Abstract
Myocilin is highly expressed in the trabecular meshwork (TM), which plays an important role in the regulation of intraocular pressure (IOP). Myocilin abnormalities may cause dysfunction of the TM, potentially leading to increased IOP. High IOP is a well-known primary risk factor for glaucoma. Myocilin mutations are common among glaucoma patients, and they are implicated in juvenile-onset open-angle glaucoma (JOAG) and adult-onset primary open-angle glaucoma (POAG). Aggregation of aberrant mutant myocilins is closely associated with glaucoma pathogenesis. The aim of the present review was to discuss the recent findings regarding the major physiological functions of myocilin, such as intra- and extracellular proteolytic processes. We also aimed to discuss the risk factors associated with myocilin and the development of glaucoma, such as misfolded/mutant myocilin, imbalance of myocilin and extracellular proteins, and instability of mutant myocilin associated with temperature. Finally, we further outlined certain issues that are yet to be resolved, which may represent the basis for future studies on the role of myocilin in glaucoma.
Key words: myocilin, function, pathogenesis, glaucoma, trabecular meshwork
1. Introduction
Glaucoma is second leading cause of blindness after cataract, and the leading cause of irreversible blindness globally (1-4). Primary open-angle glaucoma (POAG) is the most prevalent form of glaucoma (5) and is responsible for ~90% of all cases (6). The pathogenesis of glaucoma remains unknown; however, accumulating evidence indicates that genetic factors may play a causative role (7). The myocilin gene (MYOC), which encodes the secreted protein myocilin, is the first and most extensively investigated gene associated with familial forms of POAG (8,9). MYOC is detected in the aqueous humor and MYOC mutations are the most common type of mutation in patients with glaucoma (10), accounting for 10% of juvenile-onset open-angle glaucoma (JOAG) (11) and 2-4% of adult-onset POAG (12). JOAG, unlike adult-onset POAG, has a close genotype-phenotype association with regard to myocilin mutations and glaucoma, and typical signs include high intra-ocular pressure (IOP) (13,14), severe optic nerve damage and earlier age at onset (usually <40 years) (15,16), which, if left untreated, results in severe visual impairment (17).
Myocilin is ubiquitously expressed in normal tissues and organs; however, myocilin-associated disease only occurs in the eye (18). Myocilin is widely expressed in ocular tissues and highly expressed in the trabecular meshwork (TM) (19). Wild-type myocilin is secreted in the TM (9), is located in the aqueous humor and plays an important role in the regulation of IOP (20,21). High IOP is a risk factor for glaucoma (22,23) and reduction of IOP is an approach to glaucoma treatment (24). Mutant myocilin aggregation is closely associated with glaucoma (25) and it may be involved in morphological changes in the TM that may result in cell apoptosis (7). A growing body of evidence suggests that myocilin, the TM, IOP and glaucoma are closely associated.
The present review exclusively focused on the results of the latest studies that provide an insight into the physiological functions of myocilin and its role in the pathogenesis of glaucoma in the TM. An overview of the primary findings and future research topics requiring further investigation was performed in the present review.
2. Physiological functions and characteristics of myocilin
Structure of myocilin
Despite numerous studies over the 20 years since its discovery as a glaucoma-associated gene in 1997, the physiological functions and biological activities of myocilin in the TM remain poorly understood. The first report of identified myocilin mutations in autosomal dominant POAG came from Stone et al (26), and myocilin was found to map to the GLC1A locus at 1q24.3-q25.2 (OMIM: 601652). Myocilin, which encodes a 504-amino acid glycoprotein and undergoes glycosylation at amino acid residues 57-59 (27), has three exons and contains two major homology regions, the N- and C-terminus (Fig. 1) (23,26,28-35). Notably, the majority of myocilin mutations are localized in exon 3 (Fig. 1). The N-terminus of myocilin contains leucine zippers (LZ) within two coil-coil domains (35). Furthermore, the N-terminus is involved in the initial myocilin oligomerization through LZ (36), and in the extracellular interactions of myocilin with other extracellular proteins through two coil-coil domains (35,37). The C-terminus contains olfactomedin (OLF), which is important for the structure and function of myocilin (35), specifically in the process of intracellular trafficking (36). Notably, N- and C-terminus functions affect the aqueous humor outflow in the TM.
Figure 1.
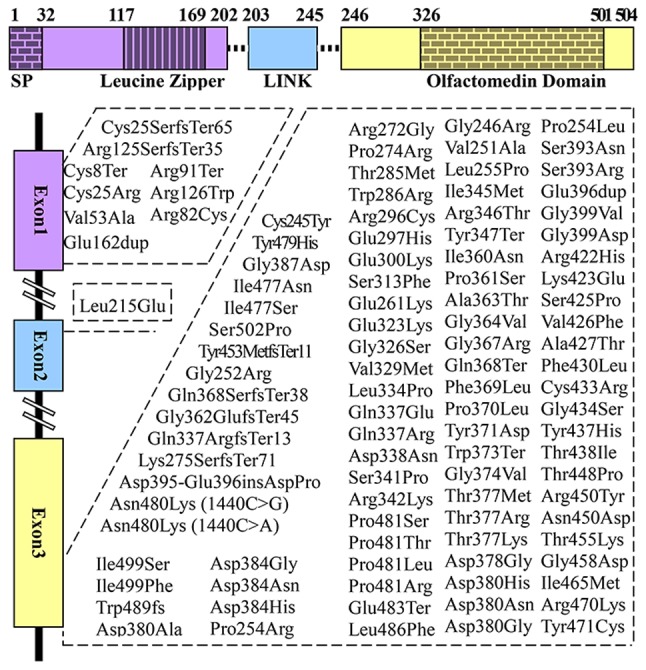
Structure of myocilin and pathogenic mutations localized in exons 1-3 (33). Three modules encoded by exons 1-3 approximately coincide with the N-terminus, LINK and C-terminus. SP, signal peptide; LINK, linker domain.
Intracellular proteolytic process
Normally, myocilin is intra-cellularly cleaved within the endoplasmic reticulum (ER) of TM cells and secreted into the aqueous humor (33,38). C-terminal myocilin fragments have been detected in the TM and the aqueous humor (23,33). N-terminal myocilin containing LZ has also been identified (39); however, this is intracellularly retained in the ER (23,33,36,40,41). N-terminal fragments can be intracellularly degraded during proteolytic processing, or they can interact with other intracellular proteins (40). Previous studies have suggested that myocilin undergoes proteolytic cleavage, and that the location of the proteolytic cleavage site is possibly between Glu214-Leu215 (39,42) or between Arg226-Ile227 (23). It was reported that myocilin fragments containing OLF did not change the outflow capacity of the aqueous humor, suggesting that both OLF and LZ fragments must coexist for myocilin to function properly in the intracellular proteolytic process (39).
Similar to myocilin, calpain II (cysteine protease) is also present within the lumen of the Golgi apparatus and the ER (43). Calpain II is required for the intracellular proteolytic cleavage of myocilin (40). The proteolytic processing of myocilin does not require the N-terminus, and two different domains of myocilin participate in the proteolytic processing through calpain II (40): i) C-terminal OLF, which likely acts as a substrate binding site recognized by calpain II; and ii) linker domain (LINK), which acts as the cleavage site (Fig. 2) (40). These findings are supported by previous studies (23,42,44), suggesting that myocilin mutations located at OLF may inhibit the proteolytic processing of myocilin. Amino acid positions mutated in OLF likely affect the structure of the myocilin binding site to calpain II (40). Interestingly, Pro370Leu, which contributes to the most severe glaucoma phenotype (44), produces the most severe inhibition of proteolytic processing. The inhibition of proteolytic processing by Glu323Lys and Asp380Ala is less severe, causing less severe glaucoma (23). However, the association of the severity of glaucoma with the inhibition of proteolytic processing remains unclear.
Figure 2.
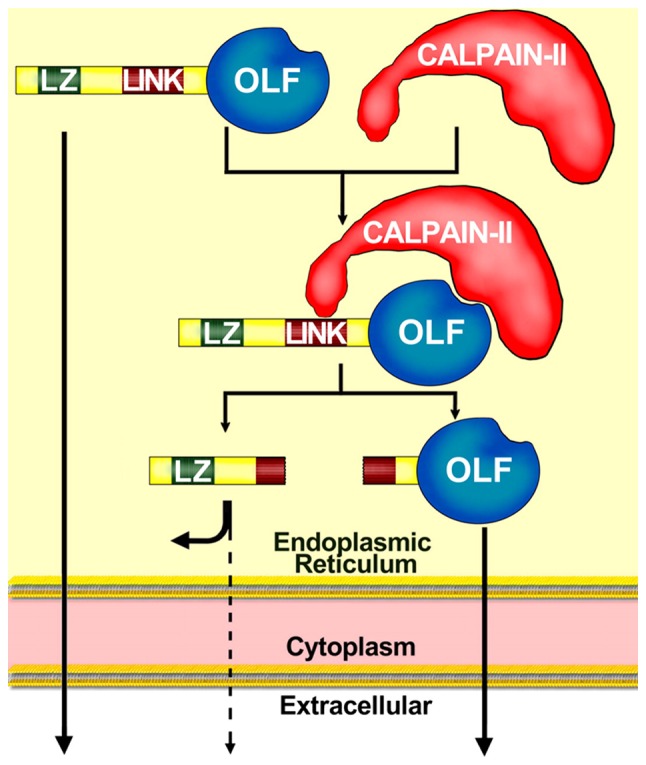
Proteolytic process of myocilin through calpain II (40). The proteo-lytic processing of myocilin is carried out by calpain II in the endoplasmic reticulum, producing two myocilin fragments: One containing LZ that is intracellularly retained and another containing OLF that is extracellularly secreted. Some full-length myocilins with LZ and OLF are also secreted. LINK contains the cleavage site. LZ, leucine zippers; OLF, olfactomedin; LINK, linker domain.
