In this multicenter study, we evaluate the performance characteristics of the Rochester and modified Philadelphia criteria for risk stratification of febrile infants with bacteremia and/or bacterial meningitis.
Abstract
Video Abstract
OBJECTIVES:
To evaluate the Rochester and modified Philadelphia criteria for the risk stratification of febrile infants with invasive bacterial infection (IBI) who do not appear ill without routine cerebrospinal fluid (CSF) testing.
METHODS:
We performed a case-control study of febrile infants ≤60 days old presenting to 1 of 9 emergency departments from 2011 to 2016. For each infant with IBI (defined as a blood [bacteremia] and/or CSF [bacterial meningitis] culture with growth of a pathogen), controls without IBI were matched by site and date of visit. Infants were excluded if they appeared ill or had a complex chronic condition or if data for any component of the Rochester or modified Philadelphia criteria were missing.
RESULTS:
Overall, 135 infants with IBI (118 [87.4%] with bacteremia without meningitis and 17 [12.6%] with bacterial meningitis) and 249 controls were included. The sensitivity of the modified Philadelphia criteria was higher than that of the Rochester criteria (91.9% vs 81.5%; P = .01), but the specificity was lower (34.5% vs 59.8%; P < .001). Among 67 infants >28 days old with IBI, the sensitivity of both criteria was 83.6%; none of the 11 low-risk infants had bacterial meningitis. Of 68 infants ≤28 days old with IBI, 14 (20.6%) were low risk per the Rochester criteria, and 2 had meningitis.
CONCLUSIONS:
The modified Philadelphia criteria had high sensitivity for IBI without routine CSF testing, and all infants >28 days old with bacterial meningitis were classified as high risk. Because some infants with bacteremia were classified as low risk, infants discharged from the emergency department without CSF testing require close follow-up.
What’s Known on This Subject:
The Rochester and modified Philadelphia criteria do not include routine cerebrospinal fluid testing. Although these criteria have high sensitivity in the risk stratification of febrile infants with serious bacterial infections, most studies had few infants with bacteremia and/or bacterial meningitis.
What This Study Adds:
The modified Philadelphia criteria were highly sensitive in the risk stratification of non–ill-appearing febrile infants with bacteremia, and no infants with bacterial meningitis were classified as low risk. Two infants ≤28 days old with meningitis were classified as low risk by the Rochester criteria.
Approximately 10% of febrile infants ≤60 days of age evaluated in the emergency department (ED) have a serious bacterial infection.1 To identify infants who may be discharged from the ED after initial evaluation, the Rochester and Philadelphia criteria2–4 are widely used to stratify febrile infants on the basis of their risk of serious bacterial infection.5 Although these criteria have a reported sensitivity of >90% in the risk stratification of febrile infants with serious bacterial infections, these studies included mostly infants with urinary tract infections (UTIs), with relatively few infants with bacteremia and/or bacterial meningitis4 (ie, invasive bacterial infection [IBI]). Therefore, data are limited regarding the precision of the Rochester and Philadelphia criteria in the risk stratification of infants with IBI.4 Additionally, with a changing epidemiology of IBI in febrile infants since the development of these criteria >2 decades ago,6,7 the performance of these criteria needs to be reevaluated periodically.4,8
Given the rarity of bacterial meningitis in febrile infants >28 days of age who appear well and the unclear benefit of routine cerebrospinal fluid (CSF) testing,9–11 some providers do not automatically perform CSF testing in this age group, although practice varies substantially across hospitals.12,13 The Rochester criteria and the modified Philadelphia criteria do not require routine CSF testing to classify febrile infants as being at a low or high risk for serious bacterial infection.2,9 Data regarding the performance characteristics of the Rochester and modified Philadelphia criteria in a large sample of febrile infants ≤60 days of age with IBI would inform the need for routine CSF testing in this population. Our objective was to evaluate the Rochester and modified Philadelphia criteria for the risk stratification of infants with IBI using a contemporary sample of febrile infants ≤60 days of age evaluated in the ED.
Methods
Study Design
We analyzed data collected for a case-control study of febrile infants ≤60 days of age evaluated in the ED at 1 of 11 children’s hospitals between July 1, 2011, and June 30, 2016. The current study was limited to the 9 hospitals containing hematology laboratories that reported band counts because these were included in both the Rochester criteria and the modified Philadelphia criteria. The study was approved by each site’s institutional review board with permission for data sharing.
Cases
Infants ≤60 days of age with IBI were identified through query of each hospital’s microbiology laboratory database or electronic medical record system for blood and/or CSF cultures positive for a pathogen (defined a priori).7 Infants were excluded if the culture with positive results was documented in the medical record to have been treated as a contaminant.7,14,15 Bacteremia was defined as growth of a pathogen in a blood culture. Bacterial meningitis was defined as growth of a pathogen in a CSF culture (with or without bacteremia) or growth of a pathogen in a blood culture with concomitant CSF pleocytosis if antimicrobial agents were administered before CSF collection. CSF pleocytosis was defined as a CSF white blood cell (WBC) count of ≥20 cells per mm3 for infants ≤28 days of age and ≥10 cells per mm3 for infants 29 to 60 days of age.16
Infants with IBI were included as case patients if they presented either from home or from an outpatient clinic to the participating hospital’s ED (ie, were not transferred from another hospital) and if the following criteria were met: (1) presence of fever (defined as a rectal temperature of ≥38.0°C at home, in an outpatient clinic, or in the ED4), (2) no ill appearance as documented on the ED physical examination,17 and (3) absence of a complex chronic condition.18,19 For all infants with IBI, medical records in the 30 days after were reviewed to assess for a diagnosis of bacterial meningitis.
