Abstract
Background:
Limited literature is available about cancer in the Appalachian Region. This is the only known analysis of all cancers for Appalachia and non-Appalachia covering 100% of the US population. Appalachian cancer incidence and trends were evaluated by state, sex, and race and compared with those found in non-Appalachian regions.
Methods:
US counties were identified as Appalachian or non-Appalachian. Age-adjusted cancer incidence rates, standard errors, and confidence intervals were calculated using the most recent data from the United States Cancer Statistics for 2004 to 2011.
Results:
Generally, Appalachia carries a higher cancer burden compared with non-Appalachia, particularly for tobacco-related cancers. For all cancer sites combined, Appalachia has higher rates regardless of sex, race, or region. The Appalachia and non-Appalachia cancer incidence gap has narrowed, with the exception of oral cavity and pharynx, larynx, lung and bronchus, and thyroid cancers.
Conclusions:
Higher cancer incidence continues in Appalachia and appears at least in part to reflect high tobacco use and potential differences in socioeconomic status, other risk factors, patient health care utilization, or provider practices. It is important to continue to evaluate this population to monitor results from screening and early detection programs, understand behavioral risk factors related to cancer incidence, increase efforts to reduce tobacco use and increase cancer screening, and identify other areas where effective interventions may mediate disparities.
Impact:
Surveillance and evaluation of special populations provide means to monitor screening and early detection programs, understand behavioral risk factors, and increase efforts to reduce tobacco use to mediate disparities.
Introduction
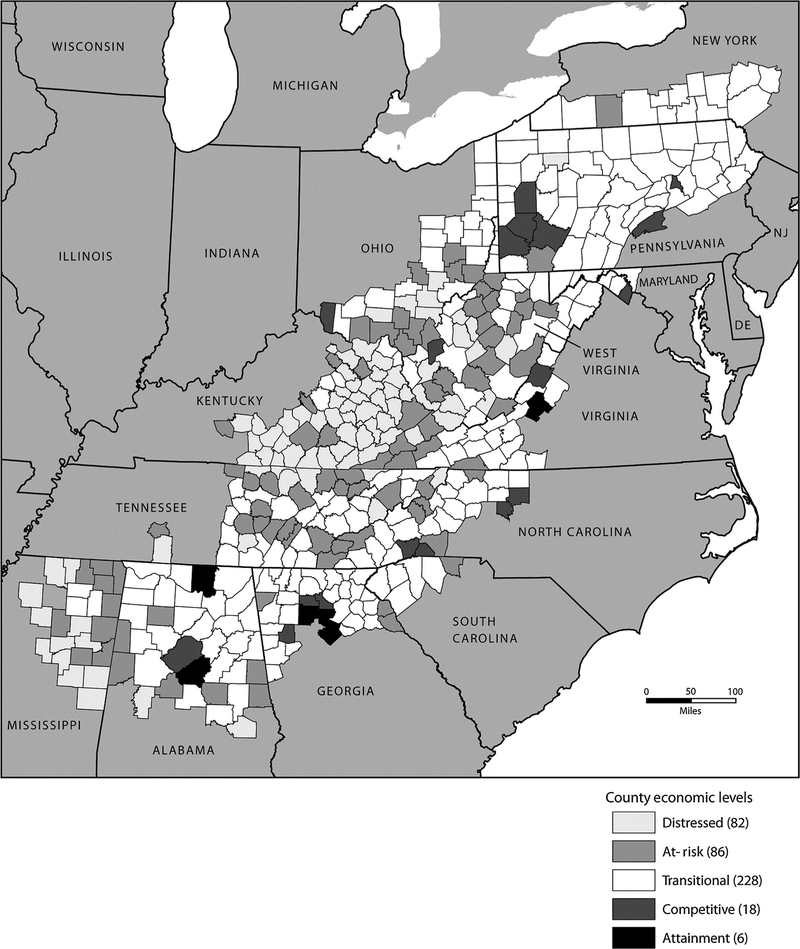
About 25 million people live in the Appalachian Region, which spans 420 counties in 13 US states (ref. 1; Fig. 1). Forty-two percent of the region’s population is rural, compared with 20% of the national population, and is primarily white non-Hispanic (approximately 85%; ref. 1). Within each Appalachian state, the minority population ranges from 4.7% in West Virginia to 38.8% in Mississippi and, within counties, ranges from 1.3% in Kentucky to 16.3% in Mississippi (2, 3). In the 1960s, the Appalachian Region had a poverty rate of 33%, improving to about 16% for the period of 2008 to 2012 (1). The Appalachian Regional Commission (ARC) categorizes the economic status of counties in the region ranging from the most economically depressed to the strongest (1). According to the ARC, 223 (53%) Appalachian counties were considered distressed in the 1960s, which decreased to 93 (22%) in 2012. Even though gains have been made overall in the region, specific Appalachian counties continue to have poverty levels of at least 20%, higher than the US average (4).
Figure 1.
County Economic Status in Appalachia, 2010–2011. Map by the ARC produced in March 2010, reproduced in August 2015 by the Centers for Disease Control and Prevention with permission. Sources, the U.S. Bureau of Labor Statistics, LAUS, 2006–2008; U.S. Bureau of Economic Analysis, REIS, 2007; and U.S. Census Bureau, 2000 Census, SF3.
Limited literature is available about cancer in the Appalachian Region, specifically about cancer incidence. One study evaluated the relationship of screening and deprivation on late-stage breast cancer in three states, another focused on cancer survivors in a single university hospital, and others evaluated cancer risk perceptions, specific screening programs, or mortality (5–15). All generally found higher cancer incidence and death rates, later-stage disease, and higher risk perception and worry about cancer recurrence. In 2007, the first comprehensive evaluation was published of cancer incidence rates (IR) in Appalachia, showing higher IRs in Appalachia than in the rest of the United States with substantial differences between regions (16). Despite many strengths, the evaluation showed that data quality—case ascertainment and missing or unknown data—varied between state and year (16). The purpose of this analysis is to update that evaluation by expanding the diagnosis years to 2004–2011.
