Abstract
Oxidative stress serves a vital function in the pathogenesis of age-related macular degeneration (AMD); genipin (GP) possesses antioxidative properties. The present study aimed to investigate the effects of GP on retinal pigment epithelial (RPE) cells induced by H2O2 and the underlying mechanism. ARPE-19 cells were subjected to H2O2 treatment to induce oxidative damage. Cell viability was determined via an MTT assay. Reactive oxygen species (ROS) levels and cell apoptosis were detected by flow cytometry. Nuclear factor-erythroid 2-related factor-2 (Nrf2) signaling-associated and the expression of apoptosis-associated factors were measured using reverse transcription-quantitative polymerase chain reaction assay and western blotting. The results revealed that 200 µM H2O2 and 30 µM GP were determined to be the optimal concentrations for subsequent experimentation. GP reversed the inhibitory effects of H2O2 by promoting cell viability, attenuating ROS accumulation and cell apoptosis, and increased the expression of Nrf2, heme oxygenase-1 (HO-1) and NAD(P)H: Quinine oxidoreductase 1 (NQO1); Nrf2 silencing inhibited HO-1 and NQO1 expression. In addition, Nrf2 silencing enhanced the effects of H2O2 by promoting ROS production and cell apoptosis. Compared with H2O2, Nrf2 silencing further decreased the expression levels of B-cell lymphoma-2 (Bcl-2), but increased that of Bcl-2-associated X protein and cleaved-caspase-3. The results of the present study revealed that Nrf2 silencing attenuated the protective effects of GP on H2O2-induced injury in ARPE-19 cells by promoting apoptosis and oxidation. Collectively, GP attenuated oxidative damage induced by H2O2 in ARPE-19 cells. Furthermore, the molecular mechanism may be associated with the Nrf2 signaling pathway. The findings of the present study nay provide insight into a potential therapeutic agent for the treatment of AMD.
Keywords: age-related macular degeneration, oxidative stress, genipin, nuclear factor-erythroid 2-related factor-2 signaling
Introduction
Age-related macular degeneration (AMD) is an age-associated macular disease, and the most common disease of blindness in people >60 years old (1). Its main feature is the retinopathy of the retina and choroid, which causes decreased visual function and reduced central vision in particular (2). Oxidative stress serves a vital role in the pathogenesis of AMD (3). Retinal pigment epithelial (RPE) cells, as the most metabolically active type of cellin eye tissue, can engulf the outer disc of the retina photoreceptor cells and produce a large number of lipid peroxides and H2O2 (4). Furthermore, the photo-oxidation effect occurs when RPE is illuminated over long durations (5). Therefore, RPE has a higher susceptibility to oxidative stress. In addition, due to aging, the resistance of the antioxidant system of RRE declines (6,7). Oxidative stress and decreased antioxidant capacity may lead to functional disorders and structural abnormalities of the RPE, which have been identified as important pathological alterations associated with AMD (3,6,7).
Genipin (GP), is a glycosidic ligand derived from iridoid glycosides and is widely distributed in plants, including Mast and Eucommia ulmoides. GP is the main metabolite of geniposide in humans or animals, and is also the main active form with pharmacokinetic function (8). Studies have demonstrated that GP has certain properties, including anti-infection, anti-inflammation, antioxidation and antitumor (9-12). In addition, GP has been widely regarded as a specific inhibitor of uncoupling protein 2 (UCP2) (13). UCP2 is a functional protein in the mitochondrial inner membrane, which regulates the proton pump of mitochondria (14). Specifically, UCP2 is involved in modulating the opening of the ion channels on the mitochondrial membrane, inhibiting the production of reactive oxygen species (ROS), there by suppressing the apoptosis of cells and damage to mitochondria (15). However, the role of GP on RPE cell injury induced by oxidative stress is unknown.
Nuclear factor-erythroid 2-related factor-2 (Nrf2), as a transcription factor, serves a vital function in opposing cell damage due to endogenous and exogenous stresses (16). It is well known that Nrf2 is a main regulator of the antioxidant reaction, which can control the antioxidant response element-regulated the expression of phase II and antioxidant enzymes, including heme oxygenase-1 (HO-1) and NAD(P)H: Quinine oxidoreductase 1 (NQO1) (17,18). It has been reported that antioxidants protect oxidative stress-induced RPE cells by activating Nrf2 signaling (19).
The present study, aimed to establish an RPE cell oxidative stress injury model; H2O2 acts as an inducer of oxidative damage to RPE cells (20). In addition, the effects of GP on H2O2-induced RPE cells were determined and whether the underlying molecular mechanism is associated with Nrf2 signaling was investigated in the present study.
Materials and methods
Cell culture and transfection
Human RPE cell lines (ARPE-19 cells) were obtained from the American Type Culture Collection (Manassas, VA, USA), which were maintained in Dulbecco’s modified Eagle’s medium/Nutrient F12 Ham (DMEM/F12; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), which contained 10% fetal bovine serum (FBS; HyClone; GE Healthcare Life Sciences, Logan, UT, USA), 100 µg/ml streptomycin and 100 U/ml penicillin (Beijing Solarbio Science & Technology, Co., Ltd., Beijing, China) in a humidified incubator with 5% CO2 at 37°C (80G-2, Shanghai Huafu Instrument Co. Ltd.).
ARPE-19 cells were transfected with pSilencer 2.1 vector (1 µg, Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing small interfering (si)RNA negative control (NC; 5′-CAC ACT GGA TGG CCT AGG AGG ATA T-3′) or siRNA Nrf2 (siNrf2;5′-CAC ACT GGA TCA GAC AGG AGG ATA T-3′) vectors using Lipofectamine® 3000 (Thermo Fisher Scientific, Inc.). After transfection, cells were incubated for 24 h and then used for subsequent experimentation; untreated cells served as the control.
Methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay
An MTT kit (Beyotime Institute of Biotechnology, Shanghai, China) was employed to measure cell viability. ARPE-19 cells were seeded into 96-well plates (2×103 cell/well) for 24 h. Cells were exposed to various concentrations of H2O2 (0, 10, 50, 100, 200, 400 and 800 µM) and GP (0, 5, 10, 30, 50 and 100 µM) for 24 h at 37°C, respectively. Other cells were transfected with NC and siNrf2 vectors as aforementioned. Then, cells were incubated with MTT solution for 4 h at 37°C. Subsequently, the supernatant was removed; dimethyl sulfoxide was then added to the cells and the optical density at 490 nm was evaluated using a microplate reader (SpectraMax iD3, Molecular Devices, LLC, Sunnyvale, CA, USA); 200 µM H2O2 was finally used to treat cells in the subsequent experiments.
Flow cytometry
For the analysis of ROS, a ROS assay kit (Beyotime Institute of Biotechnology) was employed according to the manufacturer’s protocols. In brief, cells were seeded into 6-well plates (5×104 cell/well) for 24 h. Then, cells were treated with 200 µM H2O2, 30 µM GP, NC vector or siNrf2 vector for 24 h as aforementioned. Following treatment, cells were incubated with 2′,7′-dichlorofluorescein diacetate (Sigma-Aldrich; Merck KGaA) at 37°C for 30 min and then washed with PBS for three times. The levels of ROS were determined using a MoFlo flow cytometer (Beckman Coulter, Inc., Brea, CA, USA) and the data was analyzed using SUMMIT Software V4.3 (Dako; Agilent Technologies, Santa Clara, USA).
For the cell apoptosis assay, an Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit (Dalian Meilunbio Biotechnology Co., Ltd., Dalian, China) was employed according to the manufacturer’s protocols. Briefly, cells were seeded into 6-well plates (5×104 cell/well) for 24 h. Then, cells were treated with 200 µM H2O2, 30 µM GP, NC vector and siNrf2 vector for 24 h as aforementioned. Following treatment, cells were digested with 0.25% EDTA-trypsin (Beijing Solarbio Science & Technology, Co., Ltd.) at room temperature for 2 min and resuspended in DMEM/F12. The cells were centrifuged at 1,000 × g for 5 min at 4°C, and then incubated with Annexin V-FITC and PI in darkness for 25 min at room temperature. Cell apoptosis was analyzed with a MoFlo flow cytometer (Beckman Coulter, Inc.) and the data was analyzed using SUMMIT Software V4.3. The number of apoptotic cells was calculated by adding data in the second and the fourth quadrants of the flow cytometry data.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) assay
Total RNA was extracted from cells using Trizol reagent (Thermo Fisher Scientific, Inc.) according to the manufacturer’s protocols. RNA (1 µg) was used to synthesize cDNA by using the Quantscript RT Kit (Promega Corporation, Madison, WI, USA) under the following conditions: 25°C for 10 min, 42°C for 50 min, 85°C for 15 min and 4°C for 10 min. cDNA was amplified using a SYBR Premix Taq™ II kit (Takara Bio, Inc., Otsu, Japan) on an ABI 7500 thermocycler (Applied Biosystems; Thermo Fisher Scientific, Inc.). The reaction conditions were as follows: 95°C for 3 min, followed by 30 cycles of at 95°C for 15 sec and at 62°C for 30 sec, and extension for 60 sec at 72°C. The sequences of primers were presented in Table I. GAPDH was used as an internal control. The quantification of gene expression was performed using the 2−ΔΔCq method (21).
Table I.
Sequences of the primers employed for reverse transcription-quantitative polymerase chain reaction.
| Primer | Sequence (5′-3′) |
|---|---|
| HO-1-forward | CGTTCCTGCTCAACATCCAG |
| HO-1-reverse | TGAGTGTAAGGACCCATCGG |
| NQO1-forward | AGAAAGGATGGGAGGTGGTG |
| NQO1-reverse | ATATCACAAGGTCTGCGGCT |
| Bax-forward | AACATGGAGCTGCAGAGGAT |
| Bax-reverse | CCAATGTCCAGCCCATGATG |
| Bcl-2-forward | TTCTTTGAGTTCGGTGGGGT |
| Bcl-2-reverse | CTTCAGAGACAGCCAGGAGA |
| GAPDH-forward | CCATCTTCCAGGAGCGAGAT |
| GAPDH-reverse | TGCTGATGATCTTGAGGCTG |
Bax, Bcl-2-associated X; HO-1, heme oxygenase 1; NQO1, NAD(P)H: Quinine oxidoreductase 1.
Western blot analysis
Cells were lysed with radioimmunoprecipitation assay buffer (Beijing Solarbio Science & Technology, Co., Ltd.) to obtain protein extracts. A Bradford’s protein assay kit (Beyotime Institute of Biotechnology) was employed to detect the concentrations of protein extracts. Each protein (25 µg per lane) was separated by 10% SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Merck KGaA). The membrane was blocked using tris-buffered saline with TBS solution (0.05% Tween-20) containing 5% non-fat milk for 1 h at 4°C. The membrane was incubated with anti-HO-1 (AF3169, 1:600; R&D Systems, Inc., Minneapolis, MN, USA), anti-Nrf2 (MAB3925, 1:800; R&D Systems, Inc.), anti-NQO1 (AF7567, 1:1,200; R&D Systems, Inc.), anti-cleaved-caspase-3 (AF835, 1:1,000; R&D Systems, Inc.), anti-B-cell lymphoma-2 (Bcl-2; AF810, 1:800; R&D Systems, Inc.), anti-Bcl-2 associated X (Bax; AF820, 1:1,000; R&D Systems, Inc.) and anti-GAPDH (2275-PC-100, 1:600; R&D Systems, Inc.) at 4°C overnight. Then, the membrane was washed with TBST for three times. Following washing, the membrane was incubated with corresponding secondary antibodies [rabbit anti-goat IgG-horseradish peroxidase (HRP), sc-2768, 1:6,000; mouse anti-rabbit, IgG-HRP, sc-2357, 1:7,000; donkey anti-goat IgG-HRP, sc-2020, 1:8,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 37°C for 60 min. The membrane was washed with TBST for three times. Subsequently, the proteins were detected by using an enhanced chemiluminescence detection reagent (GE Healthcare, Chicago, IL, USA)and exposed under an E-Gel Imager (Invitrogen; Thermo, Fisher, Scientific, Inc.). The blot density was analyzed by Quantity One software version 4.6.2 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
The data were presented as the mean ± standard deviation using SPSS software (version 20; IBM, Corp., Armonk, NY, USA). The differences among groups were assessed by one-way analysis of variance followed by a Tukey’s post hoc test. All the experiment was independently conducted at least for three times. P<0.05 was considered to indicate a statistically significant difference.
