Abstract
Caveolin-1 (Cav-1) expression has been shown to be associated with tumor growth and resistance to chemotherapy in pancreatic cancer. The primary aim of this study was to explore the significance of Cav-1 expression in pancreatic cancer cells as compared to fibroblasts in relation to cancer cell proliferation and chemoresistance, both in vitro and in vivo, in an immunodeficient mouse model. We also aimed to evaluate the immunohistochemical expression of Cav-1 in the epithelial and stromal component of pancreatic cancer tissue specimens. The immunohistochemical staining of poorly differentiated tissue sections revealed a strong and weak Cav-1 expression in the epithelial tumor cells and stromal fibroblasts, respectively. Conversely, the well-differentiated areas were characterized by a weak epithelial Cav-1 expression. Cav-1 downregulation in cancer cells resulted in an increased proliferation in vitro; however, it had no effect on chemoresistance and growth gain in vivo. By contrast, the decreased expression of Cav-1 in fibroblasts resulted in a growth advantage and the chemo-resistance of cancer cells when they were co-injected into immunodeficient mice to develop mixed fibroblast/cancer cell xenografts. On the whole, the findings of this study suggest that the downregulation of Cav-1 in fibroblasts is associated with an increased tumor proliferation rate in vivo and chemoresistance. Further studies are warranted to explore whether the targeting of Cav-1 in the stroma may represent a novel therapeutic approach in pancreatic cancer.
Keywords: pancreatic cancer, chemoresistance, stroma, caveolin-1, xenografts
Introduction
Pancreatic ductal adenocarcinoma (PDAC) remains one of the most lethal types of cancer with little improvement in the survival rates over the past decades. It is the fourth leading cause of cancer-related mortality in the USA and in Europe (1). The prognosis of patients with this disease remains unfavorable for all stages of the disease with the 5-year overall survival rate being <5% (2). Surgical resection, followed by adjuvant therapy, is the only radical therapeutic option; however, <15% of patients present with operable disease. Patients with advanced disease at presentation or recurrence may receive palliative chemotherapy, although the rates of response and survival benefit is modest. Aggressive tumor biology, as well as primary or secondary drug resistance (3) contribute significantly towards this dismal prognosis.
Caveolin-1 (Cav-1) is a 22-kDa molecular weight integral cell membrane scaffolding protein and a main component of caveolae, which are transmembrane microdomains composed of cholesterol and sphingolipids thus known as ‘lipid rafts’. This protein, in one of two similar isoforms (Cav-1a and Cav-1b), is associated with the processes of endo- and exocytosis, and with intracellular signal transduction mechanisms (4). Cav-1 is involved in a number of biological processes, as well as cellular transformation, tumorigenesis and metastasis (5). Cav-1 protein allows for signaling transduction events associated with the epidermal growth factor receptor (EGFR), HER2/neu, Src, the focal adhesion-associated protein kinase (FAK) and the mitogen-activated protein kinase (MAPK) (6). In addition, through its interaction with the adhesion molecules of the extracellular matrix, the integrins, it seems to be associated with the induction of the apoptosis of cancer cells (7,8).
Of note, Cav-1 appears to play a dual role in cancer biology (9). Its expression in cancer cells has been associated with an aggressive phenotype and a poor prognosis in various tumor types, including pancreatic adenocarcinoma (10-14). It has also been directly linked to the metastatic potential of pancreatic cancer cells through the regulation of epithelial to mesenchymal transition (EMT), a phenomenon closely related to the metastatic potential and chemoresistance of cancer cells (15,16). Nevertheless, conflicting results have been presented (17), with the loss of Cav-1 in the tumor stroma being associated with an adverse clinical outcome in a variety of cancer types (18-23).
Previous studies have implicated Cav-1 protein as an important factor in the development of chemo - and radio-resistance (24,25), while complex interactions seem to be involved in the role of Cav-1 in the development of multi-drug resistance (MDR) (26). To date, there are no consistent data implicating Cav-1 in the development of chemoresistance in pancreatic adenocarcinoma (27-29). Moreover, it is not clear whether the expression of Cav-1 in pancreatic cancer cells or cancer-associated fibroblasts (CAFs) is predictive of the treatment response or overall prognosis (10,21,30).
The purpose of this study was to examine the role of Cav-1 in the development of chemoresistance in pancreatic cancer. Initially, we examined the differential immunohistochemical expression of Cav-1 between tumor and stromal cells in human pancreatic cancer tissue specimens. We then examined the response of human pancreatic cancer cell lines, with differential expression levels of Cav-1, to chemotherapeutic agents both in vitro and in vivo using pancreatic cancer animal models developed in immunodeficient mice. Furthermore, and in order to shed light on the role of Cav-1 expression in the context of the tumor microenvironment, we generated and used fibroblasts with a decreased expression of Cav-1. Our results indicate that expression of Cav-1 in tumor cells per se may play a minor role in their tumorigenicity and chemoresistance. However, the decreased expression of this protein in the tumor microenvironment i.e., in fibroblasts, seems to result in increased tumorigenic properties of cancer cells together with increased chemoresistance.
Materials and methods
Materials
RPMI-1640 and DMEM were purchased from Gibco/Thermo Fisher Scientific (Athens, Greece) and L-glutamine, PBS and trypsin were purchased from GE Healthcare Life Sciences (GE Healthcare Life Sciences/Athal, Athens, Greece). Fetal bovine serum was purchased from Biowest (Biowest/Bioline Scientific, Athens, Greece) and dimethylsulphoxide (DMSO) from Eastman Kodak (Columbus, GA, USA). Trichloroacetic acid (TCA), TEMED, hydrochloric acid, SDS, hydrogen peroxide, glycerol 99.9%, sulphorodamine-B for the in vitro cytotoxic assay, NP40 and protease inhibitors were obtained from Sigma-Aldrich (Merck, Chemilab S.A., Athens, Greece). 2-β-mercaptoethanol was purchased from Merck (Chemilab S.A.) while Ponceau S staining solution and Triton X-100 were from AppliChem (AppliChem GmbH, Darmstadt, Germany). Glycine ≥99% was purchased from Roth (Karlsruhe, Germany) while protein electrophoresis markers, SDS acrylamide 30% and the Quick Start Bradford Dye reagent 1X for the measurement of protein content of our samples were purchased from Bio-Rad Laboratories Ltd. (Athens, Greece). All the chemotherapeutic agents [5-fluorouracil (5-FU), gemcitabine, doxorubicin, epirubicin, cisplatin, oxaliplatin, docetaxel and Paclitaxel] were kindly provided by the Oncology Department of the General University Hospital of Larissa, Larissa, Greece. Cell culture plastic products were all purchased from Sarstedt (Sarstedt Ltd., Athens, Greece).
Cell culture
BxPC3 (pancreatic adenocarcinoma), AsPC1 (pancreatic adenocarcinoma metastatic), PANC-1 (epithelioid carcinoma from pancreatic duct) and MIAPaCa-2 (pancreatic carcinoma) cancer cell lines were obtained from ATCC (Manassas, VA, USA). Human dermal fibroblasts were obtained originally from Thermo Fisher Scientific (Loughborough, UK). The cancer cells were adapted to proliferate in RPMI-1640 medium and the fibroblasts in DMEM, supplemented with 5% heat-inactivated fetal calf serum, 2 mM L-glutamine and antibiotics. The cultures were grown at 36.7°C in a humidified incubator with 5% CO2 atmosphere and 95% humidity.
Silencing of Cav-1 in BxPC3 cells
To minimize the differences between various cell lines, we set out to induce the stable knockdown of Cav-1 in BxPC-3 cells that naturally express high levels of Cav-1. Hence, we measured their proliferative capacity, their migratory capacity and chemosensitivity. We induced the stable knockdown through lentiviral infection, which also allowed tracking the cells containing the virus due to constitutive green fluorescent protein (GFP) expression (fluorescent in the green channel). Cav-1 expression was silenced by transduction with short hairpin RNA (shRNA) mir GIPZ lentiviral particles (Open Biosystems, Surrey, UK). The cells were seeded at 50% confluence and infected by direct contact with lentiviral particles diluted 1:50 into 1 ml of serum-free RPMI-1640 and incubated for 6 h, following which an additional 1 ml of 10% RPMI-1640 was added and the cells were incubated for a further 72 h. The transduction efficiency was evaluated by GFP co-expression by a fluorescence microscope (EVOS™ FL Imaging System; Thermo Fisher Scientific, Loughborough, UK). Stably transduced cells were then selected in media containing 1.0 µg/ml puromycin (Life Technologies/Thermo Fischer Scientific, Athens, Greece) for 10 days.
To purify further and homogenize the cellular populations, cells transduced as described above were sorted on a BD FACS-Vantage cell sorter (Becton-Dickinson, Oxford, UK) based on GFP expression. Through this procedure, employing silencing shRNA for caveolin, the cell line named BxPC3shCAV was finally generated, while a mock-transfected cell line named BxPC3mock was also generated to be used as control. These cells were used for the experiments described further in this study.
