Abstract
The human 1q21.1 deletion of ten genes is associated with increased risk of schizophrenia. This deletion involves the β-subunit of the AMP-activated protein kinase (AMPK) complex, a key energy sensor in the cell. Although neurons have a high demand for energy and low capacity to store nutrients, the role of AMPK in neuronal physiology is poorly defined. Here we show that AMPK is important in the nervous system for maintaining neuronal integrity and for stress survival and longevity in Drosophila. To understand the impact of this signaling system on behavior and its potential contribution to the 1q21.1 deletion syndrome, we focused on sleep, an important role of which is proposed to be the reestablishment of neuronal energy levels that are diminished during energy-demanding wakefulness. Sleep disturbances are one of the most common problems affecting individuals with psychiatric disorders. We show that AMPK is required for maintenance of proper sleep architecture and for sleep recovery following sleep deprivation. Neuronal AMPKβ loss specifically leads to sleep fragmentation and causes dysregulation of genes believed to play a role in sleep homeostasis. Our data also suggest that AMPKβ loss may contribute to the increased risk of developing mental disorders and sleep disturbances associated with the human 1q21.1 deletion.
Author summary
The human 1q21.1 chromosomal deletion is associated with increased risk of schizophrenia. Because this deletion affects only a small number of genes, it provides a unique opportunity to identify the specific disease-causing gene(s) using animal models. Here, we report the use of a Drosophila model to identify the potential contribution of one gene affected by the 1q21.1 deletion–PRKAB2 –to the pathology of the 1q21.1 deletion syndrome. PRKAB2 encodes a subunit of the AMP-activated protein kinase (AMPK) complex, the main cellular energy sensor. We show that AMPK deficiency reduces lifespan and causes structural abnormalities in neuronal dendritic structures, a phenotype which has been linked to schizophrenia. Furthermore, cognitive impairment and altered sleep patterning are some of the most common symptoms of schizophrenia. Therefore, to understand the potential contribution of PRKAB2 to the 1q21.1 syndrome, we tested whether AMPK alterations might cause defects in learning and sleep. Our studies show that lack of PRKAB2 and AMPK-complex activity in the nervous system leads to reduced learning and to dramatic sleep disturbances. Thus, our data links a single 1q21.1-related gene with phenotypes that resemble common symptoms of neuropsychiatric disorders, suggesting that this gene, PRKAB2, may contribute to the risk of developing schizophrenia.
Introduction
Recent genome-wide association studies have identified copy-number variants (CNVs) associated with high risk of schizophrenia and other neuropsychiatric disorders [1–4]. Sleep disturbance is one of the most common problems observed in individuals with psychiatric disorders [5]. Although the 1q21.1 CNV deletion, spanning 10 genes over 1.35 million base pairs, has been demonstrated to genetically predispose carriers to sleep disturbances, intellectual disability, autism, and schizophrenia [4], the impact of individual genes in this interval on the development of these disorders is unclear. PRKAB2, encoding a β-subunit of the AMP-activated protein kinase (AMPK) complex, is located on the 1q21.1 chromosome arm and is a promising candidate gene that may contribute to sleep disturbances and some of the behavioral effects observed in 1q21.1 patients. The AMPK complex is the main cellular energy sensor, functioning as a metabolic master switch that is required for maintenance of energy homeostasis [6]. When activated as a response to increased energy demands, by detecting increased intracellular AMP:ATP and ADP:ATP ratios, AMPK upregulates catabolic processes that generate adenosine triphosphate (ATP) while downregulating anabolic processes to maintain energy balance [7]. Interestingly, energy metabolism dysfunction is among the most consistent features observed in psychiatric disorders and has been shown to include pathways for ATP production [8]. The evolutionarily conserved AMPK complex that regulates ATP comprises a catalytic (serine/threonine kinase) α-subunit, a scaffolding β-subunit, and a regulatory γ-subunit [9, 10]. Unlike mammalian genomes, which encode multiple isoforms of each AMPK subunit (α1, α2; β1, β2; γ1, γ2) that are, at least in part, functionally redundant, the genome of the fruit fly Drosophila melanogaster contains only one gene encoding each subunit, encoded by AMPKα, alicorn (alc), and SNF4Aγ, respectively, making this organism an ideal model in which to study AMPK function [11].
While the function of AMPK in whole-body metabolism and cellular energy sensing is well-known, its role in neuronal maintenance, and the manner in which nervous-system-specific effects of AMPK manifest in global phenotypes, are poorly defined. Neurons in particular may be vulnerable to disturbances in AMPK function, as these cells have high metabolic demands and are ineffective at storing energy [12]–the human nervous system accounts for at least 20% of the body’s energy consumption while only making up 2% of the body’s mass [13]. Studies in mice have shown that AMPK is activated in the brain upon introduction of stresses such as glucose deprivation, ischaemia, and hypoxia [14, 15]. Furthermore, loss of the AMPKβ1 subunit in mice was shown to have strong effects on brain development and structure [16]. Studies using Drosophila have shown that the AMPK complex affects cellular homeostasis, longevity, and neurodegeneration [17–19]. In Drosophila, upregulation of AMPKα in the nervous system has been linked to a slowing of systemic aging and thus to prolonged lifespan [20], which indicates that AMPK function in the nervous system has a broad, systemic impact. Furthermore, alc, encoding the single Drosophila AMPKβ homolog, has been shown to have a neuroprotective role in the retina [19]. However, a more generic role for the AMPK complex in the nervous system, and any downstream impacts on behavior, remain largely unknown.
To investigate the potential contribution of PRKAB2 to the 1q21.1 CNV syndrome, and the role of AMPK signaling in neuronal maintenance and sleep regulation, we examined the function of its Drosophila ortholog, Alc/AMPKβ, in the nervous system. Here, we show that alc and AMPK signaling are required in the nervous system to maintain behaviors such as sleep and learning. Specifically, we observed reduced learning in animals with neuronal knockdown of alc. Furthermore, we find that neuronal knockdown of alc and loss of AMPK function cause severe sleep fragmentation, and animals with reduced nervous-system alc expression are vulnerable to stress induced by sleep deprivation and show no sleep rebound. We suggest that PRKAB2 is a promising candidate gene that may contribute to the behavioral disturbances seen in 1q21.1 patients such as sleep disturbances and cognitive deficits related to learning.
Results
Reduced alc function in the nervous system shortens overall lifespan and increases sensitivity to starvation
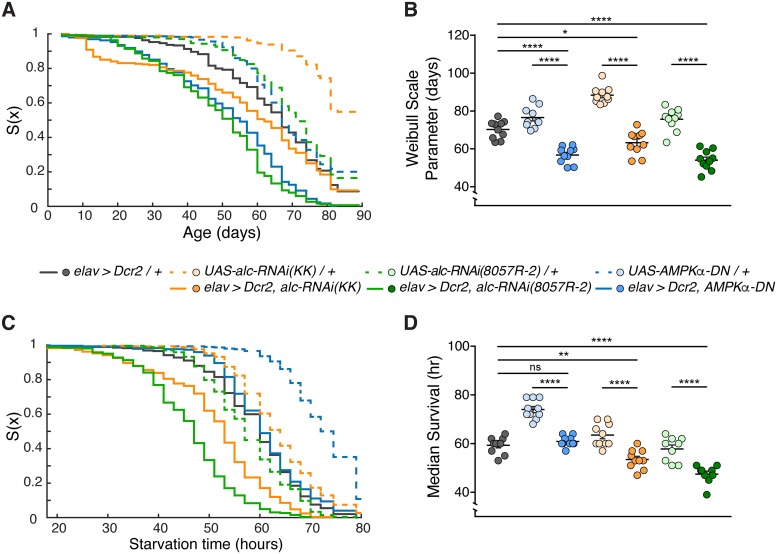
Although AMPK activity has been linked to lifespan, the effect on longevity of reducing AMPK signaling specifically in the brain has not been reported. To determine whether neuronal reduction of AMPK signaling impacts overall physiology and health in Drosophila, we used the pan-neuronal elav-GAL4 (elav>) driver to express either RNAi against alc (alc-RNAi; two independent constructs, in conjunction with Dicer-2) or overexpression of a kinase-dead dominant-negative form of the AMPKα subunit (AMPKα-DN) [17], and we monitored survival for 89 days. Both manipulations resulted in reduced survival compared to the driver (elav>Dcr-2/+) and corresponding RNAi (alc-RNAi/+) and AMPKα-DN/+ controls (Fig 1A). To quantify lifespan, we fit the survival curves for individual experimental vials to a Weibull distribution to obtain a Weibull scale parameter that is analogous to the median survival but takes into account the increasing risk of mortality in aging systems (S1 Fig). Using this measurement, we found a significant reduction of lifespan in animals with nervous-system-specific knockdown of alc or overexpression of AMPKα-DN, conditions that reduce neuronal AMPK signaling (Fig 1B). This shows that effects of AMPK on lifespan can be attributed to its function in the nervous system.
Fig 1. Loss of AMPK in the nervous system reduces overall lifespan.
(A) Survival graph showing overall survival for an 89-day period, for male flies raised and kept on standard food. Results obtained from 10 replicates per genotype, each containing 30 flies. (B) Quantification of overall survival. Knockdown of alc or overexpression of AMPKα-DN in the nervous system reduces overall longevity. Survival analyzed using Kaplan-Meier nonparametric method. Survival quantified by fitting each replicate by a Weibull distribution function, right-censoring animals alive at end of experiment. (C) Survival graph showing overall survival for an 80-hour period, for 3-5-day-old male flies raised on normal food and starved on 2% agar alone. Results were obtained from 9–10 replicates per genotype, each containing 30 flies (n > 270). (D) Quantification of survival following starvation. Pan-neuronal knockdown of alc increases sensitivity to starvation. For controls, lines were crossed to w1118. Error bars indicate SEM. Kruskal-Wallis test with Dunn’s post-hoc testing was used to determine statistical significance: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, versus the control.
Several studies have reported an increased sensitivity to starvation in addition to decreased longevity in animals with reduced AMPK signaling [17, 21]. This effect has been speculated to involve AMPK-regulated metabolic pathways in adipose tissue, muscle, and intestine [22]. To investigate whether this reduced survival under starvation stress can be attributed to effects of AMPK in the nervous system, we measured the survival of animals with reduced neuronal AMPK signaling (knockdown of alc or overexpression of AMPKα-DN) under starvation. As we did in normal-lifespan measurements, we observed reduced starvation survival with pan-neuronal alc-RNAi-mediated knockdown compared to controls (Fig 1C and 1D). Although animals expressing AMPKα-DN in the nervous system exhibited reduced survival compared to the AMPKα-DN/+ control, we observed no difference compared to the elav>Dcr-2/+ driver control. Together our results show that AMPK-mediated neuronal homeostasis plays a significant role in promoting organismal longevity and resistance to starvation. For the subsequent experiments, we chose to use the UAS-alc-RNAi (KK) line, which reduces expression of alc by ~85% in heads, and more strongly than the alc-RNAi(8057R-2) (S2A and S2B Fig). To further assess the specificity of this RNAi towards alc, and knockdown of the AMPK complex in general, we examined levels of phosphorylated AMPKα (pAMPKα) subunit, a readout of AMPK-complex activation. We found that neuronal knockdown of alc (AMPKβ) caused a ~60% reduction in pAMPKα levels in the head, confirming that alc knockdown using this RNAi line specifically reduces AMPK activity (S2C Fig).
