A 2015–2016 population-based, cross-sectional survey was administered to a sample of US households to estimate current FA prevalence, severity, and health care use.
Abstract
Video Abstract
BACKGROUND:
Childhood food allergy (FA) is a life-threatening chronic condition that substantially impairs quality of life. This large, population-based survey estimates childhood FA prevalence and severity of all major allergenic foods. Detailed allergen-specific information was also collected regarding FA management and health care use.
METHODS:
A survey was administered to US households between 2015 and 2016, obtaining parent-proxy responses for 38 408 children. Prevalence estimates were based on responses from NORC at the University of Chicago’s nationally representative, probability-based AmeriSpeak Panel (51% completion rate), which were augmented by nonprobability-based responses via calibration weighting to increase precision. Prevalence was estimated via weighted proportions. Multiple logistic regression models were used to evaluate FA predictors.
RESULTS:
Overall, estimated current FA prevalence was 7.6% (95% confidence interval: 7.1%–8.1%) after excluding 4% of children whose parent-reported FA reaction history was inconsistent with immunoglobulin E–mediated FA. The most prevalent allergens were peanut (2.2%), milk (1.9%), shellfish (1.3%), and tree nut (1.2%). Among food-allergic children, 42.3% reported ≥1 severe FA and 39.9% reported multiple FA. Furthermore, 19.0% reported ≥1 FA-related emergency department visit in the previous year and 42.0% reported ≥1 lifetime FA-related emergency department visit, whereas 40.7% had a current epinephrine autoinjector prescription. Prevalence rates were higher among African American children and children with atopic comorbidities.
CONCLUSIONS:
FA is a major public health concern, affecting ∼8% of US children. However, >11% of children were perceived as food-allergic, suggesting that the perceived disease burden may be greater than previously acknowledged.
What’s Known on This Subject:
In 2011, food allergy (FA) was estimated to impact 8% of US children, of which nearly 40% reported a history of a severe reaction. Among specific FAs, prevalence was highest for peanut, followed by milk, shellfish, and tree nut.
What This Study Adds:
In this study, we provide updated national FA prevalence estimates and characterization of related health care use, including epinephrine prescription and emergency department visits. FA remains a substantial public health concern, with a greater perceived disease burden than previously anticipated.
Childhood food allergy (FA) is a serious,1,2 potentially life-threatening3 condition known to substantially impair quality of life among patients and their caregivers.4 Because food is integral to most social interactions, children with FA may be at risk for a severe allergic reaction at any time. Childhood FA also imposes considerable financial burden on affected families, with an estimated annual economic impact of $24.8 billion ($4184 per year per child).5 We concluded from a US population–based survey conducted by our group in 2009–2010 that childhood FA may be more prevalent and severe than previously acknowledged.6
Since 2010, numerous review articles have suggested that the population-level burden of childhood FA is growing and may be historically high.7–11 For example, the authors of a recent US study described a nearly 200% increase in food-induced anaphylaxis-related emergency department (ED) visits from 2005 to 201412 among 5 to 17-year-olds. With this growing epidemic and life-threatening nature of FAs, developing treatments and prevention strategies are critical. A recent study revealed that early introduction of peanut products may prevent peanut allergy,13 and peanut immunotherapy treatments are showing promise in phase 3 trials.14 Understanding reported prevalence, types of FA, associated symptoms and severity, diagnosis and management practices, and determinants of FA is critical for clinicians, researchers, and policymakers in their efforts to address this important public health issue.
With this study, we aim to describe the public health impact of childhood FA by studying a large, nationally representative sample of US households with children. We collected parent proxy-report data on FA prevalence, symptomatology, and health care use, both overall and for many specific FAs. Furthermore, by assessing rates of epinephrine autoinjector (EAI) possession and use, as well as FA-related ED visits, the present survey provides the most comprehensive assessment of pediatric FA severity and population-level burden to date.
Methods
A population-based survey was administered between October 2015 and September 2016 to a sample of US households. Informed consent was obtained from all participants. The Northwestern University Institutional Review Board approved all study activities.
Survey Development and Design
The present parent-report survey extended our 2009–2010 survey, which was developed by pediatricians, pediatric allergists, and survey methodologists with support from an expert panel. Expert panel review and key informant cognitive interviews (N = 40) were conducted on the original survey by using the approach described by Gupta et al.15 While the core 2009–2010 survey was kept intact, additional questions were added to the present instrument to assess emerging research issues relating to the etiology and management of FA. The revised instrument was pretested on 345 pilot interviewees. Interviewee data and feedback were reviewed and incorporated into the final 2015–2016 survey.
Outcome Measures
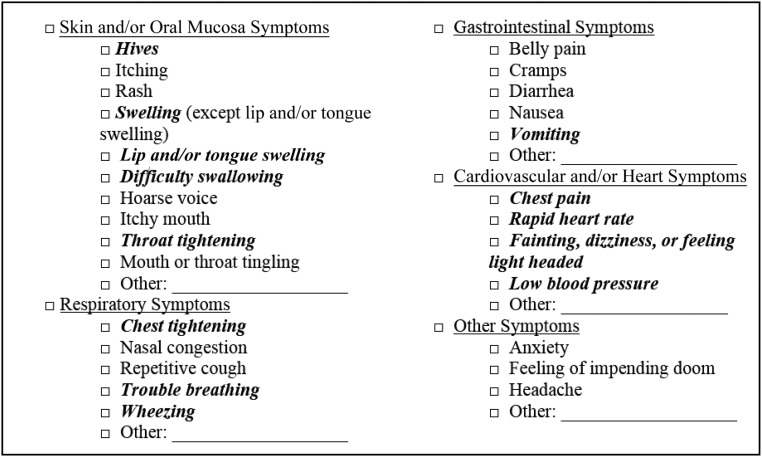
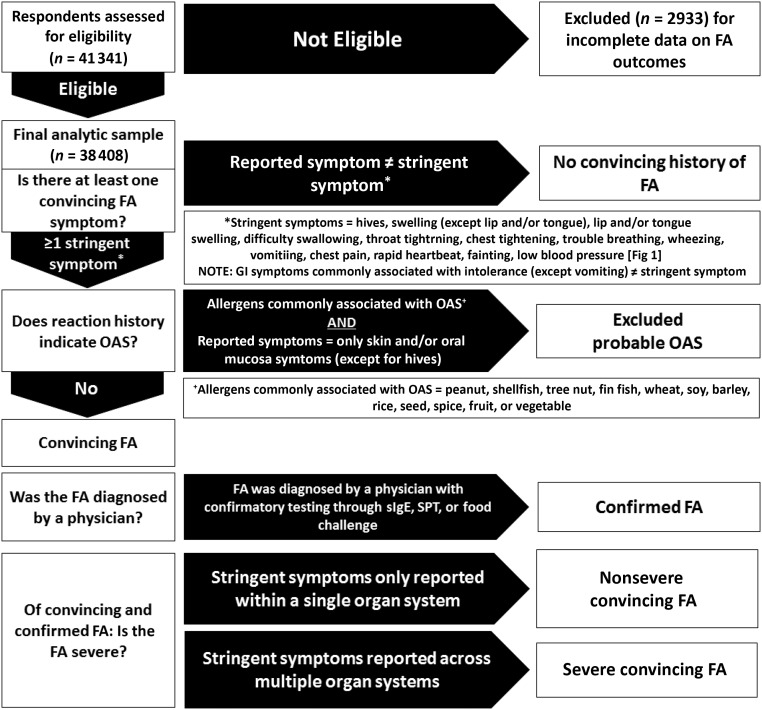
The primary outcome measure for the current study was the prevalence of overall and food-specific convincing childhood FA. Parent-reported FAs were considered convincing if the most severe reaction reported to that food included at least 1 symptom on the stringent symptom list developed by our expert panel (Fig 1). Parent-reported allergies with reaction symptoms characteristic of oral allergy syndrome (OAS) or food intolerances were not considered convincing and were categorized per the FA flowchart summarized in Fig 2, even if such allergies were physician diagnosed. For instance, individual allergies to peanut, shellfish, tree nut, fin fish, wheat, soy, barley, rice, seed, spice, fruit, or vegetables were considered indicative of OAS instead of convincing FA if their corresponding reaction symptoms were limited to the skin or oral mucosa and did not include hives. Convincing FAs for which parents reported a doctor’s diagnosis were considered physician-confirmed FAs. For each convincing allergy, a severe reaction history was indicated by the presence of multiple stringent symptoms occurring within 2 or more of the following 4 organ systems: skin or oral mucosa, gastrointestinal, cardiovascular, and respiratory. If multiple allergies were reported for a given child, each reported allergy was evaluated separately via the FA categorization flowchart. For example, if a parent reported their child had a nut allergy with a reaction history limited to oral symptoms indicative of OAS, as well as a shellfish allergy with a reaction history including throat tightening, vomiting, and hives, the child would be considered to have a single, severe shellfish allergy.
FIGURE 1.
