Abstract
The foxglove aphid, Aulacorthum solani Kaltenbach (Hemiptera: Aphididae), has become a significant pest in horticulture as it can up build high populations from 10 to 18°C. Currently, chemical control is used as no commercially available biocontrol agent is effective at these temperatures. In this study, two potential biocontrol agents were evaluated: the silverfly, Leucopis glyphinivora Tanasijtshuk (Diptera: Chamaemyiidae), and the American hoverfly, Eupeodes americanus (Wiedemann) (Diptera: Syrphidae). Active flight, oviposition, and larval voracity were tested at 12, 15, and 18°C. The proportion of individuals demonstrating flight decreased at 12°C for the hoverfly and decreased at 15 and 12°C for the silverfly. Delay before active flight was greater for both species at 12°C. More hoverflies laid eggs after 7 d at all temperatures (12, 15, and 18°C) compared with silverflies. Hoverflies laid a higher number of eggs than silverflies at all temperatures. When given an additional 7 d at 12°C, oviposition increased for both species. Daily aphid consumption decreased as temperature decreased for both species, but average total aphid consumption did not decrease regardless of the temperature. This means that larval voracity for both the silvery and the American hoverfly was similar at all temperatures (12, 15, and 18°C) when considering aphid development. Hoverfly larvae consumed two times more aphids than silverfly larvae at all temperatures. This study demonstrates a clear superiority of the hoverfly over the silverfly at low temperatures and identifies it as a potential biocontrol agent of the foxglove aphid.
Keywords: low temperature, horticulture, predation, oviposition, flight
The foxglove aphid, Aulacorthum solani Kaltenbach (Hemiptera: Aphididae), originates from Europe and has become a cosmopolitan pest (Blackman and Eastop 2000). It attacks many commodities such as soybeans in Korea and Japan (Takada et al. 2006), lettuce in South America (De Conti et al. 2011), and greenhouse peppers in Spain (Sanchez et al. 2007). In northeastern America, the foxglove aphid is a growing problem in horticulture (Jandricic et al. 2014). The foxglove aphid is quite polyphagous and capable of attacking many mono and dicotyledonous plant species, greenhouse vegetables, and floriculture plants (Jandricic et al. 2014). Its toxic salivary excretions lead to the deformation of leaves and fruits (Sanchez et al. 2007), and it can be a viral vector (Jandricic et al. 2010, Yovkova et al. 2013). Furthermore, the honeydew produced by the foxglove aphid supports the growth of Cladosporium, or fumagine, on leaves which disrupts photosynthesis (Richard and Boivin 1994). As agricultural production for greenhouse ornamentals in northeastern America starts in January and February, the temperature in greenhouses is kept lower to save on heating costs (University of Minnesota’s Center for Urban and Regional Affairs and Center for Sustainable Building Research 2013). Contrary to other species of aphids, the foxglove aphid has a high fecundity at 5, 10, and 20°C, where few natural enemies are effective (Jandricic et al. 2010).
Physiology, development, and behavior of insects are strongly linked to temperature (Denlinger and Lee 2010, Teets and Denlinger 2013). Many species of natural enemies do not perform well at lower temperatures due to effects on development time, longevity, mortality, and reproduction (Langer et al. 2004). Rearing method at optimal temperatures could be one explanation as this selects for individuals adapted to higher temperatures. For example, a study by Sørensen et al. (2013) has shown that cold acclimation of Adalia bipunctata L. (Coleoptera: Coccinellidae) may increase biocontrol efficiency but reduces pupal survival and heat tolerance. In a study done by Helgadóttir et al. (2017), it was shown that aphid predation was greater for Orius majusculus (Heteroptera: Anthocoridae) when reared at 20°C and lower when reared at 12°C (Helgadóttir et al. 2017).
Most commercially available biological control agents are ineffective against the foxglove aphid at low temperatures (Jandricic 2013). Aphidoletes aphidimyza Rondani (Diptera: Cecidomyiidae) becomes less efficient against this pest when temperatures are below 20°C in horticultural greenhouses (Alotaibi 2008, Lee et al. 2008). Commercially available ladybirds are not the best predators at low temperatures, with their optimal temperatures sitting higher at 23–27°C for Ad. bipunctata (Jalali et al. 2010) and 29°C for Hippodamia convergens Guérin-Méneville (Coleoptera: Coccinellidae) (Obrycki and Tauber 1982). Other common predators such as Ad. bipunctata (Schüder et al. 2004) and H. convergens (Katsarou et al. 2005) consume few aphids when temperatures fall below 15°C. Lacewings such as Chrysoperla rufilabris Burmeister or Chrysoperla carnea Stephens (Neuroptera: Chrysopidae), which are normally used as predators in temperatures ranging from 12 to 35°C, do not pupate as well in greenhouse environments and show cannibalistic behaviors (Jandricic 2013). Currently, in Québec, Aphidius ervi Haliday (Hymenoptera: Braconidae) is only used when temperatures during the day are above 20°C and above 18°C at night. Wild populations of Ap. ervi fly around 15°C despite the fact that they can oviposit at temperatures above 10°C (Langer et al. 2004); however, no such data exists on commercial populations. As discussed previously, mass reared individuals at optimal temperatures might not perform as well as at low temperatures. In greenhouses, Ap. ervi’s behavior increases the dropping frequency of the foxglove aphid, therefore leading to a higher dispersion of the pest (Gillespie and Acheampong 2012). Furthermore, Ap. ervi is commonly used for the control of A. solani in glasshouses, but aphid control is often disrupted by hyperparasitoids (Rocca and Messelink 2017). Although there has been a study on the parasitoid Praon volucre (Haliday) (Hymenoptera: Braconidae: Aphidiinae) in Brazil against the foxglove aphid, it was done at higher temperatures (18, 20, 22, 24, and 26°C; Silva et al. 2015). Globally, there is no biological control agent commercially available for use at low temperatures (10–20°C) against the foxglove aphid. Insecticides currently used against aphids in Canada on ornamental crops include Intercept 60 WP (imidacloprid), Endeavor 50 WG (pymetrozine), Dibrom (naled), and Cygon 480 (dimethoate) (Ministry of Agriculture, Food and Rural Affairs 2014). In the present study, we evaluated two potential biocontrol agents foxglove aphid.
The first potential biological control agent is the American hoverfly, Eupeodes (Metasyrphus) americanus (Wiedemann) (Diptera: Syrphidae). It is a Nearctic species that is widespread across North America (Vockeroth 1992) and a generalist aphid predator (Rojo et al. 2003). The biology of the American hoverfly has not been studied extensively; however, other syrphids that are predators of aphids are active early in the spring and late in the fall when temperatures are lower (Tamaki et al. 1967, Dixon et al. 2005). This is consistent with the thermal development threshold of other syrphid species, which is between 5 and 7°C (Honĕk and Kocourek 1988, Hart et al. 1997). Syrphidae are well adapted to aphid populations increases early in the season and can detect them before other predators (Almohamad et al. 2009). Many studies have shown that the hoverfly is an effective biological control agent against pests in greenhouses and in the field (Leroy et al. 2010). At present, no data is available on the thermal development thresholds for this species in particular; however, young larvae consume aphids in Petri dishes kept at 4°C (Y.B., unpublished data).