alc function is required for maintenance of dendritic arbors
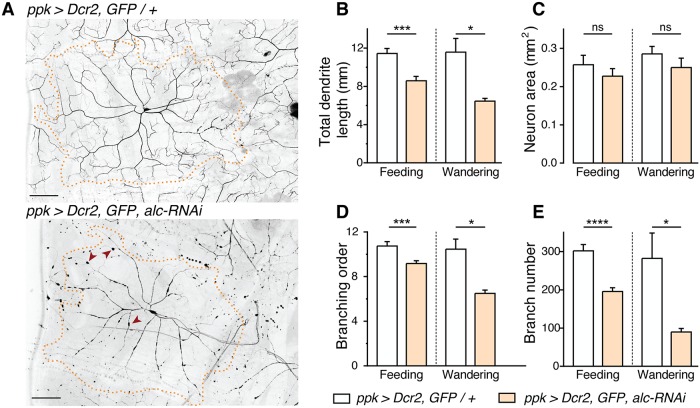
The reduced lifespan observed in animals with neuronal loss of AMPK signaling suggested that lack of this activity may lead to impaired neuronal function and maintenance. Since changes in neuronal dendritic branching and morphology are a hallmark of neuropsychiatric disorders and have been suggested to alter the function of neuronal circuitry [23–26], we chose to analyze the effect of alc manipulations on dendritic development and maintenance. In Drosophila, the class-IV dendritic arborization (da) neurons are peripheral sensory neurons that develop highly stereotyped dendritic processes, making them an ideal model for studying changes in neuronal morphology [27]. Disruption of alc has been shown to cause progressive and activity-dependent degeneration of photoreceptor neurons [19], but the impact of these disruptions upon dendritic arborization has not been explored. To determine whether alc has a role in dendrite development or maintenance, we reduced expression of alc using the da-neuron-specific pickpocket (ppk)-GAL4 (ppk>) driver line while simultaneously expressing GFP to visualize neuronal structures. We imaged individual class-IV da neurons in both large feeding (~100 hours after egg lay) and wandering (~120 hours after egg lay) 3rd-instar larvae. RNAi-mediated knockdown of alc caused a dramatic reduction of dendritic arborization in feeding larvae, reducing the total dendrite length by 25% (Fig 2A and 2B) and branching order by 14.5%, limiting dendrite structure to mainly primary and secondary branches (Fig 2C). Knockdown of alc also led to 35% fewer dendritic branches yet did not alter the total area spanned by individual neurons, compared to controls (Fig 2D and 2E). The severity of this dendritic phenotype was progressive, appearing more severe in older (wandering) animals, with a 44% reduction in total dendrite length (Fig 2B), 38% reduction in branch order (Fig 2C), and 68% reduction in branch number (Fig 2E). This observation suggests that AMPK is required for the maintenance of dendritic structures, rather than for their formation. Furthermore, we observed a “beaded” dendritic morphology as well as thinned and fragmented primary dendritic branches, which are hallmarks of dendritic degeneration. Our AMPKβ observations in da neurons are further supported by previous observations of similar dendrite-morphological abnormalities associated with loss of AMPKα and AMPKγ subunit activity in these cells [17]. Together these observations suggest that AMPK activity is necessary for the maintenance of dendritic arbors. Since proper dendritic structure is required for a functional nervous system, we then asked whether AMPK signaling, and specifically alc, might be required for proper expression of behaviors relevant to the development of neurological disorders.
Fig 2. alc/AMPKβ is required for maintenance of dendritic branching in Class-IV sensory neurons.
(A) Representative images of Class-IV pickpocket (ppk)>GFP-expressing neurons from abdominal segment A2 in control (top) and alc-RNAi knockdown third-instar larvae. Individual neurons are outlined. Arrowheads point to beaded morphology of dendrites characteristic of reduced alc. (B) Quantification of total dendrite length (mm) in both feeding (n = 10) and wandering (n = 3) third-instar larvae shows a significant decrease in total dendrite length when alc is knocked down in class-IV neurons. (C-E) Quantification shows no significant change in neuronal area (mm2) (D); however, knockdown of alc decreases both dendritic branching order (C) and branch number (E). Quantification performed using the TREES Matlab toolbox. For controls, lines were crossed to w1118. Error bars indicate SEM. Unpaired T-test was used to determine statistical significance: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, versus the control.
alc is required for learning
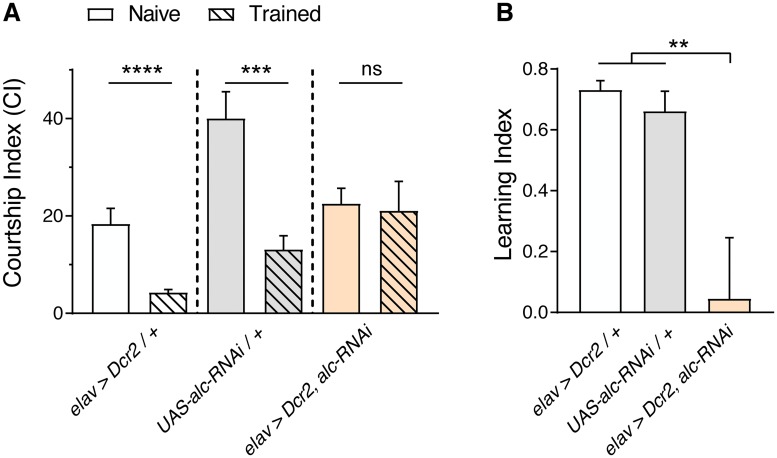
Intellectual disability and general learning disabilities are prevalent in patients carrying the 1q21.1 CNV [4, 28]. We hypothesized that alc might also be important for behaviors such as learning. In Drosophila, one simple behavioral assay that tests learning is courtship conditioning [29]. In this experiment, naïve male flies are presented with an non-receptive mated female and soon associate her mating-associated olfactory and gustatory cues with the courtship-rejection behaviors she expresses, leading the males to suppress futile courtship behavior towards other mated females in the future. To determine whether alc is required for learning following courtship conditioning, we conducted a courtship-conditioning assay with adult (4–7 day old) flies. Following a 1-hour training session in which naïve males were presented with a mated (non-receptive) female, individual naïve or trained males were introduced to a mated female. Animal pairs were video-recorded using a custom recording setup in custom chambers (S3 Fig), and stereotypical courtship behaviors were manually scored. Courtship indices (CIs) were calculated as the fraction of time each male spent courting within the 10-minute recording period. Males from control genotypes (elav>Dcr-2/+ and alc-RNAi/+) showed typical levels of courtship suppression following training, compared to naïve flies exposed to a sham training session (Fig 3A). In sharp contrast, there was no significant difference in CI between naïve and trained elav>Dcr-2,alc-RNAi flies with reduced expression of alc in the nervous system, indicating that pan-neuronal knockdown of alc results in an inability to learn to suppress courtship behavior. The percentage reduction in CI of naïve versus trained flies is scored as the learning index (LI), which is essentially zero in animals with reduced neuronal expression of alc (Fig 3B). Naïve elav>Dcr-2,alc-RNAi males display normal levels of courtship compared to controls, but fail to suppress courtship towards mated (non-receptive) females following training. This indicates that loss of alc in the nervous system specifically impairs learning and is not reflecting a defect in courtship behavior. Thus, these data suggest that neuronal expression of alc is required for learning during courtship conditioning.
Fig 3. Knockdown of alc/AMPKβ in the nervous system impairs courtship-conditioning learning.
(A) A courtship-conditioning assay was used to determine whether alc is required for courtship learning. Courtship index (percentage of time spent courting) decreased after training (with a mated female) in control animals (77% decrease for elav>Dcr-2/+ and 67% decrease for UAS-alc-RNAi/+), indicating learning in both genotypes. There was no significant difference between the performance of naïve and trained animals lacking alc pan-neuronally. Mann Whitney test was used to determine statistical significance: **p<0.01, ***p<0.001, ****p<0.0001, versus the control. Results were obtained from 11 experimental repeats for each genotype, using 3 animals trials each (n = 33 animals). (B) Calculated learning index for alc knockdown and control animals. Error bars indicate SEM. Significance was determined using a one-way ANOVA with Tukey’s post-hoc comparison: **p<0.01 versus the control. For controls, lines were crossed to w1118.
Loss of alc in the nervous system disrupts sleep architecture
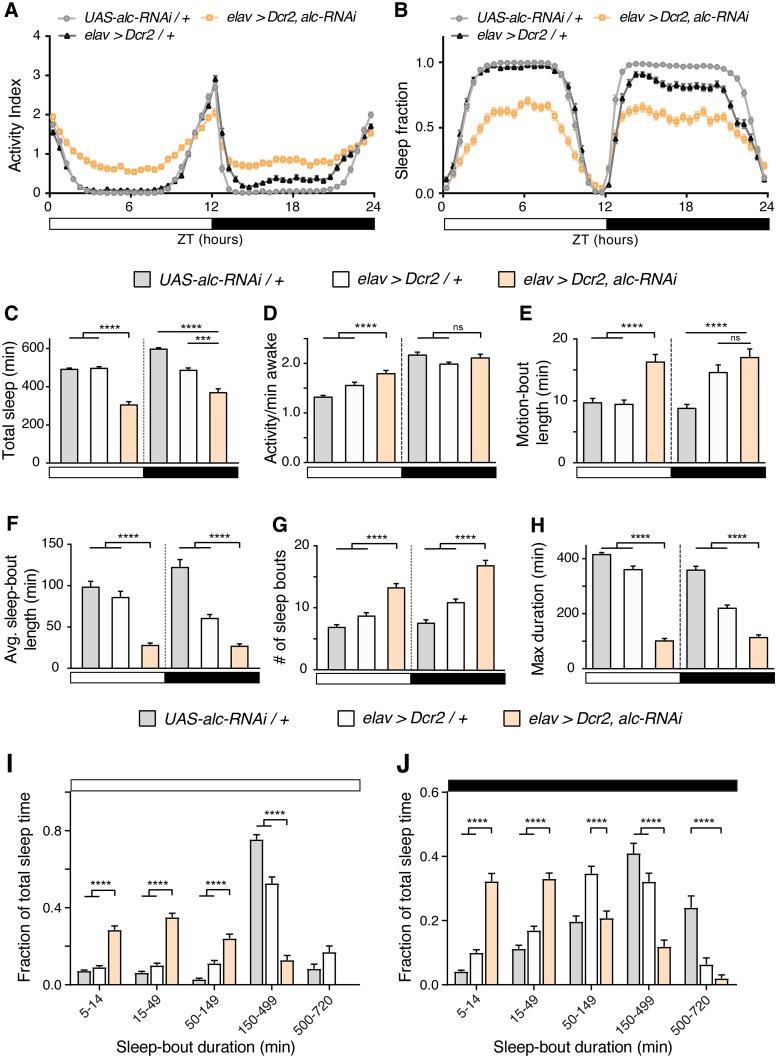
Patients with the 1q21.1 microdeletion also suffer from non-intellectual behavioral deficits, one common manifestation being disrupted sleep patterning [30]. It has been suggested that sleep is essential for the brain to replenish energy sources (ATP) depleted during wakefulness [31]. Because one of the major roles of the AMPK complex is to upregulate processes that generate ATP, we asked whether knockdown of alc in the nervous system would impair sleep consolidation. We hypothesized that if sleep drive is associated with the depletion of energy stores during metabolically demanding wakefulness, thereby stimulating AMPK-dependent signaling, levels of activated AMPK would fall during sleep as ATP levels are restored. Under this scenario, a decrease in AMPK activity would be associated with decreased sleep drive, increased wakefulness, and reduced consolidation of sleep episodes. Sleep in Drosophila can be measured using the Drosophila Activity Monitor (DAM) system (TriKinetics), an automated beam-crossing locomotion assay that is the widely accepted standard in Drosophila sleep studies. It is well established in Drosophila that periods of locomotor quiescence of longer than 5 minutes indicate a sleep-like state exhibiting the hallmarks of mammalian sleep [32, 33]. To determine whether loss of alc impairs sleep, we expressed alc-RNAi in the nervous system and monitored sleep using the DAM system in a light/dark illumination-controlled incubator. Animals with reduced alc expression in the nervous system exhibited significantly reduced overall sleep compared to control genotypes, during both day and night phases. This is also reflected in an overall increase in activity compared to controls (Fig 4A–4C). Thus reduced “sleep,” defined as it is as long-term immobility, can be indicative of a hyperactivity phenotype. To clarify whether we were observing differences in “sleep” or “activity”, we examined the properties of activity bouts during sleep. Since Drosophila adults spend circadian light transition events in an elevated state of arousal during which foraging and courtship behaviors are dominant, we separated these locomotion periods from more “steady state” periods. These transitional periods were defined as consolidated periods of activity which include the ZT 0 and ZT 12 time-points. Activity during wakeful periods (number of beam crosses per minute awake) that were not associated with light transitions (and thus reflective of sleep-time activity) was significantly increased during the light phase, but not during the dark phase (Fig 4D). Flies also demonstrated a significant increase in the duration of wakeful periods during light (compared to both controls) and dark (compared to UAS-alc-RNAi/+ control only) (Fig 4E). Interestingly, when we analyzed the periods around light-transition events we did not observe increased level or duration of activity for elav>Dcr-2,alc-RNAi flies compared to controls (S4A and S4B Fig), which indicates that the loss of alc causes a specific sleep phenotype that is not a consequence of overall hyperactivity. For Drosophila sleep studies, sucrose-based medium is generally a standard diet. However, since AMPK is involved in intracellular energy sensing, we also tested the phenotype on regular cornmeal-based food to see whether the observed effects are related to this sucrose-based diet. Nervous-system-specific alc knockdown causes a loss-of-sleep phenotype on regular cornmeal-based food, similar to what is observed on the standard sucrose-based food used for sleep studies (S4C Fig). Together these data suggest that animals with reduced expression of alc, and thus reduced AMPK activity, in the nervous system have an increased drive for wakefulness and reduced sleep drive.
