Abstract
Background
Researchers have suggested models to predict the risk of postoperative AKI (PO-AKI), but an externally validated risk index that can be practically implemented before patients undergo noncardiac surgery is needed.
Methods
We performed a retrospective observational study of patients without preexisting renal failure who underwent a noncardiac operation (≥1 hour) at two tertiary hospitals in Korea. We fitted a proportional odds model for an ordinal outcome consisting of three categories: critical AKI (defined as Kidney Disease Improving Global Outcomes AKI stage ≥2, post-AKI death, or dialysis within 90 days after surgery), low-stage AKI (defined as PO-AKI events not fulfilling the definition of critical AKI), and no PO-AKI.
Results
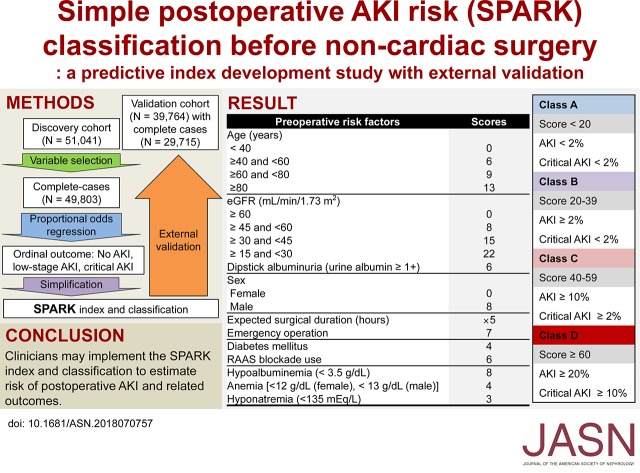
The study included 51,041 patients in a discovery cohort and 39,764 patients in a validation cohort. The Simple Postoperative AKI Risk (SPARK) index included a summation of the integer scores of the following variables: age, sex, expected surgery duration, emergency operation, diabetes mellitus, use of renin-angiotensin-aldosterone inhibitors, baseline eGFR, dipstick albuminuria hypoalbuminemia, anemia, and hyponatremia. The model calibration plot showed tolerable distribution of observed and predicted probabilities in both cohorts. The discrimination power of the SPARK index was acceptable in both the discovery (c-statistic 0.80) and validation (c-statistic 0.72) cohorts. When four SPARK classes were defined on the basis of the sum of the risk scores, the SPARK index and classes fairly reflected the risks of PO-AKI and critical AKI.
Conclusions
Clinicians may consider implementing the SPARK index and classifications to stratify patients’ PO-AKI risks before performing noncardiac surgery.
Keywords: acute kidney injury, surgery, creatinine
Visual Abstract
Postoperative AKI (PO-AKI) is a critical condition in modern medicine that is closely associated with increased risks of death and persistent renal failure.1 However, there are no general therapeutic or preventive measures for PO-AKI as the clinical cause varies according to the patient’s condition. Individualized approaches on the basis of appropriate risk stratification, early detection, and involvement of a specialist may be beneficial for the management of PO-AKI.2–7
Numerous risk factors for PO-AKI have been previously reported.1,8–20 Although each reported risk factor showed some valid association with the risk of PO-AKI, the integration of such information is challenging. Nevertheless, an established risk index that can guide clinicians stratify patients’ risks for PO-AKI is rare, particularly one that is practical for use in daily practice.21 The problem is even greater in the noncardiac surgery field in which the probability of overlooking PO-AKI is higher than in heart operations in that thorough evaluation is usually performed because of their innate high PO-AKI risk.8,22,23 Several risk prediction models have been proposed to solve this issue;1,24–27 however, studies with external validation have been scarcely reported.21 Moreover, as PO-AKI is not a homogeneous event, additional consideration of AKI severity and AKI-associated patient-oriented outcomes, such as death or dialysis, is necessary for such a prediction index but rarely done.1,21
This study aimed to develop a practical, externally validated PO-AKI risk prediction index in noncardiac surgery, which can help clinicians decide when to monitor PO-AKI or involve additional medical resources. We hypothesized that an externally validated simple risk index, which could be calculated in preoperative periods, could stratify the risks of AKI and AKI-associated adverse patient-oriented outcomes.
Methods
Ethical Approval and Reporting Guidelines
The Institutional Review Board (IRB) of Seoul National University Hospital (IRB number: H-1306–053–495) and Seoul National University Bundang Hospital (IRB number: B-1706/403–101) approved the study. The requirement for informed consent was waived as the study was a retrospective observational study without medical intervention. This study was conducted in accordance with the principles of the Declaration of Helsinki. The study was in adherence to the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) guideline.
Study Hospitals and Study Design
This was a retrospective observational cohort study performed in two government-designated, tertiary referral hospitals in Korea. The discovery cohort included adult (age ≥18 years) patients who underwent operations at Seoul National University Hospital from 2004 to 2013, and the validation cohort included adults who underwent operations at Seoul National University Bundang Hospital from 2006 to 2015. Both hospitals have over 1000 general admission beds and senior clinicians who are affiliated with Seoul National University College of Medicine. However, the two hospitals are in different administrative districts of Korea and do not share patient pools or major medical staff.
We included the first operative cases during the study period in the following five surgical departments: general surgery, orthopedic surgery, obstetrics and gynecology, neurosurgery, and urologic surgery. The exclusion criteria were as follows: (1) cardiac surgeries, (2) surgeries for deceased patients (e.g., deceased donor transplantation), (3) nephrectomy or kidney transplant recipients, (4) minor procedural operations defined as surgery duration <1 hour, (5) patients who had established or preoperative kidney dysfunction, defined as a history of RRT, preoperative serum creatinine (sCr) level ≥4 mg/dl, eGFR<15 ml/min per 1.73 m2, or baseline increment of sCr from the minimum value within 2 weeks before surgery for ≥0.3 mg/dl or ≥1.5 times, and (6) patients without baseline or follow-up sCr levels to identify PO-AKI events.
Data Collection and Variables for Model Construction
Information that could be collected or planned before surgery was included because preoperative risk classification was the purpose of the study. To further enhance the practical applications of the study findings, we categorized most of continuous variables by commonly used ranges. Detailed information regarding the collected variables is provided in Supplemental Appendix 1.
