With this population-based study, we assessed health outcomes of infants born to fertile, subfertile, and ART-treated mothers in Massachusetts.
Abstract
OBJECTIVES:
To assess the risk of adverse health outcomes for infants after assisted reproductive technology (ART)–treated and subfertile as compared with fertile deliveries.
METHODS:
Live-born singleton infants ≥23 weeks’ gestational age (GA) born in Massachusetts between July 1, 2004, and December 31, 2010, were analyzed by linking a clinical ART database with state vital records. χ2 tests were used to compare the outcomes of fertile (those without ART treatment or other indicators of infertility), subfertile (indicators of infertility, no ART), and ART-treated (linked to ART deliveries) mothers, stratified by GA. Adjusted odds ratios (aORs) and 95% confidence intervals (CIs) were calculated by using multivariate logistic regression within each GA stratum, controlling for maternal sociodemographic and health characteristics.
RESULTS:
Compared with infants of fertile mothers (n = 336 705), infants born to subfertile (n = 5043) or ART-treated (n = 8375) mothers were more likely to be preterm (aOR 1.39 [95% CI 1.26–1.54] and aOR 1.72 [95% CI 1.60–1.85], respectively) and have respiratory and gastrointestinal and/or nutritional conditions (aOR range: 1.12–1.18). When stratified by GA, infants of subfertile or ART-treated mothers were at greater risk for congenital malformations and infectious diseases as well as cardiovascular and respiratory conditions (aOR range: 1.30–2.61; 95% CI range: 1.02–4.59). Compared with infants born to subfertile mothers, infants born to ART-treated mothers were at lower risk for being small for GA and having congenital malformations and cardiovascular conditions and at higher risk for infectious disease conditions.
CONCLUSIONS:
Compared with infants born to fertile mothers, infants of subfertile and ART-treated mothers are at greater risk for adverse health outcomes at birth beyond prematurity. The occurrence and magnitude of these risks vary by GA and organ systems.
What’s Known on This Subject:
Infants born to subfertile and assisted reproductive technology–treated mothers are at greater risk for adverse birth outcomes compared with those born to fertile mothers.
What This Study Adds:
With this study, we demonstrate the variability in the risk of adverse health outcomes by maternal fertility group beyond just preterm birth and low birth weight but also, more specifically, by organ system conditions across several gestational age categories.
Assisted reproductive technology (ART) includes any fertility treatment in which eggs and embryos are handled and typically involves surgically removing eggs from a woman’s ovaries, combining them with sperm in the laboratory, and returning them to the woman’s body or donating them to another woman. Excluded are treatments in which only sperm are handled (ie, intrauterine or artificial insemination) or procedures in which only medication is administered to stimulate egg production.1,2 The use of ART has dramatically increased over time; from 2000 to 2013, ART cycles doubled in the United States, from 99 629 to 190 773, and >5 million infants now have been born with the assistance of ART worldwide, half of whom within the past 6 years.3–6
Previous studies have demonstrated higher risk for adverse birth outcomes for infants who were conceived by ART, largely driven by the prevalence of multiple gestation and subsequent greater risk for preterm birth.7,8 However, a higher risk for preterm birth and low birth weight (LBW) is also found in singleton births after ART treatment.9–11 Although there is robust evidence for adverse birth outcomes among infants conceived by ART, data on the risk for specific neonatal conditions beyond preterm birth and LBW are lacking. Furthermore, there is little research on whether maternal infertility status (without ART treatment) versus ART treatment contributes to the risk for adverse neonatal health outcomes. Although authors of previous studies have demonstrated that the underlying subfertility diagnosis has a greater and more long-lasting adverse effect for mothers12 and increases the risk for preterm birth and LBW,3,13,14 data on more specific neonatal health conditions because of maternal subfertility are sparse. Our objective in this study, therefore, was to assess the association of maternal fertility status and ART treatment on health at birth among Massachusetts singletons.
Methods
Data Sources
Data were obtained from the Massachusetts Outcome Study of Assisted Reproductive Technology (MOSART) database, which includes data from (1) the Society for Assisted Reproductive Technology Clinic Outcome Reporting System (SART CORS) database, which contains cycle-based ART data from the majority of US ART clinics, and (2) the Pregnancy to Early Life Longitudinal Data System (PELL), an ongoing population-based system that includes birth certificates, death records, and hospital use data for Massachusetts resident mothers and infants. Institutional review board approval was obtained from the Massachusetts Department of Public Health and Dartmouth College. The Society for Assisted Reproductive Technology (SART) Research Committee approved the study.
The SART CORS contains comprehensive data from >90% of US ART clinics. Data were collected and verified by SART and were reported to the Centers for Disease Control and Prevention in compliance with the Fertility Clinic Success Rate and Certification Act of 1992 (Public Law 102–493). The database includes information on demographics, ART diagnoses, treatment parameters, and pregnancy outcomes.
The PELL has linked information on >99% of all births and fetal deaths in Massachusetts since 1998 to hospital use data for women and their children. Birth defects data are linked from the Massachusetts Birth Defects Monitoring Program (MBDMP). BDMP conducts population-based active surveillance of structural birth defects among Massachusetts residents diagnosed through 1 year of age through analysis of data from delivery and specialty care hospitals, birthing centers, and vital records.
The MOSART database links the SART CORS and PELL for all children born in Massachusetts hospitals to Massachusetts resident women between July 1, 2004, and December 31, 2010. The starting date was chosen on the basis of the availability of SART CORS data (January 1, 2004) to allow us to capture any births associated with ART, and the end date reflected the latest available data from both the SART and PELL when this analysis was initiated. A deterministic 5-phase linkage algorithm was implemented, with matching being based on the infant’s date of birth, mother’s date of birth, mother’s first name and last name, and father or partner’s last name.15 The linkage rate was 89.7% overall and 95.0% for deliveries in which both the mother’s zip code and clinic were in Massachusetts.
