure that families were being asked to engage in goal setting with the providers. Examples of individualized family-driven goals included reducing bedtime anxiety and accepting medications or high fiber foods.
Constipation Goals
Across the 4 sites that focused on constipation, 93 children with ASD were identified with constipation by the presence of <3 stools per week and/or painful stools. Families were approached about participating in intensive constipation intervention and enrolled if the family agreed to work on constipation and follow-up with the clinical team on a weekly basis. Out of the 93 children with ASD who were initially identified with constipation, 82 families set intervention goals and were treated per the guidelines that were presented in the constipation practice pathway.20,26 All children were treated with a 3-pronged treatment approach of medication, diet change, and behavior change, with parental education and coaching for implementation. Initial bowel clean out was recommended to 18 patients.
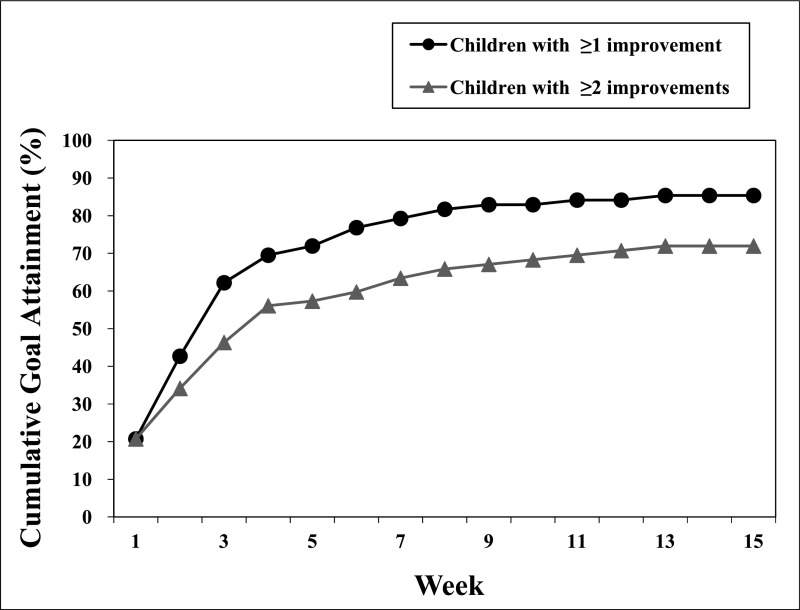
Weekly progress reports provided by the families of the 82 children with ASD were analyzed to determine the constipation improvement rate and duration to improvement. Of the 82 children with ASD, 70 children (85%) met at least 1 goal, 9 children (11%) did not achieve any goals, and 3 children (4%) were lost to follow-up. Out of the 70 intervention responders, 84% of families (n = 59) accomplished “remission” of constipation and completion of all goals. As illustrated in Fig 1, the median time for a family to meet 1 constipation-related goal was 2.5 weeks. The median time to meeting at least 2 constipation-related goals was 3 weeks. The most common goals were to increase stool frequency to ≥3 times per week and eliminate pain. Some families had additional goals, including toilet use, acceptance of medications or high fiber foods, and elimination of stimulant and laxative use.
FIGURE 1.
Duration to goal attainment in the constipation stream: run chart of the cumulative percentage of children with ASD in the constipation stream achieving goals by week (n = 82). The median time to meeting at least 1 goal was 2.5 weeks, and the median time to meeting ≥2 goals was 3 weeks.
Insomnia Goals
Across the 7 sites that focused on insomnia, 108 children with ASD were identified with insomnia in clinics; 101 of these families affirmed a desire to work on their child’s insomnia and participate in weekly follow-ups with the clinical team. The ATN insomnia practice pathway promotes the use of educational and behavioral strategies (eg, bedtime schedules) as outlined in the Sleep Education Program toolkit. If insomnia was not resolved or the family was unwilling or unable to use an educational approach, providers were guided to supplement educational intervention with medication and/or referral to a sleep specialist.
