Abstract
Background
Foot process effacement is one of the pathologic indicators of podocyte injury. However, the morphologic changes associated with it remain unclear.
Methods
To clarify the developmental process, we analyzed puromycin nephrotic podocytes reconstructed from serial focused-ion beam/scanning electron microscopy (FIB/SEM) images.
Results
Intact podocytes consisted of four subcellular compartments: cell body, primary process, ridge-like prominence (RLP), and foot process. The RLP, a longitudinal protrusion from the basal surface of the cell body and primary process, served as an adhesive apparatus for the cell body and primary process to attach to the glomerular basement membrane. Foot processes protruded from both sides of the RLP. In puromycin nephrotic podocytes, foot process effacement occurred in two ways: by type-1 retraction, where the foot processes retracted while maintaining their rounded tips; or type-2 retraction, where they narrowed across their entire lengths, tapering toward the tips. Puromycin nephrotic podocytes also exhibited several alterations associated with foot process effacement, such as deformation of the cell body, retraction of RLPs, and cytoplasmic fragmentation. Finally, podocytes were reorganized into a broad, flattened shape.
Conclusions
The three-dimensional reconstruction of podocytes by serial FIB/SEM images revealed the morphologic changes involved in foot process effacement in greater detail than previously described.
Keywords: podocyte, ultrastructure, foot process effacement, 3D electron microscopy
Visual Abstract
Podocytes, which are complicated epithelial cells specialized for glomerular ultrafiltration, contain at least three different subcellular compartments: the cell body, primary process, and foot process.1 The foot processes are the adhesive apparatus of podocytes that allow attachment to the glomerular basement membrane (GBM). Foot processes interdigitate with those of the neighboring podocytes. The primary processes are the connecting structure between the cell body and the foot processes. Conventional scanning electron microscopy (SEM) is a useful method for exploring the compartmental structures of podocytes.2–4 However, conventional SEM does not sufficiently reveal the complete architecture of individual podocytes because it does not allow observation of the basal surface of the cells.
To reveal the precise architecture of podocytes, we previously examined intact podocytes reconstructed from serial sectional images of the glomerulus acquired using focused-ion beam/scanning electron microscopy (FIB/SEM) tomography (see the details of this technique in Supplemental Figure 1).5–7 The reconstructed podocytes revealed that a more accurate structural hierarchy of subcellular compartments includes the “ridge-like prominence” (RLP), which protrudes directly from the basal surface of the cell body and primary process (Supplemental Figure 2). This subcellular compartment has been already recognized using conventional SEM and transmission electron microscopy and is referred to as the “central foot process” or “anchoring (foot) process.” 8–10 Our three-dimensional (3D) structural analysis using FIB/SEM tomography further cleared that the RLPs serve as an adhesive apparatus for the cell body and primary process to attach to the GBM and as a connecting structure for attaching the foot processes to the cell body or primary processes.6,11 Furthermore, reconstructed podocytes also clearly showed cytoplasmic arcades—the anastomosis between two primary processes from the same podocyte.5,12 Similar to primary processes, the cytoplasmic arcades also contained RLPs and foot processes (Supplemental Figures 3 and 4).
In glomerular diseases, podocytes lose the usual interdigitating pattern of foot processes between neighboring podocytes, and this alteration, known as “foot process effacement,” is regarded as a pathologic indicator of podocyte injury.12–14 The morphologic processes of foot process effacement have been elucidated to a limited extent using conventional SEM12,13,15; some morphologic aspects remain unclear owing to the technical limitations of conventional SEM.
In this study, to clarify the morphologic processes of foot process effacement, we used FIB/SEM tomography to examine diseased podocytes in puromycin aminonucleoside (PAN) nephrosis rats, an animal model of minimal change nephrotic syndrome in humans. We successfully described the morphologic processes involved in foot process effacement in greater detail than has been previously described using conventional SEM.
Methods
PAN Nephrosis
To induce PAN nephrosis, we intraperitoneally administered PAN (15 mg/100 g body wt; Sigma-Aldrich, St. Louis, MO) to 6-week-old male Wistar rats (Charles River Japan, Yokohama, Japan). Animals were perfused with 2.5% glutaraldehyde/0.1 M phosphate buffer under anesthesia with pentobarbital on day 1, 2, 4, and 8. As a control, we used 6-week-old male Wistar rats that were not administered PAN. All procedures performed on the laboratory animals were approved by the Institutional Animal Care and Use Committee of Juntendo University School of Medicine (approval no. 290213).
Conventional SEM
Conventional SEM was performed as described previously.16
FIB/SEM Tomography and 3D Reconstruction
Fixed kidney tissues were stained and embedded in epoxy resin as described previously.6 From three glomeruli in each experimental group, serial FIB/SEM images were obtained at 50-nm increments, using a backscattered electron detector at a 2.0-kV acceleration voltage using a Helios Nanolab 660 FIB/SEM (Thermo Fisher Scientific, Waltham, MA). The pixel size of each FIB/SEM image was 13.5×17.1×50 nm/pixel (width×height×depth). The pixel dimensions for a recorded image were 3072×2048 pixels. Thus, the dimension of the serial image acquired using FIB/SEM was 41.5×35.0×20–35 μm (width×height×depth). The new surface for serial FIB/SEM imaging was generated using focused-ion beam (FIB)-milling with a 0.77-nA beam current, where gallium ions were accelerated at a voltage of 30 kV. The 3D reconstruction of podocytes was performed using an AMIRA 6.1 software (Thermo Fisher Scientific).
Statistical Analyses
All measurements are shown as means±SEM. Differences were tested using ANOVA followed by the Bonferroni test as post hoc test; P<0.05 was considered statistically significant.