Cohort Selection
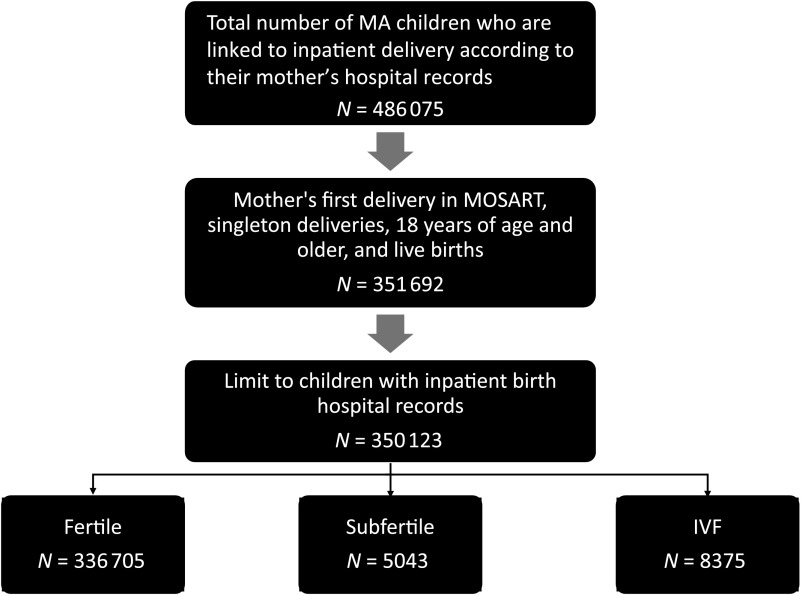
Inclusion criteria were as follows: (1) mothers’ first delivery in MOSART, regardless of parity, so as to not evaluate multiple deliveries to a single woman; (2) singleton; (3) maternal age ≥18 years; (4) live birth; and (5) infants with inpatient birth hospital records (Fig 1). Infants who were transferred to another facility after birth but before they were discharged were also included.
FIGURE 1.
Cohort selection. MA, Massachusetts.
Outcomes
By using the International Classification of Diseases, Ninth Revision (ICD-9) codes from birth hospitalization records and birth certificates, the following infant health outcomes were assessed: preterm birth (<37 weeks’ gestational age [GA]), small for gestational age (SGA), LBW (<2500 g), neonatal mortality during birth hospitalization (death between the day of delivery and the last day of hospitalization), and prolonged hospital stay (for infants ≥35 weeks’ GA, >3 days for infants born vaginally and >5 days for those born by cesarean delivery). The outcome of birth defects, obtained from the MBDMP, was categorized as chromosomal or nonchromosomal according to a previously published MOSART study.16,17 Infants who were transferred to higher levels of care after birth were included. Specific conditions from the following systems were also assessed: infectious disease, cardiovascular, respiratory, gastrointestinal and/or nutritional, neurologic, and hematologic (ICD-9 codes are shown in Supplemental Tables 4 through 10). Length of gestation was calculated on the basis of clinical estimates by the first trimester ultrasound and, when those were missing, the estimated date of the last menstrual period. Birth weights at each GA are normally distributed, and a z score (SD score) is the deviation of the value for an individual from the mean value of the reference population divided by the SD for the reference population. Birth weight z scores were calculated to evaluate the adequacy of weight for age by using Massachusetts population-based standards and were modeled as continuous and categorical variables. We generated sex-, race and/or ethnicity–, and gestation-specific birth weight means and SDs using Massachusetts data for live births from 2004 to 2010. Infants with z scores of ≤1.28 (<10th percentile for gestation) were classified as SGA.
Primary Exposure
Maternal fertility groups (fertile, subfertile, or ART treated) were the primary exposures. Women were classified as being ART treated if the delivery was linked to ART data from the SART CORS online database.
They were classified as subfertile if they had either a diagnosis of infertility (ICD-9 codes 628 and V230) on the index or previous hospitalization record, or an indication on the birth or fetal death certificate of use of non-ART medically assisted reproduction (MAR) for the index or previous deliveries.18 The term subfertility was used rather than infertility or MAR2 to indicate that this was a combination measure rather than 1 or the other of these determinations. Women who had undergone ART treatment in previous pregnancies during or preceding the MOSART study period were also defined as being subfertile for the index pregnancy. Fertile women were those in neither the ART-treated nor the subfertile groups.
Additional independent variables included maternal age, race and/or ethnicity, education, marital status, parity, insurance status, chronic and pregnancy-induced hypertension, nongestational and gestational diabetes, and year of birth.
Statistical Methods
We compared the birth outcomes of infants born to fertile, subfertile, and ART-treated mothers stratified by GA categories using χ2 statistics (α = .05). Logistic regression modeling was performed to assess the independent association between maternal fertility group and adverse birth outcomes within each GA stratum, controlling for maternal age, race and/or ethnicity, education, insurance status at birth, preexisting diabetes, preexisting hypertension, pregnancy-induced hypertension, gestational diabetes, parity, sex, birth year, and GA. Given the higher risk of adverse health outcomes in infants of younger GA, we stratified our analyses by GA categories and controlled for GA within each category for the multivariate analyses. For the outcome of prolonged infant hospital stay among infants born ≥35 weeks’ GA, we also adjusted for maternal length of hospital stay. Results, presented as adjusted odd ratios (aORs) and 95% confidence intervals (CIs), were considered significant with P values <.05 for bivariate analyses and when the 95% CIs did not include 1. All analyses were performed by using SAS software version 9.3 (SAS Institute, Inc, Cary, NC).
Results
Cohort
Our study cohort included 351 692 infants. Of these, 350 123 had birth hospitalization records and comprised our final cohort of 336 705 infants of fertile, 5403 infants of subfertile, and 8375 infants of ART-treated women (Fig 1).
There were significant differences in maternal sociodemographic and clinical characteristics among mothers in the 3 fertility groups. Compared with fertile mothers, subfertile and ART-treated mothers were more likely to be older, non-Hispanic white, more highly educated, and primiparous; have chronic hypertension, pregnancy-induced hypertension, and nongestational and gestational diabetes and other conditions; and require prolonged hospital stay (Table 1; P < .0001 for all characteristics).
TABLE 1.
