Abstract
Objective:
To evaluate the association between the presence of an atrial septal defect (ASD) and the odds of developing bronchopulmonary dysplasia (BPD) in premature infants.
Study Design:
We identified a cohort of infants with at least one echocardiogram, birth weight (BW) 501–1249 g, and gestational age (GA) 23–30 weeks discharged from the neonatal intensive care unit (NICU) from 2004–2016. We used a BPD risk estimator to calculate an infant’s predicted risk of developing BPD at 6 postnatal ages within the first 28 days of life. We examined the association between the presence of an ASD and the development of BPD using 2 multivariable logistic regression models for each BPD risk severity on each postnatal day. The first model adjusted for predicted BPD risk, and the second added therapeutic interventions for BPD.
Results:
Of 20,496 infants from 228 NICUs who met inclusion criteria, 8892 (43%) were diagnosed with BPD and 1314 (6%) had an ASD. BPD was present in 48% of infants with an ASD and 43% of infants without an ASD. In infants with an ASD, the odds ratio of developing BPD was higher after adjusting for predicted risk of BPD plus therapeutic interventions, regardless of postnatal age or predicted BPD risk severity.
Conclusions:
The presence of an ASD was associated with an increased odds of BPD in this cohort. Future trials should consider ASD as a potentially modifiable risk factor in this vulnerable population.
Keywords: chronic lung disease, congenital heart disease, pulmonary hypertension
Introduction
Bronchopulmonary dysplasia (BPD) is a major cause of morbidity and mortality, affecting 10,000 to 15,000 infants annually in the United States. Premature and low birth weight infants are known to be at higher risk.1 BPD is associated with significant long-term sequelae, including chronic cardiorespiratory impairments, growth failure, and neurodevelopmental delay.2, 3 Despite significant advances in neonatal ventilation strategies and the widespread use of antenatal steroids and postnatal surfactant, the incidence of BPD in premature infants has remained high over the past 20 years.4
In addition to prematurity and low birth weight, possible risk factors for BPD include male sex, mechanical ventilation, higher exposure to supplemental oxygen, and the presence of a patent ductus arteriosus (PDA).5 Up to 70% of preterm and low birth weight infants have a PDA, which results in left-to-right shunting and increased pulmonary blood flow.6 This increased blood flow may contribute to the development of BPD by increasing alveolar and interstitial lung fluid filtration, resulting in pulmonary edema, altered respiratory mechanics, decreased lung compliance, poor gas exchange, and the need for prolonged mechanical ventilation and supplemental oxygen.7
Left-to-right shunts are highly prevalent cardiac lesions that also include atrial septal defects (ASDs), the second most common type of congenital heart disease (CHD).8, 9 Although an ASD is usually asymptomatic in otherwise healthy infants, the resultant increased pulmonary blood flow may hinder lung tissue development and increase a premature infant’s risk of developing BPD.10, 11 Investigators have postulated that atrial level shunts contribute to the progression of lung disease in premature infants, but this assumption is based on case reports and small single-center case series.12–18 The extent to which an ASD affects the risk of BPD is still largely unknown.
This study evaluates the association between the presence of an ASD and the odds of developing BPD in premature infants admitted to the neonatal intensive care unit (NICU). We hypothesized that after controlling for known risk factors, the presence of an ASD would be associated with increased odds of developing BPD.
Methods
Data Source
We used an electronic health record database that prospectively captures information generated by clinicians on all infants cared for by the Pediatrix Medical Group in 228 NICUs in North America from 2004 to 2016. This information is gathered from routine clinical care documentation, including admission notes, daily progress notes, and discharge summaries. The record consists of data on multiple aspects of care, including demographics, maternal history, medications, laboratory results, diagnoses, and procedures. These data are then transferred to the Pediatrix Clinical Data Warehouse for quality improvement and research purposes.19
Patient Population
We included infants with at least one echocardiogram performed during their hospitalization in the NICU who had a birth weight between 501 and 1249 g and a gestational age between 23 and 30 weeks to be compatible with the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network web-based BPD risk estimator.20 We excluded outborn infants with major congenital anomalies, including heart defects other than an ASD or PDA.
Definitions
We defined BPD as continuous respiratory support (supplemental oxygen, nasal cannula, high-flow nasal cannula, nasal continuous positive airway pressure, conventional mechanical ventilation, or high frequency ventilation) between 36 0/7 and 36 6/7 weeks postmenstrual age, as reported previously.21 We defined infants as having an ASD if it was documented as a clinical diagnosis in the electronic health record. We defined PDA treatment as surgical if an infant underwent PDA ligation at any time during hospitalization. We defined PDA treatment as medical if an infant was exposed to acetaminophen or ibuprofen/indomethacin after day of life 1. This age cut-off was used to exclude courses administered as prophylaxis for the prevention of intraventricular hemorrhage. We defined postnatal steroid exposure as the use of any dexamethasone, hydrocortisone, or inhaled steroids during hospitalization. We defined postnatal diuretic exposure as the use of any diuretic (acetazolamide, amiloride, bumetanide, chlorothiazide, diazoxide, ethacrynic acid, furosemide, hydrochlorothiazide, spironolactone, and metolazone) during hospitalization. We calculated the percent of days on diuretics as the number of days with at least one diuretic received divided by the duration of an infant’s hospitalization in days.