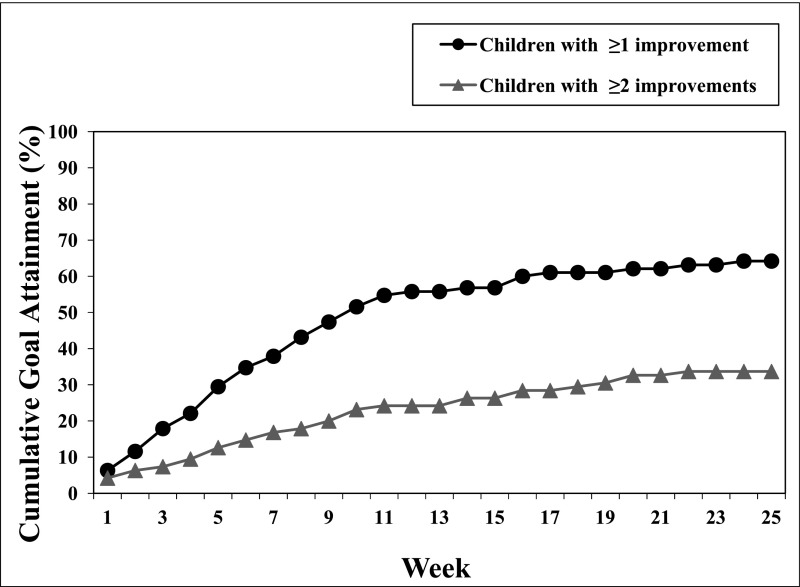
Weekly progress reports provided by the families of the 101 children with ASD were analyzed to determine the insomnia improvement rate and the duration to improvement. Per the practice pathway, 6 children (6%) required referral to sleep specialists or other providers and were excluded from additional analyses. Out of the remaining 95 children who were identified with insomnia, 63 children (66%) met at least 1 goal, 18 children (19%) did not meet any insomnia-related goals, and 14 children (15%) dropped out from the weekly follow-ups with the clinical team. Reasons for patient attrition included loss of contact and change in treatment priorities (lowered interest in treatment). Out of the 63 treatment responders, 49% (n = 31) met at least 2 goals. As illustrated in Fig 2, the median time for a family to meet 1 insomnia-related goal was 10 weeks. Common examples of insomnia-related improvements included reductions in time to fall asleep, less resistance at bedtime, and decreased nighttime awakenings. A subset of the families who had not met their prespecified goals (n = 8) nevertheless reported improvement (eg, families may be referencing partial improvement, improvement outside of their prespecified goals, or increased competency).
FIGURE 2.
Duration to goal attainment in the insomnia stream: run chart of the cumulative percentage of children with ASD in the insomnia stream achieving goals by week (n = 95). Six patients were referred to specialists and excluded from the results. The median time to meeting at least 1 goal was 10 weeks.
Insomnia Satisfaction Survey
After the intervention, 24 families completed the Insomnia Satisfaction Survey. Surveys were deidentified before reaching the clinical teams. All families reported that they were either “very satisfied” (88%) or “moderately satisfied” (13%) with the care they received for sleep issues. In terms of outcomes, 16 families (67%) reported that their child’s sleep improved a great deal, 7 families (29%) reported that their child’s sleep improved slightly, and 1 family (4%) reported that their child’s sleep had stayed the same. Families reported on insomnia-related goals across 6 areas of improvement (see Table 1). Comparable improvement was demonstrated across areas, with the most families (n = 15) reporting, “My child gets more sleep at night,” and the fewest families reporting, (n = 10) “My child is better able to sleep independently in his or her own bed.”
TABLE 1.
Caregiver-Reported Insomnia Improvements From the Insomnia Satisfaction Survey (n = 24)
| Survey Item | Endorsement, n (%) |
|---|---|
| If your child’s sleep has improved since (site) has been working intensively with you on your child’s sleep issues, please indicate how it has improved. | |
| My child falls asleep faster. | 14 (58) |
| My child fights going to bed less. | 12 (50) |
| My child wakes less often in the night. | 14 (58) |
| When my child awakes in the night, he or she is awake for less time. | 11 (46) |
| My child is better able to sleep independently in his or her own bed. | 10 (42) |
| My child gets more sleep at night. | 15 (63) |
| Total endorsement of improvement in ≥1 areas | 23 (96) |
Discussion
The Learning Collaborative standardized care of constipation and insomnia in children with ASD across 11 clinical sites by implementing the ATN constipation and insomnia practice pathways.20,21 Standardization of care was associated with at least partial constipation- or insomnia-related improvement in 75% of patients. Using the Breakthrough Series model, the Learning Collaborative refined the practice pathways by implementing change with PDSA cycles, thus developing a replicable system for other providers and settings to improve management of constipation and insomnia in children with ASD. After implementation of the practice pathways in children with ASD (n = 183), 85% of families working on constipation and 66% of families working on insomnia demonstrated improvements by meeting at least 1 intervention goal.