Fig 4. Knockdown of alc/AMPKβ in the nervous system disrupts sleep.
(A-B) Activity (A) and sleep (B) profiles over a 24-hour period for control genotypes elav>Dcr-2 (n = 128) and UAS-alc-RNAi/+ (n = 126) versus elav>Dcr-2, alc-RNAi (n = 126). All data obtained from second 24-hour cycle to allow for acclimatization. Activity and sleep are shown in bins of 30 minutes. White and black bars represent ZT time, 12 hours light and 12 hours dark, respectively. (C) Total sleep (min) in flies with pan-neuronal alc knockdown compared to controls. Total sleep is significantly reduced when alc is knocked down in the nervous system. (D) Comparison of activity outside sleep periods between alc knockdown animals and controls shows that pan-neuronal knockdown of alc increases activity during daytime, but not nighttime. (E) Motion-bout length (min) is significantly increased during the day in alc knockdown flies compared to controls. (F) Average sleep-bout length (min) is significantly reduced during both day- and night-time in alc knockdown flies. (G) The number of sleep bouts per day and night increases when alc is knocked down in the nervous system. (H) Duration of the longest sleep bout (min) is significantly shorter in alc knockdown animals than in controls. (I, J) Distribution of length of sleep bouts for control genotypes elav>Dcr-2/+ and UAS-alc-RNAi/+ versus elav>Dcr-2, alc-RNAi during the day (I) and during the night (J). Flies in which alc has been knocked down pan-neuronally only generate a small proportion of sleep bouts of >150 min, whereas the majority of sleep bouts in controls are of >150 min. For controls, lines were crossed to w1118. Error bars indicate SEM. Kruskal-Wallis test with Dunn’s post-hoc testing was used to determine statistical significance: ***p<0.001, ****p<0.0001, versus the control.
To further assess the sleep properties of animals with neuronal loss of alc function, we investigated sleep-bout duration and number. Animals with reduced alc expression in the nervous system showed an increase in sleep-bout number and a significant concomitant reduction in average sleep-bout length, indicating highly fragmented sleep (Fig 4F and 4G). The average longest single sleep bout during both day and night was also greatly reduced compared to control genotypes (Fig 4H). To more closely examine these sleep changes, we analyzed the distribution of sleep episodes by binning them by duration and calculating the time spent in each bin. Whereas control flies consolidated most of their daytime and nighttime sleep into long sleep episodes of 150–499 minutes, animals with neuronal loss of alc demonstrated a shifted sleep structure with significantly more time spent in short sleep bouts (binned into 5–14 and 15–50 minutes) (Fig 4I and 4J). In the aggregate, these data indicate that alc is necessary for proper sleep architecture and consolidation in Drosophila.
Recent studies suggest that overall metabolic state, in particular when regulated within the fat body (the adipose and liver tissue of Drosophila), may be an important regulator of complex behaviors such as sleep, reflecting the need to modulate these behaviors according to internal energy stores and availability of nutrients [34]. Ubiquitous AMPK down-regulation has previously been shown to increase overall dietary intake, yet flies with reduced AMPK activity have reduced nutrient stores and display starvation-like lipid accumulation in the fat body which suggests that they are in a persistent state of starvation [17]. Furthermore starvation is known to suppress sleep in Drosophila, which indicates a higher demand for foraging behaviors [35]. Therefore, we sought to determine whether reducing alc in the fat body might have a distinct phenotype from that which we observe in the nervous system by expressing alc-RNAi using the fat body driver line CG-GAL4 (CG>). Interestingly, we observed a reduction in sleep specifically during the day, presumably when foraging behaviors are prevalent (S4E and S4F Fig). This reduction is characterized by more sleep episodes of shorter duration (S4G and S4H Fig). However, the length of the maximum sleep-bout duration is not reduced when compared with driver control (S4I Fig). This indicates that although these animals spend more time locomoting during the day, when they do enter a consolidated sleep episode, its duration is unaltered by reduced alc in the fat body. Furthermore, total sleep and length of sleep episodes during the night are not reduced when alc is reduced in the fat body. This indicates that the sleep fragmentation that we observe in this study is specific to AMPK signaling in the nervous system and is separate from potential metabolic effects that are mediated within the fat body.
Loss of AMPK signaling causes progressively worsening sleep fragmentation
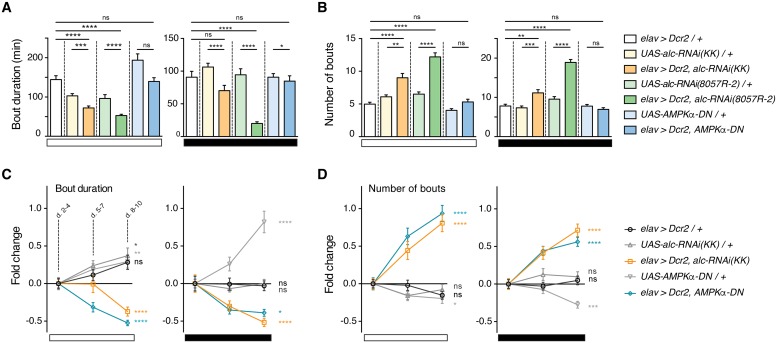
To confirm the reduced-sleep phenotype observed for pan-neuronal knockdown of alc, we used a second, independent alc-RNAi line (8057R-2) and determined average sleep-bout duration and number during both day- and night-time. We observed similar reduced sleep and sleep fragmentation characteristics with this alc-RNAi(8057R-2) line as with the alc-RNAi(KK) line (Fig 5A and 5B and S5 Fig). Furthermore, we verified that the sleep phenotype observed with the alc-RNAi(KK) line is caused specifically by loss of alc function. To do this, we tested whether the phenotype might be rescued by expression of alc in the nervous system. As expected, neuronal expression of alc rescues the loss-of-alc-function sleep phenotype, both for the reduced total sleep as well as the sleep fragmentation (S6 Fig). Together this confirms that the sleep phenotype caused by alc-RNAi in the nervous system can be attributed specifically to reduced alc expression. To further examine whether this phenotype was specifically due to altered AMPK signaling in the nervous system, we overexpressed AMPKα-DN to block AMPK-complex activity via inhibition of the alpha subunit. Surprisingly, these animals initially exhibited no significant reduction in average sleep-bout duration or number. However, over longer observation periods, sleep phenotypes arose that were similar to those displayed by alc-knockdown animals—average sleep-bout duration significantly decreased over time, while the number of sleep bouts significantly increased, during both day and night (Fig 5C and 5D). This progressive deterioration in sleep consolidation over time was specific to reduced AMPK signaling, since no significant changes in sleep-bout architecture were observed with control genotypes aside from slightly increased daytime sleep-bout duration in one control line. Thus, the loss of AMPK signaling in the nervous system causes sleep fragmentation that worsens over time. This progressive deterioration is reminiscent of the morphological phenotypes observed in larval class-IV da neurons. It is possible that a similar morphological deterioration of sleep-regulatory neurons might underlie these defects.
Fig 5. Neuronal loss of AMPK activity causes progressive sleep fragmentation.
(A-B) Quantification of average bout durations (A) and average bout numbers (B) during day and night for elav>Dcr-2/+ (n = 32), UAS-alc-RNAi(KK)/+ (n = 32), elav>Dcr-2, alc-RNAi(KK) (n = 31), UAS-alc-RNAi(8057-R2)/+ (n = 32), elav>Dcr-2, alc-RNAi(8057-R2) (n = 32), UAS-AMPKα-DN/+ (n = 32), elav>Dcr-2, AMPKα-DN (n = 31). Sleep was monitored over 10 days, and data from days 2 to 4 was used. Bout durations are significantly reduced and bout numbers significantly increased when alc is knocked down in the nervous system compared to control genotypes, for both RNAi lines. There is no initial difference in either parameter when dominant-negative AMPKα (AMPKα-DN) is overexpressed in the nervous system. (C-D) Graphical representation of the fold change in bout duration (C) and bout number (D) over time (3-day binned) during both day and night, indicated by white and black bars respectively. Shown are data for elav>Dcr-2, alc-RNAi(KK) and elav>Dcr-2, AMPKα-DN compared to control genotypes. Bout duration significantly decreases over time in both alc knockdown and AMPKα-DN overexpression flies. Bout number significantly increases over time for both genotypes. Significance between days 8–10 and days 2–4 is shown. For controls, lines were crossed to w1118. Error bars indicate SEM. Significance was determined using a Kruskal-Wallis test with Dunn’s post-hoc testing: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, versus the control.
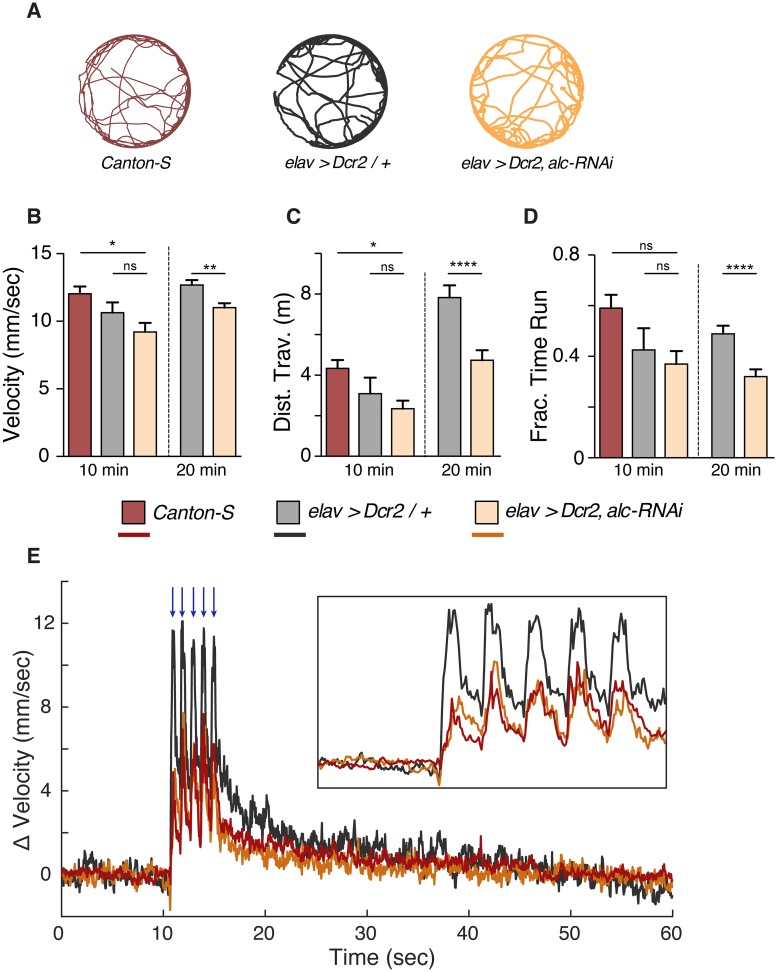
To further determine whether the fragmented-sleep phenotype of animals deficient in alc is due to hyperactive animals, we measured basal locomotion velocities. Behavior was recorded in 37-mm behavioral chambers, and spontaneous velocity was quantified using the C-trax software [36]. Example tracks for the wild-type Canton-S, control elav>Dcr-2/+, and animals with reduced alc expression in the nervous system (elav>Dcr-2,alc-RNAi) are shown in Fig 6A. Animals with reduced expression of alc in the nervous system (elav>Dcr-2,alc-RNAi) did not show elevated velocities when compared to either control and spent less time locomoting, resulting in a shorter total distance traveled over the observed 10- or 20-minute period. (Fig 6B–6D). This is consistent with the conclusion that the sleep phenotype of animals with reduced neuronal alc is due to the fragmentation of sleep episodes and not elevated activity.