Results
GP alleviates decreased viability of ARPE-19 cells induced by H2O2
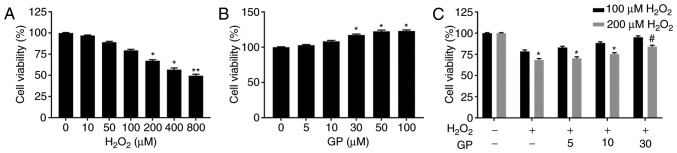
To investigate the effects of GP and H2O2 on the viability of ARPE-19 cells, an MTT assay was conducted to determine cell viability. The results revealed that cell viability was significantly suppressed in response to treatment with 200, 400 and 800 µM H2O2 compared with the control (Fig. 1A), while 30, 50 and 100 µM GP significantly promoted cell viability compared with the control; the increased cell viability at 30 µM GP was similar to that of 50 and 100 µM (Fig. 1B). Therefore, 100 and 200 µM H2O2 and 5, 10, 30 µM GP was selected to further detect the effects of GP on H2O2-treated cells. Upon treatment with H2O2 (10, 50 and 100 µM) or GP (5 and 10 µM), no notable alterations in cell viability were observed. Treatment with 200 µM H2O2 and GP (0, 5, 10 and 30 µM) exhibited significantly reduced cell viability compared with the untreated control (Fig. 1C). No significant difference was observed between the GP + 200 µM H2O2 and GP + 100 µM H2O2 groups. Thus, 30 µM GP was used to treat cells for subsequent experiments. The present study reported that cell viability was significantly increased in the 30 µM GP + 200 µM H2O2 group compared with the 200 µM H2O2 group (Fig. 1C). The results indicated that GP increased the viability of H2O2-treated cells.
Figure 1.
GP alleviates decreased viability of ARPE-19 cells induced by H2O2. (A-C) ARPE-19 cells were exposed to various concentrations of H2O2 (0, 10, 50, 100, 200, 400+ and 800 µM) and GP (0, 5, 10, 30, 50 and 100 µM). Cell viability was assessed by an MTT assay. *P<0.05, **P<0.01, vs. 0 µM group. #P<0.05, vs. 200 µM H2O2 group. n=5. GP, genipin.
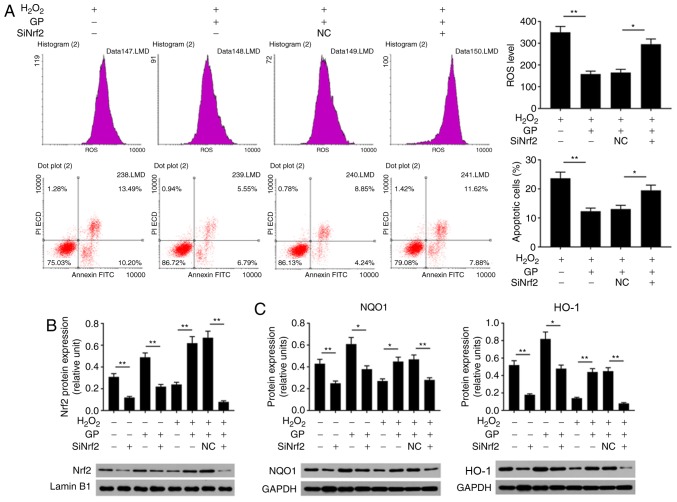
GP suppresses the effects of H2O2 on the levels of ROS and apoptosis of ARPE-19 cells by activating Nrf2 signaling
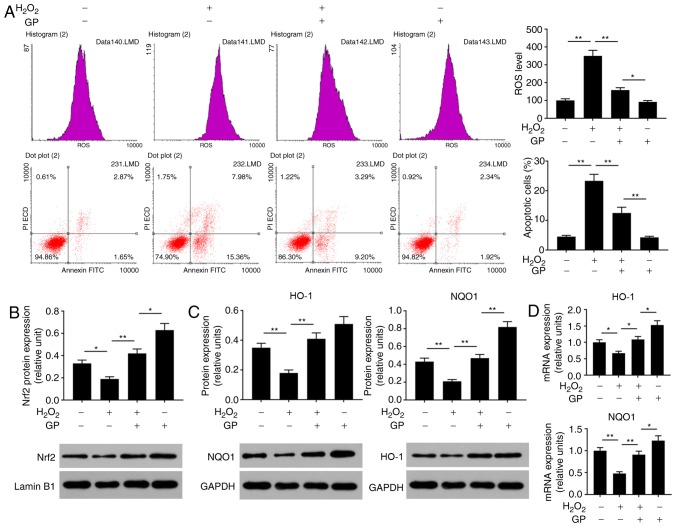
Oxidative stress is an important cause of injury to RPE cells (22). To analyze the effects of GP on H2O2-induced ARPE-19 cell injury, the ROS levels, cell apoptosis and Nrf2 signaling were detected. The data of flow cytometry revealed that ROS levels and the number of apoptotic cells were significantly enhanced in cells treated with H2O2 compared with in untreated and H2O2-induced cells treated with GP (Fig. 2A). In addition, H2O2 significantly reduced the protein expression levels of Nrf2, HO-1 and NQO1, and the mRNA expression levels of HO-1 and NQO1 compared with the control (Fig. 2B-D). On the contrary, treatment with GP significantly increased the expression of Nrf2, HO-1 and NQO1 in H2O2-induced cells compared with H2O2 treatment alone (Fig. 2B-D). The results suggested that GP suppressed H2O2-induced RPE cell injuries.
