Abstract
Approximately one third of individuals with sickle cell disease (SCD) develop chronic pain. This debilitating pain is inadequately treated because the underlying mechanisms driving the pain are poorly understood. In addition to persistent pain, SCD patients are also in a tonically pro-inflammatory state. Previous studies have revealed that there are elevated plasma levels of many inflammatory mediators including chemokine (c-c motif) ligand 2 (CCL2) in individuals with SCD. Using a transgenic mouse model of SCD, we investigated the contributions of CCL2 signaling to SCD-related pain. Inhibition of the chemokine receptor 2 (CCR2), but not CCR4, alleviated the behavioral mechanical and cold hypersensitivity in SCD. Further, acute CCR2 blockade reversed both the behavioral and the in vitro responsiveness of sensory neurons to an agonist of TRPV1, a neuronal ion channel previously implicated in SCD pain. These results provide insight into the immune-mediated regulation of hypersensitivity in SCD and could inform future development of analgesics or therapeutic measures to prevent chronic pain.
Keywords: Chemokine, sickle cell disease, CCL2, CCR2, TRPV1, cold pain, mechanical pain, mechanotransduction, cold transduction
Introduction
Sickle cell disease (SCD) is an inherited blood disorder that occurs in 1 of 400 African American births and affects approximately 100,000 Americans [11,24]. Pain is a hallmark of SCD and the most common reason for emergency department visits among this patient population [62]. In addition to the severe episodic pain experienced during acute vaso-occlusive crises, many individuals with SCD also develop chronic persistent pain. In fact, 30% of adults with SCD report pain every day, and 55% of all days include either chronic or acute pain. The chronic pain has attributes of both spontaneous and stimulus-evoked pain [1,2,50]. Many of these individuals are sensitive to cold water or cold temperatures, and innocuous mechanical stimuli including light brushing of the skin or increased wind speed can be perceived as painful [41]. Transgenic SCD mice also display clear persistent hypersensitivity to mechanical and thermal stimuli, even in absence of an experimenter-induced vaso-occlusive crisis, thereby recapitulating the heightened cutaneous sensitivity observed in patients with SCD. [9,12,23,26,31]. Using these animal models, many attempts have been made at defining the neuronal mechanisms underlying chronic SCD pain. With the field’s increased understanding however, it has become clear that additional non-neuronal processes, including chronic inflammation, likely contribute to the observed altered somatosensation [2].
Recent studies suggest that both peripheral and central sensitization contribute to the altered somatosensation observed in SCD [14,26]. Peripherally, isolated sensory neurons from SCD mice show sensitization to mechanical stimulation which is mediated in part by the ion channel transient receptor potential vanilloid 1 (TRPV1) [26]. Chronic inflammation resulting from repeated vaso-occlusive events might be sensitizing this channel or others that are important in the generation or perpetuation of pain in both SCD patients and model organisms. Chemokine (c-c motif) ligand 2 (CCL2), also known as monocyte chemoattractant protein-1 (MCP-1), is a pro-inflammatory mediator that is elevated in SCD patients both during and between crises [47]. Evidence from other pain models indicates that CCL2 signaling at its primary receptor CCR2, can enhance neuronal excitability and nociception [7,37,66]. Recently, CCL2 was also shown to mediate cold allodynia in neuropathic pain conditions [46,65].
Although research efforts have explored the contributions of inflammatory mediators to the endothelial and vascular function in SCD, the roles of these mediators in the generation and maintenance of SCD pain is not known [17]. Therefore, we sought to address the role of CCL2 in the persistent mechanical and cold sensitization observed in SCD mice. Specific contributions of CCR2 and CCR4, the two primary receptors for CCL2, signaling were assessed using behavioral assays [19]. Behavioral findings were further explored at the cellular level using calcium imaging. Finally, the link between CCL2 signaling and TRPV1 sensitization was investigated. Collectively, these findings provide novel insight into the contribution of inflammatory mediators on pain in SCD.
Methods
Animals
An a priori decision was made to include two transgenic mouse models for behavioral studies: a model of severe sickle cell disease and a model of sickle cell trait. The Berkeley sickle mice (Berk SS) contain no murine globin genes, but rather carry a transgene driving expression of human α globin and sickle β globin [Tg(Hu-miniLCRα1GγAγδβS)]; thus circulating RBCs contain only human sickle hemoglobin [44]. Many physiological characteristics of severe human SCD are recapitulated in these mice including sickled red blood cells, hemolytic anemia, organ damage, and increased pain behaviors [26,31,34,44]. Heterozygous Townes mice (Townes AS) were also used [48]; these knockout-knockin animals contain no murine globins. Instead, these animals contain one copy of normal adult human β globin and one copy of adult human sickle β globin, in addition to normal human α globin within the mouse globin locus. As such, Townes AS mice model sickle cell trait in humans, and portray a mild phenotype of several components of SCD pathology [64]. Since Berk and Townes mice both contain considerable genetic background from C57BL/6 and 129 mice, B6;129 hybrid animals were used as a shared control. Male mice were used for all experiments unless otherwise stated; mice were bred in-house and were 8–16 weeks of age at testing since we and others have shown that the severity of pain behaviors increases in SCD mice with age [12,63]. All procedures and protocols were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin.
CCL2 ELISA
Plasma CCL2 levels were assessed in Berk SS and B6;129 mice. Trunk blood was obtained via cardiac puncture from isoflurane-anesthetized animals. Sodium citrate coated syringes were used to remove blood, which was stored on ice after collection. Blood samples were centrifuged for 15 min at 1500 rpm and 4°C. The plasma supernatant was stored at −80°C until use. CCL2 concentrations were measured in plasma using a mouse CCL2 specific ELISA kit (Affymetrix MCP-1 ELISA Ready-SET-Go!, Santa Clara, CA) and compared to CCL2 standards.