Materials and Methods
We analyzed data submitted in November 2013 to the Centers for Disease Control and Prevention’s (CDC’s) National Program of Cancer Registries (NPCR) or the National Cancer Institute’s (NCI’s) Surveillance, Epidemiology, and End Results (SEER) program for the United States Cancer Statistics Web-based report. Together, NPCR and SEER cover 100% of the US population. Cancer registries in each Appalachian state for 2004 to 2011 met six data quality publication criteria related to case ascertainment and missing or unknown data (http://www.cdc.gov/cancer/npcr/uscs/technical_notes/criteria.htm; ref. 17).
The Appalachian Region was defined using the counties identified by the ARC (1). All other counties in the United States and the District of Columbia were considered non-Appalachia. Counties were further defined as Northern (Maryland, New York, Pennsylvania, and Ohio), Central (Kentucky, North Carolina, Tennessee, Virginia, and West Virginia), or Southern (Alabama, Georgia, Mississippi, and South Carolina). Both Appalachian and non-Appalachian counties were grouped by an ARC-designated economic status: distressed (most economically depressed), at- risk (at risk of becoming economically distressed), transitional (transitioning between strong and weak economies), competitive (able to compete in the national economy), and attainment (economically strongest; Fig. 1; ref. 1). Counties designated as competitive or attainment were combined and used as the referent group when evaluating IRs by economic status.
SEER*Stat 8.1.5 was used to calculate age-adjusted IRs, standard errors, 95% confidence intervals, rate ratios, and annual percent change for selected cancers defined by the SEER primary site groups (2). Comparisons were made between Appalachia and non-Appalachia by state, region, sex, race, and economic status for 2004 to 2011. Incidence trends for 2001 to 2011 were analyzed for selected cancer sites for stability and accuracy in reporting the annual percent change and to show differences between current and previously published rates.
Results
A demographic description of the dataset is shown in Table 1. Cases from US Appalachia are primarily white (92%) and non-Hispanic (99%) with a higher percentage from lower economic status counties.
Table 1.
Demographic characteristics, Appalachiana and US non-Appalachian regionsb, invasive cancers and female breast in situ cancers, males and females, all races, 2004–2011
| US Appalachia | US non-Appalachia | |||
|---|---|---|---|---|
| N | Percent | N | Percent | |
| Total cases | 1,165,379 | 9 | 11,287,410 | 91 |
| Region | ||||
| Southern | 304,313 | 26 | 542,105 | 5 |
| Central | 424,871 | 36 | 838,566 | 7 |
| Northern | 430,992 | 37 | 1,766,651 | 16 |
| Remaining US | 0 | 8,140,088 | 72 | |
| Sex | ||||
| Male | 594,535 | 51 | 5,647,745 | 50 |
| Female | 570,844 | 49 | 5,639,665 | 50 |
| Race | ||||
| White | 1,074,510 | 92 | 9,516,558 | 84 |
| Black | 76,382 | 7 | 1,218,034 | 11 |
| Other/unknown | 14,487 | 1 | 552,818 | 5 |
| Ethnicityc | ||||
| Hispanic | 9,190 | 1 | 795,383 | 7 |
| Non-Hispanic | 1,156,189 | 99 | 10,492,027 | 93 |
| Age, years | ||||
| 0–19 | 8,910 | 1 | 107,869 | 1 |
| 20–44 | 80,970 | 7 | 839,182 | 7 |
| 45–64 | 430,975 | 37 | 4,238,389 | 38 |
| 65+ | 644,524 | 55 | 6,065,060 | 54 |
| County economic status | ||||
| Competitive/attainment | 169,570 | 15 | 3,201,857 | 28 |
| Transitional | 764,629 | 66 | 6,548,648 | 58 |
| At-risk | 137,061 | 12 | 863,960 | 8 |
| Distressed | 84,993 | 7 | 334,948 | 3 |
Appalachia includes all Appalachian counties in Alabama, Georgia, Kentucky, Maryland, Mississippi, New York, North Carolina, Ohio, Pennsylvania, South Carolina, Tennessee, Virginia, and West Virginia.
US non-Appalachia includes all counties not in Appalachia.
Ethnicity is not mutually exclusive from race.
Sex
Table 2 shows IRs for selected primary cancer sites for males by Appalachian region compared with US non-Appalachia. Confidence intervals for the IRs shown in Table 2 are provided in Supplementary Table S1. All cancer sites, colon and rectum, larynx, and lung and bronchus IRs are higher across the regions when compared with the US non-Appalachian IRs. The only primary cancer site that is consistently lower than US non-Appalachia is liver and bile duct. For Southern Appalachia, the IRs are higher than US non-Appalachia for seven cancer sites, including prostate which is higher than those for Central and Northern Appalachia. When compared with US non-Appalachia, IRs in Central Appalachia are higher for 10 cancer sites and 14 cancer sites in Northern Appalachia. Even though the Northern region lung and bronchus IR is higher than the US non-Appalachian rate, it is lower than the IRs in the Southern and Central regions.
Table 2.
