Abstract
CD28 and CTLA-4 are members of a family of Immunoglobulin-related receptors that are responsible for various aspects of T cell immune regulation. The family includes CD28, CTLA-4 and ICOS as well as other proteins including PD-1, BTLA and TIGIT. These receptors have both stimulatory (CD28, ICOS) as well as inhibitory roles (CTLA-4, PD-1, BTLA and TIGIT) in T cell function. Increasingly these pathways are targeted as part of immune modulatory strategies to treat cancers, referred to generically as immune checkpoint blockade, and conversely to treat autoimmunity and CTLA-4 deficiency. Here we focus on the biology of the CD28/CTLA-4 pathway as a framework for understanding the impacts of therapeutic manipulation of this pathway.
1. Molecular and cell biology of the CTLA-4 pathway
CTLA-4 and CD28 share two ligands, CD80 and CD86
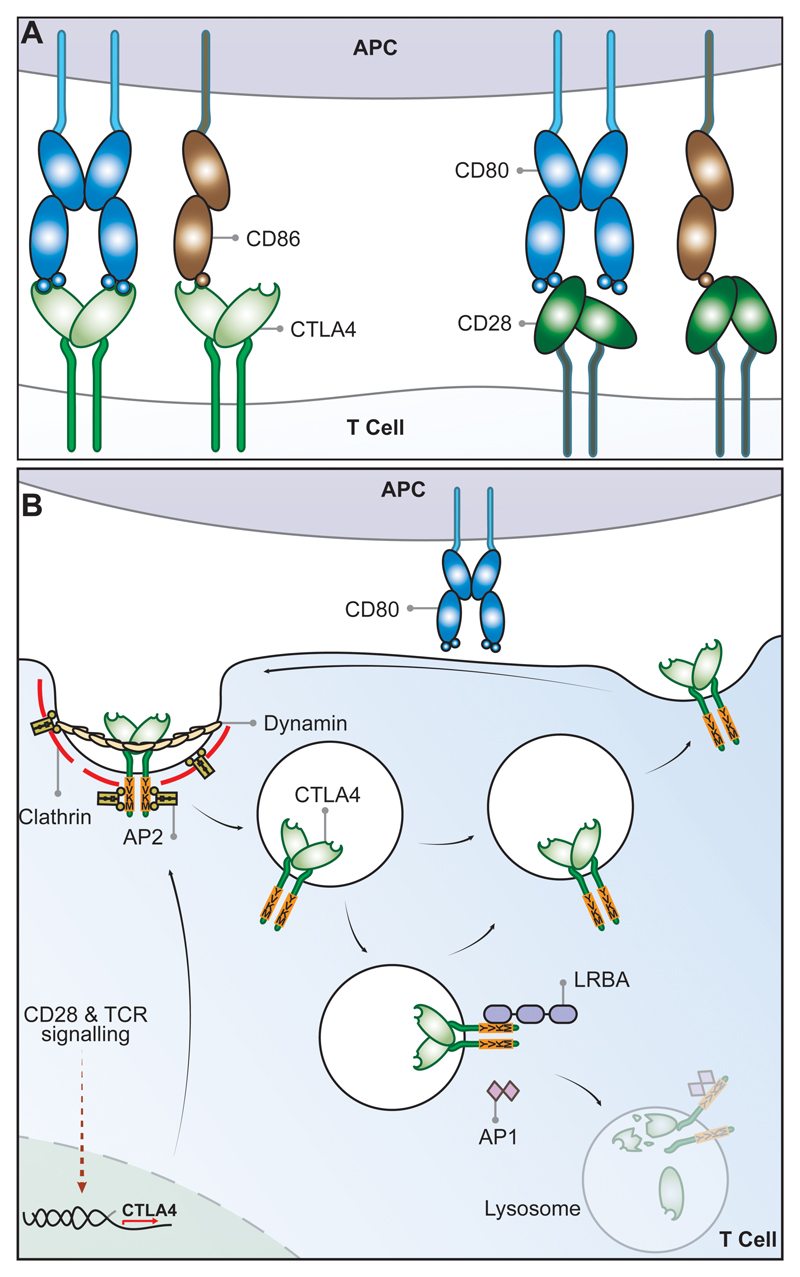
Cytotoxic T Lymphocyte antigen 4 (CTLA-4) (CD152) and CD28 are homologous receptors expressed by both CD4+ and CD8+ T cells, which mediate opposing functions in T cell activation. Both receptors share a pair of ligands expressed on the surface of antigen presenting cells (APCs). CD28 interacts with the CD80 dimer with relatively high affinity and the CD86 monomer with lower affinity, mediating T cell co-stimulation in conjunction with T cell receptor (TCR) signals. In contrast, interactions of the ligands with CTLA-4 serve to inhibit T cell responses, although the precise mechanisms are not fully understood. CTLA-4 interacts with both ligands with higher affinity and avidity than CD28 1–3 with CTLA-4-CD80 forming the highest avidity interaction and CD28-CD86 the weakest (Figure 1A). Amongst several possibilities, this raises the concept that CTLA-4 can compete with CD28 for ligand binding and thereby act as an antagonist of CD28-mediated co-stimulation 4,5. These interactions are thought to take place at the immune synapse between T cells and APCs where CTLA-4 has been shown to recruit CD80 thereby limiting its interactions with CD28 6,7.
Figure 1. Schematic of CTLA-4 cell biology.
(A) CTLA-4 and CD28 receptors share two ligands CD80 and CD86. CD80 is a dimeric high affinity ligand and CD86 is a monomeric lower affinity ligand for both receptors. CTLA-4 has a higher affinity and avidity for CD80 than CD86. The relative affinities go from high to low from left to right. (B) CTLA-4 expressed in T-cells is highly endocytic. CTLA-4 is constitutively expressed in Treg or induced following T cell activation via CD28 and TCR signaling. In the absence of the ligand, CTLA-4 is mainly found in intracellular compartments following clathrin-mediated endocytosis mediated through CTLA-4 interaction with the AP2 molecule. The AP2 (μ2 subunit) binds to the tyrosine-based (YVKM) motif of the cytoplasmic domain of CTLA-4 and mediates rapid internalization. LRBA and AP1 proteins have also been found to bind to the YVKM motif on CTLA-4, which appear to impose different fates on CTLA-4. LRBA may mediate recycling of CTLA-4 to the plasma membrane, whereas AP1 may mediate CTLA-4 trafficking to lysosomal compartments resulting in subsequent degradation.
Whilst the biophysical characteristics of CD80 and CD86 are well defined, their functional differences are less clear and the ligands are often referred to together as B7 molecules or CD80/CD86. Both ligands are found on antigen presenting cells such as dendritic cells and B cells, although they have different expression patterns, inducibility and kinetics 8–10. Knockout mice show impaired immune responses consistent with reduced CD28 co-stimulation, with CD86 deficiency arguably demonstrating a more severe phenotype 9. There is considerable variation in ligand expression making generalisation difficult, CD86 is often expressed constitutively on dendritic cells (DC) and is induced to high levels following inflammatory stimuli. In contrast CD80 is thought to be upregulated later by DC. Human peripheral blood monocytes express CD86 and not CD80, whereas some human T cells can express both CD80 and CD86 under different conditions of activation 11,12. Thus, at present, differences between the two ligands revolve mostly around different expression patterns, whereas clear functional distinctions have yet to emerge.
Cell biology of CD28
Perhaps the most surprising feature of CD28 and CTLA-4 is that whilst they share the same ligands, they have opposing functions. CD28 is the archetypal co-stimulatory molecule involved in the activation of T cells upon binding to either CD80 or CD86. Co-stimulatory signals from the CD28 cytoplasmic tail result from interactions with a number of signaling molecules including the p85 subunit of PI3 kinase, the Src family kinase Lck, IL-2 inducible family kinase Itk, protein kinase C theta and adaptor proteins such as Grb2 and GADS 13–16. These signals result in the activation of transcription factors such as NF-κB and AP-1, which are important in functional outcomes including IL-2 production and T cell survival. The ability of CD28 to activate NF-κB and AP-1 pathways aligns well with the concept that AP-1 is important in avoiding unresponsive exhausted states 17 which can occur in the absence of CD28 costimulation. Indeed, there is considerable overlap between the exhausted gene signature seen in CD8 T cells, resulting from an inability to recruit AP-1, and with that seen in CD4 T cell anergy 17.
