Abstract
Background
Lengths of hospital stay (LoS) after childbirth that are too long have a number of health, social and economic drawbacks. For this reason, in several high-income countries LoS has been reduced over the past decades and early discharge (ED) is increasingly applied to low-risk mothers and newborns.
Methods
We conducted a population-based study investigating LoS after chilbirth across all 12 maternity centres of Friuli Venezia-Giulia (FVG), North-Eastern Italy, using a database capturing all registered births in the region from 2005 to 2015 (11 years). Adjusting for clinical factors (clinical conditions of the mother and the newborn), socio-demographic bakground and obstetric history with multivariable logistic regression, we ranked facility centres for LoS that were longer than our proposed ED benchmarks (defined as >2 days for spontaneous vaginal deliveries and >3 days for instrumental vaginal deliveries). The reference was hospital A, a national excellence centre for maternal and child health.
Results
The total number of births examined in our database was 109,550, of which 109,257 occurred in hospitals. During these 11 years, the number of births significantly diminished over time, and the pooled mean LoS for spontaneous vaginal deliveries in the whole FVG was 2.9 days. There was a significantly decreasing trend in the proportion of women remaining admitted more than the respective ED cutoffs for both delivery modes. The percentage of women staying longer that the ED benchmarks varied extensively by facility centre, ranging from 32% to 97% for spontaneous vaginal deliveries and 15% to 64% for instrumental vaginal deliveries. All hospitals but G were by far more likely to surpass the ED cutoff for spontaneous deliveries. As compared with hospital A, the most significant adjusted ORs for LoS overcoming the ED thresholds for spontaneous vaginal deliveries were: 89.38 (78.49–101.78); 26.47 (22.35–31.36); 10.42 (9.49–11.44); 10.30 (9.45–11.21) and 8.40 (7.68–9.19) for centres B, D, I, K and E respectively. By contrast the OR was 0.77 (95%CI: 0.72–0.83) for centre G. Similar mitigated patterns were observed also for instrumental vaginal deliveiries.
Conclusions
For spontaneous vaginal deliveries the mean LoS in the whole FVG was shorter than 3.4 days, the average figure most recently reported for the whole of Italy, but higher than other countries’ with health systems similar to Italy’s. Since our results are controlled for the effect of all other factors, the between-hospital variability we found is likely attributable to the health care provider itself. It can be argued that some maternity centres of FVG may have had ecocomic interest in longer LoS after childbirth, although fear of medico-legal backlashes, internal organizational malfunctions of hospitals and scarce attention of ward staff on performance efficiency shall not be ruled out. It would be therefore important to ensure higher level of coordination between the various maternity services of FVG, which should follow standardized protocols to pursue efficiency of care and allow comparability of health outcomes and costs among them. Improving the performance of FVG and Italian hospitals requires investment in primary care services.
Background
Albeit birth is a wellness and natural event (not an illness), almost all babies in high-middle income countries are delivered in hospital, where postnatal care is provided with the goal of monitoring and treating the mother and the newborn for eventual complications. Hospital postnatal care includes also necessary support to the woman for her transition home, counseling on breastfeeding and health promotion indications [1].
There is no consensus around appropriate length of hospital stay (LoS) after childbirth; the only recommendation (based on weak evidence) is from the World Health Organization (WHO), according to which all women should remain admitted at least 24h postpartum [2]. This recommendation aims to ensure that the mother and the newborn, particularly in low-income countries, remain long enough in hospital to be appropriately monitored by skilled birth attendants, in the event of serious complications requiring emergency care. The first 24h post-partum in fact poses the greatest risk of fatal events (often not predictable) both for the mother and the newborn [3].
However, in high-income countries the perspective is almost overturned, as hospitalization after childbirth may also present some downsides. For instance, hospital postnatal stays that are too long may expose the mother and the newborn to risk of nosocomial infections, which increases with LoS [4]. Extended LoS could also have an impact on family ties, as the partner is often involved and sibling competition could be triggered [4]. Longer LoS may also cause in the mother sleeping disorders, stress, breastfeeding issues and dissatisfaction toward the health care service received [5,6]. Finally, LoS that are long can also have a negative economic impact on hospital performance and sustainability of health systems, especially those funded by central taxation [7]. Therefore, to contain unnecessary days of LoS, postnatal care has been going through considerable evolution in the Western World over the past 30 years [8].
In particular, the average LoS after childbirth has been progressively shortened to improve patient satisfaction and reduce health care costs associated with childbirth. Concepts such as early discharge (ED) have taken place in several high-income countries, where the average LoS for spontaeous vaaginal deliveries (SVD) is now 48 hours or less [4,9] and discharge even within a few hours after birth is not uncommon nowadays [10]. Despite an increase in medical interventions during childbirth and more complex needs of pregnant women, there is in fact evidence that in several high-income countries low-risk mothers and newborn are being discharged as early as 4–6 h after childbirth [11–13]. For instance (although it may be a special case) the Duchess of Cambridge of the UK was reportedly been discharged from S. Mary’s Hospital in London 24h, 10h and 7h after delivering her three “royal babies” respectively [14].
There is no standardized definition of ED, as what is considered ED in one country may not be considered ED in another [15–17]. However, the most shared approach comes from the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists, which in 1992 defined early postnatal discharge as a LoS less than 48h for women who had SVD and less than 96h for those who delivered by cesarean section (CS) [18].
There is considerable variability of LoS across the globe and ED is not applied systematically in all high-income countries, as a reflection not only of cultural and political diversity, but also difference in health systems [16]. Variability in LoS is also observed within Europe, with post-communist Eastern European countries reporting average LoS of 5–6 days for SVD, which considerably contrasts with figures from Western European countries. The former reports are likely the aftermath of previous communist health care policies still continuing nowadays [4,19]. However, even within Western Europe the situation is far from uniform. Whilst central European countries with Bismarckian health systems based upon social insurances (France, Luxembourg, Austria, Belgium, etc.) reported average LoS of around 4 days for SVD, in countries characterized by Beveridgean health systems founded upon central taxation (UK, Ireland, Netherlands, Sweden, Italy, etc.) the average LoS was about 2 days or even less [4,19,20]. Moreover, even within Beveridgean health systems, countries like Greece and Italy had an average LoS for SVD of 4.0 and 3.4 days respectively, which questions the performance and efficiency of the respective health systems [4,19].
A number of factors are reported to influence LoS according to the open literature: delivery mode, type of birth attendant, maternal age, parity, gestational age, birth-weight, multiple birth, infant survival status, analgesia, labour induction, maternal smoking, maternal body mass index (BMI), marital status, parents’ nationality, parental employment status, and others [4,8,17].
In view of the above, since ED is not applied in Italy nor in other European countries, we conducted a population based study in Friuli Venezia Giulia (FVG), a region of North-Eastern Italy, describing LoS after vaginal births and associated factors from 2005 to 2015, comparing the performance of the various FVG maternity centres to inform policy makers about the potential determinants of ED. We used a database capturing all registered births in the region during these 11 years to examine LoS by SVD as well as instrumental vaginal deliveries (IVD). Observations related to CS will be presented in another study.
Methods
This study employed a cross-sectional design to investigate LoS and associated factors from 2005–2015 in FVG, a region of North-Eastern Italy with an approximately 1.22 million resident population, of which roughly 50% are females [21]. FVG has one of the most advanced information health system in Italy and has been historically at the forefront of health innovation in central Europe, so it is an ideal setting to experiment and evaluate potential new health care policies [22, 23].
The database
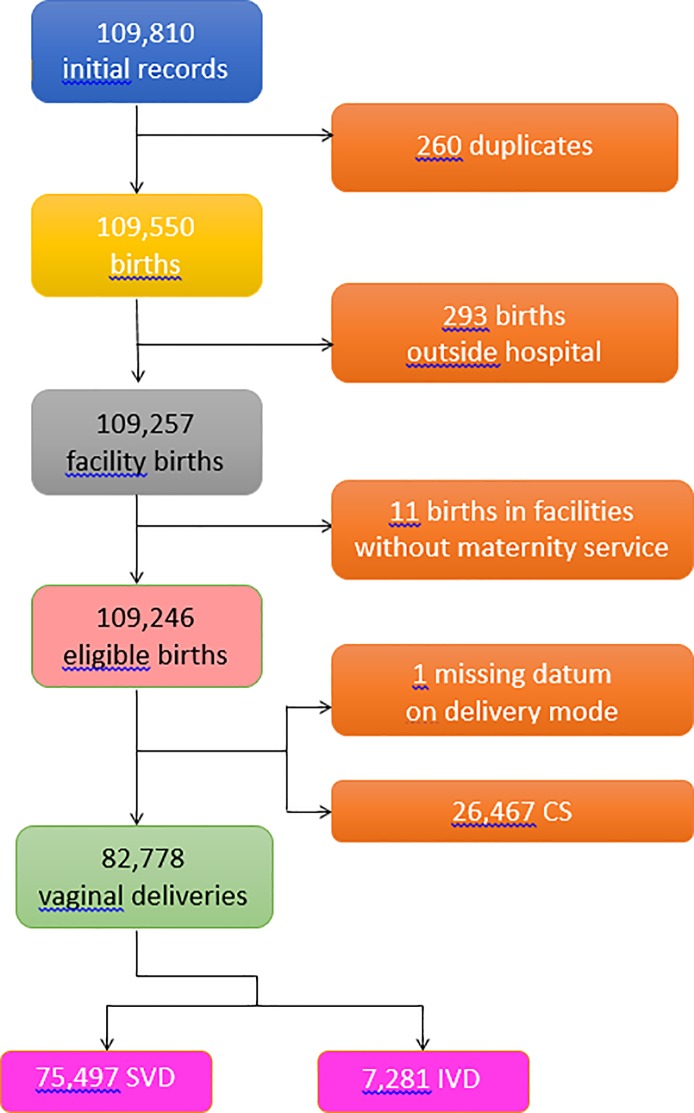
Data analyzed in the present study were extracted from the Regional Repository of FVG, a database anonymously storing administrative data from the Italian National Health Service (NHS) [21]. Fig 1 shows the flowchart displaying the various criteria applied to the initial database to obtain the final number of hospital records available for the analysis.