List of allergic reaction symptoms highlighting stringent symptoms indicative of convincing FA. All symptoms lister are offered as answer choices in the survey. Symptoms in bold italics comprised our expert panel’s stringent symptom list. A convincing FA required the patient report of at least 1 stringent symptom during a child’s most severe reaction to a given food. A severe reaction consisted of a parent report of at least 2 stringent symptoms from 2 different body systems during a child’s most severe reaction to a given food.
FIGURE 2.
Convincing, physician-confirmed, and severe childhood FA categorization flowchart. GI, gastrointestinal; SPT, skin prick test; sIgE, allergen-specific immunoglobulin E.
Study Participants, Survey Weighting, and Statistical Analysis
Eligible study participants included adults (≥18 years old) able to complete the survey in English or Spanish via Web or telephone who resided in a US household. As in the 2009–2010 survey, this study relied on a nationally representative household panel to support population-level inference.6 Study participants were first recruited from the NORC at the University of Chicago’s probability-based AmeriSpeak Panel, where a survey completion rate of 51.2% was observed (7218 responses of 14 095 invitees). Each child was assigned a base, study-specific sampling weight equal to their responding parent’s nonresponse-adjusted AmeriSpeak sampling weight. Parental weights were reconciled with external population totals associated with age, sex, education, race and/or ethnicity, housing tenure, telephone status, and census division via iterative proportional fitting to improve external validity. Child-specific weights were further adjusted to account for random selection of up to 3 children or households and raked to external pediatric population totals. To increase precision of estimates where data are scarce (such as for the prevalence of rare allergies within specific age groups) data gleaned from population-weighted AmeriSpeak responses were augmented with calibration-weighted, nonprobability-based responses obtained through Survey Sampling International (SSI). Here, state-of-the-art estimation methods were used to minimize both the bias and variance of resulting estimates to a greater degree than independent analysis of either sample permits. The final, combined sample weight was derived by applying an optimal composition factor that minimizes mean square error associated with FA prevalence estimates.16,17 In total, surveys were completed by 51 819 US households. Participants received $5 at survey completion.
Weighted proportions were calculated to estimate prevalence. Covariate-adjusted weighted logistic regression models compared relative prevalence by sample characteristics. Robust SEs accounted for household-level clustering.
Results
Parent-reported data were collected for 41 341 children; 2933 children were excluded because of incomplete data on FA outcomes. Sociodemographic characteristics of the omitted children did not significantly differ from the final analytic sample of 38 408 children.
Demographic Characteristics
Half (51.1%) of the population-weighted sample was male. Race and/or ethnicity was mutually exclusive, with 52.8% white, non-Hispanic; 24.1% Hispanic, 13.2% African American, non-Hispanic; and 3.2% Asian American, non-Hispanic (Table 1). Rates of physician-diagnosed atopic conditions were significantly higher (P < .05) among children with convincing FA compared with other children.
TABLE 1.
Demographic Distribution of Current Childhood FA Versus All Children
| Variable | Population-Weighted Frequency % (95% CI) | ||
|---|---|---|---|
| All Children | Children With FA | P | |
| Race and/or ethnicity | |||
| Asian American, non-Hispanic | 3.2 (2.8–3.8) | 2.8 (2.2–3.5) | .20 |
| African American, non-Hispanic | 13.2 (12.3–14.2) | 15.4 (13.1–18.1) | .06 |
| White, non-Hispanic | 52.8 (51.2–54.4)* | 48.3 (45.0–51.7)* | <.01* |
| Hispanic | 24.1 (22.5–25.7) | 26.5 (23.2–30.0) | .14 |
| Multiracial or other | 6.6 (6.1–7.3) | 7.1 (5.7–8.7) | .56 |
| Sex | |||
| Female | 48.9 (47.8–50.0) | 48.2 (45.0–51.5) | .68 |
| Male | 51.1 (50.0–52.2) | 51.8 (48.6–55.0) | |
| Age, y | |||
| 0 | 5.3 (4.8–5.9)* | 1.9 (1.5–2.5)* | <.001* |
| 1 | 4.9 (4.4–5.3) | 5.6 (4.3–7.3) | .28 |
| 2 | 5.7 (5.3–6.3) | 7.5 (5.4–10.4) | .09 |
| 3–5 | 16.2 (15.5–17.0) | 17.8 (15.3–20.5) | .22 |
| 6–10 | 27.9 (26.9–28.8) | 29.1 (26.5–31.9) | .34 |
| 11–13 | 16.6 (15.9–17.4) | 16.5 (14.3–18.9) | .88 |
| 14–17 | 23.4 (22.4–24.4) | 21.6 (19.3–24.2) | .17 |
| Household income, $ | |||
| <25 000 | 16.1 (14.9–17.3) | 15.4 (13.0–18.1) | .55 |
| 25 000–49 999 | 22.2 (20.9–23.5) | 23.2 (20.6–26.1) | .43 |
| 50 000–99 999 | 31.1 (29.8–32.5) | 31.5 (28.6–34.5) | .82 |
| 100 000–149 999 | 19.2 (18–20.5) | 20.4 (17.4–23.7) | .40 |
| >150 000 | 11.4 (10.3–12.6) | 9.6 (7.6–11.9) | .12 |
| Geographic region | |||
| West | 24.4 (22.9–25.9) | 22.4 (19.7–25.4) | .17 |
| Midwest | 20.6 (19.5–21.7) | 19.1 (16.8–21.7) | .23 |
| South | 38.4 (36.9–40.0) | 39.9 (36.6–43.2) | .37 |
| Northeast | 16.1 (15.0–17.2) | 17.7 (15.1–20.7) | .21 |
| Physician-diagnosed comorbid conditions | |||
| Asthma | 12.2 (11.4–13.0)* | 32.6 (29.5–35.9)* | <.001* |
| Atopic dermatitis and/or eczema | 5.9 (5.3–6.5)* | 14.9 (12.5–17.7)* | <.001* |
| Eosinophilic esophagitis | 0.2 (0.1–0.2)* | 0.7 (0.4–1.1)* | <.001* |
| Allergic rhinitis | 12.8 (12.0–13.6)* | 30.4 (27.6–33.4)* | <.001* |
| Insect sting allergy | 2.2 (1.9–2.6)* | 6.4 (5.3–7.8)* | <.001* |
| Latex allergy | 1.0 (0.8–1.3)* | 6.6 (4.8–9.0)* | <.001* |
| Medication allergy | 4.2 (3.7–4.7)* | 10.1 (8.2–12.3)* | <.001* |
| Urticaria and/or chronic hives | 0.5 (0.4–0.6)* | 1.9 (1.4–2.6)* | <.001* |
| Other chronic condition | 3.2 (2.8–3.7)* | 7.2 (5.9–8.9)* | <.001* |
Two-sided P < .05
Prevalence
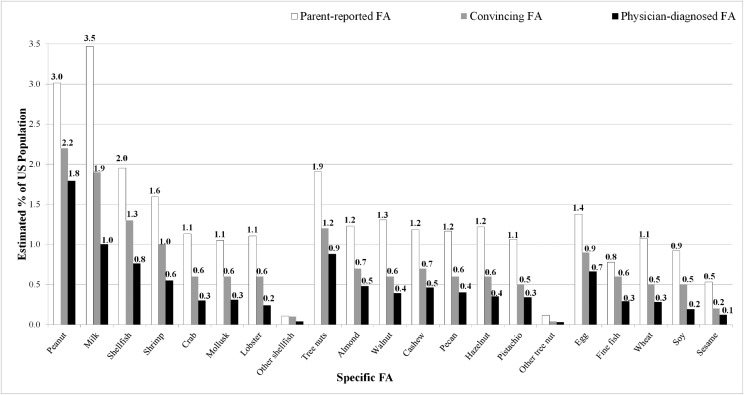
The estimated prevalence of childhood FA was 7.6% (95% confidence interval [CI]: 7.1–8.1) (Table 2), with 40% of children with FA reporting multiple FAs. Peanut (2.2%) and milk (1.9%) allergies were the most common food-specific FAs. When treated as single allergens, shellfish (1.3%), and tree nut (1.2%) were the next most common allergens, followed by egg (0.9%) and fin fish (0.6%). Among specific shellfish allergies, shrimp (1.0%) was most prevalent followed by crab (0.6%), mollusks (0.6%), and lobster (0.6%). Among specific tree nuts, rates of almond (0.7%), cashew (0.7%), walnut (0.6%), pecan (0.6%), and hazelnut (0.6%) allergies were similar. Sesame allergy prevalence was estimated at 0.2%. An estimated 61.1% of children with convincing FA had at least 1 physician-confirmed FA. Overall, 11.4% of children's caregivers reported a current FA before stringent symptom criteria were used to filter out those lacking a convincing history of IgE-mediated FA.
TABLE 2.