Controls
Each case patient was matched by hospital and date of visit to 2 febrile infant controls. Potentially eligible controls were identified through a query of the Pediatric Health Information System database for infants ≤60 days of age with an ED visit to a participating hospital during the 5-year study period and who had urine and blood cultures obtained. A query of the electronic medical record system was performed at 1 site that did not contribute ED data to the Pediatric Health Information System. For each case patient at a participating site, infants with the closest date of visit to the case patient were selected as potential controls; if >2 infants were eligible on the basis of visit proximity, a random number generator was used to select which controls to include.
Medical records were reviewed for each potential control to determine the presence of fever and to confirm eligibility. Controls were eligible if (1) they met the same inclusion criteria as case patients, (2) their blood and/or CSF culture did not have growth of a pathogen, and (3) they had not received antibiotics within 7 days before the ED visit. For all controls, medical records for the 30 days after the ED visit were reviewed to ensure that the infant was not diagnosed with an IBI. If a potentially eligible control was determined to be ineligible after a medical record review, an infant with the next closest date of visit was selected, with the process repeated until an eligible control was identified. Febrile infants with UTIs7,20,21 but without IBI were eligible for inclusion as controls.
Data Collection
For each case patient and control, we extracted the following data: demographics (age and sex); past medical history (including gestational age); clinical appearance and presence of a localized infection; complete blood count, urinalysis, and CSF cell count; and bacterial culture results (urine, blood, and CSF). Study investigators at each site entered data into a secure Research Electronic Data Capture tool hosted at Yale University.22
Rochester and Modified Philadelphia Criteria
Table 1 lists the components of the Rochester and modified Philadelphia criteria that classified an infant as low risk.9,23 For both criteria, the definition of a normal urinalysis was based on currently used urine dipstick and microscopy parameters.20 Band counts require performance of a manual differential on a complete blood count. Because performance of a manual differential is usually reflexively triggered by specific parameters set on a hospital’s automated hematology analyzer,24 the band count was recorded as 0 if only an automated differential was performed, and no bands were reported. The immature-to-total (I/T) neutrophil ratio was defined as the percentage of bands divided by the percentage of total neutrophils on a complete blood count. Infants were excluded from the primary analyses if data were missing for any component of the Rochester or modified Philadelphia criteria (eg, gestational age, urinalysis, or peripheral WBC count).23
TABLE 1.
Low-Risk Components for the Rochester and Modified Philadelphia Criteria
| Components | Rochester | Modified Philadelphia |
|---|---|---|
| Demographics | N/Aa | Age >28 d |
| Past medical history | Previously healthyb | Previously healthyb |
| Physical examination | No skin or soft tissue infection | No skin or soft tissue infection |
| Laboratory | Normal urinalysisc; peripheral WBC count of ≥5000 and ≤15 000; absolute band count of ≤1500 bands per μL | Normal urinalysisc; peripheral WBC count of ≥5000 and ≤15 000; I/T ratio of <0.2d |
N/A, not applicable.
Rochester criteria include infants ≤60 d of age without an age cutoff to define low risk.
Gestational age ≥37 wk; no previous ED visit, hospitalization, or evaluation for fever; no previous IBI or treatment with antibiotics; no other significant past medical history.
Urine dipstick with no or trace leukocyte esterase, negative nitrites, and urine microscopy, with ≤5 WBCs per HPF or ≤5 WBCs per mm3 on an enhanced urinalysis.
Bands-to-total neutrophil ratio.
Statistical Analyses
Categorical variables were described by using frequencies and percentages, and distributions were compared by using a χ2 test. For the Rochester and modified Philadelphia criteria, sensitivity and specificity were calculated and reported with 95% confidence intervals (CIs). Because both the Rochester and Philadelphia criteria have historically used ≤10 WBCs per high-power field (HPF) or ≤10 WBCs per mm3 on an enhanced urinalysis2,9,23 to define a normal urinalysis, analyses were repeated by using this definition. Additionally, because the modified Philadelphia criteria were developed for infants ≤56 days of age,9 analyses were repeated after limiting the sample to infants in this age range. Because of concerns regarding the introduction of bias on the basis of the exclusion of infants with IBI, we also calculated the sensitivity of the Rochester and modified Philadelphia criteria after the inclusion of infants with missing data.
Statistical analyses were performed by using Stata data analysis and statistical software version 15.0 (Stata Corp, College Station, TX). A 2-tailed P value < .05 was considered statistically significant.
