Abstract
Background
In the United States, incidence of ESRD is 1.5 times higher in men than in women, despite men’s lower prevalence of CKD. Prior studies, limited by inclusion of small percentages of minorities and other factors, suggested that men have more rapid CKD progression, but this finding has been inconsistent.
Methods
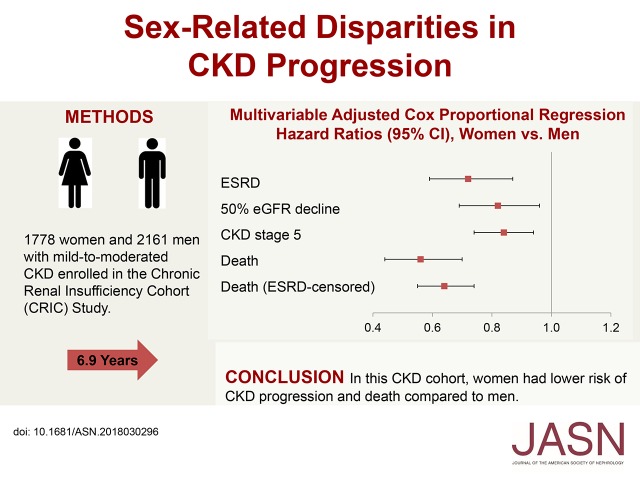
In our prospective investigation of sex differences in CKD progression, we used data from 3939 adults (1778 women and 2161 men) enrolled in the Chronic Renal Insufficiency Cohort Study, a large, diverse CKD cohort. We evaluated associations between sex (women versus men) and outcomes, specifically incident ESRD (defined as undergoing dialysis or a kidney transplant), 50% eGFR decline from baseline, incident CKD stage 5 (eGFR<15 ml/min per 1.73 m2), eGFR slope, and all-cause death.
Results
Participants’ mean age was 58 years at study entry; 42% were non-Hispanic black, and 13% were Hispanic. During median follow-up of 6.9 years, 844 individuals developed ESRD, and 853 died. In multivariable regression models, compared with men, women had significantly lower risk of ESRD, 50% eGFR decline, progression to CKD stage 5, and death. The mean unadjusted eGFR slope was −1.09 ml/min per 1.73 m2 per year in women and −1.43 ml/min per 1.73 m2 per year in men, but this difference was not significant after multivariable adjustment.
Conclusions
In this CKD cohort, women had lower risk of CKD progression and death compared with men. Additional investigation is needed to identify biologic and psychosocial factors underlying these sex-related differences.
Keywords: Epidemiology and outcomes, chronic kidney disease, mortality risk, ESRD
Visual Abstract
The prevalence of CKD is higher in women compared with men, and this difference has been consistent over time (from 13.7% versus 9.8% in 1988–1994 to 15.4% versus 12.8% in 2011–2012).1 In contrast, the lifetime risk of ESRD has been found to be up to 50% higher in men compared with women across racial/ethnic groups.2 These findings suggest that women may have slower kidney function decline compared with men or that they are more likely to die before progressing to ESRD.
Several clinical studies evaluating CKD progression in men versus women have reported conflicting results. In some studies, women seem to have slower progression of CKD compared with men.3–5 In contrast, other studies have found no difference or even a faster progression of CKD in women compared with men.6 Few clinical studies have evaluated the potential reasons for sex-related disparities in CKD progression. It has been postulated that sex disparities in CKD progression could be attributed to multiple factors, including sex hormones, renal hemodynamics, and renal mass differences between men and women.7–12 Lifestyle factors, such as dietary intake of protein13 and smoking,14 have also been proposed as potential mediators of sex differences in CKD progression.7 Sex-related differences in health care utilization15,16 and risk factor control17 may also influence outcomes in women versus men with CKD. Previous studies have been limited by inclusion of small percentages of racial/ethnic minorities who are over-represented in the United States ESRD population,18 small sample sizes, retrospective design, or limited duration of follow-up.5,6
We evaluated sex-related differences in CKD progression and potential explanatory factors for these differences in the Chronic Renal Insufficiency Cohort (CRIC) Study, a large, diverse, and well phenotyped CKD cohort.
Methods
Study Population
The CRIC Study is an ongoing multicenter, prospective, observational study of risk factors for progression of CKD and its complications. The design, methods, and baseline characteristics of study participants have been previously published.19–21 In brief, the CRIC included 3939 adults with mild-to-moderate CKD recruited from May 2003 through March 2008 at seven United States clinical centers based in Chicago, Illinois; Ann Arbor, Michigan; Philadelphia, Pennsylvania; Baltimore, Maryland; New Orleans, Louisiana; Cleveland, Ohio; and Oakland, California. The study was designed to recruit similar numbers of men and women. Main inclusion criteria were age 21–74 years old and eGFR of 20–70 ml/min per 1.73 m2 at enrollment. Exclusion criteria were inability to consent, institutionalization, pregnancy, dialysis treatment for longer than 1 month, polycystic kidney disease, organ or bone marrow transplant, immunosuppressive drugs for kidney disease in the past 6 months, chemotherapy within 2 years, current participation in another research study, New York Heart Association class 3 or 4 heart failure, cirrhosis, HIV infection, multiple myeloma, or renal cell carcinoma. The study protocol was approved by the Institutional Review Board of all participating centers, and it is in accordance with the Declaration of Helsinki. All participants provided informed consent.
