Preeclampsia is a pregnancy-specific hypertensive disorder that presents with systemic symptoms, and commonly proteinuria, after 20 weeks of gestation. It may occur either de novo or superimposed on chronic hypertension. Other hypertensive disorders of pregnancy (HDP) include chronic and gestational hypertension 1. Eclampsia is the convulsive form of preeclampsia that affects 0.1% of all pregnancies. First descriptions of preeclampsia/eclampsia date back to 400 BC and focused on headaches and convulsions, representing central nervous system (CNS) signs and symptoms of preeclampsia and eclampsia, respectively 2. Critical clinical findings of preeclampsia, such as proteinuria and elevated blood pressure, were not described until the 1800’s. Consequently, from ancient times through the 19th century, practicing physicians and scholars concentrated on understanding the underlying pathophysiology of eclamptic seizures and improving treatment. Bloodletting, use of opiates, warm baths, and hastening delivery were therapeutic options that developed over time. These efforts culminated in the introduction of magnesium sulfate for seizure prevention in the 20th century, the therapeutic approach which is currently accepted as standard of care. The immediate CNS involvement and complications of preeclampsia/eclampsia have been recognized for years; however, recognition of the long-term consequences of preeclampsia/eclampsia, in the form of cardiovascular and cerebrovascular disease, and impaired cognition dates back only a few decades.
In this issue, Dayan et al explore the relationship between preeclampsia and cognitive function after a long period of follow up 3. They used data from the Coronary Artery Risk Development In young Adults (CARDIA) cohort, which included healthy individuals between the ages of 18–30 years who were followed for 25 years. Neurocognitive measurements were made at the conclusion of follow up. They matched 193 women with preeclampsia to 375 women with normotensive pregnancies based on date of delivery within a range of 5 years. Women were included if they had at least one pregnancy and completed neurocognitive testing, and they were excluded if they had histories of prior major depression, neurodegenerative disease, epilepsy, multiple sclerosis or cancer (including brain tumors). The authors used standardized testing of neurocognitive functions that have been used previously in similar studies. The mean age at cognitive measurement was 49.3 years. The authors found that women with preeclampsia had lower scores for psychomotor speed and executive function, but the relationship lost statistical significance after adjustments for age, body mass index, hypertension, depression and level of education. There was no difference between the two groups for the learning and memory faculty. The strength of this study stems from the large number of subjects included from a diverse population in the US. The authors accounted for cumulative duration of hypertension, used validated cognitive measurements, and had a long period of follow up. The implications of the presented results need to be viewed/ discussed in the context of the current state of knowledge regarding preeclampsia, cardiovascular risk and future cognition.
It has been increasingly recognized that HDP in general, and preeclampsia, in particular, are sex-specific risk factors that are associated with an array of maternal morbidities that occur later in life. Indeed, the American Heart Association and the American Stroke Association released guidelines that focus on cardiovascular disease (CVD)4 and stroke prevention in women and recommend documenting preeclampsia as a risk factor for both conditions5. One possible explanation for these associations (Figure 1) is that preeclampsia and CVD (including stroke) share common risk factors, including obesity, diabetes, and hyperlipidemia, that may lead to preeclampsia and CVD at different times during a woman’s life. Under those circumstances, pregnancy may serve as a “stress test” that women with underlying unfavorable cardiovascular profiles may fail by developing preeclampsia, which, in turn, heralds elevated CVD risk later in life. Alternatively, preeclampsia itself may induce metabolic and vascular changes that may increase the CVD risk in women years after their affected pregnancies. It has been increasingly recognized that CVD increases the risks for both Alzheimer’s disease and vascular dementia. Thus, it is possible that women with a history of HDP also have an increased risk of dementia through their increased risks for CVD later in life. In eclampsia, a direct injury to cerebral vasculature in the form of posterior reversible encephalopathy syndrome (PRES) -characterized by neuroimaging findings of vasogenic edema in posterior circulation of the brain- has been associated with persistent imaging findings of infarction in up to a fourth of eclamptic women 6. This raises the intriguing possibility that CNS vascular damage at delivery may contribute to increased risks for stroke and cognitive decline later in life. The association between cognitive impairment and HDP was first identified on the basis of subjective complaints. New studies are finding objective evidence that stems from neurocognitive testing and brain imaging findings.