In addition to the activation of signaling pathways such as PI3K, AP-1, NF-κB and others, CD28 is increasingly thought to be important in remodeling of the actin cytoskeleton. CD28 activates actin-regulators such as Vav-1, cofilin-1 and RLTPR 18 and actin remodeling initiated by co-stimulation has been shown to be required for full TCR signaling 19. The close link between CD28 and the actin cytoskeleton has been further highlighted in a recent study characterizing CD28 interaction with capZIP, a regulator of the actin cytoskeleton. This demonstrated capZIP requirement for CD28 co-stimulation-dependent IL-2 production 20. Effects on gene regulation are also triggered via the CD28-inducible transcription factor DEC1, required for efficient regulation of CD4+ T cell activation pathways 21 and the expression of histone acetyl transferase Ezh2, which is responsible for maintaining the regulatory T cell (Treg) program 22. Thus CD28 signaling pathways are important for both effector T cell activation and Treg function.
Cell biology of CTLA-4
Whilst CD28 is constitutively expressed on the plasma membrane and co-stimulates T cells, CTLA-4 is predominantly found in intracellular vesicles in FoxP3+ Treg cells or activated conventional T cells. This localization is due to the constitutive endocytosis of CTLA-4 from the plasma membrane and results in approximately 90% of CTLA-4 being intracellular 23,24. The endocytosis of CTLA-4 is extremely rapid, with more than 80% of surface CTLA-4 being internalised within five minutes 25. Once internalised, CTLA-4 molecules appear to be either recycled to the plasma membrane or degraded in lysosomal compartments (Figure 1B), however, the details of this process and its functional significance are not yet fully understood.
The post-endocytic fate and control of CTLA-4 trafficking is still poorly characterized, although experiments show that CTLA-4 lacking its 36-amino acid cytoplasmic tail is predominantly located at the cell surface 26. A number of studies have therefore focused on identifying partners that interact with the cytoplasmic tail of CTLA-4 and on defining the role of its cytoplasmic domain. CTLA-4 endocytosis is dependent on clathrin due to its interaction with the µ2 subunit of the clathrin adaptor protein complex AP2 24,27 and is also dependent upon dynamin. AP2 targets the cytoplasmic tyrosine containing YVKM motif of CTLA-4 and can be disengaged when this motif is tyrosine-phosphorylated upon T cell activation 24,28. However, the immunological settings where the CTLA-4–AP-2 interaction is disrupted are unclear since activated T cells and Treg continue to endocytose CTLA-4 25. The cytoplasmic domain sequence and trafficking behavior of CTLA-4 are highly conserved in mammals whereas some animals such as fish lack this endocytic motif 5,29,30 resulting in predominantly surface expression. This suggests that endocytosis may be a later adaptation of CTLA-4. Overall, CD28 and CTLA-4 differ profoundly in sub-cellular location despite binding to the same ligands and, given the conservation of these features, it seems likely they are pivotal to the functioning of the system.
The significance of CTLA-4 trafficking was recently highlighted in patients with Lipopolysaccharide-responsive and beige-like anchor protein (LRBA) deficiency 31. This BEACH domain containing protein 32 appears to regulate CTLA-4 turnover and it co-localizes predominantly with recycling (Rab11+) endosomes 31. Loss of LRBA leads to increased CTLA-4 degradation with associated autoimmunity, indicating that LRBA may inhibit CTLA-4 trafficking to lysosomes and promote its recycling. This may be achieved through LRBA binding to the YVKM consensus sequence of CTLA-4 (Figure 1B). Additional control of CTLA-4 trafficking and cellular distribution may also be mediated via interaction with the TRIM/LAX/Rab8 complex, which is involved in the post-Golgi transport of CTLA-4 to the cell surface 33. More recently, CTLA-4 interactions involving PKC-eta and PIX-PAK pathway have also been reported. These interactions appear to modulate Treg-APC interactions: PKC-eta deficient Treg are associated with impaired depletion of CD86 from APCs by Treg and increased Treg motility 34.
Trans-endocytosis: a cell-extrinsic molecular mechanism of CTLA-4 function
A number of different models have been proposed to explain the mechanism of CTLA-4 function. The simplest of these involves competition between CD28 and CTLA-4 for ligand binding 4, which has been proposed alongside other cell-intrinsic inhibitory signaling models 26,35,36. However, whilst CD4 T cells from CTLA-4 deficient mice have a hyper-activated, disease-causing phenotype, the presence CTLA-4 expressing cells in chimeras can prevent disease and normalise the phenotype of CTLA-4 deficient cells via a dominant, cell-extrinsic mechanism 37.
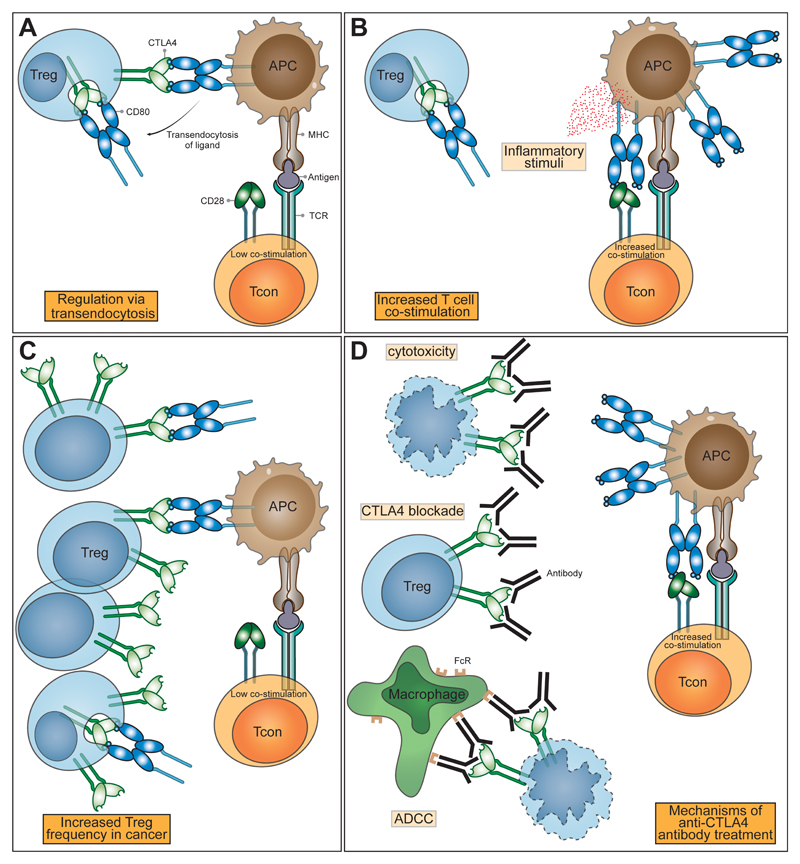
One molecular mechanism that is consistent with a dominant cell-extrinsic role for CTLA-4 is the physical capture of CD80 and CD86, and their subsequent removal from APCs: a process known as trans-endocytosis 38 (Figure 2A). Trans-endocytosis requires T cell recognition of peptide and represents an antigen specific mechanism for controlling CD80 and CD86 expression levels on APC. Whilst the molecular mechanisms underlying trans-endocytosis itself are still largely unknown, the CTLA-4 pathway is not the only receptor-ligand pair that utilises such a pathway. Other examples include the Notch-Delta pathway 39,40, where binding of Delta (ligand) allows subsequent removal of Notch (receptor) from the adjacent cell. Similar examples operating in the absence of ectodomain cleavage, include the gap junction protein connexin-43 41, adhesion junctions via VE-cadherin 42 receptor tyrosine-kinases Eph-ephrin interaction 43 and CD47-SHPS-1 44. Although these examples are present in varied biological systems, their overall similarity suggests that a conserved molecular machinery capable of removing membrane bound molecules from neighboring cells exists.