Fig 1. Flowchart displaying the various criteria applied to the initial database to obtain the final number of hospital records available for the analysis.
For our study we used the information collected by the Certificate of Delivery Care (CEDAP, Italian acronym) from all 12 hospitals with maternity services of FVG during 2005–2015. CEDAP is a formatted questionnaire filled up by trained health care personnel collecting clinical and personal information on women and newborn. Copy of CEDAP can be seen as a supplementary file (S1 Fig). The 12 regional maternity centres were anonymized and named by alphabetic letter from A to L.
Approval to conduct the study was granted by the Regional Health Authority of Friuli Venezia Giulia.
Length of stay
LoS (measured in days) was calculated by subtracting the date of birth from the date of hospital discharge.
Conceptual framework
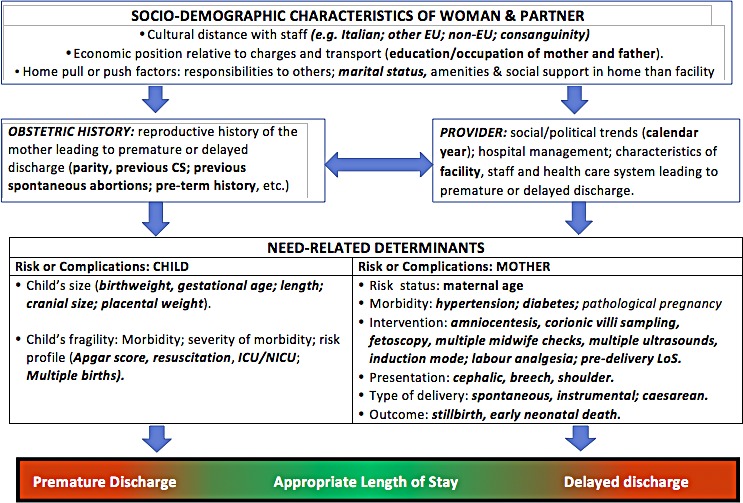
We devised a conceptual framework combining our knowledge, the existing literature, and our reasoning to disentangle the relationships between various explanatory factors and LoS [24–26]. Conceptual frameworks lay out factors and concept domains as well as construct presumed relationships between determinants [24–26]. A previous model used four categories to describe determinants of LoS [27]: patient; healthcare providers; social/family environment; healthcare system. Our conceptual framework identified 5 broad domains of potential determinants of LoS (Fig 2):
Fig 2. Conceptual framework explaining the relationship between various determinants and LoS.
Health care setting and calendar year;
Maternal health factors;
- Clinical factors of the child:
-
3.1Child’s size;
-
3.2Child’s fragility;
-
3.1
Socio-demographic background;
Obstetric history.
Variables
The following factors were used as explanatory variables in the analysis.
Health care setting
Table 1 shows the setting (hospitals) and the timeframe (calendar year) of the present investigation.
Table 1. Distibution of length of stay (LoS) after childbirth by healthcare setting and calendar year.
Number; mean LoS (M) ± standard deviation (SD); row percentage (row %). NA = Not applicable.
| FACTORS | STRATA | ALL BIRTHS | VAGINAL DELIVERY MODE | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SPONTANEOUS (N = 75,497) | INSTRUMENTAL (N = 7,281) | ||||||||||||||||||
| Number | M ± SD (days) |
LoS (days) | LoS (days) | ||||||||||||||||
| M ± SD | ≤1 | 2 | 3 | 4 | 5 | 6+ | >2 | M ± SD | ≤1 | 2 | 3 | 4 | 5 | 6+ | >3 | ||||
| Row % | Row % | ||||||||||||||||||
|
Calendar Year |
2005 | 10,177 | 3.5 ± 1.5 | 2.9 ±1.0 | 1.7 | 31.0 | 50.0 | 11.9 | 3.3 | 2.2 | 67.3 | 3.6 ± 1.7 | 0.9 | 16.0 | 43.4 | 23.6 | 8.0 | 8.2 | 39.8 |
| 2006 | 10,470 | 3.4 ± 1.4 | 2.9 ±1.0 | 1.6 | 32.9 | 48.2 | 12.0 | 3.1 | 2.1 | 65.5 | 3.3 ± 1.1 | 0.3 | 17.6 | 48.0 | 23.6 | 6.1 | 4.5 | 34.2 | |
| 2007 | 10,652 | 3.4 ± 1.4 | 2.9 ± 1.0 | 1.0 | 34.6 | 48.3 | 11.8 | 2.7 | 1.6 | 64.4 | 3.4 ± 1.3 | 0.6 | 15.6 | 49.9 | 21.7 | 8.1 | 4.1 | 33.9 | |
| 2008 | 10,478 | 3.4 ± 1.4 | 2.9 ± 1.0 | 1.2 | 32.7 | 50.1 | 10.9 | 3.3 | 1.9 | 66.2 | 3.4 ± 1.2 | 0.6 | 14.7 | 48.2 | 23.1 | 7.5 | 5.9 | 36.5 | |
| 2009 | 10,492 | 3.4 ± 1.5 | 2.9 ± 1.2 | 1.3 | 33.0 | 49.0 | 11.5 | 3.0 | 2.2 | 65.7 | 3.3 ± 1.0 | 0.3 | 18.8 | 48.5 | 22.3 | 7.0 | 3.2 | 32.4 | |
| 2010 | 10,406 | 3.4 ± 1.4 | 2.9 ± 1.0 | 1.2 | 21.2 | 51.2 | 11.8 | 2.8 | 1.8 | 67.6 | 3.4 ± 1.3 | 0.4 | 15.3 | 51.5 | 21.5 | 7.3 | 4.0 | 32.8 | |
| 2011 | 9,792 | 3.4 ± 1.5 | 2.9 ± 1.2 | 1.2 | 32.3 | 48.6 | 12.3 | 3.5 | 2.1 | 66.5 | 3.3 ± 1.3 | 0.6 | 19.5 | 47.3 | 22.6 | 5.6 | 4.3 | 32.6 | |
| 2012 | 9,747 | 3.3 ± 1.3 | 2.9 ± 1.0 | 1.0 | 34.5 | 47.7 | 12.0 | 3.0 | 1.8 | 64.5 | 3.3 ± 1.4 | 0.5 | 19.0 | 52.3 | 18.6 | 4.6 | 4.9 | 28.1 | |
| 2013 | 9,289 | 3.3 ± 1.5 | 2.9 ± 1.1 | 1.1 | 35.2 | 47.4 | 11.7 | 30 | 1.8 | 63.7 | 3.2 ± 1.2 | 0.8 | 25.1 | 47.6 | 15.8 | 6.5 | 4.2 | 26.6 | |
| 2014 | 9,095 | 3.2 ± 1.5 | 2.8 ± 1.1 | 1.3 | 42.3 | 42.8 | 10.2 | 2.9 | 1.6 | 57.4 | 3.2 ± 1.1 | 0.5 | 23.8 | 46.5 | 20.5 | 5.2 | 3.5 | 29.2 | |
| 2015 | 8,659 | 3.2 ± 1.4 | 2.8 ± 1.0 | 1.6 | 40.9 | 43.0 | 10.3 | 2.8 | 1.7 | 57.5 | 3.2 ± 1.2 | 1.0 | 23.1 | 49.7 | 17.7 | 4.7 | 3.8 | 26.2 | |
|
Hospital (Missing: 193) |
A | 19,059 | 3.1 ± 1.7 | 2.5 ± 1.1 | 3.1 | 58.9 | 32.0 | 3.7 | 1.1 | 1.1 | 38.0 | 3.1 ± 1.3 | 1.3 | 27.5 | 51.7 | 12.3 | 3.1 | 4.0 | 19.5 |
| B | 18,380 | 3.9 ± 1.3 | 3.5 ± 1.0 | 0.4 | 2.6 | 54.6 | 32.1 | 7.3 | 3.1 | 97.0 | 3.9 ± 1.0 | 0.0 | 0.6 | 35.5 | 45.2 | 12.9 | 5.8 | 63.9 | |
| C | 8,840 | 3.1 ± 1.0 | 2.8 ± 0.8 | 0.3 | 28.8 | 64.7 | 4.1 | 1.1 | 1.0 | 70.9 | 3.2 ± 0.8 | 0.0 | 0.7 | 75.4 | 10.3 | 3.5 | 2.2 | 16.0 | |
| D | 3,330 | 4.0 ± 1.5 | 3.4 ± 1.1 | 0.5 | 7.1 | 61.1 | 23.9 | 4.4 | 3.1 | 92.5 | 3.8 ± 1.2 | 0.9 | 4.4 | 36.0 | 42.1 | 10.5 | 6.1 | 58.8 | |
| E | 6,673 | 3.5 ± 1.4 | 3.1 ± 1.0 | 0.8 | 20.4 | 63.9 | 9.0 | 2.7 | 3.3 | 78.3 | 3.7 ± 1.0 | 0.5 | 6.5 | 61.0 | 16.5 | 6.1 | 9.4 | 32.0 | |
| F | 5,723 | 3.2 ± 1.2 | 2.7 ± 0.8 | 0.8 | 38.6 | 53.9 | 4.9 | 1.4 | 0.7 | 60.6 | 3.0 ± 1.0 | 0.6 | 28.7 | 56.0 | 9.7 | 1.7 | 3.4 | 14.7 | |