Results
Structural Alterations in PAN Nephrotic Podocytes Observed by Conventional SEM
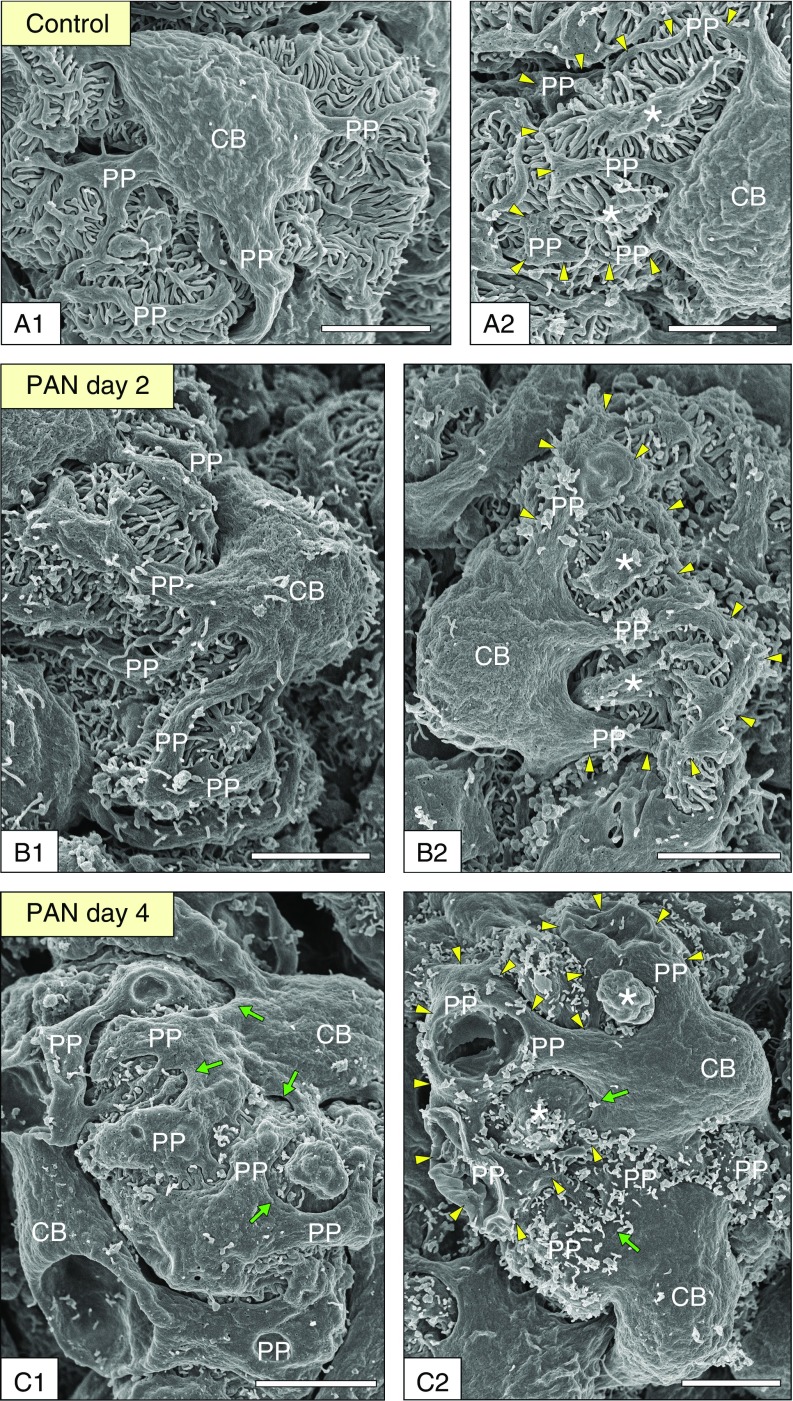
To observe the outline of the structural alterations in PAN nephrotic podocytes, we first observed them using conventional SEM (Figure 1). In control (normal) podocytes, the cell body projected primary processes, which protruded numerous fine foot processes that interdigitated with those of the neighboring podocytes (Figure 1, A1 and A2). Some primary processes bifurcated two or three times—we referred to the portions distal to the first bifurcation as primary processes, and not as secondary and tertiary processes in this study.
Figure 1.
Conventional SEM is useful for observing the alterations in the luminal surface structure of podocytes. (A1 and A2) Healthy (control) podocytes. The cell body projected primary processes, which protruded numerous fine foot processes that interdigitated with those of the neighboring podocytes. Some primary processes bifurcated two or three times—we referred to the portions distal to the first bifurcation as primary processes, and not as secondary and tertiary processes in this study. (B1 and B2) Preproteinuria phase (day 2). Numerous microvilli and bleblike protrusions appeared on the cell body and primary processes. (C1 and C2) Overt proteinuria phase (day 4). The primary processes were flattened. The periphery of the cell body extended between the flattened primary processes similar to the webbing of waterfowl (green arrows). The space between the flattened primary processes narrowed considerably. The cytoplasmic arcades and their parental primary processes also became more recognizable (arrowheads in A2, B2, and C2). Asterisks indicate regions surrounded by the cytoplasmic arcade and its parental primary processes. Scale bars, 5 μm. CB, cell body; PP, primary process.
In the preproteinuria phase (day 2), numerous fine foot processes were still found between the primary processes (Figure 1B1). Numerous microvilli and bleblike protrusions appeared on the cell body and primary processes (Figure 1, B1 and B2). In the overt proteinuria phase (day 4), the primary processes became irregular in shape and were significantly broadened compared with those of the control rats (Figure 1, C1 and C2, Supplemental Figure 5); thus, the space between the primary processes narrowed considerably.
Moreover, the periphery of the cell body extended between the primary processes to form what appeared to be webbing, creating broad and flattened podocytes (arrows in Figure 1, C1 and C2). The foot processes were almost hidden under the deformed cell bodies and primary processes; thus, it was difficult to clarify the morphologic processes of the foot process effacement only using conventional SEM, although this method was useful for observing the alterations in the luminal surface structure of cell bodies and primary processes. To overcome this problem, we next analyzed the podocytes reconstructed from the serial FIB/SEM images.
Foot Process Effacement in PAN Nephrotic Podocytes Revealed by FIB/SEM Tomography
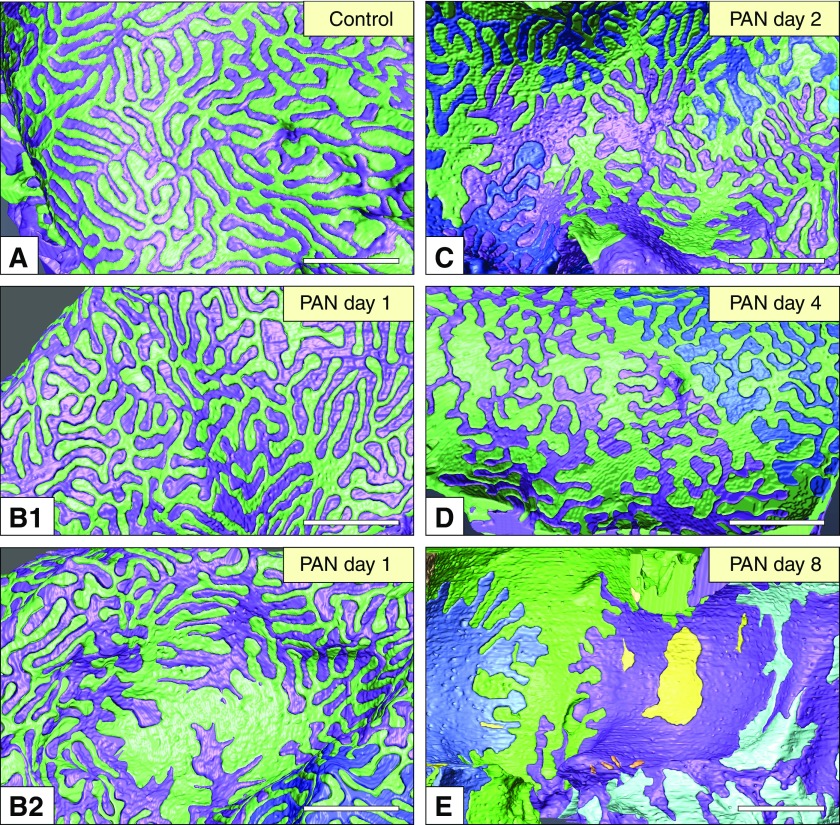
Reconstructed podocytes could be observed from any perspective, and the basal view was particularly useful in understanding the alterations in the foot processes (Figure 2). From the reconstructed podocytes, we found two forms of foot process effacement, type-1 and type-2 retractions, during the progression of PAN nephrosis (Figure 3). In normal podocytes, the foot processes exhibited a relatively uniform width at the base, except at the enlarged terminal portions (Figure 2A). In type-1 retraction, the foot processes were shortened and lost their width uniformity but maintained their rounded tips (Figure 3, A1–A3 and C). The loss of width uniformity and shortening were found in most of the foot processes on day 1 (Figures 2, B1, B2, and 3E), indicating that type-1 retraction had already initiated from as early as day 1.