The second potential biocontrol agent, the silverfly, Leucopis glyphinivora Tanasijtshuk (Diptera: Chamaemyiidae), is a Holarctic species, prevalent in the Palearctic region from Spain and France to Israel (Beschovski and Merz 1998, Kahanpää 2014), Middle East (Satar et al. 2015), Afghanistan, and the Far East. This species has also been recorded in the Nearctic region in California (Kaiser et al. 2007). Leucopis glyphinivora shares the same biology as Leucopis annulipes Zetterstedt (Diptera: Chamaemyiidae). Larvae have good mobility and are generalist furtive aphid predators (Fréchette et al. 2008). It has been shown that they consume many species of aphids (Satar et al. 2015). The silverfly’s development threshold is unknown, but adult Leucopis pinicola Malloch (Diptera: Chamaemyiidae) can be found in Northeastern Ohio during the first week of May (Sluss and Foote 1973). We can therefore assume that some species of this genus have a good tolerance to cold. Preliminary observations lead to the belief that the silverfly could be efficient at low temperatures (Y.B., unpublished data).
The aim of the present study was to assess the potential of two new candidate biological control agents, the American hoverfly, E. americanus, and the silverfly, L. glyphinivora, for the suppression of the foxglove aphid, A. solani, at low temperatures. Both species were selected because 1) they belong to a genera and family with low thermal thresholds, 2) they are polyphagous, 3) they are indigenous (or were naturalized a long time ago), and 4) it is possible to rear them in a laboratory. Active flight, female oviposition, and larval voracity for both predators were evaluated and compared at low temperatures.
Materials and Methods
Insect Rearing
All insect rearings were carried out at the Université du Québec à Montréal (UQÀM) in the Laboratoire de Lutte Biologique. Foxglove aphids (A. solani) were provided by Dr. Rose Buitenhuis for the Vineland Research and Innovation Center, Vineland Stations, ON, Canada. Insects were reared on Aristotle green pepper plants, Capsicum annuum L. (Solanaceae) in a 35 × 35 × 35 cm cage kept in a growth chamber at 18°C, with a 16:8 (L:D) photoperiod and 60% relative humidity (RH).
Hoverfly (E. americanus) rearings were established from at least 100 individuals collected on Phlox sp. in Sainte-Agathe-de-Lotbinière (N 46° 23′ 726″, W 71° 21′ 446″), QC, Canada, in 2014. Rearing of individuals is based on the Frazer method (Frazer 1972). Adults were kept in a 81 × 53 × 60 cm rearing cage covered in muslin which was kept in a greenhouse at 22°C during the day, 19°C at night and at 60% RH. Inside the rearing cage, a single flowering plant of sweet alyssum, Lobularia maritima L. (Brassicaceae), was kept in a 10 × 10 cm plastic pot and replaced when necessary. Adults were fed with a sugar:water mixture (1:10 v/v) in a solo cup with a dental cotton roll protruding from the lid and with an artificial flower consisting of a round cotton makeup remover saturated with a honey:water mixture (1:2 v/v) and covered with wildflower bee pollen. These were replaced twice a week. Green pepper plants, C. annuum, infested with foxglove aphids were introduced into the rearing cage three times a week to allow oviposition. The eggs were collected by cutting the area of the leaf where they were laid then placed inside Petri dishes on top of round cotton makeup removers dampened with water. Petri dishes were kept in a refrigerator at 4°C, 60% RH, and larvae were collected once a week. Larvae were transferred in the same type of cages as the foxglove aphids and put in a growth chamber set at 24°C, 16:8 (L:D), and 70% RH. These cages contained potato plants, Solanum tuberosum L. (Solanaceae), infested with green peach aphids, Myzus persicae Sulzer (Hemiptera: Aphididae). Adults that emerged from the cages were then collected and introduced in the rearing cage kept in the greenhouse as described previously. Rearings were refreshed yearly with new wild individuals to keep genetic variability sufficient.
Silverfly (L. glyphinivora Tanasijtshuk) rearings were established from at least 100 individuals collected on apple trees located in Montreal (N 45° 51′, W 73°56′), QC, Canada, in 2009. Identification was done by Dr. Stephen D. Gaimari from the California Department of Food and Agriculture, Sacramento, CA. Captured individuals were reared on potato plants inoculated with M. persicae following the methodology by Gaimari and Turner (1996). Rearings were also refreshed yearly with new wild individuals. The colony was maintained in the same type of cages as the green peach aphids and kept in the same growth chamber as the hoverflies. Individuals were fed with a sugar:water mixture (1:10 v/v) in a solo cup and with an artificial flower as described previously and brewer’s yeast. These were replaced twice a week. Solo cup plastic lids were also covered with dry brewer’s yeast and replaced once a week.
Experiment on Active Flight Capacity at Low Temperatures
Active flight capacity can be defined as when an insect moves its wings to achieve displacement (Brodsky 1994). This capacity was compared between the American hoverfly and the silverfly at 12, 15, and 18°C. At each temperature, a minimum of 16 males and 16 females aged of 18–36 h from each species were individually placed in the center of a 30 × 30 × 27 cm Plexiglas box with two 16 × 16 cm openings. The box was placed in a Conviron growth chamber, Model E15 at 700 LUX and 60% RH. A HOBO Data Logger Pro v2 Internal Temperature/Relative Humidity Data Logger (Model U23-001) was then used to measure the temperature inside the box. Insects were acclimated before starting the test by individually placing them in film canisters for at least 30 min outside of the test box. After this period, the canisters were placed inside the test box and then opened. Each individual was observed for a period of 10 min. The presence or absence of active flight, as well as the delay from introduction of the insect in the box to the observation of active flight, was noted. The duration of flight was not recorded because it was not of interest to us but rather the capacity to fly. The average temperature was 17.9 ± 0.5°C for tests at 18°C, 14.6 ± 0.5°C for tests at 15°C, and 11.8 ± 0.6°C for tests at 12°C.
Experiment on Oviposition at Low Temperatures
The number of eggs laid by American hoverfly and silverfly females was measured at 12, 15, and 18°C. At each temperature, 20 females and 20 males from each species aged less than 24 h were collected from the colony established in the laboratory. To evaluate the age of individuals, larvae were introduced in cages with potato plants and M. persicae only. The cages were followed daily and any individuals that emerged within a 24-h period were used for testing. One male and one female were introduced in a 45 × 18 cm transparent plastic cylinder with a muslin lid and two 12 × 12 cm ventilated windows. Each cylinder contained a green pepper plant with six to eight leaves inoculated with 200 foxglove aphids of different stages, collected with an aspirator. Adults were fed with a sugar: water mixture (1:10 v/v) in a solo cup and with an artificial flower as described previously. For silverflies, a mixture of brewer’s yeast and water (1:3) was added to the round cotton makeup remover and a solo cup lid filled with dry brewer’s yeast was placed at the bottom of the green pepper plant. White sugar was also put on the cylinder lids and vaporized with water twice a day. The cylinders were placed in a Conviron growth chamber for 7 d at 12, 15, and 18°C and 14 d at 12°C, 16:8 (L:D), 50% RH. Oviposition was evaluated only for 7 d and not for the life’s duration because our objective was to evaluate if hoverflies were able to oviposit at 12, 15, and 18°C. Extending the duration of the test, especially at 18°C, would have led to plant collapse by creating unsustainably large aphid populations. Test length was extended to 14 d at 12°C to account for any prolongation of egg maturation at this temperature. The number of eggs laid and the number of larvae on the plant were counted at the end of each trial, with larvae being counted as eggs for analyses. A HOBO Data Logger was used to measure the temperature inside the growth chamber, and temperature was attributed randomly to each growth chamber at each repetition.