Study Outcome
A PO-AKI event was defined according to the sCr criteria of the Kidney Disease: Improving Global Outcomes guidelines,28 using peak sCr level within 2 weeks after surgery.2,18,29 The term “PO-AKI” included all AKI events regardless of AKI severity. To cover the severity and patient-oriented outcomes of PO-AKI, we defined an ordinal outcome for predictive model building that included the following three outcome categories: no AKI, low-stage AKI, and critical AKI. Among patients with PO-AKI, critical AKI was defined by merging events of AKI stage ≥2 and AKI that consequently led to post-AKI death or dialysis within 90 days. As some patients died or started RRT outside the study hospitals, we reviewed a national death database from Statistics Korea and the national dialysis registry maintained by the Korean Society of Nephrology and identified the outcomes.30 The other patients who developed stage 1 PO-AKI but without critical AKI events were included in the low-stage AKI category of the ordinal outcome.
Variable Selection
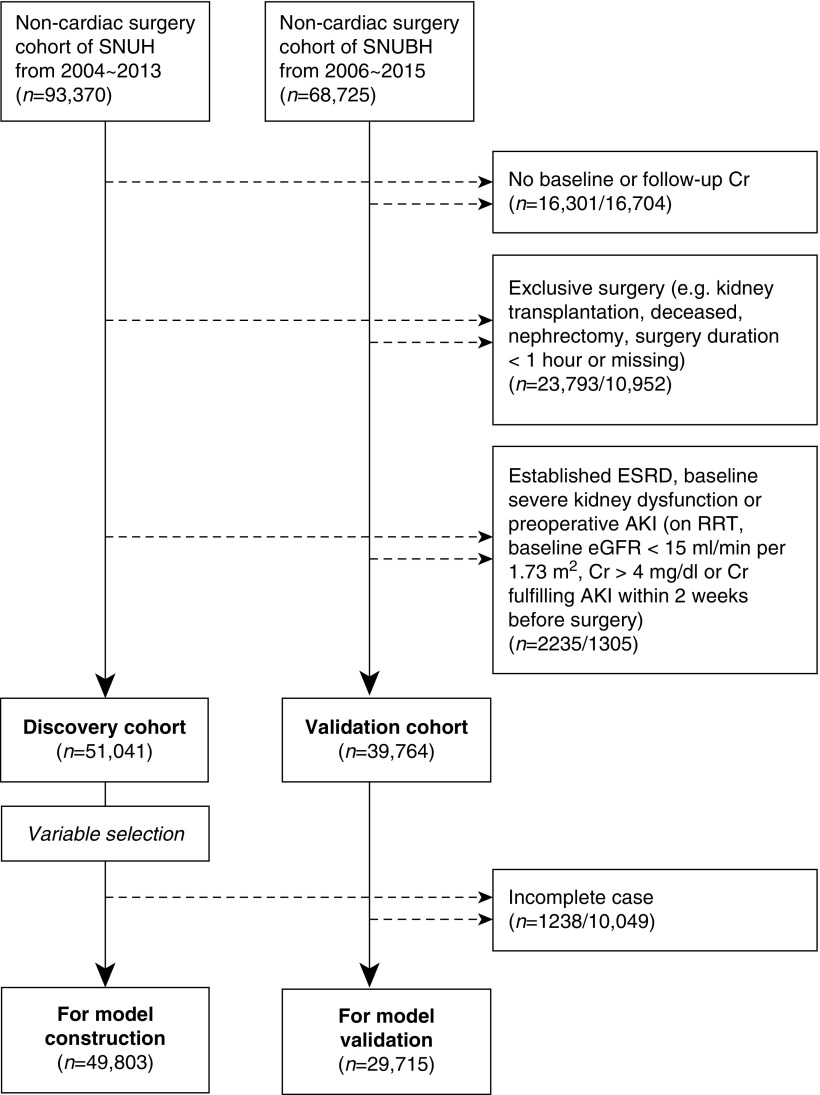
First, variable selection process was performed in the discovery cohort (Figure 1). A univariable cumulative logistic regression analysis with the binary outcome defined with different thresholds in the ordinal outcome was performed to identify the variables that violated the parallel regression assumption. The parallel regression assumption was checked by inspecting the direction and size of the model coefficients, rather than a statistical test, which is commonly anticonservative in a large dataset.31,32 Next, we fitted a multivariable proportional odds model with the ordinal outcome and only variables shown to have an independent, statistically significant association with the ordinal outcome remained. Lastly, we reduced the number of variables included in the model according to the absolute sizes of the model coefficients, and the variables that had relatively low effect sizes were excluded. After the above variable selection, further process was performed with the patients without any missing values in the selected variables.
Figure 1.
Study population. Cr, creatinine; SNUBH, Seoul National University Bundang Hospital; SNUH, Seoul National University Hospital.
Simple Postoperative AKI Risk Index and Classification
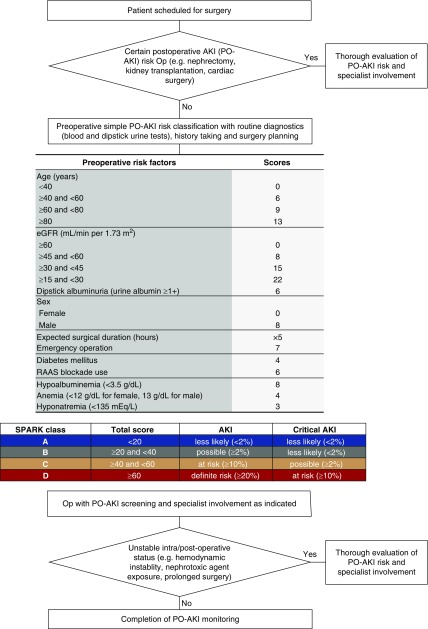
Additional simplification was performed to construct the Simple Postoperative AKI Risk (SPARK) index. After checking the calibration of the simple model, we multiplied the coefficients to set the highest sum of the model coefficients in the discovery cohort as 100 and rounded each coefficient to an integer to produce the SPARK index. Finally, to produce a comprehensive classification that could be easily interpreted in practice, cut-off values were identified in the discovery cohort to define four classes. The cut-off value for class A/B was defined to suggest a threshold for PO-AKI screening with high sensitivity (90%), whereas the threshold for class B/C was to suggest a value with high specificity (90%) for PO-AKI. Lastly, within patients with a higher SPARK index than the cut-off values for B/C, we additionally determined cut-off values for class C/D, and a threshold value with high specificity (90%) for critical AKI was selected to define class D. Considering practical issues, we rounded the threshold values up to the nearest 10.