Figure 2.
Reconstruction of podocytes based on FIB/SEM tomography is useful for observing the alterations in the basal surface structure of podocytes. Individual podocytes are shown in different colors. The basal view of the reconstructed podocytes is useful to analyze structural alterations in the foot processes. (A) In healthy (control) podocytes, foot processes exhibited a uniform width. (B1 and B2) PAN nephrotic podocytes lost this uniformity in the foot processes from day 1. The uniformity in width was further lost on (C) days 2 and (D) 4. (E) The podocytes formed a large adhesive surface on day 8, although short interdigitating processes remained. The yellow masses represent the CFs of podocyte (E), which are frequently found in the PAN nephrotic glomeruli. Scale bar, 2 μm.
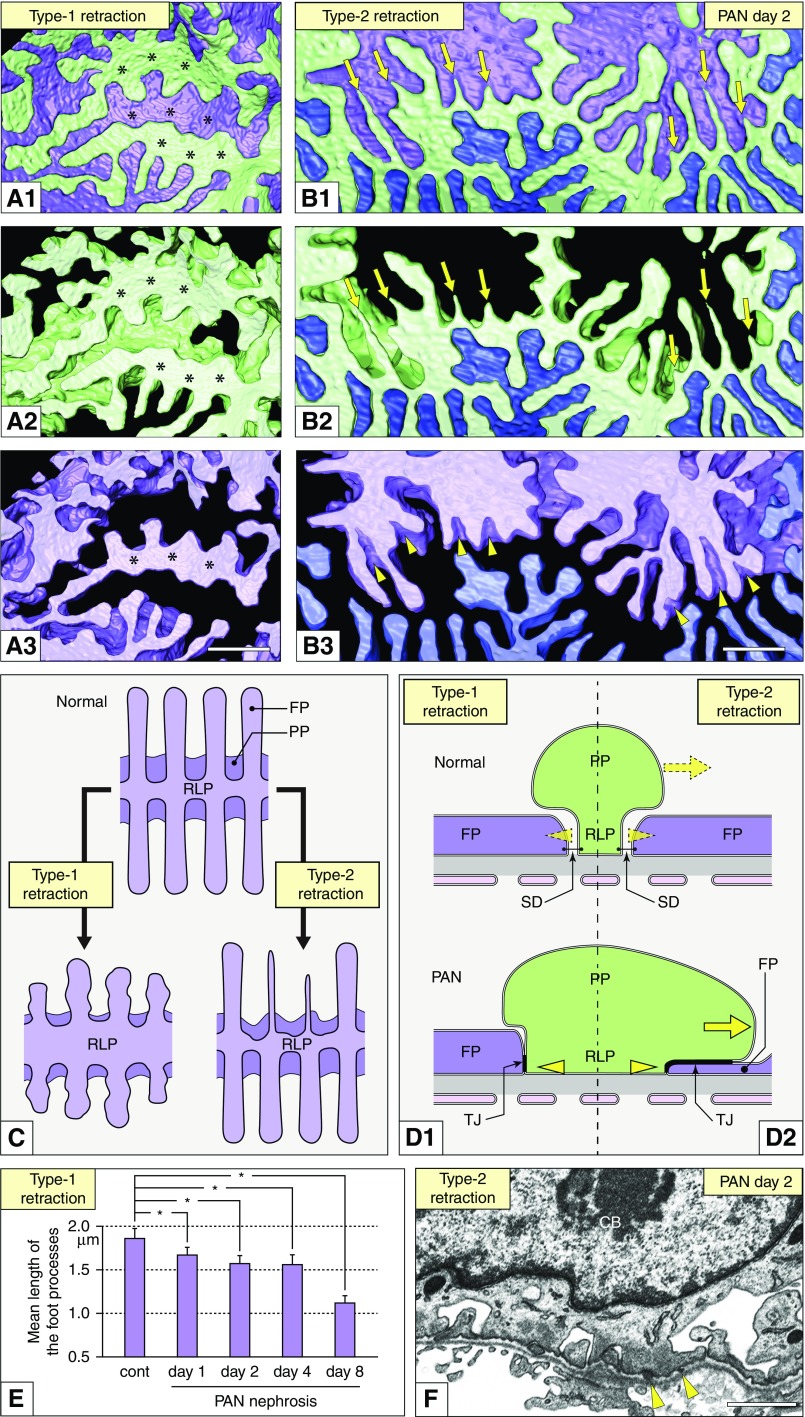
Figure 3.
Foot process effacement progressed in two ways in PAN nephrosis. (A1–A3 and B1–B3) Basal view. (A1–A3) Type-1 retraction. The foot processes of the neighboring green and purple podocytes retracted simultaneously but maintained their rounded tips. The RLPs of both podocytes also widened (asterisks) and prevented exposure of the GBM. (B1–B3) Type-2 retraction. Some foot processes narrowed over their total length and tapered toward the tip (green podocyte, arrows in B1 and B2). The neighboring purple podocyte displayed an overhang on the retracting foot processes of the green podocyte (arrowheads in B3). (C) Schematic drawings representing the two types of foot process effacement (basal view). (D1 and D2) Schematic drawings representing alterations in the podocyte next to the retracting podocyte (cross section). The purple podocyte exhibits foot process retraction. In both type-1 and type-2 retraction, the neighboring (green) podocyte widened its RLP (arrowheads in D1). In type-2 retraction, the green podocyte further extended its primary process over the retracting foot process as a roof (arrows in D2). The slit diaphragms are replaced by the TJs. (E) Mean length of foot processes significantly decreases by type-1 retraction from day 1 with progression of PAN nephrosis: control, 1.86±0.08 μm; day 1, 1.67±0.06 μm; day 2, 1.57±0.08 μm; day 4, 1.56±0.07 μm; day 8, 1.12±0.05 μm (n=60 foot processes from three podocytes). Values are means ±SEM. Differences were tested using ANOVA followed by the Bonferroni test as post hoc test. *P<0.05. (F) FIB/SEM image of type-2 retracting foot processes shown in (B1 and B2) (arrowheads). Scale bars, 1 μm. CB, cell body; FP, foot process; PP, primary process.
In type-2 retraction, the foot processes were shortened, extremely narrowed across their entire lengths, and tapered toward the tips (Figure 3, B1–B3, C, and F). Type-2 retraction was also observed on day 1 (Figure 2B2); however, unlike type-1 retraction, it was found only in some foot processes. At this point, it was not clear whether the two types of foot process retraction progressed independently or they represented two different phases of the same phenomenon of foot process effacement.