Figure 2. CTLA-4 function and the impact of immune checkpoint blockade.
(A) In health, regulatory T cells express CTLA-4, which binds CD80 and CD86 expressed on antigen presenting cells (APCs). CTLA-4 binds to CD80 and CD86 with higher affinity and avidity than does CD28, preventing Tcon stimulation through CD80/CD86 interaction with CD28. Removal of CD80/CD86 ligands by trans-endocytosis results in impaired costimulation of T cells via CD28, resulting in immune regulation. (B) When the immune system is stimulated in the presence of inflammatory/innate immune stimuli, APCs up-regulate the expression of CD80/CD86 overcoming their control by Treg, enabling co-stimulation and the proliferation of T cells. (C) In the tumour microenvironment, CD80/CD86 are controlled by Treg and the abundance of Treg leads to the suppression of immune responses. (D) Anti-CTLA-4 antibodies bind to CTLA-4 molecules with high affinity leading to Treg depletion or functional blockade resulting in enhanced T cell activation and immunological responses to cancer. The impacts of CTLA-4 blockade can be mediated by a variety of mechanisms: prevention of trans-endocytosis increasing CD80/CD86 levels on APCs, direct Treg cytotoxicity and antibody dependent cellular cytotoxicity mediated by FcR-IV expressing intra-tumoural macrophages. NB only CD80 is shown here for clarity.
In summary, CTLA-4 is a highly endocytic molecule that binds to two distinct ligands, CD80 and CD86, leading to their removal from opposing cells. By utilizing trans-endocytosis CTLA-4 can act as an immune regulatory mechanism by directly reducing the ability of APC to stimulate via CD28. This concept helps explain why a stimulatory and an inhibitory receptor share the same ligands and embraces the endocytic nature of CTLA-4. Taken together, this model suggests that the CD28/CTLA-4 system functions as a rheostat that can tune T cell activation up or down.
2. CD28 and CTLA-4: a volume control on T cell responses?
The use of a random repertoire of antigen receptors by T cells provides effective coverage for the recognition of evolving pathogens. However, it generates a significant problem: that of preventing such receptors recognising self-tissues. Such self-reactivity can lead to potentially fatal autoimmune conditions including type-I diabetes, inflammatory bowel disorders, autoimmune cytopaenias and others. Whilst T cell selection in the thymus can remove some of these self-reactive specificities, it is incomplete for a variety of reasons 45–47. Therefore significant numbers of potentially autoreactive T cells are present in the circulation of healthy individuals, at frequencies similar to other antigen specificities 48. Indeed recent evidence suggests that tolerance to some tissue-restricted self-antigens cannot be achieved by deletion and relies on the presence of regulatory T cells 49. Thus, whilst thymic selection mitigates T cell self-reactivity, additional controls are required that involve the CD28/CTLA-4 system.
CD28 function: Turning up the volume
The requirement for CD28 to co-stimulate T cell activation emerged from studies by Schwartz and Jenkins 50. Here, antigen specific T cells entered an unresponsive state, termed T cell anergy, in response to TCR stimuli in the absence of a “second signal”. This signal was subsequently identified as coming from the CD28 receptor interacting with CD80 or CD86. Since these ligands are up-regulated by a variety of inflammatory signals, (Figure 2B) CD28 signaling provides information on the inflammatory context or “danger” accompanying TCR recognition. Importantly, CD28 co-stimulation appears to be additive with signals via the TCR enhancing commitment to, as well as more rapid, cell division 51. Moreover, the requirement for CD28 co-stimulation is related to strength of TCR signaling 52 such that strong TCR stimulation requires less co-stimulation 53–55. Thus, weak TCRs (such as those that are self-reactive) may be effectively controlled by constraining the availability of CD28 co-stimulation. These concepts relating to control of CD28 co-stimulation become significant when considering how CTLA-4 might function to quantitatively limit CD28 signaling 56 thereby controlling self-reactive and tumour-reactive T cells in the absence of inflammation.
The importance of CD28 signaling in driving T cell proliferative responses has been strikingly reinforced by the recent identification of CD28 mutations in T cell leukaemias that increase its affinity for ligands. Here, point mutations with increased ligand affinity as well as direct CTLA-4-CD28 fusions have been observed generating high affinity CD28 variants 57,58, which presumably are less effectively out-competed by CTLA-4. To date, a large body of work indicates that CD28 signals drive critical T cell effector functions by increasing the magnitude of T cell responses, stimulating metabolic changes 59,60 as well as enhancing T cell differentiation 61,62 and survival 63. CD28 signals also contribute to enhanced cytokine production, influence T cell migration 64 and memory T cell responses to infections 65,66. Given these potent activating functions, it is apparent that effective control of CD28 co-stimulation is essential and can be provided by CTLA-4.
CTLA-4: Turning down the volume
The observation of fatal autoimmunity in CTLA-4 knockout mice 67,68 resulting from the release of self-reactive T cells 69 illustrated that CTLA-4 was a critical negative regulator of T cell responses. T cells isolated from knockout mice showed high levels of activation markers including CD25. Whilst the initial interpretation of this activated T cell phenotype focused on the possibility that CTLA-4 mediated an inhibitory signal preventing T cell activation 70, accumulating evidence now points to an important role for CTLA-4 in mediating the suppressive functions of Treg. Indeed early experiments indicated that the major autoimmune phenotype of CTLA-4 deficient mice could be prevented by the presence of CTLA-4 expressing cells in mixed chimeras 37. This observation has been supported by a number of other experiments (see 5) and recently a series of conditional and inducible CTLA-4 deletion experiments have confirmed that the major phenotype is consistent with an effector function for CTLA-4 on Treg 71–73. These data demonstrate that CTLA-4 on Treg can control the activity of other cells (such as APCs or naïve T cells) thereby, controlling fatal autoimmunity (Figure 2A).
Whilst, CTLA-4 on Treg is clearly important in preventing autoimmunity, its expression is also induced on activated T cells. Evidence from knockout mice demonstrates a more severe phenotype in complete CTLA-4 knockouts compared to Treg specific knockouts, indicating an important role for CTLA-4 in conventional T cells 72. However, the function of CTLA-4 in non-Treg cells can also be cell extrinsic, suggesting they might use the same mechanism as Treg 74,75 and activated Tcon can carry out trans-endocytosis 38. Nonetheless, cell-intrinsic functions of CTLA-4 controlling the homeostatic expansion of conventional T cells have been observed in vivo, although in vitro responses to stimulation were found to be similar between WT and knockout conventional T cells 73.
It is also clear that CTLA-4 and CD28 directly influence Treg homeostasis. For example, CD28-deficient mice, or those where ligands are blocked, have reduced Treg numbers due to altered Treg generation and peripheral survival 76–79. Conversely, the loss of CTLA-4 from Treg promotes their expansion via increased CD28 signaling 80,81. Thus, both CD28 and CTLA-4 profoundly impact the generation, maintenance and function of Treg. This intimate connection between CD28 and CTLA-4 function means that it is difficult to understand manipulation of one receptor without considering the impact on the other. Indeed, it is arguable that understanding the impact of CTLA-4 manipulation is best appreciated in terms of its ultimate effect on CD28 - CD80/CD86 interactions.
3. Targeting the CTLA-4 pathway: lessons from the clinic
Targeting the CD28/CTLA-4 pathway using antibodies and fusion proteins is of considerable interest in the treatment of cancer and autoimmune diseases. Since CTLA-4 limits immune responses to self-tissues, augmenting this pathway represents a treatment option for autoimmunity whereas suppressing this has the potential to stimulate anti-self-responses towards tumours. The potential for the immunological rejection of cancer by harnessing immune responses was first realised using anti-CTLA-4 antibodies, and has resulted in exponential activity in this area 82.