Results
Study Sample
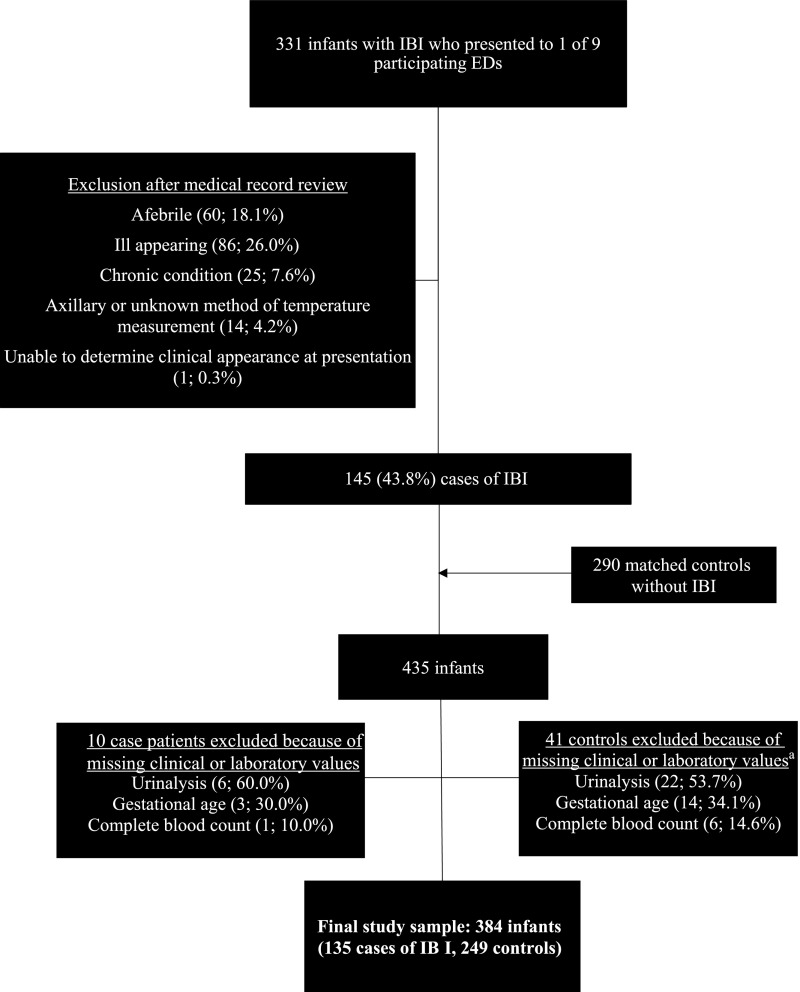
During the 5-year study period, 331 infants with IBI were evaluated in the EDs of the 9 participating hospitals. Eighty-six infants (26.0%) were excluded because of ill appearance, including 26 febrile infants who had bacterial meningitis. Of the infants with IBI who did not appear ill, 145 met inclusion criteria as case patients and were matched to 290 controls. Because of missing data on clinical or laboratory components of the Rochester and/or modified Philadelphia criteria, 10 case patients and 41 controls were excluded, resulting in a final study sample of 135 case patients and 249 controls (Fig 1). Among the 135 infants with IBI, 118 (87.4%) had bacteremia without meningitis, and 17 (12.6%) had bacterial meningitis (with or without bacteremia). Of the 17 infants with bacterial meningitis, 7 (41.2%) were >28 days of age.
FIGURE 1.
Study population. a One infant was missing a urinalysis and complete blood count.
Sensitivity and Specificity of the Rochester and Modified Philadelphia Criteria
A higher proportion of febrile infants with IBI were ≤28 days of age and had abnormal laboratory parameters compared with matched controls (Table 2). Sixty-one infants with IBI (45.2%) had a UTI compared with 15 controls (6.0%). Overall, 25 infants with IBI (18.5%; 95% CI: 12.4%–26.1%) were classified as low risk per the Rochester criteria compared with 11 infants (8.1%; 4.1%–14.1%) who were classified as low risk per the modified Philadelphia criteria (difference: 10.4%; 95% CI: 2.2%–18.6%).
TABLE 2.
Characteristics of Case Patients and Controls
| Characteristic | Case Patients (N = 135), n (%) | Controls (N = 249), n (%) | P |
|---|---|---|---|
| Demographics | |||
| Age group, d | .01 | ||
| ≤28 | 68 (50.4) | 92 (37.0) | |
| 29–60 | 67 (49.6) | 157 (63.1) | |
| Female sex | 61 (45.2) | 102 (41.0) | .42 |
| Parameters of Rochester criteria | |||
| Low risk | 25 (18.5) | 149 (59.8) | <.001 |
| Previously healthya | 107 (79.3) | 206 (82.7) | .40 |
| Normal urinalysisb | 61 (45.2) | 219 (88.0) | <.001 |
| Peripheral WBC ≥5000 and ≤15 000 | 86 (63.7) | 200 (80.3) | <.001 |
| Normal absolute band countc | 110 (81.5) | 240 (96.4) | <.001 |
| Parameters of modified Philadelphia criteria | |||
| Low risk | 11 (8.1) | 86 (34.5) | <.001 |
| Previously healthya | 107 (79.3) | 206 (82.7) | .40 |
| Normal urinalysisb | 61 (45.2) | 219 (88.0) | <.001 |
| Peripheral WBC ≥5000 and ≤15 000 | 86 (63.7) | 200 (80.3) | <.001 |
| Normal I/T ratiod | 103 (76.3) | 234 (94.0) | <.001 |
Gestational age ≥37 wk; no previous ED visit, hospitalization, or evaluation for fever; no previous IBI or treatment with antibiotics; no other significant past medical history.
