Abstract
The widespread adoption of high-resolution manometry (HRM) has led to a restructuring in the classification of esophageal motility disorder classification summarized in the Chicago Classification, currently in version 3.0. It has become apparent that the cardinal feature of achalasia, impaired lower esophageal sphincter relaxation, can occur in several disease phenotypes: without peristalsis, with premature (spastic) distal esophageal contractions, with panesophageal pressurization, or even with preserved peristalsis. Furthermore, despite these advances in diagnostics, no single manometric pattern is perfectly sensitive or specific for idiopathic achalasia and complimentary assessments with provocative maneuvers during HRM or interrogating the esophagogastric junction with the functional luminal imaging probe during endoscopy can be useful in clarifying equivocal or inexplicable HRM findings. Using these tools, we have come to conceptualize esophageal motility disorders as characterized by obstructive physiology at the esophagogastric junction, smooth muscle esophagus, or both. Recognizing obstructive physiology as a primary target of therapy has become particularly relevant with the development of a minimally invasive technique for performing a calibrated myotomy of the esophageal circular muscle, the POEM procedure. Now and going forward, optimal management is to render treatment in a phenotype-specific manner: e.g. POEM calibrated to patient-specific physiology for spastic achalasia and spastic disorders of the smooth muscle esophagus, more conservative strategies (pneumatic dilation) for the disorders limited to the sphincter.
Keywords: esophageal motility disorders, high-resolution manometry, dysphagia, esophagus
The new millennium brought much change to the world of esophageal motility: major changes in instrumentation, diagnostic algorithms, and therapeutics. Basically, if you have an old book, you can throw it away. High-resolution manometry (HRM) is now firmly ensconced in the clinical paradigm with the Chicago Classification (CC) v3.0 [1] being part of the daily dialogue. Per Oral Endoscopic Myotomy (POEM) has become the go-to intervention for both achalasia and distal esophageal spasm in many leading centers [2]. Functional Luminal Imaging Probe (FLIP) measurements are being used to calibrate therapeutic interventions and to provide alternative diagnostic criteria in equivocal HRM cases [3]. And then there is the opiate epidemic, creating a new family of syndromes that we are only beginning to appreciate [4]. None of this existed a decade ago. It is from this landscape that we write this Perspective.
Contemplating the evolution of motility diagnostics, HRM has proven to be a truly disruptive technology, fostering a series of innovations and revelations. The most fundamental innovation was changing the presentation format of motility data from line tracings to pressure topography plots. Alternatively called isobaric contour plots or, Clouse Plots [5, 6], pressure topography plots use a coordinate system of time on the x-axis, sensor position on the y-axis, and pressure values as spectral color within that grid. Apart from elegantly condensing an enormous dataset into a single image, key advantages of this format are that it makes sphincters, propagated or non-propagated contractions, luminal pressure gradients, and isobaric regions within the esophagus visually obvious and easily interrogated with software tools. Hence, in a way that was never achieved with conventional manometry, HRM revealed clinically important patterns of obstructive physiology, both at the esophagogastric junction (EGJ) and along the esophageal lumen. Although understanding its full relevance is still a work in progress, the concept of obstructive physiology as a fundamental abnormality of esophageal motility disorders has already substantially morphed their clinical management.
A New Perspective on Esophageal Motility Disorders: Obstructive Physiology
The CC of esophageal motility disorders was built around three key metrics derived from pressure topography plots: the integrated relaxation pressure (IRP), the distal contractile integral (DCI), and the distal latency (DL) [1]. From the beginning of the CC, it was proposed that the analysis of HRM studies be hierarchical, beginning at the EGJ and proceeding proximally. This was in recognition of the fundamental importance of outflow obstruction, manifest by an IRP greater than the upper limit of normal, as a driver of symptoms and a determinant of proximal contractility. The hallmark disease with EGJ outflow obstruction is achalasia, defined by the combination of impaired lower esophageal sphincter (LES) relaxation and absent peristalsis. However, it has since become clear that obstructive physiology occurs in several syndromes besides classic achalasia [7]. In fact, obstructive physiology can be a function of the EGJ, the distal esophagus, or both.