Sensitivity Analysis and Other Statistical Investigations
The other statistical investigation methods including sensitivity analyses are presented in Supplemental Appendix 2.
Results
Characteristics of the Studied Cohorts
A total of 162,095 patients were screened for inclusion in the study, which was the sum of 93,370 and 68,725 surgery cases examined at Seoul National University Hospital and Seoul National University Bundang Hospital, respectively (Figure 1). After the exclusion criteria were applied, 51,041 and 39,764 patients were screened for model construction in the discovery and validation cohorts, respectively. The number of patients with low-stage AKI and critical AKI were 2132 (4.2%) and 605 (1.2%) in the discovery cohort. Among the discovery cohort patients with critical AKI, 511 (1.0%), 167 (0.3%), and 88 (0.2%) patients with PO-AKI had AKI stage ≥2, post-AKI death, and dialysis within 90 days, respectively. The incidences of the adverse outcomes were slightly higher in the validation cohort, as 1774 (4.5%) and 727 (1.8%) patients had low-stage AKI and critical AKI. The incidences of AKI stage ≥2, post-AKI death, and dialysis within 90 days were 644 (1.6%), 176 (0.4%), and 64 (0.2%), respectively, in the validation cohort. The other characteristics of the two cohorts differed significantly (Table 1), as the validation cohort consisted of older, more frequently male patients. Obstetrics and gynecologic operations were relatively common in the discovery cohort, but orthopedic operations represented a large portion of surgeries in the validation cohort. Significant differences were also identified regarding baseline laboratory values and medication usage.
Table 1.
Characteristics of the discovery and validation cohorts
| Variables | Discovery Cohort (n=51,041) | Validation Cohort (n=39,764) | P Value |
|---|---|---|---|
| Demographics | |||
| Age, yr | 56 [44–66] | 60 [48–70] | <0.001 |
| <40 | 9206 (18.0%) | 5559 (14.0%) | |
| ≥40 to <60 | 20,877 (40.9%) | 13,492 (33.9%) | |
| ≥60 to <80 | 19,684 (38.6%) | 18,698 (47.0%) | |
| ≥80 | 1274 (2.5%) | 2015 (5.1%) | |
| Sex | <0.001 | ||
| Women | 28,306 (55.5%) | 18,706 (47.0%) | |
| Men | 22,735 (44.5%) | 21,058 (53.0%) | |
| Body mass index, kg/m2 | 23.8 [21.7–26.0] | 24.1 [21.9–26.4] | <0.001 |
| <18.5 | 1919 (3.9%) | 854 (3.8%) | |
| ≥18.5 to <30 | 45,098 (91.5%) | 20,624 (90.9%) | |
| ≥30 | 2294 (4.7%) | 1218 (5.4%) | |
| Preexisting comorbidities | |||
| Heart disease | 1629 (3.2%) | 1163 (2.9%) | 0.02 |
| Hypertension | 9824 (19.2%) | 9161 (23.0%) | <0.001 |
| Diabetes mellitus | 3956 (7.8%) | 3581 (9.0%) | <0.001 |
| Surgery characteristics | |||
| Departments | <0.001 | ||
| General surgery | 22,447 (44.0%) | 14,733 (37.1%) | |
| Neurosurgery | 5063 (9.9%) | 3842 (9.7%) | |
| Obstetrics and gynecology | 7894 (15.5%) | 908 (2.3%) | |
| Orthopedics | 11,372 (22.3%) | 16,823 (42.3%) | |
| Urologic surgery | 4265 (8.4%) | 3458 (8.7%) | |
| Surgery duration, h | 2.2 [1.5–3.3] | 2.5 [1.7–3.6] | <0.001 |
| Expected surgery duration, h | 2.5 [2.0–3.0] | 3.0 [2.0–4.0] | <0.001 |
| Anesthesia type | <0.001 | ||
| General | 43,921 (86.6%) | 30,570 (76.9%) | |
| Nongeneral | 6789 (13.4%) | 9194 (23.1%) | |
| Emergency operation | 732 (1.4%) | 1965 (4.9%) | <0.001 |
| BP before operation, mm Hg | |||
| Systolic BP | 124 [113–135] | 128 [116–142] | <0.001 |
| Diastolic BP | 77 [70–85] | 73 [65–81] | <0.001 |
| Normotensive | 36,976 (75.0%) | 22,934 (57.7%) | |
| Hypertensive (systolic BP ≥140 or diastolic BP ≥90) | 10,206 (20.7%) | 11,472 (28.9%) | |
| Hypotensive (systolic BP <90 or diastolic BP <60) | 2134 (4.3%) | 5358 (13.5%) | |
| Medication usage | |||
| Renin-angiotensin-aldosterone system blockade | 2881 (5.6%) | 2915 (7.3%) | <0.001 |
| Laboratory findings | |||
| eGFR, ml/min per 1.73 m2 | 82.1 [71.4–95.1] | 87.7 [73.1–99.7] | <0.001 |
| No CKD or CKD stage 1 or 2 (≥60) | 46,971 (92.0%) | 35,881 (90.2%) | |
| CKD stage 3A (≥45 and <60) | 3226 (6.3%) | 2918 (7.3%) | |
| CKD stage 3B (≥30 and <45) | 641 (1.3%) | 703 (1.8%) | |
| CKD stage 4 (≥15 and <30) | 203 (0.4%) | 262 (0.7%) | |
| Dipstick albuminuria (≥1+) | 4682 (9.3%) | 2169 (7.0%) | <0.001 |
| White blood cell count, /mm2 | 6100 [5000–7500] | 6500 [5400–8000] | <0.001 |
| Reference range (4000∼10,000) | 43,262 (84.8%) | 33,902 (85.3%) | |
| Leukopenia (<4000) | 3934 (7.7%) | 1806 (4.5%) | |
| Leukocytosis (>10,000) | 3823 (7.5%) | 4033 (10.1%) | |
| Hemoglobin, g/dl | 13.2 [12.1–14.4] | 13.6 [12.4–14.8] | <0.001 |
| Anemia (<12 for women, <13 for men) | 14,177 (27.8%) | 9212 (23.2%) | <0.001 |
| Platelet, ×103/μl | 200 [152–256] | 239 [199–284] | <0.001 |
| Thrombocytopenia (<10) | 4160 (8.2%) | 493 (1.2%) | <0.001 |
| Albumin, g/dl | 4.2 [3.9–4.5] | 4.3 [4.0–4.5] | <0.001 |
| Hypoalbuminemia (<3.5) | 5148 (10.1%) | 2679 (6.8%) | <0.001 |
| Sodium, mEq/L | 140 [139–142] | 141 [139–142] | <0.001 |
| Normonatremia (135∼145) | 48,288 (94.8%) | 31,156 (92.8%) | |
| Hyponatremia (<135) | 1291 (2.5%) | 986 (2.9%) | |
| Hypernatremia (>145) | 1361 (2.7%) | 1418 (4.2%) | |
| Potassium, mEq/L | 4.2 [4.0–4.4] | 4.2 [4.0–4.5] | <0.001 |
| Normokaleima (3.5∼5.5) | 49,711 (97.6%) | 32,649 (97.3%) | |
| Hypokalemia (<3.5) | 1016 (2.0%) | 711 (2.1%) | |
| Hyperkalemia (>5.5) | 213 (0.4%) | 200 (0.6%) |
Categorical variables were shown as n (%) and continuous variables as medians [interquartile ranges].