Prevalence of Most Common FAs by Age
| All Children | <1 y | 1 y | 2 y | 3–5 y | 6–10 y | 11–13 y | 14–17 y | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Any FA | 7.6 | 7.1–8.1 | 2.8 | 2.1–3.7 | 8.8 | 6.7–11.4 | 10.0 | 7.2–13.7 | 8.3 | 7.1–9.8 | 8.0 | 7.2–8.9 | 7.5 | 6.5–8.8 | 7.1 | 6.3–7.9 |
| Peanut | 2.2 | 2.0–2.5 | 0.6 | 0.3–0.9 | 2.2 | 1.5–3.0 | 2.4 | 1.6–3.6 | 2.1 | 1.7–2.6 | 2.6 | 2.2–3.1 | 2.3 | 1.6–3.2 | 2.1 | 1.6–2.7 |
| Tree nut | 1.2 | 1.1–1.4 | 0.2 | 0.1–0.6 | 0.7 | 0.4–1.1 | 1.1 | 0.5–2.2 | 1.3 | 1.0–1.8 | 1.4 | 1.1–1.7 | 1.6 | 1.2–2.1 | 0.9 | 0.7–1.2 |
| Walnut | 0.6 | 0.5–0.8 | 0.1 | 0.0–0.3 | 0.1 | 0.1–0.3 | 0.6 | 0.2–1.8 | 0.6 | 0.4–1.0 | 1.0 | 0.7–1.3 | 0.7 | 0.5–1.0 | 0.4 | 0.3–0.6 |
| Almond | 0.7 | 0.6–0.8 | 0.2 | 0.1–0.5 | 0.4 | 0.2–0.7 | 0.8 | 0.3–2.0 | 0.7 | 0.5–1.0 | 0.8 | 0.6–1.0 | 0.7 | 0.5–0.9 | 0.6 | 0.5–0.9 |
| Hazelnut | 0.6 | 0.5–0.7 | 0.0 | 0.0–0.2 | 0.4 | 0.2–0.7 | 0.6 | 0.2–1.8 | 0.6 | 0.4–1.1 | 0.7 | 0.5–1.0 | 0.8 | 0.5–1.2 | 0.4 | 0.3–0.5 |
| Pecan | 0.6 | 0.5–0.7 | 0.0 | N/A | 0.4 | 0.2–0.7 | 0.7 | 0.2–1.9 | 0.5 | 0.4–0.8 | 0.8 | 0.6–1.0 | 0.7 | 0.5–1.0 | 0.5 | 0.4–0.8 |
| Cashew | 0.7 | 0.6–0.8 | 0.1 | 0.0–0.5 | 0.4 | 0.2–0.7 | 0.9 | 0.4–2.0 | 0.6 | 0.4–0.9 | 0.8 | 0.6–1.0 | 0.9 | 0.7–1.3 | 0.5 | 0.4–0.7 |
| Pistachio | 0.5 | 0.4–0.6 | 0.1 | 0.0–0.2 | 0.4 | 0.2–0.7 | 0.6 | 0.2–1.8 | 0.4 | 0.2–0.6 | 0.7 | 0.5–0.9 | 0.8 | 0.5–1.1 | 0.4 | 0.2–0.5 |
| Other tree nut | 0.0 | 0.0–0.1 | 0.0 | N/A | 0.0 | 0.0–0.2 | 0.0 | N/A | 0.1 | 0.0–0.4 | 0.0 | 0.0–0.1 | 0.1 | 0.0–0.1 | 0.0 | 0.0–0.1 |
| Milk | 1.9 | 1.7–2.3 | 1.5 | 0.9–2.3 | 3.3 | 2.1–5.2 | 4.3 | 2.2–8.3 | 2.8 | 2.1–3.8 | 1.9 | 1.5–2.5 | 1.1 | 0.8–1.6 | 1.1 | 0.9–1.4 |
| Shellfish | 1.3 | 1.1–1.5 | 0.2 | 0.1–0.5 | 0.4 | 0.3–0.8 | 1.1 | 0.6–2.2 | 1.1 | 0.7–1.7 | 1.5 | 1.1–1.9 | 1.5 | 1.2–1.9 | 1.5 | 1.1–2.0 |
| Shrimp | 1.0 | 0.8–1.1 | 0.0 | 0.0–0.2 | 0.3 | 0.1–0.5 | 0.7 | 0.3–1.6 | 0.9 | 0.5–1.4 | 1.1 | 0.9–1.5 | 1.2 | 0.9–1.5 | 1.1 | 0.9–1.4 |
| Lobster | 0.6 | 0.5–0.7 | 0.2 | 0.1–0.4 | 0.3 | 0.2–0.6 | 0.4 | 0.1–1.5 | 0.5 | 0.3–1.1 | 0.6 | 0.4–1.0 | 0.6 | 0.4–0.8 | 0.6 | 0.5–0.9 |
| Crab | 0.6 | 0.5–0.8 | 0.1 | 0.0–0.4 | 0.2 | 0.1–0.4 | 0.1 | 0.0–0.3 | 0.7 | 0.4–1.4 | 0.7 | 0.5–1.0 | 0.7 | 0.5–0.9 | 0.8 | 0.6–1.0 |
| Mollusk | 0.6 | 0.5–0.8 | 0.1 | 0.0–0.4 | 0.2 | 0.1–0.4 | 0.6 | 0.2–1.6 | 0.6 | 0.3–1.2 | 0.7 | 0.5–1.0 | 0.6 | 0.5–0.9 | 0.8 | 0.5–1.3 |
| Other shellfish | 0.1 | 0.0–0.1 | 0.0 | N/A | 0.0 | 0.0–0.2 | 0.2 | 0.0–1.3 | 0.0 | 0.0–0.1 | 0.1 | 0.0–0.2 | 0.1 | 0.0–0.2 | 0.1 | 0.0–0.2 |
| Egg | 0.9 | 0.7–1.1 | 0.4 | 0.2–0.7 | 2.0 | 1.0–4.1 | 1.4 | 0.8–2.4 | 1.3 | 0.8–1.9 | 0.9 | 0.6–1.3 | 1.0 | 0.7–1.4 | 0.5 | 0.3–0.7 |
| Fin fish | 0.6 | 0.4–0.9 | 0.1 | 0.0–0.2 | 0.6 | 0.2–1.3 | 0.6 | 0.2–1.6 | 0.5 | 0.2–1.1 | 0.6 | 0.4–0.9 | 0.5 | 0.4–0.8 | 0.6 | 0.4–0.9 |
| Wheat | 0.5 | 0.4–0.7 | 0.4 | 0.2–1.1 | 0.5 | 0.2–1.3 | 1.0 | 0.4–2.2 | 0.5 | 0.2–1.5 | 0.5 | 0.3–0.8 | 0.5 | 0.3–0.7 | 0.4 | 0.3–0.6 |
| Soy | 0.5 | 0.4–0.6 | 0.4 | 0.2–0.8 | 1.5 | 0.5–3.9 | 0.9 | 0.4–1.9 | 0.6 | 0.2–1.6 | 0.5 | 0.3–0.8 | 0.3 | 0.2–0.5 | 0.2 | 0.1–0.3 |
| Sesame | 0.2 | 0.2–0.3 | 0.1 | 0.1–0.3 | 0.4 | 0.2–1.1 | 0.2 | 0.0–1.2 | 0.2 | 0.1–0.4 | 0.3 | 0.2–0.4 | 0.1 | 0.1–0.3 | 0.1 | 0.1–0.3 |
| Prevalence of specific allergies among children with FA | ||||||||||||||||
| Peanut | 29.0 | 26.3–32.0 | 20.2 | 12.1–31.6 | 24.6 | 16.8–34.5 | 24.5 | 15.2–37.1 | 25.1 | 20.0–31.1 | 32.8 | 28.2–37.8 | 30.5 | 23.1–39.1 | 29.5 | 23.8–36.1 |
| Tree nut | 15.8 | 13.9–17.9 | 9.0 | 4.0–18.9 | 8.0 | 4.8–13.1 | 10.9 | 5.3–21.3 | 15.9 | 11.6–21.5 | 17.6 | 14.3–21.6 | 21.3 | 16.3–27.4 | 13.3 | 10.4–17.0 |
| Walnut | 8.3 | 7.0–9.9 | 3.9 | 1.3–11.0 | 1.6 | 0.6–4.0 | 6.1 | 2.0–17.0 | 7.2 | 4.6–11.1 | 12.3 | 9.3–16.0 | 9.1 | 6.5–12.6 | 6.2 | 4.5–8.6 |
| Almond | 8.7 | 7.5–10.2 | 5.6 | 1.9–15.5 | 4.6 | 2.5–8.6 | 8.2 | 3.3–18.9 | 8.1 | 5.6–11.6 | 9.9 | 7.6–12.8 | 9.1 | 6.6–12.5 | 8.9 | 6.5–12.2 |
| Hazelnut | 7.7 | 6.3–9.4 | 1.6 | 0.4–6.5 | 4.4 | 2.4–8.1 | 6.4 | 2.2–17.4 | 7.5 | 4.4–12.6 | 9.4 | 6.8–12.9 | 10.2 | 6.6–15.5 | 5.5 | 3.9–7.8 |
| Pecan | 7.9 | 6.6–9.4 | 0.0 | N/A | 4.5 | 2.3–8.4 | 6.8 | 2.4–17.8 | 6.5 | 4.3–9.8 | 9.8 | 7.4–12.8 | 9.3 | 6.6–13.0 | 7.5 | 5.1–10.7 |
| Cashew | 8.6 | 7.3–10.1 | 3.3 | 0.6–15.6 | 4.0 | 2.1–7.6 | 8.6 | 3.7–19.0 | 7.4 | 5.1–10.5 | 9.5 | 7.2–12.5 | 12.2 | 8.7–16.8 | 7.4 | 5.4–10.0 |
| Pistachio | 6.7 | 5.5–8.1 | 2.3 | 0.7–7.2 | 4.4 | 2.2–8.5 | 6.2 | 2.1–17.1 | 4.6 | 2.8–7.3 | 8.3 | 6.0–11.2 | 10.0 | 6.8–14.5 | 5.0 | 3.5–7.2 |
| Other tree nut | 0.5 | 0.2–0.9 | 0.0 | N/A | 0.3 | 0.0–2.4 | 0.0 | N/A | 1.0 | 0.2–5.1 | 0.3 | 0.1–0.7 | 0.8 | 0.3–1.7 | 0.4 | 0.2–0.8 |
| Milk | 25.4 | 22.2–28.8 | 53.0 | 39.7–65.8 | 37.8 | 25.4–52.0 | 43.5 | 26.6–62.1 | 33.6 | 25.9–42.2 | 24.4 | 19.5–30.0 | 14.9 | 10.8–20.2 | 16.0 | 12.6–20.1 |