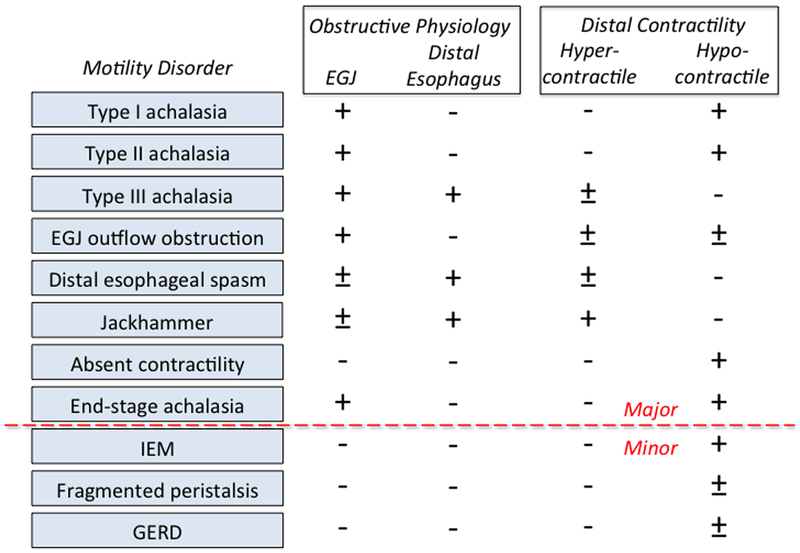
Obstructive physiology is a fundamental abnormality in esophageal motility disorders, potentially leading to the perception of pain and/or dysphagia. Why this occurs involves understanding how the peristaltic sequence couples with the EGJ to mediate bolus transit. Conceptually, esophageal transport can be deconstructed into a 4 phase process: phase 1, accommodation, during which the esophagus accepts the bolus as it is expelled from the oropharynx; phase II, compartmentalization of the bolus into the distal esophagus (beyond the transition zone) by medullary-programmed peristalsis of the proximal esophagus; phase 3, esophageal emptying, largely mediated by post-transition zone myenteric plexus programmed esophageal peristalsis; and phase 4, ampullary emptying, during which the elongated, effaced, and axially displaced LES is restored to its closed, shortened, intra-hiatal state [8]. As such, inhibition is as much a part of peristalsis as is contraction. Highlighting this, studies measuring intrabolus pressure synchronized with fluoroscopy during normal peristalsis demonstrate that during phase 2, distal esophageal intrabolus pressure actually decreases as the luminal diameter increases. This is in sharp contrast to the compartmentalized pressurization of even panesophageal pressurization that is so characteristic of the major esophageal motility disorders. With achalasia, the defining physiology necessarily includes EGJ outflow obstruction, but may or may not be accompanied by obstructive esophageal contractions [9]. With distal esophageal spasm (DES) and hypercontractility (jackhammer esophagus), the defining physiology necessarily involves obstructive esophageal contractions, but these may or may not be associated with EGJ outflow obstruction (Figure 1). In the case of DES, the obstructive characteristic of esophageal contractions is that they are premature, occurring with a DL of <4.5 seconds in the time window that should be dominated by deglutitive inhibition [10]. In the case of hypercontractility, the obstructive characteristic is that there is prolonged, concurrent contraction of essentially the entire smooth muscle segment, delaying the normal post-peristaltic recovery.
Figure 1.
Contractile and obstructing features of major esophageal motility disorders.
Figure 1 is a conceptual graphic of the defining features of esophageal motility disorders. However, the clinical challenge is in translating the conceptual into a diagnosis on a case-by-case basis. Doing so, one can encounter numerous technical and technological challenges including the performance characteristics of specific instrumentation, technically limited studies, patient intolerance of testing, skill level of the individual conducting the test, and ‘borderline’ diagnoses. Each of these issues is deserving of a treatise in its own right and all speak to the need for flexibility on the part of the practitioner in their approach to difficult cases [11]. Easy cases will always be easy, but no matter what your degree of sophistication, there will always be cases in which clinical judgment ends up the final arbiter. For a detailed discussion of many of the technical challenges of HRM interpretation, the reader is referred to a recent expert consensus on the topic [7]. Pertinent to this discussion, suffice it to say that as much as HRM has advanced the science of motility testing, it has also exposed several fundamental limitations. High on this list is that there are no biomarkers of esophageal motility disorders and, even though the underlying pathology is of a myenteric plexopathy in some cases [12], the diagnosis is not established by neuropathology. Rather, the diagnosis is established using physiological tests to implicate abnormal neuromuscular function as the cause of symptomatic dysfunction [13]. Consequently, using HRM as the ‘gold standard’ for diagnosis has inherent limitations: 1) there are always exceptions to the numerical thresholds of abnormality for the IRP, DCI, and DL; 2) other disease entities (and chronic opiate exposure) can mimic the neuromuscular dysfunction seen in esophageal motility disorders; 3) the distinction between several diagnostic entities in the CC (eg. type I and II achalasia, type III achalasia and DES, absent contractility and end stage achalasia) is gray rather than black and white; 4) motility disorders evolve over an undefined timespan at an undefined rate leaving open the possibility of ‘disease in evolution’; and 5) HRM is inherently better at quantifying contraction than detecting impaired inhibition, an equally important determinant of motility disorders. Finally, it needs mentioning that esophageal dysmotility often does not explain dysphagia, and hence, HRM will not identify the cause of dysphagia in such patients.