Variable Selection
The patient characteristics according to the studied ordinal outcome in the discovery cohort are shown in Supplemental Table 1. In the cumulative logistic regression analysis, the surgical departments, body mass index and BP categories, types of anesthesia, and hypernatremia were excluded from further model construction as they poorly met the parallel regression assumption (Supplemental Table 2). Additionally, serum potassium level categories and leukocytosis did not show significant associations with the ordinal outcome in our multivariable proportional odds model (Supplemental Table 3). Lastly, heart disease, hypertension, and leukopenia were excluded from the model as the model coefficients were relatively smaller than the others (Supplemental Table 4). The remaining variables were included for the final index construction and validation.
To be noted, a total of 49,803 and 29,715 participants in the discovery and validation cohorts, respectively, who had complete information of the finally selected variables, were used in the further analysis for simplified model building and validation (Figure 1). The number of included patients with low-stage AKI were 2062 (4.1%) and 1109 (3.7%) in the discovery and validation cohorts, respectively, without missing values. The critical AKI events occurred in the following numbers of complete cases: 563 (1.1%) in the discovery cohort and 445 (1.5%) in the validation cohort.
SPARK Index and Classification
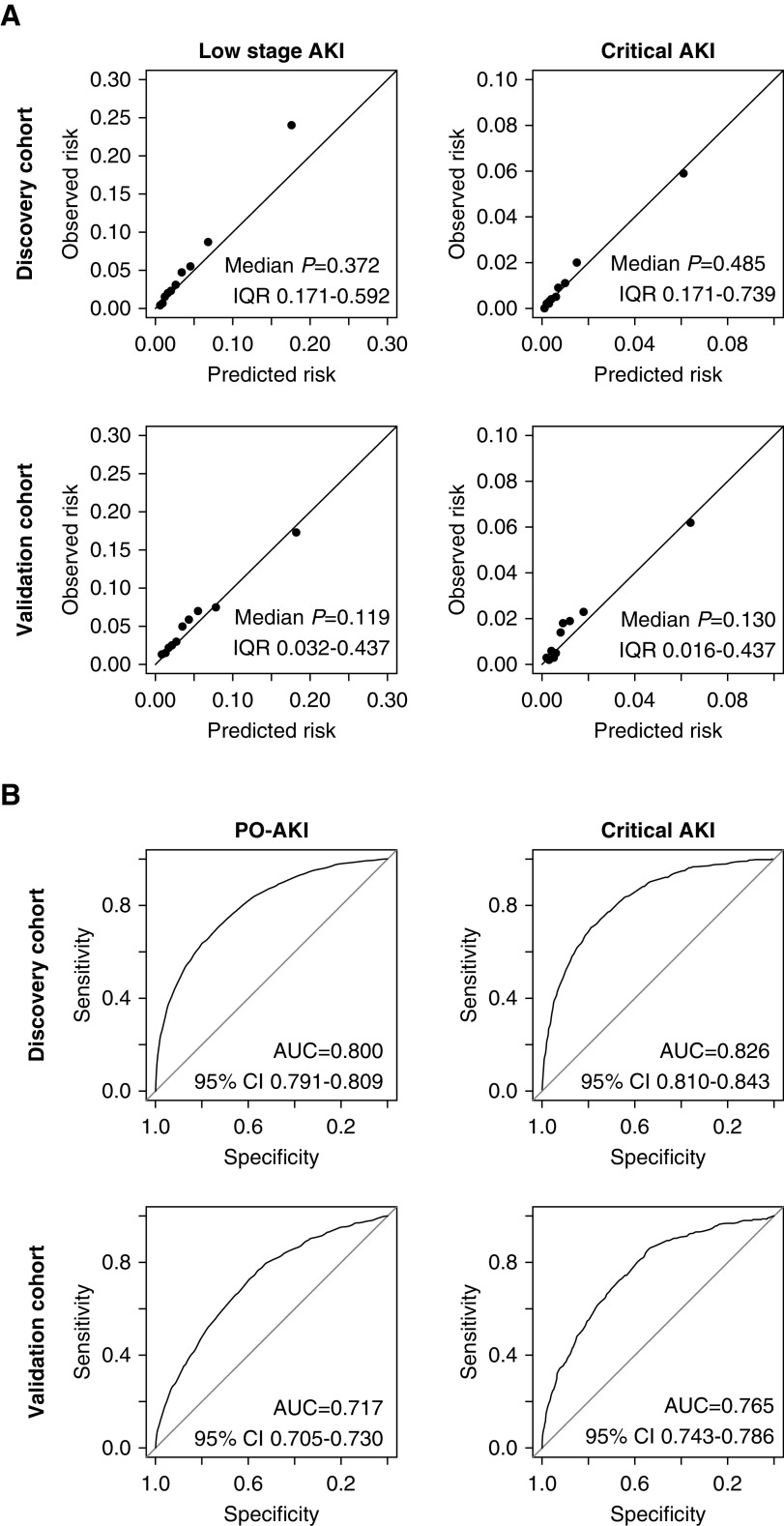
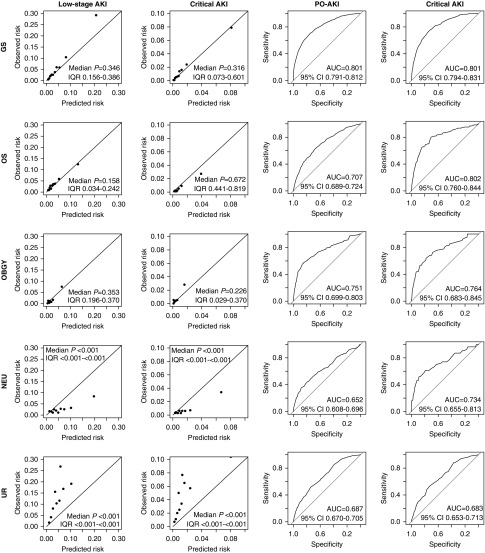
The selected variables were fitted to the ordinal outcome with proportional odds model (Supplemental Table 5). The calibration plot showed tolerable distribution of the observed and predicted probabilities, although some underestimation was observed in the high-probability range of the discovery cohort (Figure 2). The Hosmer–Lemeshow test in 1000 random subsamples with fixed sizes (n=1000) gave a median P value of 0.37 (interquartile range [IQR], 0.17–0.59, with 90.9% of the samples having P≥0.05] and 0.49 (IQR, 0.17–0.74, with 88.3% of the samples having P≥0.05) for low-stage AKI and critical AKI outcomes, respectively, in the discovery cohort.33,34 The median P values in the validation cohort from the same test also showed that the model-fit was not significantly poor (P>0.05). However, the calibration results were relatively modest in the validation cohort, as a less proportion of the subsamples showed a good model-fit. The median P value for low-stage AKI was 0.12 (IQR, 0.03–0.29, with 67.8% of the samples having P≥0.05) and that for critical AKI was 0.13 (IQR, 