| Shellfish | 16.9 | 14.8–19.2 | 7.1 | 3.0–16.0 | 5.1 | 2.8–8.9 | 11.5 | 5.8–21.5 | 13.0 | 8.4–19.6 | 18.4 | 14.6–22.9 | 20.2 | 16.0–25.2 | 21.3 | 16.7–26.8 |
| Shrimp | 12.6 | 10.9–14.5 | 1.6 | 0.4–5.4 | 3.0 | 1.5–5.7 | 7.1 | 3.0–16.1 | 10.2 | 6.4–16.1 | 14.3 | 10.9–18.4 | 15.4 | 11.8–20.0 | 15.6 | 12.4–19.4 |
| Lobster | 7.4 | 6.0–9.0 | 5.8 | 2.1–15.1 | 3.5 | 1.7–6.9 | 4.2 | 1.1–14.5 | 6.4 | 3.1–12.8 | 8.1 | 5.5–11.6 | 8.1 | 5.8–11.3 | 9.0 | 6.6–12.1 |
| Crab | 8.2 | 6.8–9.9 | 4.7 | 1.4–14.6 | 2.2 | 1.1–4.7 | 1.1 | 0.4–2.7 | 8.6 | 4.6–15.7 | 8.7 | 6.0–12.7 | 9.0 | 6.5–12.2 | 11.0 | 8.3–14.4 |
| Mollusk | 8.3 | 6.7–10.3 | 3.0 | 0.6–14.5 | 2.2 | 0.9–5.0 | 6.1 | 2.3–15.2 | 7.0 | 3.6–13.3 | 8.5 | 5.9–12.2 | 8.5 | 5.9–12.0 | 11.9 | 7.9–17.5 |
| Other shellfish | 0.8 | 0.5–1.4 | 0.0 | N/A | 0.5 | 0.1–2.2 | 1.9 | 0.3–12.4 | 0.4 | 0.2–1.1 | 0.7 | 0.2–2.2 | 0.7 | 0.2–2.5 | 1.2 | 0.5–2.7 |
| Egg | 11.9 | 9.8–14.4 | 13.5 | 7.6–23.0 | 22.8 | 12.0–39.1 | 14.1 | 7.7–24.4 | 15.0 | 10.1–21.7 | 10.8 | 7.5–15.3 | 12.8 | 9.0–17.8 | 6.6 | 4.8–9.1 |
| Fin fish | 7.1 | 5.7–8.9 | 2.6 | 0.8–7.5 | 6.4 | 2.8–13.9 | 6.0 | 2.2–15.4 | 6.2 | 2.9–13.0 | 7.8 | 5.3–11.4 | 7.1 | 5.0–10.0 | 7.9 | 5.1–12.1 |
| Wheat | 6.6 | 5.0–8.6 | 14.9 | 6.0–32.3 | 6.0 | 2.5–14.0 | 9.9 | 4.3–21.4 | 6.6 | 2.4–16.8 | 6.4 | 4.0–10.2 | 6.2 | 3.9–9.6 | 5.4 | 3.6–8.0 |
| Soy | 6.2 | 4.7–8.3 | 15.4 | 8.5–26.4 | 16.6 | 6.6–35.8 | 8.6 | 3.8–18.5 | 6.9 | 2.6–17.0 | 6.5 | 4.3–9.6 | 3.6 | 2.1–6.1 | 3.0 | 2.1–4.2 |
| Sesame | 2.7 | 2.1–3.6 | 4.6 | 1.8–10.9 | 4.9 | 1.8–12.5 | 2.3 | 0.4–11.1 | 2.7 | 1.5–5.0 | 3.3 | 2.0–5.5 | 1.8 | 0.9–3.5 | 2.1 | 1.2–3.7 |
N/A, not applicable.
Severity
Among children with convincing FA, 42.3% were estimated to have reaction symptoms indicative of a severe FA (Table 3). Severe FA was more common among children with allergy to peanut (59.2%), tree nut (56.1%), and shellfish (48.7%). Among children with convincing FA, 42.0% had been treated in the ED for a food allergic reaction at some point in their life, with 19.0% treated in the ED within the past year. However, only 40.7% of children with convincing FA reported a current prescription for an EAI. The highest rates of EAI prescription were observed for children with peanut (73.0%), tree nut (70.4%), and sesame (64.8%) allergy.
TABLE 3.
Overall and Allergy-Specific Prevalence of FA-Related Outcomes
| Proportion of Children With Each Specific FA Who Have | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Severe FA to That Food | Physician-Diagnosed FA to That Food | Multiple FAs | Current Epinephrine Prescription | One or More Lifetime ED Visits | One or More FA-Related ED Visits in the Past y | |||||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | (95% CI) | % | (95% CI) | |
| Any FA | 42.3 | 39.1–45.4 | 61.1 | 57.6–64.5 | 39.9 | 36.9–43.1 | 40.7 | 37.6–43.9 | 42.0 | 38.8–45.4 | 19.0 | 16.1–22.3 |
| Peanut | 59.2 | 53.6–64.6 | 80.9 | 77.0–84.4 | 54.8 | 49.1–60.3 | 73.0 | 68.0–77.5 | 50.4 | 44.9–55.9 | 22.9 | 19.1–27.2 |
| Tree nut | 56.1 | 50.1–61.9 | 72.9 | 66.2–78.6 | 89.5 | 84.6–93.0 | 70.4 | 64.0–76.1 | 49.4 | 43.1–55.6 | 24.4 | 19.3–30.3 |
| Walnut | 36.6 | 28.5–45.6 | 61.7 | 52.7–70.0 | 94.1 | 89.4–96.8 | 76.6 | 68.6–83.1 | 52.3 | 43.7–60.7 | 23.6 | 16.2–33.1 |
| Almond | 50.6 | 43.0–58.1 | 72.7 | 65.0–79.2 | 96.7 | 94.5–98.1 | 77.3 | 70.2–83.1 | 58.9 | 51.2–66.2 | 27.2 | 20.8–34.8 |
| Hazelnut | 46.1 | 36.1–56.4 | 60.4 | 49.7–70.2 | 95.0 | 81.9–98.7 | 72.8 | 61.6–81.7 | 53.9 | 43.7–63.7 | 25.6 | 17.5–35.8 |
| Pecan | 38.9 | 31.0–47.3 | 66.4 | 57.7–74.0 | 100.0 | N/A | 76.0 | 67.7–82.7 | 53.4 | 44.7–61.8 | 24.5 | 17.7–32.8 |
| Cashew | 42.3 | 34.8–50.2 | 70.3 | 62.6–77.0 | 95.8 | 91.8–97.9 | 77.3 | 70.0–83.2 | 52.0 | 44.1–59.8 | 24.4 | 18.2–32.0 |
| Pistachio | 37.6 | 28.9–47.1 | 65.7 | 56.6–73.7 | 98.0 | 95.5–99.2 | 81.2 | 72.5–87.6 | 53.4 | 43.9–62.6 | 22.6 | 15.4–31.8 |
| Other tree nut | 64.2 | 34.8–85.8 | 72.1 | 44.3–89.3 | 100.0 | N/A | 82.7 | 56.6–94.6 | 38.7 | 15.6–68.2 | 22.6 | 8.4–48.2 |
| Milk | 25.3 | 20.4–31.0 | 52.1 | 44.2–60.0 | 43.1 | 35.5–51.0 | 25.9 | 21.1–31.3 | 47.1 | 39.2–55.1 | 26.3 | 18.1–36.5 |
| Shellfish | 48.7 | 41.9–55.5 | 59.3 | 52.3–65.8 | 72.9 | 67.0–78.1 | 45.7 | 39.2–52.4 | 54.9 | 48.1–61.4 | 22.7 | 17.1–29.4 |
| Shrimp | 51.1 | 43.8–58.4 | 56.9 | 49.3–64.1 | 74.1 | 67.5–79.7 | 46.2 | 39.2–53.4 | 54.1 | 46.8–61.2 | 25.7 | 18.9–33.9 |
| Lobster | 42.1 | 31.9–53.1 | 43.5 | 34.3–53.2 | 96.1 | 92.2–98.1 | 43.5 | 34.0–53.4 | 62.4 | 53.1–70.9 | 28.6 | 18.6–41.3 |
| Crab | 40.6 | 31.1–51.0 | 47.1 | 37.6–56.9 | 90.9 | 82.9–95.4 | 46.4 | 36.8–56.3 | 61.2 | 51.7–70.0 | 28.2 | 19.1–39.5 |
| Mollusk | 43.8 | 32.7–55.5 | 49.4 | 38.6–60.2 | 91.0 | 86.4–94.2 | 41.5 | 31.8–51.9 | 63.8 | 54.1–72.5 | 27.1 | 17.6–39.2 |