Measurements and Variable Definition
The main predictor in this study was self-reported sex (women or men). Information on sociodemographic variables (age, race/ethnicity, education, marital status, annual household income, and health insurance), medical history (hypertension, diabetes, and cardiovascular disease), and smoking habits was obtained at baseline by self-reported questionnaires. Race/ethnicity was categorized as non-Hispanic white, non-Hispanic black, Hispanic, or other. Self-reported history of any cardiovascular disease at baseline included previous myocardial infarction, coronary revascularization, heart failure, stroke, or peripheral arterial disease. At each study visit, participants were queried about any medication use in the previous 30 days. Prior nephrology contact was determined at study entry by answering “yes” to the question: “Have you ever seen a nephrologist or kidney doctor?”22 Physical activity was self-reported using the Multi-Ethnic Study of Atherosclerosis Typical Week Physical Activity Survey.23 Anthropometric measures were assessed using standardized protocols. Body mass index (BMI; kilograms per meter2) was calculated using measured height and weight. BP measurements were obtained using the standardized American Heart Association protocol.24 A fasting blood sample was collected to measure serum creatinine, electrolytes, lipids (total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides), and plasma glucose. Serum creatinine was measured by an enzymatic method from Ortho Clinical Diagnostics through October 2008 and the Jaffe method from Beckman Coulter thereafter, and it was standardized to isotope dilution mass spectrometry–traceable values.25,26 Serum cystatin C was measured using a particle-enhanced immunonephelometric assay on the BN II System (Siemens). Urinary total protein and creatinine were measured using standard assays. Protein-to-creatinine ratios from 24-hour and spot urine specimens were highly correlated, and therefore, they were used interchangeably. Diabetes mellitus was defined by a fasting glucose ≥126 mg/dl or use of insulin or oral hypoglycemic medications, and hypertension was defined by systolic BP ≥140 mm Hg, diastolic BP ≥90 mm Hg, or use of antihypertensive medications.
Outcomes
Primary outcomes included (1) incident ESRD (defined as receipt of chronic dialysis therapy or kidney transplantation), (2) 50% decline in eGFR from baseline, and (3) incident CKD stage 5 (eGFR<15 ml/min per 1.73 m2). In addition, we evaluated the following secondary outcomes: (1) rate of decline in kidney function (eGFR slope over time) and (2) death from any cause. Ascertainment of ESRD was done through semiannual surveillance by the CRIC Study personnel supplemented by crosslinkage with the US Renal Data System, leading to no missing data for this outcome. GFR was estimated at baseline and each annual visit using a serum creatinine and cystatin C–based equation developed in a subgroup of the CRIC participants with measured iothalamate GFR.27 This equation has been shown to have superior accuracy in this cohort compared with other eGFR equations, and there was no evidence of significant bias among obese individuals (median difference [interquartile range] between measured iothalamate GFR and eGFR of 0 [−5–7] ml/min per 1.73m2) or difference in bias between women (0 [−5–7]) and men (0 [−4–6]).27 Deaths were ascertained from reports by next of kin, death certificates, hospital records, and linkage with the Social Security Death Master File. Participants were followed until the occurrence of death, withdrawal from the study, or 2013 when the database was locked for analysis.
Statistical Analyses
Descriptive statistics were summarized as mean (SD) or median (interquartile range) for continuous variables and frequency (proportion) for categorical variables. Chi-squared tests or Wilcoxon rank sum tests were used to compare categorical variables, and t tests were used to compare continuous variables. Event rates (per 100 person-years) for time-to-event outcomes were calculated as the ratio of the number of participants reaching the event divided by the total person-years of follow-up before an event or until censoring by using Poisson regression. In computations of event rates and failure-time regression analyses, follow-up times were censored at time of death or end of the follow-up period (March 31, 2013). For slope analyses, follow-up times were censored at the time of ESRD, death, or end of follow-up. Cox proportional hazards models were used to examine the association between sex and outcomes. For each outcome, we fitted five nested Cox proportional hazards models with covariates from each prior model retained as follows. Model 1 was stratified by clinical site and adjusted for age, race/ethnicity, and baseline kidney function (eGFR splines and log protein-to-creatinine ratio splines). Model 2 was additionally adjusted for other demographic and clinical care variables: education, marital status, nephrology care, and health insurance. Model 3 was additionally adjusted for lifestyle factors: smoking, physical activity, BMI, and waist circumference. Model 4 was additionally adjusted for cardiovascular risk factors: systolic BP, diabetes, prior cardiovascular disease, LDL cholesterol, HDL cholesterol, triglycerides, serum albumin, and hemoglobin. Model 5 was additionally adjusted for use of cardioprotective medications: angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, aspirin, and statin. Model 6 was additionally adjusted for markers of bone mineral metabolism: serum fibroblast growth factor 23 (FGF23), calcium, and phosphorus. These covariates, which were ascertained at the time of the baseline visit, were chosen on the basis of published clinical relevance.28–33 The order in which the covariates entered the model was chosen a priori on the basis of findings from prior studies.28–31,34 We explored effect modification by age (as a continuous variable), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, or other), BMI (as a categorical variable), and diabetes status at baseline (yes or no) by separately testing interaction terms between sex and each of these variables in the final regression model. In addition, we conducted the following sensitivity analyses: (1) death was evaluated as a competing risk for the outcome of ESRD using subdistribution hazard models,35 (2) BMI was added to the regression model as a categorical variable (<25, 25 to <30, 30 to <35, and ≥35 kg/m2) instead of as a continuous variable, and (3) ESRD was treated as a censoring event for the outcome death from any cause. Furthermore, we evaluated CKD stage at the time of death as a potential effect modifier of the association between sex and death. For eGFR slope analyses, multivariable linear mixed effects models were used to compare the eGFR mean annual change in women versus men. Serum creatinine measurements for eGFR after development of ESRD were not taken into account for these analyses. We constructed six nested models as described above for failure-time analyses. All tests were two sided, and P<0.05 was considered statistically significant for hypothesis testing. All analyses were performed using SAS 9.3 (SAS Institute, Cary, NC).