Initially, murine cancer models demonstrated that anti-CTLA-4 antibody treatment resulted in tumour regression and relative resistance to re-inoculation with cancer 83. Less immunogenic cancers were, however, unresponsive to anti-CTLA-4 therapy unless GM-CSF producing vaccines were co-administered 84. Importantly, enhanced anti-tumour responses following CTLA-4 blockade were associated with autoimmunity. This was illustrated by CD8 T cell mediated depigmentation in melanoma models 85, immune-mediated prostatitis following vaccination against prostate cancer-specific antigens 86 and the generation of anti-double stranded DNA antibodies 87. Based upon the efficacy of CTLA-4 blockade in animal models anti-CTLA-4 antibodies were developed for clinical use. Ipilimumab, a recombinant human immunoglobulin (Ig) G1 monoclonal antibody, and tremelimumab, a human IgG2 monoclonal antibody (previously known as ticilimumab) have both been trialed in a range of advanced stage cancers. It is clear that ipilimumab potently blocks the interaction of CTLA-4 with its ligands by binding to the MYPPPY motif, which is critical for this interaction 88. Reports from human trials in melanoma, non-small cell lung cancer, mesothelioma, prostate, ovarian, breast and urothelial cancer treatment have shown efficacy 89–108. Alongside the benefits in tumour control, these trials nonetheless demonstrate a broad range of immune related adverse events (irAEs) occurring in 60-65% of patients. irEAs most commonly effect the skin, gastrointestinal (GI) tract, liver and endocrine organs 109. Additionally, autoimmunity has been reported in almost all other organs including the liver, eyes, nervous system, lungs, heart, kidneys, and joints 89–108,110,111. The breadth of irAEs is therefore consistent with the biological role of CTLA-4 in the maintenance of polyclonal immune self-tolerance.
Further evidence for a role of CTLA-4 in the control of polyclonal auto-reactive T cells has come from examining the circulating TCR repertoire following anti-CTLA-4 therapy. Increased diversity of the TCR repertoire occurs following treatment 112, is associated with irAE frequency 113 and polyclonal T cell expansion predates and predicts irAEs 114. This data is consistent with the idea that CTLA-4 blockade allows the expansion and activation of new T cell clones and fits with the idea that self-reactive T cells are present in healthy individuals 48,115 and are prevented from expanding by CTLA-4-mediated control of CD80/CD86 116.
The fact that CTLA-4 blockade enables immune responses to cancers and healthy self-tissues indicates that significant recognition of self-antigens exists within our immune repertoire. This is supported by data from heterozygous loss-of-function mutations in the CTLA-4 gene. Individuals with these mutations present with an autosomal dominant syndrome of complex autoimmunity and immunodeficiency 81,117. This phenotype has many similarities to anti-CTLA-4 antibody treatment and includes enteropathy, autoimmune cytopenias, haemolytic anaemia, thyroid disease, arthritis, psoriasis, granulomatous lung disease and lymphocytic infiltration of non-immune organs. Furthermore, a second CTLA-4 deficiency syndrome caused by autosomal recessive mutations in the LRBA gene is associated with an earlier onset, but phenotypically similar autoimmune syndrome 31,118,119, consistent with the role played by LRBA in the trafficking of CTLA-4 31 (Figure 1B). The similarities in wide-ranging autoimmunity between genetic deficiency in the CTLA-4 pathway and the impact of anti-CTLA-4 treatments are striking and reaffirm the central role for CTLA-4 in the maintenance of self tolerance seen in animal studies.
The concept that CTLA-4 deficiency leads to a CD28 driven disease is also supported in humans by the effect of treatment with the CTLA-4-Ig fusion protein, abatacept. This drug has an established role in the treatment of autoimmune disorders, including rheumatoid arthritis 120, where it is viewed through the lens of blocking the CD28 co-stimulation pathway. In contrast, in CTLA-4 deficiency syndromes, abatacept is viewed as a CTLA-4 replacement therapy. Nonetheless, the functional effects are the same in that CD80 and CD86 ligand availability is diminished. The fact that CTLA-4- and LRBA-deficient patients respond to abatacept 31,121 is consistent with the concept that these diseases are driven by excessive CD28 co-stimulation and in agreement with the fundamental biology of CTLA-4, as described above.
Individuals with a mutation in a single allele of CTLA-4 are highly susceptible to autoimmunity, raising the possibility that more subtle variations in the level of CTLA-4 in the general population may influence responses to treatment. Indeed, non-pathogenic polymorphisms in CTLA-4 that alter the expression of CTLA-4 influence an individual’s lifetime risk for the development of cancer and autoimmune disorders 122. Taken together these findings illustrate that CTLA-4 expression has a pivotal role in balancing immune responses against self-tissues, and therefore in regulating both autoimmune and anti-cancer responses.
The impact of CTLA-4 blockade on Treg biology
Since Treg constitutively express CTLA-4 at high levels, anti-CTLA-4 therapy would be expected to have the greatest effect upon these cells. Data from human trials are mixed but an increased absolute number of circulating FoxP3+ CD4 Treg have been observed in some studies 104,123,124. Similarly, CTLA-4 haplo-insufficiency results in either preserved or increased Treg numbers, due to lack of CTLA-4 and resultant increased CD28 signaling 81,117,125. Indeed the role of CD28 signaling has been exploited in Treg expansion via the use of CD28-agonist antibodies. Whilst initial trials of this strategy were associated with significant inflammatory side effects, recent efforts have shown promise 126,127.
Whilst Treg express CTLA-4 at higher levels, activated conventional T cells do express CTLA-4 and there is additional evidence from cancer therapies to suggest conventional T cell CTLA-4 may have an important role in self-tolerance. Anti-CTLA-4 treatment combined with Treg depletion was shown to be more effective at inducing both anti-tumour responses and autoimmunity in animal models 128. Also, using selective blockade of CTLA-4 on Treg or conventional T cell, it was demonstrated that Treg CTLA-4 blockade alone could not induce antitumor immunity, although it could augment the anti-tumour responses induced by CTLA-4 blockade of conventional T cells 129. These experiments suggest that CTLA-4 expressed by conventional T cells is important in control of polyclonal immune self-tolerance, and the greater number of conventional T cells may compensate for their lower levels of CTLA-4 expression compared to Treg, when considering the functional impact of CTLA-4.
Despite the fact that limiting CTLA-4 function can drive an increase in Treg numbers there are indications that reducing tumour-infiltrating Tregs may be important in determining the response to immunotherapy (Figure 2C). A reduction in tumour infiltrating FoxP3+ CD4 Treg was observed in one study following anti-CTLA-4 treatment 95 (Figure 2D), although not in another 98. However, a possible mechanism of Treg control by anti-CTLA-4 therapies is Treg depletion via antibody dependent cellular cytotoxicity (ADCC). Here, anti-CTLA-4 antibodies bound by FcR-IV expressing intra-tumoural macrophages results in removal of Treg 130 (Figure 2D).
CTLA-4 and the microbiome
One interesting observation from clinical blockade of CTLA-4 is that the most frequent and earliest sites of irAE are at the microbe-rich barriers of the skin and gut 131 suggesting either that CTLA-4 has a particularly important role at these sites, or its biology is influenced by microbial exposure. Fluctuating levels of antibodies against commensal GI microbes were observed in patients following anti-CTLA-4 treatment 132 suggesting dysregulated mucosal immunity with CTLA-4 blockade. This interaction of the GI microbiome and CTLA-4 function has had considerable attention recently given that tumour control and effector immune responses following anti-CTLA-4 therapy are impaired in germ-free or antibiotic exposed mice 133. Reconstitution of the mouse GI microbiome with specific Bacteroides species or with transplantation of Bacteroides-predominant faecal microbes from patients restored anti-tumour responses. Improved tumour responses following anti-CTLA-4 therapy are associated with particular microbial populations and also with a reduced incidence of colitis in mice 133 and patients 134. Similarly, individuals with CTLA-4 deficiency also tend to experience immune targeting of microbe-rich sites, including the skin and gut, however, studies of microbiota have yet to be reported. Taken together these findings suggest that the microbial environment in the gut modulates the impact of CTLA-4 activity in the control of both distant tumour and local self-tissue responses.