Immunofluorescence
CCR2 expression was examined in lumbar DRG neurons from B6;129 and Berk SS mice; DRG were cultured as described below. After culturing, neurons were fixed in 4% paraformaldehyde. Fixed cells were blocked in 10% normal donkey serum, 5% normal goat serum, 0.2% Triton X-100 in PBS for 1 hr then incubated in a solution of the following primary antibodies for 1 hr: mouse anti-NeuN (1:500, Millipore, #MAB377), rabbit anti-CCR2 (1:50, Abcam, #ab203128) in blocking solution. Cells were washed and then incubated with the following secondary antibodies for 1 hr: donkey anti-rabbit AlexaFluor 594 (1:200, Invitrogen, #A21207), goat anti-mouse AlexaFluor 633 (1:200, Invitrogen, #A21050) in blocking solution. Coverslips were mounted and confocal images were obtained using a Nikon Eclipse E600 microscope.
Behavior
All testing was performed by experimenters blinded to mouse genotype and treatment.
Mechanical sensitivity testing
Mice were habituated to the testing environment for at least one hour prior to testing. Calibrated von Frey filaments (0.20, 0.39, 0.69, 1.57, 3.92, 5.88, 9.81, and 13.73 mN, Δ=0.27; Bioseb, Pinellas Park, FL) were used to assess mechanical sensitization. Starting with the 3.92 mN filament, filaments were applied to the center of the plantar surface of the hind paw until bent at approximately 30 degrees, for no longer than 2 s. If the animal fully raised the stimulated paw from the wire mesh testing environment before this 2 s cutoff, this was considered a withdrawal; toe flaring alone was not considered a withdrawal. If the 3.92 mN filament evoked a withdrawal response, the paw was next probed with the 1.57 mN filament; if the 3.92 mN filament did not evoke a response, the 5.88 mN filament was used. After the first change in response (i.e., animals responded to filament after not responding to previous filament or vice versa), this up-down method was employed for four additional stimulations. A 50% paw withdrawal threshold (i.e., the force that elicited paw withdrawal during 50% of trials) was calculated for both the left and right paws following previously published methods [16,20], then averaged between paws.
Cold sensitivity testing
The cold plantar assay and thermal preference tests were used to assess cold sensitivity. In the cold plantar assay, mice were habituated to the testing environment (10×10×20 cm Plexiglass chamber resting on 2.5 mm thick glass floor) for an hour prior to testing. Powdered dry ice was loaded into a 3 mL plastic syringe that had the tapered end removed; this cold probe was then applied to the underside of a glass floor plate to stimulate the plantar hind paw until the limb was withdrawn (not to exceed 20 seconds) [10]. This was repeated 5 times per paw, with a minimum of 60 seconds between applications. Withdrawal latencies were recorded for each paw and averaged between paws for an individual mouse. When the effect of intraplantar drug administration was measured, only the injected paw was assayed. As a second measure of cold sensitivity and thermal aversion, the two-temperature preference assay was used [4,39,63]. Mice were allowed to freely explore a testing chamber (TECA Corp, Chicago, IL) in which the floor was composed of two thermally regulated metal plates that were set to specific temperatures for each experiment. Mice were habituated to the apparatus for 15 minutes prior to the day of testing; this limited pre-exposure was chosen to prevent reduction in exploratory drive during the test phase. During the habituation and 5 minute baseline testing phases, both sides of the chamber were set to 30°C. During the 5 minute cold testing phase, one plate remained at 30°C while the second was held at 23°C. The percent time spent on the 23°C plate was measured.
Capsaicin test
TRPV1 sensitivity was assessed using the capsaicin test [49]. Capsaicin (160 or 16 ng in 20 μL) or phosphate-buffered saline (PBS) vehicle was injected subcutaneously into the center of the plantar surface of the hindpaw. Nociceptive behaviors (licking, flicking, or lifting) during the five minutes following paw injection were recorded and scored by an experimenter blinded to treatment.
Drug administration
The specific CCR2 antagonist, RS 504393 (3 mg/kg unless otherwise stated; also used 1 mg/kg and 10 mg/kg; Tocris, Bristol, UK), or 5% DMSO (in PBS) vehicle was injected subcutaneously into the neck 30 minutes prior to behavioral testing. This dose has previously been effective in reducing paclitaxel-induced hyperalgesia [46]. In additional experiments, intrathecal RS 504393 (3 μg/5 μL; dose effective in reducing bone cancer thermal hyperalgesia [45]) administration was performed 30 min prior to behavioral testing, or intraplantar RS 504393 (3 μg/10 μL; dose effective in reducing carrageenan-induced thermal hyperalgesia [33]) was administered 60 min prior to behavioral testing. The CCR4 antagonist C 021 dihydrochloride (3 mg/kg; Tocris) or PBS vehicle was injected subcutaneously into the neck 30 minutes prior to testing. This dose has previously been effective in protecting against the proinflammatory response associated with hepatic encephalopathy [36]. The TRPV1 antagonist A-425619 (34.5 mg/kg unless otherwise stated; also used 10.3 mg/kg and 115 mg/kg; Abbott Laboratories, Abbott Park, IL) or 10% DMSO, 34% 2-hydroxypropyl β-cyclodextran vehicle was administered intraperitoneally (i.p.) 30 min before testing. This dose has previously been effective in reducing mechanical hypersensitivity in Berk SS mice [26]. The TRPA1 antagonist HC-030031 (100 μg/30 μL) was subcutaneously injected into the hind paw 60 min prior to behavioral testing. This dose has previously been effective in reducing CFA-induced thermal hypersensitivity [32].