Invasive cancer IRsa by primary site, Appalachian Region and US non-Appalachiab, males and females, all races, 2004–2011
| Southern Appalachia |
Central Appalachia |
Northern Appalachia |
All Appalachia |
US non- Appalachia |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Males rate |
Females rate |
Males rate |
Females rate |
Males rate |
Females rate |
Males rate |
Females rate |
Males rate |
Females rate |
|
| All sites | 568.7c | 402.3c | 561.6c | 427.2c | 567.1c | 449.3c | 565.8c | 428.7c | 543.0 | 418.2 |
| Oral cavity and pharynx | 18.9c | 6.6c | 18.0c | 6.4 | 16.7 | 6.3 | 17.8c | 6.4c | 16.5 | 6.2 |
| Esophagus | 8.0c | 1.6c | 8.6 | 1.6c | 10.0g | 1.9 | 9.0c | 1.7c | 8.5 | 1.9 |
| Stomach | 8.7c | 4.4c | 8.1c | 3.9c | 10.0c | 4.2c | 9.0c | 4.2c | 9.5 | 4.7 |
| Colon and rectum | 55.0c | 39.1 | 55.5c | 41.0c | 58.0c | 43.8c | 56.6c | 41.8c | 52.4 | 39.5 |
| Liver and intrahepatic bile duct | 9.2c | 2.9c | 8.0c | 2.7c | 8.2c | 2.6c | 8.4c | 2.8c | 10.7 | 3.6 |
| Pancreas | 13.7 | 10.2c | 12.8c | 9.5c | 14.2c | 11.0c | 13.6 | 10.3c | 13.7 | 10.7 |
| Larynx | 8.5c | 1.9c | 8.8c | 2.3c | 7.4c | 1.7c | 8.2c | 2.0c | 6.6 | 1.4 |
| Lung and bronchus | 101.9c | 56.5c | 110.4c | 67.0c | 89.4c | 58.2c | 100.4c | 61.0c | 79.5 | 54.7 |
| Melanoma of the skin (whites) | 31.8c | 22.6c | 28.9c | 19.8c | 21.3c | 15.9c | 27.4 | 19.1c | 27.4 | 17.8 |
| Breast | - | 145.5c | - | 138.4c | - | 151.3c | - | 145.2c | - | 153.0 |
| Invasive | - | 117.3c | - | 115.0c | - | 121.5 | - | 118.2c | - | 122.4 |
| In situ | - | 28.2c | - | 23.4c | - | 29.7c | - | 27.0c | - | 30.5 |
| Cervix uteri | - | 8.2 | - | 8.8c | - | 7.9 | - | 8.3c | - | 7.9 |
| Corpus and uterus, NOS | - | 18.8c | - | 24.5 | - | 31.1c | - | 25.2c | - | 24.5 |
| Ovary | - | 12.4 | - | 12.4 | - | 13.4c | - | 12.7c | - | 12.3 |
| Prostate | 150.6c | - | 130.2c | - | 142.6c | - | 139.9c | - | 147.0 | - |
| Testis | 4.5c | - | 5.3 | - | 6.7c | - | 5.5 | - | 5.5 | - |
| Urinary bladder | 34.5c | 7.8c | 37.7c | 9.4 | 45.4c | 11.3c | 39.8c | 9.7c | 37.0 | 9.3 |
| Kidney and renal pelvis | 21.2 | 11.1 | 21.8c | 11.9c | 20.8 | 11.8c | 21.2 | 11.7c | 21.1 | 11.0 |
| Brain and other nervous system | 8.0 | 5.9 | 8.3c | 6.1c | 8.4c | 6.1c | 8.3c | 6.1c | 7.9 | 5.7 |
| Thyroid | 5.0c | 14.6c | 5.9 | 17.4 | 7.7g | 25.5c | 6.3c | 19.2c | 6.0 | 17.7 |
| Hodgkin Lymphoma | 3.0 | 2.2c | 2.9c | 2.3 | 3.6c | 2.9c | 3.1 | 2.5 | 3.2 | 2.5 |
| Non-Hodgkin lymphoma | 21.5c | 14.9c | 23.1 | 16.2 | 24.6c | 17.7c | 23.3 | 16.4 | 23.4 | 16.3 |
| Myeloma | 7.6 | 4.9 | 7.2 | 4.5c | 7.1c | 4.6c | 7.3c | 4.7c | 7.5 | 4.9 |
| Leukemia | 16.0c | 9.8c | 16.9 | 10.3 | 17.6c | 10.9c | 16.9 | 10.4c | 16.7 | 10.2 |
Abbreviation: NOS, not otherwise specified.
Rates are per 100,000 persons age-adjusted to the 2000 US standard population.
Southern Appalachia includes Alabama, Georgia, Mississippi, and South Carolina; Central Appalachia includes Kentucky, North Carolina, Tennessee, Virginia, and West Virginia; Northern Appalachia includes Maryland, New York, Ohio, and Pennsylvania; US non-Appalachia includes all counties not in Appalachia.
The rate ratio indicates that the rate is significantly different than the US non-Appalachia rate (P < 0.05).
IRs for selected primary cancer sites for females by the Appalachian Region compared with US non-Appalachia are shown in Table 2. The IRs for Southern Appalachia are generally lower or comparable with those for US non-Appalachia, except for four cancer sites. Invasive breast cancer IRs are statistically lower for Southern and Central Appalachia compared with US non-Appalachia. Breast in situ is lower in each region. Like males, the IRs for larynx and lung and bronchus are higher in each region compared with US non-Appalachia. In comparison with other regions, oral cavity and pharynx cancers are higher, and corpus uterus cancer is lower only in Southern Appalachia. Central Appalachia is the only region where cervical cancer IRs are significantly higher than those in US non-Appalachia.
Race
Comparing the IRs for the white population in each Appalachian region with US non-Appalachia (Table 3) shows that IRs for all cancer sites, males and females, are significantly higher for the Northern and Central regions, whereas there is no difference for the Southern region. The IR for the black population, compared with US non-Appalachia, is higher in the Southern and Northern regions. Within each Appalachian Region, the IR for the black population, when compared with the white population, is higher in the Southern and Northern regions.
Table 3.