Figure 1. Proposed pathway for preeclampsia affecting cognitive function.
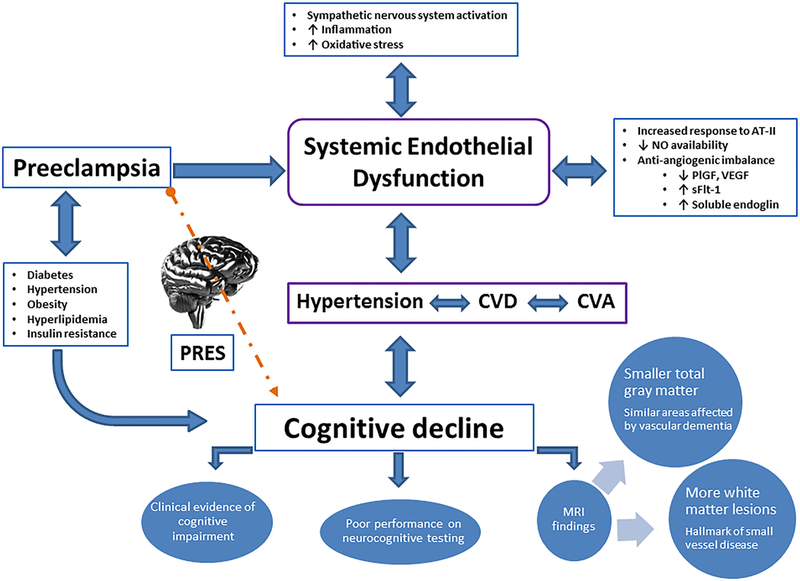
SFlt-1, soluble fms-like tyrosine kinase 1; AT-II: angiotensin II; CVD, Cardiovascular disease; CVA, cerebrovascular accidents; PRES: posterior reversible encephalopathy syndrome; MRI, Magnetic resonance imaging; VEGF, vascular endothelial growth factor; and PlGF, placental growth factor.
Using a validated questionnaire to identify women with hypertensive pregnancies, a study by Mielke et al found similar significant differences in favor of the normotensive women in terms of processing speed and executive function 7. The main difference was that in Mielke et al, the differences remained significant in favor of normotensive women after accounting for similar covariates. This difference may be related to the age at which the women were evaluated (10 years older). Using a different approach, Fields et al compared 40 women with a history of preeclampsia to a matched cohort of 40 women with a history of normotensive pregnancies for clinical evidence of cognitive impairment 8. The average age of women in both arms was ~60 years. Using a clinical approach and standard criteria for single domain mild cognitive impairment (MCI), multiple domain MCI, and dementia, more women with a history of preeclampsia met such criteria than women with a history of normotensive pregnancies.
The effects of HDP on the brain were also examined from an anatomical point of view by exploring different findings on MRI studies between normotensive and women with HDP. Several studies have alluded to the increased prevalence of white matter lesions (or hyperintensities), a marker of small vessel disease, in women with preeclampsia compared to women with normotensive pregnancies. In a study by Aukes et al, women had an average age of 37 years and underwent imaging 5 years from the index pregnancy, showing that formerly pre-eclamptic women had cerebral white matter lesions significantly more often, and more severely than controls 9. In a study by Raman et al, the effects of two exposures, a history of preeclampsia and later-onset hypertension, on total gray matter volume were assessed in women ~60 years of age 10. They found that women who had HDP and subsequently developed hypertension had smaller total gray matter volumes, particularly in the prefrontal and sensorimotor cortices, a pattern similar to vascular dementia. The evidence of smaller brain volumes was also found by Mielke et al, where the total intracranial volumes were smaller in women who had a hypertensive pregnancy compared to those who had a normotensive pregnancy 7. They also found a negative correlation between total standardized volume of brain injury (a composite measure of brain atrophy and white matter lesion volume as assessed by MRI) and both domain specific (e.g. faster processing speed) and overall cognitive function. Taken together, these data support the emerging concept of long-term adverse effects on cognition, whereby abnormal vascular factors that persist post-partum, including endothelial dysfunction, elevated body mass index, abnormal traditional and novel CVD risk factors, and cerebral ischemic abnormalities, may lead to decreased brain volumes, cognitive decline, and, ultimately, dementia.