DRG culture
Bilateral lumbar 1–6 dorsal root ganglia (DRG) were isolated from Berk SS and B6;129 control mice. Mice were deeply anesthetized with isoflurane (Midwest Veterinary Supply, Sun Prairie, WI) and euthanized via decapitation. Isolated DRG were collected into Hanks’ Balanced Salt Solution (Gibco, ThermoFisher Scientific) then transferred to a 50:50 solution of Dulbecco’s modified Eagle’s medium and Ham’s F12 medium (Gibco). DRG were chemically dissociated via incubation in 1 mg/mL collagenase type IV (Sigma Aldrich, St. Louis, MO) for 40 minutes then in 0.05% trypsin (Sigma Aldrich) for 45 minutes. All incubation steps occurred at 37°C and 5% CO2. The ganglia were washed and resuspended in a complete medium consisting of a 50:50 mixture of Dulbecco’s modified Eagle’s medium and Ham’s F12 medium supplemented with 10% heat-inactivated horse serum (Invitrogen, ThermoFisher Scientific), 2 mM L-glutamine (Invitrogen), 0.5% glucose (Sigma Aldrich), 100 units/mL penicillin (Invitrogen), and 100 μg/mL streptomycin (Invitrogen). No exogenous growth factors were added to the medium. Cells were physically dissociated via trituration, plated onto laminin-coated (Sigma) glass coverslips, and incubated at 37°C with 5% CO2 until use.
Calcium imaging
Eighteen to 30 hours after culturing, calcium imaging was performed on DRG cultures using the dual-wavelength, ratiometric calcium indicator dye, Fura-2-AM (Life Technologies, Carlsbad, CA). Coverslips were loaded with 2.5 μg/mL Fura-2 in 2% bovine serum albumin for 45 min then washed with extracellular buffer for 30 minutes. Extracellular buffer contained (in mM): 150 NaCl, 10 HEPES, 8 glucose, 5.6 KCl, 2 CaCl2, and 1 MgCl2 (all from Sigma). Coverslips were mounted onto an inverted fluorescent microscope and perfused with extracellular buffer at a rate of 6 mL/min. Fluorescence images were captured at both 340nm (Ca2+ bound Fura-2) and 380nm (unbound Fura-2) and 340/380 nm image ratios calculated. Data acquisition and analysis was performed using NIS Elements software (Nikon, Melville, NY). Responsive cells were those that exhibited a ≥20% increase in 340nm/380nm ratio from baseline measures during cold stimuli, capsaicin application, or CCL2 application. Similarly, cells were considered viable neurons if they responded to a depolarizing 50 mM KCl stimulus with ≥20% increase in 340/380 ratio. Small diameter (<27μm) cells were the focus of this study since they were previously reported as mechanically sensitized in SCD [26].
To produce a cold ramp, cells were superfused with chilled buffer (6 mL/min flow rate), gradually decreasing the exogenous temperature of the recording chamber from ~23°C to 14°C over 2 min. Recording chamber temperatures were monitored throughout imaging via a thermocouple. The percentage of cells responding at any point throughout the cold ramp and magnitude of individual cell responses was measured. Cold thresholds were defined as the temperature at which the cell first responded with a greater than 20% increase from baseline (≥ 20% ΔF/F).
To address TRPV1 sensitization, capsaicin (Fluka, Sigma) was applied to cells at concentrations of 5 nM (90 sec), 10 nM (90 sec), or 100 nM (60 sec). The CCR2 antagonist RS 504393 was used (10 μM in 0.005% DMSO unless otherwise stated; 1.0 μM and 0.1 μM were also dissolved in 0.005% DMSO; 10 μM of RS 504393 is effective in blocking CCL2-induced migration of murine macrophage cell lines [13]). RS 504393 was superfused over cells for 3 minutes prior to and during stimulation with capsaicin or cold. In other experiments, cells were superfused with 100 pM, 1 nM, or 100 nM recombinant CCL2 for 2 min to assess responsivity to this chemokine [53] (Protein Foundry, Milwaukee, WI). In time course experiments, cells were incubated with 100 nM CCL2 for 10 or 30 min prior to capsaicin application. The percentage of responsive neurons and response magnitudes to capsaicin (with and without CCR2 antagonism) and CCL2 were recorded.
Data analysis
Paw withdrawal thresholds/latencies were analyzed using a two-way ANOVA test. For von Frey dose response curve analyses, log2(withdrawal thresholds) were analyzed. Withdrawal latency to cold and percent time spent on the cold plate were compared using a t test or ANOVA where applicable. For calcium imaging experiments, the proportion of neurons responding to a given stimulus was compared between genotypes and treatment groups using a Chi square analysis followed by Bonferroni corrections. Neuronal temperature thresholds and magnitudes of calcium responses were compared using two-way ANOVA and Sidak’s multiple comparisons test. Data were analyzed using GraphPad Prism 6 (La Jolla, CA); results were considered significant when p < 0.05.
Results
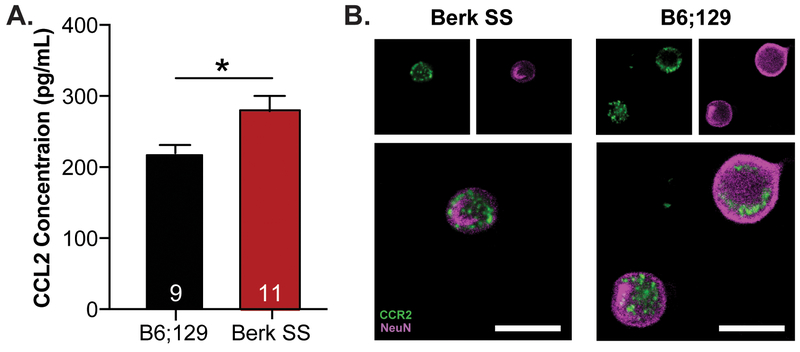
Like previous reports from SCD patient literature, SCD transgenic mice are in a tonic state of inflammation [5,15]. Specifically, circulating CCL2 was increased by ~30% in serum from Berk SS mice, a transgenic model of severe SCD, compared to B6;129 control animals (Figure 1A). The primary receptor for CCL2 is the complementarily named CCR2. CCR2 expression was assessed in DRG extracted from Berk SS and B6;129 mice via immunofluorescence; neuronal expression of CCR2 was noted in both genotypes (Figure 1B). Behavioral and cellular assays were carried out to further determine the contributions of this pro-inflammatory chemokine signaling in SCD cold and mechanical sensitization, two modalities commonly dysregulated in SCD patients.