Clarifying the Gray Zones: Provocative Stimuli and Ancillary Tests
Among potential findings in HRM studies, the detection of obstructive physiology at the EGJ is the most fundamental because it is ultimately the best therapeutic target. In the CC this is based on detection of an elevated IRP. However, no metric or technology has perfect sensitivity and specificity for detecting relevant sphincter dysfunction, and in marginal or atypical cases one has to consider all available evidence, including other studies and other metrics. There are clearly cases of achalasia with an IRP <15 mmHg, particularly in type I or advanced disease [14], and there are clearly instance in which the IRP is >15 that are not achalasia [15]. Some have proposed addressing this by lowering the threshold IRP cutoff for defining type I achalasia [16], but ultimately that only challenges the specificity of the metric as there are published cases of achalasia with IRP values as low as 3–5 mmHg [14]. Hence, in equivocal cases, or when there are conflicting findings, the International Working group on High-Resolution Manometry instead proposed to leave open the uncertainty of an achalasia diagnosis and looking for supporting evidence of functionally significant esophageal outflow obstruction [1]. This can be by demonstrating compartmentalized pressurization above the EGJ during test swallows or it can be the demonstration of esophageal pressurization during multiple rapid swallows or rapid drink challenge, two provocative tests of the integrity of deglutitive inhibition. With multiple rapid swallows, five 2-ml swallows are taken less than 4 seconds apart [17,18] and with the rapid drink challenge 200 ml of water is swallowed within 30 seconds [19, 20]. By providing a prolonged inhibitory stimulus, multiple rapid swallows are helpful in demonstrating the integrity of deglutitive inhibition in the distal esophagus, LES relaxation, and peristaltic reserve after the termination of the sequence [21]. The rapid drink challenge is most helpful by eliciting pan-esophageal pressurization, indicative of obstructive physiology at the EGJ.
Another approach to clarifying inconclusive HRM findings is to invoke ancillary tests to elicit abnormal function. The simplest of these is the timed barium esophagram wherein the patient drinks 200 ml of low-density barium over one minute in an upright posture followed by frontal x-rays 1, 2, and 5 minutes afterward. The degree of esophageal emptying is then estimated by measuring the height of the residual barium column in the esophagus. The most robust outcome measure of the timed barium esophagram is the height of the barium column at 5 minutes with the proposed critical threshold ranging from 2–5 cm [22, 23]. When a 12-mm barium tablet is used in conjunction with the timed barium esophagram, a secondary criterion of abnormality is for the tablet to become lodged at the EGJ. A positive timed barium esophagram is strong supportive evidence of functionally significant EGJ outflow obstruction and a completely normal study makes an achalasia diagnosis highly unlikely [23]. Analogous data can be obtained using high-resolution impedance manometry in an upright posture and using the impedance electrodes to ascertain the height of retained fluid in the esophagus at a 5-minute interval [24]. However, these tests are not very useful in the clarifying the significance of EGJ outflow obstruction with preserved peristalsis because bolus clearance is often uncompromised in that condition.
An alternative investigation for clarifying the significance of EGJ outflow obstruction is with the Functional Luminal Imaging Probe (FLIP) device (Medtronic, Shoreview MN). The concept of the FLIP is to measure the distensibility of the EGJ during volumetric distention. This is achieved with a trans-orally positioned FLIP probe incorporating 16 closely spaced impedance electrodes within a compliant bag. With measured volumes of conductive saline distending the bag (and distal esophagus), an electrical current runs between adjacent impedance electrodes proportionate in amperage to the cross-sectional area of the esophageal lumen separating the electrodes (impedance planimetry). The result is an 8–16 cm high-resolution measurement of luminal cross-sectional area, represented on screen as a cylinder of varying diameter surrounding the FLIP probe. When combined with measurement of the intra-bag pressure, an EGJ distensibility index (mm2/mmHg) can be calculated [25]. The conceptual advantage of the FLIP device over HRM lies in the distinction between sphincter relaxation and sphincter opening. HRM measures relaxation; the FLIP quantifies opening. Although these are usually related, it is sphincter opening that determines the volume of bolus flow through the EGJ. The EGJ distensibility index has been reported to be low in untreated achalasia patients and in patients with poor symptomatic outcomes following achalasia treatment using a cutoff value of 2.8 mm2/mmHg [26].
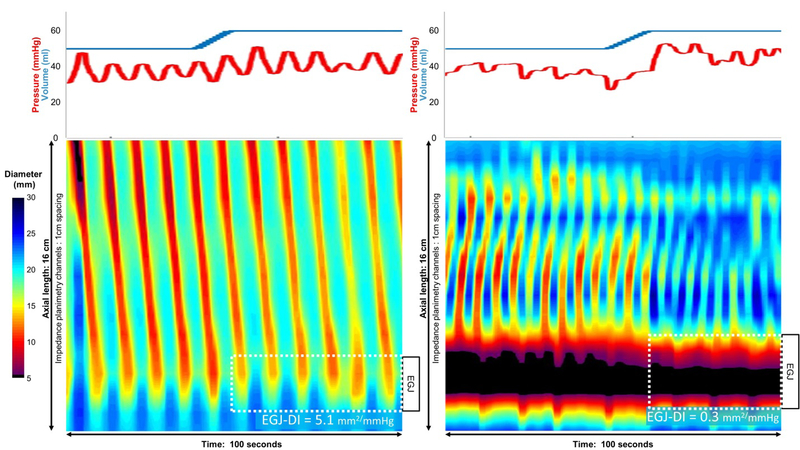
The FLIP can also assess esophageal motility during endoscopy using an emerging technology, termed Panometry [27, 28]. Panometry involves the secondary processing of FLIP data into topographic plots of esophageal regional diameter changes versus time. As such, esophageal contractions can be detected with Panometry even when they are not lumen occluding and, hence, undetectable by HRM [27]. The Panometry pattern commonly observed among controls and patients with normal peristalsis is of repetitive antegrade contractions, likely indicative of secondary peristalsis in response to sustained esophageal distension. Additionally, a unique distension-induced contractile pattern, repetitive retrograde contractions is commonly observed among patients with distal esophageal obstructive physiology and only rarely seen among asymptomatic controls (Figure 2). First described in patients with type III (spastic) achalasia, repetitive retrograde contractions have subsequently been reported among patients with non-spastic achalasia, jackhammer esophagus, eosinophilic esophagitis, reflux, and secondary hypercontractility in post-fundoplication dysphagia [28, 29]. The emerging concept is that repetitive retrograde contractions are indicative of ganglionic neural imbalance and ‘spastic-type’ motility in the distal esophagus; a manifestation of either impaired nitrergic input and premature contractions (e.g. type III achalasia) or augmented cholinergic input with hypercontractility and/or impaired deglutitive inhibition (e.g. jackhammer esophagus) [29].