| G | 9,146 | 2.8 ± 1.3 | 2.4 ± 0.9 | 1.5 | 66.0 | 25.6 | 4.0 | 1.7 | 1.2 | 32.5 | 2.8 ± 1.3 | 0.6 | 49.9 | 34.3 | 8.9 | 4.1 | 2.2 | 15.1 | |
| H | 11,681 | 3.0 ± 1.2 | 2.7 ± 0.9 | 1.1 | 39.4 | 47.4 | 9.4 | 1.7 | 0.9 | 59.4 | 3.2 ± 1.0 | 0.7 | 18.3 | 55.5 | 18.5 | 4.5 | 2.5 | 25.5 | |
| I | 6,047 | 3.6 ± 1.4 | 3.1 ± 1.0 | 1.5 | 16.5 | 63.0 | 12.9 | 3.6 | 2.5 | 82.0 | 3.5 ± 1.2 | 0.8 | 5.5 | 57.5 | 23.0 | 7.7 | 5.5 | 36.2 | |
| J | 12,035 | 3.3 ± 1.7 | 2.9 ± 1.3 | 1.2 | 43.0 | 36.5 | 11.8 | 4.1 | 3.3 | 55.8 | 3.4 ± 1.5 | 0.7 | 25.4 | 38.0 | 19.9 | 8.9 | 7.2 | 36.0 | |
| K | 8,027 | 3.5 ± 1.2 | 3.1 ± 0.9 | 0.8 | 17.7 | 63.8 | 12.4 | 3.8 | 1.7 | 81.5 | 3.4 ± 1.0 | 0.3 | 7.1 | 58.7 | 23.2 | 6.5 | 4.2 | 33.9 | |
| L | 12 | 4.9 ± 4.9 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| TOTAL | 109,246 | 2.9 ± 1.1 | 2.9 ± 1.1 | 1.3 | 34.9 | 48.0 | 11.5 | 2.5 | 1.9 | 64.4 | 3.3 ± 1.1 | 0.6 | 18.8 | 48.6 | 21.0 | 6.4 | 4.6 | 32.0 | |
Maternal health factors
Table 2 displays the classes of clinical explanatory factors related with the maternal health domain: mother’s age, hypertension/diabetes, amniocentesis, villi sample, fetoscopy, pre-delivery LoS, presentation, labour induction, neonatal status, number of obstetric checks performed, number of ultrasound (US) scans performed. In CEDAP fetal presentation is classified as follows: vertex; breech; shoulder; face; brow; Bregma; other. The group “other” was excluded from the analysis and coded as missing. A category named “cefalic” was created to incorporate vertex, face, brow and Bregma. Since shoulder presentation is incompatible with SVD and births by IVD are exceptional procedures requiring advanced obstetric craft, 39 shoulder presentations delivering by SVD and 1 shoulder presentation delivering by IVD were reclassified as cefalic. Leaving aside possible misclassification issues (which should not be ruled out), a transverse lie of the fetus will in fact ultimately result in a vaginal birth, either by spontaneous or manual version of the fetus to longitudinal lie.
Table 2. Distibution of length of stay (LoS) after childbirth by maternal health factors.
Number; mean LoS (M) ± standard deviation (SD); row percentage (row %). NA = Not applicable.
| FACTORS | STRATA | ALL BIRTHS | VAGINAL DELIVERY MODE | |||
|---|---|---|---|---|---|---|
| Number | M ± SD (days) |
SPONTANEOUS (N = 75,497) | INSTRUMENTAL (N = 7,281) | |||
| LoS >2 days (Row %) |
LoS > 3 days (Row %) |
|||||
|
Delivery mode (Missing: 1) |
Spontaneous | 75,497 | 2.9 ± 1.1 | 64.4 | ||
| Instrumental | 7,281 | 3.3 ± 1.3 | 32.0 | |||
| Caesarean | 26,467 | 4.7 ± 1.7 | ||||
|
Mother Age (years) (Missing: 32) |
15–19 | 1,254 | 3.4 ± 1.5 | 72.0 | 31.5 | |
| 20–24 | 9,485 | 3.3 ± 1.5 | 68.7 | 34.7 | ||
| 25–29 | 23,675 | 3.3 ± 1.4 | 65.6 | 27.8 | ||
| 30–34 | 38,381 | 3.3 ± 1.4 | 63.5 | 32.5 | ||
| 35–39 | 28,860 | 3.4 ± 1.5 | 62.8 | 32.6 | ||
| 40–44 | 7,214 | 3.6 ± 1.6 | 62.8 | 37.9 | ||
| 45+ | 345 | 4.3 ± 2.3 | 74.8 | 40.0 | ||
|
Hypertension/diabetes (Missing: 63) |
No | 106,690 | 3.3 ± 1.4 | 64.2 | 31.8 | |
| Yes | 2,493 | 4.5 ± 2.4 | 74.6 | 41.8 | ||
|
Villi sample (Missing: 6) |
No | 105,993 | 3.3 ± 1.5 | 64.3 | 32.0 | |
| Yes | 4,247 | 3.5 ± 1.5 | 67.5 | 32.0 | ||
|
Amniocentesis (Missing: 6) |
No | 91,986 | 3.3 ± 1.5 | 64.2 | 31.2 | |
| Yes | 17,254 | 3.5 ±1.5 | 65.5 | 36.6 | ||
| Fetoscopy(Missing: 6) | No | 108,892 | 3.3 ± 1.5 | 64.4 | 32.0 | |
| Yes | 348 | 3.4 ±1.5 | 66.8 | 37.9 | ||
|
Number of obstetric checks (Missing: 1) |
<4 | 20,856 | 3.5 ± 1.6 | 65.4 | 35.3 | |
| 4–7 | 65,800 | 3.3 ± 1.4 | 66.6 | 32.3 | ||
| 8+ | 22,589 | 3.3 ± 1.5 | 56.7 | 28.8 | ||
|
Number of US scans in pregnancy (Missing: 7) |
<4 | 19,003 | 3.1 ± 1.4 | 56.5 | 25.7 | |
| 4–5 | 52,873 | 3.3 ± 1.4 | 62.6 | 28.6 | ||
| 6+ | 37,363 | 3.6 ± 1.6 | 72.1 | 36.9 | ||
|
Analgesia (Missing: 184) |
No | 89,536 | 3.3 ± 1.5 | 63.6 | 28.5 | |
| Yes | 19,526 | 3.3 ± 1.4 | 67.7 | 38.1 | ||
|
Labour induction (Missing: 68) |
No | 81,859 | 2.9 ± 1.1 | 64.1 | 31.0 | |
| Yes | 27,319 | 4.6 ± 1.7 | 82.6 | 51.5 | ||
| Neonatal status | Liveborn | 108,944 | 3.4 ± 1.5 | 64.5 | 32.1 | |
| Stillborn | 302 | 2.8 ± 2.8 | 12.3 | 6.7 | ||
| Pre-delivery LoS (days) (Missing: 594) | <3 | 103,769 | 3.3 ± 1.4 | 64.3 | 31.8 | |
| 3–5 | 3,142 | 4.1 ± 2.0 | 68.8 | 35.6 | ||
| 6+ | 1,741 | 5.0 ± 2.9 | 69.3 | 45.8 | ||
|
Presentation (Missing:181) |
Cefalic | Spontaneous | 75,118 | 2.9 ± 1.0 | 64.4 | |
| Instrumental | 7,248 | 3.3 ± 1.3 | 32.0 | |||
| Breech | Spontaneous | 368 | 3.0 ± 1.4 | 61.0 | ||
| Instrumental | 27 | 3.8 ± 1.6 | 48.2 | |||
| Shoulder | Spontaneous | 0 | NA | NA | NA | |
| Instrumental | 0 | NA | NA | NA | ||
Child’s clinical factors fragility
We created a variable called “Child’s size” using anthropometric charts combining the distribution of four available factors: sex of child; parity, birth-weight and gestational age [28,29]. The variable was categorized in three levels: small for gestational age (SGA); appropriate for gestational age (AGA); large for gestational age (LGA).