The lessons that were learned from this initiative underscored the importance of a family-centered approach in which medical providers actively engage families and collaborate with them in the management of medical conditions. A family-centered focus may be particularly effective in this population given that families of children with ASD may face unique challenges and have different resources for treating medical concerns. When aspects of the interventions were less flexible (eg, when families chose goals from a prespecified list and were asked to use specific communication methods, etc), the Learning Collaborative reported mixed success rates and/or longer durations to goal achievements. A family-centered focus was incorporated into the practice pathways (ie, inclusion of family-driven goal setting, collaborative agreement on communication methods and schedules, etc), thus standardizing the process without being restrictive to families. High levels of treatment responsiveness were reported after implementation of family-driven goal setting. As such, standardization of care should include a scheduled collaboration with each individual family, which allows flexibility within a standardized practice that other providers can adopt.
Previous studies have indicated that medical issues that co-occur with ASD tend to be pervasive and difficult to treat consistently over time.1,6,10–12 Managing such conditions can strain families’ resources, resulting in poor treatment adherence. The use of family-driven goal setting to improve treatment adherence is consistent with a motivational interviewing27 (MI) approach. MI is a psychosocial method that is used to increase motivation and involvement and to facilitate change behavior. A critical component of MI is goal setting. Applying specific, difficult-to-attain goals has been shown to increase performance across a variety of tasks in various populations.28,29 Goal setting is widely used to promote behavior change,28 is evidence-based,30 and is regularly implemented by clinicians.31,32 Collaborative goal setting between clinician and patient can result in greater trust and improved outcomes.33,34 Applying family-driven goal setting to the practice pathways proved critical for increasing family motivation, treatment adherence, and improvements.
Although improvement rates were high, families and providers reported that intervention was time intensive particularly for meeting insomnia goals. Whereas the median time to constipation improvement was 2.5 weeks, the median time to insomnia improvement was considerably longer at 10 weeks. Insomnia treatments are not as accessible as constipation treatments (eg, laxatives, diet changes). Although insomnia was difficult to improve, family-driven goals were especially effective for insomnia, perhaps because sleep issues tend to affect the whole family. Indeed, it is possible for most families to make improvements within a relatively short time frame if they are actively engaged and collaborating with providers. Communication and follow-up were critical to managing these medical conditions. Through engagement with families to develop individualized data collection methods, the Learning Collaborative learned how to collect data on medical concerns in children with ASD, how to better help families who are struggling with data collection (eg, using flexible communication tools), and how to use data to guide intervention changes. Ultimately, this project highlights the importance of encouraging families to set the priorities for their child’s improvement and be instrumental in driving goals for improvement.
Several limitations restrict the interpretation of the results from the Learning Collaborative. Within the QI framework, data were used for driving improvement, not for research. Rates of missing data and attrition were high; consistent communication was reported to be burdensome to families given the time-intensive nature of the interventions and to providers given the demands of clinics that cater to populations with ASD (eg, long waiting lists). The Learning Collaborative conceptualized missing data as indicators of lower intervention engagement and used missing data as learning opportunities to guide the aforementioned tests of improvement.
Educating providers about constipation and insomnia in children with ASD and disseminating practical intervention systems (eg, practice pathways) is crucial to enhancing outcomes. Although specialty clinics often manage medical conditions in individuals with ASD, it is important to increase the number of primary care providers (PCPs) who have self-efficacy in these areas. The authors recommend surveying PCPs on their knowledge and comfort in handling medical concerns in children with ASD. On the basis of these results, trainings can be developed for PCPs with an emphasis on addressing common co-occurring conditions in children with ASD and working with families to implement strategies to decrease these symptoms. Finally, the authors suggest investigating the utility of dedicated clinics and staff for common medical co-occurring conditions and an autism specialist hotline for consultation. Such mechanisms, which are focused on increasing PCP knowledge and patient engagement, will likely be associated with greater trust and collaboration with families in addition to better management of common co-occurring conditions in the primary-care setting.
Acknowledgments
We thank the patients and their families for their time and participation, which contributes to the knowledge of better medical care for individuals with ASD. We thank the faculty and staff of the clinics at Glenrose Rehabilitation Hospital, Children’s Hospital of Philadelphia, Lurie Center for Autism at Massachusetts General Hospital, Thompson Center for Autism and Neurodevelopmental Disorders at the University of Missouri, University of Pittsburgh Medical Center, Holland Bloorview Kids Rehabilitation Hospital, Vanderbilt University Medical Center, Children’s Hospital Los Angeles, University of Colorado Denver School of Medicine, Arkansas Children’s Hospital, and University of Rochester for their assistance with patient management, data collection, and intervention implementation.