Data Collection
We extracted demographic variables—including gestational age, birth weight, race, sex, 5-minute Apgar score, type of delivery, and surfactant exposure—and the diagnoses of ASD and PDA from the data warehouse. Risk factors for BPD utilized in our study included the type of respiratory support, total ventilator days, and fraction of inspired oxygen content (FiO2). Therapeutic interventions for BPD identified and collected for our study included type of treatment for PDA, prenatal steroid exposure, postnatal steroid exposure, postnatal caffeine exposure, and postnatal diuretic exposure.
Statistical Analysis
We used frequencies (with percentages) and medians (with 25th and 75th percentiles) or means (with standard deviations) to describe categorical and continuous study variables, respectively. We compared the distribution of demographics, risk factors for BPD, and therapeutic interventions for BPD between infants with an ASD and those without an ASD using the chi-squared test or Wilcoxon rank sum test, where appropriate.
We applied the data collected from our database in conjunction with the NICHD Neonatal Research Network BPD risk estimator to calculate an infant’s predicted risk of developing mild, moderate, or severe BPD at postnatal ages of 1, 3, 7, 14, 21, and 28 days of life.20 The risk estimator includes birth weight, gestational age, sex, race, postnatal age, type of respiratory support, and amount of supplemental oxygen administration.
To evaluate the association between the presence of an ASD and the development of BPD, we fit separate multivariable logistic regression models with random effects for NICU site on each of the 6 postnatal days on which BPD risk was estimated. Two sets of models were built for each BPD risk severity (mild, moderate, and severe) on each of the 6 postnatal days evaluated to estimate the odds of developing BPD (for a total of 36 models). The first set (Model A) adjusted for the predicted BPD risk. The second set (Model B) adjusted for the predicted BPD risk and therapeutic interventions that may affect BPD risk, including: surgical PDA ligation (binary variable), percent of total days on diuretics (continuous variable), any prenatal steroid exposure (binary variable), and any postnatal steroid exposure (binary variable).
To assess the robustness of our analytical method, we performed a sensitivity analysis without the NICHD Neonatal Research Network BPD risk estimations. Instead, we used logistic regression for correlated data with generalized estimating equations (GEEs) adjusted for demographic variables (gestational age, birth weight, sex, race) and therapeutic interventions (surgical PDA ligation, percent of total days on diuretics, prenatal steroid exposure, and postnatal steroid exposure). We report odds ratios (ORs) with 95% confidence intervals. We defined statistical significance as a p-value <0.05. We performed all statistical analyses using Stata 15.1 (College Station, Texas). This study was approved by the Duke University Institutional Review Board.
Results
Patient Characteristics
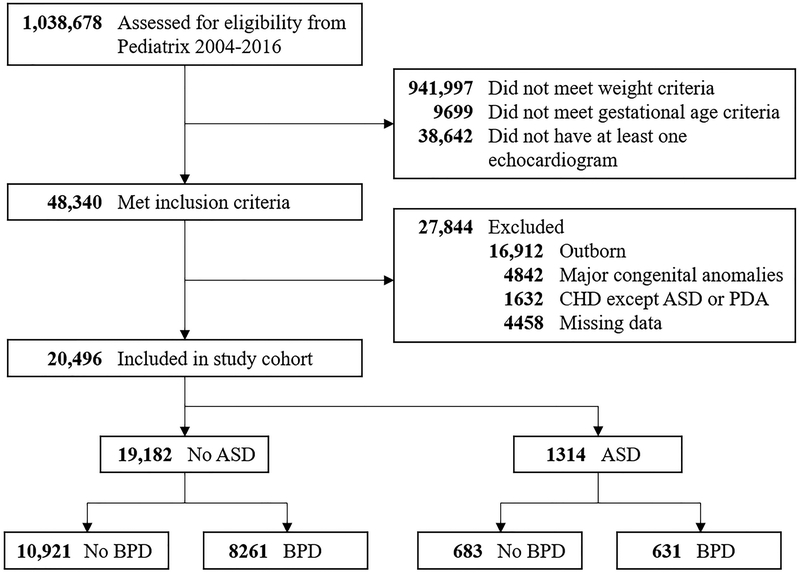
We identified 20,496 infants from 228 NICUs that met study inclusion and exclusion criteria (Figure 1; online). These infants had a median gestational age of 27 weeks (25th, 75th percentiles: 25, 28) and a median birth weight of 873 g (715, 1045). Of these infants, 16,000 (78%) required respiratory support with conventional mechanical ventilation (CMV) or high-frequency ventilation (HFV) at any time during the first 28 days of life. A total of 8892 (43%) infants were diagnosed with BPD. There were 17,209 (84%) infants with a PDA, of which 7415 (43%) were treated medically and 2945 (17%) underwent surgical ligation. We found that 17,196 (84%) infants were exposed to prenatal steroids, 7681 (37%) to postnatal steroids, and 12,307 (60%) to postnatal diuretics.
Figure 1. Flow Diagram of Infants Included in the Study.