Urine dipstick with no or trace leukocyte esterase, negative nitrites, and urine microscopy, with ≤5 WBCs per HPF or ≤5 WBCs per mm3 on an enhanced urinalysis.
Absolute band count ≤1500.
Bands-to-total neutrophil ratio <0.2.
The sensitivity of the Rochester criteria was lower than the sensitivity of the modified Philadelphia criteria (81.5% vs 91.9%; P = .01), but the specificity was higher (59.8% vs 34.5%; P < .001). The criteria performed similarly when restricted to infants 29 to 60 days of age (Table 3). Additionally, performance of the modified Philadelphia criteria was similar when limited to infants ≤56 days of age. By using the historical definition of ≤10 WBCs per HPF or ≤10 WBCs per mm3 to define a normal urinalysis, the sensitivities of both the Rochester and modified Philadelphia criteria were lower (74.8% and 88.2%, respectively), with a marginal increase in specificity (63.5% and 36.1%).
TABLE 3.
Performance Characteristics of the Rochester and Modified Philadelphia Criteria for the Identification of IBI in Febrile Infants
| Sensitivity (95% CI) | Specificity (95% CI) | |
|---|---|---|
| Infants ≤60 d of age | ||
| Rochester criteria | 81.5 (73.9–87.6) | 59.8 (53.5–66.0) |
| Modified Philadelphia criteria | 91.9 (85.9–95.9) | 34.5 (28.6–40.8) |
| Infants ≤28 d of age | ||
| Rochester criteria | 79.4 (67.9–88.3) | 64.1 (53.5–73.9) |
| Modified Philadelphia criteriaa | 100 (94.7–100) | 0 (0–3.9) |
| Infants 29–60 d of age | ||
| Rochester criteria | 83.6 (72.5–91.5) | 57.3 (49.2–65.2) |
| Modified Philadelphia criteria | 83.6 (72.5–91.5) | 54.8 (46.6–62.7) |
All infants ≤28 d of age were considered high risk per the modified Philadelphia criteria.
With the inclusion of the 10 infants with IBI who had missing data, an additional 4 infants were classified as low risk by the Rochester criteria, including 1 infant who was also classified as low risk per the modified Philadelphia criteria. When including these infants in the analysis, the sensitivity of the Rochester criteria was slightly lower overall (80.0%) and among infants 29 to 60 days of age (81.7%), whereas the sensitivity of the modified Philadelphia criteria was not materially different (91.7% overall and 83.1% among infants 29–60 days of age).
Febrile Infants With IBI Classified as Low Risk
Of the 11 infants with IBI classified as low risk per the modified Philadelphia criteria, none were diagnosed with bacterial meningitis (Table 4). Two of these infants had mild CSF pleocytosis after traumatic lumbar punctures, and neither received an antimicrobial treatment course for bacterial meningitis. The additional 14 infants with IBI who were classified as low risk per the Rochester criteria were all ≤28 days of age, including 2 infants with bacterial meningitis (Table 4). Both of these infants (≤28 days of age with bacterial meningitis) would have been classified as high risk per the modified Philadelphia criteria because of an I/T ratio of ≥0.2.
TABLE 4.