Results
Baseline Characteristics
Of the total 3939 participants in this study, 1778 were women, and 2161 were men (Table 1). The mean age at study entry was 58 years old, 42% were non-Hispanic black, and 13% were Hispanic. The mean baseline eGFR was 44 ml/min per 1.73 m2 in women and 46 ml/min per 1.73 m2 in men, and the median urine protein excretion was 113 mg/24 h in women compared with 268 mg/24 h in men. Compared with men, women were more likely to belong to a racial/ethnic minority group, have low income, and have less than a high school education. Women were also less likely to be married or live with a partner, and they were less likely to have ever seen a nephrologist. The prevalence of hypertension, cardiovascular disease, and diabetes was lower in women compared with men, although the mean systolic BP and glycosylated hemoglobin were similar between the two sexes. Moreover, women were more likely to have higher LDL and lower HDL cholesterol levels than men, but they were less likely to report statin use. Women were also less likely to report use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and aspirin than men. Furthermore, women were more likely to abstain from smoking, but they were less likely to report regular physical activity and more likely to have higher BMI and higher waist circumference compared with men. Women also had higher serum hemoglobin, calcium, phosphorus, and FGF23 than men. Seven hundred seventy-seven participants (414 men and 363 women) were excluded from multivariable regression analyses due to missing covariate data (annual household income or health insurance status [n=603], FGF23 [n=60], waist circumference [n=48], or other covariates [n=66]). Compared with individuals included in the final regression model (n=3162), those excluded due to missing covariate data (n=777) were of similar age (57.5 versus 58.3 years old), were less likely to be non-Hispanic white (32% versus 44%), had lower baseline eGFR (42 versus 46 ml/min per 1.73 m2), and had higher median proteinuria (232 versus 174 mg/24 h; P<0.05 for the former three comparisons).
Table 1.
Baseline characteristics of the Chronic Renal Insufficiency Cohort participants by sex
| Characteristics | Women, n=1778 | Men, n=2161 |
|---|---|---|
| Demographic characteristics | ||
| Age, yr, mean (SD) | 58.0 (11.2) | 58.3 (10.9) |
| Age categories, yr, n (%) | ||
| 21–45 | 242 (13.9) | 296 (14.0) |
| 46–60 | 664 (38.1) | 777 (36.8) |
| 61–74 | 836 (48.0) | 1039 (49.2) |
| Race/ethnicity, n (%) | ||
| Non-Hispanic white | 656 (36.9)a | 982 (45.4) |
| Non-Hispanic black | 844 (47.5) | 806 (37.3) |
| Hispanic | 209 (11.8) | 288 (13.3) |
| Other | 69 (3.9) | 85 (3.9) |
| Annual household income <$20,000, n (%) | 660 (37.1)a | 580 (26.8) |
| Less than high school education, n (%) | 400 (22.5)a | 428 (19.9) |
| Marital status n (%) | ||
| Married/living with partner | 752 (42.3)a | 1406 (65.1) |
| Single | 305 (17.2) | 263 (12.2) |
| Widowed/divorced | 721 (40.6) | 492 (22.8) |
| Health insurance, n (%) | 1456 (81.9) | 1782 (82.5) |
| Ever seen a nephrologist (yes versus no), n (%) | 1084 (61.0)a | 1517 (70.2) |
| Clinical and laboratory characteristics | ||
| Hypertension, n (%) | 1499 (84.3)a | 1892 (87.6) |
| Diabetes, n (%) | 844 (47.5) | 1064 (49.2) |
| Cardiovascular disease, n (%) | 514 (28.9) | 802 (37.1) |
| Family history of CHD, n (%) | 932 (65.3)a | 998 (57.7) |
| Systolic BP, mm Hg, mean (SD) | 128.3 (22.7) | 128.6 (21.7) |
| Glycated hemoglobin, %, median (IQR) | 6.1 (5.6–7.3) | 6.2 (5.6–7.4) |
| LDL cholesterol, mg/dl, mean (SD) | 107.4 (36.5)a | 98.9 (34.4) |
| HDL cholesterol, mg/dl, mean (SD) | 42.7 (12.2)a | 53.4 (17.0) |
| Triglycerides, mg/dl, median (IQR) | 122.5 (86–171)a | 133 (93–197) |
| FGF23, RU/ml, median (IQR) | 165.8 (105.0–276.8)a | 132.0 (90.7–207.9) |
| Calcium, mg/dl, mean (SD) | 9.3 (0.5)a | 9.2 (0.4) |
| Phosphate, mg/dl, mean (SD) | 3.9 (0.6)a | 3.6 (0.7) |
| Albumin, g/dl, mean (SD) | 3.9 (0.45) | 4.0 (0.49) |
| Hemoglobin, g/dl, mean (SD) | 13.1 (1.8)a | 12.0 (1.5) |
| Kidney function measurements | ||
| eGFR, ml/min per 1.73 m2, mean (SD) | 43.9 (17.4) | 45.7 (16.4) |
| eGFR categories, ml/min per 1.73 m2, n (%) | ||
| <30 | 405 (22.8)a | 402 (18.6% |
| 30 to <45 | 627 (35.3) | 712 (32.9) |
| 45 to <60 | 441 (24.8) | 650 (30.1) |
| ≥60 | 305 (17.2) | 397 (18.4) |
| Urine protein, mg/24 h, median (IQR) | 113 (60–599)a | 268 (89–1200) |
| Lifestyle factors | ||
| Never smoker, n (%) | 944 (53.1)a | 837 (38.7) |
| Physical activity 30 min/d at least 5 d/wk, n (%) | 817 (46.0)a | 1185 (54.8) |
| Body mass index, kg/m2, mean (SD) | 33.2 (9.2)a | 31.1 (6.4) |
| Body mass index categories, kg/m2, n (%) | ||
| <25 | 287 (13.3)a | 343 (19.3) |
| 25–<30 | 753 (35.0)a | 372 (20.9) |
| 30–<35 | 632 (29.4)a | 393 (22.1) |
| ≥35 | 481 (22.3)a | 668 (37.6) |
| Waist circumference, cm, mean (SD) | 106.5 (15.9)a | 105.1 (19.4) |
| Ideal waist circumference, men <102 cm and women <88 cm, n (%) | 358 (20.4)a | 928 (43.6) |
| Medications, n (%) | ||
| ACE inhibitor or ARB | 1123 (63.7)a | 1566 (72.9) |
| Aspirin | 705 (40.0)a | 972 (45.3) |
| Statin | 922 (52.3)a | 1231 (57.3) |
| Achievement of guideline-recommended goals, n (%) | ||
| BP<130/80 mm Hg | 904 (51) | 1054 (49.1) |
| LDL cholesterol <100 mg/dl | 834 (47.1)a | 1233 (57.3) |
| Hemoglobin A1c <7% | 1172 (69.3) | 1411 (68.3) |
CHD, coronary heart disease; IQR, interquartile range; FGF23, fibroblast growth factor 23; ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; hemoglobin A1c, glycated hemoglobin.