Experiment on Larval Voracity at Low Temperatures
The number of aphids consumed by American hoverfly and silverfly larvae was calculated at 12, 15, and 18°C, 16:8 (L:D), 50% RH. Green pepper plants with six to eight leaves were individually placed in the same type of cylinders described earlier for the egg-laying tests. Thirty second-instar foxglove aphids from the laboratory colony were collected with an aspirator and placed on each green pepper plant. The test was divided into three treatments. In the first two treatments, a stage I larva of each species was introduced into the cylinders. The first treatment introduced the American hoverfly, and the second treatment introduced the silverfly. The third treatment was to be used as a control where no larvae were introduced into the cylinders. For each treatment, there were 30 replicates. To compare the development of the larvae with that of the foxglove aphids, the duration of the test for each temperature was set to correspond to the time required for the accumulation of 75 degree-days (DD) by the aphid (under a threshold of 3°C). Cylinders were put inside a Conviron growth chamber for 5 d at 18°C, 6 d and 6 h at 15°C, and 8 d and 8 h at 12°C. A HOBO Data Logger was used to measure the temperature inside the growth chamber, and temperatures were randomly assigned to each growth chamber. For all tests, if the temperatures measured varied ≥1°C from the required temperature, the trial was discarded. If mortality was greater than 10% in controls for any given replicate, it was discarded. At the end of each test, the number of living aphids was calculated as such: initial density (30 aphids) − number of living aphids. If a larva was feeding on an aphid at the moment of data collection, it was considered as dead. Daily consumption was also calculated as follows: total consumption/duration of the test according to the temperature tested for.
Statistical Analyses
For all experiments, statistical analyses were performed with the statistical package JMP 12 (SAS Institute Inc. 2009). For trials on active flight capacity at low temperatures, a log-likelihood ratio test was done following a chi-square test to compare proportions of individuals that exhibited flight at all temperatures. A two-way analysis of variance (ANOVA) was used for the temperature factor and the repetition factor. Males and females for both the American hoverfly and the silverfly were compared; no significant differences were observed; therefore, the data were grouped. For average delay in seconds before active flight, only insects that demonstrated active flight were considered. A Tukey’s HSD test was used to identify treatments that were significantly different from each other. For trials on total aphid consumption and for daily aphid consumption, an ANOVA was performed and blocks were compared among themselves for the same temperature. There was no significant difference between blocks for the two species studied therefore the data was grouped. For the experiment on egg-laying at low temperatures, a multiple linear regression was used to compare proportions of females that laid eggs at all temperatures. An ANOVA was performed to analyze the average number of eggs laid by females at each temperature for both test trial lengths (7 and 14 d). A Tukey’s HSD test was used to identify which treatments were significantly different from each other. Assumptions for parametric analyses were fulfilled following a Shapiro–Wilk test of normality (P > 0.05); no transformations were required.
Results
Active Flight Capacity at Low Temperatures
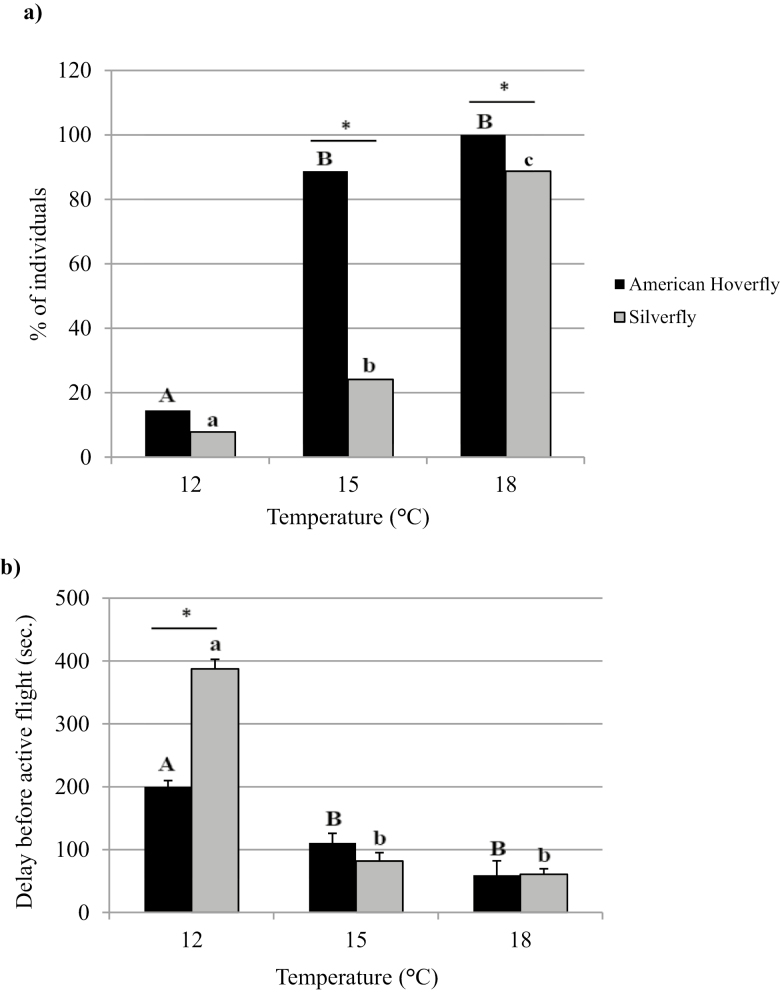
All American hoverflies demonstrated active flight at 18°C, 88.7% of individuals could fly at 15°C, and only 14.5% could fly at 12°C. For the silverfly, 88.7% demonstrated active flight at 18°C, 24.1% at 15°C, and 7.8% at 12°C (Fig. 1a). The proportion of hoverflies that demonstrated active flight was greater than the proportion of silverflies that flew at temperatures of 18°C (χ2 = 10.411, df = 1, P = 0.00131) and 15°C (χ2 = 57.805, df = 1, P < 0.0001). There was no difference between the two species at 12°C (χ2 = 1.261, df = 1, P = 0.2614).
Fig. 1.
Percentage (+SE) of individuals having demonstrated active flight (a) and average delay (+SE) in seconds before active flight (b) at 12, 15, and 18°C after a 10-min observation period for the American hoverfly, Eupeodes americanus (n = 69 at 18°C, n = 71 at 15°C, n = 62 at 12°C) and the silverfly, Leucopis glyphinivora (n = 53 at 18°C, n = 54 at 15°C, n = 51 at 12°C). Treatments with a different letter and an asterisk are significantly different (ANOVA, P < 0.05). Uppercase letters are for the American hoverfly, and lowercase letters are for the silverfly.