Figure 2.
GP suppresses the effects of H2O2 on the levels of ROS and apoptosis of ARPE-19 cells by activating Nrf2 signaling. (A) ARPE-19 cells were exposed to 200 µM H2O2 and 30 µM GP. The ROS levels and apoptosis were assessed by flow cytometry. (B and C) Protein expression levels of Nrf2, HO-1 and NQO1 were evaluated by western blotting. (D) mRNA expression levels of HO-1 and NQO1 were detected by reverse transcription-quantitative polymerase chain reaction. *P<0.05, **P<0.01. n=4. FITC, fluorescein isothiocyanate; GP, genipin; HO-1, heme oxygenase-1; NQO1, NAD(P)H: Quinine oxidoreductase; Nrf2, nuclear factor-erythroid 2-related factor-2; PI, propidium iodide; ROS, reactive oxygen species.
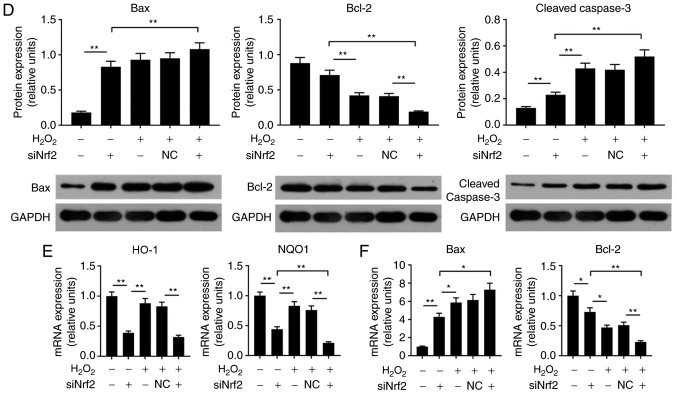
Nrf2 silencing enhances H2O2-induced damage to ARPE-19 cells
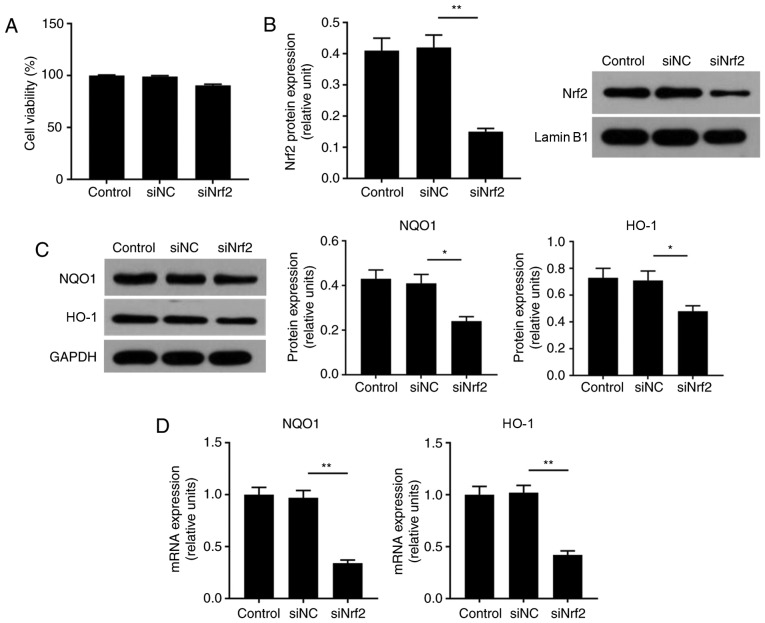
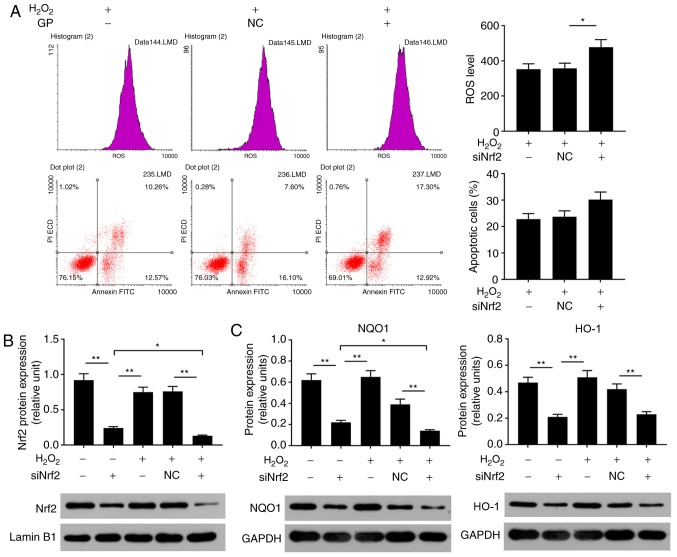
In the present study, to determine the effects of Nrf2 on ARPE cells, cell viability and the expression levels of Nrf2, HO-1 and NQO1 were respectively analyzed by an MTT assay, RT-qPCR and western blotting. When cells were transfected with siRNA Nrf2 vector, no notable alterations in cell viability were observed (Fig. 3A). As the demonstrated by western blotting, the expression levels of NQO1, HO-1 and Nrf2 proteins were significantly suppressed in the siNrf2 group compared with in the NC group (Fig. 3B and C). In addition, the mRNA expression profiles of NQO1 and HO-1 were similar to the protein expression profile in each group (Fig. 3D). In order to further investigate the effects of siNrf2 on ARPE cells induced by H2O2, the ROS levels, cell apoptosis and Nrf2 signaling were analyzed. The present study reported that siNrf2 significantly and markedly enhanced the ROS levels and apoptosis in ARPE-19 cells induced by H2O2, respectively, compared with the H2O2 + NC group (Fig. 4A). The expression levels of apoptosis-associated factors were evaluated by RT-qPCR and western blotting. SiNrf2 significantly downregulated the expression levels of Nrf2, NQO1 and HO-1 in cells treated with H2O2, compared with the H2O2 + NC group (Fig. 4B, C and E). The results also revealed that, compared with the H2O2 and H2O2 + NC groups, siNfr2-transfected cells treated with H2O2 exhibited increased Bax and cleaved-caspase-3 expression levels; the protein expression levels of Bcl-2 were significantly decreased in the H2O2 + siNrf2 group compared with the H2O2 and H2O2 + NC groups (Fig. 4D and F). These results suggested that Nrf2 knockdown enhanced H2O2-induced RPE cell injury.