P<0.05.
Outcomes
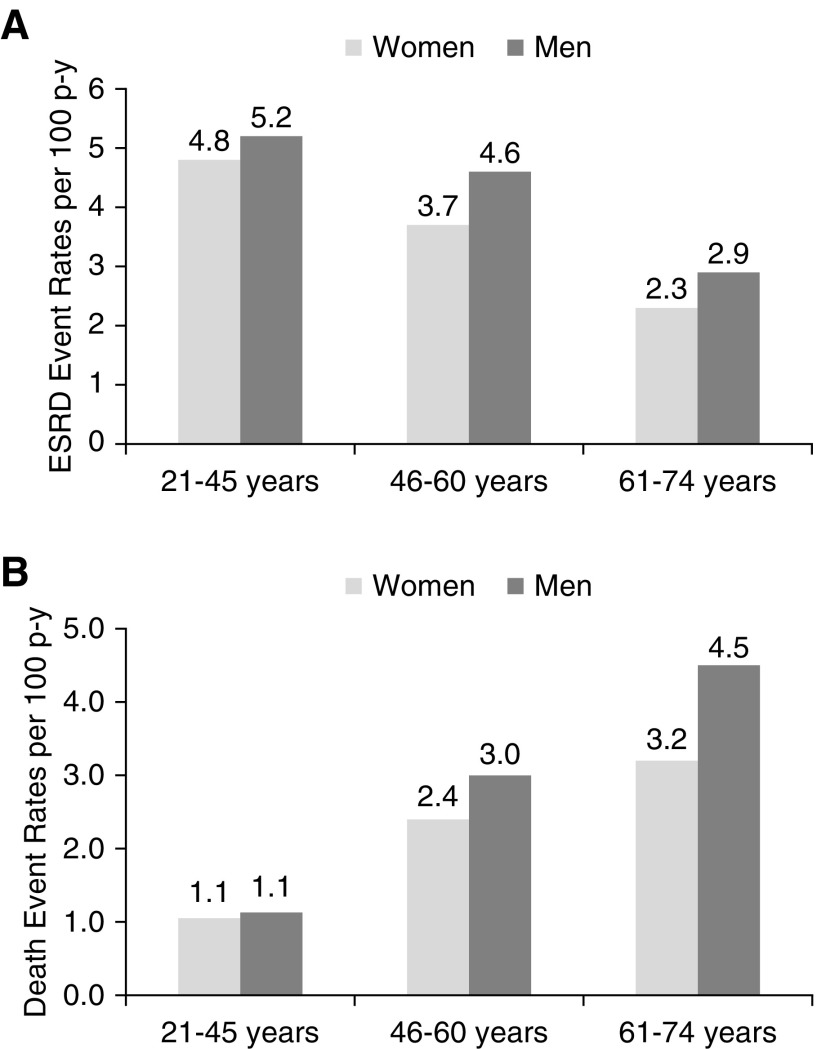
During a median follow-up of 7 years, there were 844 ESRD events (490 men and 354 women) and 853 deaths from any cause (524 men and 329 women). The rate of ESRD per 100 person-years was 3.1 in women and 3.8 in men. The corresponding rates of death were 2.6 and 3.6, respectively. These differences in event rates were observed across almost all age strata (Figure 1). ESRD rates were higher in the younger age strata (21–45 years old) compared with the older age strata (61–74 years old) (Figure 1).
Figure 1.
Unadjusted rates of ESRD and death were lower in women compared with men across age strata. Unadjusted (A) ESRD and (B) death event rates per 100 person-years of follow-up in women (light gray) versus men (dark gray) stratified by age.
Incident ESRD
In a model adjusting for age, race/ethnicity, and baseline kidney function, women had 17% lower risk of ESRD compared with men (hazard ratio [HR], 0.83; 95% confidence interval [95% CI], 0.72 to 0.96) (Table 2). This risk was attenuated but statistically significant after adjusting for additional demographic and clinical variables (HR, 0.72; 95% CI, 0.59 to 0.87). Similar results were observed for the outcomes of 50% eGFR decline (fully adjusted HR, 0.82; 95% CI, 0.69 to 0.96) and incident CKD stage 5 (fully adjusted HR, 0.84; 95% CI, 0.74 to 0.94) (Table 2). Although no significant interactions between sex and diabetes, sex and race/ethnicity, or sex and BMI were observed (P interaction >0.05), age was found to be a significant effect modifier (P interaction =0.01) (Table 2). The age strata–specific multivariable-adjusted HRs (95% CIs) for ESRD in women versus men were 1.15 (95% CI, 0.81 to 1.64) for participants ages 21–45 years old at baseline, 0.69 (95% CI, 0.52 to 0.90) for those 46–60 years old, and 0.58 (95% CI, 0.44 to 0.76) for those 61–74 years old. In sensitivity analyses evaluating death as a competing risk for ESRD, the subdistribution HRs were similar to those obtained from Cox proportional hazards models (fully adjusted subdistribution HR, 0.78; 95% CI, 0.63 to 0.97). Furthermore, adding BMI in the regression model as a categorical variable instead of a continuous variable did not modify the results (fully adjusted HR, 0.71; 95% CI, 0.59 to 0.86).
Table 2.