Figure 2.
Two images of FLIP Panometry showing repetitive antegrade cotractions (left) and repetitive retrograde contractions (right). The plots on top indicate the volume within the FLIP balloon (blue) and the corresponding pressure (red). On the topography plots time is on the x-axis, position along the 16 cm balloon on the y-axis, and spectral color indicates luminal diameter at each coordinate as per the scale. With the exception of the small blackened are on the first contraction in the left panel and the EGJ in the right panel, these are all non lumen-occluding contractions. Repetitive antegrade contractions are a normal finding and the patient on the left had normal motility on HRM. However, repetitive retrograde contractions are rarely found in normals and are usually indicative of obstructive physiology; the patient on the right had type III achalasia. The esophagogastric junction distensibility index (EGJ-DI) is measured at 60 ml distension with 2.8 mm2/mmHg being the lower limit of normal.
In summary, despite being the best available single test for demonstrating abnormal esophageal physiology, HRM findings can be equivocal or negative despite strong clinical suspicion to the contrary. In such instances, the clinician needs to investigate further, be that with provocative maneuvers or ancillary tests. The ultimate objective is to identify and localize clinically relevant obstructive physiology, recognizing that this can be limited to the EGJ or involve both the EGJ and the distal esophagus. Making this distinction has important management implications. Table 1 summarized the HRM maneuvers and ancillary tests that have been reported to help with this decision-making.
Table 1.
HRM and ancillary test findings indicative of obstructive physiology at the EGJ and distal esophagus.
| Test | EGJ outflow obstruction | Obstructive distal contractions |
|---|---|---|
| HRM |
|
|
| HRM- rapid drink challenge |
|
|
| HRM- multiple repetitive swallows |
|
|
| Timed barium esophagram |
|
|
| FLIP |
|
|
| FLIP-panometry |
|
|
| EUS or CT imaging |
|
|
HRM: high resolution manometry; EGJ: esophagogastric junction; IRP: integrated relaxation pressure; DCI: distal contractile integral;
A New Perspective on Management: Phenotype-Directed Treatment
The ideal therapy for an esophageal motility disorder would revert swallow function to normal, render the patient symptom free, and not result in pathological reflux. No current therapy for any of the esophageal motility disorders achieves all of these objectives and that is unlikely to change in the foreseeable future. Furthermore, the controlled treatment data currently available lag well behind recent advances in diagnostics. Indeed, although the original description of achalasia phenotypes noted that an important distinction among phenotypes was in the differential likelihood that they would respond to achalasia treatments (best outcomes in type II and worst in type III) [6], essentially all available controlled trials have not specified achalasia subtype. Retrospective analyses of randomized [30] and non-randomized treatment series [31, 32] have, however, confirmed the original observation of differential response rates. Furthermore, with the recognition of obstructive physiology at the EGJ or distal esophagus being important foci of treatment, several disease entities beyond classical achalasia are now being rendered treatments formerly reserved for achalasia [2]. This is particularly relevant with the widespread adoption of the POEM procedure. Not only does POEM provide an option for surgical LES myotomy with reduced operative morbidity, but it also facilitates a calibrated myotomy, potentially extending proximally to include the entire smooth muscle segment of the esophagus. Together, these developments modified treatment aims to target obstructive physiology at the EGJ or distal esophagus as identified by physiologic testing, regardless of the specific disease entity.
The concept of phenotype directed therapy is especially relevant with type III achalasia, characterized by obstructive contractility of the distal esophagus, and noted to have less robust outcomes with therapies limited to the LES [6, 30–32]. Seeking to improve on this, uncontrolled series have applied POEM to type III achalasia and gauged the length of myotomy by the length of the spastic segment seen on HRM, esophageal wall thickening on imaging studies, or intra-operative FLIP. A recent meta-analysis of these uncontrolled POEM series reported a weighted pooled response rate of 92% (CI 84–96%) in type III achalasia with a myotomy length ranging from 13–19.7 cm [33]. Similarly, a retrospective analysis comparing treatment outcomes with laparoscopic Heller myotomy (LHM) and POEM in a 49-patient multicenter analysis found POEM to be significantly more efficacious (98.0 % vs 80.8 %; P = 0.01), presumably on account of the more extensive myotomy that was done (16 cm vs 8 cm; P < 0.01) [34]. Moreover, what is effective for type III achalasia should also be effective for the other esophageal motility disorders in Figure 1 characterized by obstructive physiology of the smooth muscle esophagus: DES and jackhammer esophagus. The same POEM meta-analysis that analyzed type III achalasia outcomes reported a weighted pooled response rate to POEM of 72% (CI 55–83%) in jackhammer and 88% in DES (CI 61–97%) [33]. Notably, the DES response rate was only 4% less than that seen in type III achalasia, emphasizing the similarity of these entities when defined according to the CC.