Invasive cancer IRsa by Appalachian Region and US non-Appalachia, by race and gender, 2004–2011
| Southernb Appalachia rate (CI)c |
Centrald Appalachia rate (CI) |
Northerne Appalachia rate (CI) |
All Appalachia rate (CI) |
USf
non- Appalachia rate (CI) |
|
|---|---|---|---|---|---|
| Males and females | |||||
| White | 470.4 (468.5–472.3) | 482.0g (480.5–483.6) | 493.5g (492.0–495.1) | 484.0g (483.0–484.9) | 470.4 (470.1–470.7) |
| Black | 483.3g (478.6–488.1) | 476.9 (469.3–484.6) | 513.5g (505.2–521.9) | 488.2g (484.6–491.8) | 480.0 (479.1–480.9) |
| Males | |||||
| White | 559.0g (555.9–562.2) | 558.7g (556.2–561.1) | 561.6g (559.1–564.1) | 560.3g (558.7–561.8) | 535.9 (535.4–536.4) |
| Black | 638.7g,h (629.8–647.7) | 601.1 (587.6–614.9) | 609.1h (595.0–623.4) | 624.5g (617.9–631.1) | 604.6 (603.0–606.2) |
| Females | |||||
| White | 408.4g (406.1–410.8) | 428.3g (426.3–430.2) | 448.3g (446.2–450.4) | 431.4g (430.2–432.6) | 424.2 (423.8–424.6) |
| Black | 383.5g (378.1–388.9) | 393.4 (384.2–402.7) | 451.3g (440.8–461.9) | 398.5 (394.3–402.8) | 397.6 (396.5–398.7) |
Rates are per 100,000 persons age-adjusted to the 2000 US standard population.
Southern Appalachia includes all Appalachian counties in Alabama, Georgia, Mississippi, and South Carolina.
CI = 95% confidence interval.
Central Appalachia includes all Appalachian counties in Kentucky, North Carolina, Tennessee, Virginia, and West Virginia.
Northern Appalachia includes all Appalachian counties in Maryland, New York, Ohio, and Pennsylvania.
US non-Appalachia includes all counties not in Appalachia.
The rate ratio indicates that the rate is significantly different than the US non-Appalachia rate (P < 0.05).
The rate ratio indicates that the rate is significantly higher than the rate for the white population.
The IRs for white males are higher in each Appalachian region compared with US non-Appalachia and higher for black males in the Southern region only. The IRs for white females are higher in the Central and Northern Appalachian regions, but not the Southern region. Unlike the IRs for black males, the IRs for black females are higher only in the Northern region and lower in the Southern region.
County economic status
IRs for US Appalachia, as well as the Southern and Central regions, are significantly higher for the distressed counties compared with US non-Appalachia and are higher than the attainment/competitive counties within these regions (Table 4). US Appalachia and each region show higher rates than US non-Appalachia overall for the transitional counties. Within each Appalachian Region, there are no consistent differences when comparing the IR with the competitive/attainment counties; distressed and transitional counties in Southern Appalachia have higher IRs, whereas only the distressed counties have higher IRs in Central Appalachia, and transitional counties have significantly lower rates in Northern Appalachia. Differences were seen for the counties designated as transitional, with each region having significantly higher IRs than US non-Appalachia.
Table 4.
Invasive cancer IRsa by Appalachian Region and US non-Appalachia, by economic status, males and females, 2004–2011
| Southernb Appalachia rate (CI)c |
Centrald Appalachia rate (CI) |
Northerne Appalachia rate (CI) |
All Appalachia rate (CI) |
United Statesf non-Appalachia rate (CI) |
|
|---|---|---|---|---|---|
| Competitive/attainment | 454.1 (450.1–458.2) | 481.7 (469.7–494.0) | 505.9 (502.8–509.0)h | 486.6 (484.2–489.0) | 479.1 (478.6–479.7) |
| Distressed | 467.9g (461.2–474.6) | 503.2g (499.2–507.1) | 494.4g (491.0–497.8) | 435.0g (433.5–436.5) | |
| At risk | 452.9 (447.1–458.8) | 475.8 (472.8–478.8) | 497.8 (486.9–508.9) | 472.7g (470.2–475.3) | 467.3g (466.3–468.3) |
| Transitional | 478.8g (476.7–481.0) | 480.4 (478.5–482.3) | 493.1g (491.3–494.9) | 485.5 (484.4–486.6) | 466.5g (466.1–466.9) |
Rates are per 100,000 persons age-adjusted to the 2000 US standard population.
Southern Appalachia includes all Appalachian counties in Alabama, Georgia, Mississippi, and South Carolina.
CI = 95% confidence interval.
Central Appalachia includes all Appalachian counties in Kentucky, North Carolina, Tennessee, Virginia, and West Virginia.
Northern Appalachia includes all Appalachian counties in Maryland, New York, Ohio, and Pennsylvania.
US non-Appalachia includes all counties not in Appalachia.
The rate ratio indicates that the rate is significantly different than the US non-Appalachia rate (P < 0.05).
Statistic could not be calculated. Rates are suppressed if fewer than 16 cases are reported in the category.