Glossary
- AIR-P
Autism Intervention Research Network on Physical Health
- AS ATN
Autism Speaks Autism Treatment Network
- ASD
autism spectrum disorder
- ATN
Autism Treatment Network
- MI
motivational interviewing
- PCP
primary care provider
- PDSA
plan-do-study-act
- QI
quality improvement
Footnotes
Dr Sohl participated in the conceptualization and design of the project and drafted the initial manuscript; Ms Bellesheim participated in data analysis and interpretation and drafted and edited the manuscript; Ms Cole, Ms Klatka, and Drs Coury, Yin, Levy, Malow, Katz, and Taylor participated in the conceptualization of the project and data interpretation and edited the manuscript; Dr Guinnee participated in the conceptualization and design of the project; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the Health Resources and Services Administration of the US Department of Health and Human Services under cooperative agreement UA3 MC11054 (Autism Intervention Research Network on Physical Health). This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by, the Health Resources and Services Administration, the Department of Health and Human Services, or the US government. This work was conducted through the Autism Speaks Autism Treatment Network, serving as the Autism Intervention Research Network on Physical Health, and was funded in part by the National Institutes of Health. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Coury has received research support from Stemina and serves as a consultant to Neuren, Cognoa, and AMO Pharma; the other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Holingue C, Newill C, Lee LC, Pasricha PJ, Daniele Fallin M. Gastrointestinal symptoms in autism spectrum disorder: a review of the literature on ascertainment and prevalence. Autism Res. 2018;11(1):24–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaidez V, Hansen RL, Hertz-Picciotto I. Gastrointestinal problems in children with autism, developmental delays or typical development. J Autism Dev Disord. 2014;44(5):1117–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molloy CA, Manning-Courtney P. Prevalence of chronic gastrointestinal symptoms in children with autism and autistic spectrum disorders. Autism. 2003;7(2):165–171 [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim SH, Voigt RG, Katusic SK, Weaver AL, Barbaresi WJ. Incidence of gastrointestinal symptoms in children with autism: a population-based study. Pediatrics. 2009;124(2):680–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang LW, Tancredi DJ, Thomas DW. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J Dev Behav Pediatr. 2011;32(5):351–360 [DOI] [PubMed] [Google Scholar]
- 6.Richdale AL. Sleep problems in autism: prevalence, cause, and intervention. Dev Med Child Neurol. 1999;41(1):60–66 [DOI] [PubMed] [Google Scholar]
- 7.Krakowiak P, Goodlin-Jones B, Hertz-Picciotto I, Croen LA, Hansen RL. Sleep problems in children with autism spectrum disorders, developmental delays, and typical development: a population-based study. J Sleep Res. 2008;17(2):197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Couturier JL, Speechley KN, Steele M, Norman R, Stringer B, Nicolson R. Parental perception of sleep problems in children of normal intelligence with pervasive developmental disorders: prevalence, severity, and pattern. J Am Acad Child Adolesc Psychiatry. 2005;44(8):815–822 [DOI] [PubMed] [Google Scholar]
- 9.Souders MC, Mason TB, Valladares O, et al. Sleep behaviors and sleep quality in children with autism spectrum disorders. Sleep. 2009;32(12):1566–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauman ML. Medical comorbidities in autism: challenges to diagnosis and treatment. Neurotherapeutics. 2010;7(3):320–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coury D. Medical treatment of autism spectrum disorders. Curr Opin Neurol. 2010;23(2):131–136 [DOI] [PubMed] [Google Scholar]
- 12.Levy SE, Giarelli E, Lee LC, et al. Autism spectrum disorder and co-occurring developmental, psychiatric, and medical conditions among children in multiple populations of the United States. J Dev Behav Pediatr. 2010;31(4):267–275 [DOI] [PubMed] [Google Scholar]
- 13.Fulceri F, Morelli M, Santocchi E, et al. Gastrointestinal symptoms and behavioral problems in preschoolers with autism spectrum disorder. Dig Liver Dis. 2016;48(3):248–254 [DOI] [PubMed] [Google Scholar]