At the other end of the treatment spectrum is type II achalasia, wherein there is extreme obstructive physiology at the EGJ resulting in panesophageal pressurization with test swallows, but often remnants of normal peristalsis in the distal esophagus that become evident after treatment [35]. Hence, any treatment that relieved EGJ outflow obstruction, even if limited to the EGJ as in the case of pneumatic dilation (PD), should be effective. Consistent with that hypothesis, type II achalasia patients obtained the best treatment outcome in all of the type-specific analyses to date, regardless of the treatment. Indeed, in the European achalasia trial, which was a randomized controlled trial of PD vs LHM [36, 37], the efficacy of PD for treating type II achalasia was 100% (acknowledging acceptance of intermittent repeat pneumatic dilation) [30]. Considering that the cost of PD is substantially less than LHM and that the risk of perforation between techniques is comparable (about 1% in expert hands) [38], this argues for PD as preferable to LHM in the initial treatment of type II achalasia.
Evident from the above discussion, the optimal initial achalasia treatment likely depends on the subtype and cogent arguments can be made for POEM, LHM, and PD, all of which are relatively durable treatment options. Among these the most extensive literature, recently summarized by Pandolfino, has compared PD to LHM and concluded that both are highly efficacious, albeit best when done in expert hands [39]. On the other hand, uncontrolled outcome data have been very promising comparing POEM with LHM [2, 40]. True, POEM is relatively new, but with the pioneering Japanese center recently summarizing their experience gleaned from their first 1000 POEM procedures [41], one can hardly classify it as ‘experimental’. However, controlled data comparing POEM to either LHM or PD are still very limited. Although some are in progress, no randomized trial has yet been reported comparing POEM to LHM and only one, reported as an abstract (and now a manuscript in review), has comparing POEM to PD [42] (Table 2). A recent systematic review and meta-analysis of trials comparing POEM (1958 patients) to LHM (5834 patients) reported POEM to be more effective in relieving dysphagia (mean follow-up of 16 months), but also more likely to lead to post-procedure reflux (odds ratio of 9.31 for erosive esophagitis) [43]. The multicenter randomized controlled trial comparing POEM to PD in 133 treatment-naive achalasia patients reported 92% remission after one year follow up in the POEM cohort compared to 70% in the PD cohort (p<0.01) [42]. One perforation occurred after pneumatic dilation and no severe adverse events occurred related to POEM. Endoscopy one year after treatment found reflux esophagitis in 48% of the POEM patients (40.0% Los Angeles A or B, 8.3% C or D) compared to 13% of the PD patients (all LA A or B), p=0.02.
Table 2.
Treatment considerations for esophageal motility disorders with obstructive physiology at the EGJ with or without distal esophageal obstructive physiology.
| Syndrome | Treatment (s), comments, rationale |
|---|---|
| Type I and II achalasia |
|
| Type III achalasia |
|
| EGJ outflow obstruction |
|
| Absent contractility deemed to be achalasia |
|
| DES/Jackhammer |
|
| Opioid effect |
|
| Obstruction |
|
DES: distal esophageal spasm; EUS: endoscopic ultrasound; FLIP: functional luminal imaging probe; LHM: laparoscopic Heller myotomy; PD: pneumatic dilation; PDE-5: phosphodiesterase type 5; POEM: per-oral endoscopic myotomy; RCT: randomized controlled trials
In summary PD, LHM, and POEM can all be highly efficacious treatments for achalasia but circumstances of local expertise, achalasia subtype, and patient-specific variables (comorbidities, hiatal hernia, epiphrenic diverticulum) may make one treatment preferable to another [44, 45]. The reported success rates of PD are particularly variable among centers, with 2-year response rates ranging from 54% to 86% in two recent randomized controlled trials [36, 42], likely reflective of what constituted a “success” in each trial and balancing the aggressiveness of the dilation protocol against the associated risk of sustaining a perforation.
EGJ outflow obstruction
In addition to the three subtypes of achalasia, CC v3.0 recognizes EGJ outflow obstruction as another entity characterized by EGJ obstructive physiology. With this entity, the IRP is greater than the upper limit of normal, but there is fragmented or even normal peristalsis such that criteria for achalasia are not met. From its initial description, EGJ outflow obstruction was reported to be a heterogeneous group, only some of whom benefitted from achalasia treatments [46]. Potential etiologies include incompletely expressed or early achalasia, esophageal wall stiffness from an infiltrative disease or cancer, eosinophilic esophagitis, extrinsic vascular obstruction, sliding or paraesophageal hiatal hernia, abdominal obesity, opiate effect [47], or simply a false positive measurement. Consequently, further clinical evaluation (e.g. endoscopic ultrasound, FLIP, computerized tomography, etc) and a cautious approach to treatment are requisite. Indeed, four recent series of EGJ outflow obstruction found many cases that were minimally symptomatic or asymptomatic and that the condition resolved spontaneously 20–40% of the time [48–51]. Nonetheless, 12–40% of them ended up being treated as achalasia, albeit conservatively.