According to the foot process effacement in each podocyte, the neighboring podocytes altered their structure to maintain their intercellular junctions, including the slit diaphragm and tight junction (TJ). In both type-1 and type-2 retractions, the neighboring podocytes exhibited widened RLPs (arrowheads in Figure 3, D1 and D2). In type-2 retraction, the neighboring podocytes further extended their primary processes over the retracting foot processes as a roof (arrowheads in Figure 3B3; arrows in Figure 3D2).
Structural Alterations Associated with Foot Process Effacement Revealed by FIB/SEM Tomography
Serial FIB/SEM and 3D reconstructed images further revealed the structural alterations associated with foot process effacement, in addition to the deformation of the cell body and primary processes shown by conventional SEM.
RLP Retraction
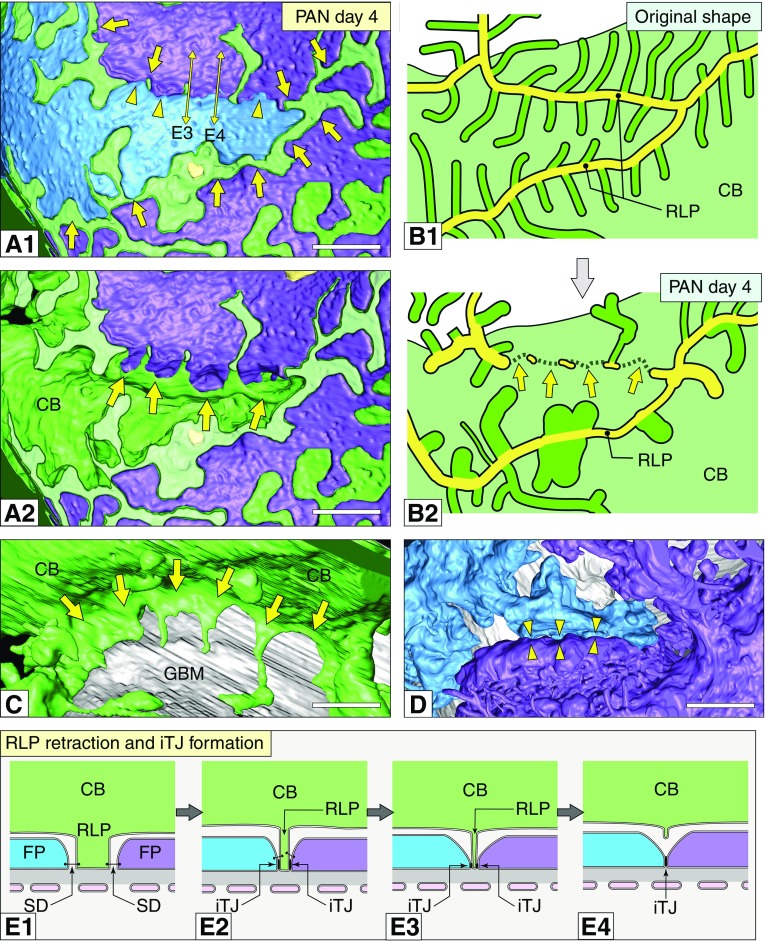
The RLPs were widened because of the foot process retraction as described above. However, some of the RLPs lost most of their foot processes and narrowed to retract (arrows in Figure 4, A1, A2, and C). Consequently, the two neighboring primary processes on both sides of the retracting RLP expanded, and thus the denuded GBM did not appear by the RLP retraction (arrowheads in Figure 4, A1 and D). These expanded primary processes were connected by a newly formed TJ (arrowhead in Figure 4, E1–E4). If the expanded primary processes were derived from the same podocyte cell body, they formed an autocellular tight junction (aTJ) to connect to each other (arrowheads in Figure 5), which has never been observed in normal podocytes. RLP retraction was observed below the cell body (Figure 4), the proximal part of the primary processes (Figure 5), and the cytoplasmic arcades (Supplemental Figure 6).
Figure 4.
Retraction of RLP progressed in association with foot process effacement. (A1) Basal view. The RLP of the green podocyte cell body lost their foot processes (arrows). This RLP was partially retracting and, thus, the neighboring purple and blue podocytes were directly contacted (arrowheads). (A2) Basal view. The blue podocyte was removed from (A1) to show the retracting RLP (arrows). (B1 and B2) Schematic diagrams showing the predicted original (B1) and observed (B2) shapes of the green podocyte shown in (A1 and A2). The yellow structures in (B1 and B2) represent the normal or deformed RLP, respectively. The retracting RLP is shown by arrows in (A2 and B2). (C) Luminal view. The retracting RLP of the green podocyte showing (A1 and A2) (arrows). (D) Luminal view. The neighboring purple and blue podocytes expanded from both sides of the retracting RLP—thus the denuded GBM do not appear by the retraction of RLP (arrowheads). (E1–E4) Schematic diagrams showing RLP retraction between two different podocytes (blue and purple) and the associated intercellular tight junction (iTJ) formation by the retraction. (E3 and E4) represent the sections at the sites indicated by the two double arrows in (A1). Scale bars, 500 nm. CB, cell body; FP, foot process; SD, slit diaphragm.
Figure 5.
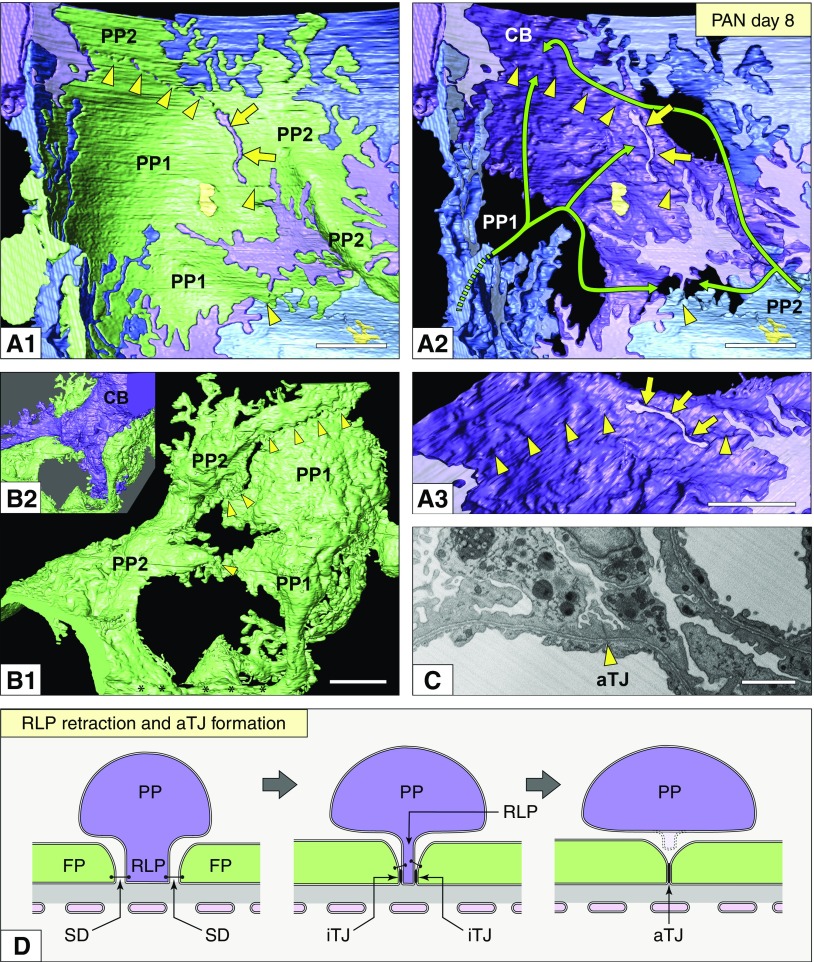
Podocytes formed aTJs in association with foot process effacement. (A1–A3) Basal view. (A1) The aTJs existed between two deformed primary processes of the green podocyte (PP1 and PP2) (arrowheads in A1)—these aTJs were presumably formed by the RLP retraction in the purple podocyte. Arrows indicate remaining RLPs. (A2) The green podocyte was removed from (A1), and its position is indicated by the green lines. Yellow arrows, the remaining RLP; arrowheads, the sites of aTJs between deformed primary processes of the green podocyte. It was not determined whether the three blue regions belonged to the same podocyte or not. (A3) Magnification of the remaining RLP shown in (A2) (arrows). (B1 and B2) Luminal view of the same green podocyte shown in (A1). Arrowheads indicate the aTJs between PP1 and PP2 (B1). The cell body of the purple podocyte (CB) was positioned above the aTJ of the green podocyte (B2). (C) FIB/SEM image showing an aTJ of the green podocyte (arrows). (D) Schematic diagrams showing aTJ formation associated with RLP retraction. Scale bars, 1 μm. FP, foot process; iTJ, intercellular tight junction; PP, primary process; SD, slit diaphragm.