Recent US population based studies indicate that almost two-thirds of individuals diagnosed with Alzheimer’s disease (AD) are women with some studies also suggesting that women have a faster rate of cognitive and functional decline after a diagnosis of AD 11. Therefore, it is critically important to understand the sex-specific risk factors that affect prevalence, clinical presentation, and prognoses of the affected individuals in order to develop sex-specific screening and treatment strategies. The study of Dayan et al significantly contributes to the current understanding of preeclampsia as a sex-specific risk factor for cognitive decline. First, it provides evidence that a history of preeclampsia predicts future cognitive decline in women starting at the age 50 years, i.e., at an age when cognitive reserve is relatively well-preserved. Second, their results suggest that several metabolic and psychosocial factors may mediate the association between preeclampsia and cognitive impairment. An important corollary to these observations is that treatment of these modifiable risk factors may provide primary prevention of cognitive decline in women with a history of HDP.
The study of Dayan et al sets the stage for future studies, which should be adequately powered and designed to address additional mediators-and potential therapeutic targets- between HDP and future cognitive decline. In addition, while preeclampsia has primarily been linked with an increased risk of dementia through vascular pathways, recent evidence suggests that preeclampsia may share common pathways with AD. Accumulation of the β-amyloid peptide, a hallmark of brain pathology in AD, has been demonstrated in placental tissue from pre-eclamptic pregnancies 12. The causal link between these aggregates and immediate and long-term morbidity related to preeclampsia requires further investigation.
Acknowledgments
Financial support: This study was supported by National Institute of Health, R01HL136348 (VDG).
Footnotes
Disclosure: None of the authors declare competing financial interests.
References
- 1.Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstetrics and gynecology. 2013;122(5):1122–1131. [DOI] [PubMed] [Google Scholar]
- 2.Bell MJ. A historical overview of preeclampsia-eclampsia. Journal of obstetric, gynecologic, and neonatal nursing : JOGNN. 2010;39(5):510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Impact of Preeclampsia on Long-term Cognitive Function Dayan N Kaur A et al. Hypertension 2018. In Press [DOI] [PubMed] [Google Scholar]
- 4.Mosca L, Banka CL, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Journal of the American College of Cardiology. 2007;49(11):1230–1250. [DOI] [PubMed] [Google Scholar]
- 5.Bushnell C, McCullough L. Stroke prevention in women: synopsis of the 2014 American Heart Association/American Stroke Association guideline. Annals of internal medicine. 2014;160(12):853–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeeman GG, Fleckenstein JL, Twickler DM, Cunningham FG. Cerebral infarction in eclampsia. American journal of obstetrics and gynecology. 2004;190(3):714–720. [DOI] [PubMed] [Google Scholar]
- 7.Mielke MM, Milic NM, Weissgerber TL, et al. Impaired Cognition and Brain Atrophy Decades After Hypertensive Pregnancy Disorders. Circulation Cardiovascular quality and outcomes. 2016;9(2 Suppl 1):S70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fields JA, Garovic VD, Mielke MM, et al. Preeclampsia and cognitive impairment later in life. American journal of obstetrics and gynecology. 2017;217(1):74.e71–74.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aukes AM, De Groot JC, Wiegman MJ, Aarnoudse JG, Sanwikarja GS, Zeeman GG. Long-term cerebral imaging after pre-eclampsia. BJOG : an international journal of obstetrics and gynaecology. 2012;119(9):1117–1122. [DOI] [PubMed] [Google Scholar]
- 10.Raman MR, Tosakulwong N, Zuk SM, et al. Influence of preeclampsia and late-life hypertension on MRI measures of cortical atrophy. Journal of hypertension. 2017;35(12):2479–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laws KR, Irvine K, Gale TM. Sex differences in Alzheimer’s disease. Current opinion in psychiatry. 2018;31(2):133–139. [DOI] [PubMed] [Google Scholar]
- 12.Buhimschi IA, Nayeri UA, Zhao G, et al. Protein misfolding, congophilia, oligomerization, and defective amyloid processing in preeclampsia. Science translational medicine. 2014;6(245):245–292. [DOI] [PubMed] [Google Scholar]