Immortalization of human dermal fibroblasts and silencing of Cav-1
For immortalization, pBabe hTERT geneticin retrovirus supernatant (Addgene, Cambridge, MA, USA) was used to transduce human dermal fibroblasts at 50% confluence. After 24 h, the cell media were changed and the cells were cultured for a further 48 h. Selection was carried out for 4 days with 1.0 µg/ml geneticin (Life Technologies; Thermo Fisher Scientific, Loughborough, UK). The hTERT-immortalized human skin fibroblasts named hhsF from hereon were used in the further experiments.
Cav-1 expression was silenced as described above. Two types of hhsF were developed: The hhsFmock and the hhsFshCAV with unaffected levels of Cav-1 or with silenced Cav-1, respectively.
Western blot analysis
For western blot analysis cancer cells were cultured in 6-well plates at inoculation densities varying from 4×106 to 6×106/ml, depending on the cell line. After 24 h, the cells were washed twice in ice-cold PBS, trypsinized, collected by gentle centrifugation, and whole cell protein extracts were prepared as previously described (31). Protein concentrations were determined with the Bradford assay, and subsequently, aliquots containing 30 µg of protein were subjected to gel electrophoresis on 10% polyacylamide SDS-gels under reducing conditions, and then transferred onto PVDF membranes (Millipore Immobilon; Merck S.A. Hellas, Athens, Greece). To confirm protein transfer, the membranes were stained with Ponceau S solution (AppliChem GmbH). Finally after the washing membranes to remove the Ponceau S, the proteins were visualized using an enhanced chemoluminescence detection system (Amersham ECL or ECL Plus, GE Healthcare Life Sciences) according to the manufacturer’s instructions. Cav-1 antibody was purchased from Santa Cruz Biotechnology (clone N-20, sc-894; Heidelberg, Germany) while, actin antibody was purchased from Sigma-Aldrich (Life Science Chemilab S.A., Athens, Greece) and GAPDH antibody from BioLegend (San Diego, CA, USA). Anti-rabbit and anti-mouse HRP conjugated secondary antibodies used were purchased from Sigma-Aldrich. Antibodies were used as follows (all were diluted in Tris-buffered saline supplemented 0.05% Tween-20 and 5% FCS): CAV-1 at 1:500 (overnight incubation at 4°C), GAPDH at 1:6,000 (2 h at room temperature), actin at 1:2,000 (2 h at room temperature), and both secondary antibodies at 1:6,000 (1 h at room temperature).
RNA isolation and RT-qPCR analysis
Total RNA was isolated using the RNeasy Mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. One microgram of total RNA from each sample was retro-transcribed to first-strand cDNA using the SuperScript III One Step RT-PCR system (Invitrogen/Thermo Fisher Scientific, Loughborough, UK). Quantitative RT-PCR was performed in triplicates using SYBR-Green RT-PCR Master Mix kit and the ABI PRISM 7500 Fast Real-Time PCR System (Applied Biosystems/Thermo Fisher Scientific, Loughborough, UK) with the following primers: Cav-1, 5′-CGACCCTAAACACCTCAACGA-3′ (forward) and 5′-TCCCTTCTGGTTCTGTCA-3′ (reverse). Quantification was performed using the comparative CT (cycle-threshold) method employing ribosomal 18S as a housekeeping gene (ΔΔCq method) (32).
Evaluation of cell proliferation [bromodeoxyuridine (BrdU) assay]
Cell proliferation was evaluated by determining the incorporation of BrdU nucleotide in actively proliferating cells, using a colorimetric ELISA (Abcam, Cambridge, UK). The assay was performed as per the manufacturer’s instructions. The colored reaction product was quantified using a spectrophotometer (microplate reader, BioTek EL-311; BioTek Instruments, Bad Friedrichshall, Germany).
Wound healing/scratch assay
To determine the effects of Cav-1 on the ability of cancer cells to migrate in vitro, we utilized the scratch assay. In the monolayer of cells covering approximately the 80% of the microtiter plate well surface, a scratch was made using a sterile 200 µl pipette tip. Following a wash with PBS and the addition of fresh growth medium, the cells were allowed to migrate for 24 or 48 h. The cells were photographed using a light microscope and a ×10 magnification (Axioplan equipped with an AxioCam, Zeiss Ltd., Cambridge, UK) at various time points i.e., 0, 5, 9 14 and 28 h to analyze the migration of the cells towards the ‘wounded’ area.
Chemotaxis/migration assay
To determine the ability of fibroblasts to induce the chemotactic migration of the BxPC3 cancer cells, 24 mm Transwell® with 8.0-µm pore polycarbonate membrane inserts (Product #3428, Corning® Transwell®; Sigma-Aldrich; Merck, Chemilab S.A.) was used. The chemotaxis/migration assay was performed as follows:
At day zero, a 24-well migration plate was inoculated with the fibroblasts (hhsFmock or hhsFshCAV) at a density of 20×103 cells/well in 500 µl of serum-free DMEM, and the cells were allowed to adapt in a 5% CO2 incubator at 36.7°C for 48 h. Subsequently, the medium was removed and DMEM supplemented with 10% FCS (10% DMEM) was added for 12 h to serum-activate the fibroblasts, as previously described (33). Thereafter, the 10% DMEM medium was removed, and the fibroblasts were washed twice with PBS and 500 µl of DMEM supplemented with 1% FCS was added. Following a further 12 h of incubation, a cell suspension of BxPC3 cells at a density of 60×104 cells/ml in serum-free media was prepared. A Transwell® insert was then applied to the fibroblast-containing wells and 250 µl of the BxPC3 cells suspended in serum-free media were then added to the insert. Wells containing 1% DMEM underneath the insert were used as negative controls in order to determine the spontaneous migration of BxPC3 cells under these conditions, while wells with 10% DMEM underneath the insert served as positive controls. Incubation continued (5% CO2, 36.7°C) for a further 12 h. Next, the media from the inside of the insert were carefully aspirated, the inserts removed from the culture plates and non-migratory cells were removed from the interior of the inserts with cotton-tipped swabs. Subsequently, the inserts were transferred to a clean well containing 400 µl of ice-cold TCA to fix the cells attached to the outer surface of the insert as described for the cytotoxicity assay. The fixed inserts were gently washed several times in distilled water, air-dried and stained with sulforhodamine B (SRB) as for the in vitro cytotoxic activity assay described below. After a second wash step to remove any unbound staining, the inserts were transferred to a clean plate containing 400 µl of unbuffered Tris to extract the dye and 200 µl of the solution was transferred to a 96-well plate and finally the OD value was measured using a micro-plate reader (Biotek EL-311; BioTek Instruments). Chemotaxis was calculated as the % OD value of the inserts as compared to inserts containing no fibroblasts (negative controls). For chemotaxis, two independent experiments were performed.
In vitro cytotoxic activity of chemotherapeutic drugs
The in vitro cytotoxic activity of all chemotherapeutics tested herein [5-fluo-rouracil (5-FU), gemcitabine, doxorubicin, epirubicin, cisplatin, oxaliplatin, docetaxel and Paclitaxel] was determined using the SRB assay, as previously described (34,35). Cell viability was assessed at the beginning of each experiment by the trypan blue dye exclusion method, and was always >97%. For the SRB assay, the cells seeded into 96-well plates in 100 µl medium at a density of 5,000 cells per well, and incubated under standard conditions for 24 h to enable the cells to resume exponential growth before addition of the compounds. In order to measure the starting cell population [time zero (Tz)], cells in one plate were fixed in situ with TCA 50%. Compounds were diluted to twice the desired final maximum test concentration (100 µM for all other drugs, but for paclitaxel and docetaxel the maximum concentrations tested were 10 µM) with complete medium and 4 or 5 additional 10-fold dilutions, depending on the drug, were prepared. Aliquots of 100 µl of these different drug dilutions were added to the appropriate microtiter wells already containing 100 µl of medium, resulting in the required final drug concentrations. Cell cultures containing DMSO alone served as negative controls, while as a positive control, control representative wells were treated with a corresponding volume of culture medium.
Following drug addition, the plates were incubated for 48 h at the conditions described above. The experiment was terminated by the addition of 50 µl of cold 50% (w/v) TCA (final concentration, 10% TCA). Incubation for 1 h at 4°C and staining with SRB was carried out as previously described (34,35). The bound stain was subsequently solubilized with a 10 mM Trizma base (Sigma-Aldrich; Merck, Chemilab) and the absorbance was read on an automated plate reader, EL-311 (BioTek Instruments), at a 530 nm wavelength.
Using the absorbance measurements [time zero (Tz), control growth (C), and test growth in the presence of drug at the 5 concentration levels (Ti)], the growth percentage of the cells was calculated for each drug concentration using the following formulas: i) [(Ti-Tz)/(C-Tz)] × 100 for concentrations for which Ti>/=Tz and ii) [(Ti-Tz)/Tz] × 100 for concentrations for which Ti<Tz.