Correlations between poor anti-tumour responses, higher peripheral Treg and gut homing lymphocyte numbers along with specific microbiome clusters have been described. In patients with a microbiome cluster associated with better clinical responses, greater up-regulation of ICOS (which is associated with more robust clinical responses) was observed on CD4 cells following treatment 134. Treg from mice raised under germ free conditions were found to be impaired in their capacity to suppress T cell proliferation and the CD25+ CD4 cells from mesenteric lymph nodes of germ free mice less frequently express FoxP3 or CTLA-4 135. Taken together these findings suggest that the microbiome is important in influencing effector and regulatory T cell responses and potentially the dependence of mucosal immune regulation upon CTLA-4. However, if CTLA-4 plays a critical role in GI immune regulation a benefit of CTLA-4-Ig might have been expected in inflammatory bowel diseases, but this has not been demonstrated 136.
Conclusions
The CTLA-4 pathway is a critical regulator of T cell responses to tissues. The sharing of ligands with the stimulatory receptor CD28 establishes a rheostat, capable of tuning T cell responses. Accordingly, decreasing CTLA-4 function by antibody blockade, or in CTLA-4 genetic disorders, increases the availability of ligands for CD28 permitting the activation of potentially self-reactive T cells and altering Treg homeostasis. Furthermore, the high level expression of CTLA-4 on Treg and the emerging cell biology of this pathway continue to offer opportunities to stimulate or inhibit T cell responses.
Acknowlegements
BR was funded by the BBSRC and NH by the Wellcome Trust PhD Programme for Clinicians.
Footnotes
Author Contributions
BR, NH and DMS co wrote the manuscript.
Conflict of Interests
The authors declare no competing financial interests.
References
- 1.Schwartz JC, Zhang X, Fedorov AA, Nathenson SG, Almo SC. Structural basis for co-stimulation by the human CTLA-4/B7-2 complex. Nature. 2001;410(6828):604–608. doi: 10.1038/35069112. [DOI] [PubMed] [Google Scholar]
- 2.Stamper CC, Zhang Y, Tobin JF, et al. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature. 2001;410(6828):608–611. doi: 10.1038/35069118. [DOI] [PubMed] [Google Scholar]
- 3.Collins AV, Brodie DW, Gilbert RJC, et al. The Interaction Properties of Costimulatory Molecules Revisited. Immunity. 2002;17(2):201–210. doi: 10.1016/s1074-7613(02)00362-x. [DOI] [PubMed] [Google Scholar]
- 4.Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7(4):445–450. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- 5.Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol. 2011;11(12):852–863. doi: 10.1038/nri3108. [DOI] [PubMed] [Google Scholar]
- 6.Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison JP. B7-1 and b7-2 selectively recruit ctla-4 and CD28 to the immunological synapse. Immunity. 2004;21(3):401–413. doi: 10.1016/j.immuni.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 7.Yokosuka T, Kobayashi W, Takamatsu M, et al. Spatiotemporal basis of CTLA-4 costimulatory molecule-mediated negative regulation of T cell activation. Immunity. 2010;33(3):326–339. doi: 10.1016/j.immuni.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Schweitzer AN, Sharpe AH. Studies using antigen-presenting cells lacking expression of both B7-1 (CD80) and B7-2 (CD86) show distinct requirements for B7 molecules during priming versus restimulation of Th2 but not Th1 cytokine production. J Immunol. 1998;161(6):2762–2771. [PubMed] [Google Scholar]
- 9.Borriello F, Sethna MP, Boyd SD, et al. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–313. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 10.Su KY, Watanabe A, Yeh CH, Kelsoe G, Kuraoka M. Efficient Culture of Human Naive and Memory B Cells for Use as APCs. J Immunol. 2016;197(10):4163–4176. doi: 10.4049/jimmunol.1502193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sansom DM, Hall ND. B7/BB1, the ligand for CD28 is expressed on repeatedly activated human T cells in vitro. Eur J Immunol. 1993;23:295–298. doi: 10.1002/eji.1830230148. [DOI] [PubMed] [Google Scholar]
- 12.Azuma M, Ito D, Yagita H, et al. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 1993;366(6450):76–79. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- 13.Boomer JS, Green JM. An enigmatic tail of CD28 signaling. Cold Spring Harb Perspect Biol. 2010;2(8) doi: 10.1101/cshperspect.a002436. a002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong KF, Yokosuka T, Canonigo-Balancio AJ, Isakov N, Saito T, Altman A. A motif in the V3 domain of the kinase PKC-theta determines its localization in the immunological synapse and functions in T cells via association with CD28. Nat Immunol. 2011;12(11):1105–1112. doi: 10.1038/ni.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esensten JH, Helou YA, Chopra G, Weiss A, Bluestone JA. CD28 Costimulation: From Mechanism to Therapy. Immunity. 2016;44(5):973–988. doi: 10.1016/j.immuni.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soskic B, Qureshi OS, Hou T, Sansom DM. A transendocytosis perspective on the CD28/CTLA-4 pathway. Adv Immunol. 2014;124:95–136. doi: 10.1016/B978-0-12-800147-9.00004-2. [DOI] [PubMed] [Google Scholar]
- 17.Martinez GJ, Pereira RM, Aijo T, et al. The transcription factor NFAT promotes exhaustion of activated CD8(+) T cells. Immunity. 2015;42(2):265–278. doi: 10.1016/j.immuni.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang Y, Cucchetti M, Roncagalli R, et al. The lymphoid lineage-specific actinuncapping protein Rltpr is essential for costimulation via CD28 and the development of regulatory T cells. Nat Immunol. 2013;14(8):858–866. doi: 10.1038/ni.2634. [DOI] [PubMed] [Google Scholar]
- 19.Tan YX, Manz BN, Freedman TS, Zhang C, Shokat KM, Weiss A. Inhibition of the kinase Csk in thymocytes reveals a requirement for actin remodeling in the initiation of full TCR signaling. Nat Immunol. 2014;15(2):186–194. doi: 10.1038/ni.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian R, Wang H, Gish GD, et al. Combinatorial proteomic analysis of intercellular signaling applied to the CD28 T-cell costimulatory receptor. Proc Natl Acad Sci U S A. 2015;112(13):E1594–1603. doi: 10.1073/pnas.1503286112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez-Llordella M, Esensten JH, Bailey-Bucktrout SL, et al. CD28-inducible transcription factor DEC1 is required for efficient autoreactive CD4+ T cell response. J Exp Med. 2013;210(8):1603–1619. doi: 10.1084/jem.20122387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DuPage M, Chopra G, Quiros J, et al. The chromatin-modifying enzyme Ezh2 is critical for the maintenance of regulatory T cell identity after activation. Immunity. 2015;42(2):227–238. doi: 10.1016/j.immuni.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linsley PS, Bradshaw J, Greene J, Peach R, Bennett KL, Mittler RS. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996;4(6):535–543. doi: 10.1016/s1074-7613(00)80480-x. [DOI] [PubMed] [Google Scholar]
- 24.Shiratori T, Miyatake S, Ohno H, et al. Tyrosine phosphorylation controls internalization of CTLA-4 by regulating its interaction with clathrin-associated adaptor complex AP-2. Immunity. 1997;6(5):583–589. doi: 10.1016/s1074-7613(00)80346-5. [DOI] [PubMed] [Google Scholar]
- 25.Qureshi OS, Kaur S, Hou TZ, et al. Constitutive clathrin-mediated endocytosis of CTLA-4 persists during T cell activation. J Biol Chem. 2012;287(12):9429–9440. doi: 10.1074/jbc.M111.304329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker LS, Sansom DM. Confusing signals: recent progress in CTLA-4 biology. Trends Immunol. 2015;36(2):63–70. doi: 10.1016/j.it.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chuang E, Alegre ML, Duckett CS, Noel PJ, Vander Heiden MG, Thompson CB. Interaction of CTLA-4 with the clathrin-associated protein AP50 results in ligand-independent endocytosis that limits cell surface expression. J Immunol. 1997;159(1):144–151. [PubMed] [Google Scholar]