Figure 1: Chemokine signaling in SCD mouse model.
(A.) Levels of circulating CCL2 were examined in Berk SS and B6;129 animals. Berk SS animals had significantly higher levels of CCL2 compared to B6;129 mice (unpaired t-test t(18)=2.552, *p=0.020; n=9–11). (B.) CCR2 expression was visualized in DRG isolated from B6;129 and Berk SS mice via immunofluorescence. CCR2 expression was noted in NeuN-labeled neurons from both B6;129 and Berk SS mice (scale bar 20 μm).
Acute blockade of CCR2 reverses cold hypersensitivity in SCD mice
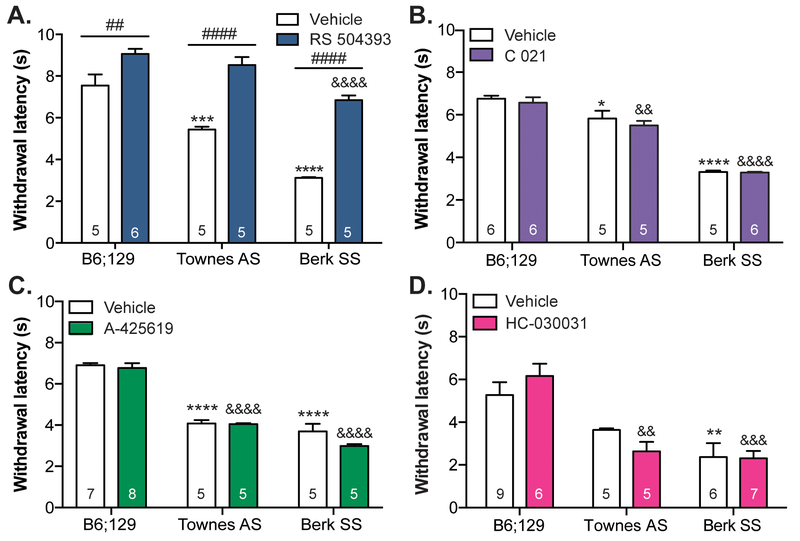
Contributions of CCL2 signaling to cold sensitivity were assessed using the dry ice cold plantar assay [10]. As previously reported using other thermal assays, both the severe sickle phenotype mice (Berk SS) and mildly affected heterozygous (Townes AS) transgenic strains exhibited increased cold sensitivity compared to B6;129 controls. Berk SS mice had significantly shorter paw withdrawal latencies than both B6;129 controls and Townes AS mice (Figure 2A). Subcutaneous administration of the specific CCR2 antagonist RS 504393 significantly increased withdrawal latencies, most notably in both transgenic strains. To determine if RS 504393 was indiscriminately decreasing cold sensitivity in all animals, a temperature preference assay was employed. In line with the cold plantar assay results, RS 504393 pre-treatment increased the amount of time Berk SS mice spent on the colder 23°C floor plate by ~45%; this drug had no effect on the amount of time that control B6;129 animals spent on the cold plate (Supplemental Figure 1). To determine if additional peripheral mechanisms were involved in SCD cold sensitivity, the CCR4 antagonist C 021, TRPV1 antagonist A-425619, and TRPA1 antagonist HC-030031 were administered prior to performing the plantar cold assay. Administration of C 021 had no effect on sickle cell-related cold hypersensitivity (Figure 2B). A similar observation was made following administration of a TRPV1 antagonist (Figure 2C) or TRPA1 antagonist (Figure 2D); the shorter withdrawal latencies in Townes AS and Berk SS mice were not significantly altered by A-425619 or HC-030031 injections respectively.
Figure 2: SCD cold behavioral sensitivity is mediated by CCR2 signaling.