Maternal Cohort Characteristics
| Demographic Characteristics | Total (N = 350 123), % | Fertile (N = 336 705), % | Subfertile (N = 5043), % | ART (N = 8375), % | P |
|---|---|---|---|---|---|
| Age, y | <.0001a | ||||
| 18–29 | 48.2 | 49.7 | 13.8 | 8.8 | |
| 30–34 | 30.2 | 30.1 | 33.4 | 32.2 | |
| 35–37 | 12.4 | 11.9 | 24.7 | 24.2 | |
| 38–40 | 6.5 | 6 | 17.5 | 19.6 | |
| 41–42 | 1.8 | 1.6 | 6.6 | 8.5 | |
| 43+ | 0.9 | 0.7 | 3.8 | 6.8 | |
| Race and/or ethnicity | <.0001a | ||||
| Hispanic | 14.2 | 14.6 | 5.2 | 3.7 | |
| Non-Hispanic white | 66.5 | 65.9 | 82.3 | 84.1 | |
| Non-Hispanic Black | 8.9 | 9.1 | 3.9 | 3.3 | |
| Asian | 8.2 | 8.2 | 7.5 | 7.9 | |
| Other non-Hispanic | 2.3 | 2.3 | 1.2 | 1.1 | |
| Education | <.0001a | ||||
| Less than high school or high school graduate | 36.3 | 37.3 | 12.9 | 10.1 | |
| Some college | 22.2 | 22.4 | 17.9 | 16.1 | |
| College graduate | 41.5 | 40.3 | 69.2 | 73.8 | |
| Parity | <.0001a | ||||
| 1 | 57.3 | 56.8 | 58.8 | 74.8 | |
| 2 | 26.6 | 26.8 | 28.1 | 19.5 | |
| 3+ | 16.1 | 16.4 | 13.1 | 5.7 | |
| Chronic hypertension | <.0001a | ||||
| No | 98.2 | 98.3 | 97.0 | 96.8 | |
| Yes | 1.8 | 1.7 | 3.0 | 3.2 | |
| Pregnancy-induced hypertension | <.0001a | ||||
| No | 90.6 | 90.7 | 88.3 | 86.6 | |
| Yes | 9.4 | 9.3 | 11.7 | 13.4 | |
| Nongestational diabetes | <.0001a | ||||
| No | 98.8 | 98.8 | 98.1 | 97.9 | |
| Yes | 1.2 | 1.2 | 1.9 | 2.1 | |
| Gestational diabetes | <.0001a | ||||
| No | 94.2 | 94.3 | 90.6 | 91.6 | |
| Yes | 5.8 | 5.7 | 9.4 | 8.4 | |
| Prolonged length of hospital stay for mothersb | <.0001a | ||||
| No | 92.3 | 92.5 | 90.3 | 86.8 | |
| Yes | 7.7 | 7.5 | 9.7 | 13.2 | |
| Year of birth | <.0001a | ||||
| 2004 | 10.2 | 10.2 | 14.3 | 5.2 | |
| 2005 | 19.7 | 19.7 | 21.9 | 17.7 | |
| 2006 | 17.7 | 17.7 | 19.6 | 16.9 | |
| 2007 | 15.3 | 15.3 | 13.4 | 16.2 | |
| 2008 | 13.5 | 13.6 | 11.2 | 14.0 | |
| 2009 | 12.3 | 12.2 | 10.3 | 14.0 | |
| 2010 | 11.3 | 11.2 | 9.3 | 16.0 | |
| Insurance at deliveryc | <.0001a | ||||
| Private | 58.6 | 57.2 | 90.1 | 95.3 | |
| Public | 40.6 | 42.0 | 9.1 | 3.5 | |
| Self-pay | 0.8 | 0.8 | 0.8 | 1.2 |
Denotes significant findings at P < .05.
Public is a composite of free care and public.
Prolonged stay is defined as >3 d for vaginal delivery or >5 d for cesarean delivery.
Bivariate Results
There were significant differences in health outcomes at birth for infants born to subfertile and ART-treated mothers compared with infants who were born to fertile mothers, with significantly higher prevalence of preterm birth, LBW, neonatal mortality, birth defects, and conditions of infectious disease and the cardiovascular, respiratory, gastrointestinal and/or nutritional, and hematologic systems. For infants born at ≥35 weeks’ GA, those born to subfertile and ART-treated mothers had a higher prevalence of prolonged hospital stay (Table 2).
TABLE 2.
Infant Outcomes by Maternal Fertility Group
| Infant Outcomes | Total (N = 350 123), % | Fertile (N = 336 705), % | Subfertile (N = 5043), % | ART (N = 8375), % | P |
|---|---|---|---|---|---|
| GA, wk | |||||
| ≤27 | 0.4 | 0.4 | 0.6 | 0.7 | <.0001a |
| 28–33 | 1.3 | 1.3 | 1.9 | 2.5 | |
| 34–36 | 4.9 | 4.8 | 6.2 | 7.5 | |
| 37–38 | 20.9 | 20.8 | 24.8 | 23.6 | |
| 39+ | 72.5 | 72.8 | 66.6 | 65.7 | |
| Birth wt, g | |||||
| ≤1000 | 0.4 | 0.4 | 0.6 | 0.7 | <.0001a |
| 1001–1500 | 0.5 | 0.5 | 0.6 | 0.9 | |
| 1501–2500 | 4.9 | 4.8 | 5.7 | 6.4 | |
| 2501+ | 94.2 | 94.3 | 93.1 | 92 | |
| Sex | |||||
| Male | 51.2 | 51.2 | 51.1 | 51.4 | .918 |
| Female | 48.8 | 48.8 | 48.9 | 48.6 | |
| SGA | |||||
| No | 91.4 | 91.4 | 92.2 | 91.7 | .1385 |
| Yes | 8.6 | 8.6 | 7.8 | 8.3 | |
| Preterm birth <37 wk | |||||
| No | 93.4 | 93.6 | 91.3 | 89.3 | <.0001a |
| Yes | 6.6 | 6.4 | 8.7 | 10.7 | |
| LBW <2500 g | |||||
| No | 94.2 | 94.3 | 93.1 | 92 | <.0001a |
| Yes | 5.8 | 5.7 | 6.9 | 8.0 | |
| Neonatal mortality | |||||
| No | 99.8 | 99.8 | 99.6 | 99.6 | .0027a |
| Yes | 0.2 | 0.2 | 0.4 | 0.4 | |
| Birth defects | |||||
| No | 98.3 | 98.4 | 97.8 | 97.9 | <.0001a |
| Yes | 1.7 | 1.6 | 2.2 | 2.1 | |
| Chromosomal birth defects | |||||
| No | 99.81 | 99.81 | 99.62 | 99.83 | .0112a |
| Yes | 0.19 | 0.19 | 0.38 | 0.17 | |
| Nonchromosomal birth defects | |||||
| No | 98.5 | 98.6 | 98.1 | 98.1 | .0003a |
| Yes | 1.5 | 1.4 | 1.9 | 1.9 | |
| Infectious disease conditions | |||||
| No | 98.8 | 98.8 | 98.6 | 98.3 | .0003a |
| Yes | 1.2 | 1.2 | 1.4 | 1.7 | |
| Cardiovascular conditions | |||||
| No | 98.3 | 98.3 | 97.6 | 97.6 | <.0001a |
| Yes | 1.7 | 1.7 | 2.4 | 2.4 | |
| Respiratory conditions | |||||
| No | 91.1 | 91.2 | 89 | 87.3 | <.0001a |
| Yes | 8.9 | 8.8 | 11 | 12.7 | |
| Gastrointestinal and/or nutritional conditions | |||||
| No | 97 | 97.1 | 95.7 | 94.9 | <.0001a |
| Yes | 3 | 2.9 | 4.3 | 5.1 | |
| Neurologic conditions | |||||
| No | 96.4 | 96.5 | 96.5 | 96.2 | .5381 |
| Yes | 3.6 | 3.5 | 3.5 | 3.8 | |
| Hematologic conditions | |||||
| No | 96.1 | 96.1 | 95.4 | 95.2 | <.0001a |
| Yes | 3.9 | 3.9 | 4.6 | 4.8 | |
| Prolonged hospital stay for kidsb | |||||
| No | 92.7 | 92.8 | 92 | 90.8 | <.0001a |
| Yes | 7.3 | 7.2 | 8 | 9.2 |
Denotes significant findings at P < .05.