Fig 6. Knockdown of alc/AMPKβ in the nervous system does not result in hyperactivity or hypersensitivity.
(A) Example tracks of individual animals over a 10-minute recording period. (B-D) Graphs showing average running velocity (>2mm/sec) (B), total distance traveled (C), and fraction of time moving (velocity > 2 mm/sec) (D) for Canton-S, elav>Dcr-2/+, and elav>Dcr-2, alc-RNAi animals for a 10 minute (n = 10) and 20 minute recording period (n = 30). Error bars indicate SEM. Mann Whitney or Kruskal-Wallis test with Dunn’s post-hoc testing was used to determine statistical significance: ***p<0.001, ****p<0.0001, versus the control. (E) Average baseline subtracted velocity for Canton-S, elav>Dcr-2/+, and elav>Dcr-2, alc-RNAi animals centered on delivery of a five pulse mechanical stimulus (blue arrows). Inset box shows a magnified view of the response. N = 80 animals for each genotype and data averaged over five pulse trains separated by 60 seconds.
Animals defective in AMPK signaling are in a state of sleep deprivation
Since defective neuronal AMPK signaling due to tissue-specific alc knockdown resulted in fragmented and reduced sleep, we suspected that these animals might therefore exhibit altered expression of sleep-stress-response genes. To assess this possibility, we performed RNA sequencing on adult head samples of animals with neuronal knockdown of alc versus controls. Amylase has been identified as a biomarker for sleep drive in Drosophila, and another, previously uncharacterized gene, heimdall, has recently been linked to animals’ response to sleep deprivation and starvation [37, 38]. Interestingly, both of these genes are significantly upregulated in the heads of alc-knockdown flies–heimdall exhibited the greatest transcriptional up-regulation of any gene, with a roughly 180-fold increase compared to control heads (Table 1 and S1 Table). Thus, alc-knockdown animals show a genetic response consistent with disrupted sleep.
Table 1. alc-regulated genes in adult male fly heads.
RNA-seq data for genes regulated in response to neuronal alc knockdown Fold change in transcript levels for elav>Dcr2, alc-RNAi(KK) compared to the elav>Dcr2/+ controls.
| Annotation | Gene Name | Fold Change | P-Value |
|---|---|---|---|
| CG4500 | heimdall | 179.53 | 8.32E-257 |
| Hsp70Bb | Heat-shock-protein-70Bb | 45.15 | 0 |
| CG30059 | - | 34.17 | 4.03E-32 |
| CR44994 | - | 33.82 | 2.66E-08 |
| Mal-A8 | Maltase A8 | 30.60 | 5.81E-12 |
| CG34040 | - | 22.44 | 6.27E-06 |
| Mal-A7 | Maltase A7 | 20.80 | 4.81E-12 |
| Cyp6d2 | Cyp6d2 | 15.82 | 7.14E-45 |
| lectin-24A | lectin-24A | 15.52 | 3.16E-07 |
| squ | squash | 11.89 | 8.54E-22 |
| Amy-p | Amylase proximal | 8.71 | 7.56E-09 |
| alc | alicorn | -2.50 | 0 |
| Obp56a | Odorant-binding protein 56a | -6.55 | 5.90E-161 |
| CG32437 | - | -6.90 | 4.60E-07 |
| CG8563 | - | -6.94 | 7.89E-15 |
| Obp99d | Odorant-binding protein 99d | -14.39 | 2.68E-36 |
| CG43291 | - | -18.69 | 9.75E-24 |
| CG12971 | - | -35.38 | 1.26E-30 |
| CG17242 | - | -40.90 | 2.58E-05 |
| CR45600 | - | -62.45 | 0 |
alc is required for initiation and maintenance of “recovery sleep” following sleep deprivation
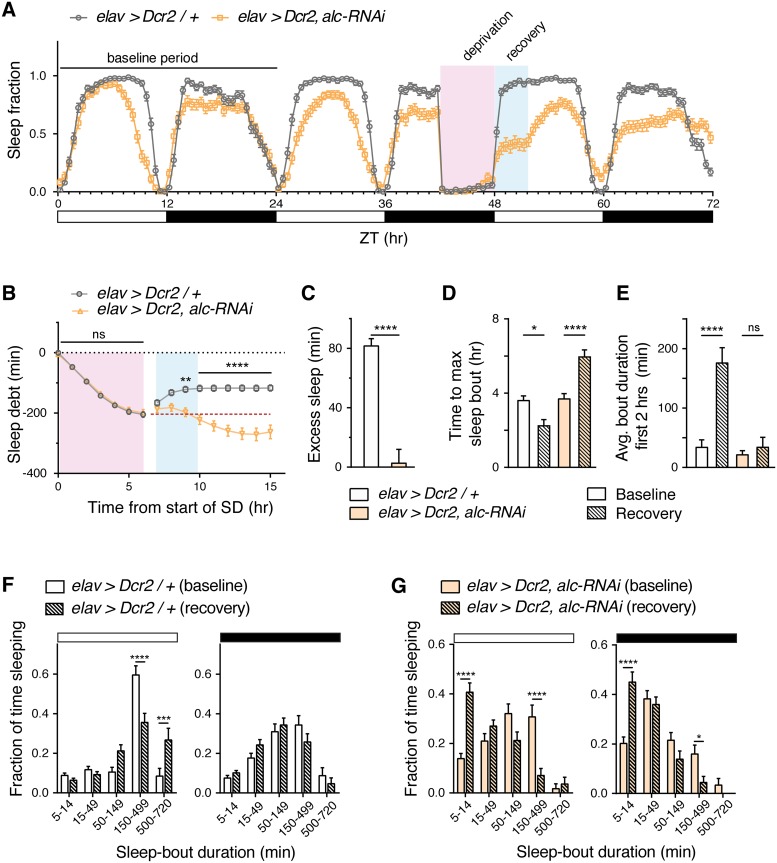
A major hallmark of sleep is homeostatic regulation, which is required for the recovery of lost sleep after sleep deprivation (SD). To investigate whether AMPK might be important for the homeostatic process of sleep regulation, we tested whether animals expressing neuronal alc RNAi were defective in “recovery sleep” following deprivation. To assess recovery sleep following SD in alc knockdown animals, we exposed elav>Dcr-2/+ control animals and elav>Dcr-2,alc-RNAi animals to mechanical SD, using a vortex mounting plate (Trikinetics) that regularly jostled the animals. Sleep deprivation was initiated during the second day of recording, for the 6 hours of the latter half of the dark phase immediately preceding “lights-on”. This resulted in similar levels of sleep loss in alc knockdown and control animals (Fig 7A and 7B, S7A Fig). However, whereas controls showed substantial subsequent sleep rebound following SD, elav>Dcr-2,alc-RNAi animals did not show any such post-SD rebound (Fig 7B and 7C, S7A and S7B Fig). Following relief from SD, control flies fell into extended sleep and entered their longest consolidated sleep episode faster (Fig 7D), with an increase in average bout duration initiated during the first two hours following SD (Fig 7E). In contrast, animals lacking alc in the nervous system took significantly longer to reach a maximally consolidated sleep bout and did not increase sleep bout duration following SD (Fig 7D and 7E). This indicates that animals with impaired neuronal AMPK signaling not only fail to initiate rebound sleep, but also seem to be generally impaired in their sleep following SD. To further examine this deprivation-dependent shift in sleep architecture, we analyzed the distribution of sleep bouts during day and night following the deprivation period. Directly following sleep deprivation, control animals spent more time in prolonged sleep episodes (500–720 min) compared to baseline periods, and returned to baseline sleep-length distribution in the following dark phase (Fig 7F). In contrast, elav>Dcr-2,alc-RNAi animals displayed more-fragmented sleep immediately following deprivation, indicative of an impaired sleep drive (Fig 7G). Surprisingly, this fragmentation persisted into the following dark phase, indicating that SD of flies with altered AMPK signaling has longer-term effects on sleep architecture (Fig 7G).
Fig 7. Alc/AMPKβ is required in the nervous system for recovery sleep following sleep deprivation.
(A) Sleep profile for control genotype elav>Dcr2/+ (n = 54) versus elav>Dcr2, alc-RNAi (n = 52) for a 72-hour period, showing sleep profiles for baseline, sleep deprivation, and recovery days. White and black bars represent ZT time, 12 hours light and 12 hours dark, respectively. A 6-hour sleep-deprivation period is illustrated using pink shading, typical recovery period illustrated with blue shading. (B) Quantification of sleep debt (min) showed that control flies recovered lost sleep in the first 3 hours following sleep deprivation. Significance determined by 2-way ANOVA with Sidak’s multiple comparisons test between control and alc-RNAi animals shown. Dashed line shows maximum sleep debt. (C) Control animals showed a significant excess of sleep (80 minutes) compared to the same time period of the baseline day while alc-RNAi animals did not. Pan-neuronal knock down of alc completely eliminates recovery sleep. (D) Time to maximum sleep bout (hours) is significantly reduced in control flies following sleep deprivation, whereas it is significantly increased in flies lacking alc in the nervous system. (E) Average bout duration in the first two hours of light phase was significantly increased during recovery in control flies, but not in alc knockdown animals. (F-G) Distribution of length of sleep bouts for control genotype elav>Dcr2/+ (F) versus elav>Dcr2, alc-RNAi (G) during the day and during the night, for baseline and recovery days. Unlike control flies, which showed an increase in long sleep bouts (>150 minutes) during the day, alc knockdown animals showed a decrease in long sleep bouts during both the day and the night, following sleep deprivation. For controls, lines were crossed to w1118. Error bars indicate SEM. For C-E, Mann Whitney test was used to determine statistical significance. For F and G, a 2-way ANOVA with Tukey’s post-hoc comparison was used: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, versus the control.
The defect in recovery sleep following sleep deprivation can be the result of animals that become hyper-aroused following mechanical perturbation. To rule out this possibility, we measured sleep latency following deprivation. We observed that both the control (elav>Dcr-2/+) and alc knockdown (elav>Dcr-2,alc-RNAi) animals enter the first sleep episode faster following deprivation, indicating that they are not hyper-aroused (S7C Fig). To further assess the sensitivity of animals with reduced alc expression, we measured the response of these animals to a mechanical vibration stimulus. While a pulse train of five consecutive (1 sec apart) vibration stimuli induced elevated locomotion in all genotypes tested, animals with reduced neuronal alc showed similar response to the Canton-S wild-type and reduced response compared to the driver control (elav>Dcr-2/+) and return to baseline velocity at a similar or increased rate compared to the two controls (Fig 6E). These results further support the conclusion that the observed sleep-rebound phenotype is due to disruption of sleep architecture and not the result of hyper-sensitivity.