Conclusions
High-resolution manometry and the CC have led to a major restructuring in the classification of esophageal motility disorders. Along with this has come the recognition that a defining feature of the major esophageal motility disorders is obstructive physiology, be that at the EGJ, the distal esophagus, or both (Figure 1). Although the CC has helped crystalize esophageal motility diagnoses, especially the varied phenotypes of achalasia, it has also led to the realization that there are circumstances beyond types I, II, and III achalasia in which therapies once reserved for achalasia are beneficial. Furthermore, since no HRM pattern or metric is absolutely sensitive or specific for idiopathic achalasia, complimentary assessments with provocative maneuvers during HRM or interrogating the EGJ with the FLIP during endoscopy can be useful in clarifying equivocal or inexplicable HRM findings. The emerging methodology of FLIP Panometry (Figure 2) is also promising in this regard. Recognizing obstructive physiology as a primary target of therapy has become particularly relevant with the development of a minimally invasive technique for performing a calibrated myotomy of the esophageal circular muscle, the POEM procedure. With the widespread adoption of POEM, there has come a shift in management strategy toward rendering treatment in a phenotype-specific manner: e.g. POEM calibrated to patient-specific physiology as defined by HRM for the spastic disorders, PD for the disorders limited to the LES. Table 2 details important treatment considerations for therapeutic interventions for the major esophageal motility disorders as defined by CC v3.0 from the perspectives of the authors. Clearly, there are substantial limitations to the current dataset in defining the optimal treatment for esophageal motility disorders as currently conceptualized and there are often major issues with the available expertise, but all things being equal, this is what we glean from the best evidence currently available.
Acknowledgments
Funding: Peter J Kahrilas and John E Pandolfino were supported by R01 DK079902 (JEP) from the US Public Health Service.
Albert J Bredenoord: Medtronic: research funding; MMS: educational and research funding
Abbreviations:
- CC
Chicago Classification
- DCI
distal contractile integral
- DL
distal latency
- DES
distal esophageal spasm
- EGJ
esophagogastric junction
- FLIP
functional luminal imaging probe
- HRM
high-resolution manometry
- IRP
integrated relaxation pressure
- LHM
laparoscopic Heller myotomy
- LES
lower esophageal sphincter
- POEM
per oral endoscopic myotomy
- PD
pneumatic dilation
Footnotes
Potential conflicts of interest
Peter J Kahrilas: No potential conflicts
Dustin A Carlson: No potential conflicts
John E Pandolfino: Medtronic, Sandhill, Torax; consulting and educational; Crospon; Stock Options
References
- 1.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015. February;27(2):160–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahrilas PJ, Katzka D, Richter JE. The use of Per-Oral Endoscopic Myotomy (POEM) in achalasia: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology 2017;153:1205–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlson DA, Lin Z, Kahrilas PJ, et al. The functional lumen imaging probe detects esophageal contractility not observed with manometry in patients with achalasia. Gastroenterology 2015;149:1742–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szigethy E, Knisely M, Drossman D. Opioid misuse in gastroenterology and non-opioid management of abdominal pain. Nat Rev Gastroenterol Hepatol 2017. November 15. doi: 10.1038/nrgastro.2017.141. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clouse RE, Staiano A. Topography of the esophageal peristaltic pressure wave. Am J Physiol 1991;261:G677–84. [DOI] [PubMed] [Google Scholar]
- 6.Clouse RE, Staiano A, Alrakawi A, Haroian L. Application of topographical methods to clinical esophageal manometry. Am J Gastroenterol 2000;95:2720–30. [DOI] [PubMed] [Google Scholar]
- 7.Kahrilas PJ, Bredenoord AJ, Fox M, et al. Expert consensus document: Advances in the management of oesophageal motility disorders with high-resolution manometry: a focus on achalasia syndromes. Nat Rev Gastroenterol Hepatol 2017. November;14(11):677–688. [DOI] [PubMed] [Google Scholar]