Extracellular proteolytic process
Although the physiological function of the intracellular proteolytic processing of myocilin remains unknown, the amount of proteolytic myocilin may be associated with the regulation of the normal TM structure through extracellular proteins, including fibrillin-1 (45), secreted protein acidic and rich in cysteine (SPARC) (34), hevin (34,46), collagen (45), optimedin (47), decorin (45), fibronectin (48) and laminin (45,49), which contribute to the regulation of aqueous humor outflow that can affect IOP (23). The identification of proteins interacting with myocilin is a possible approach to elucidating its functions, since interacting proteins are typically involved in the same physiological and pathological processes (50).
The first report of the possible role of proteolytic processing in regulating the interactions of myocilin with itself or other extracellular proteins came from Aroca-Aguilar et al (33,34), who demonstrated that homoaggregates of myocilin monomers and myocilin complexes (containing oligomers, matricellular proteins and extracellular matrix proteins) can form a dynamic extracellular network (Fig. 3) (34). The proteolytic processing of myocilin occurs in the relevant elements involved in IOP homeostasis (such as the aqueous humor and the TM); thus, the proteolytic cleavage in the dynamic myocilin network possibly participates in regulating IOP (33) through controlling IOP homeostasis (aqueous humor production and drainage). Furthermore, the myocilin network may act as a link between the extracellular matrix and matricellular proteins (34). Coincidently, OLFs in the extracellular network are similar to those resulting from intracellular proteolytic processing of myocilin through calpain II (Figs. 2 and 3) (23,34,40).
Figure 3.
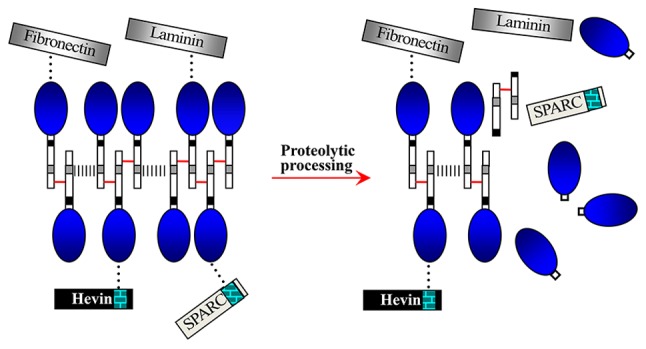
Interactions of myocilin with extracellular matrix proteins (laminin and fibronectin) and matricellular proteins (SPARC and hevin) (34). Homoaggregates of myocilin monomers covalently interact through disulfide bonds (short red lines) within LZ (12). Myocilin complexes interact by noncovalent bonds (grey dashed lines) in N-terminus (rectangle linked to the blue oval). Full-length myocilins non-covalently (black dots) interact with the extracellular calcium binding domains (brick pattern) of SPARC and hevin through OLF (blue oval). Interacting fashion of myocilin with laminin and fibronectin could be similar to that of SPARC and hevin. SPARC, secreted protein acidic and rich in cysteine; LZ, leucine zippers; OLF, olfactomedin.
Elevated IOP and overexpression of myocilin caused by inducers
Myocilin is stimulated by various inducers, including dexamethasone (DXM) (11,51-55), pentablock copolymer DXM nanoformulations (56), retinoic acid (57), transforming growth factor (TGF)-β1 (11), TGF-β2 (58), optineurin (59,60), mechanical stretch (11), rotenone (61), and hydrogen peroxide-inducible clone-5 (62). DEX is widely recognized as a major contributor to the induction of myocilin, which may cause high IOP.
Elevated IOP is a known primary risk factor for glaucoma (22,23); however, not all cases of glaucoma exhibit high IOP (63,64). Elevated IOP is caused by increased resistance to aqueous humor outflow through the TM (65,66) and accounts for visual field loss; however, its pathogenesis remains unknown (67). Homeostasis of aqueous humor drainage through the TM is essential for the maintenance of normal IOP (68). DXM-induced overexpression of myocilin in the TM cells (11,54,55) may increase IOP (69). Additionally, overexpression of myocilin induced by DXM possibly affects focal adhesion, stress fibers and actin reorganization in TM cells, which subsequently results in ER aggregation and TM cell stiffness, leading to increased outflow resistance (62) and elevated IOP. However, the results regarding the reversal of myocilin (52,70) and increased IOP (70,71) do not support the hypothesis that myocilin overexpression causes increased IOP or glaucoma; this is supported by the following data: i) Observation of an equivalent increase of IOP in MYOC-/- and wild-type mice following DXM treatment (51); and ii) observation of increased IOP and absence of myocilin overexpression following DXM treatment (71). These studies suggest that increased IOP may not be associated with myocilin overexpression.
3. Pathogenesis of mutant/misfolded myocilins
The aforementioned studies suggest that the biological functions of myocilin remain poorly understood. Similarly, the role of myocilin in the pathogenesis of glaucoma is currently unknown, although its mutations have been reported to be associated with JOAG and adult-onset POAG.
Myocilin mutations
MYOC mutations alter the myocilin protein, which affects the normal regulation of IOP and may lead to glaucoma (72). To date, 278 different myocilin mutations have been reported, among which pathogenic mutations account for 37.77% (26) (Fig. 1) (23,28-35), 9 of which have been identified in exon 1, 1 in exon 2, and 95 in exon 3. Myocilin predominantly displays two types of mutations: Missense mutations (83.8%), which are associated with JOAG and adult-onset POAG (16); and nonsense mutations (5.7%) (26). Different myocilin mutations cause POAG with varying age at onset and glaucoma phenotypes of varying severity (73). Pro370Leu is one of the most severe glaucoma phenotypes, which is involved in mitochondrial dysfunction in TM cells and may lead to apoptosis (74). Gln368Stop is the most common mutation; however, it exhibits a markedly lower penetrance for glaucoma (75).
Autosomal dominant disorders resulting from myocilin (28) may be caused by the following three pathogenic mechanisms: The dominant negative effect, gain of function, or haploinsufficiency (7,16). Some studies have demonstrated that neither haploinsufficiency (16,19,67,76) nor the dominant negative effect (77) are involved in myocilin-associated pathogenicity. However, recent findings (42,78) have suggested that the dominant negative effect may be involved in the pathogenic role of myocilin in glaucoma. Notably, the majority of experimental evidence support gain-of-function as a pathogenic mechanism involved in myocilin mutation-associated glaucoma (21,23,36,37,41,42,67,73,76,79-84).
Pathogenesis of ER stress and oxidative stress (OS) caused by mutant myocilin
Recent studies have indicated that the notable paucity of normal myocilin (16,67,76,85) or its overexpression (85) are not associated with the pathogenesis of glaucoma, and that the pathogenesis of glaucoma is dependent on the expression of mutant/misfolded myocilins (86,87). Notably, mutations may cause myocilin misfolding (88). Furthermore, the secretion of wild-type myocilin is inhibited in the presence of co-expressed mutant myocilin (36,41). The aggregation of misfolded/wild-type myocilins in the ER may be harmful for TM cells and lead to apoptosis (19,84). Previous results have suggested that the TM is several times thicker in patients with glaucoma harboring mutations compared with that in patients without myocilin mutations (7). Therefore, myocilin mutations appear to be involved in the morphological changes in the TM, which lead to cell apoptosis (7).
Mutant myocilins aggregate intracellularly in insoluble and soluble aggresomes, interact with ER proteins and promote ER stress (19,81-84). Mutant myocilins have been suggested to induce apoptosis and may contribute to TM cell dysfunction, leading to increased IOP (86,89,90). Subsequently, ER stress may cause OS (87). During ER stress, the level of reactive oxygen species (ROS) is increased (91). Excess production and an accumulation of ROS compromise reduction-oxidation balance and cause OS (91). Notably, ER stress and OS are associated events involved in the pathogenesis of various diseases (91), including glaucoma-associated TM damage and increased IOP (87,90). However, the molecular pathways that connect ER stress and OS are poorly understood. OS is caused by the excessive production and aggregation of ROS (91). The TM, which is the most sensitive to OS among the tissues in the anterior chamber of the eye (86), comes into direct contact with the ROS-containing aqueous humor (87). Furthermore, ROS alone may cause protein misfolding (86). Misfolded myocilin can render cells more sensitive to OS (87). It was recently suggested that mutant myocilin inhibited antioxidant enzymes, such as paraoxonase 2, that efficiently decrease OS and inhibit ER stress-induced apoptosis (87). Therefore, decreased antioxidative enzymes in the TM may cause ROS elevation in the aqueous humor outflow system (87).
It has been reported that OS aggravates ER stress by inducing the overproduction of misfolded proteins (92). OS has also been considered a major factor causing damage to the TM (93). To relieve the damage from ER stress and restore homeostasis, the aggregated misfolded myocilin activates the unfolded protein response (UPR), which protects the TM cells (94-96), improves the protein folding mechanism and degrades misfolded proteins (94,95). However, if ER stress persists, UPR induces cell apoptosis (19,84). In addition, mutant myocilin cannot be cleared by ER-associated degradation, which transports degraded products of misfolded myocilin to the cytosol (19), leading to deleterious aggregation of amyloid-containing myocilin (97). These studies suggest that myocilin misfolding, UPR, ROS, OS and ER stress may be related events. Notably, ER stress and OS are risk factors for glaucoma, and together with the deleterious effect of misfolded myocilin, may cause a more severe glaucoma phenotype compared with any of these factors alone. These associated events disrupt the proteostasis of myocilin, leading to an imbalance between production and clearance of misfolded myocilins.