Delays before active flight varied according to all three temperatures for the American hoverfly (F2, 141 = 15.6, P < 0.01) and the silverfly (F2, 64 = 18.8, P < 0.01; Fig. 1b). No significant difference was observed between the two species at 12°C (F2, 13 = 11.5, P = 0.57), 15°C (F2, 76 = 11.5, P = 0.63), and 18°C (F2, 116 = 11.5, P = 0.99).
Oviposition at Low Temperatures
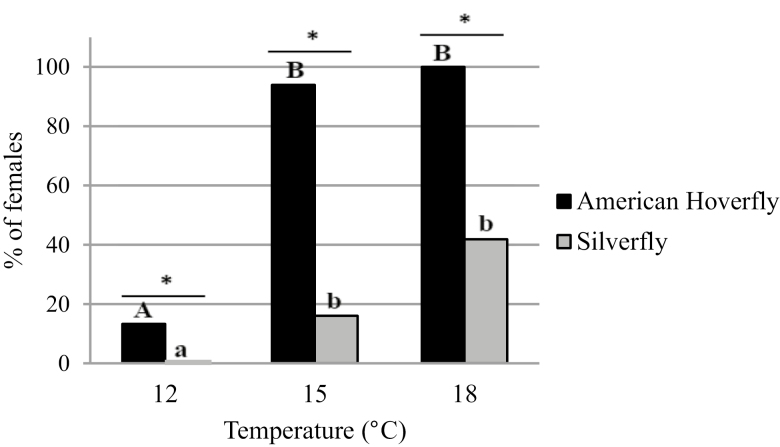
Only the tests where the female was still alive at the end of each trial were considered. Mortality for female hoverflies was 13% (after 7 d) and 24% (after 14 d) at 12°C, 13% at 15°C, and 19% at 18°C. Mortality for female silverflies was 6% (after 7 d) and 4% (after 14 d) at 12°C, 7% at 15°C, and 5% at 18°C. Temperature significantly influenced the proportion of female hoverflies and silverflies that laid eggs on the plant after 7 d (R2 = 0.9935, df = 1, P < 0.01). No female silverfly laid eggs at 12°C after 7 d. The proportion of hoverflies having laid eggs is at least four times as high as the proportion of silverflies that oviposited at 15°C (Fig. 2).
Fig. 2.
Percentage of females having laid eggs over a period of 7 d for the American hoverfly, Eupeodes americanus (n = 24 at 18°C, n = 30 at 15°C, n = 59 at 12°C) and the silverfly, Leucopis glyphinivora (n = 35 at 18°C, n = 34 at 15°C, n = 46 at 12°C). Treatments with a different letter and an asterisk are significantly different (ANOVA, P < 0.05). Uppercase letters are for the American hoverfly, and lowercase letters are for the silverfly.
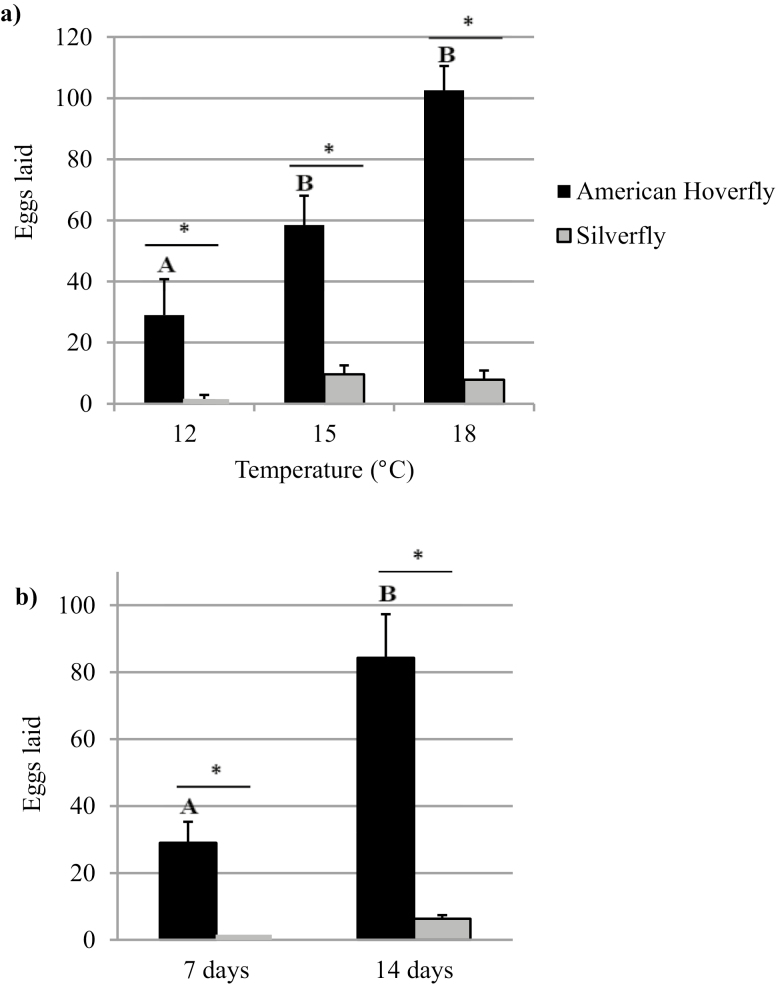
The average number of eggs laid by female hoverflies after 7 d varied significantly according to temperature (F2, 112 = 80.03, P < 0.01, Fig. 3a). The hoverfly significantly laid more eggs than the silverfly at 18°C (F2, 59 = 90.70, P ≤ 0.01), 15°C (F2, 64 = 90.70, P ≤ 0.01), and 12°C (F2, 105 = 90.70, P ≤ 0.01).
Fig. 3.
Average number of eggs (+SE) laid by female American hoverflies, Eupeodes americanus (n = 24 at 18°C, n = 30 at 15°C, n = 59 at 12°C) and silverflies, Leucopis glyphinivora (n = 35 at 18°C, n = 34 at 15°C, n = 46 at 12°C) over a period of 7 d at 12, 15, and 18°C (a) and over a period of 7 and 14 d (n = 20 for E. americanus and n = 33 for L. glyphinivora) at 12°C (b). Treatments with a different letter and an asterisk are significantly different (ANOVA, P < 0.05).
After giving females of both species an additional period of 7 d (14 d total) that did not lay any eggs after 7 d at 12°C, the average number of eggs laid significantly increased from 11.6 to 101 for the hoverfly (F2, 79 = 49.3310, P < 0.01), but the difference from 0 to 1.33 for the silverfly was not as significant (F2, 79 = 49.3310, P = 0.99, Fig. 3b). The average number of eggs laid by the American hoverfly was significantly greater than the silverfly after 7 d (F2, 105 = 84.73, P < 0.01) and 14 d at 12°C (F2, 53 = 53.12, P < 0.01). Finally, at 12°C, the average number of eggs laid by female hoverflies after 14 d was significantly greater than the average number of eggs laid by female silverflies after 7 and 14 d (F2, 79 = 49.3310, P < 0.01).