Cytoplasmic Fragment Formation
The cytoplasmic fragments (CFs) were identified as a podocyte fragment without connection to neighboring podocytes. Unlike previously discovered podocyte fragments, the CF was in contact with the GBM along with the neighboring podocyte (yellow masses in Figure 6). The CFs, which were of variable size and shape, frequently appeared both in the pro-proteinuria and overt proteinuria phases, although the dropout of an entire podocyte was not observed in this study.
Figure 6.
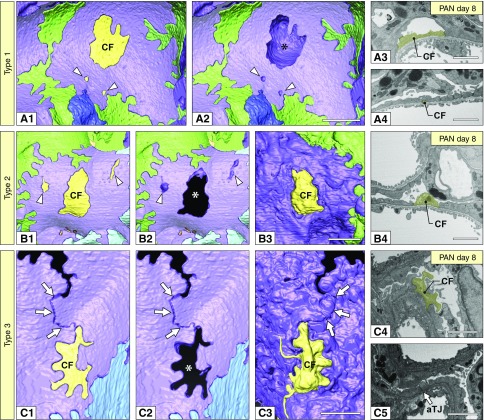
Podocyte fragmentation progressed in association with foot process effacement. Three kinds of podocyte CFs were recognized at least. (A1, A2, B1, B2, C1, and C2) Basal view. The CFs are represented as yellow masses. (A2, B2, and C2) The CFs were removed to show their positions in relation to the neighboring purple podocyte. Asterisks indicate the space for CF. (B3 and C3) Luminal view. (A1 and A2) Type-1 CFs. A large CF (CF in A1) was completely covered by a deformed purple podocyte. Arrowheads indicate two small type-1 CFs. (B1–B3) Type-2 CFs. A large CF (CF in B1) penetrated the deformed purple podocyte. Arrowheads indicate two small type-1 CFs. (C1–C3) Type-3 CFs. CF surrounded by two deformed primary processes of purple podocyte, whose distal ends were connected by an aTJ (arrows). (A3, A4, B4, and C4) FIB/SEM images of CFs shown in (A1, B1, and C1). (C5) FIB/SEM image of aTJ in purple podocyte (arrow). Scale bars, 1 μm.
The CFs were classified into three types on the basis of their positional relationship to the neighboring podocyte: type-1 CFs were covered by the deformed cell body or primary process (Figure 6, A1–A4), type-2 CFs penetrated the deformed primary process (Figure 6, B1–B4), and type-3 CFs were surrounded by two deformed primary processes (Figure 6, C1–C4), and their distal ends were connected by the aTJ (arrows in Figure 6, C1–C3 and C5).
We found several CFs that were shedding into the urinary space (Supplemental Figure 7). The adhesive area of such CF to the GBM became very small. However, there was no gap between the shedding CF and the neighboring podocytes. This finding indicated that the neighboring podocyte extended and covered the GBM in response to the shedding of CF.
Alterations of Podocyte Intercellular Junctions
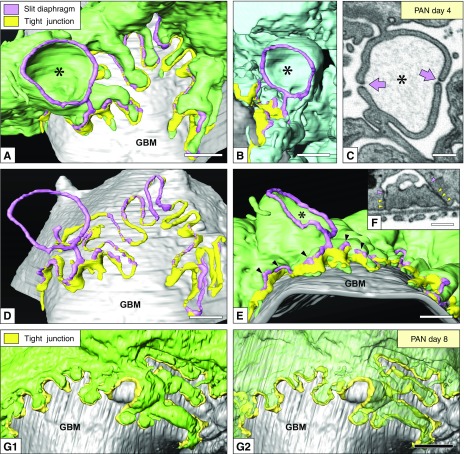
As an initial alteration, the slit diaphragm moved toward the luminal side, and the TJ appeared and coexisted beneath the slit diaphragm (arrowheads in Figure 7, E and F), as reported previously.17 This phenomenon occurred on day 1–4. Part of the slit diaphragm moved further toward the luminal side without any association with the TJ and held its position as an ectopic slit diaphragm (eSD) between the deformed primary processes. Some eSD were located between a pair of cup-shaped protrusions derived from two neighboring primary processes (Figure 7, A–E), whereas others were between the smooth surfaces of the deformed primary processes.
Figure 7.
Reconstruction of podocytes based on FIB/SEM tomography is useful for observing the alterations in the junctional structure. (A–F) Day 4. (A, B, D, and E) Luminal view. The slit diaphragm (pink) and TJ (yellow) were put on the reconstructed green podocytes. In some parts, the slit diaphragm coexisted on the TJ (arrowheads in E). Part of the slit diaphragm moved further toward the luminal side without associating with the TJ and held its position as an eSD between a pair of cup-shaped protrusions (asterisks in A, B, and E) derived from two neighboring primary processes. (C) FIB/SEM image of the cup-shaped protrusions (asterisk) linked by eSDs (arrows). (D) To clearly show the junctional apparatus, the green podocyte was removed from (A). (F) FIB/SEM image of the slit diaphragm (pink arrowheads) coexisting with the TJ (yellow arrowheads). (G1 and G2) Day 8. Luminal view. The podocytes were predominantly connected to each other by the TJs (yellow). Scale bars, 200 nm in (C and F); 500 nm in (A, B, D, E, and F2)
On day 8, the podocytes were predominantly connected to each other by the TJs, except for the eSD (Figure 7, G1 and G2). Moreover, we found some intercellular gaps without any junctional apparatuses in this stage (arrows in Figure 8, A1 and A2, Supplemental Figure 8). These intercellular gaps and microdenudation of the GBM associated with them presumably functioned as a protein-leakage pathway, which should be called the “intercellular pathway.”