| Other shellfish | 29.4 | 12.5–54.7 | 61.3 | 31.5–84.6 | 100.0 | N/A | 46.2 | 22.2–72.1 | 43.9 | 20.9–69.8 | 14.2 | 4.2–38.8 |
| Egg | 28.1 | 21.5–36.0 | 72.2 | 63.9–79.3 | 75.8 | 68.7–81.7 | 51.1 | 40.6–61.5 | 56.4 | 46.6–65.8 | 36.1 | 25.0–48.9 |
| Fin fish | 49.0 | 37.8–60.3 | 53.2 | 41.6–64.5 | 83.9 | 73.8–90.6 | 47.5 | 36.6–58.6 | 69.8 | 59.0–78.8 | 39.5 | 28.6–51.7 |
| Wheat | 36.7 | 23.5–52.3 | 54.9 | 40.6–68.5 | 66.6 | 53.0–77.8 | 35.0 | 24.5–47.1 | 43.7 | 31.2–57.1 | 20.5 | 13.3–30.3 |
| Soy | 36.8 | 24.7–50.8 | 40.7 | 28.6–54.1 | 74.7 | 62.5–84.0 | 40.9 | 27.8–55.4 | 53.5 | 38.6–67.8 | 35.1 | 22.3–50.5 |
| Sesame | 27.2 | 17.5–39.7 | 55.5 | 41.6–68.6 | 86.4 | 69.2–94.7 | 64.8 | 50.3–77.0 | 58.2 | 44.3–70.9 | 33.0 | 22.2–46.0 |
N/A, not applicable.
Associations
Adjusted odds of convincing FA are presented in Table 4. Significant differences in convincing FA prevalence were observed by race and/or ethnicity, with non-Hispanic African American children at significantly elevated risk relative to non-Hispanic white children (odds ratio [OR] = 1.4 [95% CI: 1.1–1.7]). In adjusted models, children who had ever received a physician-diagnosis of asthma (OR = 3.2 [95% CI: 2.7–3.8]), atopic dermatitis and/or eczema (OR = 1.9 [95% CI: 1.4–2.4]), allergic rhinitis (OR = 2.3 [95% CI: 1.9–2.7]), insect sting allergy (OR = 2.5 [95% CI: 1.8–3.4]), medication allergy (OR = 1.9 [95% CI: 1.4–2.4]), urticaria (OR = 2.9 [95% CI: 1.4–6.0]), or latex allergy (OR = 7.9 [95% CI: 5.5–11.3]) had increased odds of convincing FA.
TABLE 4.
Multivariate Predictors of FA Characteristics
| Convincing FA Versus No FA | Physician-Confirmed FA Versus Convincing FA | Severe FA Versus Mild-to-Moderate FA | Multiple FA Versus Single FA | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Race and/or ethnicity (versus white, non-Hispanic) | ||||||||
| Asian American, non-Hispanic | 1.0 | 0.8–1.4 | 0.7 | 0.4–1.3 | 1.0 | 0.6–1.8 | 1.9* | 1.1–3.3* |
| African American, non-Hispanic | 1.4 | 1.1–1.7 | 1.2 | 0.8–1.9 | 0.9 | 0.5–1.4 | 2.0* | 1.3–2.8* |
| Hispanic | 1.2 | 0.9–1.4 | 1.0 | 0.7–1.4 | 0.9 | 0.7–1.3 | 0.9 | 0.7–1.3 |
| Multiracial and/or other | 1.1 | 0.8–1.3 | 1.1 | 0.7–1.8 | 1.0 | 0.7–1.4 | 1.0 | 0.7–1.6 |
| Sex | ||||||||
| Female versus male | 1.0 | 0.9–1.2 | 1.0 | 0.7–1.2 | 0.6* | 0.5–0.8* | 1.1 | 0.9–1.4 |
| Age in y (versus 0–1 y) | ||||||||
| 1 | 2.8* | 1.9–4.2* | 1.3 | 0.6–2.8 | 1.1 | 0.5–2.3 | 0.6 | 0.3–1.3 |
| 2 | 3.3* | 2.0–5.3* | 0.8 | 0.3–1.7 | 0.8 | 0.4–1.6 | 0.5 | 0.2–1.2 |
| 3–5 | 2.4* | 1.7–3.3* | 0.9 | 0.4–1.8 | 1.2 | 0.6–2.2 | 0.8 | 0.4–1.7 |
| 6–10 | 1.9* | 1.4–2.6* | 1.5 | 0.8–2.9 | 0.9 | 0.5–1.6 | 0.5 | 0.2–1.1 |
| 11–13 | 1.6* | 1.1–2.2* | 1.4 | 0.7–2.6 | 1.2 | 0.6–2.2 | 0.6 | 0.3–1.3 |
| 14–17 | 1.6* | 1.1–2.2* | 1.1 | 0.6–2.1 | 1.4 | 0.7–2.5 | 0.5 | 0.2–1.0 |
| Household income (versus <$25 000/y) | ||||||||
| $25 000–$49 999 | 1.2 | 0.9–1.6 | 1.0 | 0.6–1.7 | 0.7 | 0.4–1.1 | 1.0 | 0.6–1.5 |
| $50 000–$99 999 | 1.2 | 1.0–1.5 | 1.4 | 0.9–2.2 | 0.7 | 0.5–1.1 | 1.1 | 0.7–1.6 |
| $100 000–$149 999 | 1.3 | 1.0–1.7 | 1.3 | 0.8–2.2 | 0.3* | 0.2–0.5* | 1.0 | 0.7–1.6 |
| >$150 000 | 1.0 | 0.7–1.5 | 1.3 | 0.7–2.6 | 0.7 | 0.4–1.2 | 1.3 | 0.7–2.2 |
| Geographic location (versus Midwest) | ||||||||
| West | 1.0 | 0.8–1.2 | 0.8 | 0.6–1.3 | 1.1 | 0.7–1.6 | 1.0 | 0.7–1.5 |
| South | 1.0 | 0.8–1.2 | 1.0 | 0.7–1.4 | 1.0 | 0.7–1.5 | 0.8 | 0.6–1.2 |
| Northeast | 1.1 | 0.9–1.4 | 0.7 | 0.4–1.1 | 0.8 | 0.5–1.3 | 1.2 | 0.8–1.9 |
| One or more physician-confirmed FA | — | — | — | — | 1.6* | 1.1–2.1* | 1.5* | 1.2–2.0* |
| Multiple FA versus 1 FA | — | — | 1.5* | 1.1–2.0* | 2.4* | 1.8–3.1* | — | — |
| Current epinephrine prescription | — | — | 5.1* | 3.8–6.9* | 2.6* | 1.9–3.4* | 1.1 | 0.8–1.5 |
| One or more lifetime ED visit | — | — | 1.9* | 1.4–2.5* | 1.8* | 1.4–2.3* | 1.6* | 1.3–2.1* |
| One or more severe FA | — | — | 1.6* | 1.2–2.1* | — | — | 2.4* | 1.8–3.1* |
| Physician-diagnosed comorbidities (versus absence of that condition) | ||||||||
| Asthma | 3.2* | 2.7–3.8* | 1.2 | 0.9–1.6 | 1.6* | 1.2–2.1* | 1.4* | 1.1–1.8* |
| Atopic dermatitis and/or eczema | 1.9* | 1.4–2.4* | 1.5 | 1.0–2.3 | 0.8 | 0.6–1.2 | 1.2 | 0.8–1.7 |
| Eosinophilic esophagitis | 2.5 | 0.8–7.5 | 1.7 | 0.7–4.2 | 0.6 | 0.2–2.4 | 2.7* | 1.1–6.7* |
| Allergic rhinitis | 2.3* | 1.9–2.7* | 1.7* | 1.3–2.3* | 1.1 | 0.9–1.5 | 1.5* | 1.1–1.9* |
| Insect sting allergy | 2.5* | 1.8–3.4* | 1.3 | 0.7–2.6 | 1.0 | 0.7–1.6 | 1.7* | 1.0–2.9* |
| Latex allergy | 7.9* | 5.5–11.3* | 1.5 | 0.9–2.5 | 1.1 | 0.7–1.7 | 1.2 | 0.8–1.9 |
| Medication allergy | 1.9* | 1.4–2.4* | 1.0 | 0.6–1.6 | 0.9 | 0.6–1.4 | 0.9 | 0.6–1.4 |
| Urticaria and/or chronic hives | 2.9* | 1.4–6.0* | 0.8 | 0.3–2.2 | 1.5 | 0.7–3.2 | 2.0* | 1.0–3.9* |
| Other chronic condition | 2.3* | 1.6–3.3* | 1.5 | 0.9–2.5 | 1.6* | 1.1–2.5* | 0.9 | 0.6–1.4 |
—, not applicable.