0.02–0.44, with 65.0% of the samples having P≥0.05). The c-statistics were 0.80 and 0.72 in the discovery and validation cohorts, respectively, which were in the acceptable range. After converting the model coefficients to integer scores, the final SPARK index showed fair discrimination power for both the PO-AKI (discovery cohort area under the curve [AUC], 0.80; 95% confidence interval [95% CI], 0.79 to 0.81; validation cohort AUC, 0.72; 95% CI, 0.71 to 0.73) and the critical AKI (discovery cohort AUC, 0.83; 95% CI, 0.81 to 0.84; validation cohort AUC, 0.77; 95% CI, 0.74 to 0.79) outcomes.
Figure 2.
Calibration plots and receiver operating characteristics curves showed that the calibration and discrimination of the proportional odds model and the SPARK index were in acceptable range. (A) The calibration plots of the constructed proportional odds regression model for the ordinal outcome with finally selected variables. The P values were calculated by the Hosmer–Lemeshow test to assess the goodness of fit, and values <0.05 indicate significantly poor fit. Considering the size of the studied groups, the median P values and IQRs were presented from the test results in 1000 random subsamples with fixed size (n=1000) from the according cohort. (B) The receiver operating characteristics curves showing the predictability of the SPARK indexes for PO-AKI and critical AKI events. AUC values (95% CIs) are presented in each graph.
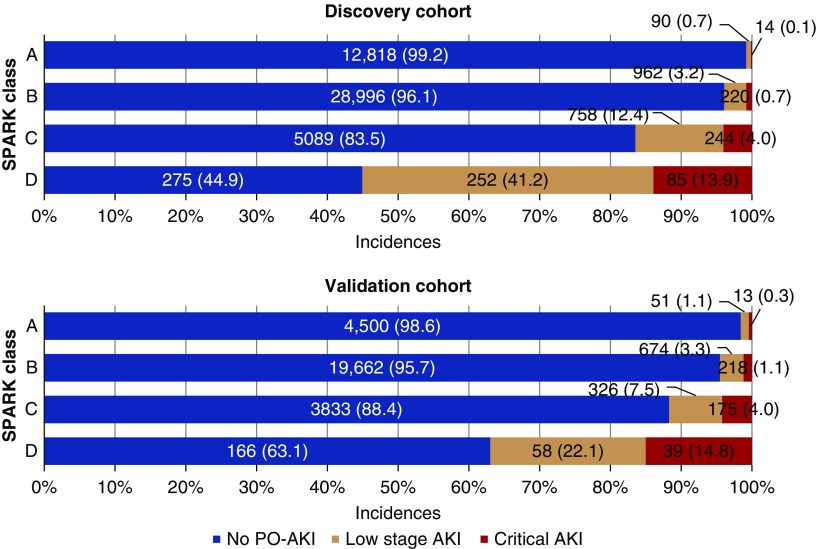
Then, we examined the sensitivity/specificity values (Supplemental Figure 1), and cut-off values of 20 and 40 were selected as the threshold values between SPARK class A/B and B/C, respectively (Supplemental Table 6). Additionally, a threshold value of 60 was identified as the threshold value between SPARK class C and D. After classification with designated cut-off values was performed, the incidences of both AKI and critical AKI showed class-dependent increments in both the discovery and validation cohorts (Figure 3). With the abovementioned results, we constructed the SPARK index and classification and suggested a perioperative AKI monitoring strategy (Figure 4).
Figure 3.
SPARK risk classification and incidences of the low-stage AKI and critical AKI showed the class-dependent increment of the incidences of low stage AKI and critical AKI outcomes. Critical AKI was defined as AKI stage ≥2, post-AKI death, or dialysis within 90 days after surgery. The AKI not fulfilling the criteria of the critical AKI events were the low-stage AKI. The top graph shows the results in the discovery cohort and the bottom graph shows the results in the validation cohort.
Figure 4.