Figure 3.
Nrf2 downregulation inhibits the expression of downstream target genes NQO1 and HO-1 in ARPE-19 cells. (A) ARPE-19 cells were transfected with siNC and siNrf2 vectors using Lipofectamine® 3000. Cell viability was analyzed by an MTT assay. (B and C) Western blotting was applied to detect the expression of Nrf2, HO-1 and NQO1 proteins. (D) Reverse transcription-quantitative polymerase chain reaction was performed to detect the expression of HO-1 and NQO1 mRNA. *P<0.05, **P<0.01. n=4. HO-1, heme oxygenase-1; NQO1, NAD(P)H: Quinine oxidoreductase; Nrf2, nuclear factor-erythroid 2-related factor-2; siRNA, small interfering RNA; siNC, siRNA negative control; siNrf2, siRNA Nrf2.
Figure 4.
Nrf2 silencing enhances H2O2-induced damage to ARPE-19 cells. (A) ARPE-19 cells were subjected to treatment with 200 µM H2O2, NC vector and siNrf2 vector. Flow cytometry was performed to analyze the levels of ROS and the number of apoptotic cells. *P<0.05 vs. H2O2 + NC group. (B) Proteins expression levels of Nrf2, were determined using western blotting. (C) The protein expression levels of NQO1 and HO-1 were detected by reverse transcription-quantitative polymerase chain reaction. (D) The protein expression levels of cleaved-caspase 3, Bax and Bcl-2 were detected by western blotting. (E) The mRNA expression levels of HO-1 and NQO1 were detected by reverse transcription-quantitative polymerase chain reaction. (F) The mRNA expression levels of Bax and Bcl-2 were detected by reverse transcription-quantitative polymerase chain reaction. *P<0.05, **P<0.01. n=4. Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X; FITC, fluorescein isothiocyanate; HO-1, heme oxygenase-1; NQO1, NAD(P)H: Quinine oxidoreductase; Nrf2, nuclear factor-erythroid 2-related factor-2; PI, propidium iodide; siRNA, small interfering RNA; NC, siRNA negative control; siNrf2, siRNA Nrf2.
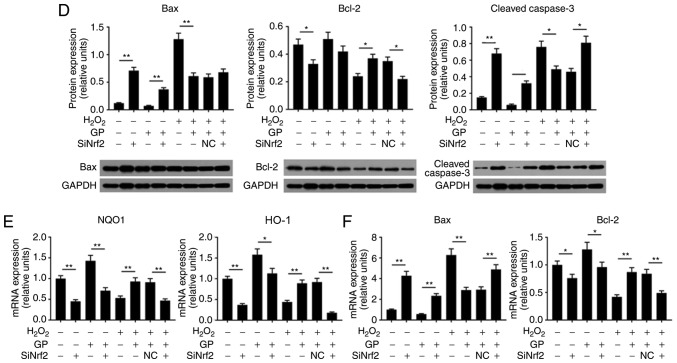
Nrf2 silencing attenuates the protective effects of GP on H2O2-induced ARPE-19 cells
In the present study, the role of Nrf2 in GP-induced-effects on H2O2-treated cells was investigated. Flow cytometry revealed that, compared with the H2O2 + GP + NC group, the ROS levels and number of apoptotic cells were significantly increased in the H2O2 + GP + siNrf2 group; GP significantly decreased ROS levels and apoptosis in H2O2-induced cells compared with H2O2 treatment alone (Fig. 5A). When cells were exposed to GP and siNrf2 vector, the protein expression levels of Nrf2, NQO1, HO-1, and Bcl-2 were significantly downregulated, while that of Bax and cleaved-caspase-3 were significantly upregulated, compared with the GP group. Additionally, compared with the H2O2 + GP + NC group, the expression of Nrf2, NQO1, HO-1, and Bcl-2 were inhibited in the H2O2 + GP + siNrf2 group; the protein expression levels of Bax and cleaved-caspase-3 were notably and significantly increased, respectively (Fig. 5B-D). The mRNA expression profile of Nrf2, NQO1, HO-1, Bcl-2 and Bax was similar to their respective trend in protein levels (Fig. 5E and F). The results of the present study suggested that the activation of Nrf2 signaling was associated with the protective effects of GP on H2O2-treated RPE cells.
Figure 5.
Nrf2 silencing attenuates the effects of GP on H2O2-induced ARPE-19 cells. (A) ARPE-19 cells were subjected to treatment with 200 µM H2O2, 30 µM GP, NC vector and siNrf2 vector. Flow cytometry was applied to analyze the levels of ROS and the number of apoptotic cells. *P<0.05, **P<0.01. (B-D) Proteins expression levels of Nrf2, HO-1, NQO1, Bax, Bcl-2 and cleaved-caspase 3 were determined by western blotting. (E and F) mRNA expression levels of HO-1, NQO1, Bax and Bcl-2 were detected by reverse transcription-quantitative polymerase chain reaction. *P<0.05, **P<0.01. n=4. Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X; FITC, fluorescein isothiocyanate; HO-1, heme oxygenase-1; NQO1, NAD(P)H: Quinine oxidoreductase; Nrf2, nuclear factor-erythroid 2-related factor-2; PI, propidium iodide; siNrf2, small interfering RNA Nrf2.