- 14.Sikora DM, Johnson K, Clemons T, Katz T. The relationship between sleep problems and daytime behavior in children of different ages with autism spectrum disorders. Pediatrics. 2012;130(suppl 2):S83–S90 [DOI] [PubMed] [Google Scholar]
- 15.Taylor MA, Schreck KA, Mulick JA. Sleep disruption as a correlate to cognitive and adaptive behavior problems in autism spectrum disorders. Res Dev Disabil. 2012;33(5):1408–1417 [DOI] [PubMed] [Google Scholar]
- 16.Goldman SE, Surdyka K, Cuevas R, Adkins K, Wang L, Malow BA. Defining the sleep phenotype in children with autism. Dev Neuropsychol. 2009;34(5):560–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malow BA, Adkins KW, Reynolds A, et al. Parent-based sleep education for children with autism spectrum disorders. J Autism Dev Disord. 2014;44(1):216–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malow BA, Katz T, Reynolds AM, et al. Sleep difficulties and medications in children with autism spectrum disorders: a registry study. Pediatrics. 2016;137(suppl 2):S98–S104 [DOI] [PubMed] [Google Scholar]
- 19.McElhanon BO, McCracken C, Karpen S, Sharp WG. Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis. Pediatrics. 2014;133(5):872–883 [DOI] [PubMed] [Google Scholar]
- 20.Furuta GT, Williams K, Kooros K, et al. Management of constipation in children and adolescents with autism spectrum disorders. Pediatrics. 2012;130(suppl 2):S98–S105 [DOI] [PubMed] [Google Scholar]
- 21.Malow BA, Byars K, Johnson K, et al. ; Sleep Committee of the Autism Treatment Network . A practice pathway for the identification, evaluation, and management of insomnia in children and adolescents with autism spectrum disorders. Pediatrics. 2012;130(suppl 2):S106–S124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Institute for Healthcare Improvement The Breakthrough Series: IHI’s collaborative model for achieving breakthrough improvement. 2003. Available at: http://www.ihi.org/resources/Pages/IHIWhitePapers/TheBreakthroughSeriesIHIsCollaborativeModelforAchievingBreakthroughImprovement.aspx. Accessed June 15, 2018
- 23.Lord C, Rutter M, DiLavore PC, et al. Autism Diagnostic Observation Schedule. 2nd ed. Torrance, CA: Western Psychological Services; 2012 [Google Scholar]
- 24.Agency for Healthcare Research and Quality; US Department of Health and Human Services CAHPS: surveys and tools to advance patient-centered care. Available at: https://cahps.ahrq.gov. Accessed November 17, 2013
- 25.Langley GJ, Moen RD, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nd ed. Hoboken, NJ: John Wiley & Sons; 2009 [Google Scholar]
- 26.National Institute for Health and Care Excellence Constipation in children and young people: diagnosis and management. Available at: https://www.nice.org.uk/guidance/cg99. Accessed March 20, 2016 [PubMed]
- 27.Miller WR. Motivational interviewing: research, practice, and puzzles. Addict Behav. 1996;21(6):835–842 [DOI] [PubMed] [Google Scholar]
- 28.Locke EA, Latham GP. Building a practically useful theory of goal setting and task motivation. A 35-year odyssey. Am Psychol. 2002;57(9):705–717 [DOI] [PubMed] [Google Scholar]
- 29.Martino S, Carroll KM, O’Malley SS, Rounsaville BJ. Motivational interviewing with psychiatrically ill substance abusing patients. Am J Addict. 2000;9(1):88–91 [DOI] [PubMed] [Google Scholar]
- 30.Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychol. 2009;28(6):690–701 [DOI] [PubMed] [Google Scholar]
- 31.Langford AT, Sawyer DR, Gioimo S, Brownson CA, O’Toole ML. Patient-centered goal setting as a tool to improve diabetes self-management. Diabetes Educ. 2007;33(suppl 6):S139–S144 [DOI] [PubMed] [Google Scholar]
- 32.Fleming SE, Boyd A, Ballejos M, et al. Goal setting with type 2 diabetes: a hermeneutic analysis of the experiences of diabetes educators. Diabetes Educ. 2013;39(6):811–819 [DOI] [PubMed] [Google Scholar]
- 33.Lafata JE, Morris HL, Dobie E, Heisler M, Werner RM, Dumenci L. Patient-reported use of collaborative goal setting and glycemic control among patients with diabetes. Patient Educ Couns. 2013;92(1):94–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCarley P. Patient empowerment and motivational interviewing: engaging patients to self-manage their own care. Nephrol Nurs J. 2009;36(4):409–413 [PubMed] [Google Scholar]