Time trends
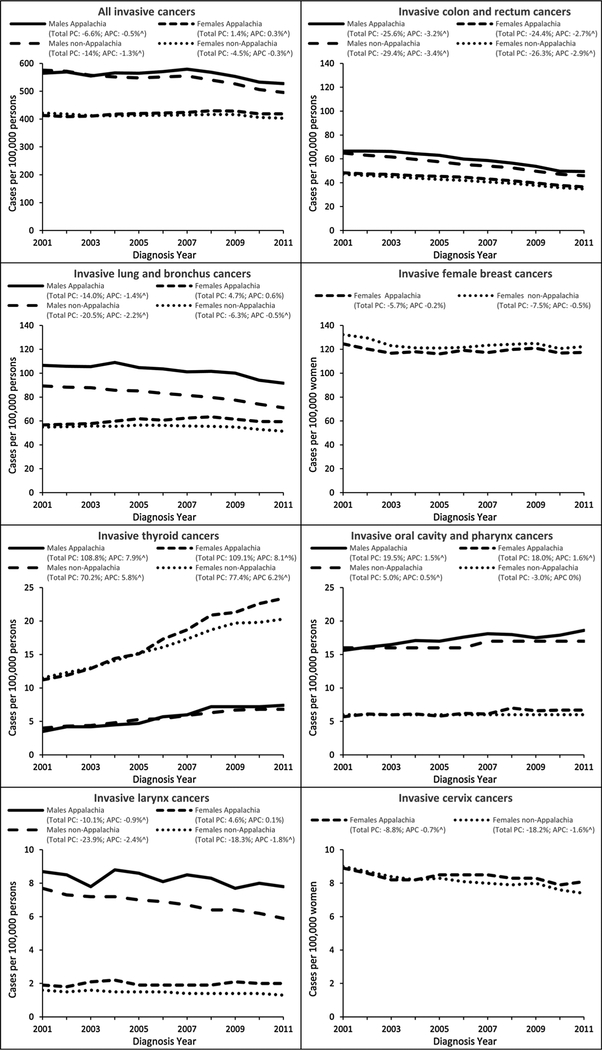
US Appalachian IRs for males steadily increased in 2001 to 2007 and then decreased in 2008 to 2011, similar to the trend seen in US non-Appalachia (Fig. 2). Overall, during the period 2001–2011, IRs for US non-Appalachian males decreased by 1.3% per year with a 14% total decrease compared with a 0.5% annual decrease and 6.6% total decrease for US Appalachian males. The IRs for US Appalachian females steadily increased during the period 2001–2008 and then decreased, whereas the rates for US non-Appalachian females increased during the period 2001–2009 and then decreased (Fig. 2). Overall, during the period 2001–2011, the total decrease for US non-Appalachian females follows a pattern similar to the US non-Appalachian males, though not to the same degree (4.5% vs. 14%, respectively).
Figure 2.
Temporal trends in invasive cancer IRs by cancer site, sex, and Appalachian status, United States, 2001–2011. Rates are per 100,000 and age-adjusted to the 2000 US standard population. Percent change (PC) was used to quantify the total percent change in rates from 2001 to 2011. Annual percent change (APC) was used to quantify the change in rates during 2001 to 2011 and was calculated using weighted least squares regression. Caret (^) denotes APC is significantly different from zero (P < 0.05). Appalachia includes select counties in Alabama, Georgia, Kentucky, Maryland, Mississippi, New York, North Carolina, Ohio, Pennsylvania, South Carolina, Tennessee, and Virginia, and all counties in West Virginia. Non-Appalachia includes all counties not in Appalachia.
Time trends varied for each primary site evaluated. Colon and rectum cancer IRs (Fig. 2) among US non-Appalachian males steadily decreased, whereas the rates among US Appalachian males increased during the period 2001–2003 and then decreased. The total decrease was similar for US non-Appalachia males (29.4%) and US Appalachia males (25.6%) with a 3.4% and 3.2% annual decrease, respectively, both significantly different. Steady decreases are seen among females in US Appalachia and US non-Appalachia with a 24.4% and 26.3% total decrease, respectively, and statistically significant 2.7% and 2.9% annual decrease, respectively.
Lung and bronchus cancer incidence (Fig. 2) among US non-Appalachian males decreased with an annual 2.2% decrease and20.5% total decrease. Among US Appalachian males, the rates decreased each year, except 2004, with a lower total decrease (14.0%) and lower, though statistically significant, annual decrease (1.4%). US non-Appalachian females had a similar pattern that was not seen among US Appalachian females. In the US non-Appalachian Region, female lung and bronchus cancer rates increased during the period 2001–2006, whereas the US Appalachian rates increased during the period 2001–2008. Also in contrast with the male rates, US non-Appalachian females had a6.3% total decrease with a statistically significant 0.5% annual decrease. Although US non-Appalachian females had an overall decrease in rates, US Appalachian females had a 4.7% total increase and non-significant 0.6% annual increase.
Female breast cancer IRs (Fig. 2) in the US non-Appalachian region had an overall 7.5% decrease with a nonsignificant 0.5% annual decrease. US Appalachian IRs varied during the period 2001–2011, but showed a 5.7% total decrease and nonsignificant0.2% annual decrease. Cervical cancer IRs (Fig. 2) show a steady decrease during the period 2001–2011 with an 18.2% total decrease in US non-Appalachia and 1.6% annual significant decrease, whereas the US Appalachia total and annual decreases are less (8.8% and 0.7%, respectively). Figure 2 shows US Appalachia trends for oral cavity and pharynx, larynx, and thyroid that differ significantly from the trends seen for US non-Appalachia. For oral cavity and pharynx, US Appalachian males and females show an overall increase of almost 20%, whereas US non-Appalachian males had a 5% increase and females had a 3% decrease. Laryngeal cancer IRs show an overall decrease for US non-Appalachian males and females as well as US Appalachian males, though not to the same degree. However, US Appalachian females show an overall increase in laryngeal cancer incidence of approximately 5%. Thyroid cancer IRs show a significant overall increase regardless of region or sex with a higher increase among US Appalachian males and females.
Discussion
Generally, the cancer incidence gap between Appalachia and non-Appalachia has narrowed over time, with Appalachia continuing to show cancer disparities. For all sites combined, Appalachia has higher rates of cancer regardless of sex, race, or region, consistent with previously reported findings (11, 16, 18–23). However, our data show that the gap has narrowed over time, with the exception of oral cavity and pharynx, larynx, lung and bronchus, and thyroid cancers. IRs reported by Wingo and colleagues showed patterns similar to those seen in this evaluation(16). Lengerich and colleagues reported no difference in IRs for all cancers and elevated rates for lung and bronchus, colon, rectum, and cervix in histologically confirmed cancers in three states (18).
The Appalachian region has a long history of poverty and has shown some improvement over the years, though few studies have examined IRs by socioeconomic status (SES; refs. 1, 5, 19). Anderson and colleagues showed that late-stage breast cancer in three states was higher in counties with high deprivation (5). Hopenhayn and colleagues reported that poverty had no association on cervical cancer rates in three states (19). An ARC publication described socioeconomic conditions in Appalachia, but did not examine cancer incidence by SES (21). Hall and colleagues discussed breast and cervical cancer screening rates among Appalachian women with less income (24). Our analysis uses the ARC economic designation for every US county to examine rates by SES, for all cancers (Table 4). The inconsistencies reported in our evaluation may be indicative of the variation in economy and health care access in the Appalachian counties. Because these may be chance findings, additional analysis is needed in this area.