- 8.Lin Z, Yim B, Nicodème F, Gawron A, et al. The four phases of esophageal bolus transit defined using high-resolution impedance manometry and fluoroscopy. Am J Physiol 2014:307:G437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandolfino JE, Kwiatek MA, Nealis T, Bulsiewicz W, Post J, Kahrilas PJ. Achalasia: A New Clinically Relevant Classification by High-Resolution Manometry. Gastroenterology 2008;135:1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandolfino JE, Roman S, Carlson D, et al. Distal esophageal spasm in high-resolution esophageal pressure topography: defining clinical phenotypes. Gastroenterology 2011;141:469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlson DA, Kahrilas PJ. How to effectively use high-resolution esophageal manometry. Gastroenterology 2016; 151:789–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahrilas PJ, Boeckxstaens G. The spectrum of achalasia: lessons from studies of pathophysiology and high-resolution manometry. Gastroenterology 2013;145(5):954–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandolfino JE, Kahrilas PJ. The second American Gastroenterological Association technical review on the clinical use of esophageal manometry. Gastroenterology 2005;128:209–229. [DOI] [PubMed] [Google Scholar]
- 14.Ponds FA, Bredenoord AJ, Kessing BF, Smout AJPM. Esophagogastric junction distensibility identifies achalasia subgroup with manometrically normal esophagogastric junction relaxation. Neurogastroenterol Motil 2017;29:e12908;DOI: 10.1111/nmo.12908. [DOI] [PubMed] [Google Scholar]
- 15.Scherer JR, Kwiatek MA, Soper NJ, Pandolfino JE, Kahrilas PJ. Functional esophagogastric junction obstruction with intact peristalsis: a heterogeneous syndrome sometimes akin to achalasia. J Gastrointest Surg 2009;13:2219–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin Z, Kahrilas PJ, Roman S, Boris L, Carlson D, Pandolfino JE. Refining the criterion for an abnormal Integrated Relaxation Pressure in esophageal pressure topography based on the pattern of esophageal contractility using a classification and regression tree model. Neurogastroenterol Motil 2012;24:e356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fornari F, Bravi I, Penagini R, Tack J, Sifrim D. Multiple rapid swallowing: a complementary test during standard oesophageal manometry. Neurogastroenterol Motil 2009;21:718–e741. [DOI] [PubMed] [Google Scholar]
- 18.Price LH, Li Y, Patel A, et al. Reproducibility patterns of multiple rapid swallows during high resolution esophageal manometry provide insights into esophageal pathophysiology. Neurogastroenterol Motil 2014;26:646–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ang D, Hollenstein M, Misselwitz B, et al. Rapid drink challenge in high-resolution manometry: an adjunctive test for detection of esophageal motility disorders. Neurogastroenterol Motil 2017;29(1) DOI: 10.1111/nmo.12902 [DOI] [PubMed] [Google Scholar]
- 20.Marin I, Cisternas D, Abrao L, et al. Normal values of esophageal pressure responses to a rapid drink challenge test in healthy subjects: results of a multicenter study. Neurogastroenterol Motil 2017;29. [DOI] [PubMed] [Google Scholar]
- 21.Shaker A, Stoikes N, Drapekin J, Kushnir V, Brunt LM, Gyawali CP. Multiple rapid swallow responses during esophageal high-resolution manometry reflect esophageal body peristaltic reserve. Am J Gastroenterol 2013;108:1706–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicodème F, de Ruigh A, Xiao Y, et al. A comparison of symptom severity and bolus retention to Chicago Classification esophageal topography metrics in achalasia. Clin Gastroenterol Hepatol 2013;11:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blonski W, Kumar A, Feldman J, Richter JE. Timed barium swallow: diagnostic role and predictive value in untreated achalasia, esophagogastric junction outflow obstruction, and non-achalasia dysphagia. Am J Gastroenterol 2018. 113(2):196–203. [DOI] [PubMed] [Google Scholar]
- 24.Cho YK, Lipowska AM, Nicodème F, et al. Assessing bolus retention in achalasia using high-resolution manometry with impedance: a comparator study with timed barium esophagram. Am J Gastroenterol 2014;109:829–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pandolfino JE, de Ruigh A, Nicodème F, et al. Distensibility of the esophagogastric junction assessed with the functional lumen imaging probe (FLIP) in achalasia patients. Neurogastroenterol Motil 2013;25:496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rohof WO, Hirsch DP, Kessing BF, et al. Efficacy of treatment for patients with achalasia depends on the distensibility of the esophagogastric junction. Gastroenterology 2012;143:328–335. [DOI] [PubMed] [Google Scholar]
- 27.Carlson DA, Lin Z, Kahrilas PJ, et al. The functional lumen imaging probe detects esophageal contractility not observed with manometry in patients with achalasia. Gastroenterology 2015;149(7):1742–51. Duplicate of reference #3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlson DA, Kahrilas PJ, Lin Z, et al. Evaluation of esophageal motility utilizing the functional lumen imaging probe. Am J Gastroenterol 2016;111(12):1726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlson DA, Kahrilas PJ, Ritter K, Lin Z, Pandolfino JE. Mechanisms of repetitive, retrograde contractions in response to sustained esophageal distension: a study evaluating patients with post-fundoplication dysphagia. Am J Physiol 2018; 314(3):G334–G340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rohof WO, Salvador R, Annese V, et al. Outcomes of treatment for achalasia depend on manometric subtype. Gastroenterology 2013;144(4):718–25. [DOI] [PubMed] [Google Scholar]
