Abstract
The endocytic pathway tightly regulates the activity of G protein-coupled receptors (GPCRs). Much of our understanding of this relationship between GPCR endocytic trafficking and signaling comes from studies done on catecholamine and opioid receptors. After ligand-induced endocytosis, a key sorting step in the endosome determines whether receptors are recycled back to the cell surface, leading to recovery of signaling, or are degraded in the lysosome, leading to desensitization. Recycling of GPCRs, unlike that of many other proteins, is an active process driven by specific sequences on the receptor and proteins that interact with this sequence. Recent data suggest that sequence-dependent recycling plays complex roles in regulating both the timing and location of GPCR signaling. This chapter will describe our current understanding of the mechanisms regulating GPCR sorting in the endosome, and discuss emerging ideas on their role in GPCR signaling, focusing on adrenergic and opioid receptors as prototypes.
1. Introduction: The endosome as a sorting station for internalized GPCRs.
Endocytic trafficking is a fundamental cellular process that regulates GPCR function. GPCR activation on the cell surface results in their removal from the cell surface by clathrin-mediated endocytosis1–3. This was first recognized by studies measuring ligand-induced desensitization of signaling, which required receptor phosphorylation, binding of beta-arrestins, and endocytosis. Endocytosed GPCRs are transported to the endosome, where a critical sorting step determines the further fate of GPCRs4–6. They may be either recycled to the cell surface, or be degraded in the lysosome. An established consequence of this sorting is that it directly controls the number of receptors on the cell surface, causing either recovery of sensitivity of the cell to the signal or downregulation of signaling, respectively7,8. Recent evidence, however, suggests that this post-endocytic sorting might have much more complex roles in regulating the function of many GPCRs, including the prototypic adrenergic and opioid receptors.
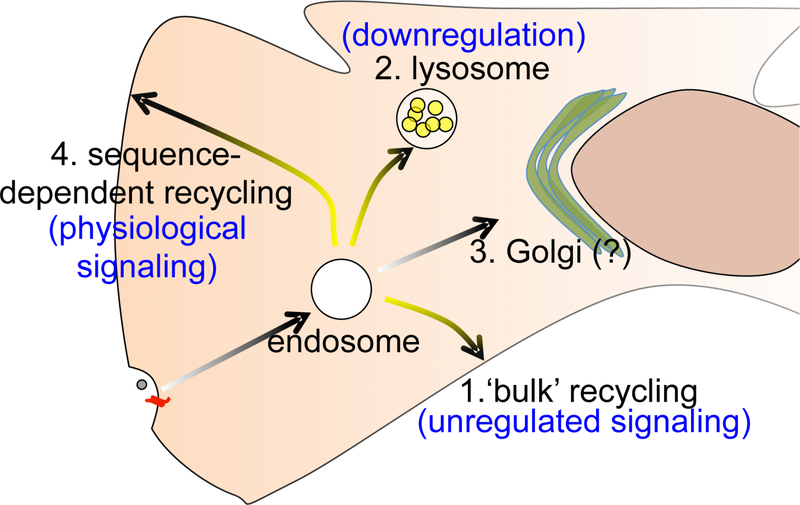
The early endosome serves as the primary sorting station for most internalized proteins including GPCRs. How endosomes sort receptors, considering the amount of membrane cargo that traffic through these dynamic organelles, is a fundamental question with many implications. There are four main pathways for internalized proteins out of the endosome (Figure 1). Proteins may be recycled by bulk membrane flow, sorted to the lysosome to be degraded, recycled via a specialized regulated “sequence-dependent” recycling pathway, or transported to the Golgi apparatus.
Figure 1.
Potential pathways for endocytosed GPCRs from the endosome. Sequence-dependent recycling is the physiological scenario for recovery of signaling, while sorting to the lysosome leads to degradation and downregulation. Bulk recycling pathway, taken normally by nutrient receptors but not GPCRs, potentially leads to unregulated signaling for GPCRs. While some proteins can travel to the Golgi apparatus, this is not established as a common route for GPCRs.
Many proteins, like the transferrin receptor (TfR), are recycled back to the cell surface as part of bulk membrane flow, apparently without any specific requirements. This “bulk recycling” was first described over 20 years ago, by Maxfield and colleagues, who labeled and tracked the membrane and fluid phase compartments of endosomes9,10. They observed that the endosome extruded narrow tubules with a large surface area (i.e., membranes) but low volume (i.e., fluids). Continued fission and recycling of these tubules provided a geometric basis for how nutrient receptors could be recycled as part of bulk membrane flow, simply because they are membrane proteins, leaving their soluble ligands in the lumen of the endosome to be eventually degraded in the lysosome9–11. Several membrane-modifying proteins responsible for membrane tubulation have been identified. Such geometric sorting likely occurs extensively along the endocytic pathway, iteratively recycling receptors from the early, recycling, and the late endosomes.
Considering that recycling is thought to be a “default” fate for membrane proteins, it is interesting that GPCRs are not commonly sorted via bulk recycling. Many GPCRs, like the delta opioid receptor (DOR), and signaling receptors like the EGF receptor, are degraded in the lysosome12,13. Degradation of these proteins takes advantage of geometric sorting. These proteins are packaged into vesicles that bud off into the interior of the endosomes, essentially partitioning the protein-containing vesicles into the fluid phase of these endosomes, which eventually mature into or fuse with lysosomes14–16. For GPCRs that recycle, this is a regulated process that requires specific protein sequences and interactions17–30. Mutation of these sequences direct GPCRs to the lysosome, reiterating that their recycling is not simply a function of them being membrane proteins17,22,25. This chapter will discuss our current understanding of GPCR sorting between the degradative and recycling pathways, using adrenergic and opioid receptors as model receptors.
2. Mechanisms of sequence-dependent GPCR sorting
2.1. GPCRs are sorted into lysosomes by multiple mechanisms
The first conceptual step in GPCR sorting to the lysosome is to segregate receptors away from bulk recycling, by packaging them into intralumenal vesicles (ILVs). The process of generating vesicles that pinch off to the interior of the vesicles, called involution, is a topologically highly interesting process, as this involves the generation of vesicles away from the cytoplasm, unlike with the “classical” coat-mediated vesicle transport processes like endocytosis. Most of our understanding of protein sorting to lysosomes comes from studies done in yeast. Genetic and biochemical studies of protein sorting to the vacuole, a compartment homologous to the mammalian lysosome, have identified the ESCRT proteins as the primary protein machinery responsible for this process. The current views on the mechanisms of how ESCRT proteins induce negative curvature and generate ILVs are discussed in structural detail in several recent authoritative reviews31–34.