Febrile Infants With IBI Classified as Low Risk per the Rochester and Modified Philadelphia Criteria
| Age, d | Urinalysis WBC | Peripheral WBC | I/T Ratioa | Absolute Band Count | CSF WBC Count | CSF RBC Count | Urine Culture | Blood Culture | CSF Culture |
|---|---|---|---|---|---|---|---|---|---|
| Infants ≤28 d of ageb | |||||||||
| 3 | 0–5 | 8.6 | 0 | 0 | 35 | 3180 | No growth | Enterococcus faecalis | No growth |
| 4 | 0–5 | 9.5 | 0.10 | 760 | 10 | 7150 | No growth | Escherichia coli | No growth |
| 10 | 0–5 | 10.6 | 0.02 | 212 | 16 | 13 | No growth | GBS | No growth |
| 11 | 0–5 | 13.0 | 0.15 | 1404 | 198 | 273 000 | No growth | Group A Streptococcus | No growth |
| 13 | Not performedc | 7.6 | 0 | 0 | 31 | 29 590 | No growth | Klebsiella pneumoniae | No growth |
| 17 | 0–5 | 11.2 | 0 | 0 | Not obtained | Not obtained | No growth | Salmonella spp | No growth |
| 18 | Not performedc | 9.6 | 0 | 0 | 9 | 4010 | No growth | Group A Streptococcus | No growth |
| 18 | 0–5 | 6.9 | 0.25 | 1035 | 1385 | 16 | No growth | Paenibacillus | Paenibacillus |
| 19 | 0–5 | 10.3 | 0 | 0 | 5 | 88 | Klebsiella oxytoca | K oxytoca | No growth |
| 19 | 0–5 | 9.2 | 0.12 | 644 | 3 | 4 | No growth | GBS | No growth |
| 22 | 0–5 | 6.7 | 0.20 | 1146 | 1024 | 46 305 | Contaminant | Streptococcus gallolyticus | S gallolyticus |
| 24 | 0–5 | 11.8 | 0 | 0 | 6 | 738 | No growth | GBS | No growth |
| 24 | 0–5 | 9.1 | 0 | 0 | 120 | 2585 | No growth | GBS | No growth |
| 28 | Not performedc | 11.4 | 0 | 0 | 0 | 81 500 | No growth | Enterobacter | No growth |
| Infants 29–60 d of aged | |||||||||
| 29 | 0–5 | 7.2 | 0.02 | 72 | Not obtained | Not obtained | No growth | GBS | No growth |
| 29 | 0–5 | 9.6 | 0 | 0 | 20 | 28 000 | No growth | Salmonella spp | No growth |
| 38 | 0–5 | 14.7 | 0 | 0 | 2 | 0 | No growth | GBS | No growth |
| 40 | Not performedc | 11.4 | 0 | 0 | 0 | 6750 | No growth | E faecalis | No growth |
| 41 | 0–5 | 6.5 | 0 | 0 | 4 | 0 | No growth | GBS | No growth |
| 47 | 0–5 | 9.4 | 0 | 0 | 1 | 1 | No growth | GBS | No growth |
| 50 | 0–5 | 10.4 | 0.10 | 830 | 1 | 13 | No growth | GBS | No growth |
| 50 | 0–5 | 9.6 | 0 | 0 | 4 | 1 | No growth | GBS | No growth |
| 53 | 0–5 | 6.7 | 0 | 0 | 4 | 36 | Contaminant | Salmonella spp | No growth |
| 55 | 0–5 | 11.1 | 0.15 | 1221 | 80 | 55 590 | No growth | GBS | No growth |
| 60 | 0–5 | 8.1 | 0 | 0 | 1 | 0 | No growth | E coli | No growth |
GBS, group B Streptococcus; RBC, red blood cell.
Bands-to-total neutrophil ratio.
Low risk per the Rochester criteria only.
Urine dipstick with negative leukocyte esterase and negative nitrites.
Low risk per the Rochester and modified Philadelphia criteria.
Of the 4 infants with IBI who had missing data and were classified as low risk per the Rochester criteria, 2 infants (ages 13 and 40 days) had group B streptococcal meningitis; both infants had a missing urinalysis. The 40-day-old infant had a peripheral WBC count of 15 000 cells per μL and an absolute band count of 1200 bands per μL, with an I/T ratio of 0.2. The 1 infant classified as low risk per the modified Philadelphia criteria was a 55-day-old infant with group B streptococcal bacteremia and mild CSF pleocytosis after a traumatic lumbar puncture; the infant was not treated for bacterial meningitis.
Discussion
In this validation study that included a large multicenter sample of febrile infants with IBI who did not appear ill, the modified Philadelphia criteria were highly sensitive in the risk stratification of infants with IBI. Importantly, no infants with bacterial meningitis were classified as low risk. Although the Rochester criteria had a similar sensitivity for IBI in febrile infants >28 days of age, 2 infants ≤28 days of age with bacterial meningitis were classified as low risk. Our findings support the use of the modified Philadelphia criteria without routine CSF testing for febrile infants in the second month of life.