Perioperative PO-AKI risk evaluation strategy on the basis of the suggested SPARK classification. Op, operation; RAAS, renin-angiotensin-aldosterone system.
Sensitivity Analyses
To inspect whether a significant bias was present due to our exclusion criteria, sensitivity analyses were performed. The discriminative power of the SPARK index in an imputed dataset including cases with missing values was acceptable in the discovery cohort (c-statistic, 0.80; n=51,041). However, the power was marginal in the validation cohort with higher proportion of missing values, mainly in the dipstick urine albuminuria variable (c-statistic, 0.70; n=39,764). When actual surgery durations were included instead of the expected ones, no prominent decrement of the powers was shown (c-statistic, 0.81 in the discovery cohort; n=49,803; and c-statistic, 0.72 in the validation cohort; n=29,715). Then, we tested whether evident differences were present according to the time-eras after combining the discovery and validation cohorts, and no prominent differences or drop in c-statistics were observed in three eras of the study (c-statistic, 0.75 in 2004–2007; n=18,560; c-statistic, 0.78 in 2008–2011; n=34,016; and c-statistic, 0.77 in 2012–2015; n=26,942). Finally, we excluded those who had any identifiable preoperative creatinine elevation of ≥0.3 mg/dl or ≥1.5-fold from the minimum value within 3 months before surgery regardless of the intervals, to control the potential bias from inclusion of subacute or chronically progressive kidney injury before operation. Even in the analysis, the discriminative power of the SPARK index remained in the acceptable range (c-statistic, 0.79 in the discovery cohort; n=48,124; and c-statistic, 0.71 in the validation cohort; n=29,315).
Performance of SPARK Index and Classification in Each Surgical Department
When we merged the cases without missing values in the discovery and validation cohorts, certain differences regarding clinical characteristics were presented between the departments (Supplemental Table 7). The results of the performance of the simplified proportional odds model and the SPARK index in each department are shown in Figure 5. The calibration results showed that the overall calibration was tolerable in the general surgery and orthopedic surgery, and was of modest degree in the obstetrics and gynecology surgery. However, a prominent overestimation was identified in the neurosurgery department, whereas our model underestimated the risks of adverse outcomes in the urology operations. This was similar with the discriminative power of the SPARK index, as the neurosurgery and urology surgery were the departments in which the index showed relatively poor discrimination performance, with AUC values under 0.7. Still, when SPARK classification was applied, the prominent class-dependent increment of adverse outcomes was again observed (Supplemental Table 8).
Figure 5.
Calibration plots and receiver operating characteristics curves of each surgery departments showed the discrimination/calibration powers were acceptable in GS, OS, and OBGY, but were poor in NEU and UR departments. The P values in the calibration plots were calculated by the Hosmer–Lemeshow test to assess the goodness of fit, and values <0.05 indicate significantly poor fit. Considering the size of the studied groups, the median P values and IQRs were presented from the test results in 1000 random subsamples with fixed size (n=1000) from the according patient group. The receiver operating characteristics curves showing the predictability of the SPARK indexes for PO-AKI and critical AKI events are also shown in the right side. AUC values (95% CIs) are presented in each AUC graph. GS, general surgery; NEU, neurosurgery; OS, orthopedic surgery; OBGY, obstetrics and gynecology; UR, urologic surgery.
Discussion
By this predictive index development study, we constructed a simple PO-AKI risk classification system for noncardiac surgeries that could be implemented in preoperative periods. The resulting SPARK index and classifications, which uses patients’ age, sex, and nine additional variables that could be readily collected or estimated before surgery, fairly reflected the risk of PO-AKI, AKI severity, and consequent death or dialysis with simple risk scores.
There are limited externally validated PO-AKI risk classification systems in noncardiac surgery era, although PO-AKI is recognized as a critical event affecting patients’ prognosis in overall major surgeries.21 The main strength of this study is that we developed PO-AKI risk index with variables that could be easily collected during preoperative evaluation and that the external validation was performed. Moreover, we included severe AKI, death, and dialysis in our prediction modeling and predicted the risks of AKI and related critical outcomes with a single index score. In addition to the previous studies regarding AKI risk estimation,24,26,27,35 our study suggested the risk index to predict PO-AKI and related outcomes in noncardiac surgery, which can be implemented when surgery duration is estimated and laboratory variables, mostly with routine diagnostics, are available. As we intended to include only variables that could be collected before surgery, the SPARK index could be useful for patient consultation or planning risk-based PO-AKI monitoring and management strategy before performing noncardiac operations. In exchange for the practicality, certain sacrifices were made in terms of robustness during our modifications; however, the overall predictability of the index was acceptable considering that not all AKI risk could be determined in the preoperative period.
On the basis of our SPARK index and classification system, we suggest the preoperative PO-AKI prediction protocol shown in Figure 4. Patients with a definite risk of PO-AKI (e.g., cardiac surgery, nephrectomy) who were not included in this study should always undergo thorough preoperative evaluation and monitoring of PO-AKI regardless of the risk index. Other patients could be calculated for their SPARK index scores and classified. As class A patients had low risks of PO-AKI, they may skip sCr follow-up in the postoperative period, if the other aspects of their clinical course are stable. However, for patients with class B–D, PO-AKI monitoring may be considered because PO-AKI is possible. For class C or D patients with certain PO-AKI risks, additional efforts should be paid to control the risk factors and an intervention such as “AKI care bundle” would be helpful in guiding clinicians to manage the potential critical AKI events.6,7 Finally, for class D patients, the need for thorough evaluation and monitoring of PO-AKI is further emphasized, and explaining to the patients with their existing critical AKI risks before surgery would be important. In addition, a specialist consultation may be helpful for the patient group with a high risk of PO-AKI or critical AKI events.2–5 On the other hand, clinicians should bear in mind that our risk classification system does not include intra- or postoperative instability. Therefore, those with additional nephrotoxic agent exposure, longer surgical duration than expected, or unstable medical status during surgery should be considered for additional risks of PO-AKI.