To determine whether the apparent detrimental effect of sleep deprivation impacts survival, we monitored sleep-deprived animals for 24 hours following sleep deprivation. We observed increased mortality of elav>Dcr-2,alc-RNAi animals following sleep deprivation compared to both un-deprived age-matched elav>Dcr-2,alc-RNAi animals and SD and non-SD driver controls (S8A Fig). To control for a possible elevated sensitivity to mechanical stress, we performed a mechanical-stress assay in which flies were exposed to prolonged repeated mechanical perturbation, and stress-induced mortality was assessed [39]. Knockdown of alc in the nervous system did not result in a higher incidence of lethality over this period (S8B Fig), which confirms that the observed lethality following sleep deprivation and the corresponding deterioration of sleep architecture is the result of sleep loss rather than physical weakness of the animals. Together, these data indicate that alc knockdown animals are incapable of consolidating rebound sleep and are highly sensitive to sleep deprivation, with long-lasting detrimental effects following SD. Our findings indicate that AMPK activity is required for rebound sleep following deprivation, indicating that the AMPK complex is involved in the homeostatic regulation of sleep. According to this view, AMPK activity would increase over periods of wakefulness, when higher neuronal energy demands deplete cellular energy stores. Consistent with this, we observed upregulation of pAMPKα levels in heads of control animals after 6 hours of SD, whereas this signal did not increase in animals with reduced neuronal alc expression (S8C Fig). Elevated pAMPKα levels were also observed in wild-type (Canton S) flies after SD, confirming AMPK-complex activation following periods of SD in wild-type animals.
Discussion
The shortening of sleep-bout duration and the associated increase in their number is indicative of a defect in sleep maintenance, in which sleep is both reduced and fragmented. Furthermore, perturbations of sleep structure are a hallmark of many neuropsychiatric and neurodegenerative disorders, and this type of perturbation is associated with the 1q21.1 deletion, which includes PRKAB2/AMPKβ2 [1–4]. Here we show that Alc/AMPKβ and AMPK signaling are necessary for sleep maintenance, with reduced AMPK-complex activity resulting in fragmented sleep episodes. Our findings indicate that animals with disrupted neuronal AMPK signaling have an impaired ability to maintain the sleep state. The loss of post-deprivation rebound sleep in animals lacking neuronal AMPK activity further suggests an effect on sleep homeostasis. Because sleep serves beneficial biological functions, the persistent state of reduced sleep might also explain the shortened lifespan of flies with reduced AMPK signaling in the nervous system. The role of AMPK in stress resistance is well established [11]; however, its role in the regulation of sleep and in the response to sleep deprivation has not been previously reported. The disruption of sleep parameters following deprivation, and the associated high level of mortality, indeed indicates a detrimental effect that may be associated with lack of rebound sleep. As increased neuronal activity under reduced AMPK signaling has been shown to result in degeneration of retinal photoreceptive neurons [19], it is possible that elevated activity during sleep deprivation, without the neuroprotective mechanisms of AMPK, leads to some degree of excitotoxicity. In any case, our Drosophila data suggest that AMPKβ2 may underlie or contribute to the phenotypes observed in human 1q21.1 CNV syndromes. This provides a molecular mechanism for understanding the link between the deletion and increased risk of developing mental disorders associated with the 1q21.1 CNV, which may be important for development of new strategies aimed at treating both mental disorders and sleep disturbances.
Neuronal AMPK activity affects lifespan and stress resistance
AMPK signaling has been shown to play a critical role in aging and lifespan determination [40]. Upregulation of AMPK signaling in Drosophila has been shown to extend lifespan by mediating effects specifically in the intestine and the brain [20]. Furthermore, tissue-specific RNAi-mediated knockdown of AMPKα in the fat body and muscle was shown to reduce lifespan [41, 42]. We find that AMPK signaling in the nervous system affects longevity and survival under starvation stress. This indicates that non-metabolic pathways may contribute to the impairment of survival upon loss of AMPK signaling. As non-cell-autonomous mechanisms have been implicated in the regulation of longevity upon enhanced AMPK signaling in the brain [20], it is possible that similar mechanisms are involved in the lifespan reduction we observe. Interestingly, reduction of AMPK signaling in neuroendocrine cells releasing the energy-mobilizing Adipokinetic Hormone resulted in a significant extension of life span under starvation [43]. An intriguing possibility is that the starvation sensitivity that we observed with nervous-system AMPK loss is mediated through effects on neuromodulatory or neurohormonal systems.
AMPK is required for neuronal maintenance
The cellular mechanisms by which altered metabolism brings about neuropathology are not clear. It is well established that neuronal morphogenesis defects may result from mitochondrial dysfunction in various cell types [44, 45]. As mitochondria and AMPK are both involved in maintaining levels of ATP, defects in these systems may phenocopy each other. In fact, it has been shown that loss of AMPK activity enhances neurodegeneration in Drosophila models of mitochondrial abnormalities [46]. Normal neuronal activity and synaptic transmission involve molecular and cellular processes, such as maintenance of resting membrane potential and generation of action potentials, transporter activity, transmitter synthesis, and vesicle transport and dynamics, that are energy-intensive. Therefore, a disruption of restorative AMPK signaling that is required to replenish energy stores could result in a state of neuronal energy depletion, which could lead to progressive degeneration. Here we show that knockdown of alc results in progressive reduction of the dendritic arbors of class-IV da neurons, a phenotype consistent with reduced AMPK activity [17, 47]. Reducing AMPK signaling by knockdown of alc/AMPKβ specifically affects terminal dendrite branching, not the distance (reach) from the soma. Our observations are consistent with previous reports showing that altered AMPK signaling results in neurodegeneration [48, 49]. RNAi-mediated knockdown of the α- or γ-subunits of the AMPK complex in class-IV da neurons in Drosophila larvae causes aberrant dendrite morphology, indicative of faulty neuronal development, neuronal damage, and degeneration. In sum, this suggests that neuronal loss of AMPK activity is associated with progressive neurodegeneration, originating with insufficient energy to maintain neuronal structures. These results are also consistent with the emerging role of AMPK in neurodegenerative diseases such as Alzheimer’s, Parkinson’s and Huntington’s [50].
Requirement of Alc/AMPKβ for learning
The role of AMPK in mediating the synaptic plasticity that underlies learning and memory consolidation is unclear. One possibility to explain our observations would be that the neuronal connections necessary for associative learning in the mushroom body of the insect brain [51] have degenerated, thus resulting in a system incapable of forming, retaining, or recalling memories. Another possibility is that loss of AMPK may impair cAMP second-messenger signaling, which underlies the neuronal plasticity necessary for learning and memory [52, 53]. In mammalian adipocytes, AMPK has been shown to be activated by intracellular cAMP levels [54]; it is possible that a similar mechanism is present in neurons and is involved in mediating neuronal activity and plasticity. Interestingly, in mice, treatment with the AMPK agonist AICAR increases spatial memory in a Morris water maze [55, 56]. Our results suggest that Alc/AMPKβ and thus the AMPK complex are required for learning, although further experiments need to be conducted to evaluate the role of reduced AMPK signaling in the formation and maintenance of memory. Interestingly, it has been shown that sleep deprivation has a negative effect on learning and memory in several animal models, which opens the possibility that the role of AMPK in memory is associated with its role in sleep [57–59]. A persistent state of sleep deprivation in animals deficient in AMPK signaling may explain their inability to learn to repress courtship upon rejection. Other learning paradigms need to be tested to determine if this is a general effect on the association center of the Drosophila brain and to rule out contribution from potential sensory defects that would lead to inability to sense olfactory mating cues.
The role of AMPK in homeostatic sleep regulation
Sleep is a highly conserved animal behavior, and humans spend roughly one-third of their life sleeping [60, 61]. Sleep and wakefulness are under the control of circadian and homeostatic processes. The circadian clock determines the timing and rhythmic nature of sleep onset, whereas homeostatic mechanisms are involved in sensing sleep drive and provide increasing sleep pressure as a function of time spent awake. Neurons have high demands for ATP, the major form of cellular energy, and low capacity to store nutrients [62], which has led to the hypothesis that sleep is required to replenish neuronal energy that is depleted during wakefulness [63]. This theory suggests that energy levels are reflected in glycogen and adenosine changes accumulated during metabolically demanding wakefulness and that these molecules play key roles in homeostatic sleep regulation [64]. Consistent with this notion, glycogen levels are indeed affected by the rest and wake cycle and drop after short periods of rest deprivation in Drosophila [65]. Adenosine is a breakdown product reflecting the depletion of ATP, the primary energy currency used by brain cells [66]. Consistent with the idea that sleep is necessary to reestablish energy stores, ATP has been shown in rats to increase during the initial hours of sleep when neuronal activity is low [31]. If the homeostat senses sleep drive by measuring energy levels, the molecular mechanism of the homeostat must involve an energy sensor that is activated by low cellular energy levels and initiates processes that restore energy levels to relieve sleep pressure after a nap. As the major cellular energy sensor activated by low energy levels, AMPK promotes processes that replenish energy levels [67]. Furthermore, the AMPKβ subunit contains a glycogen-binding domain that likely enables the AMPK complex to sense energy levels in the form of both ATP and glycogen in the nervous system [68]. If the AMPK complex is involved in homeostatic sleep regulation, then rebound sleep following sleep deprivation would not occur without this complex. Our results indicate that neuronal loss of AMPK affects sleep regulation and leads to loss of rebound sleep following deprivation, providing evidence that AMPK is indeed involved in homeostatic sleep regulation. It will be of great interest to determine whether AMPK is part of the mechanism of sleep homeostasis and its role in psychiatric disorders characterized by sleep disturbances.
Materials and methods
Drosophila strains and maintenance
Drosophila larvae and adults of mixed sexes were raised on standard cornmeal medium (Nutri-Fly “Bloomington” formulation) under a 12:12 light/dark cycle at 25 °C with 60% humidity, unless otherwise stated. Fly lines Cg-GAL4 (#7011), elav-GAL4; UAS-Dicer-2 (Dcr-2) (#25750), ppk-GAL4 (#32078), UAS-mCD8::GFP (#5137), UAS-AMPKα-K56R (UAS-AMPKα-DN, #50760), CG-GAL4 (#7011), and Canton S were obtained from Bloomington Drosophila Stock Center (BDSC; Bloomington, IL). UAS-alc-RNAi(KK) (#109325) and the w1118 (#60000) genetic background line were procured from Vienna Drosophila Resource Center (VDRC; Vienna, Austria). UAS-alc-RNAi(8057-R2) was obtained from the NIG-Fly stock center (Mishima, Shizuoka, Japan). UAS-alc was a generous gift [19]. We used a cross to w1118, the isogenic genetic background of the RNAi line and the genetic background for most fly lines, as controls.
Lifespan measurements
For starvation-survival experiments, thirty 3-5-day-old adult males were placed in vials containing 2% agar in water. Survival was assessed every 2–4 hours during the main course of the experiment for 9–10 replicate vials for each cross until all animals were dead. For each vial, the median survival was calculated in MatLab (The MathWorks, Inc., Natick, MA) as the time-point when the cumulative survivor function using the Kaplan-Meier method fell below 50%. For lifespan experiments, thirty male flies were collected upon eclosion into vials containing normal diet. Ten replicates for each cross were used, and the animals were transferred to fresh vials every 2 to 3 days. During each transfer, the numbers of dead animals left behind and carried over were recorded. Escaped flies or accidental deaths during transfer were recorded as censored. Longevity was monitored for 89 days. Survival data was analyzed for each vial in MatLab using the Kaplan-Meier nonparametric method accounting for censored data. As some control vials did not reach 50% mortality, to quantify survival, the Weibull distribution function was fit to the data, right-censoring the animals still alive at the end of the experiment. This analysis extends exponential distributions of failure (death) probability to allow for the increasing hazard rates associated with aging systems [69]. The scale parameter was determined for each vial and used for comparisons.
Visualization of class-IV dendritic-arborization (da) neurons
Feeding and wandering third-instar larvae were selected and anesthetized by exposure to chloroform for 1 minute in a sealed container. Larvae were mounted in 90% glycerol, and GFP fluorescence in live animals was imaged. One individual GFP-labelled neuron, located in segment A2, was imaged per larva. Larvae were imaged at 20X using a Zeiss 780 LSM confocal microscope. Z-stacks were processed using FIJI (NIH) software [70], and analysis was performed using the TREES toolbox in MatLab [71].