Table 3 displays the classes of clinical factors of the child, in particular:
Table 3. Distibution of length of stay (LoS) after childbirth by clinical factors of the child.
Number; mean LoS (M) ± standard deviation (SD); row percentage (row %).
| FACTORS | STRATA | ALL BIRTHS | VAGINAL DELIVERY MODE | |||
|---|---|---|---|---|---|---|
| Number | M ± SD (days) |
SPONTANEOUS (N = 75,497) | INSTRUMENTAL (N = 7,281) | |||
| LoS > 2 days (Row %) |
LoS > 3 days (Row %) |
|||||
| CHILD’S SIZE FACTORS | ||||||
|
Gestational age (weeks) |
<29 | 563 | 4.7 ± 3.5 | 47.6 | 0 | |
| 29–32 | 1,130 | 4.6 ± 2.4 | 47.2 | 57.1 | ||
| 33–36 | 6,217 | 4.6 ± 2.2 | 76.9 | 56.2 | ||
| 37–40 | 82,637 | 3.3 ± 1.3 | 63.9 | 31.5 | ||
| 41+ | 18,699 | 3.2 ± 1.3 | 64.2 | 31.1 | ||
|
Birthweight (gr) (Missing: 5) |
<1000 | 525 | 4.8 ± 2.8 | 49.0 | 52.9 | |
| 1,000–1,499 | 668 | |||||
| 1,500–1,999 | 1,330 | |||||
| 2,000–2,499 | 4,524 | 4.6 ± 2.2 | 78.1 | 42.8 | ||
| 2,500–3,999 | 95,954 | 3.3 ± 1.3 | 64.3 | 31.5 | ||
| 4,000–4,499 | 6,576 | 3.3 ± 1.3 | 62.2 | 35.1 | ||
| 4,500+ | 664 | |||||
|
Placenta weight (gr) (Missing: 172) |
<500 | 22,862 | 3.5 ± 1.7 | 68.1 | 32.5 | |
| 500–599 | 35,744 | 3.2 ± 1.3 | 63.7 | 31.2 | ||
| 600–999 | 49,048 | 3.3 ± 1.4 | 63.1 | 32.3 | ||
| 1,000–1,500 | 1,420 | 4.9 ± 2.1 | 70.8 | 57.1 | ||
| Child’s size * | SGA | 9,122 | 3.7 ± 1.7 | 68.6 | 34.4 | |
| AGA | 88,138 | 3.3 ± 1.4 | 63.8 | 30.9 | ||
| LGA | 11,986 | 3.4 ± 1.4 | 66.0 | 38.1 | ||
| CHILD’S FRAGILTY FACTORS | ||||||
|
Apgar score 1 minute |
<7 | 6,807 | 4.0 ± 2.3 | 65.8 | 32.9 | |
| 7+ | 102,439 | 3.3 ± 1.4 | 64.3 | 31.9 | ||
|
Apgar score 5 minute |
<8 | 2,386 | 4.1 ± 2.6 | 56.7 | 38.3 | |
| 8+ | 106.860 | 3.3 ± 1.4 | 64.5 | 31.8 | ||
|
ICU admission (Missing: 221) |
No | 103,900 | 3.3 ± 1.4 | 64.4 | 32.1 | |
| Yes | 5,125 | 4.5 ± 2.5 | 62.8 | 31.5 | ||
|
Resuscitation (Missing: 54) |
No | 106,774 | 3.3 ± 1.4 | 64.4 | 32.0 | |
| Yes | 2,418 | 4.5 ± 2.7 | 63.5 | 33.1 | ||
| Multiple births (Missing: 898) | Singleton | Female | 51,806 | 3.3 ± 1.4 | 64.3 | 31.1 |
| Male | 54,797 | |||||
| Twins or more | 1,745 | 5.2 ± 2.0 | 74.0 | 58.3 | ||
* SGA = Small for Gestational Age; AGA = Appropriate for Gestational Age; LGA = Large for Gestational Age
Child’s size factors: gestational age; birthweight; placenta weight; child’s size; and
Child’s fragility factors: Apgar score at 1 minute; Apgar score at 5 minutes; resuscitation; intensive care unit admission (ICU); multiple birth.
Obstetric history
Table 4 displays the classes of factors pertaining with the obstetric history of the woman: previous livebirths; previous CS; pervious stillbirths; previous pre-term births; previous spontaneous abortions; previous neonatal deaths.
Table 4. Distribution of length of stay (LoS) after childbirth by socio-demographic and obstetric history factors.
Number (N); mean LoS (M) ± standard deviation (SD); row percentage (row %).
| FACTORS | STRATA | ALL BIRTHS | VAGINAL DELIVERY MODES | ||||
|---|---|---|---|---|---|---|---|
| Number | M ± SD (days) |
SPONTANEOUS (N = 75,497) | INSTRUMENTAL (N = 7,281) | ||||
| LoS > 2 days (Row %) |
LoS > 3 days (Row %) |
||||||
| SOCIO-DEMOGRAPHIC FACTORS | |||||||
|
Father’s age (years) (Missing: 1,949) |
15–19 | 199 | 3.3 ± 1.3 | 74.7 | 36.4 | ||
| 20–24 | 2,798 | 3.3 ± 1.4 | 67.9 | 34.3 | |||
| 25–29 | 12,982 | 3.3 ± 1.4 | 66.2 | 28.1 | |||
| 30–34 | 31,601 | 3.3 ± 1.4 | 65.5 | 31.6 | |||
| 35–39 | 34,560 | 3.3 ± 1.5 | 63.3 | 33.6 | |||
| 40–44 | 17,866 | 3.4 ± 1.5 | 62.3 | 31.3 | |||
| 45–49 | 5,353 | 3.5 ± 1.5 | 63.1 | 32.2 | |||
| 50–54 | 1,361 | 3.5 ± 1.7 | 61.6 | 37.5 | |||
| 55+ | 577 | 3.5 ± 1.5 | 66.6 | 42.9 | |||
|
Mother’ snationality (Missing: 116) |
EU | Italian | 86,083 | 3.3 ± 1.4 | 63.9 | 32.1 | |
| Non-Italian | 5,983 | 3.2 ± 1.2 | 63.9 | 27.6 | |||
| Non-EU | 17,064 | 3.5 ± 1.7 | 67.1 | 33.2 | |||
|
Marital status (Missing: 8,155) |
Not married | 12,036 | 3.4 ± 1.6 | 62.1 | 28.9 | ||
| Married | 70,340 | 3.3 ± 1.5 | 63.5 | 33.3 | |||
| Separated | 1,136 | 3.5 ± 1.9 | 58.9 | 33.7 | |||
| Widow | 82 | ||||||
| Divorced | 669 | ||||||
| Living together | 16,846 | 3.3 ± 1.4 | 67.2 | 30.5 | |||
|
Mother’s education (Missing: 24) |
University or more | 29,150 | 3.3 ± 1.4 | 64.5 | 35.4 | ||
| Secondary | 52,988 | 3.3 ± 1.4 | 65.0 | 31.3 | |||
| Junior Secondary | 25,107 | 3.5 ± 1.6 | 62.6 | 29.1 | |||
| Primary/none | 1,977 | 3.6 ± 1.7 | 67.4 | 33.3 | |||
|
Father’s education (Missing: 6,772) |
University or more | 18,542 | 3.4 ± 1.5 | 64.1 | 36.3 | ||
| Secondary | 51,356 | 3.3 ± 1.4 | 64.6 | 33.1 | |||
| Junior Secondary | 30,767 | 3.3 ± 1.5 | 63.2 | 30.1 | |||
| Primary/none | 1,809 | 3.5 ± 1.6 | 67.5 | 32.2 | |||
|
Mother’s occupation (Missing: 34,592) |
Self-e/Enterpreneur | 9,037 | 3.3 ± 1.4 | 64.4 | 33.6 | ||
| Manager | 2,145 | 3.4 ± 1.3 | 69.0 | 45.7 | |||
| Employed-Clerk | 31,002 | 3.3 ± 1.4 | 65.7 | 32.0 | |||
| Blue Collar | 12,836 | 3.4 ± 1.4 | 66.7 | 32.2 | |||
| Other (employed) | 19,634 | 3.3 ± 1.4 | 60.6 | 29.4 | |||
|
Father’s occupation (Missing: 10,867) |
Self-e/Enterpreneur | 22,100 | 3.3 ± 1.4 | 63.9 | 33.2 | ||
| Manager | 3,338 | 3.5 ± 1.4 | 69.8 | 38.6 | |||
| Employed-Clerk | 22,53 | 3.3 ± 1.5 | 63.4 | 34.8 | |||
| Blue Collar | 32,812 | 3.4 ± 1.5 | 65.9 | 31.3 | |||
| Other (employed) | 17,592 | 3.3 ± 1.5 | 60.2 | 30.4 | |||
| Consaguinity | No | 109,110 | 3.4 ± 1.5 | 64.4 | 32.1 | ||
| Yes | 147 | 3.0 ± 1.3 | 48.2 | 22.2 | |||
| OBSTETRIC HISTORY FACTORS | |||||||
|
Previous livebirths |
0 | 58,217 | 3.6 ± 1.5 | 75.3 | 34.8 | ||
| 1 | 39,805 | 3.1 ± 1.3 | 54.6 | 20.2 | |||
| 2 | 8,644 | 3.1 ± 1.4 | 50.1 | 16.1 | |||
| 3 | 1,820 | 2.6 ± 1.6 | 48.4 | 12.5 | |||
| 4+ | 755 | 3.5 ± 1.5 | 46.9 | 0.0 | |||
|
Previous stillbirths |
0 | 108,502 | 3.3 ± 1.5 | 64.4 | 32.1 | ||
| 1+ | 744 | 3.8 ± 1.6 | 66.2 | 25.0 | |||
|
Previous caesarean sections |
0 | 100,003 | 3.3 ± 1.4 | 64.6 | 32.6 | ||
| 1 | 8,097 | 3.9 ± 1.5 | 57.0 | 21.3 | |||
| 2+ | 1,146 | 4.3 ± 1.4 | 85.3 | 25/0 | |||
|
Previous pre-term babies (Missing: 1,144) |
0 | 105,774 | 2.3 ± 1.5 | 64.2 | 32.0 | ||
| 1 | 2,041 | 3.5 ± 1.7 | 63.3 | 28.2 | |||
| 2+ | 287 | 3.7 ± 1.7 | 61.3 | 30.0 | |||
|
Previous spontaneous abortions |
0 | 92,694 | 3.4 ± 1.5 | 65.7 | 32.8 | ||
| 1 | 12,555 | 3.2 ± 1.5 | 56.3 | 25.8 | |||
| 2 | 2,897 | 3.3 ± 1.5 | 57.4 | 30.6 | |||
| 3+ | 1,099 | 3.5 ± 1.7 | 61.7 | 29.3 | |||
|
Previous neonatal deaths |
0 | 108,923 | 3.3 ± 1.5 | 64.4 | 32.0 | ||
| 1+ | 323 | 3.7 ± 1.7 | 61.8 | 37.5 | |||
Socio-demographic background
Table 4 displays classes of socio-demographic factors: father’s age; mother’s nationality; marital status of the woman; mother’s education; mother’s occupation; father’s education; father’s occupation; consanguinity.