Larval Voracity at Low Temperatures
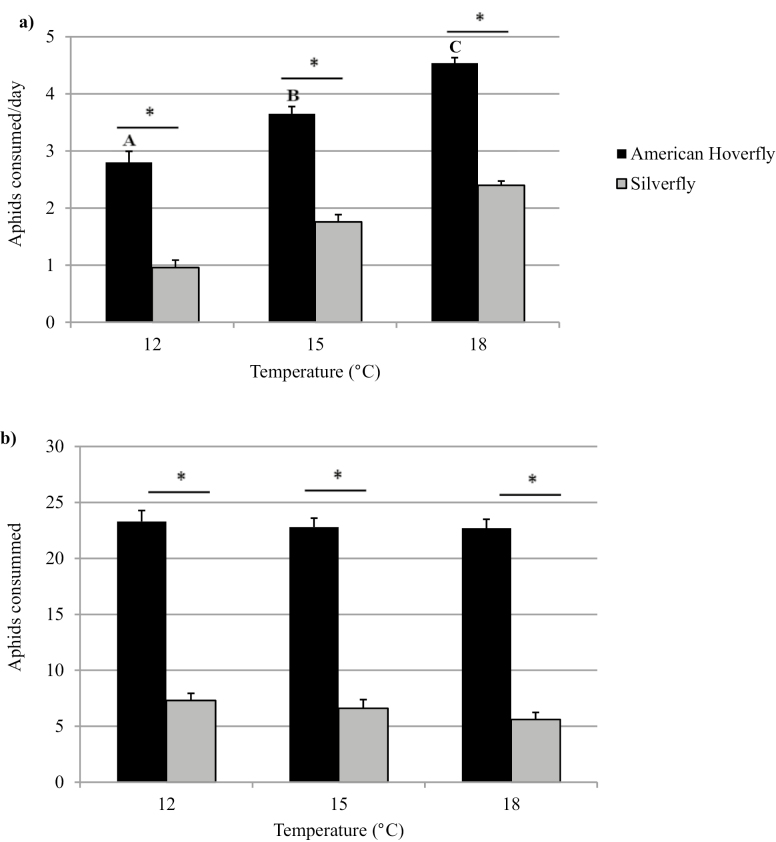
Daily consumption of aphids by silverfly increased with temperature, from 1.92 at 12°C to four aphids per day at 18°C. The highest daily consumption for the hoverfly was 5.8 aphids per day at 18°C followed by 4.8 aphids per day at 15°C and 3.6 aphids per day at 12°C (Fig. 4a). Average daily aphid consumption was at least two times greater for the hoverfly when compared with the silverfly (F2, 239 = 175.55, P < 0.01). Daily aphid consumption was significantly greater for the hoverfly when compared to the silverfly at 18°C (F2, 83 = 235.05, P < 0.01), 15°C (F2, 73 = 235.05, P < 0.01), and 12°C (F2, 83 = 235.05, P < 0.01).
Fig. 4.
Average daily consumption (a) and average total consumption (b) of foxglove aphids (+SE) by American hoverfly, Eupeodes americanus (n = 32 at 18°C, n = 32 at 15°C, n = 32 at 12°C) and silverfly, Leucopis glyphinivora (n = 51 at 18°C, n = 41 at 15°C, n = 51 at 12°C) larvae. Treatments with a different letter and an asterisk are significantly different (ANOVA, P < 0.05).
Total aphid consumption, when the duration of the tests was equal to a 75 DD development for the aphid, was significantly higher for the American hoverfly at all temperatures and was at least three times greater than the silverfly (F2, 239 = 166.13, P < 0.01, Fig. 4b). Total aphid consumption was significantly greater for the hoverfly when compared with the silverfly at 18°C (F2, 83 = 222.38, P < 0.01), 15°C (F2, 73 = 222.38, P < 0.01), and 12°C (F2, 83 = 222.38, P < 0.01).
Discussion
In accordance with to what occurs with most invertebrates, performances of both the American hoverfly and the silverfly were negatively affected at low temperatures. However, although the silverfly is clearly not an adequate potential biological control agent against the foxglove aphid at low temperatures, the American hoverfly demonstrated a high potential and should be considered for testing its efficacy in commercial cropping systems in North America.
This is the first study to look at the efficiency of the American hoverfly against the foxglove aphid in terms of active flight, oviposition, and voracity at low temperatures. Hoverflies were more efficient in terms of active flight at low temperatures when compared with silverflies. They are also the only predator currently employed for the control of M. persicae on peach trees in the Yakima Valley, WA, in September and October when temperatures were lower (Tamaki et al. 1967).
Considering oviposition performance, the delay before ovarian or egg maturation may be longer at 12°C, which may explain why females of both species laid a higher number of eggs after 14 d compared with 7 d. During this study, mating capacity was also evaluated alongside egg-laying capacity, as virgin predators were used for measuring fecundity.
Considering larval voracity, American hoverfly larvae consumed almost three times more aphids at all temperatures when compared with the silverfly. At the end of each test, hoverfly individuals were still at the third larval stage. Of the three larval stages, more than 80% of consumption is done at the third larval stage for Syrphidae in general (Schneider 1969). Hopper et al. (2011) had shown that larvae of different syrphid species have a daily aphid consumption rate higher than other groups of aphidophagous predators. Moreover, lifetime larval consumption was greater at lower temperatures for the hoverfly Melangyna viridiceps Macquart and Symosyrphus grandicornis Macquart (Soleyman-Nezhadiyan and Laughlin 1998) and Syrphus ribesii L. (Diptera: Syrphidae) (Sundby 1966) due to longer development time at cooler temperatures. We should therefore expect similar results with E. americanus. In this study, daily aphid consumption corresponding to an accumulation of 75 DD for aphids neither decreased or increased at all temperatures tested for both the American hoverfly and the silverfly. Thus, the impact of the predator was the same, regardless of drops in temperature, according to a standardized development time of the focal pest.
The silverfly, while not as efficient at low temperatures against the foxglove aphid, might still be an interesting candidate biological control agent for greenhouse vegetables at higher temperatures. The silverfly is a furtive predator, meaning that it can develop within an aphid colony and generate a lesser number of defensive behaviors within these colonies (Fréchette et al. 2008). This type of furtive behavior also generates a dilution effect (Lucas and Brodeur 2001) and a selfish herd effect (Dumont et al. 2015), which decrease intraguild predation on the furtive predator. Because the silverfly is less vulnerable to intraguild predation due to dilution and the selfish herd effect, it may be successfully used jointly with other biocontrol agents. Also, some species of the Leucopis Meigen genus are efficient in glasshouse environments (Gaimari and Turner 1996) and prey upon numerous aphid species in around 20 genera (Satar et al. 2015).
Other biocontrol agents have been tested against aphids at low temperatures. A similar study by Langer et al. (2004) done in Belgium evaluated the oviposition (2, 4, 6, 8, 10, 15, and 20°C) and flight (10, 12, 14, and 16°C) in opaque cylinders of four parasitoid species (Aphidius rhopalosiphi, Ap. ervi, P. volucre, and P. gallicum). Another approach for controlling aphids at low temperatures could be the use of generalist predators, such as mirid predatory bugs (Messelink et al. 2015), that can survive well in the absence of aphids and at low temperatures. This enables them to respond faster to new aphid colonies than syrphids, which need to first oviposit, and in cases of low temperatures, require additional days before the eggs hatch and predation can begin. The constraint is that in Canada, only a single mirid species, Dicyphus hesperus, is available commercially to control whiteflies (McGregor et al. 1999), thrips (Shipp and Wang 2006), and the green peach aphid (McGregor and Gillespie 2004).