ASD, atrial septal defect; BPD, bronchopulmonary dysplasia; CHD, congenital heart disease; PDA, patent ductus arteriosus
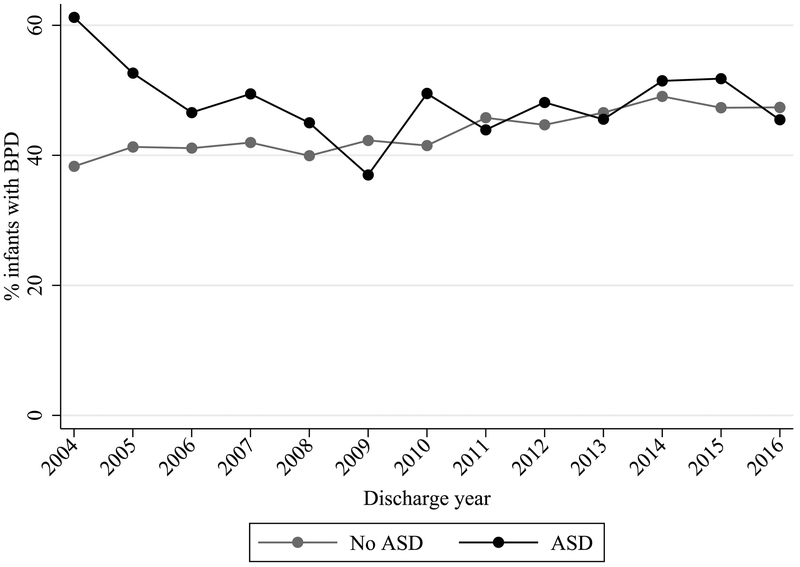
A total of 1314 (6%) infants in the study cohort were diagnosed with an ASD (Table 1) at a median postnatal age of 19 days (25th, 75th percentiles: 6, 49). These infants had a median gestational age of 27 weeks (25, 28) and a median birth weight of 885 g (725, 1060). We found that 48% of infants with an ASD versus 43% of infants without an ASD were diagnosed with BPD (p<0.001). This difference was most pronounced early in the course of the study (Figure 2; online).
Table 1.
Infant characteristics
| Characteristic | ASD, n (%) | |
|---|---|---|
| No (n= 19,182) |
Yes (n= 1314) |
|
| Gestational age (weeks) | ||
| <26 | 6076 (32) | 353 (27) |
| 26–28 | 9945 (52) | 730 (56) |
| 29–30 | 3161(16) | 231(18) |
| Birth weight (g) | ||
| 501–750 | 5979 (31) | 386 (29) |
| 751–1000 | 7329 (38) | 502 (38) |
| 1001–1250 | 5874(31) | 426 (32) |
| Small for gestational age | 2889(15) | 220(17) |
| Race | ||
| White | 9594 (50) | 658(50) |
| Black | 5809 (30) | 364 (28) |
| Hispanic | 3779 (20) | 292 (22) |
| Male | 9957 (52) | 606 (46) |
| 5-minute Apgar | ||
| 0–3 | 971 (5) | 60(5) |
| 4–6 | 4138 (22) | 284 (22) |
| 7–10 | 13,766 (73) | 954 (73) |
| Cesarean section | 14,324 (75) | 1039 (79) |
| Necrotizing enterocolitis | 1623 (8) | 94(7) |
| BPD diagnosis | 8261 (43) | 631(48) |
| Postnatal surfactant exposure | 15,778 (82) | 1064(81) |
ASD, atrial septal defect; BPD, bronchopulmonary dysplasia
Figure 2. Prevalence of BPD by Discharge Year and ASD Diagnosis.
ASD, atrial septal defect; BPD, bronchopulmonary dysplasia
Comparison of Known Risk Factors for BPD in Infants with and without an ASD
We found that known risk factors for BPD were common in our cohort of infants (Table 2). Infants with an ASD were less likely to require invasive mechanical ventilation with CMV or HFV during the first 28 days of life, compared with infants without an ASD (74% vs. 78%, p<0.001). Infants with an ASD had a lower median FiO2 requirement and were less frequently diagnosed with a PDA compared with infants without an ASD. Both groups of infants spent a similar median number of days on a ventilator and a similar number of median days with an FiO2 requirement >21%.
Table 2.
Risk factors for BPD in infants with and without an ASD
| Risk Factor | ASD | |
|---|---|---|
| No (n= 19,182) |
Yes (n= 1314) |
|
| Respiratory support, n (%) | ||
| HFV | 6421 (33) | 404(31) |
| CMV | 8612(45) | 563(43) |
| CPAP | 3283 (17) | 303 (23) |
| Cannula/Hood | 718(4) | 40(3) |
| Room air | 148(1) | 4(0) |
| Days on ventilator, median (25th, 75th %ile) | 10(2,31) | 9 (2, 33) |
| FiO2 (%), median (25th, 75th %ile) | 23 (21, 30) | 21 (21, 30) |
| Days with FiO2 >21%, median (25th, 75th %ile) | 41(11,78) | 42(11,82) |
| PDA diagnosis | 16,144(84) | 1065 (81) |
ASD, atrial septal defect; BPD, bronchopulmonary dysplasia; CMV, conventional mechanical ventilation; CPAP, continuous positive airway pressure; FiO2, fraction of inspired oxygen; HFV, high-frequency ventilation; PDA, patent ductus arteriosus
Comparison of Therapeutic Interventions for BPD in Infants with and without an ASD
Infants in our cohort were exposed to multiple interventions that may prevent or treat BPD (Table 3). Infants with an ASD were equally likely to undergo medical PDA treatment and surgical PDA ligation compared with infants without an ASD. A higher proportion of infants with an ASD were exposed to caffeine, whereas the same proportion of infants in each group were exposed to prenatal steroids, postnatal steroids, and diuretics. Infants with an ASD had a longer mean duration of exposure and higher mean percent of total days on diuretics, as compared with infants without an ASD.