Statistical analysis
The mean and proportion of LoS longer than our proposed ED benchmarks were calculated for all available factors and for both modes of vaginal deliveries.
We ran two sets of logistic regression analyses to identify factors associated with LoS longer than the ED cutoffs, defined as > 2 days for SVD and >3 days for IVD. We chose the most widely adopted ED benchmarks for SVD. We proposed a 3 days ED threshold for IVD, as a measure of compromise between SVD (2 days) and CS (4 days) cutoffs recommended by the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists [18].
Hospital A was chosen as reference since it is a national excellence centre for maternal and child health. Moreover hospital A managed a higher volume of births (N = 19,059) in FVG during 2005–2015, had the second shortest mean LoS for SVD and the third shortest mean LoS for IVD.
Initially a logistic regression was run for each factor in turn, using LoS as endpoint and adjusting only for hospital. Significant factors and potential confounders were then selected to be included in the final multiple logistic regression model.
Some factors were deliberately dropped from the final multivariate logistic model for the following different reasons:
Apgar score at 1 minute and resuscitation due to collinearity with Apgar score at 5 minutes and intensive care unit (ICU) admission respectively, which both had stronger effect size and we thought they were more plausible to be retained in the final model;
child’s size, due to collinearity with birthweight and gestational age, both with stronger effect size;
previous spontaneous abortions, as the relative effect was not consistent across the two vaginal delivery modes;
father’s education, father’s occupation, marital status and pre-term history, since in addition of being affected by a large number of missing values, their significance was inconsistent across the two vaginal delivery modes and their effect size was negligible.
Results were expressed as odds ratios (OR) with 95% confidence interval (95%CI), comparing each stratum specific to the baseline reference category. Since the percentage of missing values was less than 10% for each factor analyzed, complete case analysis was adopted. Although the two multiple logistics models were adjusted for all factors, only the estimates for hospitals and calendar year are displayed in this paper.
Sensitivity analysis was then fitted by excluding marital status and pre-term history from both final multivariable logistic regression models.
Population attributable risks (PAR) is an epidemiologic measure widely used to evaluate the impact of modifiable factors on study populations, assuming there is a perfect intervention to remove such factors [30]. PAR (percentage variation) with 95%CI were therefore calculated for each maternity centre in the ideal scenario of having the same performance as hospital A during calendar year 2015 for both delivery modes. In addition to the latter criteria, PAR was also calculated assuming each hospital had the same performance of A during 2015 and considering only low risk pregnancies (low risk conditions for the mother as well as the newborn with potential impact on LoS), which we defined as pregnancies undergoing SVD and meeting simultaneously all the following criteria:
Maternal age < 35 years;
Mother without hypertension/diabetes;
Singleton birth;
Gestational age: 37–40 weeks;
Birthweight: 2,500–3,999 g;
Pre delivery LoS ≤2 days;
No labour induction;
Apgar score at 1 minute ≥7;
Apgar score at 5 minute ≥8;
Child not admitted to ICU;
No resuscitation performed.
In addition to all the above criteria, our definition of low risk pregnancies in the calculation of PAR for IVD excluded also women administered with labor analgesia.
Consideration of the most recent estimates of deliveries (calendar year 2015) could allow to envisage future patterns of LoS.
Stata 14.2 (College Station, Texas, USA) was employed for the analysis.
Results
A total of 109,810 birth records were available in our database. After excluding 260 duplicates, 293 births outside hospital and 11 births in minor centers without maternity services, we were left with a final number of 109,246 hospital birth records for the analysis (Fig 1). The total number of SVD in FVG during 2005–2015 was 75,497 (69.1%), total IVD were 7,281 (6.7%) and CS were 26,467 (24.2%). As mentioned earlier, CSs will not be be treated in this study.
In addition to the relevant number of births, Tables 1–4 display the distribution of LoS by explanatory factors in terms of mean and proportion of stays longer than the ED benchmarks for both vaginal delivery modes (spontaneous as well as instrumental).
Table 1 shows the mean LoS and the proportion of LoS > ED according to calendar year (upper panel) and facility centre (lower panel). The latter variations were considerably larger than the former ones for both SVD as well as IVD. The mean LoS of all hospitals consistently exceeded our proposed ED benchmarks for both delivery modes, with the only exception being hospital G for IVD.
Table 2 shows mild variation of mean LoS and proportion of LoS > ED among the strata of each explanatory factor, in particular maternal age, hypertension/diabetes, pre-delivery LoS, presentation, neonatal status.
Table 3 shows that the mean LoS was higher for gestational age less than 36 weeks, for birthweight less than 2,000 gr and for placentas weighing more than 1 Kg. Big placenta weights may mask the influence of multiple birth, which also showed a high mean LoS and high proportion LoS longer than ED.
Table 4 shows minor changes in mean LoS and probability of surpassing the ED cutoffs for socio-demographic factors of parents and major differences for obstetric history factors, in particular with increasing parity and with history of CS.
Table 5 shows factors associated with LoS longer than our proposed ED benchmarks, based on multivariable logistic regression analysis. Only estimates for hospital and calendar year (both adjusted for all other factors) are displayed, The adjusted OR of having a LoS higher than 2 days for SVD ranged from 2.39 (95%CI: 2.24–2.55) for hospital J up to 89.38 (95%CI: 78.49–101.78) for hospital B. Although with attenuated effect size, similar patterns were also observed for IVD. Moreover, a significant decreasing trend over time of LoS>ED was observed in the whole FVG for both delivery modes.
Table 5. Multiple logistic regression analysis.
Outcome: length of hospital htay (LoS) longer than ED benchmarks (2 days for spontaneous vaginal deliveries; 3 days for instrumental vaginal deliveries). Effect estimates for hospital and calendar year adjusted for all other factors. Adjusted odds ratios (aOR*) and population attributable risks (PAR1$, PAR2,** PAR3,$ PAR4**) with 95% confidence intervals (95%CI). NA = Not available; observations = complete (case analysis) observations.