Cold sensitivy was assessed in Berk SS and B6;129 controls using the plantar dry ice assay. (A.) Both transgenic strains exhibited marked cold sensitivty (two-way ANOVA significant main effect of genotype F2,24 = 54.37, p<0.0001; Bonferroni multiple comparison test B6;129 vehicle vs. Townes AS vehicle ***p=0.0002, B6;129 vehicle vs. Berk SS vehicle ****p<0.0001; n=5–6). Subcutaneous neck administration of the CCR2 antagonist RS 504393 (3 mg/kg) increased withdrawal latencies, most significantly in Townes AS and Berk SS mice (two-way ANOVA significant main effect of RS 504393 F1,24 = 114.7, p<0.0001 and genotype x RS 504393 interaction F2,24=6.626, p=0.0051; Bonferroni multiple comparison test B6;129 vehicle vs. B6;129 RS 504393 ##p=0.0050, Townes AS vehicle vs. Townes AS RS 504393 #### p<0.0001, Berk SS vehicle vs. Berk SS RS 504393 #### p<0.0001, B6;129 RS 504393 vs. Berk SS RS 504393 &&&&p<0.0001). (B.) Subcutaneous administration of the CCR4 antagonist C 021 (3 mg/kg) did not alleviate cold hypersensitivity observed in Townes AS and Berk SS mice (two-way ANOVA significant main effect of genotype F2,27=139.6, p<0.0001; Bonferroni multiple comparison test B6;129 vehicle vs. Townes AS vehicle *p=0.0119, B6;129 vehicle vs. Berk SS vehicle ****p<0.0001, B6;129 C 021 vs. Townes AS C 021 &&p=0.0039, B6;129 C 021 vs. Berk SS C 021 &&&&p<0.0001; n=5–6). (C.) Similarly, i.p. administartion of the TRPV1 antagonist A-425619 (34.5 mg/kg) did not alleviate SCD-related cold hypersensitivity exhibited by Townes AS and Berk SS mice (two-way ANOVA significant main effect of genotype F2,28=165.0, p<0.0001; Bonferroni multiple comparison test B6;129 vehicle vs. Townes AS vehicle ****p<0.0001, B6;129 vehicle vs. Berk SS vehicle ****p<0.0001, B6;129 A-425619 vs. Townes AS A-425619 &&&&p<0.0001, B6;129 A-425619 vs. Berk SS A-425619 &&&&p<0.0001; n=5–8). (D.) Intraplantar administration of the TRPA1 antagonist HC-030031 (100 μg/ 30 μL) also had no effect on Townes AS or Berk SS cold hypersensitivity (two-way ANOVA significant main effect of genotype F2,32=23.63, p<0.0001; Bonferroni multiple comparison test B6;129 vehicle vs. Berk SS vehicle **p=0.0038, B6;129 HC-030031 vs. Townes AS HC-030031 &&p=0.019, B6;129 HC-030031 vs. Berk SS HC-030031 &&&p=0.0002; n=5–9).
Acute inhibition of CCR2 does not alter the enhanced cold responsiveness of SCD sensory neurons
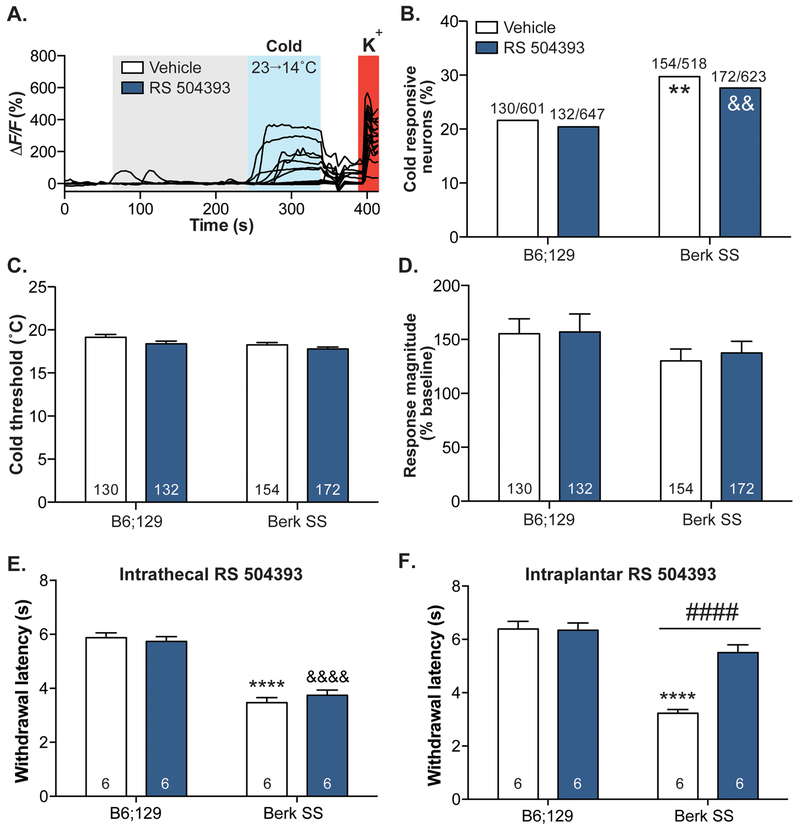
To investigate the cellular mechanisms underlying sickle cell related cold sensitivity, calcium imaging was performed on small-diameter (<27μm) neurons isolated from the lumbar DRG of Berk SS mice. After incubation in the CCR2 antagonist RS 504393 or vehicle, neurons were exposed to a gradual decrease in extracellular buffer temperature of ~10°C over 2 min (Figure 3A).
Figure 3: CCR2 is not involved in SCD neuronal cold sensitization.
Calcium imaging was used to assess cold sensitivity on a neuronal level. (A.) Isolated neurons from Berk SS and B6;129 mice were washed with vehicle or RS 504393 then exposed to a cold ramp. Neurons that exhibited calcium transients to extracellular K+ were considered viable. (B.) A higher proportion of Berk SS neurons responded to cold stimuli; exposure to RS 504393 did not decrease the percentage of Berk SS cold responders (Chi-square Γ2(3, 2,389)=19.39, p=0.0002; Fisher’s exact test B6;129 vehicle vs. Berk SS vehicle **p=0.0024, B6;129 RS 504393 vs. Berk SS RS 504393 &&p=0.0030, Berk SS vehicle vs. Berk SS RS 504393 p=0.6265). (C.) RS 504393 did not significantly change the cold threshold of either Berk SS or B6;129 neurons (two-way ANOVA significant main effect of genotype F1,563=4.671, p=0.0311, significant main effect of RS 504393 F1,563=7.002, p=0.0084; Bonferroni multiple comparisons test B6;129 vehicle vs. Berk SS vehicle p=0.0669, B6;129 vehicle vs. B6;129 RS 504393 p=0.1640, Berk SS vehicle vs. Berk SS RS 504393 p=0.3964). (D.) Similarly, RS 504393 had no effect on the response magnitude of either Berk SS or B6;129 neurons (two-way ANOVA no significant main effects). (E.) Intrathecal administration of RS 504393 (3 μg/5 μL) had no effect on paw withdrawal latencies of B6;129 or Berk SS mice during plantar cold testing (two-way ANOVA significant main effect of genotype F1,20=145.4, p<0.0001; Bonferroni multiple comparisons test B6;129 vehicle vs. Berk SS vehicle ****p<0.0001, B6;129 RS 504393 vs. Berk SS RS 504393 &&&&p<0.0001; n=6). (F.) Conversely, subcutaneous intraplantar administration of RS 504393(3 μg /10 μL) significantly increased Berk SS withdrawal latencies to cold stimuli while having no effect in B6;129 mice (two-way ANOVA significant main effect of genotype F1,19=56.69, p<0.0001, RS 504393 F1,19=17.53, p=0.0005, and genotype x RS 504393 interaction F1,19=19.15, p=0.0003; Bonferroni multiple comparison test B6;129 vehicle vs. Berk SS vehicle ****p<0.0001, Berk SS vehicle vs. Berk SS RS 504393 ####p<0.0001; n=6).