Table 3.
Therapeutic interventions for BPD in infants with and without an ASD
| Therapy | ASD | |
|---|---|---|
| No (n= 19,182) |
Yes (n= 1314) |
|
| PDA treatment, n (%) | ||
| Medical | 6928 (36) | 487 (37) |
| Surgical | 2775 (14) | 170(13) |
| Prenatal steroids, n (%) | 16,099 (84) | 1097 (83) |
| Postnatal steroids, n (%) | 7215 (38) | 466(35) |
| Postnatal caffeine, n (%) | 17,001(89) | 1236 (94) |
| Postnatal diuretics, n (%) | 11,485(60) | 822 (63) |
| Total days of diuretic exposure, mean (SD) | 13.7(25.8) | 16.3 (28.6) |
| Percent of total days on diuretics, mean (SD) | 13.5 (21.8) | 14.5(22.1) |
ASD, atrial septal defect; BPD, bronchopulmonary dysplasia; PDA, patent ductus arteriosus; SD, standard deviation
Predicted Probability of Developing Specific BPD Risk by Postnatal Age
The predicted probability of BPD in our cohort was high (Table 4; online). The total number of infants decreased by the end of the risk estimation period—from 19,186 on day of life 1 to 18,854 on day of life 28—due to transfer, discharge, or death. The median predicted probability of developing mild BPD was lowest on day of life 1 and highest on day of life 21. The predicted risk of developing moderate BPD was also lowest in the immediate postnatal period and reached a peak on day of life 14. The predicted probability of developing severe BPD was highest on day of life 7, but lowest toward the end of the risk estimation period.
Table 4.
Predicted probability of BPD by postnatal age
| Postnatal Age (days) | N | BPD Risk Severity*, median probability (25th, 75th percentiles) | ||
|---|---|---|---|---|
| Mild BPD Risk | Moderate BPD Risk | Severe BPD Risk | ||
| 1 | 19,186 | 0.23(0.13,0.30) | 0.24(0.14,0.31) | 0.11(0.05,0.17) |
| 3 | 19,478 | 0.39(0.28,0.46) | 0.24(0.14,0.31) | 0.13(0.06,0.21) |
| 7 | 19,511 | 0.25(0.15,0.36) | 0.27(0.20,0.31) | 0.29(0.12,0.44) |
| 14 | 19,226 | 0.43(0.27,0.53) | 0.39(0.22,0.54) | 0.03 (0.02, 0.05) |
| 21 | 19,011 | 0.45(0.30,0.54) | 0.33(0.19,0.49) | 0.05 (0.03, 0.07) |
| 28 | 18,854 | 0.38(0.26,0.54) | 0.38(0.18,0.63) | 0.03 (0.02, 0.05) |
BPD, bronchopulmonary dysplasia
Derived from NICHD (Eunice Kennedy Shriver National Institute of Child Health and Human Development) BPD risk estimator which includes: gestational age, birth weight, race, sex, postnatal age, daily ventilator support and highest fraction of inspired oxygen (FiO2).
Association between the Presence of an ASD and the Development of BPD
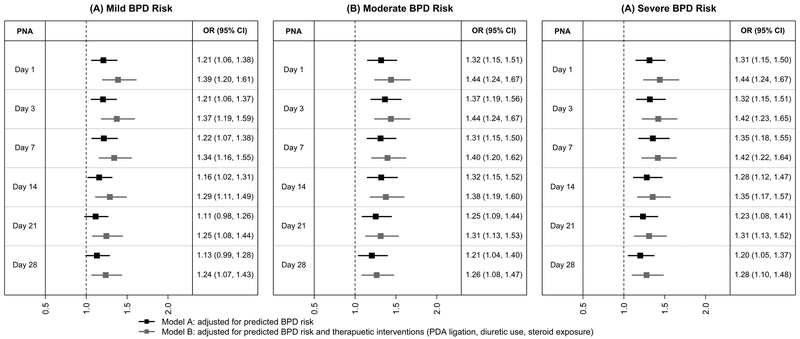
Adjusting for the predicted risk of BPD (Model A), infants with an ASD were more likely to develop BPD compared with infants without an ASD (Figure 3). This association was true for all postnatal ages (day 1, 3, 7, 14, 21, and 28) at the time of BPD risk prediction or predicted BPD risk severity (mild, moderate, or severe), except for mild BPD risk at postnatal ages 21 and 28. Adjusting for both the predicted risk of BPD and therapeutic interventions (Model B), infants with an ASD remained more likely to develop BPD (Figure 3). This association was true regardless of postnatal age or the predicted BPD risk severity. The results were similar in the logistic regression for correlated data model, with a significant association between the presence of an ASD and the risk of BPD in all models, but with decreasing magnitude of the association from day of life 1 (OR 1.37 [95% CI: 1.20–1.57]) to day of life 28 (OR 1.23 [95% CI: 1.07–1.40]).