Prolonged stay is defined as >3 d for vaginal delivery or >5 d for cesarean delivery; limited analysis to those whose GA is ≥35 wk with known data on mode of delivery and birth hospital records.
Multivariate Results
Table 3 show aORs and 95% CIs for the entire cohort and as stratified by GA categories. For each outcome, infants born to fertile or subfertile mothers serve as the reference.
TABLE 3.
Infant Outcomes by Maternal Fertility Group and GA Category
| Outcome | Fertility Group | GA Categories | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | ≤27 wk | 28–33 wk | 34–36 wk | 37–38 wk | 39+ wk | ||||||||
| aOR | 95% CI | aOR | 95% CI | aOR | 95% CI | aOR | 95% CI | aOR | 95% CI | aOR | 95% CI | ||
| SGA | Fertile | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Subfertile | 1.00 | 0.9–1.11 | — | — | 0.86 | 0.33–2.2 | 1.29 | 0.85–1.96 | 1.03 | 0.83–1.29 | 0.98 | 0.87–1.11 | |
| ART | 0.99 | 0.91–1.07 | 0.97 | 0.24–3.91 | 0.39a | 0.16–0.92a | 0.64a | 0.44–0.95a | 0.89 | 0.74–1.06 | 1.07 | 0.97–1.18 | |
| Fertile versus subfertile | 1.00 | 0.9–1.11 | — | — | 1.17 | 0.45–3.00 | 0.78 | 0.51–1.19 | 0.97 | 0.78–1.20 | 1.02 | 0.9–1.16 | |
| Subfertile | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |
| ART | 0.98 | 0.87–1.12 | — | — | 0.45 | 0.13–1.55 | 0.50a | 0.29–0.87a | 0.86 | 0.65–1.13 | 1.09 | 0.93–1.27 | |
| Preterm birth <37 wkb | Fertile | 1.00 | Reference | — | — | — | — | — | — | — | — | — | — |
| Subfertile | 1.39a | 1.26–1.54a | |||||||||||
| ART | 1.72a | 1.60–1.85a | |||||||||||
| Subfertile | 1.00 | Reference | |||||||||||
| ART | 1.23a | 1.09–1.40a | |||||||||||
| LBW <2500 g | Fertile | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Subfertile | 1.09 | 0.94–1.28 | — | — | 0.75 | 0.35–1.64 | 1.11 | 0.86–1.44 | 1.10 | 0.87–1.39 | 1.08 | 0.73–1.60 | |
| ART | 0.97 | 0.86–1.09 | — | — | 0.93 | 0.49–1.78 | 1.00 | 0.83–1.20 | 0.93 | 0.77–1.12 | 0.95 | 0.69–1.32 | |
| Subfertile | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |
| ART | 0.88 | 0.73–1.07 | — | — | 1.24 | 0.47–3.25 | 0.90 | 0.66–1.22 | 0.84 | 0.63–1.13 | 0.88 | 0.54–1.45 | |
| Neonatal mortality | Fertile | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Subfertile | 1.23 | 0.67–2.25 | 1.61 | 0.57–4.56 | 1.05 | 0.24–4.53 | 2.14 | 0.5–9.16 | — | — | — | — | |
| ART | 0.98 | 0.61–1.57 | 1.01 | 0.45–2.27 | 0.61 | 0.18–2.05 | 1.97 | 0.58–6.70 | 1.38 | 0.39–4.92 | 0.46 | 0.06–3.44 | |
| Subfertile | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |
| ART | 0.80 | 0.38–1.67 | 0.63 | 0.18–2.26 | 0.58 | 0.09–3.64 | 0.92 | 0.15–5.59 | — | — | — | — | |
| Birth defects | Fertile | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Subfertile | 1.20 | 0.99–1.46 | 1.43 | 0.3–6.86 | 1.95 | 1.00–3.79 | 1.24 | 0.72–2.16 | 1.06 | 0.72–1.57 | 1.18 | 0.90–1.54 | |
| ART | 1.04 | 0.89–1.22 | 0.59 | 0.12–2.87 | 0.66 | 0.33–1.33 | 1.4 | 0.94–2.07 | 0.95 | 0.69–1.31 | 1.05 | 0.84–1.31 | |
| Subfertile | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |
| ART | 0.86 | 0.68–1.10 | 0.42 | 0.05–3.54 | 0.34a | 0.13–0.85a | 1.12 | 0.58–2.16 | 0.90 | 0.55–1.47 | 0.89 | 0.63–1.25 | |
| Chromosomal abnormalities | Fertile | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Subfertile | 1.21 | 0.79–1.85 | — | — | 2.76 | 0.89–8.52 | 1.09 | 0.39–3.04 | 0.53 | 0.2–1.44 | 1.60 | 0.87–2.96 | |
| ART | 0.62a | 0.41–0.96a | — | — | 0.25 | 0.03–1.93 | 1.41 | 0.71–2.82 | 0.40a | 0.18–0.92a | 0.47 | 0.21–1.08 | |
| Subfertile | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |
| ART | 0.52a | 0.29–0.92a | — | — | 0.09a | 0.01–0.87a | 1.29 | 0.4–4.19 | 0.76 | 0.21–2.7 | 0.30a | 0.11–0.8a | |
| Nonchromosomal abnormalities | Fertile | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Subfertile | 1.21 | 0.97–1.5 | 1.45 | 0.3–7.1 | 1.68 | 0.75–3.77 | 1.31 | 0.68–2.5 | 1.28 | 0.84–1.96 | 1.11 | 0.82–1.5 | |
| ART | 1.17 | 0.99–1.39 | 0.68 | 0.14–3.3 | 0.84 | 0.4–1.78 | 1.36 | 0.85–2.19 | 1.23 | 0.87–1.73 | 1.16 | 0.92–1.46 | |
| Subfertile | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |
| ART | 0.97 | 0.74–1.27 | 0.47 | 0.06–4.04 | 0.50 | 0.17–1.44 | 1.04 | 0.48–2.26 | 0.96 | 0.57–1.62 | 1.04 | 0.72–1.51 | |