Among the final included variables in the SPARK index, age and sex were demographic factors that showed significance in most medical situations. Expected surgery duration and emergency operation may partially represent clinical severity of patients and surgeries. Usage of renin-angiotensin-aldosterone system blockades and included laboratory variables with high index scores, interestingly, were closely related to baseline kidney function, as eGFR and dipstick albuminuria were the most important parameters that define stages of CKD. Therefore, our simplified PO-AKI classification integrates the representative findings of patients’ demographics, clinical severity, surgical difficulty, and underlying kidney function in preoperative periods. On the other hand, as the effect sizes were largely different according to the variables and the number of included variables may still be large to use in everyday practice, an additional simplification may be considered. Furthermore, as most variables could be extracted from an electronic medical record, one could plan an automatic calculation for PO-AKI risks when attending clinicians input the expected surgery duration and prescribed medications when planning a surgery.
The relatively poor accuracy of the SPARK index and classification in the neurosurgery and urology operations should be recognized. Given that the neurosurgery was the field with the longest surgery duration but with the lowest AKI incidence, an unexpected event during surgery might have been the major trigger of PO-AKI, rather than the predictable risk factors before surgery. In addition, direct manipulation of the urinary system in urological operations would certainly affect patients’ renal function and cause the high incidence of PO-AKI in the field. Our results not only show the limitation of the index in the two surgical departments but also imply that PO-AKI in those fields might not be easily predictable before performing surgery. As the SPARK classification fairly stratified the PO-AKI incidences even in the urology and neurosurgery departments, the index might still be used to quantify the underlying risks, but additional caution should be paid for any extra intra- or postoperative factors that may be related to kidney injury in those fields.
Several limitations of this study should be overcome in future research. First, further external validation in different societies is necessary to confirm the generalizability of our results because our validation cohort was from the same country. In addition, the included long study period encourages additional study to validate whether the index could be usefully applied to ongoing clinical era. Moreover, it should be tested whether the index could be applied to other surgery categories, which were not included in this study. In cases where the index power is decreased in other clinical settings, a recalibration may be considered.26 Second, being a retrospective study, our identification of pre- and postoperative AKI was limited as repetitive kidney function measurement was not routinely performed. The exclusion of those without available perioperative sCr values, possible inclusion of undiagnosed preoperative AKI, and usage of the 2-week criterion to include peak sCr values and secure sufficient patient numbers might have caused a potential selection bias. Third, as the validity of expected surgery duration could be impaired by subjective bias, different from other measured actual values, correct estimation is warranted to increase the accuracy of the SPARK index. Centers with limited availability regarding the expected surgery duration may find it difficult to apply the risk prediction index. Lastly, the designated threshold for building the final classifications may not be the same in other hospitals depending on the available medical resources.
In conclusion, the developed SPARK index and classification fairly predicted the risk of PO-AKI and related patient-oriented outcomes with a simple summation of risk scores. Clinicians may consider implementing the index system before performing a noncardiac surgery.
Disclosures
None.
Supplementary Material
Acknowledgments
We acknowledge Eunjeong Kang, Seoul National University and Hyunjin Cho, Seoul National University Bundang Hospital, for data acquisition.
The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. S.P., H.C., S.W.P., S.K., and H.L. contributed to the conception and design of the study. S.P., H.C., K.S.K., H.J.Y., J.P., Y.C., Suehyun L., J.H.K., H.J.C., S.K., D.K.K., K.W.J., and Y.S.K. advised on all statistical aspects and interpreted the data. S.P., H.C., J.P., Y.C., and H.L. performed the statistical analysis, assisted by S.W.P. and Soojin L. S.P., H.J.C., S.W.P., S.L., D.K.K., K.W.J., Y.S.K. and H.L. drafted the manuscript, and other authors participated critically during the revision before final submission. All authors reviewed the manuscript and approved the final version to be published.
This study was supported by a grant from the Ministry of Health and Welfare, Republic of Korea (grant number HI16C2221, HI18C1604).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Postoperative AKI—Prevention Is Better than Cure?,” on pages 4–6.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018070757/-/DCSupplemental.
Supplemental Material
Supplemental Table 1. Characteristics of the discovery cohort according to studied ordinal outcome.
Supplemental Table 2. Cumulative logistic regression analysis result for variable selection.
Supplemental Table 3. Proportional odds model for variable selection.
Supplemental Table 4. Comparing model coefficients for variable selection.
Supplemental Table 5. The model coefficients of the finally selected variables in the proportional odds model.
Supplemental Table 6. Sensitivity and specificity for PO-AKI and critical AKI in discovery and validation cohorts according to suggested threshold values.
Supplemental Table 7. Distribution of the selected variables in the composite complete-case dataset according to the surgery departments.
Supplemental Table 8. Distribution of studied outcomes in each surgical department in the composite dataset according the SPARK classification.
Supplemental Figure 1. Sensitivity and specificity plot for SPARK index.
Supplemental Appendix 1. Detailed information regarding the collected variables.
Supplemental Appendix 2. Supplemental statistical analysis method.