The main sorting signal that targets proteins to the lysosome is the addition of ubiquitin to cytoplasmic lysine residues of proteins. Early evidence for the importance of ubiquitination in GPCR trafficking came from studies on the yeast GPCR Ste2, for which ubiquitination promoted transport to the vacuole. Since then, ubiquitination has been shown to promote lysosomal targeting of many membrane proteins in mammalian cells, including the EGF receptor13,35. In the case of many of these proteins, ubiquitin interacts with the ubiquitin-interacting motif (UIM) of Hrs, an endosomal protein often termed ESCRT-0. Hrs then transfers these proteins to Tsg101, an ESCRT-I component that also has a UIM. This localizes the cargo in ESCRT domains, which allows them to be incorporated in ILVs. While many GPCRs are known to be ubiquitinated, the role of these interactions in its lysosomal targeting has been shown only for CXCR436. The role of ubiquitination in GPCR trafficking is discussed much more extensively elsewhere in this book.
Receptor ubiquitination or ESCRTs, however, are not required for sorting all GPCRs to lysosomes. A mutant of the DOR lacking all cytoplasmic lysines is endocytosed and trafficked to the lysosome12. This still requires Hrs and Tsg101, suggesting ubiquitin-independent binding of GPCRs with ESCRT37. The protease activated receptor PAR1, on the other hand, can be sorted to the lysosome independent of both ubiquitination and ESCRTs38. Instead, this sorting depends on the endosomal protein sorting nexin-1 (SNX1)39. Additionally, members of the family of GPCR-associated sorting proteins (GASP1 and GASP2) and Beclin-2 have been implicated in degradation of DOR40,41. While both SNX1 and GASP proteins interact with several GPCRs, including DOR, the exact mechanism by which they sort GPCRs into the lysosome is still not well understood42. Our current understanding is that these proteins may play a role in downregulation of receptors under conditions of chronic agonist stimulation.
2.2. GPCRs Contain Diverse Recycling Sequences
It has been appreciated for over 30 years that adrenergic receptors recycle to the surface, based on the reappearance of activity after agonist-induced desensitization2,43,44. Over the years, however, it has become clear that the simple model of bulk recycling cannot explain GPCR recycling, mostly from research on the beta adrenergic receptors. The recycling of the beta-2 adrenergic receptor (B2AR) depends on a specific sequence on its C-terminus that interacts with post-synaptic density 95/disc large/zonula occludins-1 (PDZ) -domain containing proteins17. Similar sequences that conform to classical type I PDZ-ligand sequences have been identified on several GPCRs, including the related beta-1 adrenergic receptor (B1AR) and the kappa opioid receptor (KOR), over the past decade19,22,29. For many of these receptors, these sequences are required for the recycling of these receptors, and are also sufficient, as transplanting these sequences onto a non-recycling receptor like the DOR allows the receptor to recycle45. Alpha adrenergic receptors also contain PDZ ligands, although they are more diverse, and their role in recycling is less well understood46. One critical feature of these PDZ-ligand sequences is that they need to be on the C-terminal tail of the receptors. The terminal peptide binds in an antiparallel fashion in a hydrophobic cleft formed by a beta strand, a loop, and an alpha helix in the PDZ protein, with the free carboxyl group occupying a hydrophobic pocket47. In addition, sequence-comparisons have identified many internal PDZ ligands on GPCRs that might be involved in receptor recycling48,49.
How do PDZ-ligand sequences mediate adrenergic receptor recycling? For the B2AR, a complement of proteins that bind the PDZ ligand, including NHERF-1, NHERF-2, PDZK1, and MAGI-3, was identified soon after the identification of the sequence17,50. Similarly, several proteins, such as PIST, MAGI-2, MAGI-3, PSD-95, and SAP97 that bind the PDZ ligand sequence of the B1AR have also been identified19,20,29. It is interesting that these two related receptors bind distinct complements of PDZ proteins. SAP97 has been implicated as the main protein that mediates B1AR recycling, by directly interacting with the PDZ ligand and recruiting the A-Kinase Anchoring Protein-79 (AKAP-79) which phosphorylates the B1AR on a residue that contributes to recycling29. Similarly, B2AR also interacts with AKAP-12 (Gravin), which might recruit c-Src to this complex51.
However, while these protein interactions have been delineated, we have only recently started to understand how GPCRs are recycled by these interactions. Some of the key breakthroughs came from recent advances in live cell imaging that allow for direct visualization of receptor sorting and recycling in the endosome. These studies showed that the recycling of the B2AR is mediated by specific microdomains on the endosome, distinct from those that mediate bulk recycling52. These sequence-dependent recycling microdomains are marked by a specialized actin cytoskeleton and specific membrane modifying proteins such as sorting nexins and retromer53,54. This was exciting, since it challenged the traditional view that all recycling tubule populations at the early endosome have the same sorting kinetics and trafficking machinery52. A variety of proteins have been localized to this specific actin/sorting nexin/retromer tubular (ASRT) microdomain on the endosome52,54,55. A global analysis of plasma membrane proteins after depletion of SNX27 or retromer components showed a reduction in surface levels of over a 100 proteins, suggesting that this is a general recycling pathway used by many proteins56.
Evidence suggests that the primary role of the PDZ interactions is to link the receptor to these ASRT domains. Disrupting these interactions or depleting components of the endosomal actin cytoskeleton, retromer complex, or SNX27 inhibited B2AR recycling, similar to mutating the PDZ ligand or depleting the PDZ proteins52–54. Conversely, replacing the PDZ domain with an actin-binding domain from ezrin was sufficient to confer recycling to DOR and B2AR57. The facts that actin-binding is required and sufficient for recycling, and that several GPCRs have the ability to bind to PDZ-domain proteins, suggests a conserved role of the PDZ-linked actin cytoskeleton in endosomal sorting of GPCRs.