Pathogenesis of the co-aggregation of glucose-regulated protein (Grp) 94 with mutant myocilin
It has been reported that the inhibition of Grp94 is an effective approach to the treatment of glaucoma (79,98), supporting the co-aggregation of Grp94 with mutant myocilin and leading to retention within the ER (Fig. 4) (80). Not only does Grp94 accelerate the myocilin-OLF aggregation rate, but it also enhances co-precipitation with OLF (79,80,99). Therefore, Grp94 inhibition facilitates mutant myocilin clearance as an anti-glaucoma therapy (79,97,100). 4-Br-BnIm, a Grp94 inhibitor, significantly clears aggregated myocilin caused by overexpression mutations (97), and alleviates mutant myocilin-induced toxicity against TM cells (27,79) via a secondary autophagic pathway to facilitate clearance (27). When forced to misfold and aggregate, wild-type myocilin becomes a client of Grp94 and sensitized to 4-Br-BnIm (79). Recent studies (12,101) reported that myocilin aggregates were cleared by a ubiquitin-proteasome and autophagy lysosomal pathways under normal homeostatic conditions. However, when myocilin is mutated, autophagy is activated due to dysfunction of the proteasomal degradation pathway (12), and mutant myocilin is preferably degraded by autophagy (101). Grp94 inhibitors prevent Grp94 from aggregating with mutant myocilin, which induces a secondary autophagic pathway to promote clearance of abnormal myocilin (80). Grp94 is also a regulator of UPR (79). Therefore, a reduction in Grp94 may affect UPR, which induces TM cell death under persistent ER stress due to abnormal aggregations of Grp94 and misfolded myocilin.
Figure 4.
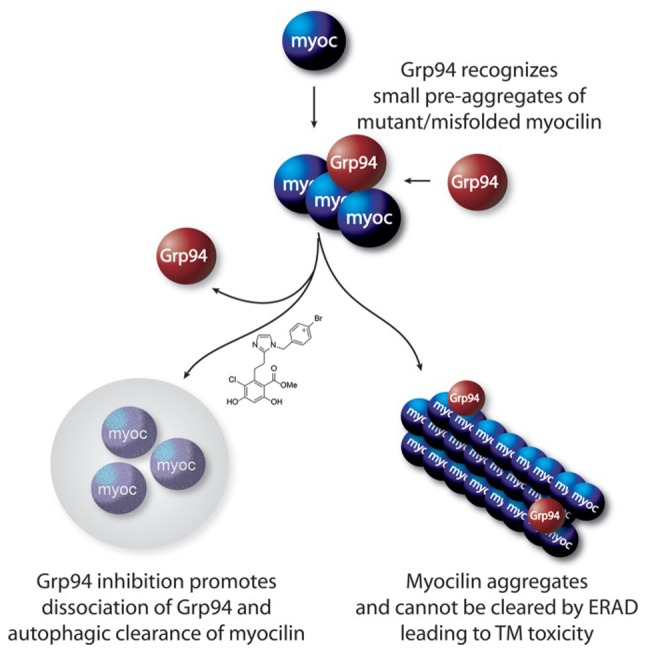
Co-aggregation of Grp94 with mutant/misfolded myocilin (80). Grp94, glucose-regulated protein 94.
Myocilin and the interleukin (IL)-1/nuclear factor (NF)-κB inflammatory stress signaling pathway
Activation of IL-1/NF-κB inflammatory stress is a defining characteristic of high-tension glaucoma (102). The release of potent proinflammatory IL-1, including IL-1β, may lead to inflammation (103) through activation of the NF-κB pathway, which induces an inflammatory response (104). It has been demonstrated that chronic low-grade inflammation causes ocular hypertension (105) and is also involved in the pathogenesis of glaucoma (103). Mutant myocilins aggregate within the TM cells and activate the NF-κB signaling pathway, significantly inducing the expression of IL-1 and IL-1β; however, extracellular mutant myocilin does not activate the NF-κB signaling pathway (106). Therefore, intracellular aggregations of mutant myocilins may induce the overexpression of IL-1 via activating NF-κB, leading to chronic inflammation that can cause elevated IOP.
Interestingly, IL-1 has additional beneficial effects on glaucoma, including reducing IOP through stimulation of matrix metalloproteinase (MMP) expression (106) and inhibiting apoptosis caused by OS through NF-κB (102). IOP and OS are associated with the aggregation of misfolded/mutant myocilins (86). Notably, IL-1 was increased by 10-fold in TM cells harboring Gln368Stop; however, the increase was only 6-fold in cells harboring Try437His (106). These findings suggest that abnormal aggregation of myocilin mutations may induce OS and upregulate IL-1. However, the amount of IL-1 induced by Gln368Stop is notably higher compared with that induced by Try437His. Coincidently, Try437His causes a severe glaucoma phenotype; however, Gln368Stop only causes a moderately severe glaucoma phenotype. This may be explained by the greater extent of IOP reduction and inhibition of the apoptotic process caused by OS through IL-1 via Gln368Stop compared with Try437His in the short-term. This also explained the finding that Gln368Stop is the most common mutation; however, it revealed a markedly lower penetrance for glaucoma caused by high IOP.
4. Imbalance of pathogenic myocilin and extracellular proteins
Glaucoma-associated TM damage is involved in various biochemical and morphological changes, including aberrant extracellular matrix protein aggregation and cell apoptosis (107). Synthesis and degradation of the extracellular matrix are in a dynamic balance that is constantly remodeled by proteolysis and protein deposition (52). Certain diseases, including glaucoma, may result from disruption of this dynamic balance (107). Recently, the crystal structure of myocilin-OLF was elucidated, and it indicated that myocilin-OLF belongs to the five-bladed β-propeller family (108), which is a known hub for protein-protein interactions. To date, a number of extracellular proteins interacting with myocilin have been identified, including tissue inhibitor of matrix metalloproteinase (TIMP3) (8,50), fibronectin (107), flotillin-1 (109) and hevin (46).
Effect of pathogenic myocilin on MMPs and MMP inhibitors
MMPs have multiple physiological functions, among which cell migration and proliferation have been linked to myocilin (110,111). Changes in MMP activity are involved in the pathogenesis of glaucoma (112). Notably, MMP2 activity may be associated with the regulation of IOP, which may be predominantly associated with the TM, where myocilin and TIMP3 coexist (50). MMP2, which is abundant in the TM (8), is involved in the breakdown of extracellular matrix (52), which facilitates aqueous humor outflow (8). It has also been demonstrated that myocilin mutations or MYOC null can enhance inhibition of MMP2 by TIMP3 (8,50). An imbalance between MMPs and TIMP within the TM can cause POAG (113). Of note, mutant myocilins can also reduce the active forms of MMP2 (107). Therefore, the effect of mutant myocilin on MMP2 activation disrupts the balance between MMPs and TIMPs, leading to glaucoma (96). In addition, reduction of MMP2 inhibits the decomposition of the extracellular matrix, disrupting its homeostasis, which may change the TM morphology and function, leading to increased IOP.
Effect of pathogenic myocilin on fibronectin, flotillin-1 and hevin
Fibronectin is a key component of the extracellular matrix of the TM, and is also an important mediator involved in extracellular matrix formation, which regulates aqueous humor outflow (114). Aberrant aggregation of the extracellular matrix in the TM reduces aqueous humor outflow and increases IOP in POAG (107). Overexpression of fibronectin and increased ER stress markers c/ebphomologous protein (CHOP) and Grp78 coexist in mice harboring MYOC-Tyr437His (107). Furthermore, another study reported that mutant myocilins increased CHOP and Grp78 in the TM (96). Myocilin, fibronectin, CHOP and Grp78 expression levels were increased following DXM treatment (115), which was similar to the overexpression of myocilin and fibronectin observed following DXM-Ac treatment (51). Recent studies have suggested that a reduction of ER stress through sodium 4-phenylbutyrate decreases the DXM-induced increase of IOP (115) and fibro-nectin aggregation caused by mutant myocilin (107) in TM cells. Therefore, myocilin, fibronectin, CHOP, Grp78 and the extracellular matrix may be considered as elements implicated in the pathogenesis of glaucoma in the TM. Additionally, mutant myocilin induces aberrant aggregation of the extracellular matrix in the ER of TM cells, which may contribute to decreased aqueous humor outflow and increased IOP (107). Furthermore, failure of the TM to reduce ER stress caused by mutant myocilin accounts for the induction of CHOP, which may also be associated with apoptosis and increased IOP in the TM cells (96).
Flotillin-1, a structural protein of lipid rafts, interacts with myocilin (109). Myocilin mutations, including Gly364Val, Tyr437His and Lys423Glu, which are scattered in the OLF, fail to interact with flotillin-1 (109), suggesting that myocilin-OLF may be required for the interaction. Although the role of the interaction remains to be elucidated, loss of interaction with flotillin-1 due to myocilin mutations may be a pathogenic mechanism underlying the development of POAG (109). Hevin is a matricellular protein involved in the assembly of the extracellular matrix (116). Mutant myocilin causes intracellular aggregation of hevin and also affects hevin secretion (46). Hevin coexpressed with Pro370Leu, which is also known as a severe glaucoma mutation, aggravates the impairment of hevin secretion (46). These findings indicate that mutant myocilins affect the interactions with extracellular proteins, leading to disruption of extracellular matrix homeostasis, which may be involved in the pathogenesis of glaucoma.