- 28.Zhang Y, Allison JP. Interaction of CTLA-4 with AP50, a clathrin-coated pit adaptor protein. Proc Natl Acad Sci U S A. 1997;94(17):9273–9278. doi: 10.1073/pnas.94.17.9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernard D, Riteau B, Hansen JD, et al. Costimulatory receptors in a teleost fish: typical CD28, elusive CTLA4. J Immunol. 2006;176(7):4191–4200. doi: 10.4049/jimmunol.176.7.4191. [DOI] [PubMed] [Google Scholar]
- 30.Kaur S, Qureshi OS, Sansom DM. Comparison of the intracellular trafficking itinerary of ctla-4 orthologues. PLoS One. 2013;8(4):e60903. doi: 10.1371/journal.pone.0060903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lo B, Zhang K, Lu W, et al. AUTOIMMUNE DISEASE. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science. 2015;349(6246):436–440. doi: 10.1126/science.aaa1663. [DOI] [PubMed] [Google Scholar]
- 32.Cullinane AR, Schaffer AA, Huizing M. The BEACH is hot: a LYST of emerging roles for BEACH-domain containing proteins in human disease. Traffic. 2013;14(7):749–766. doi: 10.1111/tra.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banton MC, Inder KL, Valk E, Rudd CE, Schneider H. Rab8 binding to immune cell-specific adaptor LAX facilitates formation of trans-Golgi network-proximal CTLA-4 vesicles for surface expression. Mol Cell Biol. 2014;34(8):1486–1499. doi: 10.1128/MCB.01331-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kong KF, Fu G, Zhang Y, et al. Protein kinase C-eta controls CTLA-4-mediated regulatory T cell function. Nat Immunol. 2014;15(5):465–472. doi: 10.1038/ni.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudd CE. The reverse stop-signal model for CTLA4 function. Nat Rev Immunol. 2008;8(2):153–160. doi: 10.1038/nri2253. [DOI] [PubMed] [Google Scholar]
- 36.Bour-Jordan H, Esensten JH, Martinez-Llordella M, Penaranda C, Stumpf M, Bluestone JA. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/ B7 family. Immunol Rev. 2011;241(1):180–205. doi: 10.1111/j.1600-065X.2011.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bachmann MF, Kohler G, Ecabert B, Mak TW, Kopf M. Cutting edge: lymphoproliferative disease in the absence of CTLA-4 is not T cell autonomous. J Immunol. 1999;163(3):1128–1131. [PubMed] [Google Scholar]
- 38.Qureshi OS, Zheng Y, Nakamura K, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332(6029):600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kramer H. RIPping notch apart: a new role for endocytosis in signal transduction? Sci STKE. 2000;2000(29):pe1. doi: 10.1126/stke.2000.29.pe1. [DOI] [PubMed] [Google Scholar]
- 40.Meloty-Kapella L, Shergill B, Kuon J, Botvinick E, Weinmaster G. Notch ligand endocytosis generates mechanical pulling force dependent on dynamin, epsins, and actin. Dev Cell. 2012;22(6):1299–1312. doi: 10.1016/j.devcel.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Falk MM, Kells RM, Berthoud VM. Degradation of connexins and gap junctions. FEBS Lett. 2014;588(8):1221–1229. doi: 10.1016/j.febslet.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakurai T, Woolls MJ, Jin SW, Murakami M, Simons M. Inter-cellular exchange of cellular components via VE-cadherin-dependent trans-endocytosis. PLoS One. 2014;9(6):e90736. doi: 10.1371/journal.pone.0090736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marston DJ, Dickinson S, Nobes CD. Rac-dependent trans-endocytosis of ephrinBs regulates Eph-ephrin contact repulsion. Nat Cell Biol. 2003;5(10):879–888. doi: 10.1038/ncb1044. [DOI] [PubMed] [Google Scholar]
- 44.Kusakari S, Ohnishi H, Jin FJ, et al. Trans-endocytosis of CD47 and SHPS-1 and its role in regulation of the CD47-SHPS-1 system. J Cell Sci. 2008;121(Pt 8):1213–1223. doi: 10.1242/jcs.025015. [DOI] [PubMed] [Google Scholar]
- 45.Malhotra D, Linehan JL, Dileepan T, et al. Tolerance is established in polyclonal CD4(+) T cells by distinct mechanisms, according to self-peptide expression patterns. Nat Immunol. 2016;17(2):187–195. doi: 10.1038/ni.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hogquist KA, Jameson SC. The self-obsession of T cells: how TCR signaling thresholds affect fate 'decisions' and effector function. Nat Immunol. 2014;15(9):815–823. doi: 10.1038/ni.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sewell AK. Why must T cells be cross-reactive? Nature reviews Immunology. 2012;12(9):669–677. doi: 10.1038/nri3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. Virus-specific CD4(+) memory-phenotype T cells are abundant in unexposed adults. Immunity. 2013;38(2):373–383. doi: 10.1016/j.immuni.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Legoux FP, Lim JB, Cauley AW, et al. CD4+ T Cell Tolerance to Tissue-Restricted Self Antigens Is Mediated by Antigen-Specific Regulatory T Cells Rather Than Deletion. Immunity. 2015;43(5):896–908. doi: 10.1016/j.immuni.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 51.Gett AV, Hodgkin PD. A cellular calculus for signal integration by T cells. Nat Immunol. 2000;1(3):239–244. doi: 10.1038/79782. [DOI] [PubMed] [Google Scholar]
- 52.Marchingo JM, Kan A, Sutherland RM, et al. T cell signaling. Antigen affinity, costimulation, and cytokine inputs sum linearly to amplify T cell expansion. Science. 2014;346(6213):1123–1127. doi: 10.1126/science.1260044. [DOI] [PubMed] [Google Scholar]
- 53.Gardner DH, Jeffery LE, Soskic B, et al. 1,25(OH)2D3 Promotes the Efficacy of CD28 Costimulation Blockade by Abatacept. Journal of immunology. 2015;195(6):2657–2665. doi: 10.4049/jimmunol.1500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kundig TM, Shahinian A, Kawai K, et al. Duration of TCR stimulation determines costimulatory requirement of T cells. Immunity. 1996;5(1):41–52. doi: 10.1016/s1074-7613(00)80308-8. [DOI] [PubMed] [Google Scholar]
- 55.Viola A, Lanzavecchia A. T cell activation determined by T cell receptor number and tunable thresholds. Science. 1996;273:104–106. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]
- 56.Hou TZ, Qureshi OS, Wang CJ, et al. A transendocytosis model of CTLA-4 function predicts its suppressive behavior on regulatory T cells. J Immunol. 2015;194(5):2148–2159. doi: 10.4049/jimmunol.1401876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rohr J, Guo S, Huo J, et al. Recurrent activating mutations of CD28 in peripheral T-cell lymphomas. Leukemia. 2016;30(5):1062–1070. doi: 10.1038/leu.2015.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kataoka K, Nagata Y, Kitanaka A, et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet. 2015;47(11):1304–1315. doi: 10.1038/ng.3415. [DOI] [PubMed] [Google Scholar]
- 59.Frauwirth KA, Riley JL, Harris MH, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16(6):769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 60.Gubser PM, Bantug GR, Razik L, et al. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat Immunol. 2013;14(10):1064–1072. doi: 10.1038/ni.2687. [DOI] [PubMed] [Google Scholar]
- 61.Wang CJ, Heuts F, Ovcinnikovs V, et al. CTLA-4 controls follicular helper T-cell differentiation by regulating the strength of CD28 engagement. Proc Natl Acad Sci U S A. 2015;112(2):524–529. doi: 10.1073/pnas.1414576112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khattri R, Auger JA, Griffin MD, Sharpe AH, Bluestone JA. Lymphoproliferative disorder in CTLA-4 knockout mice is characterized by CD28-regulated activation of Th2 responses. J Immunol. 1999;162(10):5784–5791. [PubMed] [Google Scholar]