In vivo experiments
To generate human-to-mouse pancreatic cancer xenografts, 5×106 cells from exponentially growing cultures of BxPC3mock or BxPC3shCAV cells were injected subcutaneously, according to the British practice of bilateral implants, at the axillary region of the rear flanks into 6-8-week-old (mean weight, 20 g) female NOD.CB17-Prkdcscid/J (NOD/SCID) mice from our animal facility (EL 42 BIO_BR01). During experimentation, all animals were kept in the Animal Unit of the Department of Pharmacology (University of Thessaly, Larissa Greece; EL42 BIO_EXP03) under specific pathogen-free (SPF) conditions, a 12-h/12-h light/dark, a temperature of 21°C and a relative humidity 50% and allowed access to water and food ad libitum. For the co-injection of fibroblasts with BxPC3 (3×105) cells at the exponential growth phase, hhsFmock or hhsFshCAV fibroblasts were co-injected with BxPC3 cells at 1:1 or 1:3 ratios, respectively in plain DMEM, subcutaneously at the axillary region of the rear flanks of mice (two injections per mouse as described above for the generation of BxPC3mock or BxPC3shCAV xenografts). In this experiment a group of mice received 3×105 fibroblasts alone, as well, to determine whether the transformed fibroblasts had any tumorigenic capacity. Each group consisted of 5 mice of matching age and weight. The mice were then followed for the development of tumors. At the end of the experiment, which varied depending on the specific group (i.e., for the generation of BxPC3mock or BxPC3shCAV the experiment ended at day 65 post-tumor cell inoculation, for the development of co-injected xenografts experiments ended at days 26-28 post-tumor cell inoculation) the mice were euthanized (age, 12-16 weeks; mean weight, 20-22 g) and the tumors were removed and weighed. Where tumor volume was used this was calculated according to the formula [(axb2)/2], where a=length and b=width of the tumor as measured with a Vernier’s caliper (measurements were performed twice a week).
Animals treated with gemcitabine received an intraperitoneal injection/week at a 100 mg/kg dose until the end of experiment. Treatment began 4 days after cell implantation. Weight loss (assessed twice weekly), neurological disorders, behavioral and dietary changes were also recorded as indicators of drug toxicity.
The handling and experimentation of the animals were conducted in accordance with the Greek laws (PD 56/2013 and Circular 2215/117550/2013) and the guidelines of the European Union (2013/63/EU) under a licenced protocol approved by the IACUC and Greek authorities (Lisence no. 5542/228006, IACUC; Professor Dr N. Pitsikas, Dr A. Zacharioudaki, Dr J. Chloptsios and Dr A. Konstantinidis).
Immunohistochemistry
A total of 11 de-identified FFPE (formalin-fixed paraffin-embedded) blocks of pancreatic cancer tissues, obtained from the archive of the Pathology Department of The Leeds Teaching Hospitals Trust (Leeds, UK) were stained for Cav-1. All patients provided informed consent prior to the collection of the samples and all procedures were approved by and were in compliance with the ethical standards of the Leeds Teaching Hospitals NHS Trust (R&I Ref. no. CO18/113235). The differential expression of Cav-1 between the tumor and stroma in poorly differentiated (PD) and well/moderately differentiated (WD) areas was assessed by a pancreatic pathologist (CV). As previously described by Witkiewicz et al (30), Cav-1 expression was evaluated as follows: 0 for no staining; 1 for weak and/or focal (<10% of the cells) staining; 2 for moderate or strong staining (10-50% of the cells); and 3 for moderate or strong staining (>50% of the cells).
Immunohistochemical analysis (IHC) of human and xenograft pancreatic cancer tissues was performed on 3-µm-thick paraffin-embedded sections using a rabbit poly-clonal anti-Cav-1 antibody (dilution 1:200, anti-cav-1 N-20 rabbit; sc-894; Santa Cruz Biotechnology). Antigen retrieval was performed according to the suppliers’ instructions. The immunoreaction was detected using a goat anti-rabbit-ALP (Menarini Diagnostics, Winnersh-Wokingham, UK) followed by 3,3-diaminobenzidine tetrahydrochloride (DAB; Vector Laboratories, Peterborough, UK). Negative controls were processed by the omission of primary antibody. For histological evaluation, sections were stained with hematoxylin and eosin (H&E; Thermo Fisher Scientific, Loughborough, UK). The sections were imaged using an Axioplan Zeiss light microscope (Carl Zeiss Ltd., Cambridge, UK) equipped with an AxioCam digital camera.
Statistical analysis
Statistical analysis was performed using a Student’s t-test or one-way ANOVA with Holm-Sidak as a post hoc test where multiple comparisons were carried out using GraphPad Prism 6.0 software (GraphPad Software, La Jolla, USA). Data are presented as the means ± SD. Statistical significance was set at P<0.05.
Results
IHC reveals the variable expression of Cav-1 in the epithelial and stromal component of pancreatic cancer tissue specimens
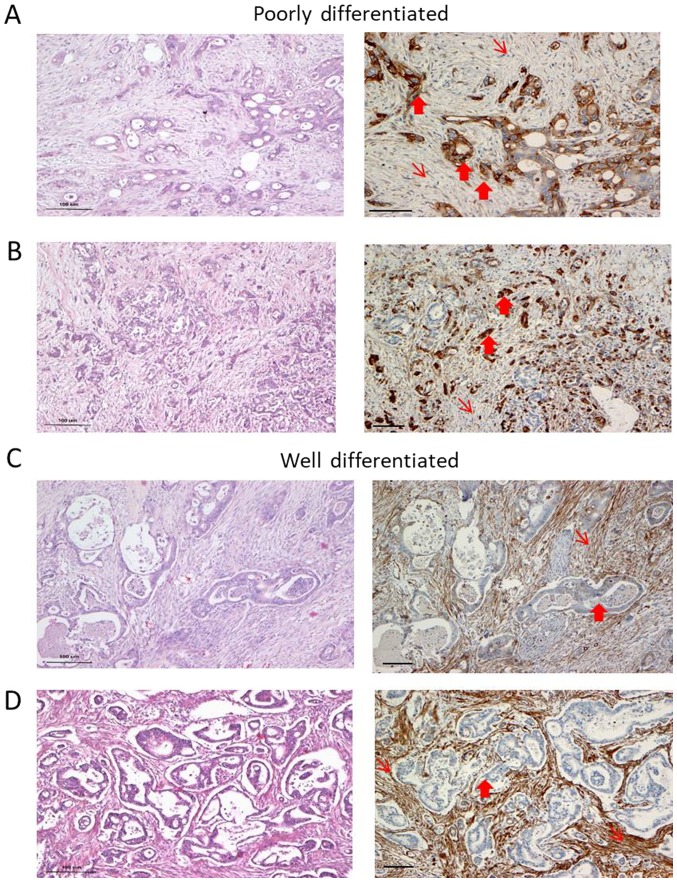
We observed heterogeneity in Cav-1 expression with respect to the degree of differentiation. In the well-differentiated (WD) areas of the samples, Cav-1 staining in the cancer cells was negative or exhibited a focal/weak protein expression in 9 out of the 11 cases. By contrast, the stroma of the WD areas exhibited a strong expression of Cav-1 (Fig. 1 and Table I). Cancer cells in the poorly differentiated (PD) areas were moderately to strongly Cav-1-positive in 6 out of the 7 cases examined. Immunostaining was predominantly cytoplasmic, while membranous staining was apparent only in the tumor cells with clear cell morphology. The fibroblasts were weakly stained in the PD areas, particularly in areas where cancer cells were strongly positive (Fig. 1A and C). There seemed to be an inverse pattern of Cav-1 expression, with strong staining in the cancer cells and weak or no staining in the stroma-associated fibroblasts or vice versa (Table I).
Figure 1.
(A-D) Caveolin-1 (Cav-1) expression in pancreatic cancer cases. (A and B) Cav-1 expression in poorly differentiated pancreatic cases with high expression in the cancer cells (thick arrows) and no expression in the stroma (thin arrows). (C and D) In well-differentiated areas of the same tumors shown in (A and B), the opposite pattern was observed i.e., a strong expression in the stroma, whereas the cancer cells were negative (representative areas of this pattern are shown). H&E staining of these regions is also presented (left panels). (A and C) case 1; (B and D) case 10 (see Table I). Scale bar, 100 µm.
Table I.