Two-sided P < .05
Among children with 1 or more convincing FAs, having multiple FAs, a current epinephrine prescription, a history of 1 or more lifetime FA-related ED visits, a severe reaction history, and comorbid allergic rhinitis were each associated with the presence of 1 or more physician-confirmed FAs. Having multiple FAs, a current epinephrine prescription, a history of 1 or more lifetime FA-related ED visits, or comorbid asthma were also significantly associated with increased odds of a severe allergic reaction history.
In Table 5, we present factors associated with having a current epinephrine prescription, reporting 1 or more lifetime FA-related ED visit and reporting 1 or more FA-related ED visit within the past year. Children who had 1 or more lifetime ED visits or severe FA had significantly elevated odds of having a current epinephrine prescription, as did children with peanut or pistachio allergy, whereas children allergic to milk had significantly reduced odds. Children with multiple or more severe FAs, milk or fin fish allergies, or comorbid asthma, insect allergy, or urticaria had significantly greater odds of 1 or more lifetime FA-related ED visits.
TABLE 5.
Multivariate Predictors of Epinephrine Prescription, Lifetime ED Visits, and Last Year ED Visits
| Current Epinephrine Prescription | Lifetime ED Visits | Last Year ED Visits | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Race and/or ethnicity (versus white, non-Hispanic) | ||||||
| Asian American, non-Hispanic | 1.3 | 0.7–2.5 | 0.9 | 0.6–1.6 | 1.0 | 0.5–2.0 |
| African American, non-Hispanic | 0.8 | 0.5–1.2 | 1.3 | 0.9–1.8 | 1.4 | 0.8–2.3 |
| Hispanic | 0.8 | 0.6–1.2 | 1.5* | 1.1–2.1* | 1.1 | 0.7–1.6 |
| Multiracial and/or other | 0.9 | 0.6–1.5 | 0.9 | 0.5–1.6 | 1.4 | 0.8–2.7 |
| Sex | ||||||
| Female versus male | 1.1 | 0.9–1.5 | 0.9 | 0.7–1.2 | 1.0 | 0.7–1.3 |
| Age in y (versus 0–1 y) | ||||||
| 1 | 0.9 | 0.4–2.0 | 1.2 | 0.5–2.9 | 0.9 | 0.4–2.1 |
| 2 | 0.7 | 0.3–1.6 | 3.4* | 1.3–9.0* | 2.0 | 0.7–5.6 |
| 3–5 | 1.3 | 0.7–2.6 | 1.8 | 0.9–3.5 | 0.8 | 0.4–1.6 |
| 6–10 | 1.0 | 0.5–1.9 | 2.8* | 1.4–5.4* | 0.7 | 0.3–1.4 |
| 11–13 | 1.0 | 0.5–2.0 | 1.8 | 0.9–3.5 | 0.4* | 0.2–0.8* |
| 14–17 | 0.8 | 0.4–1.6 | 2.1* | 1.1–4.1* | 0.5* | 0.2–0.9* |
| Household income (versus <$25 000/y) | ||||||
| $25 000–$49 999 | 0.7 | 0.5–1.1 | 0.9 | 0.6–1.4 | 0.4* | 0.3–0.7* |
| $50 000–$99 999 | 1.1 | 0.8–1.7 | 1.0 | 0.7–1.5 | 0.6* | 0.4–1.0* |
| $100 000–$149 999 | 1.2 | 0.8–1.9 | 0.9 | 0.5–1.5 | 0.6 | 0.3–1.1 |
| >$150 000 | 1.3 | 0.7–2.3 | 0.6* | 0.3–1.0* | 0.4* | 0.2–0.7* |
| Geographic location (versus Midwest) | ||||||
| West | 0.9 | 0.7–1.4 | 1.3 | 0.9–1.9 | 1.8* | 1.1–2.9* |
| South | 1.1 | 0.8–1.5 | 0.8 | 0.6–1.2 | 0.9 | 0.6–1.4 |
| Northeast | 1.8* | 1.1–2.7* | 0.9 | 0.6–1.3 | 1.2 | 0.7–2.0 |
| One or more physician-diagnosed FA | 4.3* | 3.2–5.8* | 1.8* | 1.3–2.4* | 1.4 | 0.9–2.0 |
| Multiple FA versus 1 FA | 0.8 | 0.6–1.2 | 1.5* | 1.1–2.0* | 1.5* | 1.0–2.1* |
| Has epinephrine | — | — | 1.5* | 1.2–2.0* | 1.4 | 1.0–2.0 |
| One or more lifetime ED visit | 1.6* | 1.2–2.0* | — | — | — | — |
| One or more severe FA | 2.1* | 1.6–2.7* | 1.9* | 1.4–2.4* | 1.0 | 0.8–1.4 |
| Specific FAs (versus absence of that allergy) | ||||||
| Peanut | 3.9* | 2.9–5.2* | 0.9 | 0.7–1.3 | 1.0 | 0.8–1.4 |
| Tree nut | 1.6 | 0.9–2.9 | 0.5* | 0.3–0.9* | 1.2 | 0.7–2.2 |
| Almond | 1.0 | 0.5–1.8 | 1.6 | 1.0–2.7 | 1.0 | 0.5–1.7 |
| Walnut | 1.4 | 0.7–2.7 | 0.9 | 0.5–1.5 | 0.7 | 0.4–1.2 |
| Cashew | 1.3 | 0.7–2.5 | 1.1 | 0.6–1.9 | 1.2 | 0.7–2.2 |
| Hazelnut | 0.7 | 0.3–1.4 | 1.2 | 0.7–2.2 | 1.2 | 0.7–2.3 |
| Pistachio | 2.5* | 1.3–5.0* | 0.9 | 0.5–1.7 | 0.6 | 0.3–1.2 |
| Pecan | 0.8 | 0.4–1.5 | 1.0 | 0.5–1.8 | 1.0 | 0.5–2.0 |
| Other tree nut | 1.3 | 0.3–6.8 | 0.6 | 0.1–2.8 | 0.9 | 0.1–8.9 |
| Sesame | 1.4 | 0.6–3.2 | 1.3 | 0.7–2.3 | 1.5 | 0.8–2.9 |
| Milk | 0.5* | 0.3–0.6* | 1.6* | 1.1–2.3* | 1.7* | 1.1–2.6* |
| Egg | 1.3 | 0.8–2.1 | 1.2 | 0.8–1.8 | 1.7* | 1.0–2.6* |
| Finfish | 0.8 | 0.4–1.4 | 2.2* | 1.3–3.6* | 2.2* | 1.3–3.7* |
| Shellfish | 1.1 | 0.4–3.4 | 1.2 | 0.6–2.3 | 0.5 | 0.3–1.1 |
| Shrimp | 1.4 | 0.5–4.1 | 0.7 | 0.4–1.3 | 2.0 | 1.0–4.0 |
| Lobster | 0.7 | 0.3–1.6 | 0.9 | 0.5–1.6 | 0.5 | 0.3–1.1 |
| Crab | 1.7 | 0.6–4.9 | 1.5 | 0.8–2.9 | 2.1 | 1.0–4.4 |
| Mollusk | 0.4 | 0.2–1.0 | 1.4 | 0.8–2.4 | 1.2 | 0.6–2.3 |
| Other shellfish | 0.9 | 0.2–3.9 | 0.3* | 0.1–1.0* | 0.4 | 0.1–2.0 |
| Soy | 0.7 | 0.4–1.1 | 1.2 | 0.7–2.0 | 1.6 | 0.9–2.8 |
| Wheat | 0.7 | 0.4–1.2 | 0.8 | 0.4–1.6 | 0.7 | 0.4–1.2 |
| Physician-diagnosed comorbidities (versus absence of that condition) | ||||||
| Asthma | 1.2 | 0.9–1.6 | 2.1* | 1.6–2.7* | 1.8* | 1.3–2.5* |
| Atopic dermatitis and/or eczema | 1.4 | 1.0–2.0 | 0.6* | 0.4–0.9* | 0.8 | 0.5–1.2 |
| Eosinophilic esophagitis | 3.1 | 1.0–10.0 | 2.5 | 0.8–7.3 | 3.6* | 1.1–11.1* |
| Allergic rhinitis | 0.9 | 0.7–1.2 | 0.9 | 0.7–1.2 | 0.8 | 0.6–1.1 |
| Insect sting allergy | 1.6 | 0.9–2.8 | 1.6* | 1.0–2.4* | 0.8 | 0.5–1.2 |
| Latex allergy | 2.6* | 1.5–4.6* | 0.9 | 0.5–1.6 | 1.2 | 0.8–2.0 |
| Medication allergy | 1.8* | 1.2–2.7* | 1.0 | 0.7–1.6 | 0.7 | 0.5–1.1 |
| Urticaria and/or chronic hives | 0.6 | 0.2–2.0 | 2.5* | 1.2–5.5* | 2.5* | 1.1–6.0* |
| Other chronic condition | 1.6* | 1.0–2.6* | 0.8 | 0.5–1.3 | 0.4* | 0.2–0.8* |
—, not applicable.