- 63.Boise LH, Minn AJ, Noel PJ, et al. CD28 costimulation can promote T cell survival by enhancing expression of Bcl-Xl. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 64.Jain N, Miu B, Jiang JK, et al. CD28 and ITK signals regulate autoreactive T cell trafficking. Nature medicine. 2013;19(12):1632–1637. doi: 10.1038/nm.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ndlovu H, Darby M, Froelich M, et al. Inducible deletion of CD28 prior to secondary nippostrongylus brasiliensis infection impairs worm expulsion and recall of protective memory CD4(+) T cell responses. PLoS Pathog. 2014;10(2):e1003906. doi: 10.1371/journal.ppat.1003906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frohlich M, Gogishvili T, Langenhorst D, Luhder F, Hunig T. Interrupting CD28 costimulation before antigen rechallenge affects CD8(+) T-cell expansion and effector functions during secondary response in mice. Eur J Immunol. 2016;46(7):1644–1655. doi: 10.1002/eji.201546232. [DOI] [PubMed] [Google Scholar]
- 67.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3(5):541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 68.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270(5238):985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 69.Ise W, Kohyama M, Nutsch KM, et al. CTLA-4 suppresses the pathogenicity of self antigen-specific T cells by cell-intrinsic and cell-extrinsic mechanisms. Nat Immunol. 2010;11(2):129–135. doi: 10.1038/ni.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee KM, Chuang E, Griffin M, et al. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282(5397):2263–2266. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- 71.Klocke K, Sakaguchi S, Holmdahl R, Wing K. Induction of autoimmune disease by deletion of CTLA-4 in mice in adulthood. Proc Natl Acad Sci U S A. 2016;113(17):E2383–2392. doi: 10.1073/pnas.1603892113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 73.Paterson AM, Lovitch SB, Sage PT, et al. Deletion of CTLA-4 on regulatory T cells during adulthood leads to resistance to autoimmunity. J Exp Med. 2015;212(10):1603–1621. doi: 10.1084/jem.20141030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang CJ, Kenefeck R, Wardzinski L, et al. Cutting edge: cell-extrinsic immune regulation by CTLA-4 expressed on conventional T cells. J Immunol. 2012;189(3):1118–1122. doi: 10.4049/jimmunol.1200972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Corse E, Allison JP. Cutting edge: CTLA-4 on effector T cells inhibits in trans. J Immunol. 2012;189(3):1123–1127. doi: 10.4049/jimmunol.1200695. [DOI] [PubMed] [Google Scholar]
- 76.Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol. 2005;6(2):152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 77.Zhang R, Huynh A, Whitcher G, Chang J, Maltzman JS, Turka LA. An obligate cell-intrinsic function for CD28 in Tregs. J Clin Invest. 2013;123(2):580–593. doi: 10.1172/JCI65013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gogishvili T, Luhder F, Goebbels S, Beer-Hammer S, Pfeffer K, Hunig T. Cell-intrinsic and -extrinsic control of Treg-cell homeostasis and function revealed by induced CD28 deletion. Eur J Immunol. 2013;43(1):188–193. doi: 10.1002/eji.201242824. [DOI] [PubMed] [Google Scholar]
- 79.Salomon B, Lenschow DJ, Rhee L, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12(4):431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 80.Schmidt EM, Wang CJ, Ryan GA, et al. Ctla-4 controls regulatory T cell peripheral homeostasis and is required for suppression of pancreatic islet autoimmunity. J Immunol. 2009;182(1):274–282. doi: 10.4049/jimmunol.182.1.274. [DOI] [PubMed] [Google Scholar]
- 81.Schubert D, Bode C, Kenefeck R, et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med. 2014;20(12):1410–1416. doi: 10.1038/nm.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 83.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 84.van Elsas A, Hurwitz AA, Allison JP. Combination Immunotherapy of B16 Melanoma Using Anti–Cytotoxic T Lymphocyte–Associated Antigen 4 (Ctla-4) and Granulocyte/Macrophage Colony-Stimulating Factor (Gm-Csf)-Producing Vaccines Induces Rejection of Subcutaneous and Metastatic Tumors Accompanied by Autoimmune Depigmentation. The Journal of Experimental Medicine. 1999;190(3):355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Elsas A, Sutmuller RPM, Hurwitz AA, et al. Elucidating the Autoimmune and Antitumor Effector Mechanisms of a Treatment Based on Cytotoxic T Lymphocyte Antigen-4 Blockade in Combination with a B16 Melanoma Vaccine: Comparison of Prophylaxis and Therapy. The Journal of Experimental Medicine. 2001;194(4):481–490. doi: 10.1084/jem.194.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hurwitz AA, Foster BA, Kwon ED, et al. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60(9):2444–2448. [PubMed] [Google Scholar]
- 87.Lute KD, May KF, Lu P, et al. Human CTLA4 knock-in mice unravel the quantitative link between tumor immunity and autoimmunity induced by anti–CTLA-4 antibodies. Blood. 2005;106(9):3127–3133. doi: 10.1182/blood-2005-06-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ramagopal UA, Liu W, Garrett-Thomson SC, et al. Structural basis for cancer immunotherapy by the first-in-class checkpoint inhibitor ipilimumab. Proc Natl Acad Sci U S A. 2017;114(21):E4223–e4232. doi: 10.1073/pnas.1617941114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hodi FS, Mihm MC, Soiffer RJ, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(8):4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(14):8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Attia P, Phan GQ, Maker AV, et al. Autoimmunity Correlates With Tumor Regression in Patients With Metastatic Melanoma Treated With Anti–Cytotoxic T-Lymphocyte Antigen-4. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(25):6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maker AV, Phan GQ, Attia P, et al. Tumor Regression and Autoimmunity in Patients Treated With Cytotoxic T Lymphocyte–Associated Antigen 4 Blockade and Interleukin 2: A Phase I/II Study. Annals of surgical oncology. 2005;12(12):1005–1016. doi: 10.1245/ASO.2005.03.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sanderson K, Scotland R, Lee P, et al. Autoimmunity in a Phase I Trial of a Fully Human Anti-Cytotoxic T-Lymphocyte Antigen-4 Monoclonal Antibody With Multiple Melanoma Peptides and Montanide ISA 51 for Patients With Resected Stages III and IV Melanoma. Journal of Clinical Oncology. 2005;23(4):741–750. doi: 10.1200/JCO.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 94.Comin-Anduix B, Lee Y, Jalil J, et al. Detailed analysis of immunologic effects of the cytotoxic T lymphocyte-associated antigen 4-blocking monoclonal antibody tremelimumab in peripheral blood of patients with melanoma. Journal of Translational Medicine. 2008;6:22–22. doi: 10.1186/1479-5876-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liakou CI, Kamat A, Tang DN, et al. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci U S A. 2008;105(39):14987–14992. doi: 10.1073/pnas.0806075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ménard C, Ghiringhelli F, Roux S, et al. CTLA-4 Blockade Confers Lymphocyte Resistance to Regulatory T-Cells in Advanced Melanoma: Surrogate Marker of Efficacy of Tremelimumab? Clinical Cancer Research. 2008;14(16):5242–5249. doi: 10.1158/1078-0432.CCR-07-4797. [DOI] [PubMed] [Google Scholar]
- 97.Camacho LH, Antonia S, Sosman J, et al. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J Clin Oncol. 2009;27(7):1075–1081. doi: 10.1200/JCO.2008.19.2435. [DOI] [PubMed] [Google Scholar]
- 98.Ribas A, Comin-Anduix B, Economou JS, et al. Intratumoral Immune Cell Infiltrates, FoxP3, and Indoleamine 2,3-Dioxygenase in Patients with Melanoma Undergoing CTLA4 Blockade. Clinical Cancer Research. 2009;15(1):390–399. doi: 10.1158/1078-0432.CCR-08-0783. [DOI] [PubMed] [Google Scholar]