Caveolin-1 expression in patient archival samples.
| Poorly differentiated | Moderately/well differentiated | |||
|---|---|---|---|---|
|
| ||||
| Case no. | Cav-1 expression
|
Cav-1 expression
|
||
| Cancer | Stroma | Cancer | Stroma | |
| 1 | 3 | 1 | 1 | 3 |
| 2 | 2 | 2 | 0 | 2 |
| 3 | No PD areas | 2 | 3 | |
| 4 | No PD areas | 2 | 1 but scarce stroma | |
| 5 | No PD areas | 1 | 3 | |
| 6 | 1 | 3 | 0 | 3 |
| 7 | 3 | 2 | 0 | 1 but scarce stroma |
| 8 | 3 | 1 | 1 | 3 |
| 9 | 2 | 2 | 1 | 3 |
| 10 | 3 | 1 | 1 | 3 |
| 11 | No PD areas | 0 | 3 | |
Caveolin-1 expression in cancer cells and stroma in well and poorly differentiated areas of pancreatic cancer in 11 archival samples. For details please see the Materials and methods, immunohistochemistry. Cav-1 staining was graded as follows: 0, negative; 1, weak and/or focal (<10%); 2, moderate or strong (10-50%); 3, moderate or strong >50%. Cav-1, caveolin-1, PD, poorly differentiated.
Generation of pancreatic cancer cells and fibroblasts in which Cav-1 is knocked down
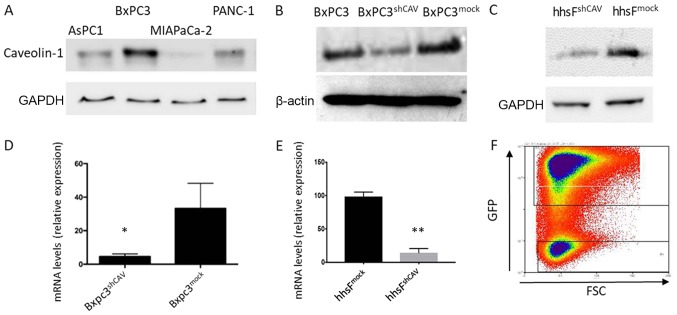
In order to select the most appropriate cell line for our hypothesis, we screened several pancreatic cell lines for Cav-1 expression (Fig. 2A) and we found that the BxPC3 cells expressed the highest levels of Cav-1. We thus selected these cells for use in the subsequent experiments. The lentivirus-induced introduction of shRNA into the BXPC3 cells resulted in a decreased mRNA and protein expression of Cav-1, as shown in Fig. 2B and D. The concomitant introduction of GFP allowed for the discrimination and separation of cells with the highest viral integration/expression and consequent lower Cav-1 expression (named BxPC3shCAV) with the aid of cell sorting (Fig. 2F). As a control, the BxPC3mock cell line was generated with unaffected levels of Cav-1. Lentiviral particles expressing scrambled shRNA (mock) did not alter the Cav-1 levels, confirming that the decreased expression of Cav-1 in BxPC3shCAV was due to the specific effects of shRNA (Fig. 2B and D). Fibroblasts with silenced (hhsFshCAV) or unaffected (hhsFmock) Cav-1 expression were generated from hTERT immortalized human skin fibroblasts following the methodology described above for the BxPC3 cells (Fig. 2C and E).
Figure 2.
Generation of cells with a modified expression of caveolin-1. (A) Differential caveolin-1 expression in expression in 4 cells lines tested. Differential protein expression and relative mRNA levels of caveolin-1 in (B and D) BxPC3 cells and in (C and E) hTERT human skin fibroblasts. (F) Gating strategy for sorting the transfected cells. BxPC3shCAV, BxPC3 cells transfected with shRNA against caveolin-1; BxPC3mock, BxPC3 cells transfected with a sequence that was not expressed; hhsFshCAV, hTERT human skin fibroblasts transfected with shRNA against caveolin-1; hhsFmock, hTERT human skin fibroblasts transfected with a sequence that was not expressed; GFP, green fluorescent protein; FSC, forward scattering. *P<0.05 and **P<0.01
Downregulation of Cav-1 expression results in a marginal increase in DNA synthesis and tumor cell proliferation, and in the increased migration/motility of BxPC3 cells
We first examined whether Cav-1 downregulation in pancreatic cancer cells affected the proliferation rate of these cells. By the SRB method and the BrdU DNA incorporation method, beginning with an inoculation density of 5,000 cells/well, a moderate increase in DNA synthesis (Fig. 3A) and in the proliferation of BxPC3shCAV as compared to BxPC3 and BxPC3mock cells were observed (Fig. 3B). These results suggest that Cav-1 may, to a certain extent, act as negative regulator of the growth of pancreatic cancer cells under the prevailing experimental conditions. Furthermore, as shown in Fig. 3C, the downregulation of Cav-1 in the BxPC3 cells resulted in a statistically significant increase in the migratory capacity of the BxPC3shCAV cells as compared to the BxPC3mock cells (P<0.01), suggesting again that Cav-1 may act as a brake on the proliferative and migratory capacity of pancreatic cancer cells.
Figure 3.
Silencing of caveolin-1 in BxPC3 cells results in a moderate increase in cellular proliferation and the increased migration of BxPC3 cells. A total of 5,000 cells/well for each cell line were inoculated and either (A) the DNA synthesis rate by the incorporation of BrdU or (B) the total cell population using the SRB assay, were determined after 48 h of incubation. Bars show the means of 3 independent experiments run in triplicate. (A) **P<0.001 vs. BxPC3 and BxPC3mock, one-way ANOVA, Holm-Sidak post hoc test. (B) **P<0.001 vs. BxPC3 and #P<0.05 vs. BxPC3mock, one-way ANOVA, Holm-Sidak post hoc test. x-axis labels are the same for A and B. (C) Percentage free area for the wound healing/scratch assay at various time points. Bars represent the means of 2 independent experiments. **P<0.01, ***P<0.005 and ###P<0.005 vs. BxPC3mock at 5 h. (D-G) Representative images of the wound healing/scratch assay for (D and F) BxPC3mock or (E and G) BxPC3shCAV cells at time 0 (D and E, respectively) and 28 h later (F and G, respectively). The red dotted line shows the borders of the cell population at the site of the scratch. Photos are representative of 1 out of 2 experiments.
Cav-1 downregulation in fibroblasts increases the migratory and chemotactic ability of BxPC3 cells
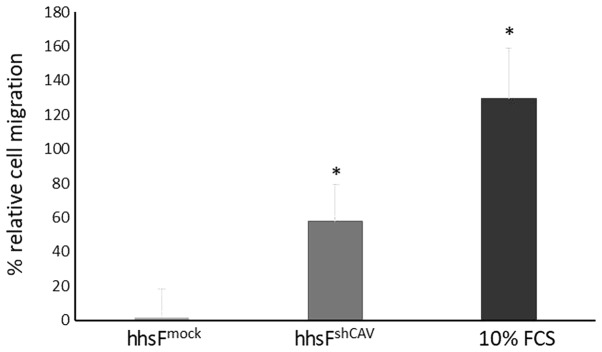
To further determine the effects of Cav-1 levels not in the cancer cells, but in the context of the tumor microenvironment, a Transwell migration/chemotaxis assay was performed (Fig. 4) where the effect of fibroblasts in which Cav-1 was silenced on the chemotactic migration of normal (non-Cav 1 silenced) BxPC3 cells was examined. In this experiment, the silencing of Cav-1 in the fibroblasts (hhsFshCAV) resulted in an increased migration of BxPC3 cancer cells (60±21%) through the micropores of the Transwell as compared to the mock-infected fibroblasts (hhsFmock, P<0.05, paired t-test). These results suggest that the absence of Cav-1 in fibroblasts may favor the metastatic potential of tumor cells in the context of the tumor microenvironment. As the tumor cells were not in contact with the fibroblasts, the secretion of agents from the fibroblasts may have acted as chemoattractants to induce the migration of tumor cells towards the surrounding stroma.
Figure 4.
Downregulation of caveolin-1 in fibroblasts potentiates the migratory capability of the cancer cells. Changes in the Transwell chemo-taxis/migration assay for BxPC3 cancer cells in the presence of fibroblasts. Bars represent the percentage increase in the migrated population as compared to cells migrating in DMEM supplemented with 1% FCS in the absence of fibroblasts. The mean of 2 independent experiments ± SD is shown. *P<0.05 vs. BxPC3mock in a pairwise t-test.