Discussion
Oxidative stress is one of the most important mechanisms underlying the pathogenesis of AMD (3). Oxidative damage of RPE is a key process in the pathogenesis of AMD (4). H2O2 can induce the production of ROS in cells, leading to oxidative damage (23). Exogenous H2O2 treatment is a simple and feasible cell model for studying RPE oxidative damage, which can effectively simulate the process of oxidative damage of RPE in AMD (24,25). Therefore, in the present study, H2O2 was selected as an inducer of oxidative damage to ARPE-19 cells. Additionally, 200 µM H2O2 was identified as the optimal concentration to generate a cellular oxidative damage model.
GP has been demonstrated to prevent or treat cardiovascular diseases, diabetes, nervous system diseases, pathogenic infections and inflammation (26-30). Lin et al (28) reported that GP protected renal tissue from oxidative stress-associated injury by suppressing oxidative stress and apoptosis. Shin and Lee (29) proposed that GP improved age-associated insulin resistance by reducing oxidative stress in LO2 cells; however, the role of GP in AMD remains unclear. In the present study, it was suggested that GP exerted a protective effect on H2O2-induced oxidative damage of ARPE-19 cells. The results of the present study revealed that GP (30, 50 and 100 µM) significantly increased the viability of ARPE-19 cells, indicating that the treatment of GP may induce some survival signals in ARPE-19 cells; the increased cell viability in response to 30 µM GP was similar to that of 50 and 100 µM. Therefore, low, moderate and high concentrations of GP (5, 10 and 30 µM) were selected to analyze cell viability inhibited by H2O2. The data demonstrated that 30 µM GP significantly enhanced cell viability. Hence, 30 µM GP was determined to be the optimal concentration for analysis in the present study. The observations of the present study suggested that GP exerted a protective effect on H2O2-induced oxidative damage of ARPE-19 cells by enhancing cell viability.
Oxidative damage is caused by the imbalance of the intracellular redox state, which generates a large amount of active oxygen and produces free radicals (31). Providing the concentration of ROS exceeds the clearance capacity of the body, tissue or cell damage may occur (32,33). It has been reported that antioxidant genes and drugs inhibit H2O2-induced oxidative damage by decreasing ROS activity (26,34-37). Studies have demonstrated that oxidative stress injury is one of the important factors of apoptosis (38-40). Radi et al (39) have reported that taxifolin alleviated H2O2-induced oxidative stress injury of ARPE-19 cells by inhibiting apoptosis. Therefore, ROS levels and the apoptosis of ARPE-19 cells treated with H2O2 and GP were analyzed by flow cytometry in the present study. Similar to previous reports (34,36,37,41), the results of the present study revealed that GP significantly reduced the levels of ROS and apoptosis in ARPE-19 cells induced by H2O2. In addition, GP significantly suppressed the expression of Bax and cleaved-caspase 3, and promoted Bcl-2 expression. These observations indicated that GP opposed the effects of H2O2 on ARPE-19 cell injury via anti-apoptosis and antioxidation.
Nrf2 signaling serves a key role in regulating antioxidant enzymes, and is also an important part of maintaining oxidative and antioxidative homeostasis, and alleviating oxidative stress damage (42). HO-1 and NQO1 are the key downstream factors of Nrf2 signaling, and serve an important role in protecting cells from oxidative damage (43,44). Hu et al (43) indicated that microRNA-455 activated the Nrf2 signaling pathway to protect osteoblasts against H2O2-induced injury. Vurusaner et al (44) proposed that laminarin ameliorated H2O2-induced MRC-5 cell oxidative injury by promoting the Nrf2 signaling pathway (45). Previous studies have also reported the beneficial effects of the Nrf2 signaling pathway on RPE cells (46,47). In the present study, it was proposed that the potential antioxidative mechanism of GP may comprise Nrf2 signaling. The results demonstrated that GP reversed the inhibitory effects of H2O2 on the expression of Nrf2, HO-1 and NQO1 in ARPE-19 cells. In addition, the effects of Nrf2 on ARPE-19 cells were investigated in the present study. The results revealed that Nrf2 knockdown exhibited no toxicity to cells; the effects of Nrf2 knockdown may be mainly produced on the molecular level rather than at the cellular level. However, Nrf2 silencing enhanced the effects of H2O2 on inducing cell damage via increasing ROS levels and apoptosis. Furthermore, Nrf2 silencing attenuated the protective effects of GP on H2O2-induced ARPE-19 cell injury by promoting apoptosis and oxidation. Therefore, the activation of Nrf2 signaling may be closely associated with the protective effects of GP. In addition, it has been reported that GP suppressed the growth of breast cancer cells by inhibiting UCP2 (14). Thus, it is possible that other signals may also associate with the protective effects of GP.