According to the present results, it remains difficult to assess the potential of the hoverfly in glasshouses based on tests in small cages and cylinders. Nevertheless, in the study presented previously (Langer et al. 2004), trends in the results obtained in cylinders (flight and oviposition activities) were confirmed in real conditions. There is still much more work to be done before commercialization. First, to validate the potential of the syrphid at low temperatures in commercial greenhouses, the RH must also be considered. For example, Braconidae were studied under constant temperatures and varying levels of RH, and it was observed that under high RH, flight capacity increased (Abraham 1975). Furthermore, it would be relevant in future studies to compare the effect of RH on oviposition of different sized auxiliaries such as the American hoverfly and the silverfly. Starý (1970) established that reproductive success of smaller insects was affected negatively by low humidity. RH has also been shown to affect performance and developmental time of immature stages of Sphaerophoria rueppellii Wiedemann (Diptera: Syrphidae) (Amoróros-Jiménez et al. 2012). Finally, since Juillet (1964) demonstrated that only extremes of RH cause drastic reductions in the flight activity of biological control agents such as parasitic wasps, we can expect that the efficiency of the hoverfly should not be potentially affected in commercial greenhouses.
Second, the present study did not look at oviposition responses to aphid colony sizes as Sutherland et al. (2001) have done for Episyrphus balteatus De Geer (Diptera: Syrphidae). Studying aphid density thresholds for the American hoverfly would allow us to better understand syrphid oviposition mechanisms and the way they can contribute to lowering foxglove aphid populations (Sutherland et al. 2001). As was done in a study by Langer et al. (2004) on oviposition at low temperatures of four aphid parasitoid species, it would also be interesting to look at offspring survival and sex ratios, the influence of male densities on female oviposition, as well as sperm fitness (see Langer et al. 2004 for four aphid parasitoid species).
Third, the efficacy of the hoverfly in a real commercial greenhouse environment must be evaluated. Preliminary results confirm the potential of the American hoverfly against the foxglove aphid on a larger scale. With these results, we will be able to estimate the time needed for aphid control at low temperatures when larvae or pupae are introduced in the system. Similar hoverflies such as Ep. balteatus and S. rueppellii have already been commercialized in Europe by Biobest Belgium NV. Episyrphus balteatus is also produced by Koppert Biological Systems (The Netherlands). However, both Ep. balteatus (Pu et al. 2017) and S. rueppellii (Barkalov 2010) are Palearctic species native to Europe and Northern Asia. Episyrphus balteatus has been shown to prey upon Sitobion avenae (F.) (Hemiptera: Aphididae) (17°C), Metopolophium dirhodum (Walker) (Hemiptera: Aphididae) (14 and 17°C), as well as Aphis fabae (Scopoli) (Hemiptera: Aphididae) (15°C) (Tenhumberg 1995). As for S. rueppellii, there is little information on the larval performance at low temperatures. However, adults are known to be active between 12 and 40°C, with an optimal range of 25–35°C (Biobest Canada Ltd.).
Finally, this study brings new knowledge on two species of candidate biological control agents that could be interesting for biological control programs. The ultimate step leading to commercialization depends on many other aspects such as host location, dispersal, rearing difficulties as well as costs for mass production, market value, and efficacy in commercial cropping systems (Tauber et al. 2000). The application of biological control has yet to be increased (Lenteren 2011), the diversity of wild biocontrol agents is underutilized, and an important gap remains to be filled (Putra and Yasuda 2006).
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. There is an overlap between this manuscript and an article found on a website. The manuscript is accessible via the link https://www.iobc-wprs.org/members/shop_en.cfmmod_Shop_detail_produkte=170.
Acknowledgments
We thank Caroline Martineau, Émilie Lemaire, and Benoit Champagne from the ‘Institut québecois du développement de l’horticulture ornementale’ (IQDHO) for their time and expertise. We also thank the entire team from the ‘Laboratoire de lutte biologique’, Sylvain Dallaire, and Jill Vandermeerschen. Finally, we thank Dr. S. D. Gaimari, California Department of Food and Agriculture, for Leucopis glyphinivora identification and rearing advice. This study was funded by the ‘Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec’, Québec, QC, Canada (UQAM 1-13-1652). E.L. and M.F. conceived and designed research. Y.B. and M.F. conducted experiments. Y.B. and M.F. analyzed data. Y.B. wrote the manuscript. E.L. supervised the study. All authors read and approved the manuscript. We declare that they have no conflict of interest.
Reference Cited
- Abraham R. 1975. Über die wirkung der temperatur auf die flugaktivität parasitischer Hymenopteren. J. Appl. Entomol. 79: 113–123. [Google Scholar]
- Almohamad R., Verheggen F. J., and Haubruge É.. 2009. Searching and oviposition behaviour of aphidophagous hoverflies (Diptera: Syrphidae): a review. Biotechnol. Agron. Soc. Envir. 13: 467–481. [Google Scholar]
- Alotaibi S. 2008. Mass production and utilization of the predatory midge, Aphidoletes aphidimyza Rondani for controlling aphids. Global J. Biotech. Biochem. 3: 1–7. [Google Scholar]
- Amoróros-Jiménez R., Pineda A., Fereres A., and Ángeles Marcos-García M.. 2012. Prey availability and abiotic requirements of immature stages of the aphid predator Sphaerophoria rueppellii. Biol. Control 63: 17–24. [Google Scholar]
- Barkalov A. V. 2010. New data on the nomenclature and fauna of the genus Sphaerophoria Le Peletier et Serville, 1828 (Diptera, Syrphidae) from Siberia and adjacent territories. Entomol. Rev. 91: 898–907. [Google Scholar]
- Beschovski V. L., and Merz B.. 1998. Contribution to the knowledge of the Chamaemyiidae (Diptera), with particular reference to the fauna of Switzerland. J. Swiss. Entomol. Soc. 71: 83–106. [Google Scholar]
- Biobest Canada Ltd 2017. Robust aphid control with predatory Sphaero phoria-System (http://www.biobestgroup.com/en/news/robust-aphid-control-with-predatory-sphaerophoria-system) (Accessed 21 July 2017).