Courtship-conditioning assay
A courtship-conditioning assay was used to assay learning in adult flies [29]. Male flies were collected upon eclosion and housed individually until the start of the experiment. Virgin females were collected upon eclosion and housed in groups of 30. Mated females were generated by housing with males for 24 hours prior to experimental start, after which the males were removed. Male flies were split into two groups, naïve flies and flies to be trained. For training, individual male flies were incubated with mated females for 1 hour prior to testing in custom 2-cm-diameter mating chambers with a food source. For imaging courtship behavior, custom chambers were laser cut from clear acrylic. Each chamber set consisted of an array of 20, 1.5-cm individual chambers allowing for testing of all conditions in parallel (S3 Fig). Channels were cut to allow for a thin separator to be inserted through all of the chambers to keep the loaded males and female separate until the start of recording. Single naïve or trained males were then transferred into a testing arena together with individual virgin females (loaded through a separate loading hole and kept separate from males). Transfer was done using gentle aspiration to avoid disturbing the animals. Once all animals were loaded, the chamber was placed on a custom-built image-acquisition setup consisting of a 20-cm diffuse infrared LED backlight (Falcon illumination FLFL-Si200-IR24) and a Basler acA2000-50gmNIR GigE camera fitted with an IR filter (S3 Fig). Once the dividers separating males and females was removed, video was recorded for 10 minutes using LabView (National Instruments, Inc., Austin, TX). All experiments were done in a climate-controlled room at 25 °C and 70% humidity. Courtship behaviours were manually scored, with the scorer blinded to the genotype. The experiment was repeated 11 times, with three trials (individual fly pairs) per experiment. Courtship indices (CIs) were calculated as the percentage of time that a male fly spends courting during a 10-minute period. The learning index (LI) was calculated as the relative reduction of CI in trained male flies compared to naïve flies.
Basal locomotion assay
For basal locomotion measurements, animals were video recorded at 15 Hz using the imaging setup described above (S3 Fig). Animals were gently aspirated into 37 mm diameter behavioral chambers and their behavior recorded for 10 or 20 minutes. Spontaneous locomotion was quantified using the Ctrax MatLab package [36]. Running velocity was determined for periods where animals moved faster than 2 mm/sec. Mechanical stimulus was delivered using a 10 mm brushless vibration motor (Precision Microdrives #910–101) driven by an Arduino Uno microcontroller interfaced with the custom imaging LabView program to deliver a train of five 500 msec stimuli at 1 Hz every 60 seconds over the 10 minute recording period. For analysis, velocities for 60 second periods were aligned by stimulus onset for the first 5 stimuli and baseline velocity (average velocity during 5-minute period preceding stimulus) was subtracted.
Sleep assays and analysis
Locomotion over a 24-hour period was measured using the Drosophila Activity Monitor (DAM) system (TriKinetics, Inc., Waltham, MA). Adult males were collected in groups of 30 upon eclosion and housed under standard conditions until experimental start. Four-to-seven-day-old males were used for experiments, housed in 65-mm glass tubes with a plug of 5% sucrose and 2% agar medium at one end. Experiments were performed under a 12-hour light/12-hour dark cycle, and activity measurements were binned in one-minute periods. Episodes of sleep were defined as at least 5 minutes of uninterrupted quiescence. Animals with less than 10 minutes of activity during either the light or dark phase were flagged as dead. All analyses of sleep- and motion-bout dynamics were done in MatLab. For sleep-deprivation experiments, flies were mounted in DAM monitors and were mechanically stimulated using a vortexer mounting plate (TriKinetics) for 2 seconds every minute, over a 6-hour period prior to lights-on. Recovery sleep from flies with >60% loss of sleep during the deprivation period was analyzed and compared to baseline conditions 24 hours prior to the commencement of sleep deprivation. Recovery sleep was defined to occur in the first three hours following the end of sleep deprivation.
Western blotting
Fly heads were homogenized in Laemmli sample buffer (#1610737, Bio-Rad, Hercules, CA). For separation of polypeptides, samples were electrophoresed through precast polyacrylamide gels (Bio-Rad, #4561094) for 1 hour in a Tris/glycine/SDS buffer. Separated proteins were transferred to a nitrocellulose membrane and were then blocked with Odyssey blocking buffer (LI-COR Lincoln, NE, #927–40000) for 1 hour prior to incubation overnight with rabbit anti-pAMPKα (1:1000, Cell Signaling technology, Danvers, MA, #2535) and mouse anti-α-Tubulin (Sigma #T9026, diluted 1:5000) antibody at 4 °C. After 3 x 15-minute washes in PBS at RT, samples were incubated with IRDye 680RD and 800CW secondary antibodies diluted 1:10,000 (LI-COR) for 30 minutes at RT. Western blots were imaged using an Odyssey Fc imaging system (LI-COR) and ImageJ was used to quantify probe signal intensity.
Quantitative PCR and RNA sequencing
For quantitative real-time PCR (qPCR), total RNA was prepared from 10 adult male heads using the RNeasy Mini Kit (Qiagen #74106) with DNase treatment (Qiagen #79254). cDNA was synthesized using the iScript Reverse Transcription Supermix for RT-PCR (Bio-Rad #1708840), and qPCR was performed using the QuantiTect SYBR Green PCR Kit (Fisher Scientific #204145) on an Mx3005P qPCR system (Agilent Technologies). Expression was normalized to RpL32. Primers: alc; GGGCGACCATCAGTACAAGT and GCGTTCTCCACGCTTTTC; RpL32, AGTATCTGATGCCCAACATCG and CAATCTCCTTGCGCTTCTTG. For RNA-sequencing transcriptomics (RNA-seq), ten adult fly heads from 4-7-day-old males were harvested for each condition, and RNA libraries were prepared for Illumina HiSeq sequencing of paired-end 100-bp reads. Triplicates were sequenced for each genotype to determine differentially expressed genes.
Mechanical stress test
The mechanical stress test was conducted on 3-day-old male flies as described [39]. For each genotype, three vials containing 10 flies each were evaluated, with genotypes masked during test and evaluation. Each vial of flies was subjected to 5 seconds of mechanical stimulation (vortexing), followed by 55 seconds of recovery. The number of upright flies was counted after this time and is represented as a percentage of total number of flies.
Supporting information
Example for all genotypes tested, showing fits of survival data, from individual vials of 30 animals, to a Weibull distribution. For controls, lines were crossed to w1118.
(TIF)
(A and B) Expression levels of alc mRNA in adult heads from animals with reduced neuronal expression of alc using two independent RNAi lines compared to controls. Data shown is relative to driver control. (C) Levels of pAMPKα are significantly reduced (-60% from controls) in adult heads when alc is knocked down pan-neuronally; α tubulin is used as a ratiometric loading control. Data from 4 experimental repeats using 8 adult heads per sample. For controls, lines were crossed to w1118. Error bars indicate SEM. Mann Whitney test was used to determine statistical significance: *p<0.05, **p<0.01, versus the control.
(TIF)
(A) Schematic diagram and components of the setup built to test courtship conditioning. (B) Schematic diagram of individual chambers laser-cut from acrylic that were used to test courtship conditioning.
(TIF)
(A) Quantification of the average motion-bout length during dark-light and light-dark transitions (isolated as the motion bout present at t = 0(24) and t = 12 hours respectively) showed no significant differences in activity between elav>Dcr-2, alc-RNAi and control genotypes. (B) Quantification of activity (mean number of beam crossings per minute) during dark-light and light-dark transition periods showed a decrease in activity for elav>Dcr-2, alc-RNAi animals compared to control genotypes. (C) Total sleep over a 24-hour period for control genotypes elav>Dcr-2 and UAS-alc-RNAi/+ versus elav>Dcr-2, alc-RNAi on sucrose-based food and regular cornmeal food. For controls, lines were crossed to w1118. (D-E) Effects of fat body knockdown of alc. Activity (A) and sleep (B) profiles over a 24-hour period for control genotypes CG>+ (n = 32) and UAS-alc-RNA/+ (n = 32) versus CG>alc-RNAi (n = 32). All data obtained from second and third 24-hour cycle. Activity and sleep are shown in bins of 30 minutes. White and black bars represent ZT time, 12 hours light and 12 hours dark, respectively. (F) Total sleep (min) in CG>alc-RNAi flies with fat body alc knockdown shows reduced day time sleep and elevated night sleep compared to controls. (G) Average sleep-bout length (min) is significantly reduced during daytime in alc knockdown flies but not during night. (H) The number of sleep bouts per day increases when alc is knocked down in the fat body while being reduced during night sleep. (I) Maximum duration of sleep when alc is reduced in the fat body is unaffected except when comparing to UAS-alc-RNAi control during the day. For controls, lines were crossed to w1118. Error bars indicate SEM. Kruskal-Wallis test with Dunn’s post-hoc testing was used to determine statistical significance: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, versus the control.
(TIF)
(A-B) Activity (A) and sleep (B) profiles over a 24-hour period for control genotypes elav>Dcr-2 (n = 32) and UAS-alc-RNAi(8057R-2)/+ (n = 32) versus elav>Dcr-2, alc-RNAi(8057R-2) (n = 32). All data obtained from second to fourth 24-hour cycle. Activity and sleep are shown in bins of 30 minutes. White and black bars represent ZT time, 12 hours light and 12 hours dark, respectively. (C) Total sleep (min) in flies with pan-neuronal alc knockdown compared to controls. Total sleep is significantly reduced compared to both controls during dark phase and to driver control in light phase when alc is knocked down in the nervous system. (D) Duration of the longest sleep bout (min) is significantly shorter in alc knockdown animals than in controls. (E, F) Distribution of length of sleep bouts for control genotypes elav>Dcr-2/+ and UAS-alc-RNAi(8057R-2)/+ versus elav>Dcr-2, alc-RNAi(8057R-2) during the day (E) and during the night (F). For controls, lines were crossed to w1118. Error bars indicate SEM. Kruskal-Wallis test with Dunn’s post-hoc testing was used to determine statistical significance: ***p<0.001, ****p<0.0001, versus the control.
(TIF)
(A-B) Activity (A) and sleep (B) profiles over a 24-hour period for control genotypes elav>Dcr-2 (n = 16) and UAS-alc-RNAi/+ (n = 16) versus alc knockdown animals (elav>Dcr-2, alc-RNAi; n = 16) and alc overexpression animals (elav>Dcr-2, alc-RNAi, alc; n = 16). All data obtained from second and third 24-hour cycle. Activity and sleep are shown in bins of 30 minutes. White and black bars represent ZT time, 12 hours light and 12 hours dark, respectively. (C) Total sleep (min) in flies with pan-neuronal alc knockdown with and without alc overexpression compared to controls. (D) Average sleep-bout length (min) is significantly reduced during both day- and night-time in alc knockdown flies and rescued with alc overexpression. (E) The number of sleep bouts per day and night increases when alc is knocked down in the nervous system and rescued with alc overexpression. For controls, lines were crossed to w1118. Error bars indicate SEM. Kruskal-Wallis test with Dunn’s post-hoc testing was used to determine statistical significance: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, versus the control.
(TIF)
(A) Quantification of sleep debt (min) showing that both control elav>Dcr2/+ (n = 32) and UAS-alc-RNAi/+ (n = 32) flies recovered lost sleep in the first 3 hours following sleep deprivation while elav>Dcr-2, alc-RNAi(KK) (n = 32) animals with neuronal alc knockdown did not. (B) Both control genotypes showed a significant excess of sleep (~80 minutes) compared to the same time period of the baseline day while pan-neuronal alc-RNAi animals did not. (C) Time to first sleep bout (hours) is significantly reduced in both elav>Dcr2/+ (n = 54) and elav>Dcr2, alc-RNAi (n = 52) flies following sleep deprivation. For controls, lines were crossed to w1118. ****p<0.0001, versus the control.
(TIF)
(A) Graph showing percentage survival and increased mortality of sleep deprived elav>Dcr2, alc-RNAi (n = 32) animals, compared to a sleep-deprived (SD) control genotype (elav>Dcr2/+, n = 32), and non-sleep deprived controls (elav>Dcr2/+, elav>Dcr2, alc-RNAi, n = 32). Survival was monitored over 24 hours post sleep deprivation, and the number of dead animals was counted. (B) Graph showing mean fraction mortality following a mechanical-stress assay (n = 3 vials of 10 animals for each genotype). Knockdown of alc in the nervous system does not increase susceptibility to mortality following mechanical stress. (C) Levels of phosphorylated AMPKα (pAMPKα) increased (normalized to alpha-Tubulin) immediately following 6 hours of sleep deprivation (SD) compared to same-time non-deprived animals for control animals (elav>Dcr-2/+ and Canton S). For controls, lines were crossed to w1118.