Although only 0.2% of febrile infants 29 to 60 days of age who present to the ED have bacterial meningitis,6 many pediatric emergency medicine clinicians routinely perform CSF testing in this age group because of the high rate of neurologic sequelae and mortality associated with this condition.25,26 However, performance of routine CSF testing in this age group is associated with higher costs, increased hospitalization rates for otherwise low-risk infants, and significant stress for parents, all without a reduction in adverse outcomes compared with selective CSF testing.10,13,27,28 Therefore, some have questioned the need for routine CSF testing in this age group. However, previous investigations in which the Rochester and modified Philadelphia criteria were evaluated included few infants with bacterial meningitis who did not appear ill.4,8,9,23
Our results inform this important issue by revealing that the modified Philadelphia criteria had high sensitivity for IBI and classified all infants with bacterial meningitis as high risk without routine CSF testing. Although 11 infants 29 to 60 days of age were classified as low risk by the modified Philadelphia criteria, all the infants had bacteremia without meningitis, and the 2 infants with CSF pleocytosis had traumatic lumbar punctures. Additionally, we excluded 26 febrile infants with bacterial meningitis who appeared ill, all of whom would have been classified as high risk by the modified Philadelphia criteria because of their ill appearance.9
The overall prevalence of IBI has been reported to be 2% in febrile infants.6 Therefore, among the thousands of febrile infants evaluated in the ED, few infants with IBI will be missed with use of these criteria. For instance, of 300 febrile infants >28 days of age who do not appear ill, 6 (2%) will have an IBI, and 1 of these 6 infants will be classified as low risk by the modified Philadelphia criteria. Therefore, only 1 of 300 febrile infants (0.3%) who do not appear ill will have an IBI, specifically with bacteremia, that will be missed with use of these criteria. Although the Rochester criteria had a similar sensitivity for IBI among infants 29 to 60 days of age, when including infants with missing data, 1 infant with bacterial meningitis in this age group was classified as low risk; our results favor the use of the modified Philadelphia criteria. However, ultimately, clinicians must balance the rarity of bacterial meningitis in febrile infants >28 days of age who do not appear ill and the risks of a lumbar puncture27,28 with the potential for serious neurologic sequelae or death if treatment is delayed.25,26 For infants treated with empirical antimicrobial therapy without performance of CSF testing, there is potential for prolonged antimicrobial therapy if the blood culture grows a pathogen and CSF pleocytosis is present on a subsequent lumbar puncture. Additionally, although infection with herpes simplex virus is rare among febrile infants >28 days age,29,30 CSF testing should be obtained in this age group if herpes simplex virus is suspected (eg, presence of vesicles or seizures).31
The prevalence of IBI is highest among febrile infants ≤28 days of age, and 1% of these infants will have bacterial meningitis.6 Although most infants in the first month of life who are classified as low risk without CSF testing will not have an IBI, 2 infants with meningitis in our study would have been missed by the Rochester criteria. Although a recently validated procalcitonin-based, low-risk algorithm that also does not include routine CSF testing (the step-by-step approach) uses an age cutoff of 21 days instead of 28 days,32 in our study, 3 infants with IBI, including 1 with bacterial meningitis, were between 22 and 28 days of age and were classified as low risk per the Rochester criteria. Therefore, caution should be exercised in applying these criteria to febrile infants ≤28 days of age.
The sensitivity and specificity of the modified Philadelphia criteria were similar to those of the step-by-step approach.32 Because procalcitonin is not currently available at some US hospitals, the modified Philadelphia criteria can be more widely implemented for the risk stratification of febrile infants. However, these criteria should be prospectively tested against the step-by-step approach in a large cohort of febrile infants. Additionally, although favorable outcomes have been reported for infants managed as outpatients with the step-by-step approach,33 future investigation is needed to evaluate outcomes with use of the modified Philadelphia criteria.
Our study has several limitations. First, data were collected through a medical record review, and clinical variables, such as clinical appearance, may not be accurately documented. However, we used a previously utilized definition of ill appearance, and we only included infants who were not ill appearing.17 Second, although our search strategy for identifying case patients made it unlikely that we missed infants with IBI, we were unable to calculate the positive and negative predictive values of the Rochester and modified Philadelphia criteria because we did not include all febrile infants at study sites. Third, only 17 infants in our sample had bacterial meningitis, reflecting the low prevalence of this condition among infants who do not appear ill. However, our study included the largest sample to date of febrile infants ≤60 days of age with IBI who did not appear ill. Additionally, although we excluded 26 infants with bacterial meningitis who appeared ill, all of these infants would have been classified as high risk by both the Rochester and modified Philadelphia criteria.9,23 Fourth, although we reviewed subsequent visits for controls, we cannot exclude the possibility that a control was diagnosed with IBI at a nonparticipating hospital. Fifth, although we excluded infants with IBI who had missing documentation of any component of the Rochester or modified Philadelphia criteria, we included these infants in a sensitivity analysis. Sixth, the band count was recorded as 0 if only an automated differential was performed on the complete blood count, and no bands were reported. Manual differentials are triggered reflexively by a hospital’s automated hematology analyzer or on request from a clinician. It is therefore possible that some low-risk infants with IBI and 0 bands would have been classified as high risk if a manual differential had been performed, which may have resulted in an underestimation of the sensitivity of the criteria. Lastly, we only included infants who presented to EDs at participating children’s hospitals, and our results may not be generalizable to other settings, particularly community-based EDs.
Conclusions
The modified Philadelphia criteria, which does not include routine CSF testing, classifies most febrile infants with IBI as high risk. Because a few infants >28 days of age with bacteremia were classified as low risk, febrile infants discharged from the ED without CSF testing should have close outpatient follow-up. Caution should be exercised in applying low risk criteria to infants ≤28 days of age. A prospective study is needed to confirm the safety of routinely omitting CSF testing in low-risk febrile infants >28 days of age.