| Infectious disease conditions | Fertile | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Subfertile | 1.07 | 0.83–1.39 | 1.59 | 0.69–3.68 | 0.77 | 0.41–1.45 | 1.14 | 0.61–2.12 | 1.16 | 0.59–2.27 | 1.19 | 0.80–1.78 | |
| ART | 1.21 | 1.00–1.45 | 1.15 | 0.62–2.14 | 1.59a | 1.12–2.28a | 0.68 | 0.39–1.19 | 1.22 | 0.72–2.08 | 1.02 | 0.74–1.42 | |
| Subfertile | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |
| ART | 1.12 | 0.82–1.53 | 0.72 | 0.27–1.97 | 2.07a | 1.03–4.14a | 0.60 | 0.27–1.35 | 1.05 | 0.46–2.42 | 0.86 | 0.52–1.42 | |
| Cardiovascular conditions | Fertile | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Subfertile | 1.19 | 0.98–1.45 | 0.72 | 0.3–1.74 | 1.18 | 0.70–1.99 | 1.30 | 0.78–2.17 | 1.50a | 1.02–2.21a | 1.18 | 0.86–1.61 | |
| ART | 1.07 | 0.92–1.25 | 1.16 | 0.62–2.17 | 0.89 | 0.61–1.3 | 1.47a | 1.03–2.10a | 0.82 | 0.55–1.23 | 1.02 | 0.78–1.33 | |
| Subfertile | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |
| ART | 0.90 | 0.70–1.15 | 1.62 | 0.57–4.61 | 0.75 | 0.41–1.40 | 1.13 | 0.62–2.05 | 0.55a | 0.32–0.94a | 0.87 | 0.58–1.29 | |
| Respiratory conditions | Fertile | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Subfertile | 1.12a | 1.02–1.24a | 2.22 | 0.59–8.37 | 1.85 | 0.93–3.70 | 1.28 | 0.99–1.63 | 1.13 | 0.93–1.38 | 1.07 | 0.93–1.24 | |
| ART | 1.18a | 1.09–1.27a | 0.99 | 0.35–2.79 | 2.61a | 1.49–4.59a | 1.43a | 1.20–1.71a | 1.01 | 0.86–1.19 | 1.12 | 1.01–1.25 | |
| Subfertile | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |
| ART | 1.05 | 0.93–1.18 | 0.45 | 0.09–2.33 | 1.41 | 0.59–3.36 | 1.12 | 0.83–1.51 | 0.89 | 0.70–1.15 | 1.04 | 0.88–1.24 | |
| Gastrointestinal and/or nutritional conditions | Fertile | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Subfertile | 1.18a | 1.01–1.38a | 1.06 | 0.43–2.66 | 1.33 | 0.87–2.03 | 0.98 | 0.71–1.37 | 1.36 | 0.98–1.90 | 1.24 | 0.96–1.60 | |
| ART | 1.15a | 1.03–1.29a | 1.46 | 0.78–2.74 | 1.23 | 0.91–1.65 | 0.96 | 0.76–1.21 | 1.05 | 0.79–1.40 | 1.09 | 0.89–1.34 | |
| Subfertile | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |
| ART | 0.97 | 0.81–1.17 | 1.38 | 0.47–4.02 | 0.92 | 0.56–1.52 | 0.98 | 0.66–1.45 | 0.77 | 0.51–1.18 | 0.88 | 0.64–1.22 | |
| Neurologic conditions | Fertile | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Subfertile | 1.04 | 0.89–1.21 | 0.58 | 0.22–1.56 | 1.26 | 0.71–2.23 | 0.60 | 0.30–1.23 | 1.08 | 0.77–1.51 | 1.12 | 0.93–1.36 | |
| ART | 0.96 | 0.85–1.08 | 0.73 | 0.38–1.41 | 0.85 | 0.55–1.32 | 0.68 | 0.42–1.08 | 0.96 | 0.73–1.26 | 1.04 | 0.89–1.21 | |
| Subfertile | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |
| ART | 0.92 | 0.76–1.12 | 1.26 | 0.40–3.98 | 0.68 | 0.34–1.36 | 1.12 | 0.48–2.58 | 0.89 | 0.58–1.36 | 0.92 | 0.73–1.17 | |
| Hematologic conditions | Fertile | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Subfertile | 1.10 | 0.95–1.27 | 1.06 | 0.39–2.88 | 1.39 | 0.89–2.18 | 1.07 | 0.70–1.63 | 1.17 | 0.87–1.56 | 1.11 | 0.90–1.36 | |
| ART | 1.04 | 0.93–1.16 | 0.72 | 0.35–1.46 | 1.32 | 0.96–1.82 | 0.85 | 0.62–1.18 | 1.00 | 0.78–1.28 | 1.04 | 0.88–1.23 | |
| Subfertile | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |
| ART | 0.94 | 0.79–1.12 | 0.68 | 0.21–2.23 | 0.95 | 0.56–1.61 | 0.80 | 0.48–1.35 | 0.86 | 0.59–1.25 | 0.94 | 0.73–1.22 | |
| Prolonged hospital stayc | Fertile | 1.00 | Reference | — | — | — | — | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Subfertile | 1.06 | 0.94–1.18 | — | — | — | — | 0.99 | 0.75–1.31 | 1.06 | 0.86–1.30 | 1.09 | 0.93–1.28 | |
| ART | 1.04 | 0.95–1.13 | — | — | — | — | 1.04 | 0.86–1.27 | 0.90 | 0.76–1.07 | 1.09 | 0.96–1.23 | |
| Subfertile | 1.00 | Reference | — | — | — | — | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |
| ART | 0.98 | 0.85–1.13 | — | — | — | — | 1.06 | 0.75–1.48 | 0.85 | 0.65–1.10 | 0.99 | 0.82–1.21 | |
All models are adjusted for maternal age, race, education, payer (private, public, or self-pay), preexisting diabetes, preexisting hypertension, pregnancy-induced hypertension, gestational diabetes, parity, sex, birth year, and GA (continuous variable). —, not applicable.