In summary, the present study proposed the novel functions of GP, which protected ARPE-19 cells against oxidative damage induced by H2O2 via promoting cell viability and suppressing ROS levels and apoptosis. The molecular mechanism was associated with the activation of Nrf2 signaling. These results suggested that GP may be considered as a therapeutic agent for the treatment and prevention of AMD.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Authors’ contributions
HZ wrote the manuscript. HZ, RW, MY and LZ performed the experiments and data analysis. HZ and LZ designed the study and contributed to manuscript revisions. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Nowak JZ. Age-related macular degeneration (AMD): Pathogenesis and therapy. Pharmacol Rep. 2006;58:353–363. [PubMed] [Google Scholar]
- 2.Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358:2606–2617. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- 3.Hernández-Zimbrón LF, Zamora-Alvarado R, Ochoa-De la Paz L, Velez-Montoya R, Zenteno E, Gulias-Cañizo R, Quiroz-Mercado H, Gonzalez-Salinas R. Age-related macular degeneration: New paradigms for treatment and management of AMD. Oxid Med Cell Longev. 2018;2018:8374647. doi: 10.1155/2018/8374647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L, Wu XW. Nobiletin protects human retinal pigment epithelial cells from hydrogen peroxide-induced oxidative damage. J Biochem Mol Toxicol. 2018;32:e22052. doi: 10.1002/jbt.22052. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Kim HJ, Sparrow JR. Quercetin and cyanidin-3-glucoside protect against photooxidation and photo-degradation of A2E in retinal pigment epithelial cells. Exp Eye Res. 2017;160:45–55. doi: 10.1016/j.exer.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dun Y, Vargas J, Brot N, Finnemann SC. Independent roles of methionine sulfoxide reductase A in mitochondrial ATP synthesis and as antioxidant in retinal pigment epithelial cells. Free Radic Biol Med. 2013;65:1340–1351. doi: 10.1016/j.freeradbiomed.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang H, Wu M, Liu Y, Song L, Li S, Wang X, Zhang YF, Fang J, Wu S. Serine racemase deficiency attenuates choroidal neovascularization and reduces nitric oxide and VEGF levels by retinal pigment epithelial cells. J Neurochem. 2017;143:375–388. doi: 10.1111/jnc.14214. [DOI] [PubMed] [Google Scholar]
- 8.Lee CH, Kwak SC, Kim JY, Oh HM, Rho MC, Yoon KH, Yoo WH, Lee MS, Oh J. Genipin inhibits RANKL-induced osteoclast differentiation through proteasome-mediated degradation of c-Fos protein and suppression of NF-κB activation. J Pharmacol Sci. 2014;124:344–353. doi: 10.1254/jphs.13174FP. [DOI] [PubMed] [Google Scholar]
- 9.Kim ES, Jeong CS, Moon A. Genipin, a constituent of Gardenia jasminoides Ellis, induces apoptosis and inhibits invasion in MDA-MB-231 breast cancer cells. Oncol Rep. 2012;27:567–572. doi: 10.3892/or.2011.1508. [DOI] [PubMed] [Google Scholar]
- 10.Lim W, Kim O, Jung J, Ko Y, Ha J, Oh H, Lim H, Kwon H, Kim I, Kim J, et al. Dichloromethane fraction from Gardenia jasminoides: DNA topoisomerase 1 inhibition and oral cancer cell death induction. Pharm Biol. 2010;48:1354–1360. doi: 10.3109/13880209.2010.483246. [DOI] [PubMed] [Google Scholar]
- 11.Machida K, Oyama K, Ishii M, Kakuda R, Yaoita Y, Kikuchi M. Studies of the constituents of Gardenia species. II. Terpenoids from Gardeniae Fructus. Chem Pharm Bull (Tokyo) 2000;48:746–748. doi: 10.1248/cpb.48.746. [DOI] [PubMed] [Google Scholar]
- 12.Zuo T, Zhu M, Xu W, Wang Z, Song H. Iridoids with genipin stem nucleus inhibit lipopolysaccharide-induced inflammation and oxidative stress by blocking the NF-κB pathway in polycystic ovary syndrome. Cell Physiol Biochem. 2017;43:1855–1865. doi: 10.1159/000484074. [DOI] [PubMed] [Google Scholar]
- 13.Hoshovs’ka IUV, Shymans’ka TV, Sahach VF. Effect of UCP2 activity inhibitor genipin on heart function of aging rats. Fiziol Zh. 2009;55:28–34. In Ukrainian. [PubMed] [Google Scholar]
- 14.Ayyasamy V, Owens KM, Desouki MM, Liang P, Bakin A, Thangaraj K, Buchsbaum DJ, LoBuglio AF, Singh KK. Cellular model of Warburg effect identifies tumor promoting function of UCP2 in breast cancer and its suppression by genipin. PLoS One. 2011;6:e24792. doi: 10.1371/journal.pone.0024792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dando I, Fiorini C, Pozza ED, Padroni C, Costanzo C, Palmieri M, Donadelli M. UCP2 inhibition triggers ROS-dependent nuclear translocation of GAPDH and autophagic cell death in pancreatic adenocarcinoma cells. Biochim Biophys Acta. 2013;1833:672–679. doi: 10.1016/j.bbamcr.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 16.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M, An C, Gao Y, Leak RK, Chen J, Zhang F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog Neurobiol. 2013;100:30–47. doi: 10.1016/j.pneurobio.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sachdeva MM, Cano M, Handa JT. Nrf2 signaling is impaired in the aging RPE given an oxidative insult. Exp Eye Res. 2014;119:111–114. doi: 10.1016/j.exer.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang KA, Wang ZH, Zhang R, Piao MJ, Kim KC, Kang SS, Kim YW, Lee J, Park D, Hyun JW. Myricetin protects cells against oxidative stress-induced apoptosis via regulation of PI3K/Akt and MAPK signaling pathways. Int J Mol Sci. 2010;11:4348–4360. doi: 10.3390/ijms11114348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Patel AK, Hackam AS. Toll-like receptor 3 (TLR3) protects retinal pigmented epithelium (RPE) cells from oxidative stress through a STAT3-dependent mechanism. Mol Immunol. 2013;54:122–131. doi: 10.1016/j.molimm.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sies H. Role of metabolic H2O2 generation: Redox signaling and oxidative stress. J Biol Chem. 2014;289:8735–8741. doi: 10.1074/jbc.R113.544635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen XD, Su MY, Chen TT, Hong HY, Han AD, Li WS. Oxidative stress affects retinal pigment epithelial cell survival through epidermal growth factor receptor/AKT signaling pathway. Int J Ophthalmol. 2017;10:507–514. doi: 10.18240/ijo.2017.04.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du L, Chen J, Xing YQ. Eupatilin prevents H2O2-induced oxidative stress and apoptosis in human retinal pigment epithelial cells. Biomed Pharmacother. 2017;85:136–140. doi: 10.1016/j.biopha.2016.11.108. [DOI] [PubMed] [Google Scholar]