Acknowledgments
We thank the following collaborators in the Febrile Young Infant Research Collaborative for their contributions as group authors for this study: Elizabeth R. Alpern, MD, MSCE, Northwestern University Feinberg School of Medicine (Chicago, IL); Whitney L. Browning, MD, Vanderbilt University School of Medicine (Nashville, TN); Elana A. Feldman, MD, Lucile Packard Children’s Hospital Stanford (Palo Alto, CA); Katie L. Hayes, BS, Children’s Hospital of Philadelphia (Philadelphia, PA); Catherine E. Lumb, BS, University of Alabama School of Medicine (Birmingham, AL); Christine E. Mitchell, BSN, Children’s Hospital of Philadelphia (Philadelphia, PA); Nipam Shah, MBBS, MPH, University of Alabama at Birmingham (Birmingham, AL); Sarah J. Shin, BSN, Children’s Hospital of Philadelphia (Philadelphia, PA); and Derek J. Williams, MD, MPH, Vanderbilt University School of Medicine (Nashville, TN).
Glossary
- CI
confidence interval
- CSF
cerebrospinal fluid
- ED
emergency department
- HPF
high-power field
- IBI
invasive bacterial infection
- I/T
immature-to-total
- UTI
urinary tract infection
- WBC
white blood cell
Footnotes
Dr Aronson conceptualized and designed the study, supervised data collection locally and nationally, performed the data analyses, interpreted the data, drafted the initial manuscript, and reviewed and revised the manuscript critically for important intellectual content; Dr Wang contributed to the design of the study, collected local data, interpreted the data, and reviewed and revised the manuscript critically for important intellectual content; Drs Shapiro and Shah contributed to the design of the study, interpreted the data, and reviewed and revised the manuscript critically for important intellectual content; Drs DePorre, McCulloh, Pruitt, Desai, Nigrovic, Marble, Leazer, Rooholamini, Sartori, Balamuth, and Woll collected local data, interpreted the data, and reviewed and revised the manuscript critically for important intellectual content; Dr Neuman contributed to the conceptualization and design of the study, collected local data, interpreted the data, and reviewed and revised the manuscript critically for important intellectual content; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported, in part, by Clinical and Translational Science Awards grants KL2 TR001862 (to Drs Aronson and Shapiro) and UL1TR0001863 (to Dr Shapiro) from the National Center for Advancing Translational Science, a component of the National Institutes of Health. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Shapiro has served as an expert witness in malpractice cases involving the evaluation of febrile children; the other authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found online at www.pediatrics.org/cgi/doi/10.1542/peds.2018-2861.
References
- 1.Huppler AR, Eickhoff JC, Wald ER. Performance of low-risk criteria in the evaluation of young infants with fever: review of the literature. Pediatrics. 2010;125(2):228–233 [DOI] [PubMed] [Google Scholar]
- 2.Dagan R, Powell KR, Hall CB, Menegus MA. Identification of infants unlikely to have serious bacterial infection although hospitalized for suspected sepsis. J Pediatr. 1985;107(6):855–860 [DOI] [PubMed] [Google Scholar]
- 3.Baker MD, Bell LM, Avner JR. Outpatient management without antibiotics of fever in selected infants. N Engl J Med. 1993;329(20):1437–1441 [DOI] [PubMed] [Google Scholar]
- 4.Hui C, Neto G, Tsertsvadze A, et al. Diagnosis and management of febrile infants (0-3 months). Evid Rep Technol Assess (Full Rep). 2012;(205):1–297 [PMC free article] [PubMed] [Google Scholar]
- 5.Meehan WP III, Fleegler E, Bachur RG. Adherence to guidelines for managing the well-appearing febrile infant: assessment using a case-based, interactive survey. Pediatr Emerg Care. 2010;26(12):875–880 [DOI] [PubMed] [Google Scholar]
- 6.Powell EC, Mahajan PV, Roosevelt G, et al. ; Febrile Infant Working Group of the Pediatric Emergency Care Applied Research Network (PECARN) . Epidemiology of bacteremia in febrile infants aged 60 days and younger. Ann Emerg Med. 2018;71(2):211–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woll C, Neuman MI, Pruitt CM, et al. ; Febrile Young Infant Research Collaborative . Epidemiology and etiology of invasive bacterial infection in infants ≤60 days old treated in emergency departments. J Pediatr. 2018;200:210–217.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garra G, Cunningham SJ, Crain EF. Reappraisal of criteria used to predict serious bacterial illness in febrile infants less than 8 weeks of age. Acad Emerg Med. 2005;12(10):921–925 [DOI] [PubMed] [Google Scholar]