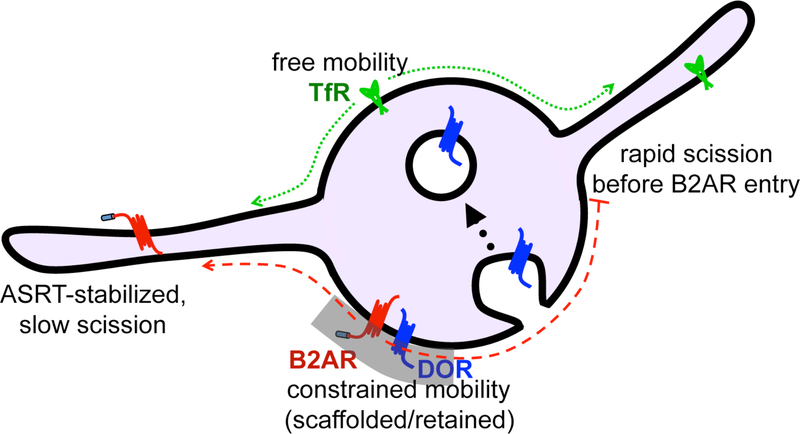
However, why and how GPCRs are excluded from bulk recycling is still an open question. Current data suggest a kinetic basis for this exclusion (Figure 2). Estimation of diffusion rates showed that the mobility of the B2AR on endosomes was highly restricted52. This might make it difficult for B2AR to diffuse into and populate the bulk recycling tubules, which undergo fast fission. The actin cytoskeleton on the ASRT domains stabilizes these domains and delays fission of these tubules. This might allow enough time for the slow-diffusing B2AR to diffuse into these tubules, where they will be concentrated by interactions with the ASRT proteins. Such a “kinetic sorting” mechanism in fact might provide a common mechanism for sorting in many different membrane compartments. Validating this model at a mechanistic level is an important future direction in our understanding of GPCR biology.
Figure 2.
A kinetic model for sorting in the endosome. The mobility of sequence-dependent recycling proteins or degraded proteins on the endosome are constrained by an unknown mechanism. This slow mobility reduces their entry into bulk recycling tubules which form and undergo scission rapidly. Bulk recycling proteins have unconstrained mobility on the endosome, which allows them to enter the short-lived bulk recycling tubules. ASRT domains are stabilized by the actin cytoskeleton, which provides sequence-dependent recycling proteins enough time to diffuse into these tubules. Degraded proteins are captured by the ESCRT machinery and packaged into intralumenal vesicles.
In contrast to beta adrenergic receptors, the opioid receptor family exhibits very diverse trafficking characteristics. The KOR requires a PDZ domain for post-endocytic recycling, similar to the adrenergic receptors22. DOR, as mentioned above, does not recycle and is degraded in lysosomes following agonist-induced endocytosis12,58. Interestingly, the mu-opioid receptor (MOR), recycles following agonist-induced internalization, but does not require a PDZ ligand sequence, like KOR and the adrenergic receptors. MOR contains a unique, seven amino acid recycling sequence in its C-terminal tail, LENLEAE. Mutation of this sequence re-routes MOR to the lysosome following endocytosis, and fusion of this sequence to the C-terminal tail of DOR is sufficient to promote its rapid recycling, and prevent lysosomal degradation of DOR25.
Although MOR’s recycling sequence was discovered a decade ago, the exact mechanism of how this sequence promotes MOR sorting and recycling remains unknown. However, a number of MOR binding partners that regulate MOR trafficking have been found. The actin-binding protein, filamin A, has been shown to interact with the C-terminal tail of MOR, and this interaction is thought to reduce MOR agonist-induced internalization59. Additionally, the dendritic spine protein, spinophilin, interacts with MOR in the striatum, and interestingly, knockout of spinophilin reduces sensitivity to morphine-induced analgesia60. Further, agonist-induced internalization of MOR is significantly reduced in spinophilin knockout cells60. Interestingly, spinophilin interacts with DOR, as well as MOR, and this interaction requires the third intracellular loop, the G protein coupling domain of GPCRs, as well as the first eighteen amino acids of the C-terminal tail, conserved between the two opioid receptors61. Additionally, this interaction seems to enhance ERK signaling through DOR, but not MOR, suggesting that the interaction with spinophilin may modulate sensitivity of MOR and DOR differentially61. Moreover, single nucleotide polymorphisms within the G protein-coupling domain of the third extracellular loop of MOR are also associated with calmodulin binding and an increase in basal MOR activity62. Interestingly, spinophilin also interacts with alpha-2 adrenergic receptors and D2 dopamine receptors, also through the third intracellular loop of these receptors63,64, suggesting that spinophilin may regulate several GPCRs, potentially through G protein coupling. The additional requirement of a conserved region of MOR and DOR C-terminal tails for spinophilin binding suggests a potential role in membrane trafficking, while the exact mechanism remains unknown.
Even though GPCR recycling uses diverse sequences and proteins, it is likely that there is a common mechanism that mediates the recycling of most GPCRs, which can be accessed by specific “recycling adaptors” that link receptors to this machinery. In support of this, disruption of Hrs inhibits the recycling of most GPCRs that recycle in a sequence-dependent manner. This role of Hrs seems to be independent of its role in ESCRT-mediated degradation of proteins, as the other components of the ESCRT machinery such as Tsg101 do not produce this phenotype65. Further, Hrs mediates its effect on B2AR recycling via its Vps27-Hrs-STAM (VHS) domain, which does not play an active role in lysosomal sorting65. Additional support for this idea comes from the identification of mutations on the B2AR that converts the receptor into a bulk recycling protein. A sequence on the proximal part of the C-terminal tail on B2AR (EKENKLL) that resembles, but is distinct from, an acidic dileucine sequence, was required for Hrs-dependence and sequence-dependence of B2AR recycling66. Similarly, mutating a phosphorylation site on B2AR, adjacent to the EKENKLL sequence, that is phosphorylated by Protein Kinase A (PKA), converts B2AR recycling to be independent of actin and the PDZ ligand sequence67,68. Identification of these specific sequences indicates that there is a specific machinery that retains B2AR on the endosome (which might constitute step 1 in the hierarchical sorting model , actively excluding it from bulk recycling. As of now, there are no known interacting partners to either of these sites. Once future studies identify candidates, we will be able to dissect out the mechanisms and function of this hierarchical sorting of GPCRs.
Another argument for common machineries mediating GPCR recycling is the involvement of general vesicle trafficking machineries in this process. For example, Rab GTPases regulate many steps in vesicle trafficking of GPCRs, such as vesicle budding, tethering, and docking69. Rab5 is a marker for the early endosome to which many GPCRs, such as the B2AR, endothelin A and B receptors, and the thyrotropin-releasing hormone receptor, localizes following agonist-induced endocytosis70–73. Overexpression of dominant negative Rab5 mutants interferes with endocytic trafficking of B2AR, the D2 dopamine receptor, neurokinin-1 receptor (NK1R), CXC chemokine receptor 2, lysophosphatidic acid-coupled/EDG-2 receptor, and the cannabinoid receptor 272,74–78. Rab5 may be involved in resensitization of B2AR and NK1R72,75. Further, Rab4 is thought to control rapid recycling from Rab5/Rab4 early endosomes to the plasma membrane, while Rab11 mediates a slower recycling pathway79. Both these have been implicated in the recycling of GPCRs as well as a variety of non-GPCR cargo, including TfR. For example, a dominant negative Rab4 or depletion of Rab4 by RNA interference inhibits rapid B2AR recycling following agonist-induced endocytosis72,67. MOR is also thought to recycle in a Rab4 dependent manner, but also recycles through a slower Rab11-mediated pathway80. Other GPCRs are thought to traffic through the slow, Rab11-dependent pathway, such as the cannabinoid receptor 2, angiotensin II type I receptor, and the M4 muscarinic acetylcholine receptor78,81,82. Together, this suggests that, in addition to regulation of GPCR recycling by C-terminal recycling sequences and their respective binding partners, GPCR post-endocytic trafficking is also subject to regulation by Rab GTPase activity at different endosomal compartments.