Figure 8.
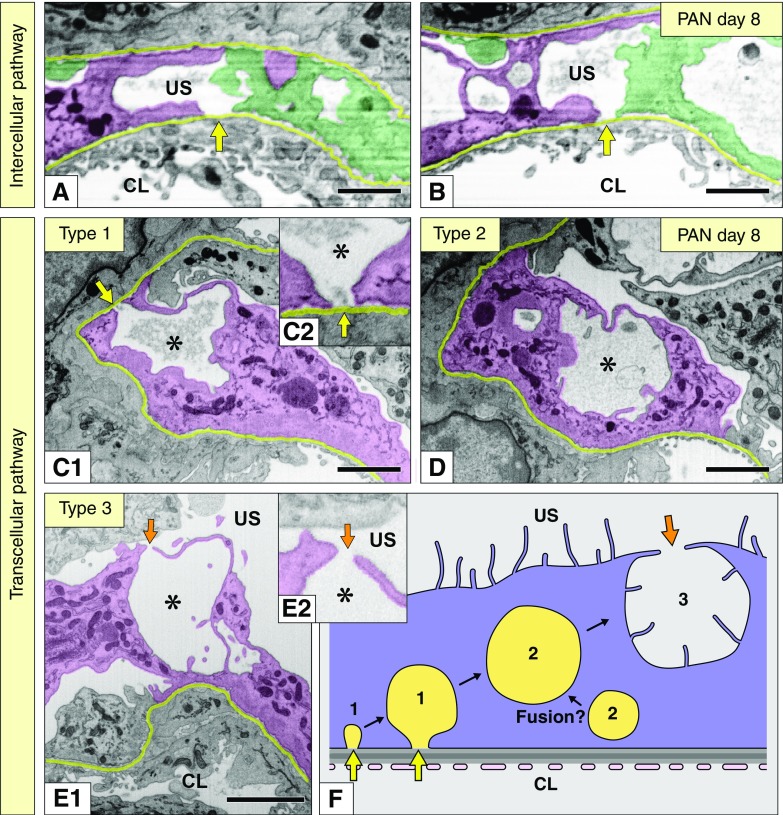
Serial FIB/SEM images revealed two kinds of leakage pathways for plasma proteins in PAN nephrosis. (A and B) Intercellular pathway shown by FIB/SEM images. This pathway was found between purple and green podocytes as the intercellular spaces/gaps without presence of any junctional apparatus (arrows). (C1, C2, D, E1, and E2) Three types of pseudocyst shown by FIB/SEM images. Pseudocysts were formed within the purple podocyte. Type-1 pseudocyst opened toward the GBM (arrow in C1); type-2 pseudocysts were completely closed (D); type-3 pseudocysts opened to the urinary space via small opening (arrow in E1). The type-1 and type-2 pseudocysts frequently contained amorphous materials, whereas type-3 pseudocysts did not contain any amorphous materials. The openings of type-1 and type-3 pseudocysts are magnified in (C2 and E2), respectively. The serial FIB/SEM images containing (C1, D, and E1) are shown in Supplemental Figures 8–10, respectively. (F) Transcellular pathway shown by a schematic diagram. This pathway is presumably formed by the transition between pseudocystic types in the order of type 1, type 2, and type 3. Asterisks indicate lumen of the pseudocysts; yellow lines indicate the GBM. Scale bars, 500 nm in (A and B); 1 μm in (C1, D, and E1). CL, capillary lumen; US, urinary space.
Pseudocyst Formation
The PAN nephrotic podocytes exhibited numerous pseudocysts within their deformed cell bodies and primary processes on day 4 and 8, when severe proteinuria was recognized (Figure 8, C1, D, and E1, Supplemental Figures 9 and 10), as described in previous electron microscopy studies.18–21
Serial FIB/SEM images revealed that the pseudocysts were topologically divided into the following three types: type-1 pseudocysts were dome-shaped and outpocketing, developing in conjunction with focal microdenudation of the GBM (Figure 8, C1 and C2, Supplemental Figure 9); type-2 pseudocysts were completely closed (Figure 8D, Supplemental Figure 9); and type-3 pseudocysts were opened to the urinary space through small channels (Figure 8, E1 and E2, Supplemental Figure 10). The type-1 and type-2 pseudocysts frequently contained amorphous materials, which is reminiscent of plasma protein aggregation due to chemical fixation, whereas type-3 pseudocysts did not contain any such amorphous materials. These pseudocysts presumably contributed to another protein-leakage pathway, which should be called “transcellular pathway” (see Discussion).
Discussion
Advantages of FIB/SEM Tomography in Structural Analysis of Podocytes
FIB/SEM tomography, including the reconstruction technique, enabled a more precise analysis of the 3D ultrastructure of healthy, developing, and diseased podocytes, as shown in this and previous studies.5–7,22
The high level of visibility of the 3D ultrastructure is the most important advantage of this method. Conventional SEM is useful for revealing the luminal surface structure of podocytes; however, it is too weak to directly observe several portions of the podocytes, such as the basal surface structures. The 3D reconstruction images of single podocytes by FIB/SEM tomography completely overcame this problem by enabling the observation of surface structures from any direction and without interpretation by neighboring podocytes or GBM. Furthermore, the color-coded reconstruction images of multi-podocytes are also valuable for analyzing the 3D mutual relationship of podocytes.
In FIB/SEM tomography, numerous serial FIB/SEM images of podocytes, which achieve quality comparable with that of conventional transmission electron microscopy images, can be easily observed. This feature is another advantage of FIB/SEM tomography in the 3D ultrastructural analysis of podocytes. By observing the serial FIB/SEM images, we could analyze two potential leakage pathways for plasma proteins in the PAN nephrotic podocytes.
Pathologic Significance of aTJ and eSD in Podocytes
The aTJ, a connection between two adjacent parts of the same cell mediated by the same adhesion complex,16 has not been reported in vertebrate podocytes. However, the PAN nephrotic podocytes formed aTJs, especially in the overt proteinuria phase. This formation of the aTJ contributes to preventing the exposure of the GBM caused by the retraction of podocyte protrusions.
In PAN nephrosis, proteinuria gradually becomes less severe over a period of several months, but glomeruli are not completely restored to normal morphology.23,24 Several possibilities have been considered regarding the fate of the podocyte aTJ after restoration. For instance, if a long aTJ persists in the recovery phase, it is highly likely to interfere with the restoration of the normal architecture of podocytes. In this study, we did not analyze the recovery phase of PAN nephrosis. In a future study, to determine the effect of aTJs on podocyte restoration, we will examine whether aTJs are retained in the recovery phase and the extent to which deformed podocytes can be restored to the normal architecture.
The eSD between the primary processes clearly do not contribute to the filtration barrier function. However, they may play a role in the pool of slit diaphragm proteins used to restore normal slit diaphragms at the base of foot processes.
Formation and Fate of CFs in PAN Nephrotic Podocytes
It is reasonable to think that the CFs were derived from the distal parts of foot processes or primary processes with their foot processes. Type-1 CFs were formed by the separation of the distal parts under the deformed cell body and primary processes of neighboring podocyte. Type-1 CFs were highly likely to necrotize under the neighboring podocyte; however, it was difficult to clarify the fate of type-1 CFs from the data obtained in this study.