Findings are significant.
Preterm is adjusted for maternal age, race, education, payer (private, public, self-pay), preexisting diabetes, preexisting hypertension, pregnancy-induced hypertension, gestational diabetes, parity, sex, and birth year.
Analysis limited to those whose GA is ≥35 wk with known data on mode of delivery and birth hospital records. Prolonged stay is defined as >3 d for vaginal delivery or >5 d for cesarean delivery for the mother.
SGA
For infants who were 28 to 33 weeks’ GA and 34 to 36 weeks’ GA, those who were conceived by ART had lower odds of being SGA compared with infants of fertile or subfertile mothers.
Preterm Birth
Compared with infants of fertile mothers, those who were born to subfertile and ART-treated mothers had higher odds of preterm birth; those who were born to ART-treated mothers had higher odds than those of subfertile mothers.
Birth Defects
For infants 28 to 33 weeks’ GA, those born to ART-treated mothers had decreased odds of birth defects compared with subfertile mothers. When stratified by chromosomal versus nonchromosomal birth defects, infants born to ART-treated mothers had lower odds of chromosomal abnormalities compared with those of fertile and subfertile mothers. For infants born at 28 to 33 weeks’ GA and ≥39 weeks’ GA, the likelihood of chromosomal birth defects was lower among ART-treated mothers compared with subfertile mothers. For infants born at 37 to 38 weeks’ GA, compared with infants who were born to fertile mothers, infants who were born to ART-treated mothers had lower odds of chromosomal birth defects. There were no differences in the likelihood of nonchromosomal birth defects.
Infectious Disease Conditions
For infants 28 to 33 weeks’ GA, those conceived by ART had higher odds of infectious disease conditions compared with infants of fertile and subfertile mothers.
Cardiovascular Conditions
For infants 34 to 36 weeks’ GA, those born to ART-treated mothers had higher odds of cardiovascular conditions compared with infants born to fertile mothers. For infants 37 to 38 weeks’ GA, those born to subfertile mothers had higher odds of cardiovascular conditions compared with those of fertile mothers. Infants born to ART-treated mothers had lower odds of cardiovascular conditions when compared with those born to subfertile mothers.
Respiratory Conditions
Compared with infants of fertile mothers, those born to subfertile and ART-treated mothers had higher odds of respiratory conditions. When stratified by GA, for infants 28 to 33 weeks’ GA and 34 to 36 weeks’ GA, those born to ART-treated mothers had higher odds of respiratory conditions compared with infants born to fertile mothers.
Gastrointestinal and/or Nutritional Conditions
Compared with infants born to fertile mothers, infants born to ART-treated and subfertile mothers had higher odds of gastrointestinal and/or nutritional conditions.
For the outcomes of LBW, neonatal mortality, neurologic conditions, hematologic conditions, and prolonged hospital stay, there was no difference among infants of the 3 fertility groups.
Autologous and Donor Cycles
We performed a sensitivity analysis to assess whether our effect estimates would differ when the ART-treated group was restricted to the use of autologous oocytes. Most of the significant differences remained. Exceptions included that the difference seen in infants who were SGA at 34 to 36 weeks’ GA and the odds of infectious disease conditions among infants 28 to 33 weeks’ GA of ART-treated mothers were no longer significant (data not shown).
Discussion
In this population-based analysis of health outcomes of infants born to fertile, subfertile, and ART-treated mothers, we found that after adjusting for key maternal and infant characteristics, the risk for being SGA, preterm birth, congenital malformations, and conditions of infectious disease and the cardiovascular, respiratory, and gastrointestinal systems varied across GA categories, although there were no differences in the risk for LBW, neonatal mortality, and conditions of the neurologic and hematologic systems.
Although it is clearly accepted that multiple gestation is a significant predictor of preterm birth and LBW, recent studies have also revealed that even among singleton births, mothers with infertility without ART treatment along with those who do undergo ART treatment are at higher risk for preterm delivery. Our results confirm the findings of these previous studies.10,14,19–21 For instance, Luke et al7 reported that among singleton deliveries, the preterm birth rate was 11.5%, which was higher than the overall preterm birth rate among singletons of 6.2% in Massachusetts during this time period (Massachusetts Department of Public Health, personal communication, 2017). In another analysis of MOSART data, Stern et al12 found that even after adjusting for plurality, the risk for preterm birth and LBW was increased for women with infertility-related diagnoses in both ART-treated and non–ART-treated women with the exception of ART-treated mothers with endometriosis.12 Additionally, Sunkara et al11 found that among singleton live births, there was a significantly higher risk of preterm birth and LBW among women with >20 oocytes after ovarian stimulation compared with women with 10 to 15 oocytes in their observational study of in vitro fertilization (IVF) cycles performed in the United Kingdom. Interestingly, in a retrospective analysis in which they compared birth outcomes of women who underwent stimulated or unstimulated IVF in a single center, Mak et al9 reported no difference in the risk of preterm birth after adjusting for potential confounders. Nevertheless, the precise biological mechanism for this disruption in term gestation remains unknown and requires continued investigation.
Among infants born at several GAs, we found that those born to ART-treated mothers were at lower risk for chromosomal abnormalities compared with infants born to subfertile mothers. There were no differences in noncongenital abnormalities. We have previously shown that infants of subfertile and ART-treated deliveries have an overall higher rate of birth defects than infants of fertile deliveries; however, much of this difference in our previous study was associated with multiple births.16 Although previous literature has also revealed that maternal subfertility conditions, as well as ART treatment practices, are associated with congenital malformations,22–25 the effect on a specific GA category has not been previously demonstrated, to our knowledge.