3. Regulation of sequence-dependent recycling by modifying steps in hierarchical sorting
Recent evidence suggests that intracellular signaling cascades can control endosomal sorting of GPCRs. This provides new explanations for how cells might coordinate the diverse cellular responses mediated by different GPCRs at physiological time scales. B2AR recycling is regulated by protein kinase A (PKA), a signaling kinase downstream of B2AR activation67,68. Further, B2AR signaling can homologously regulate its recycling through PKA phosphorylation of the C-terminal tail of B2AR. These two PKA sites on the C-terminal tail of B2AR regulate a switch in the type of recycling tubules that B2AR is sorted into at the early endosome. Increased PKA phosphorylation of B2AR, following sustained adrenergic signaling, restricts B2AR to the ASRT domain on the endosome. Conversely, non-phosphorylated B2AR can enter the non-ASRT, or bulk recycling tubules, traversed by nutrient receptors, like the transferrin receptor (TfR)68. This suggests a hierarchical sorting mechanism that allows a cell to fine-tune its responses to extracellular signals (Figure 3A). For example, in the case of sustained adrenergic signaling, restriction of B2AR to the sequence-dependent pathway by PKA phosphorylation allows a cell to quickly slow down B2AR resensitization by decreasing recycling from the endosome. It is possible that a similar mechanism exists for B1AR, considering the described role of PKA phosphorylation in B1AR recycling and resensitization29,51, but the exact mechanisms have not been addressed in detail.
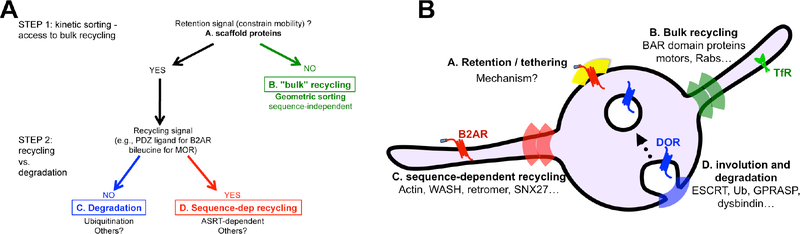
Figure 3.
A hierarchical model for GPCR sorting in the endosome. A) The first step in sorting is an unknown mechanism that tethers GPCRs on the body of the endosome, excluding them from entering bulk recycling tubules. This could be a consequence of decreased mobility, as in the kinetic sorting model described in Figure 2. Bulk recycling proteins are not tethered by this mechanism, allowing them to recycle by geometric sorting. Tethered proteins are either recycled, if they have a recycling sequence, or degraded, if they contain one of multiple signals including ubiquitination. B) The proposed protein complexes on the endosome that mediate these hierarchical steps in sorting are shown.
Considering that the ASRT domains contain a variety of protein complexes whose functions are highly regulated, these potentially provide additional control points for regulating GPCR recycling even beyond a simple switch between bulk and ASRT-dependent recycling. For example, cortactin, one of the key components of the actin cytoskeleton in ASRT domains, is regulated by c-Src phosphorylation83. This phosphorylation increases the rate of vesicle scission from these domains84. Because modifying vesicle scission affects the surface delivery of all cargo proteins that use this pathway56, it is possible that this provides a general mechanism for controlling recycling of all these proteins. Such hierarchical steps of recycling provide a number of checkpoints for signaling receptors. They can first be sorted at the early endosome according to the recycling sequence on their C-terminal, then phosphorylation of GPCRs themselves at their C-terminal tails, or components of the ASRT domains, by kinases allows the cell to alter recycling kinetics in response to diverse physiological situations (Figure 3B).
Recent work suggests that MOR recycling can also be regulated by phosphorylation by kinases downstream of GPCR activation. Much like B2AR, MOR recycling in striatal neurons is decreased by forskolin, which activated cAMP, although PKA was not directly tested in these neurons85. MOR recycling is also regulated through a heterologous pain signaling pathway in sensory neurons. Pain signaling, through activation of the neurokinin-1 receptor (NK1R) by substance P, increases MOR recycling in sensory neurons through PKC phosphorylation of two residues on the C-terminal tail of MOR, serine 363 (S363) and threonine 370 (T370)86. This is consistent with physiological and pharmacological data that PKC inhibition reduces MOR resensitization, and that PKC modulation can change behavioral correlates of opioid tolerance87,88. Interestingly, the phosphorylation state at these sites can be differentially regulated by diverse opioid agonists. S363 is constitutively phosphorylated, whether or not the receptor is bound to an opioid drug. T370, on the other hand is phosphorylated when the receptor is activated by high-efficacy opioids, like [D-Ala2, N-Me-Phe4, Gly5-ol]-Enkephalin (DAMGO) and fentanyl, but not morphine89. Further, substance P has also been shown to induce phosphorylation at MOR T37090. This heterologous regulation of MOR recycling further suggests the possibility that hierarchical sorting of GPCRs, through signaling regulation, allows the cell to control GPCR resensitization by regulating recycling kinetics in response to different physiological stimuli.
4. Relevance of sequence-dependent GPCR recycling in the endosome
The exact role of endocytic trafficking in GPCR function has been often highly debated in the past. After activation, receptors are desensitized by phosphorylation, and need to be dephosphorylated to be resensitized and made competent for ligand binding and signaling. Evidence suggests that both phosphorylation and dephosphorylation can occur at the cell surface. Endocytic trafficking might play roles in modifying the kinetics of this process, although the direction in which endocytosis drives resensitization might vary depending on the receptor and conditions6,30. Nevertheless, the long-standing model for GPCR function was that they signal primarily at the cell membrane through their heterotrimeric G proteins, after which they are desensitized. Based on this, a straightforward role for endosomal GPCR sorting, that sorting of receptors into the sequence-dependent pathway caused rapid recycling and delivery of receptors to the cell surface and led to recovery of cellular responsiveness to the same signal, has been appreciated for a while91.