Hazard ratios with 95% confidence intervals from multivariable regression analyses of the association of sex (women versus men) with renal outcomes
| Model | Variables Included in Regression Models | ESRD | 50% Decline in eGFR from Baseline | Development of CKD Stage 5 (eGFR<15 ml/min per 1.73 m2) |
|---|---|---|---|---|
| Model 1 | Clinical site, age, race/ethnicity, baseline eGFR, and urine protein-to-creatinine ratio | 0.83 (0.72 to 0.96) | 0.85 (0.75 to 0.96) | 0.93 (0.86 to 1.02) |
| Model 2 | +Education, marital status, nephrology care, health insurance | 0.82 (0.71 to 0.95) | 0.85 (0.74 to 0.97) | 0.91 (0.82 to 1.00) |
| Model 3 | +Smoking status, physical activity, BMI, waist circumference | 0.88 (0.75 to 1.04) | 0.91 (0.79 to 1.05) | 0.94 (0.85 to 1.04) |
| Model 4 | +Systolic BP, diabetes, CVD, LDL, HDL, triglycerides, albumin, and hemoglobin | 0.74 (0.61 to 0.88) | 0.82 (0.70 to 0.96) | 0.89 (0.79 to 1.00) |
| Model 5 | +ACEi/ARB, aspirin, and statin | 0.74 (0.62 to 0.89) | 0.83 (0.70 to 0.97) | 0.88 (0.78 to 0.98) |
| Model 6 | +Serum FGF23, calcium, and phosphorus | 0.72 (0.59 to 0.87) | 0.82 (0.69 to 0.96) | 0.84 (0.74 to 0.94) |
| Model 6 | P value for interaction sex × age | 0.01 | 0.03 | 0.53 |
| Age strata–specific multivariable regression analyses | ||||
| Model 6 | 21–45 yr | 1.15 (0.81 to 1.64) | 1.34 (0.96 to 1.86) | — |
| Model 6 | 46–60 yr | 0.69 (0.52 to 0.90) | 0.78 (0.62 to 0.99) | — |
| Model 6 | 61–74 yr | 0.58 (0.44 to 0.76) | 0.73 (0.57 to 0.93) | — |
BMI, body mass index; CVD, cardiovascular disease; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; FGF23, fibroblast growth factor 23; —, not applicable.
eGFR Slope
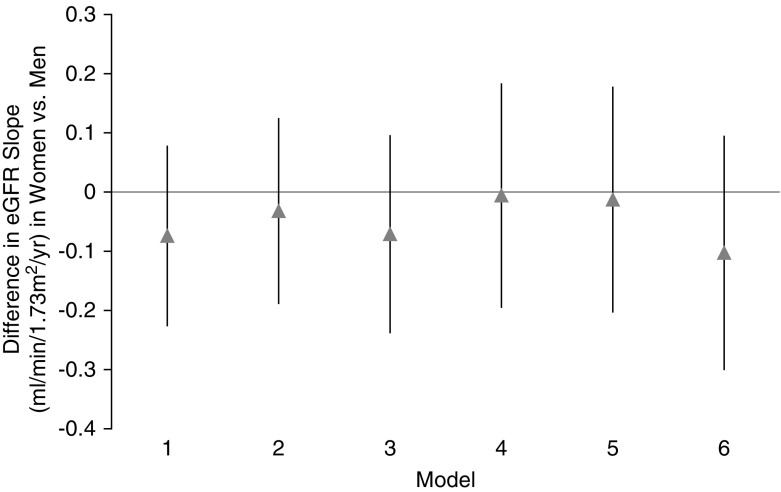
The median numbers (interquartile ranges) of creatinine measurements were six (three to eight) among women and five (three to eight) among men. The mean unadjusted eGFR slope was −1.09 ml/min per 1.73 m2 per year in women and −1.43 ml/min per 1.73 m2 per year in men (difference women versus men: 0.33 ml/min per 1.73 m2 per year; P<0.001). The corresponding fully adjusted means were −3.01 and −2.90 (difference women versus men: −0.10 ml/min per 1.73 m2 per year; P=0.31) (Figure 2).
Figure 2.
Adjusted eGFR slope was similar in women compared with men. Mixed effects models for the difference in eGFR slope in women versus men using a hierarchical modeling approach similar to failure-time regression analyses.
Death from Any Cause
In regression models adjusted for age, race/ethnicity, and baseline kidney function, the risk of death was lower in women than in men (HR, 0.69; 95% CI, 0.60 to 0.80) (Table 3), and this association was unchanged after additional adjustment for other demographic and clinical variables (HR, 0.56; 95% CI, 0.44 to 0.70). Similar results were observed when ESRD was treated as a censoring event (fully adjusted HR, 0.64; 95% CI, 0.55 to 0.74). We found no evidence of effect modification by age, race/ethnicity, diabetes status, or CKD stage at the time of death (P value for interaction >0.05).
Table 3.
Hazard ratios with 95% confidence intervals from multivariable regression analyses of the association of sex (women versus men) with all-cause mortality
| Model | Variables Included in Regression Models | Death from Any Cause | Death from Any Cause (ESRD as a Censoring Event) |
|---|---|---|---|
| Model 1 | Clinical site, age, race/ethnicity, baseline eGFR, and urine protein-to-creatinine ratio | 0.69 (0.60 to 0.80) | 0.78 (0.70 to 0.86) |
| Model 2 | +Education, marital status, nephrology care, health insurance | 0.61 (0.51 to 0.73) | 0.70 (0.62 to 0.79) |
| Model 3 | +Smoking status, physical activity, BMI, waist circumference | 0.65 (0.54 to 0.80) | 0.76 (0.66 to 0.86) |
| Model 4 | +Systolic BP, diabetes, CVD, LDL, HDL, triglycerides, albumin, and hemoglobin | 0.65 (0.52 to 0.81) | 0.67 (0.58 to 0.78) |
| Model 5 | +ACEi/ARB, aspirin, and statin | 0.65 (0.52 to 0.81) | 0.68 (0.58 to 0.78) |
| Model 6 | +Serum FGF23, calcium, and phosphorus | 0.56 (0.44 to 0.70) | 0.64 (0.55 to 0.74) |
BMI, body mass index; CVD, cardiovascular disease; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; FGF23, fibroblast growth factor 23.
Discussion
In this large and diverse CKD cohort, women had lower risk of ESRD and death, despite observed sex-related disparities in lifestyle and medical management and after extensive adjustment for sociodemographic characteristics, baseline kidney function, cardiovascular risk factors, medications, and markers of bone mineral metabolism.
We observed a number of important sex-related differences in risk factors and management at study entry. Compared with men, women were more likely to have adverse risk factors, including lower socioeconomic status and physical activity; higher BMI and waist circumference; higher serum phosphorus, FGF23, and LDL cholesterol levels; and lower HDL cholesterol and eGFR. Furthermore, women were less likely to report use of cardioprotective medications despite guideline recommendations, which is consistent with the considerable evidence of undertreatment of cardiovascular risk factors among women.36 However, women were less likely to smoke and had lower proteinuria. Despite having a less favorable risk factor profile and consistent with data from the US Renal Data System,18 women in the CRIC Study were less likely to experience ESRD and death compared with men.