Effect of Cav-1 expression on the chemosensitivity of cancer cells
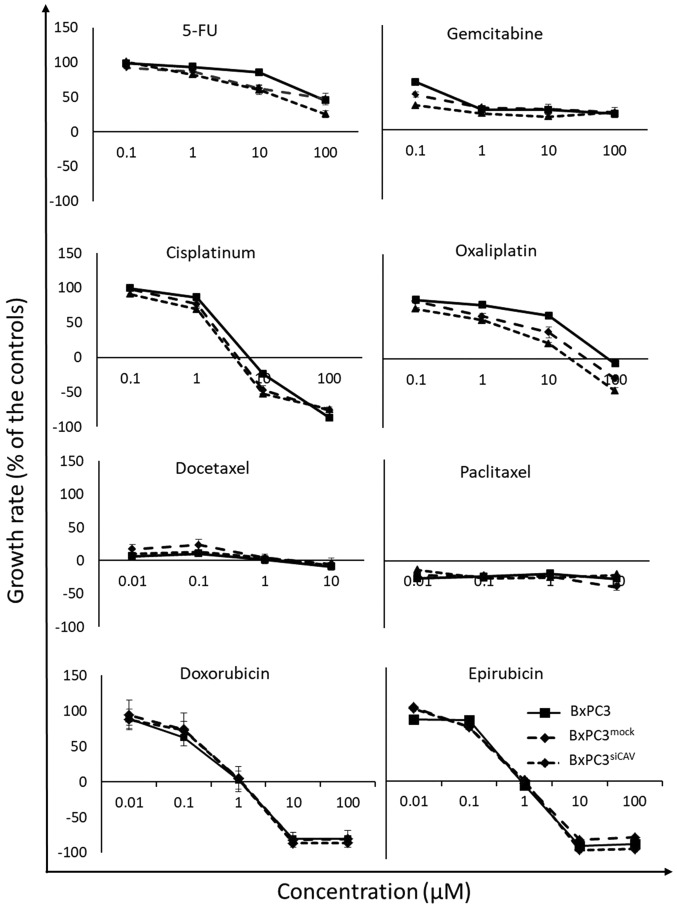
To examine the effect of Cav-1 expression on the chemo-sensitivity of human the pancreatic cancer BxPC3 cells, the BxPC3mock and BxPC3shCAV cells were exposed to various chemotherapeutic agents i.e., 5-fluorouracil (5-FU), gemcitabine, doxorubicin, epirubicin, cisplatin, oxaliplatin, docetaxel and Paclitaxel. The sensitivity of all 3 cell lines (BxPC3, BxPC3mock and BxPC3shCAV) seemed to be unaffected by the levels of expression of Cav-1 to the chemotherapeutic agents that were tested, as shown by the corresponding growth curves (Fig. 5).
Figure 5.
Downregulation of caveolin-1 in cancer cells does not affect the in vitro chemosensitivity of BxPC3 cells. The growth curves of the 3 cell lines co-cultured for 48 h with various concentrations of the drugs are presented. Each point represents the mean of 2 independent experiments run in triplicate ± SD. Negative values denote toxicity. For details on the calculation of the growth rate, please see the Materials and methods.
Decreased Cav-1 levels in the stroma promote the growth of BxPC3 tumor xenografts
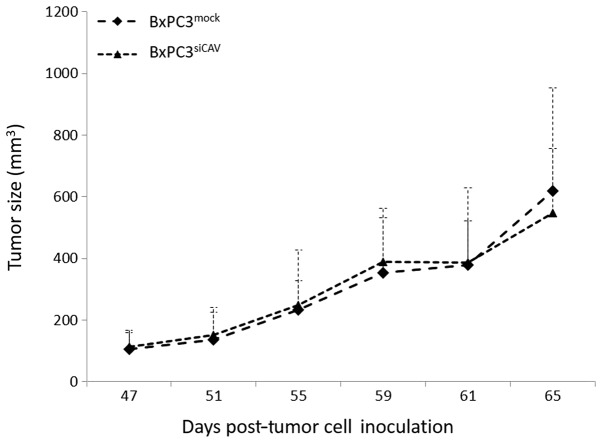
We then examined whether the protein expression levels of Cav-1 can affect the tumorigenic capacity and/or the chemoresistance of BxPC3 in xenografts. Towards this aim, we first sought to compare the tumorigenic and growth characteristics of the two cell lines, BxPC3mock and BxPC3shCAV, when inoculated into NOD/SCID mice. The two xenografts from the transfected cells demonstrated no difference between the growth rates of BxPC3mock- and BxPC3shCAV-derived tumors (Fig. 6).
Figure 6.
Downregulation of caveolin-1 in BxPC3 cells does not affect the tumor growth in the corresponding xenografts. The growth rates of the tumors developed by the subcutaneous inoculation of 5×106 cells of BxPC3mock and BxPC3shCAV in immunocompromised mice are shown. Points represent the average ± SD.
Following the in vitro observation that low levels of fibroblast Cav-1 expression resulted in increased cancer cell motility/migration (Fig. 4), we determined whether fibroblast Cav-1 expression can affect the growth of BxPC3 when grown as xenografts. As shown in Fig. 7, the co-injection of fibroblasts with BxPC3 cells significantly affected the growth of tumors in a Cav-1-dependent manner. The tumors that developed from BxPC3 cells co-injected with hhsFmock (BxPC3 + hhsFmock tumors) cells exhibited similar growth as the tumors derived from the BxPC3 cells alone. However, the co-injection of BxPC3 cells with hhsFshCAV (BxPC3 + hhsFshCAV tumors), resulted in an almost 3-fold increase in tumor weight compared to both the BxPC3- and the BxPC3 + hhsFmock-derived tumors (130±98 and 167±42 mg, respectively vs. 360±138 mg for the BxPC3 + hhsFshCAV tumors; P<0.01). At 6 days post-inoculation of 3×105 BxPC3 cells into NOD/SCID mice, at a 1:1 ratio of cancer cells:fibroblasts, the following was observed: No tumor development in the BxPC3-only inoculated mice (0/10); 1 tumor palpable in 10 mice that received BxPC3 + hhsFmock (1/10), and 3 tumors palpable in mice that were co-injected with BxPC3 and hhsFshCAV cells (3/10). A week later, at day 13, the ratio was 3/10 for BxPC3 and for BxPC3 + hhsFmock as compared to 7/10 for the BxPC3 + hhsFshCAV-derived tumors. Similarly, increasing the inoculation density of cancer cells to 1×106 (cancer cells:fibroblasts ratio: 3:1) resulted in the development of tumors in the following numbers of mice: 0/10 for BxPC3, 1/10 for BxPC3 + hhsFmock and 7/10 for BxPC3 + hhsFshCAV at day 6 and 5/10, 6/10 and 9/10 tumors at day 13, respectively (data not shown). The post-mortem weights of the tumors that had developed by day 28 post-inoculation of the cells revealed a substantial difference between the BxPC3-derived tumors that developed from the co-injection with hhsFshCAV fibroblasts as compared to the BxPC3-only and BxPC3 + hhsFmock-derived tumors, both at the cancer cells:fibroblasts ratios of 1:1 and 3:1 (P<0.05 or 0.01, respectively; Fig. 7). Mice injected with fibroblasts alone did not develop any tumors (data not shown).
Figure 7.
Downregulation of caveolin-1 in fibroblasts results in increased growth rate of cancer cells. The post-mortem tumor weights for BxPC3 cells alone or co-injected with either hhsFmock or hhsFshCAV are shown as the average of the weights of all tumors ± SD in either a ratio of (A) 1:1 or (B) 3:1 (BxPC3 vs. hhsF). (A) *P<0.05 vs. the BxPC3 and BxPC3+hhsFmock xenografts. (B) **P<0.01 vs. the BxPC3 and BxPC3+hhsFmock. Inserts show representative tumors from the 3 groups in each case. Scale bar, 1 cm.
Finally, to examine the effect of Cav-1 expression in fibroblasts on the chemosensitivity of pancreatic cancer cells exposed to gemcitabine, co-injection experiments as described above were undertaken following the administration of gemcitabine. Beginning on day 4 post-cells’ inoculation, the mice were injected intraperitoneally with either 100 mg/kg gemcitabine or an equivalent volume of saline as the control, on a weekly basis for 3 weeks (till the end of the experiment). As shown in Fig. 8, tumors that developed from the co-injection of BxPC3 and hhsFshCAV fibroblasts were substantially more resistant as compared to those developed from the co-inoculation of hhsFmock fibroblasts (P<0.01vs. BxPC3 + hhsFmock + gemcitabine). These tumors exhibited no response at all when compared to the corresponding untreated tumors (i.e., vs. BxPC3 + hhsFshCAV).
Figure 8.
Downregulation of caveolin-1 in fibroblasts results in the increased chemoresistance of BxPC3 pancreatic cancer cells to gemcitabine. The (A) volumes and (B) the post mortem tumor weights for BxPC3 cells alone or co-injected with either hhsFmock or hhsFshCAV at a ratio of 3:1 (BxPC3 vs. hhsF), treated or untreated with gemcitabine are shown as the average ± SD. *P<0.05; **P<0.01; ***P<0,001; ****P<0,0001. Insert shows representative tumors from the 4 groups as follows: (C) BxPC3 + hhsFmock; (D) BxPC3 + hhsFmock + gem-citabine; (E) BxPC3 + hhsFshCAV; (F) BxPC3 + hhsFshCAV + gemcitabine. Scale bar, 1 cm. GEM; gemcitabine.