Recent evidence suggests, however, that sequence-dependent recycling plays more complex roles in tuning both spatial and temporal characteristics of GPCR signaling. GPCRs can signal through several non-G protein signaling pathways - for example through the GPCR adaptor, beta arrestin92. Some GPCRs recruit and signal through arrestin at the endosome. Sustained signaling through G proteins at the endosome following GPCR endocytosis has also been demonstrated for some GPCRs93–95. The parathyroid hormone receptor (PTHR) continues to signal after receptor endocytosis, and different agonists differ in their ability to induce this type of signaling. Interestingly, PTHRs signaling at the endosome were also shown to associate with Gαs, challenging the traditional view that G protein coupling and signaling occurs primarily at the cell surface93,96. Further, internalized thyroid stimulating hormone receptors (TSHR) exhibit coupling to Gs and cyclic AMP (cAMP) production following internalization94.
For “conventional” GPCRs like the B2AR, however, G protein signaling at endosomes has remained controversial, in part because the signaling profiles of B2AR are much faster compared to receptors like the PTHR, and because traditional signaling readouts could not discriminate between the cell surface and subcellular compartments as signaling sources. Recent breakthroughs have generated a GFP-tagged nanobody biosensor that specifically recognizes the activated form of the Gα stimulatory protein (Gαs). This sensor, which provides spatial resolution, showed that B2AR can activate Gαs at early endosomes97. This presents the first clear demonstration that even conventional GPCRs, where the focus has largely been on signaling from the cell surface, can induce G protein signaling cascades from endosomal compartments. Following up on this work, the von Zastrow group has also shown that cell surface and endosomal G protein signaling activated distinct transcriptional profiles98, suggesting that spatial encoding of where the cAMP signaling was generated could produce diverse cellular responses. While still early, this emerging body of work provides a new perspective on the role of endocytic trafficking in GPCR function. Direct visualization of active conformations of B2AR and Gs proteins on the endosome suggests a transformative idea that endocytosis and subsequent endosomal sorting of GPCRs, in addition to deciding whether GPCRs are degraded at the lysosome or recycled to the cell surface, could also regulate diverse intracellular signaling cascades at the early endosome following agonist activation.
5. Summary and perspectives
The post-endocytic sorting of internalized adrenergic and opioid receptors between the recycling and degradative pathways determines whether receptors are delivered back to the cell surface or degraded in the lysosome. GPCR recycling is an active process that requires specific sequences on the receptor tails. Several proteins that interact with these sequences and drive receptors into the sequence-dependent recycling pathway have been identified. Considering that many membrane proteins can recycle as part of bulk membrane flow, how and why GPCRs are excluded from bulk recycling is a clear area that needs further investigation.
A well-appreciated role for post-endocytic sorting is that it controls receptor signaling by determining the number of signaling receptors that are recycled to the cell surface. Accumulating data in the recent years, however, suggest that post-endocytic sorting plays more complex roles in regulating receptor function. Recycling of GPCRs is mediated by specific microdomains on the endosome that are physically separate from domains that mediate bulk membrane recycling. GPCR recycling domains also serve as organizing centers for specific signaling complexes. Importantly, signals originating from the surface and endosomes induce distinct downstream responses, suggesting that GPCR signaling is spatially encoded. The cytoplasmic interactions of GPCRs, by determining receptor localization in these microdomains, might directly determine this spatial encoding, and therefore might provide control points for the cell to precisely modulate both the spatial and temporal characteristics of signaling. We have only recently begun to identify signaling pathways that modify these interactions and regulate receptor signaling. As future studies identify more examples of signaling-mediated regulation of these machineries, especially in physiologically relevant systems, we will be able to build a better picture of how cross-talk between multiple signaling pathways allow cells to generate an integrated response in complex signaling environments.
References
- 1.Yoburn BC, Purohit V, Patel K, Zhang Q. Opioid agonist and antagonist treatment differentially regulates immunoreactive mu-opioid receptors and dynamin-2 in vivo. Eur J Pharmacol. 2004;498(1–3):87–96. [DOI] [PubMed] [Google Scholar]
- 2.Von Zastrow M, Kobilka BK. Ligand-regulated internalization and recycling of human beta 2-adrenergic receptors between the plasma membrane and endosomes containing transferrin receptors. J Biol Chem. 1992;267(5):3530–3538. [PubMed] [Google Scholar]
- 3.Lamb ME, De Weerd WF, Leeb-Lundberg LM. Agonist-promoted trafficking of human bradykinin receptors: arrestin- and dynamin-independent sequestration of the B2 receptor and bradykinin in HEK293 cells. Biochem J. 2001;355(Pt 3):741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3(9):639–650. [DOI] [PubMed] [Google Scholar]
- 5.Von Zastrow M Mechanisms regulating membrane trafficking of G protein-coupled receptors in the endocytic pathway. Life Sci. 2003;74(2–3):217–224. [DOI] [PubMed] [Google Scholar]
- 6.Marchese A, Paing MM, Temple BRS, Trejo J. G protein-coupled receptor sorting to endosomes and lysosomes. Annu Rev Pharmacol Toxicol. 2008;48:601–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorkin A, Von Zastrow M. Signal transduction and endocytosis: close encounters of many kinds. Nat Rev Mol Cell Biol. 2002;3(8):600–614. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez VA, Arttamangkul S, Dang V, et al. mu-Opioid receptors: Ligand-dependent activation of potassium conductance, desensitization, and internalization. J Neurosci. 2002;22(13):5769–5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn KW, McGraw TE, Maxfield FR. Iterative fractionation of recycling receptors from lysosomally destined ligands in an early sorting endosome. J Cell Biol. 1989;109(6 Pt 2):3303–3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayor S, Presley JF, Maxfield FR. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J Cell Biol. 1993;121(6):1257–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao M, Maxfield FR. Characterization of rapid membrane internalization and recycling. J Biol Chem. 2000;275(20):15279–15286. [DOI] [PubMed] [Google Scholar]