Type-2 CFs were presumably derived from the distal parts of the podocytes in the fenestration surrounded by the cytoplasmic arcade and its parental primary processes. The cytoplasmic arcade possessed RLPs that continued and joined those of the parental primary processes; thus, the fenestration was also surrounded by a continuous RLP. The continuous RLP possessed one narrow channel to allow the neighboring podocyte to enter the fenestration (yellow lines in Supplemental Figure 3). The neighboring podocyte broke off at the narrow channel, converting the part in the fenestration to a type-2 CF. Thus, the distal regions of podocytes that entered into the fenestration can be regarded as a “fragile region” in podocytes.
Type-3 CFs were presumably formed by the separation of the distal parts of podocytes that were located between two primary processes from the same neighboring podocyte. After the separation, the primary processes next to the CF expanded and connected with the aTJ.
Most of the type-2 and type-3 CFs were highly likely to shed into the urinary space. In this study, we found three shedding type-2 CFs, which were shed into the urinary space without forming intercellular gaps. This finding indicated that the shedding CFs did not contribute to forming the bare area of the GBM as a leakage pathway for plasma proteins.
Some of the CFs appeared viable in the FIB/SEM images. The PAN nephrotic podocytes showed upregulated expression of connexin 43, a gap junction protein.25 If gap-junction communication was established between such viable CFs and neighboring podocytes by connexin 43, this would contribute to the survival of the CFs.
Protein Leakage Pathways in PAN Nephrosis
In this FIB/SEM analyses, at least one protein leakage pathway, the intercellular pathway, was determined in the PAN nephrotic podocytes. The intercellular pathway consisted of the intercellular gaps without any junctional structures (slit diaphragm and TJ) between deformed podocytes and the microdenudation of GBM at the intercellular gaps.
Kriz and colleagues13,26,27 proposed that the pseudocysts provide a communicating system of extracellular spaces through which the filtrate pass to directly reach the urinary space. However, we scarcely found any pseudocysts that directly communicated the microdenudation of GBM and urinary space in the PAN nephrotic podocytes. The pseudocysts exhibited three kinds of state; if the pseudocysts shifted between the types in order of type 1, type 2, and type 3, as shown in Figure 8F, they were regarded as another pathway of protein leakage, which may be called “transcellular pathway,” as described by Venkatachalam et al.21
More specifically, the transcellular pathway is a consequence of the following phenomena: (1) the deformed cell body and primary processes partially detach from the GBM as the initial step of this pathway; (2) type-1 pseudocysts are subsequently formed and developed by the filtration pressure on the detached basal surface of podocyte; (3) developed type-1 pseudocysts lose the orifice from which the filtrate entered and become type-2 pseudocysts, and type-1 and type-2 pseudocysts contained amorphous materials, which were highly likely to represent the plasma proteins leaking from the denuded area of GBM; (4) type-2 pseudocysts coalesce with each other into large pseudocysts; (5) enlarged type-2 pseudocysts open to the urinary space via newly formed small channels and become type-3 pseudocysts; and (6) the leaked plasma proteins within the pseudocysts are released from the small channels into the urinary space.
Limitations of 3D Ultrastructural Analysis Using FIB/SEM Tomography
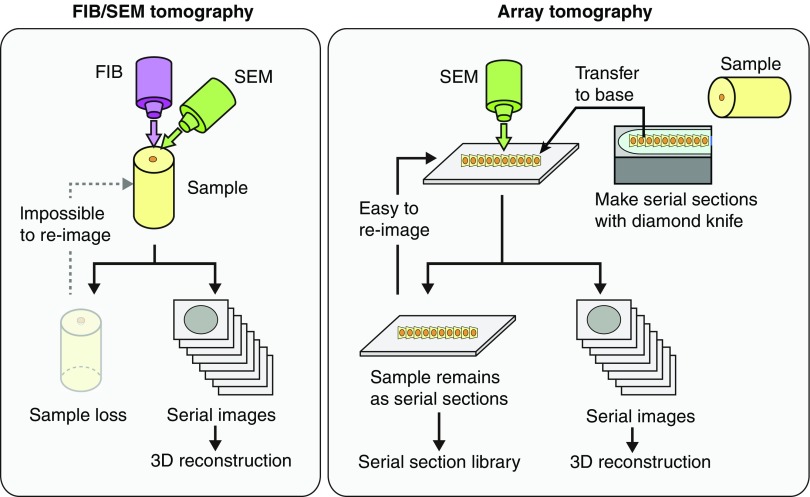
FIB/SEM tomography is a powerful tool for precisely analyzing the 3D ultrastructure of podocytes; however, there are several technical disadvantages to this method. Firstly, the imaged region is completely lost because of FIB-milling. Moreover, the nonimaged region adjacent to the imaged region is severely damaged during scanning for the positional correction of FIB-milling. This damage makes it impossible to obtain FIB/SEM images with sufficient quality for the 3D reconstruction of foot processes. Therefore, we hesitated to adapt FIB/SEM tomography for irreplaceable specimens such as renal biopsy specimens from patients. Array tomography is a potential solution to this problem, and it was used to obtain serial images from serial ultrathin sections mounted on bases, such as silicon wafer and glass plate (Figure 9).28–31 These serial ultrathin sections are physically stable and repeatedly observable. For long-term future studies, we aim to establish a “serial ultrathin section library” of renal biopsy specimens and to analyze them using array tomography.
Figure 9.
Future evolution of three-dimensional ultrastructural analysis of human biopsy samples. In FIB/SEM tomography, the imaged region of sample is completely lost because of FIB-milling (left panel). Array tomography is a potential solution to this problem (right panel). In this method, serial sectional SEM images are obtained from ultrathin serial sections mounted on a base. These ultrathin serial sections are physically stable and repeatedly observable.
In conclusion, FIB/SEM tomography was shown to be a powerful tool for elucidating the 3D architecture of podocytes. Using this method, we analyzed and described the morphologic processes involved in foot process effacement more clearly and precisely than that reported previously. The morphologic information revealed in this study will be valuable for the 3D structural analyses of podocytes in other animal and human glomerular diseases.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors wish to thank Mr. Takanobu Ishimura (Maxnet Co., Ltd., Tokyo, Japan) for giving a technical lecture on the reconstruction software and Mr. Kota Kato (Juntendo University) for three-dimensional (3D) printing. This study was supported, in part, by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT; nos. 15K18960 and 17K08521, to K.I.) and Grants-in-Aid from the Foundation of Strategic Research Projects in Private Universities from the MEXT (nos. S1311011 and S1101009, to Juntendo University).
K.I. designed the experiments. K.I. and S.K. obtained the serial focused-ion beam/scanning electron microscopy images. K.I., T.M., Y.K., and M.K. performed 3D reconstruction. K.I. and T.S. analyzed the experimental data. K.I. prepared the figures and wrote the manuscript text.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018020139/-/DCSupplemental.
Supplemental Material
Supplemental Figure 1. 3D reconstruction of podocytes using FIB/SEM tomography.
Supplemental Figure 2. Structural hierarchy of podocyte subcellular compartments.