Two-sided P < .05
Discussion
Based on population-weighted estimates obtained from this nationally representative, parent-reported sample, an estimated 7.6% of US children have an FA, corresponding to ∼5.6 million children. Moreover, an estimated 42.3% of children with an FA have a history of at least 1 severe food allergic reaction, and 39.9% have multiple FAs. At least 1 lifetime FA-related visit to the ED was reported by an estimated 42.0% of children with FA, with 19.0% of children reporting at least 1 FA-related ED visit in the last year. Furthermore, these data indicate that only 40.7% of food-allergic children in the United States have a current prescription for an EAI.
The relative prevalence of specific parent-reported FAs observed in the current survey indicates that peanut remains the most common FA in the United States (affecting ∼1.6 million children), followed by milk (1.4 million), shellfish (1 million), tree nut (0.9 million), egg (0.6 million), fin fish (0.4 million), wheat (0.4 million), soy (0.4 million), and sesame (0.15 million). Among shellfish allergies, shrimp allergy was most common, followed by crab, mollusk, and lobster. These findings are consistent with past research suggesting that crustacea elicit more allergic reactions than mollusks.18,19 Specific tree nut allergy prevalence rates, however, did not significantly differ. These findings contrast with previous studies in which researchers reported walnut and cashew allergies to be substantially more prevalent than other tree nut allergies, including pecan and almond.20–22 Moreover, sesame was the ninth most common allergen in our study, with a prevalence of 0.2%. This is consistent with previous studies in which researchers reported sesame allergy prevalence of 0.1% to 0.2% in the United States and Canada.20,23,24 Because the prevalence and severity of sesame allergy appears comparable to allergens for which labeling is currently mandated, these findings suggest that including sesame under allergen labeling laws in the United States may be warranted, as is already the case in Canada, the European Union, Australia, and Israel.11
Although the current 7.6% FA prevalence estimate is similar to the 8.0% estimate published by our group in 2011, it is important to note that the current study used more stringent criteria to define “convincing” allergies, thus precluding direct comparison of the estimates. The updated criteria (most notably the exclusion of children reporting only nonsystemic, oropharyngeal reaction symptoms and those whose symptoms were limited to the gastrointestinal system, even in cases of physician-diagnosed FA) reflect recent advances in understanding of the key differential diagnoses of OAS and specific food intolerances, respectively.25 If we applied 2011 criteria for a convincing FA to the present data, estimated current FA prevalence would be higher. However, even if identical criteria were used at both waves, temporal trends in FA prevalence are almost certainly influenced by growing FA awareness and diagnosis among both patients and clinicians.26 Such increased awareness may contribute to higher observed rates of both convincing and physician-diagnosed allergy.
Although these findings suggest that ∼8% of US children have an FA, the estimated prevalence of current FA (parent-reported rate before exclusion of nonconvincing symptoms) was 11.4%. This discrepancy underscores the importance of improving patient access to physicians trained in the accurate diagnosis of FA to prevent placing families under the social, emotional, and economic burden of unnecessarily avoiding foods to which they are not truly allergic. Reactions to food may actually be intolerances or OAS, which are difficult for parents to decipher on their own. Additionally, positive test results without a reaction history may mislabel children as having an FA when they may be able to tolerate the food. Indeed, just over two-thirds of children with convincing FA in our study had received a physician diagnosis of FA. Among convincing allergies, peanut had the highest rates of physician diagnosis (81%), whereas soy had the lowest rates (41%). As evident in Fig 3, the proportion of children experiencing severe reactions as well as the proportion parent-reported FAs that were classified as convincing or physician-confirmed also varied substantially by allergen. Additionally, these data suggest that >40% of children with FAs in the United States have been treated in the ED for their FA, with just under 20% reporting an FA-related ED visit within the past year. These data are consistent with recent reports suggesting that ED admission rates for anaphylaxis are rising, particularly among children.12,27,28
FIGURE 3.
Comparing rates of parent-reported versus convincing versus physician-confirmed FAs.
Approximately 40% of children with an FA in the current study reported current EAI prescriptions, ranging from ∼70% of children with peanut and tree nut allergies to 26% of children with milk allergy. All allergens can cause severe, potentially life-threatening reactions, so all FA patients require counseling on proper anaphylaxis management. Low EAI prescription rates have been reported previously.29 Additionally, previous research suggests that even with a current prescription, families of children with FAs frequently fail to fill and refill EAI prescriptions as recommended.30,31 The low rate of epinephrine prescriptions observed in our study suggests that efforts to improve physician evaluation and/or diagnosis of an FA and appropriate prescription of EAIs may be warranted.
As in our 2011 prevalence study6 and previous analysis of NHANES data32 non-Hispanic African American children were more likely to have an FA than non-Hispanic white children. African American children were also more likely to have multiple FAs than children of other racial and/or ethnic groups with FA. These findings suggest that the racial disparities observed in other atopic conditions (eg, asthma) may also exist in the context of FAs.32,33 It is noteworthy that this observed difference in FA prevalence between non-Hispanic African American and white children persisted even after accounting for atopic comorbidities and other covariates, including household income. Consequently, additional research is necessary to better understand the etiology of these racial differences, particularly in the context of FA.
Finally, peanut allergy prevalence in our study was 2.2%, slightly higher than the 2.0% estimated by our group in 2011, the 2.0% estimated by Bunyavanich et al34 in 2014 among a northeastern US cohort, and higher than Sicherer et al’s20 national estimate of 1.4% in 2008. Although our data suggest that the burden of childhood peanut allergy has increased over the past decade, the authors of a recent study demonstrated that introducing peanut-containing foods alongside typical complementary foods between 4 and 11 months can achieve relative reductions in peanut allergy risk of up to 80%13 among high-risk infants. Consequently, the National Institute of Allergy and Infectious Diseases–sponsored 2017 “Addendum Guidelines for the Prevention of Peanut Allergy” were released, which guide clinicians in promoting early peanut introduction for primary peanut allergy prevention.35 Therefore, if the “Prevention of Peanut Allergy” guidelines are broadly implemented, the age-specific peanut allergy prevalence estimates reported in this study may provide important baseline reference points for future work.
Although double-blinded placebo-controlled oral food challenges remain the current “gold standard” for FA diagnosis, such methods were not employed in the current study because of their expense, impracticality, and concerns about nonparticipation bias. As in past work20 to strengthen the rigor of our parent-reported questionnaire, stringent criteria were established in collaboration with an expert panel to exclude FAs where corresponding symptom report was not consistent with immunoglobulin E–mediated FA. Nevertheless, by relying exclusively on parent-report and not directly observing symptoms immediately after allergenic food protein consumption, misestimation of true FA prevalence and symptomatology remains possible. However, it is important to recognize the use of survey-based approaches given their ability to capture patients with FAs who may not receive formal evaluations or diagnoses.
Conclusions
These data suggest that childhood FA is a significant public health issue resulting in relatively high rates of severe allergic reactions and ED use. Previous findings of racial differences in FA prevalence were also supported here with elevated rates identified among non-Hispanic African American children. Overall, these findings provide critical epidemiologic information that improves understanding of the public health impact of childhood FA.
Acknowledgments
We thank Michael Dennis, PhD, and Nada Ganesh, PhD, of NORC at the University of Chicago; Ozge Nur Aktas, MD, Lauren Kao, MA, and the members of our expert panel.