- 12.Tanowitz M, Von Zastrow M. Ubiquitination-independent trafficking of G protein-coupled receptors to lysosomes. J Biol Chem. 2002;277(52):50219–50222. [DOI] [PubMed] [Google Scholar]
- 13.Huang F, Zeng X, Kim W, et al. Lysine 63-linked polyubiquitination is required for EGF receptor degradation. Proc Natl Acad Sci U S A. 2013;110(39):15722–15727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babst M A protein’s final ESCRT. Traffic. 2005;6(1):2–9. [DOI] [PubMed] [Google Scholar]
- 15.Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol. 2007;23:519–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piper RC, Lehner PJ. Endosomal transport via ubiquitination. Trends Cell Biol. 2011;21(11):647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature. 1999;401(6750):286–290. [DOI] [PubMed] [Google Scholar]
- 18.Cong M Binding of the beta 2 Adrenergic Receptor to N-Ethylmaleimide-sensitive Factor Regulates Receptor Recycling. J Biol Chem. 2001;276(48):45145–45152. [DOI] [PubMed] [Google Scholar]
- 19.Hu LA. beta 1-Adrenergic Receptor Association with PSD-95. Inhibition of receptor internalization and facilitation of beta 1-adrenergic receptor interaction with n-methyl-d-aspartate receptors. J Biol Chem. 2000;275(49):38659–38666. [DOI] [PubMed] [Google Scholar]
- 20.He J, Bellini M, Inuzuka H, et al. Proteomic analysis of beta1-adrenergic receptor interactions with PDZ scaffold proteins. J Biol Chem. 2006;281(5):2820–2827. [DOI] [PubMed] [Google Scholar]
- 21.Gage RM, Kim KA, Cao TT, von Zastrow M. A transplantable sorting signal that is sufficient to mediate rapid recycling of G protein-coupled receptors. J Biol Chem. 2001;276(48):44712–44720. [DOI] [PubMed] [Google Scholar]
- 22.Li J-G, Chen C, Liu-Chen L-Y. Ezrin-Radixin-Moesin-binding Phosphoprotein-50/Na+/H+ Exchanger Regulatory Factor (EBP50/NHERF) Blocks U50,488H-induced Down-regulation of the Human kappa Opioid Receptor by Enhancing Its Recycling Rate. J Biol Chem. 2002;277(30):27545–27552. [DOI] [PubMed] [Google Scholar]
- 23.Hirakawa T, Galet C, Kishi M, Ascoli M. GIPC binds to the human lutropin receptor (hLHR) through an unusual PDZ domain binding motif, and it regulates the sorting of the internalized human choriogonadotropin and the density of cell surface hLHR. J Biol Chem. 2003;278(49):49348–49357. [DOI] [PubMed] [Google Scholar]
- 24.Hu LA, Chen W, Martin NP, Whalen EJ, Premont RT, Lefkowitz RJ. GIPC Interacts with the 1-Adrenergic Receptor and Regulates 1-Adrenergic Receptor-mediated ERK Activation. J Biol Chem. 2003;278(28):26295–26301. [DOI] [PubMed] [Google Scholar]
- 25.Tanowitz M, von Zastrow M. A novel endocytic recycling signal that distinguishes the membrane trafficking of naturally occurring opioid receptors. J Biol Chem. 2003;278(46):45978–45986. [DOI] [PubMed] [Google Scholar]
- 26.Galet C, Hirakawa T, Ascoli M. The postendocytotic trafficking of the human lutropin receptor is mediated by a transferable motif consisting of the C-terminal cysteine and an upstream leucine. Mol Endocrinol. 2004;18(2):434–446. [DOI] [PubMed] [Google Scholar]
- 27.Huang P, Steplock D, Weinman EJ, et al. Opioid Receptor Interacts with Na+/H+-exchanger Regulatory Factor-1/Ezrin-Radixin-Moesin-binding Phosphoprotein-50 (NHERF-1/EBP50) to Stimulate Na+/H+ Exchange Independent of Gi/Go Proteins. J Biol Chem. 2004;279(24):25002–25009. [DOI] [PubMed] [Google Scholar]
- 28.Vargas GA, Von Zastrow M. Identification of a novel endocytic recycling signal in the D1 dopamine receptor. J Biol Chem. 2004;279(36):37461–37469. [DOI] [PubMed] [Google Scholar]
- 29.Gardner LA, Naren AP, Bahouth SW. Assembly of an SAP97-AKAP79-cAMP-dependent protein kinase scaffold at the type 1 PSD-95/DLG/ZO1 motif of the human beta(1)-adrenergic receptor generates a receptosome involved in receptor recycling and networking. J Biol Chem. 2007;282(7):5085–5099. [DOI] [PubMed] [Google Scholar]
- 30.Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol. 2008;48:537–568. [DOI] [PubMed] [Google Scholar]
- 31.Hurley JH. The ESCRT complexes. Crit Rev Biochem Mol Biol. 2010;45(6):463–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hurley JH, Hanson PI. Membrane budding and scission by the ESCRT machinery: it’s all in the neck. Nat Rev Mol Cell Biol. 2010;11(8):556–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shields SB, Piper RC. How ubiquitin functions with ESCRTs. Traffic. 2011;12(10):1306–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henne WM, Stenmark H, Emr SD. Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harb Perspect Biol. 2013;5(9). doi: 10.1101/cshperspect.a016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dores MR, Trejo J. Ubiquitination of G protein-coupled receptors: functional implications and drug discovery. Mol Pharmacol. 2012;82(4):563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verma R, Marchese A. The Endosomal Sorting Complex Required for Transport Pathway Mediates Chemokine Receptor CXCR4 Promoted Lysosomal Degradation of the mTOR Antagonist DEPTOR. J Biol Chem. 2015. doi: 10.1074/jbc.M114.606699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henry AG, White IJ, Marsh M, von Zastrow M, Hislop JN. The role of ubiquitination in lysosomal trafficking of δ-opioid receptors. Traffic. 2011;12(2):170–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dores MR, Chen B, Lin H, et al. ALIX binds a YPX(3)L motif of the GPCR PAR1 and mediates ubiquitin-independent ESCRT-III/MVB sorting. J Cell Biol. 2012;197(3):407–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gullapalli A, Wolfe BL, Griffin CT, Magnuson T, Trejo J. An essential role for SNX1 in lysosomal sorting of protease-activated receptor-1: evidence for retromer-, Hrs-, and Tsg101-independent functions of sorting nexins. Mol Biol Cell. 2006;17(3):1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whistler JL, Enquist J, Marley A, et al. Modulation of Postendocytic Sorting of G Protein–Coupled Receptors. Science. 2002;297. [DOI] [PubMed] [Google Scholar]