In line with behavioral results, a larger proportion of Berk SS neurons were sensitive to the cold ramp stimulus when compared to B6;129 neurons (Figure 3B). In contrast with behavioral data, incubation with RS 504393 did not decrease the heightened proportion of cold-sensitive neurons in Berk SS cultures. As additional measures of cold responsivity, the cold response thresholds and magnitude were also analyzed. The temperature at which isolated neurons from Berk SS and control mice first responded to cold was not significantly different (~18.7°C in both cohorts; Figure 3C). Similarly, the response magnitude of cold-sensitive neurons did not differ between Berk SS and B6;129 cultures (Figure 3D). CCR2 inhibition had no effect on either cold threshold or response magnitude (Figure 3C–D).
Since subcutaneous administration of RS 504393 significantly decreased Berk SS cold hypersensitivity (Figure 2A), but direct administration of the compound on Berk SS DRG neurons had no effect on cold responsiveness, behavioral cold sensitivity was tested following intrathecal administration of the CCR2 antagonist to determine if SCD cold sensitivity was mediated through spinal cord (central nervous system) CCR2 activity. Spinal administration of RS 504393 had no effect on Berk SS cold hypersensitivity (Figure 3E), despite the efficacy of this intrathecal dose in other pain models. Conversely, local subcutaneous administration of RS 504393 in the plantar surface of the hind paw significantly increased Berk SS withdrawal latencies to cold stimulation (Figure 3F). Collectively, these data suggest that acute CCR2 inhibition meditates sickle cell disease-related cold hypersensitivity at the peripheral tissue level, and likely via non-neuronal cell types.
Acute blockade of CCR2 or TRPV1 reverses mechanical hypersensitivity in SCD mice
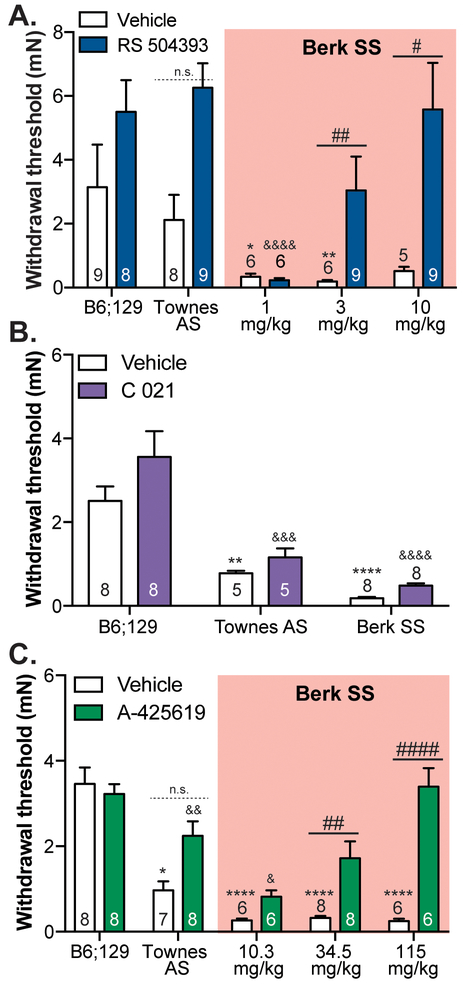
Both SCD patients and transgenic mouse models display persistent mechanical hypersensitivity [26]. To determine the contributions of CCL2 signaling to this sensory surplus, von Frey filaments were used to assess mechanical paw sensitivity following administration of the CCR2 antagonist RS 504393 or the CCR4 antagonist C 021. Subcutaneous administration of 3 mg/kg or 10 mg/kg RS 504393 significantly increased withdrawal thresholds in Berk SS mice (Figure 4A). Conversely, subcutaneous administration of C 021 had no effect on the pronounced hypersentivity displayed by Townes AS and Berk SS mice (Figure 4B).
Figure 4: SCD mechanical behavioral sensitivity is mediated by CCR2 and TRPV1 signaling.