Figure 3. Adjusted Odds Ratio of BPD in Infants with an ASD Compared with Infants without an ASD by Postnatal Age (PNA) and Predicted BPD Risk.
ASD, atrial septal defect; BPD, bronchopulmonary dysplasia
Discussion
To our knowledge, this is the first study to investigate the association between the presence of an ASD and the development of BPD in premature infants. We found that infants with an ASD had increased odds of developing BPD after adjusting for the predicted risk of BPD, as determined by the NICHD BPD risk estimator and therapeutic interventions for BPD (surgical PDA ligation, percent of total days on diuretics, prenatal steroid exposure, and postnatal steroid exposure).20 Furthermore, the odds of developing BPD in infants with an ASD were similar regardless of postnatal age and the predicted BPD risk severity (mild, moderate, or severe). These findings suggest that the presence of an ASD may represent a previously unidentified risk factor for the development of BPD in premature infants.
BPD is the most prevalent and serious consequence of preterm birth, predisposing infants to high morbidity and mortality beyond the neonatal period.1 Survivors of BPD are at risk for several adverse health outcomes, including chronic respiratory and cardiovascular impairments and long-term neurodevelopmental disabilities.2 The incidence of BPD in premature and low birth weight infants has continued to remain high over the past 2 decades.4 This suggests that despite advances in the recognition and management of BPD, underappreciated risk factors and missed opportunities for intervention likely remain.22–24
The presence of an ASD could represent one such risk factor. Several authors have previously hypothesized that atrial level defects might contribute to the development of BPD.12–17 These theories are based on inferences from small single-center case series of ASD closures in former premature infants with lung disease. A multicenter prospective cohort study of 3684 premature and low birth weight infants found that those diagnosed with CHD by ICD-9 codes had a much higher odds of developing BPD compared with those without CHD.25 Although 22 of the 71 infants with CHD had an ASD, the study did not assess the relative contributions of each CHD lesion to the odds of developing BPD.
The presence of an ASD leads to multiple pathologic alterations of pulmonary physiology that may predispose infants to developing BPD. First, an ASD can result in excessive pulmonary blood flow secondary to left-to-right shunting. Since premature infants possess low plasma oncotic pressure and high capillary permeability, increased pulmonary blood flow can cause elevated alveolar and interstitial fluid filtration rates, pulmonary edema, and poor pulmonary mechanics.26, 27 Second, the transudation of plasma proteins into the lung interstitium from increased fluid filtration rates may inhibit surfactant function and increase alveolar surface tension.28 This can have adverse effects on alveolarization, postnatal alveolar development, and lung tissue growth.23 Third, premature infants have poor ventricular compliance as compared with term infants.6 Therefore, right ventricular (RV) over-distention secondary to left-to-right shunting from an ASD may impact left ventricular (LV) filling via ventricular cross-talk. This can lead to increased LV end-diastolic pressures and further contribute to pulmonary edema. Lastly, exposure of underdeveloped pulmonary vessels to excessive pulmonary blood flow and the resulting increase in pressure can halt pulmonary angiogenesis and lead to vascular remodeling with intimal fibrosis and medial hypertrophy.29, 30 These above mechanisms can lead to impaired pulmonary mechanics, poor lung compliance, higher pulmonary vascular resistance, abnormal pulmonary vasoreactivity, inadequate gas exchange, and the need for prolonged mechanical ventilation and supplemental oxygen—all hallmark signs of BPD.31 Given that an ASD is the second most prevalent congenital heart defect, targeting these mechanisms may provide immense potential for the development of novel and/or modified interventions to reduce the overall incidence of BPD in premature infants.8, 9
Early ASD closure in premature infants maybe helpful in eliminating excessive pulmonary blood flow and halting the progression of BPD. In single-center case reports and case series, infants undergoing interventional device closure of an ASD had improved respiratory outcomes, a reduced need for mechanical ventilation, and a decreased need for diuretics.10–18 A majority of these procedures were performed safely and efficaciously on former premature infants with severe BPD. However, all of these infants underwent device closure after prolonged hospitalization on mechanical respiratory support. One of the reasons for this delay was likely to allow for adequate growth in order to decrease the procedural risks and accommodate the size of current ASD closure devices. It is very possible that the harmful effects of left-to-right shunting on the pathogenesis of BPD had already taken place in these infants. Therefore, the development of novel ASD devices that are suitable in size for younger and smaller premature infants could have a tremendous impact on the risk of BPD. This could prove to be an extremely impactful area of research, especially given that current preventative and therapeutic interventions have not shown to improve the rates of BPD.4