Supplemental Figure 3. Cytoplasmic arcade in normal podocyte (I).
Supplemental Figure 4. Cytoplasmic arcade in normal podocyte (II).
Supplemental Figure 5. Alteration in mean width of primary processes in PAN nephrotic podocytes.
Supplemental Figure 6. aTJ in PAN nephrotic podocytes.
Supplemental Figure 7. Shedding CF in PAN nephrotic podocyte.
Supplemental Figure 8. Intercellular pathways for plasma protein leakage in PAN nephrotic podocytes.
Supplemental Figure 9. Type-1 and type-2 pseudocysts in PAN nephrotic podocyte.
Supplemental Figure 10. Type-3 pseudocyst shown in PAN nephrotic podocyte.
References
- 1.Kriz W, Kaissling B: Structural organization of the mammalian kidney. In: The Kidney, Physiology and Pathophysiology, 3rd Ed., edited by Seldin DW, Giebisch G, Philadelphia, Lippincott Williams & Wilkins, 2000, pp 587–654 [Google Scholar]
- 2.Andrews PM: Scanning electron microscopy of human and rhesus monkey kidneys. Lab Invest 32: 510–518, 1975 [PubMed] [Google Scholar]
- 3.Bulger RE, Siegel FL, Pendergrass R: Scanning and transmission electron microscopy of the rat kidney. Am J Anat 139: 483–501, 1974 [DOI] [PubMed] [Google Scholar]
- 4.Fujita T, Tokunaga J, Miyoshi M: Scanning electron microscopy of the podocytes of renal glomerulus. Arch Histol Jpn 32: 99–113, 1970 [DOI] [PubMed] [Google Scholar]
- 5.Ichimura K, Kakuta S, Kawasaki Y, Miyaki T, Nonami T, Miyazaki N, et al.: Morphological process of podocyte development revealed by block-face scanning electron microscopy. J Cell Sci 130: 132–142, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Ichimura K, Miyazaki N, Sadayama S, Murata K, Koike M, Nakamura K, et al.: Three-dimensional architecture of podocytes revealed by block-face scanning electron microscopy. Sci Rep 5: 8993, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ichimura K, Sakai T: Evolutionary morphology of podocytes and primary urine-producing apparatus. Anat Sci Int 92: 161–172, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neal CR, Crook H, Bell E, Harper SJ, Bates DO: Three-dimensional reconstruction of glomeruli by electron microscopy reveals a distinct restrictive urinary subpodocyte space. J Am Soc Nephrol 16: 1223–1235, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Takahashi-Iwanaga H: Comparative anatomy of the podocyte: A scanning electron microscopic study. Microsc Res Tech 57: 196–202, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Neal CR: Podocytesc … What’s under yours? (podocytes and foot processes and how they change in nephropathy). Front Endocrinol (Lausanne) 6: 9, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burghardt T, Hochapfel F, Salecker B, Meese C, Gröne HJ, Rachel R, et al.: Advanced electron microscopic techniques provide a deeper insight into the peculiar features of podocytes. Am J Physiol Renal Physiol 309: F1082–F1089, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Inokuchi S, Sakai T, Shirato I, Tomino Y, Koide H: Ultrastructural changes in glomerular epithelial cells in acute puromycin aminonucleoside nephrosis: A study by high-resolution scanning electron microscopy. Virchows Arch A Pathol Anat Histopathol 423: 111–119, 1993 [DOI] [PubMed] [Google Scholar]
- 13.Kriz W, Shirato I, Nagata M, LeHir M, Lemley KV: The podocyte’s response to stress: The enigma of foot process effacement. Am J Physiol Renal Physiol 304: F333–F347, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Shirato I: Podocyte process effacement in vivo. Microsc Res Tech 57: 241–246, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Inokuchi S, Shirato I, Kobayashi N, Koide H, Tomino Y, Sakai T: Re-evaluation of foot process effacement in acute puromycin aminonucleoside nephrosis. Kidney Int 50: 1278–1287, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Dong HM, Ichimura K, Sakai T: Structural organization of hepatic portal vein in rat with special reference to musculature, intimal folds, and endothelial cell alignment. Anat Rec (Hoboken) 293: 1887–1895, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Caulfield JP, Reid JJ, Farquhar MG: Alterations of the glomerular epithelium in acute aminonucleoside nephrosis. Evidence for formation of occluding junctions and epithelial cell detachment. Lab Invest 34: 43–59, 1976 [PubMed] [Google Scholar]
- 18.Kanwar YS, Rosenzweig LJ: Altered glomerular permeability as a result of focal detachment of the visceral epithelium. Kidney Int 21: 565–574, 1982 [DOI] [PubMed] [Google Scholar]
- 19.Messina A, Davies DJ, Dillane PC, Ryan GB: Glomerular epithelial abnormalities associated with the onset of proteinuria in aminonucleoside nephrosis. Am J Pathol 126: 220–229, 1987 [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan GB, Karnovsky MJ: An ultrastructural study of the mechanisms of proteinuria in aminonucleoside nephrosis. Kidney Int 8: 219–232, 1975 [DOI] [PubMed] [Google Scholar]
- 21.Venkatachalam MA, Karnovsky MJ, Cotran RS: Glomerular permeability. Ultrastructural studies in experimental nephrosis using horseradish peroxidase as a tracer. J Exp Med 130: 381–399, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribeiro C, Neumann M, Affolter M: Genetic control of cell intercalation during tracheal morphogenesis in Drosophila. Curr Biol 14: 2197–2207, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Rasch R, Nyengaard JR, Marcussen N, Meyer TW: Renal structural abnormalities following recovery from acute puromycin nephrosis. Kidney Int 62: 496–506, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Uchida K, Suzuki K, Iwamoto M, Kawachi H, Ohno M, Horita S, et al.: Decreased tyrosine phosphorylation of nephrin in rat and human nephrosis. Kidney Int 73: 926–932, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Yaoita E, Yao J, Yoshida Y, Morioka T, Nameta M, Takata T, et al.: Up-regulation of connexin43 in glomerular podocytes in response to injury. Am J Pathol 161: 1597–1606, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kriz W, Lemley KV: A potential role for mechanical forces in the detachment of podocytes and the progression of CKD. J Am Soc Nephrol 26: 258–269, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kriz W, Hähnel B, Hosser H, Rösener S, Waldherr R: Structural analysis of how podocytes detach from the glomerular basement membrane under hypertrophic stress. Front Endocrinol (Lausanne) 5: 207, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Micheva KD, Smith SJ: Array tomography: A new tool for imaging the molecular architecture and ultrastructure of neural circuits. Neuron 55: 25–36, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wacker I, Schroeder RR: Array tomography. J Microsc 252: 93–99, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Koga D, Kusumi S, Watanabe T: Backscattered electron imaging of resin-embedded sections. Microscopy (Oxf) 67: 196–206, 2018 [DOI] [PubMed] [Google Scholar]
- 31.Koike T, Kataoka Y, Maeda M, Hasebe Y, Yamaguchi Y, Suga M, et al.: A device for ribbon collection for array tomography with scanning electron microscopy. Acta Histochem Cytochem 50: 135–140, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.