Taken together, these results demonstrate that although the levels of Cav-1 seem to have a minor effect on cellular proliferation and none regarding the chemosensitivity of BxPC3 cancer cells in vitro, the lack of Cav-1 expression in fibroblasts within the cancer microenvironment may affect more substantially both the growth and chemoresistance of the tumor cells.
Immunohistochemical analysis of xenografts does not identify differences in the amount of stroma
To determine the contribution of the stroma to the tumor weight in xenografts from the co-injection experiments of explanted xenografts, IHC was performed. The amount of stroma did not differ across the experimental conditions, indicating that differences in tumor size are not attributed to differences in stromal content (data now shown).
Discussion
In this study, we initially observed that there was an inverse association between Cav-1 expression in cancer cells and stromal fibroblasts. Despite the small number of cases, it was consistently observed that in areas of well-differentiated tumors, fibroblasts stained strongly for Cav-1, while cancer cells were negative or stained only weakly. By striking contrast, in areas of poorly differentiated tumors, the cancer cells exhibited a high expression of Cav-1 and were surrounded by fibroblasts with a low Cav-1 expression. These results are in accordance with those of previous studies, which linked Cav-1 expression in cancer cells to poor differentiation (30,36). However, to the best of our knowledge, this is the first study showing an opposite pattern of Cav-1 expression between tumor and stromal cells in pancreatic cancer. These results are in agreement with those of a previous study on colorectal cancer, reporting the differential expression of Cav-1 between stroma and cancer cells (37). Alshenawy and Ali reported that the overexpression of Cav-1 in cancer cells was associated with adverse prognostic features, while a higher stromal expression was associated with small non-metastasizing tumors and thus a better prognosis (37). The exact mechanisms behind this observation are not yet clear; however, it has been suggested that regional differences in hypoxia or acidity may explain tumor heterogeneity (38,39).
To determine whether this differential expression of Cav-1 in cancer cells and the surrounding stroma have an influence on tumor biology, we examined the effects of decreased levels of Cav-1 expression on cell proliferation and chemosensitivity in a human pancreatic cancer cell line with a high Cav-1 expression. Four commonly used human pancreatic cancer cell lines, i.e., BxPC3 (moderate to poor differentiation) (40), AsPC1, PANC-1 and MIAPaCa-2 (all 3 of poor differentiation) were tested for caveolin expression (Fig. 1A). In our laboratory, BxPC3 cells were found to express the highest levels of Cav-1 with the MIAPaCa-2 cells showing minimal expression. We herein though need to point out that the data from previous studies regarding the expression of Cav-1 in MIAPaCa-2 cells have been controversial, as in the study by Salem et al (29), it was reported a low expression of the protein, in agreement with our data, while Chatterjee et al (36) reported high levels of Cav-1 expression in MIAPaCa-2 cells. In studies using mice to compare these cell lines for their tumorigenicity (xenograft studies), it was suggested that BxPC3 may be more aggressive as compared to PANC1, AsPC1 and MIAPaCa-2 cells (40). Thus, we decided to proceed with the BxPC3 cell line in this study.
The results revealed that the decreased expression of Cav-1 only marginally increased the cellular proliferation and DNA synthesis, suggesting that Cav-1 may act as a tumor suppressor to a certain extent. The downregulation of the protein resulted in the increased migratory capability of the BxPC3 cancer cell line in the wound healing assay. However, we need to point out herein that this effect may be the result of the increased proliferation of the cells.
Our results are in agreement with those reported in other studies regarding the role of Cav-1 in the migration and invasion of pancreatic cancer cell lines (16,29,41). In support of the inhibitory effects of Cav-1 on the migration and invasion of pancreatic cancer cells, Han and Zhu (41) reported that the knockdown of Cav-1 promoted the activity of matrix metalloprotease (MMP)2 and 9. However, these observations are not supported by Chatterjee et al (36), who reported opposite results, suggesting that the overexpression of Cav-1 may act as a promoting factor for cancer cell proliferation, invasion and migration. The use of different cell lines or other methods to down- or upregulate Cav-1 and to address migration and invasion may potentially explain these differences.
Cav-1 expression in pancreatic cancer cells has been related to a decreased sensitivity to ionizing radiation (42), while chemosensitivity is preserved through EMT inhibition (29). In this study, a variety of chemotherapeutic agents commonly used in pancreatic cancer were tested in vitro. It was demonstrated that lower levels of the protein did not affect the in vitro chemosensitivity of the BxPC3 cells to all the tested chemotherapeutics. Finally, in an effort to shed some light on the capacity of the Cav-1 to affect tumorigenicity of cancer cells, we performed an in vivo experiment by injecting BxPC3mock and BxPC3shCAV cells into SCID mice. This experiment clearly demonstrated that Cav-1 expression in the injected cells played no role in overall tumor growth.
Tumor development involves a stromal microenvironment that contains fibroblasts, macrophages and other cell types. It is now widely accepted that cancer-associated fibroblasts play a major role in both tumor initiation and progression. In this context, it has been proposed that the absence of Cav-1 may be a characteristic of a cancer-associated fibroblast phenotype (43,44). Pancreatic cancer stromal cells have been shown to express low levels of Cav-1 (11), and in a cohort of 45 patients, the expression levels of this protein were found to be related with the TNM stage, HER-2/neu amplification and the overall prognosis of the disease (21).
We then set out to determine whether differences in Cav-1 expression in the tumor microenvironment have a direct influence on the phenotype of pancreatic cancer cells. Human dermal fibroblasts, manipulated to express decreased levels of Cav-1 compared to the control, induced the increased invasion of pancreatic cancer cells in vitro. Since the two types of cells were not in direct contact, these findings suggest that the increased invasiveness of the tumor cells may be driven by secreted chemotactic agents. Such an effect has already been demonstrated in breast cancer (45); however, to the best of our knowledge, this is the first report to demonstrate that Cav-1 silencing in fibroblasts may directly regulate the invasiveness of pancreatic tumor cells in a paracrine manner. In support of this observation, it has been suggested that cancer-associated fibroblasts (CAFs) with a decreased expression of Cav-1 may provide nutritional support to the cancer cells by the phenomenon of reverse Warburg effect (46).
Subsequently, we examined the in vivo effect of Cav-1 silencing in fibroblasts. From these in vivo experiments it was evident that the cancer cells gained developmental advantage when they were co-injected with fibroblasts that had a decreased expression of Cav-1. Indeed, an almost 3-fold increase in tumor weight was observed in the tumors that were developed by the co-injection of fibroblasts with silenced Cav-1 and BxPC3 cells, as compared to BxPC3- and hhsFmock-derived tumors. These results outweighed the need to perform co-culture experiments in vitro. Similar results have been reported by Capozza et al (47) in a murine model of melanoma, in which the downregulation of Cav-1 in CAFs promoted the development of melanoma cells through paracrine Sonic Hedgehog signaling. Similarly, Bonuccelli et al (45) reported that fibroblasts with silenced Cav-1 expression enhanced the growth of MDA-MB231 breast cancer xenografts.
Once we obtained clear evidence that the downregulation of Cav-1 expression in fibroblasts could provide a growth advantage to cancer cells in vivo, we set out to determine whether the absence of Cav-1 in fibroblasts of the tumor microenvironment may play a role in the development of chemoresistance. According to our findings, the downregulation of Cav-1 in stromal fibroblasts triggered the development of chemoresistance when mice were treated with gemcitabine. Indeed, tumors derived from the inoculation with fibroblasts with silenced Cav-1 expression and BxPC3 cells alone did not respond to gemcitabine chemotherapy. These data suggest a possible link between stromal Cav-1 expression and chemo-resistance to gemcitabine, which is a standard drug used in the treatment of pancreatic cancer. In accordance to our initial clinical observation, we could argue that hhsFshCAV represent the stroma in the poorly differentiated pancreatic cancer area and the fibroblasts with a decreased expression of Cav-1 provide survival gain and chemoresistance to the pancreatic cancer cells.
The stroma has been regarded for a long time as a potential barrier to the diffusion of chemotherapeutic agents into tumors. Strategies to deplete the tumor-related stroma have therefore been considered as a rational approach to improving response to chemotherapy (48). Unfortunately, this approach has not been translated into successful clinical trials. Recent studies have actually suggested that stroma depletion may accelerate cancer development, thus reducing survival (49,50). Furthermore, the recent observation that CAFs lead the scavenging of gemcitabine is a further possible mechanism of treatment failure in pancreatic cancer (51). Methods to restore the stromal expression of Cav-1 (52) may represent another potential therapeutic strategy for pancreatic cancer by increasing the sensitivity to chemotherapy.
Despite some limitations to our study, mainly pertaining to the use of dermal immortalized fibroblasts instead of CAFs and the use of a single cell line, our data support the hypothesis that Cav-1 expression in the stromal component of pancreatic cancer may influence cancer cell growth and the development of resistance to chemotherapy. Nevertheless, xenograft models with the use of non-tumor associated fibroblasts have been used successfully for the study of chemotherapy resistance (53). Our group aims to further shed light onto the role of Cav-1 in pancreatic cancer by developing patient derived xenografts and trying to isolate cancer-associated normal fibroblasts that could be used to address the role of Cav-1 in clinically more relevant settings.