Our findings are similar to those of previous studies, including the Modification of Diet in Renal Disease37 and the African American Study of Hypertension and Kidney Disease,38 which have shown lower rates of ESRD in women compared with men.3–5 Furthermore, a meta-analysis of 68 studies including 11,345 patients with nondiabetic kidney disease concluded that men with kidney disease from various etiologies were more likely to progress to ESRD than women.5 We found that sex-related disparities in incident ESRD were not accounted for by differences in the multiple demographic and clinical variables ascertained in this study. Potential explanations for these findings include the protective effect of endogenous estrogens and/or the deleterious effects of testosterone, sex differences in nitric oxide metabolism, and the differential effect of sex on lifestyle and traditional risk factors.39–41 In addition, factors related to access to care and other psychosocial factors that were incompletely measured in our study might also play a role. The lower risk of incident ESRD in women could be explained in part by the fact that women might be more likely to opt for conservative treatment rather than dialysis. A national survey study in Australia reported that, among patients with CKD stage 5, those who chose conservative care as planned initial treatment were more likely to be women than those who started dialysis therapy.42 We did not explore this possibility in our study. However, this would not explain the observed lower risk of incident CKD stage 5 or 50% eGFR decline from baseline among women in our study.
Similar to prior studies, the incident ESRD rate in our study was lower among older individuals.3,28,43 Furthermore, in our cohort, age was a significant effect modifier of the association between sex and ESRD. Although the risk of ESRD was lower among older women compared with older men, this risk seemed to be higher among younger women compared with younger men, although the association was not statistically significant. Although we did not adjust for menopausal status, it is likely that most women in this age strata (21–45 years old) were premenopausal and therefore, had circulating endogenous estrogens. Our findings are in contrast with the hypothesized protective effect of endogenous estrogens on CKD progression, which is on the basis of animal studies.44–46 Future studies evaluating the role of endogenous estrogens on CKD progression among women with CKD are warranted.
Prior studies have suggested that the association between sex and kidney function is modified by diabetes, whereby the lower risk of ESRD in women versus men is more apparent in nondiabetic kidney disease than in diabetic kidney disease.5,47,48 It has been proposed that potential mechanisms for the absence of a clear sex difference in the setting of diabetes may be alterations in sex hormone levels present in patients with this condition.47 However, we found no evidence of significant effect modification by diabetes on the association between sex and ESRD. Reasons for the discrepancy are unclear but could be due to differences in regression modeling approaches.
We report a steeper decline in eGFR over time in men compared with women. However, in contrast with results from our failure-time analyses, after adjusting for age, race/ethnicity, and baseline kidney function, this difference became statistically nonsignificant. One potential explanation could be the phenomenon of informative censoring or missing not at random, which may occur in longitudinal studies when some participants have unobserved eGFR values (in our study, more men than women) due to events, such as initiation of dialysis, kidney transplant, or death.49,50 In addition, it is possible that greater prevalence or earlier development of uremic symptoms in men may prompt earlier initiation of dialysis than in women.11 However, this information is not available in our study. Furthermore, earlier initiation of dialysis among men would not explain the discrepancy in findings between eGFR slope and the other failure-time outcomes, which were evaluated (incident CKD and 50% decline).
We also found that women were less likely to die than men. This survival advantage was present at every age strata, and it was not attenuated by traditional or nontraditional risk factors. Similar to our findings, prior studies have shown a lower risk of death among women with CKD compared with men with CKD,3,51,52 and this has been attributed to the sex differences in health behaviors as well as the effects of sex hormones, where estrogens may play a protective effect on the vasculature and testosterone has a deleterious effect.53
The findings of our study need to be interpreted in light of its limitations. The CRIC Study did not use a population-based sampling strategy; therefore, it is possible that the risk for CKD progression differs between men and women enrolled in the study compared with those not enrolled. For instance, it is well recognized that CKD awareness is lower in women compared with men54 and that women are less likely to undergo CKD screening or have nephrology contact after receiving a diagnosis of CKD22,55; therefore, our clinic-based sample of women might have had a lower risk profile compared with women who were not recruited. Moreover, we did not include measurements of endogenous sex hormones, which might play a role in CKD progression, and important variables, such as the cause of CKD and the type of diabetes mellitus, were not collected in the CRIC Study. Furthermore, we did not conduct joint modeling of survival outcomes and longitudinal data to take into account the cause of unobserved eGFR values in individuals who developed ESRD or died; as mentioned above, this limitation might have contributed to the finding of nonsignificant eGFR slope differences in women versus men after covariate adjustment. Finally, our findings are subject to residual confounding and bias that may occur in observational studies, especially because baseline characteristics differ between women and men. Despite these limitations, our study also has important strengths: the large sample size, which included similar proportions of men and women, and a diverse racial/ethnic population with a wide age range and broad set of underlying causes of CKD. We had extensive annual data collection of clinical and laboratory measurements that are often not available in population-based studies. Moreover, we evaluated multiple definitions of CKD progression, which allowed our findings to be compared with those from other studies. Finally, the follow-up of the CRIC participants is very complete (90% retained and actively under study as of the year 5 visit), and linkage with the US Renal Data System and national death databases maximized capture of primary study end points.
In summary, our results highlight a sex-related disparity in CKD progression and mortality. Although there were no significant differences in eGFR slopes between women and men after adjusting for demographic and clinical factors, women had lower risk of ESRD and death than men. The role of other biologic and psychosocial factors needs to be further investigated in this population.
Disclosures
None.