- 9.Scarfone R, Murray A, Gala P, Balamuth F. Lumbar puncture for all febrile infants 29-56 days old: a retrospective cohort reassessment study. J Pediatr. 2017;187:200–205.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chua KP, Neuman MI, McWilliams JM, Aronson PL; Febrile Young Infant Research Collaborative . Association between clinical outcomes and hospital guidelines for cerebrospinal fluid testing in febrile infants aged 29-56 days. J Pediatr. 2015;167(6):1340–1346.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aronson PL, McCulloh RJ, Tieder JS, et al. ; Febrile Young Infant Research Collaborative . Application of the Rochester criteria to identify febrile infants with bacteremia and meningitis [published online ahead of print February 5, 2018]. Pediatr Emerg Care. doi: 10.1097/PEC.0000000000001421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aronson PL, Thurm C, Alpern ER, et al. ; Febrile Young Infant Research Collaborative . Variation in care of the febrile young infant <90 days in US pediatric emergency departments [published correction appears in Pediatrics. 2015;135(4):775]. Pediatrics. 2014;134(4):667–677 [DOI] [PubMed] [Google Scholar]
- 13.Aronson PL, Thurm C, Williams DJ, et al. ; Febrile Young Infant Research Collaborative . Association of clinical practice guidelines with emergency department management of febrile infants ≤56 days of age. J Hosp Med. 2015;10(6):358–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biondi E, Evans R, Mischler M, et al. Epidemiology of bacteremia in febrile infants in the United States. Pediatrics. 2013;132(6):990–996 [DOI] [PubMed] [Google Scholar]
- 15.Leazer R, Erickson N, Paulson J, et al. Epidemiology of cerebrospinal fluid cultures and time to detection in term infants. Pediatrics. 2017;139(5):e20163268. [DOI] [PubMed] [Google Scholar]
- 16.Kestenbaum LA, Ebberson J, Zorc JJ, Hodinka RL, Shah SS. Defining cerebrospinal fluid white blood cell count reference values in neonates and young infants. Pediatrics. 2010;125(2):257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baskin MN, Goh XL, Heeney MM, Harper MB. Bacteremia risk and outpatient management of febrile patients with sickle cell disease. Pediatrics. 2013;131(6):1035–1041 [DOI] [PubMed] [Google Scholar]
- 18.Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6). Available at: www.pediatrics.org/cgi/content/full/107/6/e99 [DOI] [PubMed] [Google Scholar]
- 19.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tzimenatos L, Mahajan P, Dayan PS, et al. ; Pediatric Emergency Care Applied Research Network (PECARN) . Accuracy of the urinalysis for urinary tract infections in febrile infants 60 days and younger. Pediatrics. 2018;141(2):e20173068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schroeder AR, Newman TB, Wasserman RC, Finch SA, Pantell RH. Choice of urine collection methods for the diagnosis of urinary tract infection in young, febrile infants. Arch Pediatr Adolesc Med. 2005;159(10):915–922 [DOI] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaskiewicz JA, McCarthy CA, Richardson AC, et al. ; Febrile Infant Collaborative Study Group . Febrile infants at low risk for serious bacterial infection–an appraisal of the Rochester criteria and implications for management. Pediatrics. 1994;94(3):390–396 [PubMed] [Google Scholar]
- 24.Guarner J, Atuan MA, Nix B, et al. Process to evaluate hematological parameters that reflex to manual differential cell counts in a pediatric institution. Clin Lab. 2010;56(1–2):21–27 [PubMed] [Google Scholar]
- 25.Lebel MH, McCracken GH Jr. Delayed cerebrospinal fluid sterilization and adverse outcome of bacterial meningitis in infants and children. Pediatrics. 1989;83(2):161–167 [PubMed] [Google Scholar]
- 26.Okike IO, Johnson AP, Henderson KL, et al. ; neoMen Study Group . Incidence, etiology, and outcome of bacterial meningitis in infants aged <90 days in the United Kingdom and Republic of Ireland: prospective, enhanced, national population-based surveillance. Clin Infect Dis. 2014;59(10):e150–e157 [DOI] [PubMed] [Google Scholar]
- 27.Pingree EW, Kimia AA, Nigrovic LE. The effect of traumatic lumbar puncture on hospitalization rate for febrile infants 28 to 60 days of age. Acad Emerg Med. 2015;22(2):240–243 [DOI] [PubMed] [Google Scholar]
- 28.Paxton RD, Byington CL. An examination of the unintended consequences of the rule-out sepsis evaluation: a parental perspective. Clin Pediatr (Phila). 2001;40(2):71–77 [DOI] [PubMed] [Google Scholar]
- 29.Long SS, Pool TE, Vodzak J, Daskalaki I, Gould JM. Herpes simplex virus infection in young infants during 2 decades of empiric acyclovir therapy. Pediatr Infect Dis J. 2011;30(7):556–561 [DOI] [PubMed] [Google Scholar]
- 30.Cruz AT, Freedman SB, Kulik DM, et al. ; HSV Study Group of the Pediatric Emergency Medicine Collaborative Research Committee . Herpes simplex virus infection in infants undergoing meningitis evaluation. Pediatrics. 2018;141(2):e20171688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimberlin DW, Lin CY, Jacobs RF, et al. ; National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group . Natural history of neonatal herpes simplex virus infections in the acyclovir era. Pediatrics. 2001;108(2):223–229 [DOI] [PubMed] [Google Scholar]
- 32.Gomez B, Mintegi S, Bressan S, Da Dalt L, Gervaix A, Lacroix L; European Group for Validation of the Step-by-Step Approach . Validation of the “Step-by-Step” approach in the management of young febrile infants. Pediatrics. 2016;138(2):e20154381. [DOI] [PubMed] [Google Scholar]
- 33.Mintegi S, Gomez B, Martinez-Virumbrales L, Morientes O, Benito J. Outpatient management of selected young febrile infants without antibiotics. Arch Dis Child. 2017;102(3):244–249 [DOI] [PubMed] [Google Scholar]