| FACTORS | STRATA | VAGINAL DELIVERY MODE | |||||
|---|---|---|---|---|---|---|---|
| SPONTANEOUS | INSTRUMENTAL | ||||||
| aOR (95%CI) (LoS >2 vs. ≤ 2) (73,281 observations) |
PAR1 (95%CI) | PAR2 (95%CI) | aOR (95%CI) (LoS >3 vs. ≤ 3) (7,050 observations) |
PAR3 (95%CI) | PAR4 (95%CI) | ||
| HOSPITAL | A | reference | reference | reference | reference | reference | reference |
| B | 89.38 (78.49; 101.78) | +64.5% (+63.4%; +65.6%) | +65.8% (+64.6%; +67.0%) | 7.90 (6.38; 9.78) | +44.8% (+41.0%; +48.5%) | +43.2% (+39.4%; +46.9%) | |
| C | 4.86 (4.51; 5.23) | +37.5% (+35.9%; +39.0%) | +38.1% (+36.5%; +39.7%) | 0.83 (0.59; 1.17) | -0.0% (-5.2%; +4.3) | -0.3% (-4.3%; +3.7.%) | |
| D | 26.47 (22.35; 31.46) | +59.0 (+57.5%; +60.6%) | +60.2% (+58.6%; +61.8%) | 7.85 (5.08; 12.12) | +44.7% (+35.1%; +53.4%) | +43.0 (+33.0%; +52.1%) | |
| E | 8.40 (7.68; 9.19) | +46.7% (+45.1%; +48.2%) | +47.5 (+45.9%; +49.1%) | 2.21 (1.67; 2.94) | +16.1% (+10.7%; +21.5%) | +14.5% (+9.5%; +19.4%) | |
| F | 2.93 (2.69; 3.20) | +27.4% (+25.6%; +29.3%) | +27.9% (+26.0%; +29.8%) | 0.79 (0.58; 1.08) | -1.1% (-5.2%; +3.1%) | -1.0 (-4.3%; +2.8%) | |
| G | 0.77 (0.72; 0.83) | -1.0 (-2.2; +1.0%) | -1.0% (-2.1%; +1.0%) | 0.72 (0.56; 0.95) | -2.2% (-5.7%; +1.4%) | -1.7 (-4.7%; +1.3%) | |
| H | 2.78 (2.61; 2.96) | +26.3% (+24.8; +27.7%) | +26.7% (+25.2%; +28.2%) | 1.53 (1.21; 1.94) | +9.0% (+5.0%; +13.0%) | +7.9% (+4.4%; +11.4%) | |
| I | 10.42 (9.49; 11.44) | +49.7% (+48.2%; +51.2%) | +50.6% (+49.0%; +52.1%) | 2.85 (2.15; 3.78) | +21.5% (+15.8%; +27.1%) | +19.6% (+14.2%; +24.9%) | |
| J | 2.39 (2.24; 2.55) | +23.1% (+21.6%; +24.6%) | +23.5% (+22.0%; +25.0%) | 2.56 (2.03; 3.23) | +19.2% (+14.8; +23.5%) | +17.3% (+13.4%; +21.3%) | |
| K | 10.30 (9.45 11.21) | +49.5% (+48.1%; +51.0) | +50.4% (+48/9%; +51.9%) | 2.41 (1.88; 3.10) | +17.9% (+13.2%; +22.5%) | +16.1% (+11.8%; +20.4%) | |
| L | NA | NA | NA | NA | NA | NA | |
| Calendar year (2005–2015) | 0.96 (0.95; 0.96) | 0.97 (0.95; 0.99) | |||||
* Multiple logistic regression model adjusted for: Health care setting and time-frame factors (hospital and calendar year); Maternal health factors (mother’s age; hypertension/diabetes; amniocentesis; number of obstetric checks; number of ultrasound scans performed; no labour induction; labour analgesia; neonatal status; presentation; pre-delivery LoS); Child’s fragility factors (Apgar score at 5 minutes; ICU admission; multiple birth); Child’s size factors (gestational age; birthweight; placenta weight); Obstetric history factors (parity; history of caesarean sections); Socio-demographic factors (father’s age; mother’s nationality; mother’s educational level)
$ Population Attributable Risk 1 (PAR 1) and 3 (PAR 3). Proportional variation of LoS < ED after childbirth in the ideal scenario each hospital would be performing as hospital A during calendar year 2015
** Population Attributable Risk 2 (PAR 2) and 4 (PAR4). Proportional variation of LoS < ED after childbirth in the ideal scenario each hospital would be performing as hospital A during calendar year 2015. Estimations of PAR2 and PAR4 calculated only for low risk pregnancies, defined as conditions of the mother and/or the newborn simultaneously meeting all the following criteria: for spontaneous vaginal deliveries (PAR 2): mother’s age<35; no resuscitation performed; child not admitted to ICU; singleton birth; Apgar score at 1 minute ≥7; Apgar score at 5 minutes ≥8; no labour induction; no women affected by hypertension/diabetes; birthweight: 2,500–3,999gr; gestational age: 37–40 weeks; pre delivery LoS ≤2 days; for instrumental vaginal deliveries (PAR 4): in addition to all above criteria, the calculation of PAR4 was restricted to women not administered with labour analgesia.
As can be seen also from Table 5, in the ideal scenario each hospital would be performing as hospital A during calendar year 2015, a significant increase in ED rate for SVD would be seen for all hospitals but G (which conversely would have a slightly smaller, non-significant ED rate). The proportional increase in LoS<ED for SVD would range from 23.1% (centre J) up to 64.5% (centre B), and would be +59.0%, +49.7%, +49.5%, +46.7%, +37.5%, +27.4% and +26.3% for centres D, I, K, E, C, F and H respectively (PAR1). Almost overlapping figures of PAR for SVD were obtained by restricting the analysis to low risk pregnancies (PAR2). For IVD, the pattern of PAR (PAR3 and PAR4) was rather similar to SVDs’, although slightly mitigated (Table 5).
S1 Table shows the results of the sensitivity analysis. The removal of pre-term history and marital status from the final logistic regression model had little impact on the effect size of all other factors.
Discussion
Key findings
This population-based study analyzed all hospital births in FVG from 2005 to 2015. During these 11 years, the number of births in the region significantly diminished over time and the pooled mean LoS was 2.9 days for SVD and 3.3 days for IVD.
In FVG we found a decreasing trend over time in the proportion of women staying longer than the respective ED benchmarks for SVD as well as IVD. Nonetheless, the average regional LoS was still consistently higher than the ED benchmarks for both delivery modes. After removing the effect of all other factors, all regional hospitals but G were by far more likely than the referral hospital A to overcome the ED benchmarks for SVD. A similar, mitigated pattern was observed also for IVD.
In the ideal scenario of performing as hospital A during calendar year 2015, all FVG hospitals but G would have a significant increase in ED rate for both delivery modes, reaching the percentage of 64.5% and 59.0% for SVD in centres B and D, and being about 50% for facilities E, I and K (PAR1). Similar attenuated patterns were observed also for IVD (PAR 3). Almost the same figures were found in low risk pregnancies for SVD as well as IVD (PAR 2 and PAR 4). Hospital A managed the highest volume of SVD in FVG during 2005–2015 (17.8% = 13,445/75,383). However, despite having the second shortest mean LoS for SVD (2.5 days), 38.0% (= 5,090/13,409) women still remained admitted more than 2 days following childbirth (data not shown in any tables). The latter proportion reduced to 34.6% (= 1,925/5,568) among the subgroup of low risk pregnancies, and was still 34.0% (= 2,710/ 7,982) by including also women in the age band 35–39 years (relaxed definition of low risk pregnancies). Therefore, there seems to be room for substantial performance improvement in FVG hospitals, particularly for SVD.
Generalizability
The pooled mean LoS for SVD was 2.9 days in FVG, shorter than the average figures most recently reported for the whole of Italy (3.4 days) [4,19]. However, Organization for Economic Cooperation and Development (OECD) data from other countries with Beveridgean health systems similar to Italy’s reported average LoS for SVD shorter than FVG, in particular Sweden (2.3 days), Ireland (2.0 days), the Netherlands (1.9 days) and the UK (1.5 days) [19].
Since our results were controlled for the effect of all other factors, the between-hospital variability we found is likely attributable to the single health care provider itself. The Diagnosis Related Group (DRG) system, introduced in Italy in 1995, was designed to improve hospital efficiency and contain average LoS, a goal generally attained in several high income countries, including Italy [31]. However, despite the prospective payment health system based upon capitation grants in place in Italy, it can be argued that some maternity centres of FVG may still have had convenience in extended hospitalizations after childbirth, to show higher daily rates of bed occupancy as a measure of health care activity to negotiate their allocated budgets with the regional government. Although, fear of medico-legal backlashes, internal organizational malfunctions of hospital and scarce attention of ward staff on performance efficiency shall not be ruled out [31–34].
Variation in ED rates by region and matenity centre also exists elsewhere. For instance, in England it is estimated that currently almost 30% women remain hospitalized more than 2 days following childbirth [35]. In a recent Danish study on 2,786 pregnant women, the proportion discharged within 48h was almost 60%, with 25% of them being discharged between 13h and 50h after childbirth, and 34% remaining admitted for less than 12h (very early discharge, VED). Seventy percent of the latter Danish women expected ED, hinting that this approach has become established in Denmark [8]. ED and VED are the norm in Scandinavia [15] and are also common in many other high-income countries such as Australia, Canada, UK and Ireland [16,36]. In a recent Italian study, a significant increase in ED for SVD was found. However, the definition of ED threshold adopted was 3 days, hence longer than ours [17]. The latter Italian study reported higher odds of ED with increasing number of hospital births on the birthday of the index child, suggesting that ED may become feasible if higher hospital efficiency is needed in terms of bed turnover [17]. A higher number of birth per day generally determines shorter LoS, but seems not to translate into increased readmission rates [37].