When we limited our analysis to infants of ≥35 weeks’ GA, we found that when maternal length of stay was not included in the model, only infants of ≥39 weeks’ GA born to ART-treated women were at higher risk of prolonged hospitalization compared with infants born to fertile mothers. With maternal length of stay added to the models, there was no difference across fertility groups, highlighting that ART-treated mothers required longer birth hospitalizations, possibly related to their underlying subfertility diagnoses and potential delivery complications that then increased the length of stay for their infants. In one of the few studies that assessed hospital length of stay among infants of fertile and ART-treated mothers, Ericson et al26 analyzed both singletons and multiples from birth to 14 days of life, demonstrating that in the postnatal period, length of hospitalization was longer for infants born to ART-treated women. When limited to term infants, the risk for longer length of stay was attenuated but remained significant. However, maternal length of stay after delivery was not included in their analyses, which we have demonstrated is a significant factor that is associated with the infant’s length of hospitalization.
The variation by fertility status and across gestational categories in risk for infectious disease and cardiovascular, respiratory, and gastrointestinal and/or nutritional conditions has not been previously reported, to our knowledge. It is possible that the multiple comparisons made in our analyses may result in false-positive associations. However, the robust estimates in the risk for certain conditions (infectious disease and respiratory complications) and the lack of significant associations in other systems (neurologic and hematologic conditions) reveal a developmental component to the risk for certain organ system conditions in fetal life. Further investigation is needed to better understand the biological disruptions that may occur in fetal organ development among subfertile and ART-treated mothers and to assess potential effects of underlying maternal conditions on these outcomes.
Researchers in a recent meta-analysis assessed whether maternal and infant outcomes varied by ART treatment with oocyte donation, conventional IVF and intracytoplasmic sperm injection, or spontaneous conception.27 In the pooled results, when compared with conventional IVF and intracytoplasmic sperm injection, infants conceived with oocyte donation were more likely to be preterm and of LBW. The results revealed only minor differences from our analysis containing combined autologous and donor cycles.
There are several limitations to our study. First, given that the MOSART database is composed of vital statistics and hospital-level administrative discharge codes, some parameters were not available for study, namely, those related to specific MAR techniques used for treated mothers and for the prepared sperm in the subfertile group, which may influence neonatal health outcomes and initial zygosity. For instance, as demonstrated in animal models, ovulation induction led to loss of imprinted DNA methylation in mouse blastocysts to the same extent as with the use of in vitro follicle culture.28 For human sperm, previous researchers have demonstrated decreased methylation after swim-up preparation.29 Second, BMI, which was shown to be significantly associated with a number of adverse neonatal outcomes, was not available.30–32 Moreover, data related to pregnancy course, such as fetal growth and uteroplacental Doppler ultrasound data, were not available. Third, data on paternal infertility were unavailable for linkage. Finally, given that the cohort included only births to Massachusetts resident mothers, findings may not be generalizable to a broader population.
Conclusions
Our study revealed that infants born as singletons to primiparous, subfertile, and ART-treated mothers are at greater risk for being born preterm and having chromosomal abnormalities and conditions of infectious disease and in the cardiovascular, respiratory, and gastrointestinal and/or nutritional systems, but we saw no difference in the risk for LBW, neonatal mortality, neurologic and hematologic abnormality, and length of birth hospitalization. Our population-based study of neonatal health outcomes is one of the first to reveal the variability in risk of adverse health outcomes beyond preterm birth and LBW but also, more specifically, by organ system conditions across several GA categories. With this approach, we offer more detailed associations between maternal fertility and the receipt of treatment along the continuum of fetal organ development and subsequent infant health conditions. Future studies will be needed to better understand the biological mechanisms that underlie these findings. Moreover, longer-term follow-up of infants conceived through MAR and ART will be needed to understand their health outcomes into later childhood and adulthood.
Acknowledgments
The SART thanks all of its members for providing clinical information to the SART CORS database for use by patients and researchers. Without the efforts of our members, this research would not have been possible.
Glossary
- aOR
adjusted odds ratio
- ART
assisted reproductive technology
- CI
confidence interval
- GA
gestational age
- ICD-9
International Classification of Diseases, Ninth Revision
- IVF
in vitro fertilization
- LBW
low birth weight
- MAR
medically assisted reproduction
- MBDMP
Massachusetts Birth Defects Monitoring Program
- MOSART
Massachusetts Outcome Study of Assisted Reproductive Technology
- PELL
Pregnancy to Early Life Longitudinal Data System
- SART
Society for Assisted Reproductive Technology
- SART CORS
Society for Assisted Reproductive Technology Clinic Outcome Reporting System
- SGA
small for gestational age
Footnotes
Dr Hwang conceptualized and designed the study, analyzed and interpreted data, and drafted the initial manuscript; Drs Dukhovny, Missmer, Diop, DeClercq, and Stern conceptualized and designed the study, analyzed and interpreted data, and critically reviewed the manuscript; Ms Gopal and Dr Cabral conducted the analysis, interpreted the data, and critically reviewed the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by the National Institutes of Health grant R01HD067270. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Centers for Disease Control and Prevention What is assisted reproductive technology? Available at: https://www.cdc.gov/art/whatis.html. Accessed May 1, 2017
- 2.Zegers-Hochschild F, Adamson GD, Dyer S, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108(3):393–406 [DOI] [PubMed] [Google Scholar]