The presence of an untreated ASD in premature infants at risk for BPD can result in the development of pulmonary hypertension (PH) and a higher probability of mortality. Infants with BPD have been shown to have significantly higher odds of PH compared with infants without BPD.32–34 Furthermore, infants with both BPD and PH have almost 5-times higher odds of mortality than those infants with BPD and no PH.35–37 Multiple recent studies have reported that infants with BPD and atrial level shunt lesions are at an even higher risk for PH.38–40 A single-center retrospective study of 383 preterm infants diagnosed with BPD found an almost 4-fold increased odds of PH in the atrial shunt group compared with those without an atrial shunt.38 Additionally, a single-center prospective echocardiogram PH screening program in 159 premature and low birth weight infants with BPD found that those with PH were more than twice as likely to have an ASD.39
The strengths of this study include its large sample size and the ability to adjust for previously described risk factors and therapeutic interventions for BPD. Our cohort included data from 228 NICUs across North America, including both academic and regional sites. It has been previously reported in a number of studies that differential NICU practices between hospitals has a significant impact on the rate of BPD occurrence in premature infants.41–44 As a result, we performed our regression analyses using random effects for NICU site to account for between-hospital variability. There are numerous risk factors that are known to alter the risk of developing BPD, including prolonged respiratory support, birth weight, gestational age, race, and sex.45–48 In order to account for all these relevant risk factors, we used the NICHD BPD risk estimator algorithm to calculate our cohort’s predicted risk of developing mild, moderate, or severe BPD at various postnatal ages (days 1, 3, 7, 14, 21, and 28).20 By using these predicted values in our multivariate regression models, we were able to adjust for the variable contributions of each risk factor and their relative importance at different postnatal ages. Infants in our study were exposed to multiple interventions to prevent or treat BPD (Table 3). In order to ensure that these therapies did not confound the association between the presence of an ASD and the development of BPD, we accounted for surgical PDA ligation, percent of total days on diuretics, prenatal steroid exposure, and postnatal steroid use in our regression models.
The limitations of this study included those inherent to a retrospective study using a large electronic health record database. While our data source contains a broad range of maternal and neonatal clinical variables, there may exist other unobserved risk factors that could have contributed to variability. For instance, certain characteristics of the infants’ ASDs were not available from the Pediatrix database, including ASD size, location, number, hemodynamic properties, and degree/direction of shunting. The presence of an ASD was identified solely based on clinical documentation from the electronic heath record database, and therefore we cannot verify whether the diagnosis was made based on echocardiogram reports alone.
Future studies assessing the structural and hemodynamic characteristics of a premature infant’s ASD would be helpful in determining whether there is a subset of infants who would benefit from more aggressive medical or surgical treatment to halt or prevent the progression of BPD and associated PH.
Conclusion
In this large multicenter retrospective study, we found that premature and low birth weight infants with an ASD had an increased odds of developing BPD compared with those infants without an ASD. Early therapeutic interventions in this population should be strongly considered in order to prevent, reverse, or stop the progression of BPD and pulmonary vascular remodeling that can lead to long-term cardiorespiratory consequences.
Acknowledgments
Funding/Support: This work was funded, in part, by a grant from the National Institute of Child Health and Human Development (NICHD) (5R25HD076475–05).
Footnotes
Conflict of Interest (COI) Disclosures:
Dr. Kumar receives support for research from the National Institutes of Health (NIH) (5T32HD043029–15 and 4T32HD043029–14). Dr. Thompson receives support for research from the NIH (4T32HD043029–14). Dr. Greenberg receives salary support for research from the NICHD (HHSN275201000003I), National Institute of Allergy and Infectious Diseases (NIAID) (HHSN272201300017I), and Food and Drug Administration (FDA) (HHSF223201610082C). Dr. Smith receives salary support for research from the NIH (1R21HD080606–01A1), NICHD (HHSN275201000003I), ECHO Program (1U2COD023375–01), and FDA (1R18FD005292–01); he also receives research support from industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). Dr. Benjamin Jr receives salary support from the NIH (2K24HD058735–06), NICHD (HHSN275201000003I), NIAID (HHSN272201500006I), ECHO Program (1U2COD023375–01), and National Center for Advancing Translational Sciences (NCATS) (1U24TR001608–01); he also receives research support from Cempra Pharmaceuticals (subaward to HHSO100201300009C) and industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). Dr. Laughon receives support for research from the FDA (R01FD005101), National Heart, Lung, and Blood Institute (NHLBI) (R34HL124038), ECHO Program (U2COD023375), NICHD (U10HD040492), and the U.S. government for his work in neonatal clinical pharmacology (Government Contract HHSN267200700051C). Dr. Hornik receives salary support for research from the NICHD (1K23HD090239) and the U.S. government for his work in pediatric and neonatal clinical pharmacology (Government Contract HHSN267200700051C). He also receives research support from industry for pediatric drug development (https://www.dcri.org/about-us/conflict-of-interest/). The remaining authors have nothing to disclose.