Prospects
Italian women are still referred almost exclusively to hospitals both for pre-natal and post-natal care. Much of this care could instead be managed by health districts through general practitioners and community midwives, who could also provide home visits, especially during postnatal care [38]. This could also pave the way for optional home births, a practice rather popular in the Netherlands but still culturally unaccepted in Italy [39,40]. Effective postnatal care following ED would need adequate investment in community services to ensure continuity of care [36,41,42]. Northern European countries have invested more in midwifery care [36,38,40]. For instance, Scandinavian countries employ models of care based upon birth centres [15,40]. These centres (designed to simulate a domestic environment) are managed by midwives, welcome parental involvement, encourage natural childbirth and minimize postnatal facility stay. Midwives provide antenatal, perinatal, and postnatal home care. Standard care involving obstetricians is offered only in case of complications or if the woman desires analgesia [40]. In the UK prenatal care until childbirth is managed by midwives and women have the option to deliver in hospital or in midwifery led units, where the new mothers are discharged usually within 6 to 48 hours following delivery, receiving four to five postnatal midwifery home visits afterwards [36]. In some areas of Italy, experimental programs are being conducted where ED is accompanied by follow-up phone calls to monitor the postnatal conditions of the woman and early pediatric care is provided [17].
The FVG Regional Health System (RHS) has recently been revised to increase quality and efficiency of the health care delivered. A higher level of coordination among health services was introduced with the aim of overcoming fragmentation and meaningless competition between health-care facilities. Maternity services are now required to ensure at least 1,000 births per year in order to maintain their status and all complex cases should be forwarded to referral centres. The revised RHS has allocated substantial resources to set up intermediate health services (country hospitals, community rehabilitation centres and care homes) for cost-effective management of long-term conditions following “protected” discharge from hospitals [43]. Nonetheless, this integrated system of secondary and primary care services was not extended to maternal and child health. Considering child delivery is also one of the major causes of hospitalization in high-income countries, an organizational revision of the RHS is recommended [17].
Strengths and limitations
The database we analyzed is highly reliable, since it contains administrative hospital data collected by trained health care staff. Any long LoS is therefore very unlikely to be an outlier, but rather reflects the clinical condition of the woman requiring extended hospitalization. Nevertheless, the proportion of women remaining admitted for more than 10 days was just 0.4% (= 422/108,469) in our study. Since it comprises all births records of FVG, the design and methods of this study are strong. The size of the database and the large number of variables available allows this work to bring important and accurate conclusions. Furthermore, the percentage of missing values was negligible, and mainly pertained to socio-demographic factors. It can be argued that a proportion of women may have been reluctant to disclose some personal information. Completeness of clinical data was instead very close to 100%.
Although this study focused on a particular Italian region (FVG), we believe our findings can be to some extent generalized to other Italian and European regions with health systems similar to FVG. We employed an ED threshold for IVD which has not been validated yet, but we believe it makes sense, since it is a compromise between the internationally recognized ED cutoffs for SVD and CS. Moreover, the methodology we proposed to contrast hospital performance is sound as it took into account a considerable number of determinants, including clinical factors, which could also be applied in the evaluation of different types of health systems. The innovative conceptual framework we devised to explain the relationship between various determinants and LoS is a significant advance from previous models [26].
Conclusions
Despite a decreasing trend in hospitalization length for both vaginal delivery modes over time, women probably still stayed longer than needed in FVG hospitals. In low risk pregnancies, ED followed by community midwifery care may be a realistic and acceptable alternative model than standard delivery. Offering women the option of ED (in line with other European countries with healthcare systems similar to Italy’s) could not only meet the respectful preference of a proportion of Italian women, but could also reduce the risk of nosocomial infections, increase patient satisfaction with the health care services, prevent stress and sleeping disorders in the woman, contribute to improved performance of Italian hospitals and, ultimately, also contain healthcare costs associated with childbirth [44]. ED followed by domiciliary care is more cost-effective than standard delivery [38,45,46]. Furthermore, although the impact of ED on maternal and newborn outcomes is still inconclusive [33,35,47–50], there is evidence that ED is safe for full-term as well as early term babies [37].
The systematic methodology we proposed for FVG could be applied for confirmatory studies in other geographical areas, possibly at country level. In the future it would be useful to correlate our findings (and eventual applications of ED policies) with cost effective analysis and maternal/child health outcomes, employing also patient satisfaction surveys.
Supporting information
(DOC)
Sensitivity model A: multiple logistic regression analysis adjusted for hospital and all other factors Including pre-term history and marital status. Sensitivity model B: multiple logistic regression analysis adjusted for hospital and all other factors, but pre-term history and marital status. Odds ratio (OR) with 95% confidence interval (95%CI); LoS = length of hospital stay; NA = not available; observations = complete (case analysis) observations.
(DOCX)
Data Availability
This study analyzed third party data, extracted from the Regional Repository of Friuli Venezia Giulia (FVG), a database anonymously storing potentially sensitive information. The authors do not have special privileges to access these data, which can be obtained also by others, subject to permission from the Regional Health Authority of FVG. Contact: Epidemiology & Health Information Service; Central Health Directorate; Health & Social Integration; Social & Family Policies; Via Pozzuolo 330, 33100, Udine, Italy. Tel: +39 0432 805661; email: salute@certregione.fvg.it.
Funding Statement
This research was supported by Special programs 5 ‰ for health research 2014 of IRCCS Burlo Garofolo. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization (2002). Essential Antenatal, Perinatal and Postpartum Care. Available from: http://www.euro.who.int/__data/assets/pdf_file/0013/131521/E79235.pdf (last accessed on 9 May 2018).
- 2.World Health Organization (2013). WHO recommendations on postnatal care of the mother and newborn. Geneva: World Health Organization. [PubMed] [Google Scholar]
- 3.Chervenak FA, McCullough LB, Brent RL, Levene MI, Arabin B. Planned home birth: the professional responsibility response. Am J Obstet Gynecol. 2013; 208:31–38. 10.1016/j.ajog.2012.10.002 [DOI] [PubMed] [Google Scholar]
- 4.Campbell O, Cegolon L, McLeod D, Benova L. Length of Stay After Childbirth in 92 Countries and Associated Factors in 30 Low- and Middle- Income Countries: Compilation of Reported Data and a Cross-sectional Analysis from Nationally Representative Surveys. PLoS Medicine. 2016: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Britton JR, Britton HL, Beebe SA. Early discharge of the term newborn: a continued dilemma. Pediatrics. 1994; 94(3):291–5. [PubMed] [Google Scholar]
- 6.Hellman LM, Kohl SG, Palmer J. Early hospital discharge in obstetrics. Lancet. 1962; 1:227–232. [DOI] [PubMed] [Google Scholar]
- 7.WHO Europe (2012). Modern health care delivery systems, care coordination and the role of hospitals. Available from: http://www.euro.who.int/__data/assets/pdf_file/0008/158885/BRU-report-Modern-health-care-delivery-systems.pdf?ua=1 (last accessed on 9 May 2018).
- 8.Nilsson IMS, Kronborg H, Knight CH, Strandberg-Larsen K. Early discharge following birth–What characterises mothers and newborns? Sexual & Reproductive Healthcare. 2017; 11: 60–68. [DOI] [PubMed] [Google Scholar]
- 9.Brown S, Small R, Argus B, Davis PG, Krastev A. Early postnatal discharge from hospital for healthy mothers and term infants. Cochrane Database Syst Rev, Issue 3, 2009. Art. No.: CD002958. [DOI] [PubMed] [Google Scholar]
- 10.Sievertsen HH, Wüst M. Discharge on the day of birth, parental response and health and schooling outcomes. J Health Econ. 2017. [DOI] [PubMed] [Google Scholar]
- 11.Jones E, Taylor B, MacArthur C, Pritchett R, Cummins C. The effect of early postnatal discharge from hospital for women and infants: a systematic review protocol. Systematic Reviews (2016) 5:24 10.1186/s13643-016-0193-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Royal College of Midwife (2014). Pressure Points—Postnatal Care Planning. Available from: https://www.rcm.org.uk/sites/default/files/Pressure%20Points%20-%20Postnatal%20Care%20Planning%20-%20Web%20Copy.pdf (last accessed on 9 May 2018).
- 13.Knight M, Kenyon S, Brocklehurst P, Neilson J, Shakespeare J, Kurinczuk J, et al. Saving lives, improving mothers’ care—lessons learned to inform future maternity care from the UK and Ireland Confidential Enquires into Maternal Deaths and Morbidity 2009–2012 Oxford: National Perinatal Epidemiology Unit, University of Oxford; 2014. [Google Scholar]
- 14.USA Today (2018). How does Duchess Kate do it? From giving birth to camera-ready in heels in under 8 hours. Available from: https://eu.usatoday.com/story/life/2018/04/23/how-does-duchess-kate-do/543533002/ (last accessed on 4 June 2018).
- 15.Lindgren C. Aftercare of Newborn infants in a patient hotel. Tidsskr Nor Laegenforen. 2000; 120 (12): 1409–11. [PubMed] [Google Scholar]
- 16.Fink A. Early hospital discharge in maternal and newborn care. J Obstet Gynecol Neonatal Nurs 2011; 40:149–56. 10.1111/j.1552-6909.2011.01225.x [DOI] [PubMed] [Google Scholar]
- 17.Pertile R, Pavanello L, Soffiati M, Manica L, Piffer S. Length of stay for childbirth in Trentino (North-East of Italy): the impact of maternal characteristics and organizational features of the maternity unit on the probability of early discharge of healthy, term infants. Eur J Pediatr. 2017. [DOI] [PubMed] [Google Scholar]
- 18.Grullon KE, Grimes DA. The safety of early postpartum discharge: a review and critique. Obstetr Gynecol. 1997;90(5):861–5. [DOI] [PubMed] [Google Scholar]
- 19.OECD (2016). Health at a Glance: Europe 2016—State of Health in the EU Cycle. Available from: https://www.oecd.org/els/health-systems/Health-at-a-Glance-Europe-2016-CHARTSET.pdf (last accessed on 9 May 2018).