Mechanical sensitivity was assessed in Berk SS and B6;129 controls using the von Frey up-down method. (A.) Subcutaneous neck administration of RS 504393 (3 mg/kg and 10 mg/kg) significantly increased withdrawal thresholds Berk SS mice to a level commensurate with B6;219 controls (two-way ANOVA significant main effect of RS 504393 F1,63=30.50, p<0.0001, significant main effect of RS 504393 x genotype interaction F4,63=2.535, p=0.0488; Bonferroni multiple comparisons test B6;129 vehicle vs. Berk SS 1 mg/kg vehicle *p=0.0237, B6;129 vehicle vs. Berk SS 3 mg/kg vehicle **p=0.0069, Berk SS 3 mg/kg vehicle vs. Berk SS 3 mg/kg RS 504393 ##p=0.0095, Berk SS 10 mg/kg vehicle vs. Berk SS 10 mg/kg RS 504393 #p=0.0327, B6;129 RS 504393 vs. Berk SS 1 mg/kg RS 504393 &&&&p<0.0001, B6;129 vehicle vs. Berk SS 3 mg/kg RS 504393 p>0.05, B6;129 vehicle vs. Berk SS 10 mg/kg RS 504393 p>0.05 ). 3 mg/kg RS 504393 had a noticable, though statistically insignifcant, effect on Townes AS wihtdrawal thresholds (Bonferroni mutliple comarisons test Townes AS vehicle vs. Townes AS RS 504393 p=0.0796; n=5–9). (B.) Conversely, subcutaenous administration of C 021 (3 mg/kg) had no effect on the mechanical hypersensitivity observed in transgenic SCD mice (two-way ANOVA significant main effect of genotype F2,36=38.34, p<0.0001; Bonferroni multiple comparisons test B6;129 vehicle vs. Townes AS vehicle **p=0.0054, B6;129 vehicle vs. Berk SS vehicle ****p<0.0001, B6;129 C 021 vs. Townes AS C 021 &&&p=0.0001, B6;129 C 021 vs. Berk SS C 021 &&&&p<0.0001; n=5–8). (C.) Like the CCR2 antagonist, i.p. injections of the TRPV1 antagonist A-425619 significantly raised withdrawal thresholds in Berk SS mice in a dose-dependent fashion (two-way ANOVA significant main effect of genotype F4,61=15.14, p<0.0001, significant main effect of A-425619 F1,61=30.94, p<0.0001, significant main effect of genotype x A-425619 interaction F4,61=8.326, p<0.0001; Bonferroni multiple comparisons test B6;129 vehicle vs. Townes AS vehicle *p=0.0478, B6;129 vehicle vs. each Berk SS vehicle group ****p<0.0001, Berk SS 34.5 mg/kg vehicle vs. Berk SS 34.5 mg/kg A-425619 ##p=0.0090, Berk SS 115 mg/kg vehicle vs. Berk SS 115 mg/kg A-425619 ####p<0.0001, B6;129 A-425619 vs. Townes AS A-425619 &&p=0.0086, B6;129 A-425619 vs. 10.3 mg/kg A-425619 &p=0.0275, B6;129 vehicle vs. Berk SS 34.5mg/kg A-425619 p>0.05, B6;129 vehicle vs. Berk SS 115 mg/kg A-425619 p>0.05; n=6–8).
Previous reports have linked increased TRPV1 activity with the heightened mechanical sensitivity observed in transgenic SCD models [26]. Confirmation of this association was demonstrated following i.p. administration of the TRPV1 antagonist A-425619. Significant increases in paw withdrawal threshold were noted in Berk SS mice following injection of 34.5 mg/kg or 115 mg/kg A-425619 (Figure 4C). Collectively these data suggest that signaling through both TRPV1 and CCR2 is important for sickle cell-related mechanical hypersensitivity.
Small-diameter sensory neurons of SCD mice are sensitized to TRPV1 activation
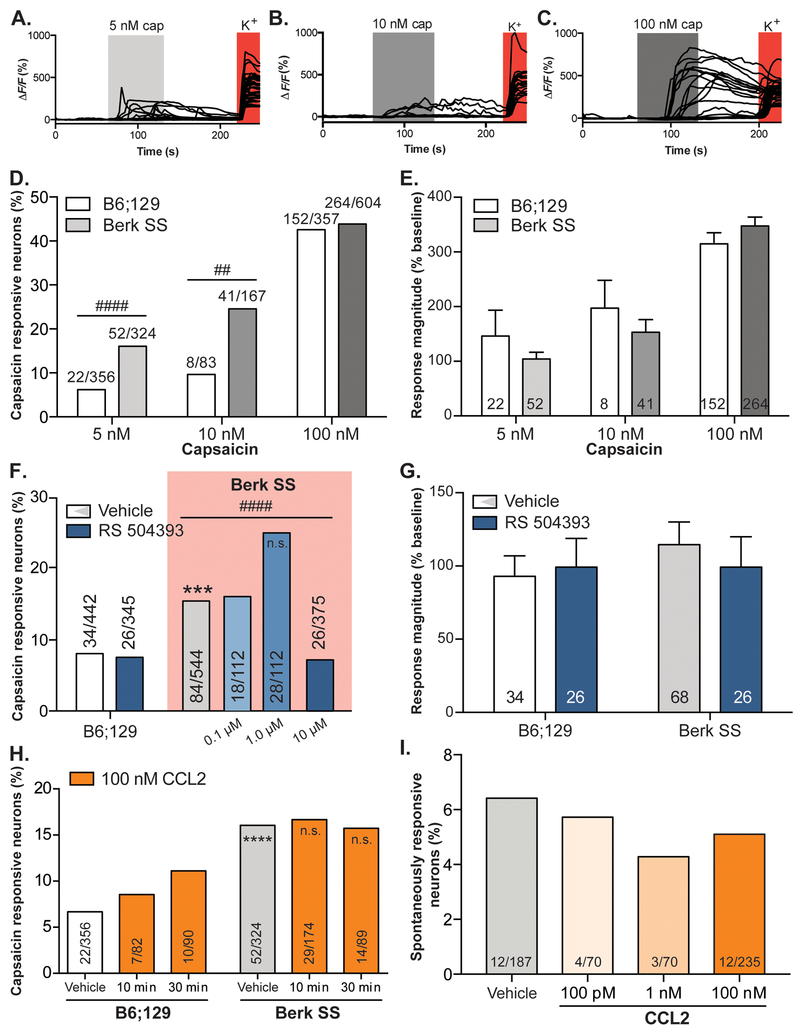
After demonstrating an association between TRPV1 activity and behavioral correlates of ongoing sickle cell disease pain, calcium imaging was used to assess the cellular underpinnings of this relationship. Again, small-diameter neurons were isolated from lumbar DRG of Berk SS and B6;129 mice. TRPV1 activity in individual neurons was assessed by responsiveness to 5 nM (Figure 5A), 10 nM (Figure 5B), or 100 nM capsaicin (Figure 5C), an exogenous TRPV1 ligand. No difference was observed between the proportion of B6;129 and Berk SS neurons responsive to the high concentration (100 nM) of capsaicin; however, increased proportions of Berk SS neurons responded to lower (5 nM and 10 nM) capsaicin concentrations (Figure 5D). While increasing concentrations of capsaicin elicited larger responses in agonist-sensitive neurons, the magnitude of these responses did not differ between Berk SS and control neurons (Figure 5E).