5. Association of glaucoma pathogenesis with the crystal structure and stability of myocilin
Crystal structure of myocilin
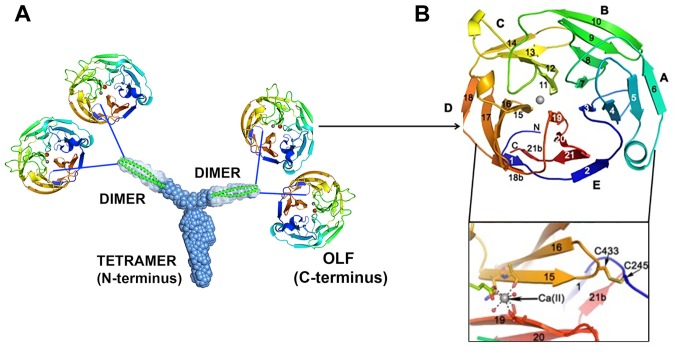
A better understanding of the conformation of myocilin can provide a structural basis for investigating myocilin mis-/unfolding in myocilin-associated glaucoma and, in turn, result in a better understanding of myocilin-associated glaucoma pathogenesis. The myocilin N-terminus has a unique tripartite architecture, including a Y-shaped parallel dimer-of-dimers with distinct tetramer and dimer regions. Furthermore, full-length myocilin should also be branched, with two pairs of C-terminal OLFs (Fig. 5A) (117). MYOC-Glu396Asp has discontinuous D-18/D-18b and E-21/ E-21b strands (Fig. 5B) (108,117) that are unlike the continuous D-18 and E-21 strands in wild-type myocilin. Accumulating evidence has revealed that pathogenic myocilin mutations are associated with intracellular aggregation propensity and thermal stability (19,32,37,41,42,82,108,118,119). Three destabilizing regions are localized in myocilin-OLF (108): i) Loop B-10/C-11 and cation-π; ii) hydrophobic β-sheet belt; and iii) Ca2+ site environs (Fig. 5B).
Figure 5.
Structure of myocilin (108,117). (A) The N-terminus has a Y-shaped parallel (117). (B) The crystal of myocilin-OLF is a five-bladed β-propeller, and each blade is composed of four antiparallel β-strands, arranged radically around a central water-filled cavity (108). The blades in OLF are notably asymmetric. Nearly half of the toroid-shaped molecule is occupied by blades D and E. The myocilin-OLF propeller has five blades (A, B, C, D and E). A disulfide bond and calcium-binding site are located at the bottom face of the propeller, and a single disulfide bond is observed between Cys433 located within Loop D-15/D-16 and Cys245 at the N-terminus prior to the start of E-1. Tyr 437 further stabilizes the region near the single disulfide bond. Unlike other amyloid-like proteins (120), the effect of disulfide bonds on the stability of wild-type myocilin is not significant.
Structural location and stability of myocilin mutations
The largest number of mutations are observed in core β-sheet belts (~40%), loop B-10/C-11 and cation-π (~33%) (108), particularly the most destabilized myocilin-OLF mutations, such as Trp286Arg, Tyr437His, Lys423Glu, Pro370Leu, Ile477Asn and Ile477Ser (118,119). The myocilin-OLF mutations exhibit changes in the side chains. The most destabilizing mutations also exhibit other characteristics: i) Lower melting temperature (Table I) (108); ii) insoluble aggregates (118); and iii) earlier age at diagnosis. In contrast to the most destabilizing mutations, the most stabilizing myocilin-OLF mutations, Ala427Thr and Ala445Val (118), exhibit similar stability and structure to those of wild-type myocilin-OLF. Furthermore, the difference in their melting temperature from that of wild-type myocilin-OLF (52.7±0.8°C) is only within 2°C, and they are soluble (118). The trend in melting temperature follows the general stability of myocilin-OLF mutations and the severity of their aggregation. Additionally, this instability may cause structural changes in myocilin-OLF, resulting in mis-/unfolding. Notably, higher proportion of mis-/unfolded myocilin has been associated with greater exposure of interior hydrophobic regions and more severe intracellular aggregation propensity (118). Intracellular aggregation of mutant myocilin deforms TM cells (84) and changes the size of pores between the cells, through which aqueous humor outflows. Dysfunction of TM cells may contribute to the pathogenesis of glaucoma (41,84).
Table I.
Structural location and stability of myocilin mutations.
| Mutations | Melting temperature (°C) | Structural location | Solubility | Mean age at diagnosis, years | Secretion | Mean maximum IOP (mmHg) | (Refs.) | |
|---|---|---|---|---|---|---|---|---|
| 37°C | 30°C | |||||||
| Wild-type | 52.7±0.8 | NA | Soluble | NA | +++ | +++ | NA | (37,118) |
| Trp286Arg | NA | β-sheet belt | NA | 27.5±13.4 | No | No | 39.5±31.8 | (26,80,97,108,118,121) |
| Pro370Leu | NA | Loop B-10/C-11, cation-π | Insoluble | 13.3±2.4 | No | Little | 49±8.5 | (15,19,26,82,108,118) |
| Lys423Glu | 34.2±0.4 | Loop B-10/C-11, cation-π | Insoluble | 28.8±8.1 | No | No | 36±8.5 | (26,77,108,118) |
| Ile477Asn | 37.7±0.8 | β-sheet belt | Insoluble | 20.7±1.3 | No | Little | 44±0 | (26,79,82,97,108,118) |
| Ile477Ser | 39.7±0.2 | β-sheet belt | Insoluble | 33±0 | No | +++ | NA | (26,108,118) |
| Tyr437His | 40.3±0.4 | β-sheet belt | Insoluble | 19.9±0 | No | Little | 43.7±0 | (26,80,82,97,108,118) |
| Cys433Arg | 40.4±0.4 | β-sheet belt | Insoluble | 22.8 ± 8.9 | No | No | 39.1 ± 12.6 | (26,108,118) |
| Arg272Gly | 41.0±0.3 | β-sheet belt | NA | 33±0 | No | ++ | 62±0 | (26,108,118,122) |
| Ser502Pro | 41.0±0.3 | β-sheet belt | Insoluble | 19±0 | No | No | NA | (26,108,118) |
| Val426Phe | 41.5±0.1 | Loop B-10/C-11, cation-π | Insoluble | 18.6±1.4 | Little | +++ | 43±0 | (26,108,118,122,123) |
| Asn480Lys | 42.4±0.2 | β-sheet belt | Insoluble | 25.4±3.1 | Little | ++ | 37.7±12.4 | (26,80,97,108,118) |
| Gly367Arg | 42.7±0.1 | Loop B-10/C-11, cation-π | Insoluble | 26.6±5.5 | No | +++ | 51±0 | (26,28,108,118) |
| Ile499Phe | 42.8±0.1 | β-sheet belt | Partially insoluble | 30.9±7.6 | Little | +++ | 31±6.6 | (26,82,108,118) |
| Gly252Arg | 43.0±0.2 | β-sheet belt | Insoluble | 35±7.3 | No | +++ | 62±0 | (26,108,118,122) |
| Glu323Lys | 44.0±0.5 | Loop B-10/C-11, cation-π | Insoluble | 19±0 | Little | +++ | 43±0 | (26,108,118,122) |
| Thr377Met | 44.3±0.3 | Loop B-10/C-11, cation-π | Partially insoluble | 21.4±5.0 | + | +++ | 44±0 | (26,82,108,118,121,122) |
| Gly364Val | 45.0±0.4 | Loop B-10/C-11, cation-π | Partially insoluble | 34±0 | + | +++ | 36±0 | (26,82,108,118,121) |
| Pro481Leu | 45.5±0.4 | Ca2+ site environs | Insoluble | 33±0 | No | ++ | 46±0 | (26,108,118,121) |
| Asp380Ala | 46.6±0.3 | Ca2+ site environs | Partially insoluble | 11.9±3.5 | Little | +++ | 34.2±17.9 | (26,82,108,118) |
| Ala427Thr | 50.5±0.2 | Ca2+ site environs | Soluble | 61±21.2 | +++ | NA | 31.8±6.1 | (26,108,118,123) |
| Ala445Val | 54.2±0.2 | NA | Soluble | 51.5±16.3 | +++ | NA | 24±0 | (26,108,118,121) |
IOP, intraocular pressure; NA, not available.
Myocilin mutations at a moderate level of stability (Table I) renew the secretion through shifting temperature from 37 to 30°C (37,82,118), demonstrating their temperature-sensitive secretion. Trimethylamine N-oxide and sarcosine also stabilize myocilin mutations through shifting their melting temperatures to near that of wild-type myocilin (119). Thr377Met, Ile499Phe and Gly364Val, which are abundantly secreted at 30°C, are associated with less severe glaucoma (82). Interestingly, Pro370Leu and Ile477Asn, which are associated with a more severe glaucoma phenotype (82,118), are not secreted at higher levels when the temperature changes (82). Thus, temperature-sensitive secretion may be a notable property of these moderate myocilin mutations (82). A temperature of 30°C is a condition known to facilitate protein folding (37,42). Therefore, this favorable temperature promotes the correct folding of myocilin into its native form. Taken together, these studies demonstrated that the stability of myocilin mutations may be associated with their pathogenicity. Notably, the less stable myocilin mutations were associated with the more severe glaucoma phenotype and were less sensitive to temperature.
6. Conclusion
Based on the aforementioned evidence, although myocilin mutations have been reported to be associated with JOAG and adult-onset POAG, the physiological functions and pathogenicity of myocilin remain elusive. Thus far, there are several possible points of view on the pathogenic potential of myocilin: i) Myocilin misfolding/unfolding; (ii) overexpression of myocilin; iii) co-aggregation of Grp94; iv) disruption of extracellular matrix homeostasis caused by mutant myocilin; v) OS, ER stress and IL-1/NF-κB inflammatory stress; and vi) instability resulting from conformational disorders caused by mutant myocilin. However, certain suggestions require further investigation, particularly those with contradicting conclusions. Furthermore, unsolved or partially solved problems require further research, as they could direct future studies on myocilin and contribute to novel therapeutic approaches to the treatment of myocilin-associated glaucoma.