(TIF)
Control elav>Dcr2/+ animals were compared to elav>Dcr2, alc-RNAi(KK) animals with neuronal knockdown of alc.
(XLS)
Acknowledgments
We thank Annika Groenborg-Forsingdal for assistance with analysis of RNA-seq samples. We also thank David Mouritzen for his help, and Michael J. Texada for reading and editing the manuscript.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by the Danish Council for Independent Research, Medical Sciences grant 4183-00312 to KR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fejgin K, Nielsen J, Birknow MR, Bastlund JF, Nielsen V, Lauridsen JB, et al. A mouse model that recapitulates cardinal features of the 15q13.3 microdeletion syndrome including schizophrenia- and epilepsy-related alterations. Biol Psychiatry. 2014;76(2):128–37. 10.1016/j.biopsych.2013.08.014 . [DOI] [PubMed] [Google Scholar]
- 2.Malhotra D, Sebat J. CNVs: harbingers of a rare variant revolution in psychiatric genetics. Cell. 2012;148(6):1223–41. 10.1016/j.cell.2012.02.039 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ingason A, Giegling I, Cichon S, Hansen T, Rasmussen HB, Nielsen J, et al. A large replication study and meta-analysis in European samples provides further support for association of AHI1 markers with schizophrenia. Hum Mol Genet. 2010;19(7):1379–86. Epub 2010/01/15. 10.1093/hmg/ddq009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stefansson H, Rujescu D, Cichon S, Pietilainen OP, Ingason A, Steinberg S, et al. Large recurrent microdeletions associated with schizophrenia. Nature. 2008;455(7210):232–6. Epub 2008/08/01. 10.1038/nature07229 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szelenberger W, Soldatos C. Sleep disorders in psychiatric practice. World Psychiatry. 2005;4(3):186–90. . [PMC free article] [PubMed] [Google Scholar]
- 6.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nature reviews Molecular cell biology. 2007;8(10):774–85. 10.1038/nrm2249 . [DOI] [PubMed] [Google Scholar]
- 7.Hardie DG, Carling D, Gamblin SJ. AMP-activated protein kinase: also regulated by ADP? Trends Biochem Sci. 2011;36(9):470–7. 10.1016/j.tibs.2011.06.004 . [DOI] [PubMed] [Google Scholar]
- 8.Zuccoli GS, Saia-Cereda VM, Nascimento JM, Martins-de-Souza D. The Energy Metabolism Dysfunction in Psychiatric Disorders Postmortem Brains: Focus on Proteomic Evidence. Front Neurosci. 2017;11:493 10.3389/fnins.2017.00493 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardie DG, Schaffer BE, Brunet A. AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol. 2016;26(3):190–201. 10.1016/j.tcb.2015.10.013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riek U, Scholz R, Konarev P, Rufer A, Suter M, Nazabal A, et al. Structural properties of AMP-activated protein kinase: dimerization, molecular shape, and changes upon ligand binding. J Biol Chem. 2008;283(26):18331–43. 10.1074/jbc.M708379200 . [DOI] [PubMed] [Google Scholar]
- 11.Sinnett SE, Brenman JE. The Role of AMPK in Drosophila melanogaster. EXS. 2016;107:389–401. 10.1007/978-3-319-43589-3_16 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardie DG, Frenguelli BG. A neural protection racket: AMPK and the GABA(B) receptor. Neuron. 2007;53(2):159–62. 10.1016/j.neuron.2007.01.004 . [DOI] [PubMed] [Google Scholar]
- 13.Harris JJ, Jolivet R, Attwell D. Synaptic energy use and supply. Neuron. 2012;75(5):762–77. 10.1016/j.neuron.2012.08.019 . [DOI] [PubMed] [Google Scholar]
- 14.Culmsee C, Monnig J, Kemp BE, Mattson MP. AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci. 2001;17(1):45–58. 10.1385/JMN:17:1:45 . [DOI] [PubMed] [Google Scholar]
- 15.McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem. 2005;280(21):20493–502. 10.1074/jbc.M409985200 . [DOI] [PubMed] [Google Scholar]
- 16.Dasgupta B, Milbrandt J. AMP-activated protein kinase phosphorylates retinoblastoma protein to control mammalian brain development. Dev Cell. 2009;16(2):256–70. 10.1016/j.devcel.2009.01.005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson EC, Kazgan N, Bretz CA, Forsberg LJ, Hector CE, Worthen RJ, et al. Altered metabolism and persistent starvation behaviors caused by reduced AMPK function in Drosophila. PLoS One. 2010;5(9). 10.1371/journal.pone.0012799 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poels J, Spasic MR, Gistelinck M, Mutert J, Schellens A, Callaerts P, et al. Autophagy and phagocytosis-like cell cannibalism exert opposing effects on cellular survival during metabolic stress. Cell Death Differ. 2012;19(10):1590–601. 10.1038/cdd.2012.37 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spasic MR, Callaerts P, Norga KK. Drosophila alicorn is a neuronal maintenance factor protecting against activity-induced retinal degeneration. J Neurosci. 2008;28(25):6419–29. 10.1523/JNEUROSCI.1646-08.2008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ulgherait M, Rana A, Rera M, Graniel J, Walker DW. AMPK modulates tissue and organismal aging in a non-cell-autonomous manner. Cell reports. 2014;8(6):1767–80. 10.1016/j.celrep.2014.08.006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tohyama D, Yamaguchi A. A critical role of SNF1A/dAMPKalpha (Drosophila AMP-activated protein kinase alpha) in muscle on longevity and stress resistance in Drosophila melanogaster. Biochem Biophys Res Commun. 2010;394(1):112–8. 10.1016/j.bbrc.2010.02.126 . [DOI] [PubMed] [Google Scholar]
- 22.Dasgupta B, Chhipa RR. Evolving Lessons on the Complex Role of AMPK in Normal Physiology and Cancer. Trends Pharmacol Sci. 2016;37(3):192–206. 10.1016/j.tips.2015.11.007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaaro-Peled H, Ayhan Y, Pletnikov MV, Sawa A. Review of pathological hallmarks of schizophrenia: comparison of genetic models with patients and nongenetic models. Schizophr Bull. 2010;36(2):301–13. Epub 2009/11/12. 10.1093/schbul/sbp133 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dierssen M, Ramakers GJ. Dendritic pathology in mental retardation: from molecular genetics to neurobiology. Genes Brain Behav. 2006;5 Suppl 2:48–60. Epub 2006/05/10. 10.1111/j.1601-183X.2006.00224.x . [DOI] [PubMed] [Google Scholar]
- 25.Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14(3):285–93. Epub 2011/02/25. 10.1038/nn.2741 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57(1):65–73. Epub 2000/01/13. . [DOI] [PubMed] [Google Scholar]
- 27.Corty MM, Matthews BJ, Grueber WB. Molecules and mechanisms of dendrite development in Drosophila. Development. 2009;136(7):1049–61. Epub 2009/03/10. 10.1242/dev.014423 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brunetti-Pierri N, Berg JS, Scaglia F, Belmont J, Bacino CA, Sahoo T, et al. Recurrent reciprocal 1q21.1 deletions and duplications associated with microcephaly or macrocephaly and developmental and behavioral abnormalities. Nat Genet. 2008;40(12):1466–71. 10.1038/ng.279 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siegel RW, Hall JC. Conditioned responses in courtship behavior of normal and mutant Drosophila. Proc Natl Acad Sci U S A. 1979;76(7):3430–4. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haldeman-Englert CR, Jewett T. 1q21.1 Recurrent Microdeletion In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, et al. , editors. GeneReviews((R)). Seattle (WA) 1993. [Google Scholar]
- 31.Dworak M, McCarley RW, Kim T, Kalinchuk AV, Basheer R. Sleep and brain energy levels: ATP changes during sleep. J Neurosci. 2010;30(26):9007–16. 10.1523/JNEUROSCI.1423-10.2010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, Sehgal A, et al. Rest in Drosophila is a sleep-like state. Neuron. 2000;25(1):129–38. . [DOI] [PubMed] [Google Scholar]
- 33.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287(5459):1834–7. Epub 2000/03/10. . [DOI] [PubMed] [Google Scholar]
- 34.Yurgel ME, Shah KD, Brown EB, Burns C, Bennick RA, DiAngelo JR, et al. Ade2 Functions in the Drosophila Fat Body To Promote Sleep. G3 (Bethesda). 2018. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keene AC, Duboue ER, McDonald DM, Dus M, Suh GS, Waddell S, et al. Clock and cycle limit starvation-induced sleep loss in Drosophila. Curr Biol. 2010;20(13):1209–15. 10.1016/j.cub.2010.05.029 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Branson K, Robie AA, Bender J, Perona P, Dickinson MH. High-throughput ethomics in large groups of Drosophila. Nature methods. 2009;6(6):451–7. 10.1038/nmeth.1328 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seugnet L, Boero J, Gottschalk L, Duntley SP, Shaw PJ. Identification of a biomarker for sleep drive in flies and humans. Proc Natl Acad Sci U S A. 2006;103(52):19913–8. 10.1073/pnas.0609463104 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thimgan MS, Seugnet L, Turk J, Shaw PJ. Identification of genes associated with resilience/vulnerability to sleep deprivation and starvation in Drosophila. Sleep. 2015;38(5):801–14. 10.5665/sleep.4680 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaw PJ, Tononi G, Greenspan RJ, Robinson DF. Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature. 2002;417(6886):287–91. 10.1038/417287a . [DOI] [PubMed] [Google Scholar]
- 40.Burkewitz K, Zhang Y, Mair WB. AMPK at the nexus of energetics and aging. Cell Metab. 2014;20(1):10–25. 10.1016/j.cmet.2014.03.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin WS, Chen JY, Wang JC, Chen LY, Lin CH, Hsieh TR, et al. The anti-aging effects of Ludwigia octovalvis on Drosophila melanogaster and SAMP8 mice. Age (Dordr). 2014;36(2):689–703. 10.1007/s11357-013-9606-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stenesen D, Suh JM, Seo J, Yu K, Lee KS, Kim JS, et al. Adenosine nucleotide biosynthesis and AMPK regulate adult life span and mediate the longevity benefit of caloric restriction in flies. Cell Metab. 2013;17(1):101–12. 10.1016/j.cmet.2012.12.006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braco JT, Gillespie EL, Alberto GE, Brenman JE, Johnson EC. Energy-dependent modulation of glucagon-like signaling in Drosophila via the AMP-activated protein kinase. Genetics. 2012;192(2):457–66. 10.1534/genetics.112.143610 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dickey AS, Strack S. PKA/AKAP1 and PP2A/Bbeta2 regulate neuronal morphogenesis via Drp1 phosphorylation and mitochondrial bioenergetics. J Neurosci. 2011;31(44):15716–26. 10.1523/JNEUROSCI.3159-11.2011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsuyama T, Tsubouchi A, Usui T, Imamura H, Uemura T. Mitochondrial dysfunction induces dendritic loss via eIF2alpha phosphorylation. J Cell Biol. 2017;216(3):815–34. 10.1083/jcb.201604065 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ng CH, Guan MS, Koh C, Ouyang X, Yu F, Tan EK, et al. AMP kinase activation mitigates dopaminergic dysfunction and mitochondrial abnormalities in Drosophila models of Parkinson’s disease. J Neurosci. 2012;32(41):14311–7. 10.1523/JNEUROSCI.0499-12.2012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swick LL, Kazgan N, Onyenwoke RU, Brenman JE. Isolation of AMP-activated protein kinase (AMPK) alleles required for neuronal maintenance in Drosophila melanogaster. Biol Open. 2013;2(12):1321–3. 10.1242/bio.20136775 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cook M, Mani P, Wentzell JS, Kretzschmar D. Increased RhoA prenylation in the loechrig (loe) mutant leads to progressive neurodegeneration. PLoS One. 2012;7(9):e44440 10.1371/journal.pone.0044440 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tschape JA, Hammerschmied C, Muhlig-Versen M, Athenstaedt K, Daum G, Kretzschmar D. The neurodegeneration mutant lochrig interferes with cholesterol homeostasis and Appl processing. EMBO J. 2002;21(23):6367–76. 10.1093/emboj/cdf636 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marinangeli C, Didier S, Vingtdeux V. AMPK in Neurodegenerative Diseases: Implications and Therapeutic Perspectives. Curr Drug Targets. 2016;17(8):890–907. . [DOI] [PubMed] [Google Scholar]