REFERENCES
- 1.Horbar JD, Carpenter JH, Badger GJ, Kenny MJ, Soll RF, Morrow KA, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics 2012;129:1019–26. [DOI] [PubMed] [Google Scholar]
- 2.Doyle LW, Anderson PJ. Long-term outcomes of bronchopulmonary dysplasia. Semin Fetal Neonatal Med. 2009;14:391–5. [DOI] [PubMed] [Google Scholar]
- 3.Short EJ, Kirchner HL, Asaad GR, Fulton SE, Lewis BA, Klein N, et al. Developmental sequelae in preterm infants having a diagnosis of bronchopulmonary dysplasia: analysis using a severity-based classification system. Arch Pediatr Adolesc Med 2007;161:1082–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 2015;314:1039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen EA, Schmidt B. Epidemiology of bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol 2014;100:145–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clyman RI. The role of patent ductus arteriosus and its treatments in the development of bronchopulmonary dysplasia. Semin Perinatol 2013;37:102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bancalari E, Claure N, Gonzalez A. Patent ductus arteriosus and respiratory outcome in premature infants. Biol Neonate 2005;88:192–201. [DOI] [PubMed] [Google Scholar]
- 8.Triedman JK, Newburger JW. Trends in congenital heart disease: the next decade. Circulation 2016;133:2716–33. [DOI] [PubMed] [Google Scholar]
- 9.van der Linde D, Konings EEM, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJM, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol 2011;58:2241–7. [DOI] [PubMed] [Google Scholar]
- 10.Lammers A, Hager A, Eicken A, Lange R, Hauser M, Hess J. Need for closure of secundum atrial septal defect in infancy. J Thorac Cardiov Sur 2005;129:1353–7. [DOI] [PubMed] [Google Scholar]
- 11.Wood AM, Holzer RJ, Texter KM, Hill SL, Gest AL, Welty SE, et al. Transcatheter elimination of left-to-right shunts in infants with bronchopulmonary dysplasia is feasible and safe. Congenit Heart Dis 2011;6:330–7. [DOI] [PubMed] [Google Scholar]
- 12.Motz R, Grassl G, Trawoger R. Dependence on a respiratory ventilator due to an atrial septal defect. Cardiol Young 2000;10:150–2. [DOI] [PubMed] [Google Scholar]
- 13.Bishnoi RN, Everett AD, Ringel RE, Owada CY, Holzer RJ, Chisolm JL, et al. Device closure of secundum atrial septal defects in infants weighing less than 8 kg. Pediat Cardiol 2014;35:1124–31. [DOI] [PubMed] [Google Scholar]
- 14.Lim DS, Matherne GP. Percutaneous device closure of atrial septal defect in a premature infant with rapid improvement in pulmonary status. Pediatrics 2007;119:398–400. [DOI] [PubMed] [Google Scholar]
- 15.Thomas VC, Vincent R, Raviele A, Diehl H, Qian H, Kim D. Transcatheter closure of secundum atrial septal defect in infants less than 12 months of age improves symptoms of chronic lung disease. Congenit Heart Dis 2011;7:204–11. [DOI] [PubMed] [Google Scholar]
- 16.Wyss Y, Quandt D, Weber R, Stiasny B, Weber B, Knirsch W, et al. Interventional closure of secundum type atrial septal defects in infants less than 10 kilograms: indications and procedural outcome. J Interv Cardiol 2016;29:646–53. [DOI] [PubMed] [Google Scholar]
- 17.Zussman ME, Freire G, Cupp SD, Stapleton GE. Closure of a secundum atrial septal defect in two infants with chronic lung disease using the Gore HELEX Septal Occluder. Cardiol Young 2016;26:79–83. [DOI] [PubMed] [Google Scholar]
- 18.Diab KA, Cao QL, Bacha EA, Hijazi ZM. Device closure of atrial septal defects with the Amplatzer septal occluder: safety and outcome in infants. J Thorac Cardiov Sur 2007;134:960–6. [DOI] [PubMed] [Google Scholar]
- 19.Spitzer AR, Ellsbury DL, Handler D, Clark RH. The Pediatrix BabySteps Data Warehouse and the Pediatrix QualitySteps improvement project system--tools for “meaningful use” in continuous quality improvement. Clin Perinatol 2010;37:49–70. [DOI] [PubMed] [Google Scholar]
- 20.Laughon MM, Langer JC, Bose CL, Smith PB, Ambalavanan N, Kennedy KA, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med 2011;183:1715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trembath A, Hornik CP, Clark R, Smith PB, Daniels J, Laughon M. Comparative effectiveness of surfactant preparations in premature infants. J Pediatr 2013;163:955–60.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keszler M, Sant’Anna G. Mechanical ventilation and bronchopulmonary dysplasia. Clin Perinatol 2015;42:781–96. [DOI] [PubMed] [Google Scholar]
- 23.Jobe AH. Antenatal factors and the development of bronchopulmonary dysplasia. Semin Neonatol 2003;8:9–17. [DOI] [PubMed] [Google Scholar]
- 24.Rojas-Reyes MX, Morley CJ, Soll R. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev 2012:CD000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polito A, Piga S, Cogo PE, Corchia C, Carnielli V, Da Frè M, et al. Increased morbidity and mortality in very preterm/VLBW infants with congenital heart disease. Intens Care Med 2013;39:1104–12. [DOI] [PubMed] [Google Scholar]