In Appalachia, elevated rates of tobacco-related cancers are particularly apparent for both men and women (25, 26). Lung and bronchus, oral cavity and pharynx, and larynx cancers were higher among both men and women in Appalachia. Additional tobacco-related cancers that were elevated in Appalachia regions are urinary bladder, esophagus (men only), colon and rectum, cervix, and kidney and renal pelvis (women only). Other studies have shown varying results (16, 18, 19, 23). CDC data show that among both males and females, the average smoking prevalence is higher for states in each Appalachian region compared with the United States (20% among males and 16% among females): 27% and 20% Southern region; 25% and 20% Central region; and 22% and 20% Northern region, among males and females, respectively(27). The higher smoking prevalence may, in part, contribute to the higher IRs seen for these tobacco-related cancers. It is important to note that many of these tobacco-related cancers (e.g., esophagus, colon and rectum, and kidney and renal pelvis) are also related to obesity and physical activity, illustrating the multifactorial aspect of cancer (28). CDC data also show that the Appalachian states generally have higher obesity rates and higher percentage of adults reporting no leisure-time physical activity.
Differences in cancer incidence are often attributed to differences in risk factors between groups, but can also be the result of differences in detection rates. The U.S. Preventive Services Task Force (USPSTF) currently recommends population-based screening for colorectal cancer, female breast cancer, and cervical cancer, and lung cancer in those at high risk (29). The Appalachian Region has IRs for each of these cancers that are not consistent with US non-Appalachia. It is well documented that cancer screening rates are lower in Appalachia compared with US non-Appalachia (5, 11, 12, 18, 19, 22–24, 30, 31). Screening for colon and rectum and cervical cancer can result in the prevention of invasive cancer through the removal of precancerous lesions so lower cancer incidence is often seen as a result of effective screening for these cancers. Colon and rectum cancers are higher in Appalachia for both men and women, regardless of region, except for women in the Southern region where rates are consistent with non-Appalachia. Various studies have found lower colorectal cancer screening rates in Appalachia areas, or among persons residing in rural areas (12, 30, 31). Cervical cancers are also higher in Appalachia overall and appear to be driven by increased rates primarily in Central Appalachia. Horner and colleagues and Hopenhayn and colleagues reported lower cervical cancer screening percentages among rural and Appalachian populations (11, 19). Hall and colleagues demonstrated that cervical cancer screening percentages were approximately 3% lower for Appalachian women (24). If screening for these cancers is suboptimal, our study may be underestimating the already high cancer burden in Appalachia.
Female-invasive and in-situ breast cancer is lower in Appalachia, regardless of region, similar to other results (5, 16, 21, 24). Lung and bronchus cancer screening for certain at-risk groups was recommended in 2013; however, given increased tobacco use in Appalachia, it will be important to monitor differences in IRs before and after this recommendation.
Prostate cancer has a higher incidence in Appalachia than non-Appalachia possibly due to differences in risk factors or detection rates (32, 33). Prostate IRs are known to be higher in the black population than the white population, approximately 64% higher (17, 34–36). Black males in Southern Appalachia represent approximately 30% of the male population, compared with 13% in US non-Appalachia, and may, in part, contribute to the higher prostate IR (3). Compared with the Southern region and US non-Appalachia, prostate cancer is significantly lower in Central and Northern Appalachia where black males represent approximately 15% and 17%, respectively, of each region’s male population. Cook and colleagues examined prostate cancer incidence by race and US Census Division showing that the divisions with the highest black: white rate ratios encompass the Appalachian region; the two divisions in Cook’s analysis with the highest rate ratios include Southern Appalachia (34). CDC data show that nine Appalachian states have PSA screening rates, among men age 40 and older, above the US rate of 57% and that a higher percentage of black men had a PSA within the past 2 years (37). This suggests that black men may be more likely to have PSA screening which may be a result of the known elevated rate of prostate cancer in the black population (17). In turn, the increased PSA screening percentages may, in part, contribute to the higher prostate cancer IRs. PSA screening recommendations have varied over the years. The USPSTF, in 2008, had no recommendations on screening for men under age 75 and recommended no screening for men age 75 and older (38). Li and colleagues found that PSA screening among black males, ages 40 to 49, was 15% higher than white males for the period 2000–2008 and suggested that the USPSTF recommendations had little effect (39). Since 2012, the USPSTF recommends against PSA screening among men of all ages (40).
The observation that thyroid cancer is higher among both men and women in Appalachia could also be due to more detection among the urban Appalachia population. Studies have suggested that the approximate 3-fold increase in thyroid cancer in the United States over the past few decades is due to increased diagnostic procedures and/or awareness, whereas others suggest an actual increase in the disease (3, 41–44). Davies and Welch also report that detection of thyroid cancer and access to medical care are strongly related (43). Given that the Appalachian Region generally has less access to care, primarily in the rural areas, it would be expected that thyroid cancer IRs would be low. The thyroid IR among Appalachian males is higher than seen for non-Appalachia, though the difference is not statistically significant. This difference is led by the statistically significantly higher IR in Northern Appalachia, whereas the IRs in Southern and Central Appalachia are lower. This pattern is also seen among Appalachian females. A brief review shows that urban areas have higher IRs and may, in part, contribute to the higher rates overall, where access to medical care is more readily available. This may lead to more thyroid cancers identified incidentally which, according to various studies, may represent approximately 15% to 24% of thyroid cancers (42).