References
- 1.Biteker M, Dayan A, Tekkeşin AI, Can MM, Taycı İ, İlhan E, et al.: Incidence, risk factors, and outcomes of perioperative acute kidney injury in noncardiac and nonvascular surgery. Am J Surg 207: 53–59, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Park S, Baek SH, Ahn S, Lee KH, Hwang H, Ryu J, et al.: Impact of electronic Acute Kidney Injury (AKI) alerts with automated nephrologist consultation on detection and severity of AKI: A quality improvement study. Am J Kidney Dis 71: 9–19, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Soares DM, Pessanha JF, Sharma A, Brocca A, Ronco C: Delayed nephrology consultation and high mortality on acute kidney injury: A meta-analysis. Blood Purif 43: 57–67, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Ponce D, Zorzenon CP, dos Santos NY, Balbi AL: Early nephrology consultation can have an impact on outcome of acute kidney injury patients. Nephrol Dial Transplant 26: 3202–3206, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Meier P, Bonfils RM, Vogt B, Burnand B, Burnier M: Referral patterns and outcomes in noncritically ill patients with hospital-acquired acute kidney injury. Clin J Am Soc Nephrol 6: 2215–2225, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Göcze I, Jauch D, Götz M, Kennedy P, Jung B, Zeman F, et al.: Biomarker-guided intervention to prevent acute kidney injury after major surgery: The prospective randomized bigpAK study. Ann Surg 267: 1013–1020, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Meersch M, Schmidt C, Hoffmeier A, Van Aken H, Wempe C, Gerss J, et al.: Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: The PrevAKI randomized controlled trial. Intensive Care Med 43: 1551–1561, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grams ME, Sang Y, Coresh J, Ballew S, Matsushita K, Molnar MZ, et al.: Acute kidney injury after major surgery: A retrospective analysis of veterans health administration data. Am J Kidney Dis 67: 872–880, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kendale SM, Lapis PN, Melhem SM, Blitz JD: The association between pre-operative variables, including blood pressure, and postoperative kidney function. Anaesthesia 71: 1417–1423, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Kim K, Bang JY, Kim SO, Kim S, Kim JU, Song JG: Association of preoperative hypoalbuminemia with postoperative acute kidney injury in patients undergoing brain tumor surgery: A retrospective study. J Neurosurg 128: 1115–1122, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Dreischulte T, Morales DR, Bell S, Guthrie B: Combined use of nonsteroidal anti-inflammatory drugs with diuretics and/or renin-angiotensin system inhibitors in the community increases the risk of acute kidney injury. Kidney Int 88: 396–403, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Lapi F, Azoulay L, Yin H, Nessim SJ, Suissa S: Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: Nested case-control study. BMJ 346: e8525, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SW, Baek SH, Ahn SY, Na KY, Chae DW, Chin HJ, et al.: The effects of pre-existing hyponatremia and subsequent-developing acute kidney injury on in-hospital mortality: A retrospective cohort study. PLoS One 11: e0162990, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park S, Baek SH, Lee SW, Lee A, Chin HJ, Na KY, et al.: Elevated baseline potassium level within reference range is associated with worse clinical outcomes in hospitalised patients. Sci Rep 7: 2402, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danziger J, Chen KP, Lee J, Feng M, Mark RG, Celi LA, et al.: Obesity, acute kidney injury, and mortality in critical illness. Crit Care Med 44: 328–334, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajan S, Babazade R, Govindarajan SR, Pal R, You J, Mascha EJ, et al.: Perioperative factors associated with acute kidney injury after partial nephrectomy. Br J Anaesth 116: 70–76, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Karkouti K, Yip P, Chan C, Chawla L, Rao V: Pre-operative anaemia, intra-operative hepcidin concentration and acute kidney injury after cardiac surgery: A retrospective observational study. Anaesthesia 73: 1097–1102, 2018 [DOI] [PubMed] [Google Scholar]
- 18.Park S, Lee S, Lee A, Paek JH, Chin HJ, Na KY, et al.: Preoperative dipstick albuminuria and other urine abnormalities predict acute kidney injury and patient outcomes. Surgery 163: 1178–1185, 2018 [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Che M, Xie B, Xue S, Yan Y: Preoperative serum cystatin C combined with dipstick proteinuria predicts acute kidney injury after cardiac surgery. Ren Fail 36: 1497–1503, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Han SS, Ahn SY, Ryu J, Baek SH, Kim KI, Chin HJ, et al.: U-shape relationship of white blood cells with acute kidney injury and mortality in critically ill patients. Tohoku J Exp Med 232: 177–185, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Wilson T, Quan S, Cheema K, Zarnke K, Quinn R, de Koning L, et al.: Risk prediction models for acute kidney injury following major noncardiac surgery: Systematic review. Nephrol Dial Transplant 31: 231–240, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP: A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol 16: 162–168, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Wijeysundera DN, Karkouti K, Dupuis JY, Rao V, Chan CT, Granton JT, et al.: Derivation and validation of a simplified predictive index for renal replacement therapy after cardiac surgery. JAMA 297: 1801–1809, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Rueggeberg A, Boehm S, Napieralski F, Mueller AR, Neuhaus P, Falke KJ, et al.: Development of a risk stratification model for predicting acute renal failure in orthotopic liver transplantation recipients. Anaesthesia 63: 1174–1180, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Sanchez EQ, Gonwa TA, Levy MF, Goldstein RM, Mai ML, Hays SR, et al.: Preoperative and perioperative predictors of the need for renal replacement therapy after orthotopic liver transplantation. Transplantation 78: 1048–1054, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Bell S, Dekker FW, Vadiveloo T, Marwick C, Deshmukh H, Donnan PT, et al.: Risk of postoperative acute kidney injury in patients undergoing orthopaedic surgery--development and validation of a risk score and effect of acute kidney injury on survival: Observational cohort study. BMJ 351: h5639, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kheterpal S, Tremper KK, Heung M, Rosenberg AL, Englesbe M, Shanks AM, et al.: Development and validation of an acute kidney injury risk index for patients undergoing general surgery: Results from a national data set. Anesthesiology 110: 505–515, 2009 [DOI] [PubMed] [Google Scholar]
- 28.KDIGO Work Group : KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 6, 2012 [Google Scholar]
- 29.Mizuguchi KA, Mitani A, Waikar SS, Ireland P, Panizales C, Deluke G, et al.: Use of postoperative creatinine to predict sustained kidney injury in patients undergoing mesothelioma surgery. Clin J Am Soc Nephrol 7: 1071–1078, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin DC, Yun SR, Lee SW, Han SW, Kim W, Park J, et al.: Lessons from 30 years’ data of Korean end-stage renal disease registry, 1985-2015. Kidney Res Clin Pract 34: 132–139, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’ Connell AA: Logistic Regression Models for Ordinal Response Variables, Thousand Oaks, California, SAGE Publications, 2009 [Google Scholar]
- 32.Ananth CV, Kleinbaum DG: Regression models for ordinal responses: A review of methods and applications. Int J Epidemiol 26: 1323–1333, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Kramer AA, Zimmerman JE: Assessing the calibration of mortality benchmarks in critical care: The Hosmer-Lemeshow test revisited. Crit Care Med 35: 2052–2056, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Paul P, Pennell ML, Lemeshow S: Standardizing the power of the Hosmer-Lemeshow goodness of fit test in large data sets. Stat Med 32: 67–80, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Malhotra R, Kashani KB, Macedo E, Kim J, Bouchard J, Wynn S, et al.: A risk prediction score for acute kidney injury in the intensive care unit. Nephrol Dial Transplant 32: 814–822, 2017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.