The functions of myocilin through interactions of its binding partners should be further investigated, as interacting proteins may have similar biological functions. In addition, it is necessary to gain further insight into the proteolytic processes of myocilin, as this may contribute to an effective approach to breaking down abnormal aggregations of myocilin. Furthermore, a more detailed understanding of the structural basis of myocilin stability will be valuable for elucidating the roles of conformational disorders, such as misfolding or unfolding, in myocilin-associated glaucoma. Notably, this may help elucidate the role of myocilin mutations in the pathogenesis of glaucoma. Additionally, the association of glaucoma phenotype severity with the secretion (associated with temperature, stability and insolubility) of mutant myocilin should be emphasized, as unraveling these elusive associations may contribute to understanding the pathogenesis underlying abnormal aggregations of mutant myocilin. Future studies regarding myocilin should focus on the deleterious effect of myocilin misfolding on OS and ER stress in the TM, which are a series of associated events, each of which causes the next, leading to further injury to the TM. Furthermore, these effects are associated with increased IOP, which is a known primary risk factor for glaucoma.
Acknowledgments
The authors would like to thank Xin Wang in the Library of Qiqihar Medical University for the careful edits and the knowledge promoted and supported by the Heilongjiang Province Philosophy and Social Science Research Planning project (16TQB04).
Abbreviations
- JOAG
juvenile-onset open-angle glaucoma
- POAG
primary open-angle glaucoma
- UPR
unfolded protein response
- ER
endoplasmic reticulum
- TGF
transforming growth factor
- CHOP
c/ebp-homologous protein
- Grp78
glucose-regulated protein 78
- IOP
intraocular pressure
- OLF
olfactomedin
- LINK
linker domain
- OS
oxidative stress
- IL-1
interleukin-l
- ROS
reactive oxygen species
- ERAD
ER-associated degradation
- Grp94
glucose-regulated protein 94
- NF-κB
nuclear factor κB
- MMP
matrix metalloproteinase
- LZ
leucine zippers
- PBA
sodium 4-phenylbutyrate
- MYOC
myocilin
- TM
trabecular meshwork
- SPARC
secreted protein acidic and rich in cysteine
- TIMP3
tissue inhibitor of matrix metalloproteinase 3
Funding
The present study was supported by a grant from Taizhou Science and Technology Support Projects for Social Development (2016) of Taizhou Science and Technology Bureau (SSF20160112).
Availability of data and materials
Not applicable.
Authors’ contributions
HW, ML, ZZ, HX, XC and YJ designed the article, contributed to the conception of the study and critically revised the article for important intellectual content. HW and YJ drafted the article. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Khawaja AP, Cooke Bailey JN, Wareham NJ, Scott RA, Simcoe M, Igo RP, Jr, Song YE, Wojciechowski R, Cheng CY, Khaw PT, et al. Genome-wide analyses identify 68 new loci associated with intraocular pressure and improve risk prediction for primary open-angle glaucoma. Nat Genet. 2018;50:778–782. doi: 10.1038/s41588-018-0126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang X, Huai G, Wang H, Liu Y, Qi P, Shi W, Peng J, Yang H, Deng S, Wang Y. Mutual regulation of the Hippo/Wnt/LPA/TGF-β signaling pathways and their roles in glaucoma (Review) Int J Mol Med. 2018;41:1201–1212. doi: 10.3892/ijmm.2017.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rangachari K, Bankoti N, Shyamala N, Michael D, Sameer Ahmed Z, Chandrasekaran P, Sekar K. Glaucoma Pred: Glaucoma prediction based on myocilin genotype and phenotype information. Genomics. 2018:S0888–S7543. 30087–30089. doi: 10.1016/j.ygeno.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Narooie-Nejad M, Rasouli A, Mousavi M, Rohani MR. Study of MYOC gene mutation in POAG patients in zahedan iran. Clin Lab. 2017;63:1283–1291. doi: 10.7754/Clin.Lab.2017.161109. [DOI] [PubMed] [Google Scholar]
- 5.Rasnitsyn A, Doucette L, Seifi M, Footz T, Raymond V, Walter MA. FOXC1 modulates MYOC secretion through regulation of the exocytic proteins RAB3GAP1, RAB3GAP2 and SNAP25. PLoS One. 2017;12:e0178518. doi: 10.1371/journal.pone.0178518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma S, Bollinger KE, Kodeboyina SK, Zhi W, Patton J, Bai S, Edwards B, Ulrich L, Bogorad D, Sharma A. Proteomic alterations in aqueous humor from patients with primary open angle glaucoma. Invest Ophthalmol Vis Sci. 2018;59:2635–2643. doi: 10.1167/iovs.17-23434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamanaka T, Kimura M, Sakurai T, Ishida N, Yasuda J, Nagasaki M, Nariai N, Endo A, Homma K, Katsuoka F, et al. A histologic categorization of aqueous outflow routes in familial open-angle glaucoma and associations with mutations in the MYOC gene in japanese patients. Invest Ophthalmol Vis Sci. 2017;58:2818–2831. doi: 10.1167/iovs.16-20646. [DOI] [PubMed] [Google Scholar]
- 8.Fini ME. Another piece of the puzzle: MYOC and myocilin glaucoma. Invest Ophthalmol Vis Sci. 2017;58:5319. doi: 10.1167/iovs.17-23045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donegan RK, Lieberman RL. Discovery of molecular therapeutics for glaucoma: Challenges successes and promising directions. J Med Chem. 2016;59:788–809. doi: 10.1021/acs.jmedchem.5b00828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katoli P, Godbole A, Romanowski MJ, Clark K, Meredith E, Saenz-Vash V, Wang YK, Lewicki N, Nguyen AA, Lynch JM. Full-length myocilin protein is purified from mammalian cells as a dimer. Protein Expr Purif. 2018;147:38–48. doi: 10.1016/j.pep.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Faralli JA, Clark RW, Filla MS, Peters DM. NFATc1 activity regulates the expression of myocilin induced by dexamethasone. Exp Eye Res. 2015;130:9–16. doi: 10.1016/j.exer.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu Y, Shen X, Shyam R, Yue BY, Ying H. Cellular processing of myocilin. PLoS One. 2014;9:92845. doi: 10.1371/journal.pone.0092845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta V, Somarajan BI, Gupta S, Chaurasia AK, Kumar S, Dutta P, Gupta V, Sharma A, Tayo BO, Nischal K. The inheritance of juvenile onset primary open angle glaucoma. Clin Genet. 2017;92:134–142. doi: 10.1111/cge.12906. [DOI] [PubMed] [Google Scholar]
- 14.Mauri L, Uebe S, Sticht H, Vossmerbaeumer U, Weisschuh N, Manfredini E, Maselli E, Patrosso M, Weinreb RN, Penco S, et al. Expanding the clinical spectrum of COL1A1 mutations in different forms of glaucoma. Orphanet J Rare Dis. 2016;11:108. doi: 10.1186/s13023-016-0495-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C, Xie L, Wu Z, Cao Y, Zheng Y, Pang CP, Zhang M. Detection of mutations in MYOC OPTN NTF4 WDR36 and CYP1B1 in Chinese juvenile onset open-angle glaucoma using exome sequencing. Sci Rep. 2018;8:4498–4505. doi: 10.1038/s41598-018-22337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiggs JL, Vollrath D. Molecular and clinical evaluation of a patient hemizygous for TIGR/MYOC. Arch Ophthalmol. 2001;119:1674–1678. doi: 10.1001/archopht.119.11.1674. [DOI] [PubMed] [Google Scholar]
- 17.Gupta V, Ganesan VL, Kumar S, Chaurasia AK, Malhotra S, Gupta S. Visual disability among juvenile open-angle glaucoma patients. J Glaucoma. 2018;27:e87-e89. doi: 10.1097/IJG.0000000000000887. [DOI] [PubMed] [Google Scholar]
- 18.Borrás T. The effects of myocilin expression on functionally relevant trabecular meshwork genes: A mini-review. J Ocul Pharmacol Ther. 2014;30:202–212. doi: 10.1089/jop.2013.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Vollrath D. Reversal of mutant myocilin non-secretion and cell killing: Implications for glaucoma. Hum Mol Genet. 2004;13:1193–1204. doi: 10.1093/hmg/ddh128. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez H, Millar JC, Curry SM, Clark AF, McDowell CM. BMP and activin membrane bound inhibitor regulates the extracellular matrix in the trabecular meshwork. Invest Ophthalmol Vis Sci. 2018;59:2154–2166. doi: 10.1167/iovs.17-23282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain A, Zode G, Kasetti RB, Ran FA, Yan W, Sharma TP, Bugge K, Searby CC, Fingert JH, Zhang F, et al. CRISPR-Cas9-based treatment of myocilin-associated glaucoma. Proc Natl Acad Sci USA. 2017;114:11199–11204. doi: 10.1073/pnas.1706193114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JH, Caprioli J. Intraocular pressure fluctuation: Is it important. J Ophthalmic Vis Res. 2018;13:170–174. doi: 10.4103/jovr.jovr_35_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aroca-Aguilar JD, Sánchez-Sánchez F, Ghosh S, Coca-Prados M, Escribano J. Myocilin mutations causing glaucoma inhibit the intracellular endoproteolytic cleavage of myocilin between amino acids Arg226 and Ile227. J Biol Chem. 2005;280:21043–21051. doi: 10.1074/jbc.M501340200. [DOI] [PubMed] [Google Scholar]