Glossary
- CI
confidence interval
- EAI
epinephrine autoinjector
- ED
emergency department
- FA
food allergy
- OAS
oral allergy syndrome
- OR
odds ratio
- SSI
Survey Sampling International
Footnotes
Dr Gupta conceptualized and designed the study and oversaw data acquisition, analysis, and interpretation; Mr Warren participated in study design, was responsible for data analysis and interpretation, and drafted the manuscript; Dr Smith participated in study design and was responsible for data analysis and interpretation; Mr Blumenstock participated in study design and participated in data analysis and interpretation; Ms Jiang contributed to the study design, participated in the interpretation of data, and drafted the manuscript; Drs Davis and Nadeau consulted on study design and contributed to interpretation of data; and all authors reviewed and/or revised the manuscript and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by the National Institute of Allergy and Infectious Disease (R21AI135702 [Principal Investigator: Dr Gupta]), Sean N. Parker Center for Allergy and Asthma Research at Stanford University, Aimmune Therapeutics, and Denise and Dave Bunning. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Rudders SA, Arias SA, Camargo CA Jr. Trends in hospitalizations for food-induced anaphylaxis in US children, 2000-2009. J Allergy Clin Immunol. 2014;134(4):960–962.e3 [DOI] [PubMed] [Google Scholar]
- 2.Jones SM, Burks AW. Food allergy. N Engl J Med. 2017;377(12):1168–1176 [DOI] [PubMed] [Google Scholar]
- 3.Bock SA, Muñoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001-2006. J Allergy Clin Immunol. 2007;119(4):1016–1018 [DOI] [PubMed] [Google Scholar]
- 4.Warren CM, Otto AK, Walkner MM, Gupta RS. Quality of life among food allergic patients and their caregivers. Curr Allergy Asthma Rep. 2016;16(5):38. [DOI] [PubMed] [Google Scholar]
- 5.Gupta R, Holdford D, Bilaver L, Dyer A, Holl JL, Meltzer D. The economic impact of childhood food allergy in the United States [published correction appears in JAMA Pediatr. 2013;167(11):1083]. JAMA Pediatr. 2013;167(11):1026–1031 [DOI] [PubMed] [Google Scholar]
- 6.Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128(1). Available at: www.pediatrics.org/cgi/content/full/128/1/e9 [DOI] [PubMed] [Google Scholar]
- 7.Sampson HA. Food allergy: past, present and future. Allergol Int. 2016;65(4):363–369 [DOI] [PubMed] [Google Scholar]
- 8.Tang ML, Mullins RJ. Food allergy: is prevalence increasing? Intern Med J. 2017;47(3):256–261 [DOI] [PubMed] [Google Scholar]
- 9.Sicherer SH, Sampson HA. Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol. 2018;141(1):41–58 [DOI] [PubMed] [Google Scholar]
- 10.Keet CA, Savage JH, Seopaul S, Peng RD, Wood RA, Matsui EC. Temporal trends and racial/ethnic disparity in self-reported pediatric food allergy in the United States. Ann Allergy Asthma Immunol. 2014;112(3):222–229.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stallings VA, Oria MP, eds; National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board; Committee on Food Allergies: Global Burden, Causes, Treatment, Prevention, and Public Policy . Finding a Path to Safety in Food Allergy: Assessment of the Global Burden, Causes, Prevention, Management, and Public Policy. Washington, DC: National Academies Press (US); 2017 [PubMed] [Google Scholar]
- 12.Motosue MS, Bellolio MF, Van Houten HK, Shah ND, Campbell RL. Increasing emergency department visits for anaphylaxis, 2005-2014. J Allergy Clin Immunol Pract. 2017;5(1):171–175.e3 [DOI] [PubMed] [Google Scholar]
- 13.Du Toit G, Roberts G, Sayre PH, et al. ; LEAP Study Team . Randomized trial of peanut consumption in infants at risk for peanut allergy [published correction appears in N Engl J Med. 2016;375(4);398]. N Engl J Med. 2015;372(9):803–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones SM, Beyer K, Burks AW, et al. Efficacy and safety of AR101 in peanut allergy: results from a phase 3, randomized, double-blind, placebo-controlled trial (PALISADE). J Allergy Clin Immunol. 2018;141(2):AB400. [DOI] [PubMed] [Google Scholar]
- 15.Gupta RS, Kim JS, Springston EE, Pongracic JA, Wang X, Holl J. Development of the Chicago Food Allergy Research Surveys: assessing knowledge, attitudes, and beliefs of parents, physicians, and the general public. BMC Health Serv Res. 2009;9:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fahimi M, Barlas FM, Thomas RK, Buttermore N. Scientific surveys based on incomplete sampling frames and high rates of nonresponse. Surv Pract. 2015;8(6):1–13 [Google Scholar]
- 17.DiSogra C, Cobb C, Chan E, Dennis JM. Calibrating nonprobability Internet samples with probability samples using early adopter characteristics. In: Proceedings of the American Statistical Association, Section on Survey Research. Joint Statistical Meetings (JSM); August 3, 2011; Miami Beach, FL [Google Scholar]
- 18.Sicherer SH, Muñoz-Furlong A, Sampson HA. Prevalence of seafood allergy in the United States determined by a random telephone survey. J Allergy Clin Immunol. 2004;114(1):159–165 [DOI] [PubMed] [Google Scholar]
- 19.Moonesinghe H, Mackenzie H, Venter C, et al. Prevalence of fish and shellfish allergy: a systematic review. Ann Allergy Asthma Immunol. 2016;117(3):264–272.e4 [DOI] [PubMed] [Google Scholar]
- 20.Sicherer SH, Muñoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125(6):1322–1326 [DOI] [PubMed] [Google Scholar]
- 21.McWilliam V, Koplin J, Lodge C, Tang M, Dharmage S, Allen K. The prevalence of tree nut allergy: a systematic review. Curr Allergy Asthma Rep. 2015;15(9):54. [DOI] [PubMed] [Google Scholar]
- 22.Weinberger T, Sicherer S. Current perspectives on tree nut allergy: a review. J Asthma Allergy. 2018;11:41–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ben-Shoshan M, Harrington DW, Soller L, et al. A population-based study on peanut, tree nut, fish, shellfish, and sesame allergy prevalence in Canada [published correction appears in J Allergy Clin Immunol. 2011;127(3):840]. J Allergy Clin Immunol. 2010;125(6):1327–1335 [DOI] [PubMed] [Google Scholar]
- 24.Soller L, Ben-Shoshan M, Harrington DW, et al. Adjusting for nonresponse bias corrects overestimates of food allergy prevalence. J Allergy Clin Immunol Pract. 2015;3(2):291–293.e2 [DOI] [PubMed] [Google Scholar]
- 25.Sampson HA, Aceves S, Bock SA, et al. ; Joint Task Force on Practice Parameters; Practice Parameter Workgroup . Food allergy: a practice parameter update-2014. J Allergy Clin Immunol. 2014;134(5):1016–1025.e43 [DOI] [PubMed] [Google Scholar]
- 26.McGowan EC, Peng RD, Salo PM, Zeldin DC, Keet CA. Changes in food-specific IgE over time in the National Health and Nutrition Examination Survey (NHANES). J Allergy Clin Immunol Pract. 2016;4(4):713–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dyer AA, Lau CH, Smith TL, Smith BM, Gupta RS. Pediatric emergency department visits and hospitalizations due to food-induced anaphylaxis in Illinois [published correction appears in Ann Allergy Asthma Immunol. 2015;115(5):458]. Ann Allergy Asthma Immunol. 2015;115(1):56–62 [DOI] [PubMed] [Google Scholar]
- 28.Liew WK, Williamson E, Tang ML. Anaphylaxis fatalities and admissions in Australia. J Allergy Clin Immunol. 2009;123(2):434–442 [DOI] [PubMed] [Google Scholar]
- 29.Waserman S, Avilla E, Ben-Shoshan M, Rosenfield L, Adcock AB, Greenhawt M. Epinephrine autoinjectors: new data, new problems. J Allergy Clin Immunol Pract. 2017;5(5):1180–1191 [DOI] [PubMed] [Google Scholar]
- 30.Kaplan MS, Jung SY, Chiang ML. Epinephrine autoinjector refill history in an HMO. Curr Allergy Asthma Rep. 2011;11(1):65–70 [DOI] [PubMed] [Google Scholar]
- 31.Song TT, Worm M, Lieberman P. Anaphylaxis treatment: current barriers to adrenaline auto-injector use. Allergy. 2014;69(8):983–991 [DOI] [PubMed] [Google Scholar]
- 32.Liu AH, Jaramillo R, Sicherer SH, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2010;126(4):798–806.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahdavinia M, Fox SR, Smith BM, et al. Racial differences in food allergy phenotype and health care utilization among US children. J Allergy Clin Immunol Pract. 2017;5(2):352–357.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bunyavanich S, Rifas-Shiman SL, Platts-Mills TAE, et al. Peanut allergy prevalence among school-age children in a US cohort not selected for any disease. J Allergy Clin Immunol. 2014;134(3):753–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Togias A, Cooper SF, Acebal ML, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J Allergy Clin Immunol. 2017;139(1):29–44 [DOI] [PMC free article] [PubMed] [Google Scholar]