- 99.Carthon B, Wolchok JD, Yuan J, et al. Pre-operative CTLA-4 blockade: tolerability and immune monitoring in the setting of a pre-surgical clinical trial. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16(10):2861–2871. doi: 10.1158/1078-0432.CCR-10-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vonderheide RH, LoRusso PM, Khalil M, et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res. 2010;16(13):3485–3494. doi: 10.1158/1078-0432.CCR-10-0505. [DOI] [PubMed] [Google Scholar]
- 102.Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11(2):155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 103.Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30(17):2046–2054. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 104.Calabrò L, Morra A, Fonsatti E, et al. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. The Lancet Oncology. 2013;14(11):1104–1111. doi: 10.1016/S1470-2045(13)70381-4. [DOI] [PubMed] [Google Scholar]
- 105.Slovin SF, Higano CS, Hamid O, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Annals of Oncology. 2013;24(7):1813–1821. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(7):700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. New England Journal of Medicine. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. New England Journal of Medicine. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 109.Boutros C, Tarhini A, Routier E, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13(8):473–486. doi: 10.1038/nrclinonc.2016.58. [DOI] [PubMed] [Google Scholar]
- 110.Johnson DB, Balko JM, Compton ML, et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med. 2016;375(18):1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kitchlu A, Fingrut W, Avila-Casado C, et al. Nephrotic Syndrome With Cancer Immunotherapies: A Report of 2 Cases. Am J Kidney Dis. 2017 doi: 10.1053/j.ajkd.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 112.Cha E, Klinger M, Hou Y, et al. Improved Survival with T Cell Clonotype Stability After Anti–CTLA-4 Treatment in Cancer Patients. Science translational medicine. 2014;6(238):238ra270–238ra270. doi: 10.1126/scitranslmed.3008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Oh DY, Cham J, Zhang L, et al. Immune Toxicities Elicted by CTLA-4 Blockade in Cancer Patients Are Associated with Early Diversification of the T-cell Repertoire. Cancer Res. 2017;77(6):1322–1330. doi: 10.1158/0008-5472.CAN-16-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Subudhi SK, Aparicio A, Gao J, et al. Clonal expansion of CD8 T cells in the systemic circulation precedes development of ipilimumab-induced toxicities. Proc Natl Acad Sci U S A. 2016;113(42):11919–11924. doi: 10.1073/pnas.1611421113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yu W, Jiang N, Ebert PJ, et al. Clonal Deletion Prunes but Does Not Eliminate Self-Specific alphabeta CD8(+) T Lymphocytes. Immunity. 2015;42(5):929–941. doi: 10.1016/j.immuni.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maeda Y, Nishikawa H, Sugiyama D, et al. Detection of self-reactive CD8(+) T cells with an anergic phenotype in healthy individuals. Science. 2014;346(6216):1536–1540. doi: 10.1126/science.aaa1292. [DOI] [PubMed] [Google Scholar]
- 117.Kuehn HS, Ouyang W, Lo B, et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 2014;345(6204):1623–1627. doi: 10.1126/science.1255904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lopez-Herrera G, Tampella G, Pan-Hammarstrom Q, et al. Deleterious mutations in LRBA are associated with a syndrome of immune deficiency and autoimmunity. Am J Hum Genet. 2012;90(6):986–1001. doi: 10.1016/j.ajhg.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gamez-Diaz L, August D, Stepensky P, et al. The extended phenotype of LPS-responsive beige-like anchor protein (LRBA) deficiency. J Allergy Clin Immunol. 2016;137(1):223–230. doi: 10.1016/j.jaci.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 120.Kremer JM, Westhovens R, Leon M, et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349(20):1907–1915. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]
- 121.Lee S, Moon JS, Lee CR, et al. Abatacept alleviates severe autoimmune symptoms in a patient carrying a de novo variant in CTLA-4. J Allergy Clin Immunol. 2016;137(1):327–330. doi: 10.1016/j.jaci.2015.08.036. [DOI] [PubMed] [Google Scholar]
- 122.Sun T, Hu Z, Shen H, Lin D. Genetic polymorphisms in cytotoxic T-lymphocyte antigen 4 and cancer: the dialectical nature of subtle human immune dysregulation. Cancer Res. 2009;69(15):6011–6014. doi: 10.1158/0008-5472.CAN-09-0176. [DOI] [PubMed] [Google Scholar]
- 123.Kavanagh B, O'Brien S, Lee D, et al. CTLA4 blockade expands FoxP3(+) regulatory and activated effector CD4(+) T cells in a dose-dependent fashion. Blood. 2008;112(4):1175–1183. doi: 10.1182/blood-2007-11-125435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Calabro L, Morra A, Fonsatti E, et al. Efficacy and safety of an intensified schedule of tremelimumab for chemotherapy-resistant malignant mesothelioma: an open-label, single-arm, phase 2 study. Lancet Respir Med. 2015;3(4):301–309. doi: 10.1016/S2213-2600(15)00092-2. [DOI] [PubMed] [Google Scholar]
- 125.Hou TZ, Verma N, Wanders J, et al. Identifying functional defects in patients with immune dysregulation due to LRBA and CTLA-4 mutations. Blood. 2017;129(11):1458–1468. doi: 10.1182/blood-2016-10-745174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tabares P, Berr S, Romer PS, et al. Human regulatory T cells are selectively activated by low-dose application of the CD28 superagonist TGN1412/TAB08. Eur J Immunol. 2014;44(4):1225–1236. doi: 10.1002/eji.201343967. [DOI] [PubMed] [Google Scholar]
- 127.Hunig T. The rise and fall of the CD28 superagonist TGN1412 and its return as TAB08: a personal account. Febs j. 2016;283(18):3325–3334. doi: 10.1111/febs.13754. [DOI] [PubMed] [Google Scholar]
- 128.Sutmuller RPM, van Duivenvoorde LM, van Elsas A, et al. Synergism of Cytotoxic T Lymphocyte–Associated Antigen 4 Blockade and Depletion of Cd25(+) Regulatory T Cells in Antitumor Therapy Reveals Alternative Pathways for Suppression of Autoreactive Cytotoxic T Lymphocyte Responses. The Journal of Experimental Medicine. 2001;194(6):823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206(8):1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Simpson TR, Li F, Montalvo-Ortiz W, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210(9):1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Weber JS, Dummer R, de Pril V, Lebbe C, Hodi FS. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119(9):1675–1682. doi: 10.1002/cncr.27969. [DOI] [PubMed] [Google Scholar]
- 132.Berman D, Parker SM, Siegel J, et al. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immunity : a Journal of the Academy of Cancer Immunology. 2010;10:11. [PMC free article] [PubMed] [Google Scholar]
- 133.Vétizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science (New York, NY) 2015;350(6264):1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dubin K, Callahan MK, Ren B, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun. 2016;7 doi: 10.1038/ncomms10391. 10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ostman S, Rask C, Wold AE, Hultkrantz S, Telemo E. Impaired regulatory T cell function in germ-free mice. Eur J Immunol. 2006;36(9):2336–2346. doi: 10.1002/eji.200535244. [DOI] [PubMed] [Google Scholar]
- 136.Sandborn WJ, Colombel JF, Sands BE, et al. Abatacept for Crohn's disease and ulcerative colitis. Gastroenterology. 2012;143(1):62–69.e64. doi: 10.1053/j.gastro.2012.04.010. [DOI] [PubMed] [Google Scholar]