In conclusion, the data presented herein suggest that Cav-1 expressed in the stroma rather than in the tumor cells has an impact on tumor development and chemoresistance in pancreatic cancer. However, further studies are warranted to investigate whether the manipulation of Cav-1 expression in CAFs may represent a valid and effective therapeutic approach in pancreatic cancer.
Acknowledgments
Not applicable.
Funding
Dr Kamposioras was supported by the European Society of Medical Oncology (ESMO) Translational Research Fellowship. This study was supported by the Hellenic Society of Medical Oncology (HeSMO) Fellowship program.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
KK, KD, AA, FDG, CNP, NS, GV, SPP conceived and designed the experiments. KK, CT, CV, AD, VL, GM performed the experiments. KK, KD, FDG, CV, NS analyzed the data. KK, KD, FDG CP, NS, GV, SPP wrote and modified the manuscirpt. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
All patients provided informed consent prior to the collection of the samples and all procedures were approved by and were in compliance with the ethical standards of the Leeds Teaching Hospitals NHS Trust (R&I Ref. no. CO18/113235). The handling and experimentation of the animals were conducted in accordance with the Greek laws (PD 56/2013 and Circular 2215/117550/2013) and the guidelines of the European Union (2013/63/EU) under a licenced protocol approved by the IACUC and Greek authorities (Lisence no. 5542/228006, IACUC; Professor Dr N. Pitsikas, Dr A. Zacharioudaki, Dr J. Chloptsios and Dr A. Konstantinidis).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2014. Ann Oncol. 2014;25:1650–1656. doi: 10.1093/annonc/mdu138. [DOI] [PubMed] [Google Scholar]
- 2.Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goéré D, Seufferlein T, Haustermans K, Van Laethem JL, Conroy T, et al. ESMO Guidelines Committee: Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v56–v68. doi: 10.1093/annonc/mdv295. [DOI] [PubMed] [Google Scholar]
- 3.Andersson R, Aho U, Nilsson BI, Peters GJ, Pastor-Anglada M, Rasch W, Sandvold ML. Gemcitabine chemoresistance in pancreatic cancer: Molecular mechanisms and potential solutions. Scand J Gastroenterol. 2009;44:782–786. doi: 10.1080/00365520902745039. [DOI] [PubMed] [Google Scholar]
- 4.Williams TM, Lisanti MP. The Caveolin genes: From cell biology to medicine. Ann Med. 2004;36:584–595. doi: 10.1080/07853890410018899. [DOI] [PubMed] [Google Scholar]
- 5.Chen T, Liu L, Xu HX, Wang WQ, Wu CT, Yao WT, Yu XJ. Significance of caveolin-1 regulators in pancreatic cancer. Asian Pac J Cancer Prev. 2013;14:4501–4507. doi: 10.7314/APJCP.2013.14.8.4501. [DOI] [PubMed] [Google Scholar]
- 6.Boscher C, Nabi IR. Caveolin-1: Role in cell signaling. Adv Exp Med Biol. 2012;729:29–50. doi: 10.1007/978-1-4614-1222-9_3. [DOI] [PubMed] [Google Scholar]
- 7.Del Pozo MA, Schwartz MA. Rac, membrane heterogeneity, caveolin and regulation of growth by integrins. Trends Cell Biol. 2007;17:246–250. doi: 10.1016/j.tcb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Wary KK, Mariotti A, Zurzolo C, Giancotti FG. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell. 1998;94:625–634. doi: 10.1016/S0092-8674(00)81604-9. [DOI] [PubMed] [Google Scholar]
- 9.Goetz JG, Lajoie P, Wiseman SM, Nabi IR. Caveolin-1 in tumor progression: The good, the bad and the ugly. Cancer Metastasis Rev. 2008;27:715–735. doi: 10.1007/s10555-008-9160-9. [DOI] [PubMed] [Google Scholar]
- 10.Suzuoki M, Miyamoto M, Kato K, Hiraoka K, Oshikiri T, Nakakubo Y, Fukunaga A, Shichinohe T, Shinohara T, Itoh T, et al. Impact of caveolin-1 expression on prognosis of pancreatic ductal adenocarcinoma. Br J Cancer. 2002;87:1140–1144. doi: 10.1038/sj.bjc.6600619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanase CP, Dima S, Mihai M, Raducan E, Nicolescu MI, Albulescu L, Voiculescu B, Dumitrascu T, Cruceru LM, Leabu M, et al. Caveolin-1 overexpression correlates with tumour progression markers in pancreatic ductal adenocarcinoma. J Mol Histol. 2009;40:23–29. doi: 10.1007/s10735-008-9209-7. [DOI] [PubMed] [Google Scholar]
- 12.Nam KH, Lee BL, Park JH, Kim J, Han N, Lee HE, Kim MA, Lee HS, Kim WH. Caveolin 1 expression correlates with poor prognosis and focal adhesion kinase expression in gastric cancer. Pathobiology. 2013;80:87–94. doi: 10.1159/000341685. [DOI] [PubMed] [Google Scholar]
- 13.Han F, Zhang J, Shao J, Yi X. Caveolin-1 promotes an invasive phenotype and predicts poor prognosis in large cell lung carcinoma. Pathol Res Pract. 2014;210:514–520. doi: 10.1016/j.prp.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Ando T, Ishiguro H, Kimura M, Mitsui A, Mori Y, Sugito N, Tomoda K, Mori R, Harada K, Katada T, et al. The overexpression of caveolin-1 and caveolin-2 correlates with a poor prognosis and tumor progression in esophageal squamous cell carcinoma. Oncol Rep. 2007;18:601–609. [PubMed] [Google Scholar]
- 15.Huang C, Qiu Z, Wang L, Peng Z, Jia Z, Logsdon CD, Le X, Wei D, Huang S, Xie K. A novel FoxM1-caveolin signaling pathway promotes pancreatic cancer invasion and metastasis. Cancer Res. 2012;72:655–665. doi: 10.1158/0008-5472.CAN-11-3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin M, DiVito MM, Merajver SD, Boyanapalli M, van Golen KL. Regulation of pancreatic cancer cell migration and invasion by RhoC GTPase and caveolin-1. Mol Cancer. 2005;4:21. doi: 10.1186/1476-4598-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye Y, Miao SH, Lu RZ, Zhou JW. Prognostic value of caveolin-1 expression in gastric cancer: A meta-analysis. Asian Pac J Cancer Prev. 2014;15:8367–8370. doi: 10.7314/APJCP.2014.15.19.8367. [DOI] [PubMed] [Google Scholar]
- 18.Witkiewicz AK, Dasgupta A, Sotgia F, Mercier I, Pestell RG, Sabel M, Kleer CG, Brody JR, Lisanti MP. An absence of stromal caveolin-1 expression predicts early tumor recurrence and poor clinical outcome in human breast cancers. Am J Pathol. 2009;174:2023–2034. doi: 10.2353/ajpath.2009.080873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma X, Liu L, Nie W, Li Y, Zhang B, Zhang J, Zhou R. Prognostic role of caveolin in breast cancer: A meta-analysis. Breast. 2013;22:462–469. doi: 10.1016/j.breast.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Z, Han FH, Yang SB, Hua LX, Wu JH, Zhan WH. Loss of stromal caveolin-1 expression in colorectal cancer predicts poor survival. World J Gastroenterol. 2015;21:1140–1147. doi: 10.3748/wjg.v21.i4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shan T, Lu H, Ji H, Li Y, Guo J, Chen X, Wu T. Loss of stromal caveolin-1 expression: A novel tumor microenvironment biomarker that can predict poor clinical outcomes for pancreatic cancer. PLoS One. 2014;9:e97239. doi: 10.1371/journal.pone.0097239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia Y, Wang N, Wang J, Tian H, Ma W, Wang K, Tan B, Zhang G, Yang S, Bai B, et al. Down-regulation of stromal caveolin-1 expression in esophageal squamous cell carcinoma: A potent predictor of lymph node metastases, early tumor recurrence, and poor prognosis. Ann Surg Oncol. 2014;21:329–336. doi: 10.1245/s10434-013-3225-x. [DOI] [PubMed] [Google Scholar]