- 51.Guven-Ozkan T, Davis RL. Functional neuroanatomy of Drosophila olfactory memory formation. Learn Mem. 2014;21(10):519–26. 10.1101/lm.034363.114 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davis RL, Cherry J, Dauwalder B, Han PL, Skoulakis E. The cyclic AMP system and Drosophila learning. Mol Cell Biochem. 1995;149–150:271–8. . [DOI] [PubMed] [Google Scholar]
- 53.Louis T, Stahl A, Boto T, Tomchik SM. Cyclic AMP-dependent plasticity underlies rapid changes in odor coding associated with reward learning. Proc Natl Acad Sci U S A. 2018;115(3):E448–E57. 10.1073/pnas.1709037115 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Omar B, Zmuda-Trzebiatowska E, Manganiello V, Goransson O, Degerman E. Regulation of AMP-activated protein kinase by cAMP in adipocytes: roles for phosphodiesterases, protein kinase B, protein kinase A, Epac and lipolysis. Cell Signal. 2009;21(5):760–6. 10.1016/j.cellsig.2009.01.015 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kobilo T, Guerrieri D, Zhang Y, Collica SC, Becker KG, van Praag H. AMPK agonist AICAR improves cognition and motor coordination in young and aged mice. Learn Mem. 2014;21(2):119–26. 10.1101/lm.033332.113 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kobilo T, Yuan C, van Praag H. Endurance factors improve hippocampal neurogenesis and spatial memory in mice. Learn Mem. 2011;18(2):103–7. 10.1101/lm.2001611 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alvarenga TA, Patti CL, Andersen ML, Silva RH, Calzavara MB, Lopez GB, et al. Paradoxical sleep deprivation impairs acquisition, consolidation, and retrieval of a discriminative avoidance task in rats. Neurobiol Learn Mem. 2008;90(4):624–32. 10.1016/j.nlm.2008.07.013 . [DOI] [PubMed] [Google Scholar]
- 58.Krishnan HC, Gandour CE, Ramos JL, Wrinkle MC, Sanchez-Pacheco JJ, Lyons LC. Acute Sleep Deprivation Blocks Short- and Long-Term Operant Memory in Aplysia. Sleep. 2016;39(12):2161–71. 10.5665/sleep.6320 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li X, Yu F, Guo A. Sleep deprivation specifically impairs short-term olfactory memory in Drosophila. Sleep. 2009;32(11):1417–24. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Potdar S, Sheeba V. Lessons from sleeping flies: insights from Drosophila melanogaster on the neuronal circuitry and importance of sleep. Journal of neurogenetics. 2013;27(1–2):23–42. 10.3109/01677063.2013.791692 . [DOI] [PubMed] [Google Scholar]
- 61.Greenspan RJ, Tononi G, Cirelli C, Shaw PJ. Sleep and the fruit fly. Trends Neurosci. 2001;24(3):142–5. . [DOI] [PubMed] [Google Scholar]
- 62.Magistretti PJ, Allaman I. A cellular perspective on brain energy metabolism and functional imaging. Neuron. 2015;86(4):883–901. 10.1016/j.neuron.2015.03.035 . [DOI] [PubMed] [Google Scholar]
- 63.Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45(4):347–60. . [DOI] [PubMed] [Google Scholar]
- 64.Petit JM, Burlet-Godinot S, Magistretti PJ, Allaman I. Glycogen metabolism and the homeostatic regulation of sleep. Metab Brain Dis. 2015;30(1):263–79. 10.1007/s11011-014-9629-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zimmerman JE, Mackiewicz M, Galante RJ, Zhang L, Cater J, Zoh C, et al. Glycogen in the brain of Drosophila melanogaster: diurnal rhythm and the effect of rest deprivation. J Neurochem. 2004;88(1):32–40. . [DOI] [PubMed] [Google Scholar]
- 66.Landolt HP. Sleep homeostasis: a role for adenosine in humans? Biochem Pharmacol. 2008;75(11):2070–9. 10.1016/j.bcp.2008.02.024 . [DOI] [PubMed] [Google Scholar]
- 67.Hardie DG. AMPK—sensing energy while talking to other signaling pathways. Cell Metab. 2014;20(6):939–52. 10.1016/j.cmet.2014.09.013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McBride A, Hardie DG. AMP-activated protein kinase—a sensor of glycogen as well as AMP and ATP? Acta Physiol (Oxf). 2009;196(1):99–113. 10.1111/j.1748-1716.2009.01975.x . [DOI] [PubMed] [Google Scholar]
- 69.Weibull W. A Statistical Distribution Function of Wide Applicability. Journal of Applied Mechanics. 1951;18:293–7. [Google Scholar]
- 70.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nature methods. 2012;9(7):676–82. 10.1038/nmeth.2019 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cuntz H, Forstner F, Borst A, Hausser M. One rule to grow them all: a general theory of neuronal branching and its practical application. PLoS Comput Biol. 2010;6(8). 10.1371/journal.pcbi.1000877 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Example for all genotypes tested, showing fits of survival data, from individual vials of 30 animals, to a Weibull distribution. For controls, lines were crossed to w1118.
(TIF)
(A and B) Expression levels of alc mRNA in adult heads from animals with reduced neuronal expression of alc using two independent RNAi lines compared to controls. Data shown is relative to driver control. (C) Levels of pAMPKα are significantly reduced (-60% from controls) in adult heads when alc is knocked down pan-neuronally; α tubulin is used as a ratiometric loading control. Data from 4 experimental repeats using 8 adult heads per sample. For controls, lines were crossed to w1118. Error bars indicate SEM. Mann Whitney test was used to determine statistical significance: *p<0.05, **p<0.01, versus the control.
(TIF)
(A) Schematic diagram and components of the setup built to test courtship conditioning. (B) Schematic diagram of individual chambers laser-cut from acrylic that were used to test courtship conditioning.
(TIF)
(A) Quantification of the average motion-bout length during dark-light and light-dark transitions (isolated as the motion bout present at t = 0(24) and t = 12 hours respectively) showed no significant differences in activity between elav>Dcr-2, alc-RNAi and control genotypes. (B) Quantification of activity (mean number of beam crossings per minute) during dark-light and light-dark transition periods showed a decrease in activity for elav>Dcr-2, alc-RNAi animals compared to control genotypes. (C) Total sleep over a 24-hour period for control genotypes elav>Dcr-2 and UAS-alc-RNAi/+ versus elav>Dcr-2, alc-RNAi on sucrose-based food and regular cornmeal food. For controls, lines were crossed to w1118. (D-E) Effects of fat body knockdown of alc. Activity (A) and sleep (B) profiles over a 24-hour period for control genotypes CG>+ (n = 32) and UAS-alc-RNA/+ (n = 32) versus CG>alc-RNAi (n = 32). All data obtained from second and third 24-hour cycle. Activity and sleep are shown in bins of 30 minutes. White and black bars represent ZT time, 12 hours light and 12 hours dark, respectively. (F) Total sleep (min) in CG>alc-RNAi flies with fat body alc knockdown shows reduced day time sleep and elevated night sleep compared to controls. (G) Average sleep-bout length (min) is significantly reduced during daytime in alc knockdown flies but not during night. (H) The number of sleep bouts per day increases when alc is knocked down in the fat body while being reduced during night sleep. (I) Maximum duration of sleep when alc is reduced in the fat body is unaffected except when comparing to UAS-alc-RNAi control during the day. For controls, lines were crossed to w1118. Error bars indicate SEM. Kruskal-Wallis test with Dunn’s post-hoc testing was used to determine statistical significance: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, versus the control.
(TIF)
(A-B) Activity (A) and sleep (B) profiles over a 24-hour period for control genotypes elav>Dcr-2 (n = 32) and UAS-alc-RNAi(8057R-2)/+ (n = 32) versus elav>Dcr-2, alc-RNAi(8057R-2) (n = 32). All data obtained from second to fourth 24-hour cycle. Activity and sleep are shown in bins of 30 minutes. White and black bars represent ZT time, 12 hours light and 12 hours dark, respectively. (C) Total sleep (min) in flies with pan-neuronal alc knockdown compared to controls. Total sleep is significantly reduced compared to both controls during dark phase and to driver control in light phase when alc is knocked down in the nervous system. (D) Duration of the longest sleep bout (min) is significantly shorter in alc knockdown animals than in controls. (E, F) Distribution of length of sleep bouts for control genotypes elav>Dcr-2/+ and UAS-alc-RNAi(8057R-2)/+ versus elav>Dcr-2, alc-RNAi(8057R-2) during the day (E) and during the night (F). For controls, lines were crossed to w1118. Error bars indicate SEM. Kruskal-Wallis test with Dunn’s post-hoc testing was used to determine statistical significance: ***p<0.001, ****p<0.0001, versus the control.
(TIF)
(A-B) Activity (A) and sleep (B) profiles over a 24-hour period for control genotypes elav>Dcr-2 (n = 16) and UAS-alc-RNAi/+ (n = 16) versus alc knockdown animals (elav>Dcr-2, alc-RNAi; n = 16) and alc overexpression animals (elav>Dcr-2, alc-RNAi, alc; n = 16). All data obtained from second and third 24-hour cycle. Activity and sleep are shown in bins of 30 minutes. White and black bars represent ZT time, 12 hours light and 12 hours dark, respectively. (C) Total sleep (min) in flies with pan-neuronal alc knockdown with and without alc overexpression compared to controls. (D) Average sleep-bout length (min) is significantly reduced during both day- and night-time in alc knockdown flies and rescued with alc overexpression. (E) The number of sleep bouts per day and night increases when alc is knocked down in the nervous system and rescued with alc overexpression. For controls, lines were crossed to w1118. Error bars indicate SEM. Kruskal-Wallis test with Dunn’s post-hoc testing was used to determine statistical significance: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, versus the control.
(TIF)
(A) Quantification of sleep debt (min) showing that both control elav>Dcr2/+ (n = 32) and UAS-alc-RNAi/+ (n = 32) flies recovered lost sleep in the first 3 hours following sleep deprivation while elav>Dcr-2, alc-RNAi(KK) (n = 32) animals with neuronal alc knockdown did not. (B) Both control genotypes showed a significant excess of sleep (~80 minutes) compared to the same time period of the baseline day while pan-neuronal alc-RNAi animals did not. (C) Time to first sleep bout (hours) is significantly reduced in both elav>Dcr2/+ (n = 54) and elav>Dcr2, alc-RNAi (n = 52) flies following sleep deprivation. For controls, lines were crossed to w1118. ****p<0.0001, versus the control.
(TIF)
(A) Graph showing percentage survival and increased mortality of sleep deprived elav>Dcr2, alc-RNAi (n = 32) animals, compared to a sleep-deprived (SD) control genotype (elav>Dcr2/+, n = 32), and non-sleep deprived controls (elav>Dcr2/+, elav>Dcr2, alc-RNAi, n = 32). Survival was monitored over 24 hours post sleep deprivation, and the number of dead animals was counted. (B) Graph showing mean fraction mortality following a mechanical-stress assay (n = 3 vials of 10 animals for each genotype). Knockdown of alc in the nervous system does not increase susceptibility to mortality following mechanical stress. (C) Levels of phosphorylated AMPKα (pAMPKα) increased (normalized to alpha-Tubulin) immediately following 6 hours of sleep deprivation (SD) compared to same-time non-deprived animals for control animals (elav>Dcr-2/+ and Canton S). For controls, lines were crossed to w1118.
(TIF)
Control elav>Dcr2/+ animals were compared to elav>Dcr2, alc-RNAi(KK) animals with neuronal knockdown of alc.
(XLS)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.