- 41.He C, Wei Y, Sun K, et al. Beclin 2 functions in autophagy, degradation of G protein-coupled receptors, and metabolism. Cell. 2013;154(5):1085–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heydorn A, Søndergaard BP, Ersbøll B, et al. A library of 7TM receptor C-terminal tails. Interactions with the proposed post-endocytic sorting proteins ERM-binding phosphoprotein 50 (EBP50), N-ethylmaleimide-sensitive factor (NSF), sorting nexin 1 (SNX1), and G protein-coupled receptor-associated sorting protein (GASP). J Biol Chem. 2004;279(52):54291–54303. [DOI] [PubMed] [Google Scholar]
- 43.Staehelin M, Hertel C. [3H]CGP-12177, a beta-adrenergic ligand suitable for measuring cell surface receptors. J Recept Res. 1983;3(1–2):35–43. [DOI] [PubMed] [Google Scholar]
- 44.Hertel C, Staehelin M. Reappearance of beta-adrenergic receptors after isoproterenol treatment in intact C6-cells. J Cell Biol. 1983;97(5 Pt 1):1538–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gage RM, Matveeva EA, Whiteheart SW, von Zastrow M. Type I PDZ ligands are sufficient to promote rapid recycling of G Protein-coupled receptors independent of binding to N-ethylmaleimide-sensitive factor. J Biol Chem. 2005;280(5):3305–3313. [DOI] [PubMed] [Google Scholar]
- 46.Chen Z, Hague C, Hall RA, Minneman KP. Syntrophins regulate alpha1D-adrenergic receptors through a PDZ domain-mediated interaction. J Biol Chem. 2006;281(18):12414–12420. [DOI] [PubMed] [Google Scholar]
- 47.Karthikeyan S, Leung T, Birrane G, Webster G, Ladias JA. Crystal structure of the PDZ1 domain of human Na(+)/H(+) exchanger regulatory factor provides insights into the mechanism of carboxyl-terminal leucine recognition by class I PDZ domains. J Mol Biol. 2001;308(5):963–973. [DOI] [PubMed] [Google Scholar]
- 48.Paasche JD, Attramadal T, Kristiansen K, et al. Subtype-specific sorting of the ETA endothelin receptor by a novel endocytic recycling signal for G protein-coupled receptors. Mol Pharmacol. 2005;67(5):1581–1590. [DOI] [PubMed] [Google Scholar]
- 49.Romero G, von Zastrow M, Friedman PA. Role of PDZ proteins in regulating trafficking, signaling, and function of GPCRs: means, motif, and opportunity. Adv Pharmacol. 2011;62:279–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hall RA, Premont RT, Chow CW, et al. The beta2-adrenergic receptor interacts with the Na+/H+-exchanger regulatory factor to control Na+/H+ exchange. Nature. 1998;392(6676):626–630. [DOI] [PubMed] [Google Scholar]
- 51.Tao J, Wang H-Y, Malbon CC. Src docks to A-kinase anchoring protein gravin, regulating beta2-adrenergic receptor resensitization and recycling. J Biol Chem. 2007;282(9):6597–6608. [DOI] [PubMed] [Google Scholar]
- 52.Puthenveedu MA, Lauffer B, Temkin P, et al. Sequence-dependent sorting of recycling proteins by actin-stabilized endosomal microdomains. Cell. 2010;143(5):761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lauffer BEL, Melero C, Temkin P, et al. SNX27 mediates PDZ-directed sorting from endosomes to the plasma membrane. J Cell Biol. 2010;190(4):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Temkin P, Lauffer B, Jäger S, Cimermancic P, Krogan NJ, von Zastrow M. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat Cell Biol. 2011;13(6):715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gallon M, Cullen PJ. Retromer and sorting nexins in endosomal sorting. Biochem Soc Trans. 2015;43(1):33–47. [DOI] [PubMed] [Google Scholar]
- 56.Steinberg F, Gallon M, Winfield M, et al. A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat Cell Biol. 2013;15(5):461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lauffer BEL, Chen S, Melero C, Kortemme T, von Zastrow M, Vargas GA. Engineered protein connectivity to actin mimics PDZ-dependent recycling of G protein-coupled receptors but not its regulation by Hrs. J Biol Chem. 2009;284(4):2448–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsao PI, von Zastrow M. Type-specific sorting of G protein-coupled receptors after endocytosis. J Biol Chem. 2000;275(15):11130–11140. [DOI] [PubMed] [Google Scholar]
- 59.Onoprishvili I, Andria ML, Kramer HK, Ancevska-Taneva N, Hiller JM, Simon EJ. Interaction between the mu opioid receptor and filamin A is involved in receptor regulation and trafficking. Mol Pharmacol. 2003;64(5):1092–1100. [DOI] [PubMed] [Google Scholar]
- 60.Charlton JJ, Allen PB, Psifogeorgou K, et al. Multiple actions of spinophilin regulate mu opioid receptor function. Neuron. 2008;58(2):238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fourla D-D, Papakonstantinou M-P, Vrana S-M, Georgoussi Z. Selective interactions of spinophilin with the C-terminal domains of the δ- and μ-opioid receptors and G proteins differentially modulate opioid receptor signaling. Cell Signal. 2012;24(12):2315–2328. [DOI] [PubMed] [Google Scholar]
- 62.Wang D, Quillan JM, Winans K, Lucas JL, Sadée W. Single nucleotide polymorphisms in the human mu opioid receptor gene alter basal G protein coupling and calmodulin binding. J Biol Chem. 2001;276(37):34624–34630. [DOI] [PubMed] [Google Scholar]
- 63.Richman JG, Brady AE, Wang Q, Hensel JL, Colbran RJ, Limbird LE. Agonist-regulated Interaction between 2-Adrenergic Receptors and Spinophilin. J Biol Chem. 2001;276(18):15003–15008. [DOI] [PubMed] [Google Scholar]
- 64.Smith FD, Oxford GS, Milgram SL. Association of the D2 dopamine receptor third cytoplasmic loop with spinophilin, a protein phosphatase-1-interacting protein. J Biol Chem. 1999;274(28):19894–19900. [DOI] [PubMed] [Google Scholar]
- 65.Hanyaloglu AC, McCullagh E, von Zastrow M. Essential role of Hrs in a recycling mechanism mediating functional resensitization of cell signaling. EMBO J. 2005;24(13):2265–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hanyaloglu AC, von Zastrow M. A novel sorting sequence in the beta2- adrenergic receptor switches recycling from default to the Hrs-dependent mechanism. J Biol Chem. 2007;282(5):3095–3104. [DOI] [PubMed] [Google Scholar]
- 67.Yudowski GA, Puthenveedu MA, Henry AG, von Zastrow M. Cargo-mediated regulation of a rapid Rab4-dependent recycling pathway. Mol Biol Cell. 2009;20(11):2774–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vistein R, Puthenveedu MA. Reprogramming of G protein-coupled receptor recycling and signaling by a kinase switch. Proc Natl Acad Sci U S A. 2013;110(38):15289–15294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tuvim MJ, Adachi R, Hoffenberg S, Dickey BF. Traffic control: Rab GTPases and the regulation of interorganellar transport. News Physiol Sci. 2001;16:56–61. [DOI] [PubMed] [Google Scholar]