- 23.Zhao X, He Y, Gao J, Fan L, Li Z, Yang G, Chen H. Caveolin-1 expression level in cancer associated fibroblasts predicts outcome in gastric cancer. PLoS One. 2013;8:e59102. doi: 10.1371/journal.pone.0059102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakatani K, Wada T, Nakamura M, Uzawa K, Tanzawa H, Fujita S. Expression of caveolin-1 and its correlation with cisplatin sensitivity in oral squamous cell carcinoma. J Cancer Res Clin Oncol. 2005;131:445–452. doi: 10.1007/s00432-004-0662-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tirado OM, MacCarthy CM, Fatima N, Villar J, Mateo-Lozano S, Notario V. Caveolin-1 promotes resistance to chemotherapy-induced apoptosis in Ewing’s sarcoma cells by modulating PKCalpha phosphorylation. Int J Cancer. 2010;126:426–436. doi: 10.1002/ijc.24754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, Wang N, Liu P, Peng F, Tang H, Chen Q, Xu R, Dai Y, Lin Y, Xie X, et al. Caveolin-1, a stress-related oncotarget, in drug resistance. Oncotarget. 2015;6:37135–37150. doi: 10.18632/oncotarget.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hehlgans S, Cordes N. Caveolin-1: An essential modulator of cancer cell radio-and chemoresistance. Am J Cancer Res. 2011;1:521–530. [PMC free article] [PubMed] [Google Scholar]
- 28.Hehlgans S, Eke I, Storch K, Haase M, Baretton GB, Cordes N. Caveolin-1 mediated radioresistance of 3D grown pancreatic cancer cells. Radiother Oncol. 2009;92:362–370. doi: 10.1016/j.radonc.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Salem AF, Bonuccelli G, Bevilacqua G, Arafat H, Pestell RG, Sotgia F, Lisanti MP. Caveolin-1 promotes pancreatic cancer cell differentiation and restores membranous E-cadherin via suppression of the epithelial-mesenchymal transition. Cell Cycle. 2011;10:3692–3700. doi: 10.4161/cc.10.21.17895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witkiewicz AK, Nguyen KH, Dasgupta A, Kennedy EP, Yeo CJ, Lisanti MP, Brody JR. Co-expression of fatty acid synthase and caveolin-1 in pancreatic ductal adenocarcinoma: Implications for tumor progression and clinical outcome. Cell Cycle. 2008;7:3021–3025. doi: 10.4161/cc.7.19.6719. [DOI] [PubMed] [Google Scholar]
- 31.Dimas K, Hatziantoniou S, Tseleni S, Khan H, Georgopoulos A, Alevizopoulos K, Wyche JH, Pantazis P, Demetzos C. Sclareol induces apoptosis in human HCT116 colon cancer cells in vitro and suppression of HCT116 tumor growth in immunodeficient mice. Apoptosis. 2007;12:685–694. doi: 10.1007/s10495-006-0026-8. [DOI] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Iyer VR, Eisen MB, Ross DT, Schuler G, Moore T, Lee JC, Trent JM, Staudt LM, Hudson J, Jr, Boguski MS, et al. The transcriptional program in the response of human fibroblasts to serum. Science. 1999;283:83–87. doi: 10.1126/science.283.5398.83. [DOI] [PubMed] [Google Scholar]
- 34.Tsimplouli C, Demetzos C, Hadzopoulou-Cladaras M, Pantazis P, Dimas K. In vitro activity of dietary flavonol congeners against human cancer cell lines. Eur J Nutr. 2012;51:181–190. doi: 10.1007/s00394-011-0204-5. [DOI] [PubMed] [Google Scholar]
- 35.Dimas K, Demetzos C, Vaos B, Marselos M, Kokkinopoulos D. Cytotoxic and antiproliferative effects of heptaacetyltiliroside on human leukemic cell lines. Leuk Res. 1999;23:1021–1033. doi: 10.1016/S0145-2126(99)00124-1. [DOI] [PubMed] [Google Scholar]
- 36.Chatterjee M, Ben-Josef E, Thomas DG, Morgan MA, Zalupski MM, Khan G, Andrew Robinson C, Griffith KA, Chen CS, Ludwig T, et al. Caveolin-1 is associated with tumor progression and confers a multi-modality resistance phenotype in pancreatic cancer. Sci Rep. 2015;5:10867. doi: 10.1038/srep10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alshenawy HA, Ali MA. Differential caveolin-1 expression in colon carcinoma and its relation to E-cadherin-β-catenin complex. Ann Diagn Pathol. 2013;17:476–482. doi: 10.1016/j.anndiagpath.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Erkan M, Kurtoglu M, Kleeff J. The role of hypoxia in pancreatic cancer: A potential therapeutic target. Expert Rev Gastroenterol Hepatol. 2016;10:301–316. doi: 10.1586/17474124.2016.1117386. [DOI] [PubMed] [Google Scholar]
- 39.Verbeke C. Morphological heterogeneity in ductal adenocarcinoma of the pancreas - Does it matter. Pancreatology. 2016;16:295–301. doi: 10.1016/j.pan.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Deer EL, González-Hernández J, Coursen JD, Shea JE, Ngatia J, Scaife CL, Firpo MA, Mulvihill SJ. Phenotype and genotype of pancreatic cancer cell lines. Pancreas. 2010;39:425–435. doi: 10.1097/MPA.0b013e3181c15963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han F, Zhu HG. Caveolin-1 regulating the invasion and expression of matrix metalloproteinase (MMPs) in pancreatic carcinoma cells. J Surg Res. 2010;159:443–450. doi: 10.1016/j.jss.2009.03.079. [DOI] [PubMed] [Google Scholar]
- 42.Cordes N, Frick S, Brunner TB, Pilarsky C, Grützmann R, Sipos B, Klöppel G, McKenna WG, Bernhard EJ. Human pancreatic tumor cells are sensitized to ionizing radiation by knockdown of caveolin-1. Oncogene. 2007;26:6851–6862. doi: 10.1038/sj.onc.1210498. [DOI] [PubMed] [Google Scholar]
- 43.Di Vizio D, Morello M, Sotgia F, Pestell RG, Freeman MR, Lisanti MP. An absence of stromal caveolin-1 is associated with advanced prostate cancer, metastatic disease and epithelial Akt activation. Cell Cycle. 2009;8:2420–2424. doi: 10.4161/cc.8.15.9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinez-Outschoorn UE, Pavlides S, Whitaker-Menezes D, Daumer KM, Milliman JN, Chiavarina B, Migneco G, Witkiewicz AK, Martinez-Cantarin MP, Flomenberg N, et al. Tumor cells induce the cancer associated fibroblast phenotype via caveolin-1 degradation: Implications for breast cancer and DCIS therapy with autophagy inhibitors. Cell Cycle. 2010;9:2423–2433. doi: 10.4161/cc.9.12.12048. [DOI] [PubMed] [Google Scholar]
- 45.Bonuccelli G, Whitaker-Menezes D, Castello-Cros R, Pavlides S, Pestell RG, Fatatis A, Witkiewicz AK, Vander Heiden MG, Migneco G, Chiavarina B, et al. The reverse Warburg effect: Glycolysis inhibitors prevent the tumor promoting effects of caveolin-1 deficient cancer associated fibroblasts. Cell Cycle. 2010;9:1960–1971. doi: 10.4161/cc.9.10.11601. [DOI] [PubMed] [Google Scholar]
- 46.Pavlides S, Tsirigos A, Vera I, Flomenberg N, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, Pestell RG, et al. Loss of stromal caveolin-1 leads to oxidative stress, mimics hypoxia and drives inflammation in the tumor microenvironment, conferring the ‘reverse Warburg effect’: A transcriptional informatics analysis with validation. Cell Cycle. 2010;9:2201–2219. doi: 10.4161/cc.9.11.11848. [DOI] [PubMed] [Google Scholar]
- 47.Capozza F, Trimmer C, Castello-Cros R, Katiyar S, Whitaker- Menezes D, Follenzi A, Crosariol M, Llaverias G, Sotgia F, Pestell RG, et al. Genetic ablation of Cav1 differentially affects melanoma tumor growth and metastasis in mice: Role of Cav1 in Shh heterotypic signaling and transendothelial migration. Cancer Res. 2012;72:2262–2274. doi: 10.1158/0008-5472.CAN-11-2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hessmann E, Patzak MS, Klein L, Chen N, Kari V, Ramu I, Bapiro TE, Frese KK, Gopinathan A, Richards FM, et al. Fibroblast drug scavenging increases intratumoural gemcitabine accumulation in murine pancreas cancer. Gut. 2018;67:497–507. doi: 10.1136/gutjnl-2016-311954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bocci G, Fioravanti A, Orlandi P, Di Desidero T, Natale G, Fanelli G, Viacava P, Naccarato AG, Francia G, Danesi R. Metronomic ceramide analogs inhibit angiogenesis in pancreatic cancer through up-regulation of caveolin-1 and thrombos-pondin-1 and down-regulation of cyclin D1. Neoplasia. 2012;14:833–845. doi: 10.1593/neo.12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma Y, Lin Z, Fallon JK, Zhao Q, Liu D, Wang Y, Liu F. New mouse xenograft model modulated by tumor-associated fibroblasts for human multi-drug resistance in cancer. Oncol Rep. 2015;34:2699–2705. doi: 10.3892/or.2015.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.