Supplementary Material
Acknowledgments
Funding for the Chronic Renal Insufficiency Cohort Study was obtained under a cooperative agreement from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; grants U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award (CTSA) National Institutes of Health (NIH)/National Center for Advancing Translational Sciences (NCATS) grant UL1TR000003, Johns Hopkins University grant UL1 TR-000424, University of Maryland grant GCRC M01 RR-16500, the Clinical and Translational Science Collaborative of Cleveland, grant UL1TR000439 from the NCATS component of the NIH and NIH Roadmap for Medical Research, Michigan Institute for Clinical and Health Research grant UL1TR000433, University of Illinois at Chicago CTSA grant UL1RR029879, Tulane Center of Biomedical Research Excellence for Clinical and Translational Research in Cardiometabolic Diseases grant P20 GM109036, and Kaiser Permanente NIH/National Center for Research Resources University of California San Francisco-Clinical & Translational Science Institute grant UL1 RR-024131. A.C.R. is funded by NIDDK award K23DK094829. S.E.R. receives salary support from NIDDK grants R01 HL127028 and 1UC4 DK101108. J.P.L. is funded by NIDDK awards K24DK092290 and R01-DK072231-91.
The CRIC Study investigators include Lawrence J. Appel (Johns Hopkins University), Harold L. Feldman (Perelman School of Medicine, University of Pennsylvania), Alan S. Go (Kaiser Permanente Division of Research, Oakland, California), Jiang He (Tulane University), John W. Kusek (National Institute for Diabetes and Digestive and Kidney Diseases), Mahboob Rahman (Case Western Reserve University School of Medicine), Akinlolu Ojo (University of Arizona Health Sciences), and Raymond Townsend (Hospital of the University of Pennsylvania).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Contributor Information
Collaborators: Lawrence J. Appel, Harold I. Feldman, Alan S. Go, Jiang He, John W. Kusek, James P. Lash, Akinlolu Ojo, Mahboob Rahman, and Raymond R. Townsend
References
- 1.Murphy D, McCulloch CE, Lin F, Banerjee T, Bragg-Gresham JL, Eberhardt MS, et al.: Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team : Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med 165: 473–481, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albertus P, Morgenstern H, Robinson B, Saran R: Risk of ESRD in the United States. Am J Kidney Dis 68: 862–872, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eriksen BO, Ingebretsen OC: The progression of chronic kidney disease: A 10-year population-based study of the effects of gender and age. Kidney Int 69: 375–382, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Haroun MK, Jaar BG, Hoffman SC, Comstock GW, Klag MJ, Coresh J: Risk factors for chronic kidney disease: A prospective study of 23,534 men and women in Washington County, Maryland. J Am Soc Nephrol 14: 2934–2941, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Neugarten J, Acharya A, Silbiger SR: Effect of gender on the progression of nondiabetic renal disease: A meta-analysis. J Am Soc Nephrol 11: 319–329, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Jafar TH, Schmid CH, Stark PC, Toto R, Remuzzi G, Ruggenenti P, et al.: The rate of progression of renal disease may not be slower in women compared with men: A patient-level meta-analysis. Nephrol Dial Transplant 18: 2047–2053, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Silbiger SR, Neugarten J: The impact of gender on the progression of chronic renal disease. Am J Kidney Dis 25: 515–533, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Seliger SL, Davis C, Stehman-Breen C: Gender and the progression of renal disease. Curr Opin Nephrol Hypertens 10: 219–225, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Dubey RK, Jackson EK: Estrogen-induced cardiorenal protection: Potential cellular, biochemical, and molecular mechanisms. Am J Physiol Renal Physiol 280: F365–F388, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Silbiger S, Neugarten J: Gender and human chronic renal disease. Gend Med 5[Suppl A]: S3–S10, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Carrero JJ: Gender differences in chronic kidney disease: Underpinnings and therapeutic implications. Kidney Blood Press Res 33: 383–392, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Neugarten J, Golestaneh L: Gender and the prevalence and progression of renal disease. Adv Chronic Kidney Dis 20: 390–395, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Coggins CH, Breyer Lewis J, Caggiula AW, Castaldo LS, Klahr S, Wang SR: Differences between women and men with chronic renal disease. Nephrol Dial Transplant 13: 1430–1437, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Briganti EM, Branley P, Chadban SJ, Shaw JE, McNeil JJ, Welborn TA, et al.: Smoking is associated with renal impairment and proteinuria in the normal population: The AusDiab kidney study. Australian Diabetes, Obesity and Lifestyle Study. Am J Kidney Dis 40: 704–712, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Stoverinck MJ, Lagro-Janssen AL, Weel CV: Sex differences in health problems, diagnostic testing, and referral in primary care. J Fam Pract 43: 567–576, 1996 [PubMed] [Google Scholar]
- 16.Cameron KA, Song J, Manheim LM, Dunlop DD: Gender disparities in health and healthcare use among older adults. J Womens Health (Larchmt) 19: 1643–1650, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw LJ, Bairey Merz CN, Pepine CJ, Reis SE, Bittner V, Kelsey SF, et al.: WISE Investigators : Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study. Part I. Gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol 47[Suppl]: S4–S20, 2006 [DOI] [PubMed] [Google Scholar]
- 18.United States Renal Data System: 2017 Annual Data Report, 2017. Available at: https://www.usrds.org/adr.aspx. Accessed February 1, 2018
- 19.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, et al.: Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : The Chronic Renal Insufficiency Cohort (CRIC) study: Design and methods. J Am Soc Nephrol 14[Suppl 2]: S148–S153, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, et al.: Chronic Renal Insufficiency Cohort (CRIC) Study Group : Chronic Renal Insufficiency Cohort (CRIC) study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 4: 1302–1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer MJ, Go AS, Lora CM, Ackerson L, Cohan J, Kusek JW, et al.: CRIC and H-CRIC Study Groups : CKD in Hispanics: Baseline characteristics from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic-CRIC studies. Am J Kidney Dis 58: 214–227, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ricardo AC, Roy JA, Tao K, Alper A, Chen J, Drawz PE, et al.: CRIC Study Investigators : Influence of nephrologist care on management and outcomes in adults with chronic kidney disease. J Gen Intern Med 31: 22–29, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertoni AG, Whitt-Glover MC, Chung H, Le KY, Barr RG, Mahesh M, et al.: The association between physical activity and subclinical atherosclerosis: The multi-ethnic study of atherosclerosis. Am J Epidemiol 169: 444–454, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al.: Recommendations for blood pressure measurement in humans and experimental animals. Part 1. Blood pressure measurement in humans: A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 111: 697–716, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Joffe M, Hsu CY, Feldman HI, Weir M, Landis JR, Hamm LL; Chronic Renal Insufficiency Cohort (CRIC) Study Group : Variability of creatinine measurements in clinical laboratories: Results from the CRIC study. Am J Nephrol 31: 426–434, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, et al.: Chronic Kidney Disease Epidemiology Collaboration : Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53: 766–772, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, et al.: CRIC Study Investigators : Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 60: 250–261, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Hare AM, Choi AI, Bertenthal D, Bacchetti P, Garg AX, Kaufman JS, et al.: Age affects outcomes in chronic kidney disease. J Am Soc Nephrol 18: 2758–2765, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Tangri N, Stevens LA, Griffith J, Tighiouart H, Djurdjev O, Naimark D, et al.: A predictive model for progression of chronic kidney disease to kidney failure. JAMA 305: 1553–1559, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, et al.: Chronic Renal Insufficiency Cohort (CRIC) Study Group : Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunsicker LG, Adler S, Caggiula A, England BK, Greene T, Kusek JW, et al.: Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int 51: 1908–1919, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Ricardo AC, Anderson CA, Yang W, Zhang X, Fischer MJ, Dember LM, et al.: CRIC Study Investigators : Healthy lifestyle and risk of kidney disease progression, atherosclerotic events, and death in CKD: Findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 65: 412–424, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lash JP, Ricardo AC, Roy J, Deo R, Fischer M, Flack J, et al.: CRIC Study Investigators : Race/ethnicity and cardiovascular outcomes in adults with CKD: Findings from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic CRIC studies. Am J Kidney Dis 68: 545–553, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denker M, Boyle S, Anderson AH, Appel LJ, Chen J, Fink JC, et al.: Chronic Renal Insufficiency Cohort Study Investigators : Chronic Renal Insufficiency Cohort Study (CRIC): Overview and summary of selected findings. Clin J Am Soc Nephrol 10: 2073–2083, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 36.Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, et al.: WISE Investigators : Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) study. Part II. Gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol 47[Suppl]: S21–S29, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Menon V, Wang X, Sarnak MJ, Hunsicker LH, Madero M, Beck GJ, et al.: Long-term outcomes in nondiabetic chronic kidney disease. Kidney Int 73: 1310–1315, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Norris KC, Greene T, Kopple J, Lea J, Lewis J, Lipkowitz M, et al.: Baseline predictors of renal disease progression in the African American Study of Hypertension and Kidney Disease. J Am Soc Nephrol 17: 2928–2936, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carrero JJ, Hecking M, Chesnaye NC, Jager KJ: Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol 14: 151–164, 2018 [DOI] [PubMed] [Google Scholar]
- 40.Cobo G, Hecking M, Port FK, Exner I, Lindholm B, Stenvinkel P, et al.: Sex and gender differences in chronic kidney disease: Progression to end-stage renal disease and haemodialysis. Clin Sci (Lond) 130: 1147–1163, 2016 [DOI] [PubMed] [Google Scholar]
- 41.Halbesma N, Brantsma AH, Bakker SJL, Jansen DF, Stolk RP, De Zeeuw D, et al.: PREVEND Study Group : Gender differences in predictors of the decline of renal function in the general population. Kidney Int 74: 505–512, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Morton RL, Turner RM, Howard K, Snelling P, Webster AC: Patients who plan for conservative care rather than dialysis: A national observational study in Australia. Am J Kidney Dis 59: 419–427, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Evans M, Fryzek JP, Elinder C-G, Cohen SS, McLaughlin JK, Nyrén O, et al.: The natural history of chronic renal failure: Results from an unselected, population-based, inception cohort in Sweden. Am J Kidney Dis 46: 863–870, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Yanes LL, Sartori-Valinotti JC, Reckelhoff JF: Sex steroids and renal disease: Lessons from animal studies. Hypertension 51: 976–981, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Silbiger SR: Raging hormones: Gender and renal disease. Kidney Int 79: 382–384, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Doublier S, Lupia E, Catanuto P, Periera-Simon S, Xia X, Korach K, et al.: Testosterone and 17β-estradiol have opposite effects on podocyte apoptosis that precedes glomerulosclerosis in female estrogen receptor knockout mice. Kidney Int 79: 404–413, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maric C: Sex, diabetes and the kidney. Am J Physiol Renal Physiol 296: F680–F688, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen Y, Cai R, Sun J, Dong X, Huang R, Tian S, et al.: Diabetes mellitus as a risk factor for incident chronic kidney disease and end-stage renal disease in women compared with men: A systematic review and meta-analysis. Endocrine 55: 66–76, 2017 [DOI] [PubMed] [Google Scholar]
- 49.Misra M, Vonesh E, Churchill DN, Moore HL, Van Stone JC, Nolph KD: Preservation of glomerular filtration rate on dialysis when adjusted for patient dropout. Kidney Int 57: 691–696, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Shou H, Hsu JY, Xie D, Yang W, Roy J, Anderson AH, et al.: Chronic Renal Insufficiency Cohort (CRIC) Study Investigators: Analytic considerations for repeated measures of eGFR in cohort studies of CKD. Clin J Am Soc Nephrol 12: CJN.11311116, 2017 [DOI] [PMC free article] [PubMed]
- 51.John R, Webb M, Young A, Stevens PE: Unreferred chronic kidney disease: A longitudinal study. Am J Kidney Dis 43: 825–835, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Roderick PJ, Atkins RJ, Smeeth L, Mylne A, Nitsch DDM, Hubbard RB, et al.: CKD and mortality risk in older people: A community-based population study in the United Kingdom. Am J Kidney Dis 53: 950–960, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Beltrán-Sánchez H, Finch CE, Crimmins EM: Twentieth century surge of excess adult male mortality. Proc Natl Acad Sci U S A 112: 8993–8998, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coresh J, Byrd-Holt D, Astor BC, Briggs JP, Eggers PW, Lacher DA, et al.: Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999 to 2000. J Am Soc Nephrol 16: 180–188, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Gasparini A, Evans M, Coresh J, Grams ME, Norin O, Qureshi AR, et al. : Prevalence and recognition of chronic kidney disease in Stockholm healthcare. Nephrol Dial Transplant 31: 2086–2094, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.