- 20.Glied S, Smith PC. The Oxford Handbook of Health Economics. Oxford University Press, 2011. [Google Scholar]
- 21.Morassutto C, Monasta L, Ricci G, Barbone F, Ronfani L. Incidence and Estimated Prevalence of Endometriosis and Adenomyosis in Northeast Italy: A Data Linkage Study. PLoS One. 2016; PLoS One. 2016 April 21;11(4):e0154227 10.1371/journal.pone.0154227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valent F. The use of Friuli Venezia Giulia health information system to build up a regional register of individuals affected by diabetetes mellitus. Available from: http://www.epicentro.iss.it/ben/2016/ottobre/2.asp (last accessed on 4 June 2018).
- 23.Friuli Veneza Giulia Region (2012). Atlas of Social and Health Services in Friuli Venezia Giulia. Available from: http://www.aas2.sanita.fvg.it/opencms/export/sites/ass5/it/servizi_al_cittadino/foreing_people/categoria1/ENGLISH.-3-EDIZIONE.pdf (last accessed on 9 May 2018).
- 24.Novak J, Canas A. The theory underlying concept maps and how to construct and use them. 2008 Jan 22 [cited 27 Aug 2015]. Pensacola (Florida): Institute for Human and Machine Cognition. Available from: http://cmap.ihmc.us/Publications/ResearchPapers/TheoryUnderlyingConceptMaps.pdf.
- 25.Miles M, Huberman A. Qualitative data analysis: an expanded source book. 2nd edition Newbury Park (California): Sage Publications; 1994. [Google Scholar]
- 26.Victoria CG, Huttly SR, Fuchs SC, A Olinto MC. The Role of Conceptual Frameworks in Epidemiological Analysis: A Hierarchical Approach. Int J Epidemiology. 1997; 26: 224–27. [DOI] [PubMed] [Google Scholar]
- 27.Schorr E. Theoretical framework for determining hospital length of stay (LOS). BMC Proc. 2012; 6(Suppl 4):P32. [Google Scholar]
- 28.Bertino E, Spada E, Occhi L, Coscia A, Giuliani F, Gagliardi L et al. Neonatal Anthropometric Charts: The Italian Neonatal Study Compared With Other European Studies. JPGN 2010;51: 353–361. 10.1097/MPG.0b013e3181da213e [DOI] [PubMed] [Google Scholar]
- 29.International Network of Engineers and Scientists (INES). 2017. Available from: www.inescharts.com/docs/INeS_CENTILI.XLS (last accessed on 9 May 2018).
- 30.Mansournia MA, Altman DG. Population attributable fraction. BMJ. 2018; 360:k757 10.1136/bmj.k757 [DOI] [PubMed] [Google Scholar]
- 31.Busse R, Geissler A, Quentin W, Wiley M. Diagnosis-Related Groups in Europe. European Observatory on Health systems and Policies (2013). Available from: http://www.euro.who.int/__data/assets/pdf_file/0004/162265/e96538.pdf (last accessed on 27 July 2018).
- 32.Mathauer I, Wittenbecher F. Hospital payment systems based on diagnosis-related groups: experiences in low- and middle-income countries. Bull WHO. 2013. Bull World Health Organ 2013;91:746–756A. 10.2471/BLT.12.115931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Busato A, von Below G. The implementation of DRG-based hospital reimbursement in Switzerland: A population-based perspective. Health Research Policy and Systems 2010, 8:31 10.1186/1478-4505-8-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cavalieri M, Gitto L, Guccio C. Reimbursement systems and quality of hospital care: an empirical analysis for Italy. Health policy. 2013; 111: 273; 289. 10.1016/j.healthpol.2013.05.014 [DOI] [PubMed] [Google Scholar]
- 35.Health and Social Care Information Centre. Hospital Episode Statistics NHS Maternity Statistics– 2012–13. 2013. http://www.hscic.gov.uk/catalogue/PUB12744/nhs-mate-eng-2012-13-summ-repo-rep.pdf. (last accessed on 9 May 2018).
- 36.Bowers J, Cheyne H, Mould G, Page M. Continuity of care in community midwifery. Health Care Manag Sci (2015) 18:195–204. 10.1007/s10729-014-9285-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harron K, Gilbert R, Cromwell D, Oddie S, van der Meulena J. Newborn Length of Stay and Risk of Readmission. Paediatric and Perinatal Epidemiology, 2017, 31, 221–232. 10.1111/ppe.12359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bowers J, Cheyne H. Reducing the length of postnatal hospital stay: implications for cost and quality of care. BMC Health Services Research. 2016, 16:16 10.1186/s12913-015-1214-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiegers TA, Keirse MJNC, van der Zee J, et al. Outcome of planned home and planned hospital births in low risk pregnancies in the Netherlands. BMJ 1996;313:1309–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hildingsson I, Waldenstrom U, Radestad I. Swedish Women’s Interest in Home Birth and In-Hospital Birth Center. Birth. 2003. March;30(1):11–2. [DOI] [PubMed] [Google Scholar]
- 41.Sellwood M, Huertas-Ceballos A. Review of NICE guidelines on routine postnatal infant care. Arch Dis Child Fetal Neonatal Ed. 2008; 93: F10–3. 10.1136/adc.2006.111757 [DOI] [PubMed] [Google Scholar]
- 42.Sainz-Bueno JA, Romano MR, Teruel RG, Benjumea AG, Palacin AF, Gonzalez CA, et al. Early discharge from obstetrics-pediatrics at the Hospital de Valme, with domiciliary follow-up. Am J Obstetr Gynecol. 2005;193(3):714–26. [DOI] [PubMed] [Google Scholar]
- 43.FVG Regional Council (2014). Revision of the Regional Health System. Available from: http://www.consiglio.regione.fvg.it/iterdocs/Serv-LC/ITER_LEGGI/LEGISLATURA_XI/LEGGI_APPROVATE/059_060-061_LR.pdf (last accessed on 1 May 2018).
- 44.Bravo P, Uribe C, Contreras A. Early postnatal hospital discharge: the consequences of reducing length of stay for women and newborns. Rev Esc Enferm USP 2011; 45(3):758–63. [DOI] [PubMed] [Google Scholar]
- 45.Petrou S, Boulvain M, Aricot P, Borst F, Perneger T, Irion O. Home-based care after a shortened hospital stay versus hospital-based care postpartum: an economic evaluation. BJOG. 2004; 111(8):800–6. 10.1111/j.1471-0528.2004.00173.x [DOI] [PubMed] [Google Scholar]
- 46.Boulvain M, Perneger TV, Othenin-Girard V, Petrou S, Berner M, Irion O. Home-based care after a shortened hospital stay versus hospital-based care postpartum: an economic evaluation. BJOG. 2004; 111(8): 807–13. 10.1111/j.1471-0528.2004.00227.x [DOI] [PubMed] [Google Scholar]
- 47.Datar A, Sood N. Impact of postpartum hospital-stay legislation on newborn length of stay: readmission, and mortality in California. Pediatrics, 118 (2006), pp. 63–72. 10.1542/peds.2005-3044 [DOI] [PubMed] [Google Scholar]
- 48.Sánchez Luna M, Pallás Alonso CR, Botet Mussons F, Echániz Urcelay I, Castro Conde JR, Narbona E. Recomendaciones para el cuidado y atención al recién nacido sano en el parto y en las primeras horas después del nacimiento. An Pediatr (Barc). (2009); 71: 349–361. [DOI] [PubMed] [Google Scholar]
- 49.Watt S, Sword W, Krueger P. Longer postpartum hospitalization options–who stays, who leaves, what changes? BMC Pregnancy Childbirth, 5 (2005), pp. 13 10.1186/1471-2393-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paul IM, Lehman EB, Hollenbeak CS, Maisels MJ. Preventable newborn readmissions since passage of the Newborn's and Mother's Health Protection Act. Pediatrics, 118 (2006), pp. 2349–2358. 10.1542/peds.2006-2043 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Sensitivity model A: multiple logistic regression analysis adjusted for hospital and all other factors Including pre-term history and marital status. Sensitivity model B: multiple logistic regression analysis adjusted for hospital and all other factors, but pre-term history and marital status. Odds ratio (OR) with 95% confidence interval (95%CI); LoS = length of hospital stay; NA = not available; observations = complete (case analysis) observations.
(DOCX)
Data Availability Statement
This study analyzed third party data, extracted from the Regional Repository of Friuli Venezia Giulia (FVG), a database anonymously storing potentially sensitive information. The authors do not have special privileges to access these data, which can be obtained also by others, subject to permission from the Regional Health Authority of FVG. Contact: Epidemiology & Health Information Service; Central Health Directorate; Health & Social Integration; Social & Family Policies; Via Pozzuolo 330, 33100, Udine, Italy. Tel: +39 0432 805661; email: salute@certregione.fvg.it.