- Blackman R. L., and Eastop V. F. 2000. Aphids on the world’s crops: an identification and information guide, 2nd ed, p. 245 Wiley, New York. [Google Scholar]
- Brodsky A. K. 1994. The evolution of insect flight. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- De Conti B. F., Bueno V. H. P., Sampaio M. V., and Van Lenteren J. C.. 2011. Development and survival of Aulacorthum solani, Macrosiphum euphorbiae and Uroleucon ambrosiae at six temperatures. Bull. Insectol. 64: 63–68. [Google Scholar]
- Denlinger D. L., and Lee R. E. Jr. 2010. Low temperature biology of insects, pp. 1–406. Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- Dixon A. F. G., Jarošík V., and Honĕk A.. 2005. Thermal requirements for development and resource partitioning in aphidophagous guilds. Eur. J. Entomol. 102: 407–411. [Google Scholar]
- Dumont F., Lucas E., and Brodeur J.. 2015. Do furtive predators benefit from a selfish herd effect by living within their prey colony?Behav. Ecol. Sociobiol. 69: 971–976. [Google Scholar]
- Frazer B. D. 1972. A simple and efficient method of rearing aphidophagous hoverflies (Diptera: Syrphidae). J. Entomol. Soc. B. C. 69: 23–24. [Google Scholar]
- Fréchette B., Larouche F., and Lucas E.. 2008. Leucopis annulipes larvae (Diptera: Chamaemyiidae) use a furtive predation strategy within aphid colonies. Eur. J. Entomol. 105: 399–403. [Google Scholar]
- Gaimari S. D., and Turner W. J.. 1996. Methods for rearing aphidophagous Leucopis spp. (Diptera: Chamaemyiidae). J. Kans. Entomol. Soc. 69: 363–369. [Google Scholar]
- Gillespie D. R., and Acheampong S.. 2012. Dropping behaviour in Aulacorthum solani (Hemiptera: Aphididae) following attack by Aphidius ervi (Hymenoptera: Braconidae): are sticky stem bands a useful integrated pest management method?Can. Entomol. 144: 589–598. [Google Scholar]
- Hart A. J., Bale J. S., and Fenlon J. S.. 1997. Developmental threshold, day-degree requirements and voltinism of the aphid predator Episyrphus balteatus (Diptera: Syrphidae). Ann. Appl. Biol. 130: 427–437. [Google Scholar]
- Helgadóttir F., Toft S., and Sigsgaard L.. 2017. Negative effects of low developmental temperatures on aphid predation by Orius majusculus (Heteroptera: Anthocoridae). Biol. Control 114: 59–64. [Google Scholar]
- Honĕk A. and Kocourek F.. 1988. Thermal requirements for development of aphidophagous Coccinellidae (Coleoptera), Chrysopidae, Hemerobiidae (Neuroptera), and Syrphidae (Diptera): some general trends. Oecologia 76: 455–460. [DOI] [PubMed] [Google Scholar]
- Hopper J. V., Nelson E. H., Daane K. M., and Mills N. J.. 2011. Growth, development and consumption by four syrphid species associated with the lettuce aphid, Nasonovia ribisnigri, in California. Biol. Control 58: 271–276. [Google Scholar]
- Jalali M. A., Tirry L., and De Clercq P.. 2010. Effect of temperature on the functional response of Adalia bipunctata to Myzus persicae. BioControl 55: 261–269. [Google Scholar]
- Jandricic S. E. 2013. Investigations of the biology of the pest aphid Aulacorthum solani (Kaltenbach) (Hemiptera: Aphididae) and of biological control agents for control of multi-species aphid outbreaks in greenhouse floriculture crops. Ph.D. dissertation. Cornell University, Ithaca, New York, pp. 1–260. [Google Scholar]
- Jandricic S. E., Mattson N. S., Wraight S. P., and Sanderson J. P.. 2014. Within-plant distribution of Aulacorthum solani (Hemiptera: Aphididae), on various greenhouse plants with implications for control. J. Econ. Entomol. 107: 697–707. [DOI] [PubMed] [Google Scholar]
- Jandricic S. E., Wraight S. P., Bennett K. C., and Sanderson J. P.. 2010. Developmental times and life table statistics of Aulacorthum solani (Hemiptera: Aphididae) at six constant temperatures, with recommendations on the application of temperature-dependent development models. Environ. Entomol. 39: 1631–1642. [DOI] [PubMed] [Google Scholar]
- Juillet J. A. 1964. Influence of weather on flight activity of parasitic Hymenoptera. Can. J. Zool. 42: 1133–1141. [Google Scholar]
- Kahanpää J. 2014. Checklist of the fly families Chamaemyiidae and Lauxaniidae of Finland (Insecta, Diptera). Zookeys 441: 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser M. E., Noma T., Brewer M. J., Pike K. S., Vockeroth J. R., and Gaimari S. D.. 2007. Hymenopteran parasitoids and dipteran predators found using soybean aphid after its Midwestern United States invasion. Ann. Entomol. Soc. Am. 100: 196–205. [Google Scholar]
- Katsarou I., Margaritopoulos J. T., Tsitsipis J. A., Perdikis D. C., and Zarpas K. D.. 2005. Effect of temperature on development, growth, and feeding of Coccinella septempunctata and Hippodamia convergens reared on the tobacco aphid, Myzus persicae nicotianae. BioControl 50: 565–588. [Google Scholar]
- Langer A., Boivin G., and Hance T.. 2004. Oviposition, flight and walking capacity at low temperatures of four aphid parasitoid species (Hymenoptera: Aphidiinae). Eur. J. Entomol. 101: 473–479. [Google Scholar]
- Lee S. G., Kim H. H., Kim T. H., Park G-J., Kim K. H., and Kim J. S.. 2008. Development model of the foxglove aphid, Aulacorthum solani (Kaltenbach) on lettuce. Korean J. Appl. Entomol. 47: 359–364. [Google Scholar]
- Lenteren J. C. 2011. The state of commercial augmentative biological control: plenty of natural enemies, but a frustrating lack of uptake. BioControl 57: 1–20. [Google Scholar]
- Leroy P. D., Verheggen F. J., Capella Q., Francis F., and Haubruge E.. 2010. An introduction device for the aphidophagous hoverfly Episyrphus balteatus (De Geer) (Diptera: Syrphidae). Biol. Control 54: 181–188. [Google Scholar]
- Lucas E., and Brodeur J.. 2001. A fox in sheep’s clothing: furtive predators benefit from the communal defense of their prey. Ecology 82: 3246–3250. [Google Scholar]
- McGregor R. R., and Gillespie D. R.. 2004. Olfactory responses of the omnivorous generalist predator Dicyphus hesperus to plant and prey odours. Entomol. Exp. Appl. 112: 201–205. [Google Scholar]
- McGregor R. R., Gillespie D. R., Quiring D. M. J., and Foisy M. R. J.. 1999. Potential use of Dicyphus hesperus Knight (Heteroptera: Miridae) for biological control of pests of greenhouse tomatoes. Biol. Control 16: 104–110. [Google Scholar]
- Messelink G. J., Bloemhard C. M. J., Hoogerbrugge H., van Schelt J., Ingegno B. L., and Tavella L.. 2015. Evaluation of mirid predatory bugs and release strategy for aphid control in sweet pepper. J. Appl. Entomol. 139: 333–341. [Google Scholar]