- 3.Luke B. Pregnancy and birth outcomes in couples with infertility with and without assisted reproductive technology: with an emphasis on US population-based studies. Am J Obstet Gynecol. 2017;217(3):270–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sunderam S, Kissin DM, Crawford SB, et al. Assisted reproductive technology surveillance - United States, 2014. MMWR Surveill Summ. 2017;66(6):1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toner JP, Coddington CC, Doody K, et al. Society for Assisted Reproductive Technology and assisted reproductive technology in the United States: a 2016 update. Fertil Steril. 2016;106(3):541–546 [DOI] [PubMed] [Google Scholar]
- 6.Chandra A, Copen CE, Stephen EH. Infertility service use in the United States: data from the National Survey of Family Growth, 1982-2010. Natl Health Stat Rep. 2014;(73):1–21 [PubMed] [Google Scholar]
- 7.Luke B, Stern JE, Kotelchuck M, Declercq ER, Anderka M, Diop H. Birth outcomes by infertility treatment: analyses of the population-based cohort: Massachusetts Outcomes Study of Assisted Reproductive Technologies (MOSART). J Reprod Med. 2016;61(3–4):114–127 [PMC free article] [PubMed] [Google Scholar]
- 8.Luke B, Stern JE, Kotelchuck M, et al. Adverse pregnancy outcomes after in vitro fertilization: effect of number of embryos transferred and plurality at conception. Fertil Steril. 2015;104(1):79–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mak W, Kondapalli LA, Celia G, Gordon J, DiMattina M, Payson M. Natural cycle IVF reduces the risk of low birthweight infants compared with conventional stimulated IVF. Hum Reprod. 2016;31(4):789–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald SD, Han Z, Mulla S, Murphy KE, Beyene J, Ohlsson A; Knowledge Synthesis Group . Preterm birth and low birth weight among in vitro fertilization singletons: a systematic review and meta-analyses. Eur J Obstet Gynecol Reprod Biol. 2009;146(2):138–148 [DOI] [PubMed] [Google Scholar]
- 11.Sunkara SK, La Marca A, Seed PT, Khalaf Y. Increased risk of preterm birth and low birthweight with very high number of oocytes following IVF: an analysis of 65 868 singleton live birth outcomes. Hum Reprod. 2015;30(6):1473–1480 [DOI] [PubMed] [Google Scholar]
- 12.Stern JE, Luke B, Tobias M, Gopal D, Hornstein MD, Diop H. Adverse pregnancy and birth outcomes associated with underlying diagnosis with and without assisted reproductive technology treatment. Fertil Steril. 2015;103(6):1438–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luke B, Gopal D, Cabral H, Stern JE, Diop H. Adverse pregnancy, birth, and infant outcomes in twins: effects of maternal fertility status and infant gender combinations; the Massachusetts Outcomes Study of Assisted Reproductive Technology. Am J Obstet Gynecol. 2017;217(3):330.e1–330.e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Declercq E, Luke B, Belanoff C, et al. Perinatal outcomes associated with assisted reproductive technology: the Massachusetts Outcomes Study of Assisted Reproductive Technologies (MOSART). Fertil Steril. 2015;103(4):888–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotelchuck M, Hoang L, Stern JE, Diop H, Belanoff C, Declercq E. The MOSART database: linking the SART CORS clinical database to the population-based Massachusetts PELL reproductive public health data system. Matern Child Health J. 2014;18(9):2167–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liberman RF, Getz KD, Heinke D, et al. Assisted reproductive technology and birth defects: effects of subfertility and multiple births. Birth Defects Res. 2017;109(14):1144–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stern JE, Gopal D, Liberman RF, Anderka M, Kotelchuck M, Luke B. Validation of birth outcomes from the Society for Assisted Reproductive Technology Clinic Outcome Reporting System (SART CORS): population-based analysis from the Massachusetts Outcome Study of Assisted Reproductive Technology (MOSART). Fertil Steril. 2016;106(3):717–722.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Declercq ER, Belanoff C, Diop H, et al. Identifying women with indicators of subfertility in a statewide population database: operationalizing the missing link in assisted reproductive technology research. Fertil Steril. 2014;101(2):463–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinborg A, Wennerholm UB, Romundstad LB, et al. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum Reprod Update. 2013;19(2):87–104 [DOI] [PubMed] [Google Scholar]
- 20.Wennerholm UB, Henningsen AK, Romundstad LB, et al. Perinatal outcomes of children born after frozen-thawed embryo transfer: a Nordic cohort study from the CoNARTaS group. Hum Reprod. 2013;28(9):2545–2553 [DOI] [PubMed] [Google Scholar]
- 21.Malchau SS, Loft A, Henningsen AK, Nyboe Andersen A, Pinborg A. Perinatal outcomes in 6,338 singletons born after intrauterine insemination in Denmark, 2007 to 2012: the influence of ovarian stimulation. Fertil Steril. 2014;102(4):1110–1116.e2 [DOI] [PubMed] [Google Scholar]
- 22.Hansen M, Kurinczuk JJ, Milne E, de Klerk N, Bower C. Assisted reproductive technology and birth defects: a systematic review and meta-analysis. Hum Reprod Update. 2013;19(4):330–353 [DOI] [PubMed] [Google Scholar]
- 23.Davies MJ, Rumbold AR, Marino JL, et al. Maternal factors and the risk of birth defects after IVF and ICSI: a whole of population cohort study. BJOG. 2017;124(10):1537–1544 [DOI] [PubMed] [Google Scholar]
- 24.Davies MJ, Moore VM, Willson KJ, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366(19):1803–1813 [DOI] [PubMed] [Google Scholar]
- 25.Hansen M, Kurinczuk JJ, Bower C, Webb S. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med. 2002;346(10):725–730 [DOI] [PubMed] [Google Scholar]
- 26.Ericson A, Nygren KG, Olausson PO, Källén B. Hospital care utilization of infants born after IVF. Hum Reprod. 2002;17(4):929–932 [DOI] [PubMed] [Google Scholar]
- 27.Storgaard M, Loft A, Bergh C, et al. Obstetric and neonatal complications in pregnancies conceived after oocyte donation: a systematic review and meta-analysis. BJOG. 2017;124(4):561–572 [DOI] [PubMed] [Google Scholar]
- 28.Saenz-de-Juano MD, Billooye K, Smitz J, Anckaert E. The loss of imprinted DNA methylation in mouse blastocysts is inflicted to a similar extent by in vitro follicle culture and ovulation induction. Mol Hum Reprod. 2016;22(6):427–441 [DOI] [PubMed] [Google Scholar]
- 29.Kim SK, Jee BC, Kim SH. Histone methylation and acetylation in ejaculated human sperm: effects of swim-up and smoking. Fertil Steril. 2015;103(6):1425–1431 [DOI] [PubMed] [Google Scholar]
- 30.Aune D, Saugstad OD, Henriksen T, Tonstad S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. JAMA. 2014;311(15):1536–1546 [DOI] [PubMed] [Google Scholar]
- 31.Marshall NE, Guild C, Cheng YW, Caughey AB, Halloran DR. Maternal superobesity and perinatal outcomes. Am J Obstet Gynecol. 2012;206(5):417.e1–417.e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott-Pillai R, Spence D, Cardwell CR, Hunter A, Holmes VA. The impact of body mass index on maternal and neonatal outcomes: a retrospective study in a UK obstetric population, 2004-2011. BJOG. 2013;120(8):932–939 [DOI] [PubMed] [Google Scholar]