- 26.Alpan G, Scheerer R, Bland R, Clyman R. Patent ductus arteriosus increases lung fluid filtration in preterm lambs. Pediatr Res 1991;30:616–21. [DOI] [PubMed] [Google Scholar]
- 27.Alpan G, Mauray F, Clyman RI. Effect of patent ductus arteriosus on water accumulation and protein permeability in the lungs of mechanically ventilated premature lambs. Pediatr Res 1989;26:570–5. [DOI] [PubMed] [Google Scholar]
- 28.Ikegami M, Jacobs H, Jobe A. Surfactant function in respiratory distress syndrome. J Pediatr 1983;102:443–7. [DOI] [PubMed] [Google Scholar]
- 29.Bancalari E Patent ductus arteriosus and short- and long-term respiratory outcomes. Am J Perinatol 2016;33:1055–7. [DOI] [PubMed] [Google Scholar]
- 30.Broccard AF, Hotchkiss JR, Kuwayama N, Olson DA, Jamal S, Wangensteen DO, et al. Consequences of vascular flow on lung injury induced by mechanical ventilation. Am J Respir Crit Care Med 1998;157:1935–42. [DOI] [PubMed] [Google Scholar]
- 31.Gerhardt T, Bancalari E. Lung compliance in newborns with patent ductus arteriosus before and after surgical ligation. Neonatology 1980;38:96–105. [DOI] [PubMed] [Google Scholar]
- 32.Al-Ghanem G, Shah P, Thomas S, Banfield L, El Helou S, Fusch C, et al. Bronchopulmonary dysplasia and pulmonary hypertension: a meta-analysis. J Perinatol 2017;37:414–9. [DOI] [PubMed] [Google Scholar]
- 33.Mirza H, Ziegler J, Ford S, Padbury J, Tucker R, Laptook A. Pulmonary hypertension in preterm infants: prevalence and association with bronchopulmonary dysplasia. J Pediatr 2014;165:909–14.e1. [DOI] [PubMed] [Google Scholar]
- 34.Mourani PM, Sontag MK, Ivy DD, Abman SH. Effects of long-term sildenafil treatment for pulmonary hypertension in infants with chronic lung disease. J Pediatr 2009;154:379–84.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slaughter JL, Pakrashi T, Jones DE, South AP, Shah TA. Echocardiographic detection of pulmonary hypertension in extremely low birth weight infants with bronchopulmonary dysplasia requiring prolonged positive pressure ventilation. J Perinatol 2011;31:635–40. [DOI] [PubMed] [Google Scholar]
- 36.An HS, Bae EJ, Kim GB, Kwon BS, Beak JS, Kim EK, et al. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circ J 2010;40:131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics 2007;120:1260–9. [DOI] [PubMed] [Google Scholar]
- 38.Choi EK, Jung YH, Kim HS, Shin SH, Choi CW, Kim EK, et al. The impact of atrial left-to-right shunt on pulmonary hypertension in preterm infants with moderate or severe bronchopulmonary dysplasia. Pediatr Neonatol 2015;56:317–23. [DOI] [PubMed] [Google Scholar]
- 39.Weismann CG, Asnes JD, Bazzy-Asaad A, Tolomeo C, Ehrenkranz RA, Bizzarro MJ. Pulmonary hypertension in preterm infants: results of a prospective screening program. J Perinatol 2017;37:572–7. [DOI] [PubMed] [Google Scholar]
- 40.Vyas-Read S, Kanaan U, Shankar P, Stremming J, Travers C, Carlton DP, et al. Early characteristics of infants with pulmonary hypertension in a referral neonatal intensive care unit. BMC Pediatr 2017;17:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lapcharoensap W, Gage SC, Kan P, Profit J, Shaw GM, Gould JB, et al. Hospital variation and risk factors for bronchopulmonary dysplasia in a population-based cohort. JAMA Pediatr 2015;169:e143676. [DOI] [PubMed] [Google Scholar]
- 42.Lorch SA, Baiocchi M, Ahlberg CE, Small DS. The differential impact of delivery hospital on the outcomes of premature infants. Pediatrics. 2012;130:270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warner B, Musial MJ, Chenier T, Donovan E. The effect of birth hospital type on the outcome of very low birth weight infants. Pediatrics 2004;113:35–41. [DOI] [PubMed] [Google Scholar]
- 44.Guaman MC, Gien J, Baker CD, Zhang H, Austin ED, Collaco JM. Point prevalence, clinical characteristics, and treatment variation for infants with severe bronchopulmonary dysplasia. Am J Perinatol 2015;32:960–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ambalavanan N, Van Meurs KP, Perritt R, Carlo WA, Ehrenkranz RA, Stevenson DK, et al. Predictors of death or bronchopulmonary dysplasia in preterm infants with respiratory failure. J Perinatol 2008;28:420–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marshall DD, Kotelchuck M, Young TE, Bose CL, Kruyer L, O’Shea TM, et al. Risk factors for chronic lung disease in the surfactant era: a North Carolina population-based study of very low birth weight infants. Pediatrics 1999;104:1345–50. [DOI] [PubMed] [Google Scholar]
- 47.Palta M, Gabbert D, Weinstein MR, Peters ME. Multivariate assessment of traditional risk factors for chronic lung disease in very low birth weight neonates. The Newborn Lung Project. J Pediatr 1991;119:285–92. [DOI] [PubMed] [Google Scholar]
- 48.Rojas MA, Gonzalez A, Bancalari E, Claure N, Poole C, Silva-Neto G. Changing trends in the epidemiology and pathogenesis of neonatal chronic lung disease. J Pediatr 1995;126:605–10. [DOI] [PubMed] [Google Scholar]