Strengths and Limitations
This is the only analysis of Appalachia using data covering 100% of the US population and the first to report cancer incidence by economic status for the entire region. Other studies were limited to specific cancer sites and states, implied cancer incidences based on hospitalizations, or were limited to cancer mortality rates (11, 13–16, 18–24). This analysis improved upon the article by Wingo and colleagues by using a broader time period (eight versus three years) and having complete population coverage (100% vs. 88%; ref. 16). Using a broader time period and complete population coverage allows more in-depth evaluation of potential differences and may help to identify reasons for the differences.
Despite improvements upon previous analyses, this study is subject to a number of limitations. The intent of this analysis was to present a broad overview of differences in IRs for Appalachia compared with non-Appalachia, and thus may not have revealed differences for specific cancers by race and sex, particularly by economic status. The analysis was also limited to the county level with counties designated as Appalachian or non-Appalachian. This study did not fully investigate differences when designating counties as either urban or rural. Screening rates and risk factor data were only available in this analysis at the state level and may not be indicative of the actual screening or risk factor in each county. In addition, the screening and risk factor data are based on self-reported responses from the Behavioral Risk Factor Surveil-lance System (37). Another limitation is that rates were calculated for all ages and were not evaluated by age group. Where differences may not have been identified in this analysis, subsequent analyses by age group may yield different results.
Conclusions
We found that cancer IRs are generally higher in states that include Appalachia. This observation appears in part to reflect the high prevalence of tobacco use and could also reflect differences in other risk factors, patient health care utilization, or provider practices in Appalachia populations compared with the rest of the United States. Further, because the region is historically under-served and often under- or uninsured, screening for and diagnosis of cancer could be underused causing the already high burden of cancer to be underestimated. Attention to preventing tobacco use and promoting cessation might help reduce the excess cancer burden in this region. In addition, screening for breast, cervical, colorectal, and lung cancer can either prevent cancer or detect it early, potentially reducing incidence and mortality from these cancers. It is important to continue to evaluate this population to monitor potential changes in tobacco cessation, and cancer screening that may occur as the Affordable Care Act increases access to insurance coverage of these services for millions of people.
With these emerging population-based cancer surveillance data that are representative of the entire Appalachia Region, future analyses have the potential to evaluate cancer incidence at finer geographic levels and by stage distribution. More specific analysis may help to better understand behavioral risk factors and to identify areas where intervention programs may be needed to mediate disparities, such as those documented for educational attainment, and cancer screening, and early detection. Additional economic status analyses could be considered for specific cancers by race, sex, and urban–rural status and should include time trends. Initial cancer survival analyses for the Appalachian Region are under way and could continue so that progress in cancer control efforts in these populations can be more completely documented.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the contributions of the state and regional cancer registry and health department staffs for their work in collecting the data used in this study.
Grant Support
This work was supported by the Division of Cancer Prevention and Control at the National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention (all authors).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Note: Supplementary data for this article are available at Cancer Epidemiology, Biomarkers & Prevention Online (http://cebp.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Publisher's Disclaimer: Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Appalachian Regional Commission [Internet]. Washington, DC: Available from: http://www.arc.gov/index.asp. [Google Scholar]
- 2.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Populations - Total U.S. (1969–2012) <Katrina/Rita Adjustment> - Linked To County Attributes - Total U.S., 1969–2012 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released December 2013. [Google Scholar]
- 3.SEER*Stat Database: NPCR and SEER Incidence - Analytic file - 1998–2011 - jbk 082714 - Linked To County Attributes - Total U.S., 1969–2012 Counties.
- 4.County-Level Data Sets: Poverty. USDA Economic Research Service. Source: Bureau of the Census, Small Area Income and Poverty Estimates [cited 2013 Nov. 7]. Available from: http://ers.usda.gov/data-products/county-level-data-sets/poverty.aspx. [Google Scholar]
- 5.Anderson RT, Yang TC, Matthews SA, Camacho F, Kern T, Mackley HB, et al. Breast cancer screening, area deprivation, and later-stage breast cancer in Appalachia: does geography matter? Health Serv Res 2014; 49:546–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly KM, Shedlosky-Shoemaker R, Porter K, Desimone P, AndrykowskiM. Cancer recurrence worry, risk perception, and informational-coping styles among Appalachian cancer survivors. J Psychosoc Oncol 2011; 29:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanderpool RC, Huang B. Cancer risk perceptions, beliefs, and physician avoidance in Appalachia: results from the 2008 HINTS Survey. J Health Commun 2010;15 Suppl 3:78–91. [DOI] [PubMed] [Google Scholar]
- 8.Behringer B, Krishnan K. Understanding the role of religion in cancer care in Appalachia. South Med J 2011;104:295–6. [DOI] [PubMed] [Google Scholar]
- 9.Linnan LA, Weiner BJ, Bowling JM, Bunger EM. Views about secondhand smoke and smoke-free policies among North Carolina restaurant owners before passage of a law to prohibit smoking. N C Med J 2010; 71:325–33. [PubMed] [Google Scholar]
- 10.Schoenberg N, Baltisberger J, Bardach S, Dignan M. Perspectives on pap test follow-up care among rural Appalachian women. Women Health 2010;50:580–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horner MJ, Altekruse SF, Zou J, Wideroff L, Katki HA, Stinchcomb D. US geographic distribution of pre-vaccine era cervical cancer screening, incidence, stage, and mortality. Cancer Epidemiol Biomarkers Prev 2011;20:591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz ML, Reiter P, Fickle D, Heaner S, Sim C, Lehman A, et al. Community involvement in the development and feedback about a colorectal cancer screening media campaign in Ohio Appalachia. Health Promot Pract 2010;12:589–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall HI, Rogers JD, Weir HK, Miller DS, Uhler RJ. Breast and cervical carcinoma mortality among women in the Appalachian region of the U.S., 1976–1996. Cancer 2000;89:1593–602. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong LR, Thompson T, Hall HI, Coughlin SS, Steele B, Rogers JD. Colorectal carcinoma mortality among Appalachian men and women, 1969–1999. Cancer 2004;101:2851–8. [DOI] [PubMed] [Google Scholar]
- 15.Borak J, Salipante-Zaidel C, Slade MD, Fields CA. Mortality disparities in Appalachia reassessment of major risk factors. J Occup Environ Med 2012;54:146–56. [DOI] [PubMed] [Google Scholar]
- 16.Wingo PA, Tucker TC, Jamison PM, Martin H, McLaughlin C, Bayakly R, et al. Cancer in Appalachia, 2001–2003. Cancer 2008;112:181–92. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2011 Incidence and Mortality Web-based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2014. [Google Scholar]
- 18.Lengerich EJ, Tucker TC, Powell RK, Colsher P, Lehman E, Ward AJ, et al. Cancer incidence in Kentucky, Pennsylvania, and West Virginia: disparities in Appalachia. J Rural Health Winter 2005;21:39–47. [DOI] [PubMed] [Google Scholar]
- 19.Hopenhayn C, Bush H, Christian A, Shelton BJ. Comparative analysis of invasive cervical cancer incidence rates in three Appalachian states. Prev Med 2005;41:859–64. [DOI] [PubMed] [Google Scholar]
- 20.Hendryx M, O’Donnell K, Horn K. Lung cancer mortality is elevated in coal-mining areas of Appalachia. Lung Cancer 2008;62:1–7. [DOI] [PubMed] [Google Scholar]
- 21.Appalachian Regional Commission. An analysis of disparities in health status and access to health care in the Appalachian Region. 2007 [cited 2015 Feb. 25]. Available from: http://www.arc.gov/research/researchreportdetails.asp?REPORT_ID=82.