- Ministry of Agriculture, Food and Rural Affairs 2014. Guide to greenhouse floriculture production, pp. 1–162. Publication 370. Ministry of Agriculture, Food and Rural Affairs, Toronto, ON, Canada. [Google Scholar]
- Obrycki J. J., and Tauber M. J.. 1982. Thermal requirements for development of Hippodamia convergens (Coleoptera: Coccinellidae). Ann. Entomol. Soc. Am. 75: 678–683. [Google Scholar]
- Pu D-Q., Liu H.-L., Gong Y.-Y., Ji P.-C., Li Y.-J., Mou F.-S., and Wei S.-J.. 2017. Mitochondrial genomes of the hoverflies Episyrphus balteatus and Eupeodes corollae (Diptera: Syrphidae), with a phylogenetic analysis of Muscomorpha. Sci. Rep. 7: 44300; doi:10.1038/srep44300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putra N. S., and Yasuda H.. 2006. Effects of prey species and its density on larval performance of two species of hoverfly larvae, Episyrphus balteatus de Geer and Eupeodes corolla Fabricius (Diptera: Syrphidae). Appl. Entomol. Zool. 41: 389–397. [Google Scholar]
- Richard C., and Boivin G.. 1994. Maladies et ravageurs des cultures légumières au Canada: un traité pratique illustré ‘Cet ouvrage a paru en anglais sous le titre Diseases and pests of vegetable crops in Canada, publié sous la direction de Ronald J. Howard, J. Allan Garland et W. Lloyd Seaman’, p. 7 La société Canadienne de Phytopathologie et la Société d’entomologie du Canada, Ottawa, ON, Canada. [Google Scholar]
- Rocca M., and Messelink G. J.. 2017. Combining lacewings and parasitoids for biological control of foxglove aphids in sweet pepper. J. Appl. Entomol. 141: 402–410. [Google Scholar]
- Rojo S., Gilbert F., Marcos-García M. A., Nieto J. M., and Mier Durante M. P.. 2003. A world review of predatory hoverflies (Diptera, Syrphidae: Syrphinae) and their prey. Centro Iberoamericano de la Biodiversidad (CIBIO) Ediciones, Universidad de Alicante, Alicante, Spain. [Google Scholar]
- Sanchez J. A., Cánovas F., and Lacasa A.. 2007. Thresholds and management strategies for Aulacorthum solani (Hemiptera: Aphididae) in greenhouse pepper. J. Econ. Entomol. 100: 123–130. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc 2009. JMP® 8 user guide, 2nd ed SAS Institute Inc, Cary, NC. [Google Scholar]
- Satar S., Raspi A., Özdemir I., Tusun A., Karacaoğlu M., and Benelli G.. 2015. Seasonal habits of predation and prey range in aphidophagous silver flies (Diptera: Chamaemyiidae), an overlooked family of biological control agents. Bull. Insectol. 68: 173–180. [Google Scholar]
- Schneider F. 1969. Bionomics and physiology of aphidophagous Syrphidae. Annu. Rev. Entomol. 14: 103–124. [Google Scholar]
- Schüder I., Hommes M., and Larink O.. 2004. The influence of temperature and food supply on the development of Adalia bipunctata (Coleoptera: Coccinellidae). Eur. J. Entomol. 101: 379–384. [Google Scholar]
- Shipp J. L. and Wang K.. 2006. Evaluation of Dicyphus hersperus (Heteroptera: Miridae) for biological control of Frankliniella occidentalis (Thysanoptera: Thripidae) on greenhouse tomato. J. Econ. Entomol. 99: 414–420. [DOI] [PubMed] [Google Scholar]
- Silva D. B., Bueno V. H. P., Sampaio M. V., and van Lenteren J. C.. 2015. Performance of the parasitoid Praon volucre in Aulacorthum solani at five temperatures. Bull. Insectol. 68: 119–125. [Google Scholar]
- Sluss T. P., and Foote B. A.. 1973. Biology and immature stages of Leucopis pinicola and Chamaemyia polystigma (Diptera: Chamaemyiidae). Can. Entomol. 105: 1443–1452. [Google Scholar]
- Soleyman-Nezhadiyan E., and Laughlin R.. 1998. Voracity of larvae, rate of development in eggs, larvae and pupae, and flight seasons of adults of the hoverflies Melangyna viridiceps Macquart and Symosyrphus grandicornis Macquart (Diptera: Syrphidae). Aust. Entomol. 37: 243–248. [Google Scholar]
- Sørensen C. H., Toft S., and Kristensen T. N.. 2013. Cold-acclimation increases the predatory efficiency of the aphidophagous coccinellid Adalia bipunctata. Biol. Control 65: 87–94. [Google Scholar]
- Starý P. 1970. Biology of aphid parasites (Hymenoptera: Aphidiidae) with respect to integrated control. Ser. Entomol. 6: 1–643. [Google Scholar]
- Sundby R. A. 1966. A comparative study of the efficiency of three predatory insects Coccinella septempunctata L. (Coleoptera, Coccinellidae), Chrysopa carnea Steph. (Neuroptera, Chrysopidae) and Syrphus ribesii L. (Diptera, Syrphidae) at two temperatures. Entomophaga 11: 395–404. [Google Scholar]
- Sutherland J. P., M. S. Sullivan, and Poppy G. M.. 2001. Oviposition behaviour and host colony size discrimination in Episyrphus balteatus (Diptera: Syrphidae). Bull. Entomol. Res. 91: 411–418. [DOI] [PubMed] [Google Scholar]
- Takada H., Ono T., Torikura H., and Enokiya T.. 2006. Geographic variation in esterase allozymes of Aulacorthum solani (Homoptera: Aphididae) in Japan, in relation to its outbreaks on soybean. Appl. Entomol. Zool. 41: 595–605. [Google Scholar]
- Tamaki G., Landis B. J., and Weeks R. E.. 1967. Autumn populations of green peach aphid on peach trees and the role of syrphid flies in their control. J. Econ. Entomol. 60: 433–436. [Google Scholar]
- Tauber M. J., Tauber C. A., Daane K. M., and Hagen K. S.. 2000. Commercialization of predators: recent lessons from green lacewings (Neuroptera: Chrysopidae: Chrosoperla). Am. Entomol. 46: 26–38. [Google Scholar]
- Teets N. M., and Denlinger D. L.. 2013. Physiological mechanisms of seasonal and rapid cold-hardening in insects. Physiol. Entomol. 38: 105–116. [Google Scholar]
- Tenhumberg B. 1995. Estimating predatory efficiency of Episyrphus balteatus (Diptera: Syrphidae) in cereal fields. Environ. Entomol. 24: 687–691. [Google Scholar]
- University of Minnesota’s Center for Urban and Regional Affairs and Center for Sustainable Building Research 2013. Cold Climate Greenhouse Resource: A guidebook for designing and building a cold-climate greenhouse, pp. 1–65. The Regents of the University of Minnesota, Minneapolis, Minnesota. [Google Scholar]
- Vockeroth J. R. 1992. The flower flies of the subfamily Syrphinae of Canada, Alaska, and Greenland, pp. 1–456. Agriculture Canada, Ottawa, ON, Canada. [Google Scholar]
- Yovkova M., Petrović-Obradović O., Tasheva-Terzieva E., and Pencheva A.. 2013. Aphids (Hemiptera, Aphididae) on ornamental plants in greenhouses in Bulgaria. Zookeys 319: 347–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. There is an overlap between this manuscript and an article found on a website. The manuscript is accessible via the link https://www.iobc-wprs.org/members/shop_en.cfmmod_Shop_detail_produkte=170.