- 24.Jiang L, Eaves S, Dhillon N, Ranjit P. Postoperative outcomes following trabeculectomy and nonpenetrating surgical procedures: A 5-year longitudinal study. Clin Ophthalmol. 2018;12:995–1002. doi: 10.2147/OPTH.S163247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Gao Y, Hill SE, Huard DJE, Tomlin MO, Lieberman RL, Paravastu AK, Hall CK. Simulations and experiments delineate amyloid fibrilization by peptides derived from glaucoma-associated myocilin. J Phys Chem B. 2018;122:5845–5850. doi: 10.1021/acs.jpcb.8b03000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hewitt AW, Mackey DA, Craig JE. Myocilin myocilin allele-specific glaucoma phenotype database. Hum Mutat. 2008;29:207–211. doi: 10.1002/humu.20634. [DOI] [PubMed] [Google Scholar]
- 27.Stothert AR, Fontaine SN, Sabbagh JJ, Dickey CA. Targeting the ER-autophagy system in the trabecular meshwork to treat glaucoma. Exp Eye Res. 2016;144:38–45. doi: 10.1016/j.exer.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao YH, Wang YQ, Fang WF, Zhang L, Yang JH, Zhu YH. A recurrent G367R mutation in MYOC associated with juvenile open angle glaucoma in a large chinese family. Int J Ophthalmol. 2018;11:369–374. doi: 10.18240/ijo.2018.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Souzeau E, Burdon KP, Ridge B, Dubowsky A, Ruddle JB, Craig JE. A novel de novo myocilin variant in a patient with sporadic juvenile open angle glaucoma. BMC Med Genet. 2016;17:30. doi: 10.1186/s12881-016-0291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang F, Li Y, Lan L, Li B, Lin L, Lu X, Li J. Ser341 Pro MYOC gene mutation in a family with primary open-angle glaucoma. Int J Mol Med. 2015;35:1230–1236. doi: 10.3892/ijmm.2015.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Shi Y, Huang X, Li X, Ye Z, Shuai P, Qu C, Chen R, Xu J, Yang Z, et al. Identification of a novel MYOC mutation in a Chinese family with primary open-angle glaucoma. Gene. 2015;571:188–193. doi: 10.1016/j.gene.2015.06.042. [DOI] [PubMed] [Google Scholar]
- 32.Zadoo S, Nguyen A, Zode G, Hulleman JD. A novel luciferase assay for sensitively monitoring myocilin variants in cell culture. Invest Ophthalmol Vis Sci. 2016;57:1939–1950. doi: 10.1167/iovs.15-18789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aroca-Aguilar JD, Martínez-Redondo F, Sánchez-Sánchez F, Coca-Prados M, Escribano J. Functional role of proteolytic processing of recombinant myocilin in self-aggregation. Invest Ophthalmol Vis Sci. 2010;51:72–78. doi: 10.1167/iovs.09-4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aroca-Aguilar JD, Sánchez-Sánchez F, Ghosh S, Fernández-Navarro A, Coca-Prados M, Escribano J. Interaction of recombinant myocilin with the matricellular protein SPARC: Functional implications. Invest Ophthalmol Vis Sci. 2011;52:179–189. doi: 10.1167/iovs.09-4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Resch ZT, Fautsch MP. Glaucoma-associated myocilin: A better understanding but much more to learn. Exp Eye Res. 2009;88:704–712. doi: 10.1016/j.exer.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caballero M, Rowlette LL, Borras T. Altered secretion of a TIGR/MYOC mutant lacking the olfactomedin domain. Biochim Biophys Acta. 2000;1502:447–460. doi: 10.1016/S0925-4439(00)00068-5. [DOI] [PubMed] [Google Scholar]
- 37.Gobeil S, Letartre L, Raymond V. Functional analysis of the glaucoma-causing TIGR/myocilin protein: Integrity of amino-terminal coiled-coil regions and olfactomedin homology domain is essential for extracellular adhesion and secretion. Exp Eye Res. 2006;82:1017–1029. doi: 10.1016/j.exer.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Zhou T, Souzeau E, Sharma S, Landers J, Mills R, Goldberg I, Healey PR, Graham S, Hewitt AW, Mackey DA, et al. Whole exome sequencing implicates eye development the unfolded protein response and plasma membrane homeostasis in primary open-angle glaucoma. PLoS One. 2017;12:e0172427. doi: 10.1371/journal.pone.0172427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldwich A, Ethier CR, Chan DW, Tamm ER. Perfusion with the olfactomedin domain of myocilin does not affect outflow facility. Invest Ophthalmol Vis Sci. 2003;44:1953–1961. doi: 10.1167/iovs.02-0863. [DOI] [PubMed] [Google Scholar]
- 40.Sánchez-Sánchez F, Martínez-Redondo F, Aroca-Aguilar JD, Coca-Prados M, Escribano J. Characterization of the intracellular proteolytic cleavage of myocilin and identification of calpain II as a myocilin-processing protease. J Biol Chem. 2007;282:27810–27824. doi: 10.1074/jbc.M609608200. [DOI] [PubMed] [Google Scholar]
- 41.Jacobson N, Andrews M, Shepard AR, Nishimura D, Searby C, Fingert JH, Hageman G, Mullins R, Davidson BL, Kwon YH, et al. Non-secretion of mutant proteins of the glaucoma gene myocilin in cultured trabecular meshwork cells and in aqueous humor. Hum Mol Genet. 2001;10:117–125. doi: 10.1093/hmg/10.2.117. [DOI] [PubMed] [Google Scholar]
- 42.Aroca-Aguilar JD, Sánchez-Sánchez F, Martínez-Redondo F, Coca-Prados M, Escribano J. Heterozygous expression of myocilin glaucoma mutants increases secretion of the mutant forms and reduces extracellular processed myocilin. Mol Vis. 2008;14:2097–2108. [PMC free article] [PubMed] [Google Scholar]
- 43.Hood JL, Brooks WH, Roszman TL. Differential compart-mentalization of the calpain/calpastatin network with the endoplasmic reticulum and Golgi apparatus. J Biol Chem. 2004;279:43126–43135. doi: 10.1074/jbc.M408100200. [DOI] [PubMed] [Google Scholar]
- 44.Wei YT, Li YQ, Bai YJ, Wang M, Chen JH, Ge J, Zhuo YH. Pro370Leu myocilin mutation in a chinese pedigree with juvenile-onset open angle glaucoma. Mol Vis. 2011;17:1449–1456. [PMC free article] [PubMed] [Google Scholar]
- 45.Ueda J, Wentz-Hunter K, Yue BY. Distribution of myocilin and extracellular matrix components in the juxtacanalicular tissue of human eyes. Invest Ophthalmol Vis Sci. 2002;43:1068–1076. [PubMed] [Google Scholar]
- 46.Li Y, Aroca-Aguilar JD, Ghosh S, Sánchez-Sánchez F, Escribano J, Coca-Prados M. Interaction of myocilin with the C-terminal region of hevin. Biochem Biophys Res Commun. 2006;339:797–804. doi: 10.1016/j.bbrc.2005.11.082. [DOI] [PubMed] [Google Scholar]
- 47.Torrado M, Trivedi R, Zinovieva R, Karavanova I, Tomarev SI. Optimedin: A novel olfactomedin-related protein that interacts with myocilin. Hum Mol Genet. 2002;11:1291–1301. doi: 10.1093/hmg/11.11.1291. [DOI] [PubMed] [Google Scholar]
- 48.Filla MS, Liu X, Nguyen TD, Polansky JR, Brandt CR, Kaufman PL, Peters DM. In vitro localization of TIGR/MYOC in trabecular meshwork extracellular matrix and binding to fibro-nectin. Invest Ophthalmol Vis Sci. 2002;43:151–161. [PubMed] [Google Scholar]
- 49.Fautsch MP, Vrabel AM, Johnson DH. The identification of myocilin-associated proteins in the human trabecular meshwork. Exp Eye Res. 2006;82:1046–1052. doi: 10.1016/j.exer.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 50.Joe MK, Lieberman RL, Nakaya N, Tomarev SI. Myocilin regulates metalloprotease 2 activity through interaction with TIMP3. Invest Ophthalmol Vis Sci. 2017;58:5308–5318. doi: 10.1167/iovs.16-20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel GC, Phan TN, Maddineni P, Kasetti RB, Millar JC, Clark AF, Zode GS. Dexamethasone-induced ocular hypertension in mice: Effects of myocilin and route of administration. Am J Pathol. 2017;187:713–723. doi: 10.1016/j.ajpath.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li G, Cui G, Dismuke WM, Navarro I, Perkumas K, Woodward DF, Stamer WD. Differential response and withdrawal profile of glucocorticoid-treated human trabecular meshwork cells. Exp Eye Res. 2017;155:38–46. doi: 10.1016/j.exer.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Webber HC, Bermudez JY, Sethi A, Clark AF, Mao W. Crosstalk between TGFβ and Wnt signaling pathways in the human trabecular meshwork. Exp Eye Res. 2016;148:97–102. doi: 10.1016/j.exer.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raghunathan VK, Morgan JT, Park SA, Weber D, Phinney BS, Murphy CJ, Russell P. Dexamethasone stiffens trabecular meshwork trabecular meshwork cells and matrix. Invest Ophthalmol Vis Sci. 2015;56:4447–4459. doi: 10.1167/iovs.15-16739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nguyen TD, Chen P, Huang WD, Chen H, Johnson D, Polansky JR. Gene structure and properties of myocilin an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J Biol Chem. 1998;273:6341–6350. doi: 10.1074/jbc.273.11.6341. [DOI] [PubMed] [Google Scholar]
- 56.Agrahari V, Li G, Agrahari V, Navarro I, Perkumas K, Mandal A, Stamer WD, Mitra AK. Pentablock copolymer dexamethasone nanoformulations elevate MYOC: In vitro liberation, activity and safety in human trabecular meshwork cells. Nanomedicine (Lond) 2017;12:1911–1926. doi: 10.2217/nnm-2017-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prat C, Belville C, Comptour A, Marceau G, Clairefond G, Chiambaretta F, Sapin V, Blanchon L. Myocilin expression is regulated by retinoic acid in the trabecular meshwork-derived cellular environment. Exp Eye Res. 2017;155:91–98. doi: 10.1016/j.exer.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 58.Wu Y, Chen W, Guo M, He Q, Hu Y. Effects of transforming growth factor-β2 on myocilin expression and secretion in human primary cultured trabecular meshwork cells. Int J Clin Exp Pathol. 2014;7:4827–4836. [PMC free article] [PubMed] [Google Scholar]