- 22.Appalachia Community Cancer Network. Addressing the cancer burden in Appalachian communities. 2010 [cited 2015 Jan. 21]. Available from: http://www.accnweb.com/pages/cancer-burden-app.pdf.
- 23.Paskett ED, Fisher JL, Lengerich EJ, Schoenberg NE, Kennedy SK, Conn ME, et al. Disparities in underserved white populations: the case of cancer-related disparities in Appalachia. Oncologist 2011;16: 1072–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall HI, Uhler RJ, Coughlin SS, Miller DS. Breast and cervical cancer screening among Appalachian women. Cancer Epidemiol Biomarkers Prev 2002;11:137–42. [PubMed] [Google Scholar]
- 25.American Cancer Society. Cancer facts & figures 2014. Atlanta: American Cancer Society; 2014. [Google Scholar]
- 26.Underwood JM, Richards TB, Henley SJ, Momin B, Houston K, Rolle I, et al. Decreasing trend in tobacco-related cancer incidence, United States 2005–2009. J Community Health 2015;40:414–8. [DOI] [PubMed] [Google Scholar]
- 27.CDC, smoking & tobacco use, office on smoking and health [Internet; cited 2014 Oct. 7]. Available from: http://www.cdc.gov/tobacco/data_statistics/index.htm.
- 28.Eheman C, Henley SJ, Ballard-Barbash R, Jacobs EJ, Schymura MJ, Noone AM, et al. Annual Report to the nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer 2012;118:2338–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Recommendations for Primary Care Practice. U.S. Preventive Services Task Force. 2014 [cited 2015 Feb. 5]. Available from: http://www.uspreventiveservicestaskforce.org/Page/Name/recommendations.
- 30.Coughlin SS, Thompson TD. Colorectal cancer screening practices among men and women in rural and nonrural areas of the United States, 1999. J Rural Health 2004;20:118–24. [DOI] [PubMed] [Google Scholar]
- 31.Dignan M, Shelton B, Slone SA, Tolle C, Mohammad S, Schoenberg N, et al. Effectiveness of a primary care practice intervention for increasing colorectal cancer screening in Appalachian Kentucky. Prev Med 2014; 58:70–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Cancer Society. Cancer facts & figures 2015. Atlanta: American Cancer Society; 2015. [Google Scholar]
- 33.Li J, German R, King J, Joseph D, Thompson T, Wu XC, et al. Recent trends in prostate cancer testing and incidence among men under age of 50. Cancer Epidemiol 2012;36:122–7. [DOI] [PubMed] [Google Scholar]
- 34.Cook MB, Rosenberg PS, McCarty FA, Wu M, King J, Eheman C, et al. Racial disparities in prostate cancer incidence rates by census division in the United States, 1999–2008. Prostate 2015;75:758–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramsey SD, Zeliadt SB, Hall IJ, Ekwueme DU, Penson DF. On the importance of race, socioeconomic status and comorbidity when evaluating quality of life in men with prostate cancer. J Urol 2007;177:1992–9. [DOI] [PubMed] [Google Scholar]
- 36.Faisal FA, Sundi D, Cooper JL, Humphreys EB, Partin AW, Han M, et al. Racial disparities in oncologic outcomes after radical prostatectomy: long-term follow-up. Urology 2014;84:1434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention (CDC). Behavioral Risk Factor Surveillance System Survey Data. Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2012. [Google Scholar]
- 38.U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Task Force recommendation statement. Ann Intern Med 2008;149:185–91. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Berkowitz Z, Richards TB, Richardson LC. Shared decision making in prostate-specific antigen testing with men older than 70 years. J Am Board Fam Med 2013;26:401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moyer VA U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2012;157:120–34. [DOI] [PubMed] [Google Scholar]
- 41.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2020. JAMA 2006;295:2164–7. [DOI] [PubMed] [Google Scholar]
- 42.Yoo F, Chaikhoutdinov I, Mitzner R, Liao J, Goldenberg D. Characteristics of incidentally discovered thyroid cancer. JAMA Otolaryngol Head Neck Surg 2013;139:1181–6. [DOI] [PubMed] [Google Scholar]
- 43.Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 2014;140:317–22. [DOI] [PubMed] [Google Scholar]
- 44.Malone MK, Zagzag J, Ogilvie JB, Patel KN, Heller KS. Thyroid cancers detected by imaging are not necessarily small or early stage. Thyroid 2014;24:314–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.