- 26.Chen S, Sun P, Zhao X, Yi R, Qian J, Shi Y, Wang R. Gardenia jasminoides has therapeutic effects on L-NNA-induced hypertensio in vivo. Mol Med Rep. 2017;15:4360–4373. doi: 10.3892/mmr.2017.6542. [DOI] [PubMed] [Google Scholar]
- 27.Chen XL, Tang WX, Tang XH, Qin W, Gong M. Downregulation of uncoupling protein-2 by genipin exacerbates diabetes-induced kidney proximal tubular cells apoptosis. Ren Fail. 2014;36:1298–1303. doi: 10.3109/0886022X.2014.930650. [DOI] [PubMed] [Google Scholar]
- 28.Lin YH, Tsai SC, Lai CH, Lee CH, He ZS, Tseng GC. Genipin-cross-linked fucose-chitosan/heparin nanoparticles for the eradication of Helicobacter pylori. Biomaterials. 2013;34:4466–4479. doi: 10.1016/j.biomaterials.2013.02.028. [DOI] [PubMed] [Google Scholar]
- 29.Shin JK, Lee SM. Genipin protects the liver from ischemia/reperfusion injury by modulating mitochondrial quality control. Toxicol Appl Pharmacol. 2017;328:25–33. doi: 10.1016/j.taap.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Yu D, Shi M, Bao J, Yu X, Li Y, Liu W. Genipin ameliorates hypertension-induced renal damage via the angiotensin II-TLR/MyD88/MAPK pathway. Fitoterapia. 2016;112:244–253. doi: 10.1016/j.fitote.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Dias HKI, Milward M, Grant M, Chapple ILC, Griffiths HR. Sulforaphane decreases neutrophil hyperactivity by reducing intracellular oxidative stress. Free Rad Biol Med. 2012;53:S49. doi: 10.1016/j.freeradbiomed.2012.08.523. [DOI] [Google Scholar]
- 32.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slimen IB, Najar T, Ghram A, Dabbebi H, Ben Mrad M, Abdrabbah M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int J Hyperthermia. 2014;30:513–523. doi: 10.3109/02656736.2014.971446. [DOI] [PubMed] [Google Scholar]
- 34.Liu H, Liu W, Zhou X, Long C, Kuang X, Hu J, Tang Y, Liu L, He J, Huang Z, et al. Protective effect of lutein on ARPE-19 cells upon H2O2-induced G2/M arrest. Mol Med Rep. 2017;16:2069–2074. doi: 10.3892/mmr.2017.6838. [DOI] [PubMed] [Google Scholar]
- 35.Mitter SK, Song C, Qi X, Mao H, Rao H, Akin D, Lewin A, Grant M, Dunn W, Jr, Ding J, et al. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy. 2014;10:1989–2005. doi: 10.4161/auto.36184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu C, Dong Y, Liu H, Ren H, Cui Z. Hesperetin protects against H2O2-triggered oxidative damage via upregulation of the Keap1-Nrf2/HO-1 signal pathway in ARPE-19 cells. Biomed Pharmacother. 2017;88:124–133. doi: 10.1016/j.biopha.2016.11.089. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Y, Zhao KK, Tong Y, Zhou YL, Wang YX, Zhao PQ, Wang ZY. Exogenous NAD(+) decreases oxidative stress and protects H2O2-treated RPE cells against necrotic death through the up-regulation of autophagy. Sci Rep. 2016;6:26322. doi: 10.1038/srep26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Areti A, Yerra VG, Naidu V, Kumar A. Oxidative stress and nerve damage: Role in chemotherapy induced peripheral neuropathy. Redox Biol. 2014;2:289–295. doi: 10.1016/j.redox.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radi E, Formichi P, Battisti C, Federico A. Apoptosis and oxidative stress in neurodegenerative diseases. J Alzheimers Dis. 2014;42(Suppl 3):S125–S152. doi: 10.3233/JAD-132738. [DOI] [PubMed] [Google Scholar]
- 40.Su J, Shi HX, Wang LJ, Guo RX, Ren TK, Wu YB. Chemical constituents of bark of Taxus chinensis var. mairei. Zhong Yao Cai. 2014;37:243–251. In Chinese. [PubMed] [Google Scholar]
- 41.Xie X, Feng J, Kang Z, Zhang S, Zhang L, Zhang Y, Li X, Tang Y. Taxifolin protects RPE cells against oxidative stress-induced apoptosis. Mol Vis. 2017;23:520–528. [PMC free article] [PubMed] [Google Scholar]
- 42.Huang Y, Li W, Su ZY, Kong AN. The complexity of the Nrf2 pathway: Beyond the antioxidant response. J Nutr Biochem. 2015;26:1401–1413. doi: 10.1016/j.jnutbio.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu Y, Duan M, Liang S, Wang Y, Feng Y. Senkyunolide I protects rat brain against focal cerebral ischemia-reperfusion injury by up-regulating p-Erk1/2, Nrf2/HO-1 and inhibiting caspase 3. Brain Res. 2015;1605:39–48. doi: 10.1016/j.brainres.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 44.Vurusaner B, Gamba P, Gargiulo S, Testa G, Staurenghi E, Leonarduzzi G, Poli G, Basaga H. Nrf2 antioxidant defense is involved in survival signaling elicited by 27-hydroxycholesterol in human promonocytic cells. Free Radic Biol Med. 2016;91:93–104. doi: 10.1016/j.freeradbiomed.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Liu X, Liu H, Zhai Y, Li Y, Zhu X, Zhang W. Laminarin protects against hydrogen peroxide-induced oxidative damage in MRC-5 cells possibly via regulating NRF2. PeerJ. 2017;5:e3642. doi: 10.7717/peerj.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang K, Jiang Y, Wang W, Ma J, Chen M. Escin activates AKT-Nrf2 signaling to protect retinal pigment epithelium cells from oxidative stress. Biochem Biophys Res Commun. 2015;468:541–547. doi: 10.1016/j.bbrc.2015.10.117. [DOI] [PubMed] [Google Scholar]
- 47.Hu H, Hao L, Tang C, Zhu Y, Jiang Q, Yao J. Activation of KGFR-Akt-mTOR-Nrf2 signaling protects human retinal pigment epithelium cells from Ultra-violet. Biochem Biophys Res Commun. 2018;495:2171–2177. doi: 10.1016/j.bbrc.2017.12.078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.