- 59.Huang X, Li M, Guo X, Li S, Xiao X, Jia X, Liu X, Zhang Q. Mutation analysis of seven known glaucoma-associated genes in Chinese patients with glaucoma. Invest Ophthalmol Vis Sci. 2014;55:3594–3602. doi: 10.1167/iovs.14-13927. [DOI] [PubMed] [Google Scholar]
- 60.Park J, Kim M, Park CK, Chae H, Lee S, Kim Y, Jang W, Chi HY, Park HY, Park SH. Molecular analysis of myocilin and optineurin genes in Korean primary glaucoma patients. Mol Med Rep. 2016;14:2439–2448. doi: 10.3892/mmr.2016.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maurya N, Agarwal NR, Ghosh I. Low-dose rotenone exposure induces early senescence leading to late apoptotic signaling cascade in human trabecular meshwork (HTM) cell line: An in vitro glaucoma model. Cell Biol Int. 2016;40:107–120. doi: 10.1002/cbin.10561. [DOI] [PubMed] [Google Scholar]
- 62.Pattabiraman PP, Rao PV. Hic-5 regulates actin cytoskeletal reorganization and expression of fibrogenic markers and myocilin in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2015;56:5656–5669. doi: 10.1167/iovs.15-17204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wei X, Cho KS, Thee EF, Jager MJ, Chen DF. Neuroimmflammation and microglia in glaucoma: Time for a paradigm shift. J Neurosci Res. 2018 doi: 10.1002/jnr.24256. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wareham LK, Buys ES, Sappington RM. The nitric oxide-guanylate cyclase pathway and glaucoma. Nitric Oxide. 2018;77:75–87. doi: 10.1016/j.niox.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Michelessi M, Bicket AK, Lindsley K. Cyclodestructive procedures for non-refractory glaucoma. Cochrane Database Syst Rev. 2018;4:CD009313. doi: 10.1002/14651858.CD009313.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stamer WD, Acott TS. Current understanding of conventional outflow dysfunction in glaucoma. Curr Opin Ophthalmol. 2012;23:135–143. doi: 10.1097/ICU.0b013e32834ff23e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim BS, Savinova OV, Reedy MV, Martin J, Lun Y, Gan L, Smith RS, Tomarev SI, John SW, Johnson RL. Targeted disruption of the myocilin gene (Myoc) suggests that human glaucoma-causing mutations are gain of function. Mol Cell Biol. 2001;21:7707–7713. doi: 10.1128/MCB.21.22.7707-7713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Acott TS, Kelley MJ, Keller KE, Vranka JA, Abu-Hassan DW, Li X, Aga M, Bradley JM. Intraocular pressure homeostasis: Maintaining balance in a high-pressure environment. J Ocul Pharmacol Ther. 2014;30:94–101. doi: 10.1089/jop.2013.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fautsch MP, Bahler CK, Jewison DJ, Johnson DH. Recombinant TIGR/MYOC increases outflow resistance in the human anterior segment. Invest Ophthalmol Vis Sci. 2000;41:4163–4168. [PubMed] [Google Scholar]
- 70.Patel GC, Liu Y, Millar JC, Clark AF. Glucocorticoid receptor GRβ regulates glucocorticoid-induced ocular hypertension in mice. Sci Rep. 2018;8:862. doi: 10.1038/s41598-018-19262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Faralli JA, Dimeo KD, Trane RM, Peters D. Absence of a secondary glucocorticoid response in C57BL/6J mice treated with topical dexamethasone. PLoS One. 2018;13:e0192665. doi: 10.1371/journal.pone.0192665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nazir S, Mukhtar M, Shahnawaz M, Farooqi S, Fatima N, Mehmood R, Sheikh N. A novel single nucleotide polymorphism in exon 3 of MYOC enhances the risk of glaucoma. PLoS One. 2018;13:e0195157. doi: 10.1371/journal.pone.0195157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shepard AR, Jacobson N, Millar JC, Pang IH, Steely HT, Searby CC, Sheffield VC, Stone EM, Clark AF. Glaucoma-causing myocilin mutants require the Peroxisomal targeting signal-1 receptor (PTS1R) to elevate intraocular pressure. Hum Mol Genet. 2007;16:609–617. doi: 10.1093/hmg/ddm001. [DOI] [PubMed] [Google Scholar]
- 74.Guan Y, Li J, Zhan T, Wang JW, Yu JB, Yang L. Idebenone maintains survival of mutant myocilin cells by inhibiting apoptosis. Chin Med J (Engl) 2016;129:2001–2004. doi: 10.4103/0366-6999.187856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nag A, Lu H, Arno M, Iglesias AI, Bonnemaijer P, Broer L, Uitterlinden AG, Klaver CC, van Duijn C, Hysi PG, Hammond CJ. Evaluation of the myocilin mutation gln368stop demonstrates reduced penetrance for glaucoma in european populations. Ophthalmology. 2017;124:547–553. doi: 10.1016/j.ophtha.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 76.Lam DS, Leung YF, Chua JK, Baum L, Fan DS, Choy KW, Pang CP. Truncations in the TIGR gene in individuals with and without primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2000;41:1386–1391. [PubMed] [Google Scholar]
- 77.Morissette J, Clépet C, Moisan S, Dubois S, Winstall E, Vermeeren D, Nguyen TD, Polansky JR, Côté G, Anctil JL, et al. Homozygotes carrying an autosomal dominant TIGR mutation do not manifest glaucoma. Nat Genet. 1998;19:319–321. doi: 10.1038/1203. [DOI] [PubMed] [Google Scholar]
- 78.Kuchtey J, Chowdhury UR, Uptegraft CC, Fautsch MP, Kuchtey RW. A de novo MYOC mutation detected in juvenile open angle glaucoma causes non-secretion of associated with reduced myocilin protein in aqueous humor. Eur J Med Genet. 2013;56:292–296. doi: 10.1016/j.ejmg.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huard DJE, Crowley VM, Du Y, Cordova RA, Sun Z, Tomlin MO, Dickey CA, Koren J, III, Blair L, Fu H, et al. Trifunctional high-throughput screen identifies promising scaffold to inhibit Grp94 and treat myocilin-associated glaucoma. ACS Chem Biol. 2018;13:933–941. doi: 10.1021/acschembio.7b01083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stothert AR, Suntharalingam A, Huard DJ, Fontaine SN, Crowley VM, Mishra S, Blagg BS, Lieberman RL, Dickey CA. Exploiting the interaction between Grp94 and aggregated myocilin to treat glaucoma. Hum Mol Genet. 2014;23:6470–6480. doi: 10.1093/hmg/ddu367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Caballero M, Borras T. Inefficient processing of an olfactomedindeficient myocilin mutant: Potential physiological relevance to glaucoma. Biochem Biophys Res Commun. 2001;282:662–670. doi: 10.1006/bbrc.2001.4624. [DOI] [PubMed] [Google Scholar]
- 82.Vollrath D, Liu Y. Temperature sensitive secretion of mutant myocilins. Exp Eye Res. 2006;82:1030–1036. doi: 10.1016/j.exer.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 83.Yam GH, Gaplovska-Kysela K, Zuber C, Roth J. Aggregated myocilin induces russell bodies and causes apoptosis: Implications for the pathogenesis of myocilin-caused primary open-angle glaucoma. Am J Pathol. 2007;170:100–109. doi: 10.2353/ajpath.2007.060806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Joe MK, Sohn S, Hur W, Moon Y, Choi YR, Kee C. Accumulation of mutant myocilins in ER leads to ER stress and potential cytotoxicity in human trabecular meshwork cells. Biochem Biophys Res Commun. 2003;312:592–600. doi: 10.1016/j.bbrc.2003.10.162. [DOI] [PubMed] [Google Scholar]
- 85.Gould DB, Miceli-Libby L, Savinova OV, Torrado M, Tomarev SI, Smith RS, John SW. Genetically increasing Myoc expression supports a necessary pathologic role of abnormal proteins in glaucoma. Mol Cell Biol. 2004;24:9019–9025. doi: 10.1128/MCB.24.20.9019-9025.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Joe MK, Nakaya N, Abu-Asab M, Tomarev SI. Mutated myocilin and heterozygous Sod2 deficiency act synergistically in a mouse model of open-angle glaucoma. Hum Mol Genet. 2015;24:3322–3334. doi: 10.1093/hmg/ddv082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Joe MK, Tomarev SI. Expression of myocilin mutants sensitizes cells to oxidative stress-induced apoptosis: Implication for glaucoma pathogenesis. Am J Pathol. 2010;176:2880–2890. doi: 10.2353/ajpath.2010.090853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hill SE, Donegan RK. The glaucoma-associated olfactomedin domain of myocilin forms polymorphic fibrils that are constrained by partial unfolding and peptide sequence. J Mol Biol. 2014;426:921–935. doi: 10.1016/j.jmb.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zode GS, Kuehn MH, Nishimura DY, Searby CC, Mohan K, Grozdanic SD, Bugge K, Anderson MG, Clark AF, Stone EM, Sheffield VC. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J Clin Invest. 2011;121:3542–3553. doi: 10.1172/JCI58183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maddineni P, Kasetti RB, Zode GS. Methods for analyzing endoplasmic reticulum stress in the trabecular meshwork of glaucoma models. Methods Mol Biol. 2018;1695:121–134. doi: 10.1007/978-1-4939-7407-8_12. [DOI] [PubMed] [Google Scholar]