- 31.Salvador R, Costantini M, Zaninotto G, et al. The preoperative manometric pattern predicts the outcome of surgical treatment for esophageal achalasia. J Gastrointest Surg 2010;14(11):1635–45. [DOI] [PubMed] [Google Scholar]
- 32.Pratap N, Kalapala R, Darisetty S, et al. Achalasia cardia subtyping by high-resolution manometry predicts the therapeutic outcome of pneumatic balloon dilatation. J Neurogastroenterol Motil 2011;17(1):48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan AK, Kumbhari V, Ngamruengphong S, et al. Is POEM the answer for management of spastic esophageal disorders? A systematic review and meta-analysis. Dig Dis Sci 2017;62:35–44. [DOI] [PubMed] [Google Scholar]
- 34.Kumbhari V, Tieu AH, Onimaru M, et al. Peroral endoscopic myotomy (POEM) vs laparoscopic Heller myotomy (LHM) for the treatment of Type III achalasia in 75 patients: a multicenter comparative study. Endosc Int Open. 2015. June;3(3):E195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roman S, Kahrilas PJ, Mion F, et al. Partial recovery of peristalsis after myotomy for achalasia; more the rule than the exception. JAMA Surg. 2013;148:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boeckxstaens GE, Annese V, Bruley des Varannes S, et al. Pneumatic dilation versus laparoscopic Heller’s myotomy for idiopathic achalasia. N Engl J Med 2011;364:1807–16. [DOI] [PubMed] [Google Scholar]
- 37.Moonen A, Annese V, Belmans A, et al. Long-term results of the European achalasia trial: a multicenter randomised controlled trial comparing pneumatic dilation versus laparoscopic Heller myotomy. Gut 2016;65:732–9. [DOI] [PubMed] [Google Scholar]
- 38.Lynch KL, Pandolfino JE, Howden CW & Kahrilas PJ Major complications of pneumatic dilation and Heller myotomy for achalasia: single center experience and systematic review of the literature. Am J Gastroenterol 2012;107:1817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pandolfino JE, Gawron AJ. Achalasia a systematic review. JAMA 2015;313(18):1841–1852. [DOI] [PubMed] [Google Scholar]
- 40.Wei M, Yang T, Yang X, Wang Z, Zhou Z. Peroral esophageal myotomy versus laparoscopic Heller’s myotomy for achalasia: a meta-analysis. J Laparoendosc Adv Surg Tech 2015;25:123–130. [DOI] [PubMed] [Google Scholar]
- 41.Bechara R, Onimaru M, Ikeda H, Inoue H. Per-oral endoscopic myotomy, 1000 cases later: pearls, pitfalls, and practical considerations. Gastrointest Endosc 2016;84:330–338. [DOI] [PubMed] [Google Scholar]
- 42.Ponds FA, Fockens P, Neuhaus H, et al. Peroral endoscopic myotomy (POEM) versus pneumatic dilatation in therapy-naïve patients with achalasia: results of a randomized controlled trial. Gastroenterology 2017;152 (abstract). [Google Scholar]
- 43.Werner YB, Costamagna G, Swanström LL, et al. Clinical response to peroral endoscopic myotomy in patients with idiopathic achalasia at a minimum follow-up of 2 years. Gut 2016;65(6):899–906. [DOI] [PubMed] [Google Scholar]
- 44.Kahrilas PJ. Treating achalasia; more than just flipping a coin. Gut 2016;65(5):726–7. [DOI] [PubMed] [Google Scholar]
- 45.Ihara E, Muta K, Fukaura K, Nakamura K. Diagnosis and treatment strategy of achalasia subtypes and esophagogastric outflow obstruction based on high-resolution manometry. Digestion 2017;95:29–35. [DOI] [PubMed] [Google Scholar]
- 46.Scherer JR, Kwiatek MA, Soper NJ, Pandolfino JE, Kahrilas PJ. Functional esophagogastric junction obstruction with intact peristalsis: a heterogeneous syndrome sometimes akin to achalasia. J Gastrointest Surg 2009;13:2219–2225. Duplicate of #15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ratuapli SK, Crowell MD, DiBaise JK, Vela MF, Ramirez FC, et al. Opioid-induced esophageal dysfunction (OIED) in patients on chronic opioids. Am J Gastroenterol 2015;110:979–84. [DOI] [PubMed] [Google Scholar]
- 48.Van Hoeij FB, Smout AJPM, Bredenoord AJ. Characterization of idiopathic esophagogastric junction outflow obstruction. Neurogastroenterol Motil 2015;27:1310–1316. [DOI] [PubMed] [Google Scholar]
- 49.Pérez-Fernández MT, Santander C, Marinero A, Burgos-Santamaría D, Chavarría-Herbozo C. Characterization and follow-up of esopahgogastric junction outflow obstruction detected by high resolution manometry. Neurogastroenterol Motil 2016;28:116–126. [DOI] [PubMed] [Google Scholar]
- 50.Okeke FC, Raja S, Lynch KL, et al. What is the clinical significance of esophagogastric junction outflow obstruction? Evaluation of 60 patients at a tertiary referral center. Neurogastroenterol Motil 2017;29:e13061. [DOI] [PubMed] [Google Scholar]
- 51.Schupack D, Katzka DA, Geno DM, Ravi K. The clinical significance of esophagogastric junction outflow obstruction and hypercontractile esophagus in high resolution esophageal manometry. Neurogastroenterol Motil 2017. 29(10):1–9. [DOI] [PubMed] [Google Scholar]