- 70.Moore RH, Tuffaha A, Millman EE, et al. Agonist-induced sorting of human beta2-adrenergic receptors to lysosomes during downregulation. J Cell Sci. 1999;112 (Pt 3):329–338. [DOI] [PubMed] [Google Scholar]
- 71.Bremnes T, Paasche JD, Mehlum A, Sandberg C, Bremnes B, Attramadal H. Regulation and intracellular trafficking pathways of the endothelin receptors. J Biol Chem. 2000;275(23):17596–17604. [DOI] [PubMed] [Google Scholar]
- 72.Seachrist JL, Anborgh PH, Ferguson SS. beta 2-adrenergic receptor internalization, endosomal sorting, and plasma membrane recycling are regulated by rab GTPases. J Biol Chem. 2000;275(35):27221–27228. [DOI] [PubMed] [Google Scholar]
- 73.Hinkle PM, Gehret AU, Jones BW. Desensitization, trafficking, and resensitization of the pituitary thyrotropin-releasing hormone receptor. Front Neurosci. 2012;6:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iwata K, Ito K, Fukuzaki A, Inaki K, Haga T. Dynamin and rab5 regulate GRK2-dependent internalization of dopamine D2 receptors. Eur J Biochem. 1999;263(2):596–602. [DOI] [PubMed] [Google Scholar]
- 75.Schmidlin F, Dery O, DeFea KO, et al. Dynamin and Rab5a-dependent trafficking and signaling of the neurokinin 1 receptor. J Biol Chem. 2001;276(27):25427–25437. [DOI] [PubMed] [Google Scholar]
- 76.Fan G-H, Lapierre LA, Goldenring JR, Richmond A. Differential regulation of CXCR2 trafficking by Rab GTPases. Blood. 2003;101(6):2115–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Murph MM, Scaccia LA, Volpicelli LA, Radhakrishna H. Agonist-induced endocytosis of lysophosphatidic acid-coupled LPA1/EDG-2 receptors via a dynamin2- and Rab5-dependent pathway. J Cell Sci. 2003;116(Pt 10):1969–1980. [DOI] [PubMed] [Google Scholar]
- 78.Grimsey NL, Goodfellow CE, Dragunow M, Glass M. Cannabinoid receptor 2 undergoes Rab5-mediated internalization and recycles via a Rab11-dependent pathway. Biochim Biophys Acta. 2011;1813(8):1554–1560. [DOI] [PubMed] [Google Scholar]
- 79.Sönnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol. 2000;149(4):901–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang F, Chen X, Zhang X, Ma L. Phosphorylation state of mu-opioid receptor determines the alternative recycling of receptor via Rab4 or Rab11 pathway. Mol Endocrinol. 2008;22(8):1881–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li H, Li H-F, Felder RA, Periasamy A, Jose PA. Rab4 and Rab11 coordinately regulate the recycling of angiotensin II type I receptor as demonstrated by fluorescence resonance energy transfer microscopy. J Biomed Opt. 2008;13(3):031206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Volpicelli LA, Lah JJ, Fang G, Goldenring JR, Levey AI. Rab11a and myosin Vb regulate recycling of the M4 muscarinic acetylcholine receptor. J Neurosci. 2002;22(22):9776–9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martinez-Quiles N, Ho H-YH, Kirschner MW, Ramesh N, Geha RS. Erk/Src phosphorylation of cortactin acts as a switch on-switch off mechanism that controls its ability to activate N-WASP. Mol Cell Biol. 2004;24(12):5269–5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vistein R, Puthenveedu MA. Src regulates sequence-dependent beta-2 adrenergic receptor recycling via cortactin phosphorylation. Traffic. 2014;15(11):1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roman-Vendrell C, Yu YJ, Yudowski GA. Fast modulation of μ-opioid receptor (MOR) recycling is mediated by receptor agonists. J Biol Chem. 2012;287(18):14782–14791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bowman SL, Soohoo AL, Shiwarski DJ, Schulz S, Pradhan AA, Puthenveedu MA. Cell autonomous regulation of mu-opioid receptor recycling by substance P. Cell Reports, in press 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Inoue M, Ueda H. Protein kinase C-mediated acute tolerance to peripheral muopioid analgesia in the bradykinin-nociception test in mice. J Pharmacol Exp Ther. 2000;293(2):662–669. [PubMed] [Google Scholar]
- 88.Dang VC, Williams JT. Chronic morphine treatment reduces recovery from opioid desensitization. J Neurosci. 2004;24(35):7699–7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Doll C, Konietzko J, Pöll F, Koch T, Höllt V, Schulz S. Agonist-selective patterns of μ-opioid receptor phosphorylation revealed by phosphosite-specific antibodies. Br J Pharmacol. 2011;164(2):298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Illing S, Mann A, Schulz S. Heterologous regulation of agonist-independent μ-opioid receptor phosphorylation by protein kinase C. Br J Pharmacol. 2014;171(5):1330–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lefkowitz RJ, Pitcher J, Krueger K, Daaka Y. Mechanisms of beta-adrenergic receptor desensitization and resensitization. Adv Pharmacol. 1998;42:416–420. [DOI] [PubMed] [Google Scholar]
- 92.Shenoy SK, Lefkowitz RJ. Seven-transmembrane receptor signaling through beta-arrestin. Sci STKE. 2005;2005(308):cm10. [DOI] [PubMed] [Google Scholar]
- 93.Ferrandon S, Feinstein TN, Castro M, et al. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol. 2009;5(10):734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Calebiro D, Nikolaev VO, Gagliani MC, et al. Persistent cAMP-Signals Triggered by Internalized G-Protein–Coupled Receptors. von Zastrow M, ed. PLoS Biol. 2009;7(8):e1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Calebiro D, Nikolaev VO, Persani L, Lohse MJ. Signaling by internalized G-protein-coupled receptors. Trends Pharmacol Sci. 2010;31(5):221–228. [DOI] [PubMed] [Google Scholar]
- 96.Okazaki M, Ferrandon S, Vilardaga J-P, Bouxsein ML, Potts JT Jr, Gardella TJ. Prolonged signaling at the parathyroid hormone receptor by peptide ligands targeted to a specific receptor conformation. Proc Natl Acad Sci U S A. 2008;105(43):16525–16530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Irannejad R, Tomshine JC, Tomshine JR, et al. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 2013;495(7442):534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tsvetanova NG, von Zastrow M. Spatial encoding of cyclic AMP signaling specificity by GPCR endocytosis. Nat Chem Biol. 2014;10(12):1061–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]