- 91.Chong WC, Shastri MD, Eri R. Endoplasmic reticulum stress and oxidative stress: A vicious nexus implicated in bowel disease pathophysiology. Int J Mol Sci. 2017;18:E771. doi: 10.3390/ijms18040771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Plaisance V, Brajkovic S, Tenenbaum M, Favre D, Ezanno H, Bonnefond A, Bonner C, Gmyr V, Kerr-Conte J, Gauthier BR, et al. Endoplasmic reticulum stress links oxidative stress to impaired pancreatic beta-cell function caused by human oxidized LDL. PLoS One. 2016;11:e0163046. doi: 10.1371/journal.pone.0163046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao J, Wang S, Zhong W, Yang B, Sun L, Zheng Y. Oxidative stress in the trabecular meshwork (Review) Int J Mol Med. 2016;38:995–1002. doi: 10.3892/ijmm.2016.2714. [DOI] [PubMed] [Google Scholar]
- 94.Grootjans J, Kaser A, Kaufman RJ, Blumberg RS. The unfolded protein response in immunity and inflammation. Nat Rev Immunol. 2016;16:469–484. doi: 10.1038/nri.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Luo K, Cao SS. Endoplasmic reticulum stress in intestinal epithelial cell function and inflammatory bowel disease. Gastroenterol Res Pract. 2015;2015:328791. doi: 10.1155/2015/328791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peters JC, Bhattacharya S, Clark AF, Zode GS. Increased endoplasmic reticulum stress in human glaucomatous trabecular meshwork cells and tissues. Invest Ophthalmol Vis Sci. 2015;56:3860–3868. doi: 10.1167/iovs.14-16220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huard DJ, Lieberman RL. Progress toward development of a proteostasis drug for myocilin-associated glaucoma. Future Med Chem. 2018;10:1391–1393. doi: 10.4155/fmc-2018-0073. [DOI] [PubMed] [Google Scholar]
- 98.Mishra SJ, Ghosh S, Stothert AR, Dickey CA, Blagg BS. Transformation of the non-selective aminocyclohexanol-based Hsp90 inhibitor into a Grp94-seletive scaffold. ACS Chem Biol. 2017;12:244–253. doi: 10.1021/acschembio.6b00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Crowley VM, Khandelwal A, Mishra S, Stothert AR, Huard DJ, Zhao J, Muth A, Duerfeldt AS, Kizziah JL, Lieberman RL, et al. Development of glucose regulated protein 94-selective inhibitors based on the BnIm and radamide scaffold. J Med Chem. 2016;59:3471–3488. doi: 10.1021/acs.jmedchem.6b00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stothert AR, Suntharalingam A, Tang X, Crowley VM, Mishra SJ, Webster JM, Nordhues BA, Huard DJE, Passaglia CL, Lieberman RL, et al. Isoform-selective Hsp90 inhibition rescues model of hereditary open-angle glaucoma. Sci Rep. 2017;7:17951. doi: 10.1038/s41598-017-18344-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Keller KE, Wirtz MK. Working your SOCS off: The role of ASB10 and protein degradation pathways in glaucoma. Exp Eye Res. 2017;158:154–160. doi: 10.1016/j.exer.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang N, Chintala SK, Fini ME, Schuman JS. Activation of a tissue-specific stress response in the aqueous outflow pathway of the eye defines the glaucoma disease phenotype. Nat Med. 2001;7:304–309. doi: 10.1038/85446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yerramothu P, Vijay AK, Willcox MDP. Inflammasomes the eye and anti-inflammasome therapy. Eye (Lond) 2018;32:491–505. doi: 10.1038/eye.2017.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Meier-Soelch J, Jurida L, Weber A, Newel D, Kim J, Braun T, Schmitz ML, Kracht M. RNAi-based identification of gene-specific nuclear cofactor networks regulating interleukin-1 target genes. Front Immunol. 2018;9:775. doi: 10.3389/fimmu.2018.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yasuda M, Takayama K, Kanda T, Taguchi M, Someya H, Takeuchi M. Comparison of intraocular pressure-lowering effects of ripasudil hydrochloride hydrate for inflammatory and corticosteroid-induced ocular hypertension. PLoS One. 2017;12:e0185305. doi: 10.1371/journal.pone.0185305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Itakura T, Peters DM, Fini ME. Glaucomatous MYOC mutations activate the IL-1/NF-κB inflammatory stress response and the glaucoma marker SELE in trabecular meshwork cells. Mol Vis. 2015;21:1071–1084. [PMC free article] [PubMed] [Google Scholar]
- 107.Kasetti RB, Phan TN, Millar JC, Zode GS. Expression of mutant myocilin induces abnormal intracellular accumulation of selected extracellular matrix proteins in the trabecular meshwork. Invest Ophthalmol Vis Sci. 2016;57:6058–6069. doi: 10.1167/iovs.16-19610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Donegan RK, Hill SE, Freeman DM, Nguyen E, Orwig SD, Turnage KC, Lieberman RL. Structural basis for misfolding in myocilin-associated glaucoma. Hum Mol Genet. 2015;24:2111–2124. doi: 10.1093/hmg/ddu730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Joe MK, Sohn S, Choi YR, Park H, Kee C. Identification of flotillin-1 as a protein interacting with myocilin: Implications for the pathogenesis of primary open-angle glaucoma. Biochem Biophys Res Commun. 2005;336:1201–1206. doi: 10.1016/j.bbrc.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 110.Joe MK, Kwon HS, Cojocaru R, Tomarev SI. Myocilin regulates cell proliferation and survival. J Biol Chem. 2014;289:10155–10167. doi: 10.1074/jbc.M113.547091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kessenbrock K, Wang CY, Werb Z. Matrix metalloproteinases in stem cell regulation and cancer. Matrix Biol. 2015;46:184–190. doi: 10.1016/j.matbio.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.De Groef L, Van Hove I, Dekeyster E, Stalmans I, Moons L. MMPs in the neuroretina and optic nerve: Modulators of glaucoma pathogenesis and repair. Invest Ophthalmol Vis Sci. 2014;55:1953–1964. doi: 10.1167/iovs.13-13630. [DOI] [PubMed] [Google Scholar]
- 113.Ashworth Briggs EL, Toh T, Eri R, Hewitt AW, Cook AL. TIMP1 TIMP2 and TIMP4 are increased in aqueous humor from primary open angle glaucoma patients. Mol Vis. 2015;21:1162–1172. [PMC free article] [PubMed] [Google Scholar]
- 114.Filla MS, Dimeo KD, Tong T, Peters DM. Disruption of fibronectin matrix affects type IV collagen fibrillin and laminin deposition into extracellular matrix of human trabecular meshwork (HTM) cells. Exp Eye Res. 2017;165:7–19. doi: 10.1016/j.exer.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zode GS, Sharma AB, Lin X, Searby CC, Bugge K, Kim GH, Clark AF, Sheffield VC. Ocular-specific ER stress reduction rescues glaucoma in murine glucocorticoid-induced glaucoma. J Clin Invest. 2014;124:1956–1965. doi: 10.1172/JCI69774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ho H, Htoon HM, Yam GH, Toh LZ, Lwin NC, Chu S, Lee YS, Wong TT, Seet LF. Altered anterior segment biometric parameters in mice deficient in SPARC. Invest Ophthalmol Vis Sci. 2017;58:386–393. doi: 10.1167/iovs.16-20261. [DOI] [PubMed] [Google Scholar]
- 117.Hill SE, Nguyen E, Donegan RK, Patterson-Orazem AC, Hazel A, Gumbart JC, Lieberman RL. Structure and misfolding of the flexible tripartite coiled-coil domain of glaucoma-associated myocilin. Structure. 2017;25:1697–1707. doi: 10.1016/j.str.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Burns JN, Turnage KC, Walker CA, Lieberman RL. The stability of myocilin olfactomedin domain variants provides new insight into glaucoma as a protein misfolding disorder. Biochemistry. 2011;50:5824–5833. doi: 10.1021/bi200231x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Burns JN, Orwig SD, Harris JL, Watkins JD, Vollrath D, Lieberman RL. Rescue of glaucoma-causing mutant myocilin thermal stability by chemical chaperones. ACS Chem Biol. 2010;5:477–487. doi: 10.1021/cb900282e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Honda R. Role of the disulfide bond in prion protein amyloid formation: A thermodynamic and kinetic analysis. Biophys J. 2018;114:885–892. doi: 10.1016/j.bpj.2017.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fingert JH, Héon E, Liebmann JM, Yamamoto T, Craig JE, Rait J, Kawase K, Hoh ST, Buys YM, Dickinson J, et al. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum Mol Genet. 1999;8:899–905. doi: 10.1093/hmg/8.5.899. [DOI] [PubMed] [Google Scholar]
- 122.Shimizu S, Lichter PR, Johnson AT, Zhou Z, Higashi M, Gottfredsdottir M, Othman M, Moroi SE, Rozsa FW, Schertzer RM, et al. Age-dependent prevalence of mutations at the GLC1A locus in primary open-angle glaucoma. Am J Ophthalmol. 2000;130:165–177. doi: 10.1016/S0002-9394(00)00536-5. [DOI] [PubMed] [Google Scholar]
- 123.Millá E, Mañé B, Duch S, Hernan I, Borràs E, Planas E, Dias Mde S, Carballo M, Gamundi MJ, Spanish Multicenter Glaucoma Group-Estudio Multicéntrico Español de Investigación Genética del Glaucoma, EMEIGG Survey of familial glaucoma shows a high incidence of cytochrome P450 family 1 subfamily B polypeptide 1 (CYP1B1) mutations in non-consanguineous congenital forms in a Spanish population. Mol Vis. 2013;19:1707–1722. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.