Figure 5: TRPV1sensitization in SCD neurons is mediated by CCR2 activity.
Calcium imaging was used to assess TRPV1 sensitization in Berk SS and B6;129 neurons . Neurons were exposed to (A.) 5 nM, (B.) 10 nM , or (C.) 100 nM capsaicin, an exogenous TRPV1 agonist. (D.) A higher proportion of Berk SS neurons responded to 5nM and 10 nM capsaicin than B6;129 neurons; statistically equal populations responded to 100 nM capsaicin (Chi-square X 2(5, 1,891)=230.7, p<0.0001; Fisher’s exact test B6;129 5 nM vs. Berk SS 5 nM ####p<0.0001, B6;129 10 nM vs. Berk SS 10 nM ##p=0.0063, B6;129 100 nM vs. Berk SS 100 nM p=0.7368). (E.) While increasing capsicain concentrations evoked larger calcium transients, the magnitude of transients did not differ between Berk SS and B6;129 neurons (two-way ANOVA significant main effect of capsaicin concentration F2,533=10.71, ****p<0.0001, no main effect of genotype p=0.6403). (F.) Application of high concentration (10 μM) RS 504393 significnatly decreased the percentage of Berk SS neurons that responded to 5 nM capsaicin (Bonferroni-corrected p=0.0083, Chi-square Berk SS vehicle vs. Berk SS 10 μM RS 504393 X2(1, 919)=15.25, ####p<0.0001; B6;129 vehicle vs. Berk SS vehicle X2(1, 986)=15.25, ***p=0.002). Lower concentrations of RS 504393 had no effect on the number of Berk SS neurons that responded to capsaicin (Berk SS vehicle vs. Berk SS 1.0 μM RS 504393 X2(1, 656)=5.994, p=0.0144; Berk SS vehicle vs. Berk SS 1.0 μM RS 504393 X2(1, 656)=0.028, p=0.8669). (G.) Application of RS 504393 had no effect on the magnitude of response in Berk SS or B6;129 neurons to 5 nM capsaicin (two-way ANOVA no significant main effects). (H.) Similarly, incubating Berk SS and B6;129 neurons with CCL2 had no effect on the proportion of neurons activated by 5 nM capsaicin in either population (Chi-square X2(5, 1,015)=20.24, p=0.0011, Fisher’s exact test B6;129 vehicle vs. Berk SS vehicle ****p<0.0001, B6;129 vehicle vs. B6;129 CCL2 30 min p=0.1122 Berk SS vehicle vs. Berk SS CCL2 30 min p>0.9999). (I.) Spontaneous calcium transients in Berk SS neurons were not altered by incubation with 100 pM, 1 nM, or 100 nM CCL2 (Chi-square X2(3, 562)=0.5756, p=0.9020).
Given that both CCR2 and TRPV1 antagonism can rapidly alleviate behavioral mechanical sensitization, we next assessed whether direct CCR2 inhibition in cultured neurons alleviates TRPV1 sensitization. Acute incubation (3 min) with high concentrations (10 μM) of the CCR2 antagonist RS 504393 fully reduced the enhanced TRPV1 sensitization of Berk SS neurons without impairing basal sensitivity to capsaicin in control neurons (Figure 5F). Lower concentrations (1.0 and 0.1 μM of RS 504393) did not statistically alter the number of Berk SS neurons that responded to capsaicin. RS 504393 had no impact on capsaicin response magnitudes in neurons of either genotype (Figure 5G).
Whereas inhibition of CCR2 in Berk SS neurons produced functional decreases in TRPV1 sensitivity, the converse effect was not observed: application of exogenous CCL2 for 10 or 30 minutes did not further increase TRPV1 sensitivity in Berk SS neurons (Figure 5H). Control neurons display a non-significant trend toward increasing capsaicin sensitivity following different periods of CCL2 co-incubation (Figure 5H). Direct application of CCL2 did not induce calcium influx in Berk SS neurons; a similar proportion of small-diameter neurons from sickle mice exhibited spontaneous calcium transients regardless of the presence of various concentrations of exogenous CCL2 (Figure 5I).
Acute blockade of CCR2 decreases TRPV1 hypersensitivity in SCD mice
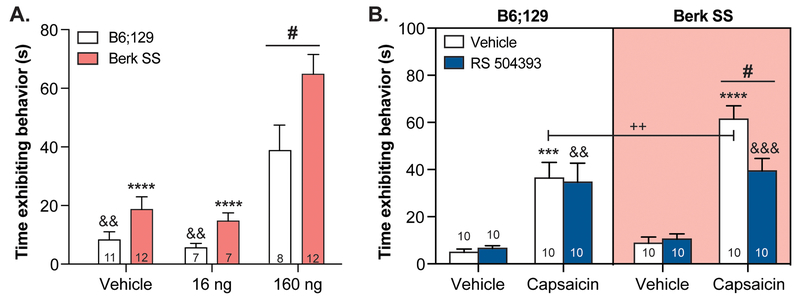
In order to determine if CCR2 signaling is mediating TRPV1 sensitization on the behavioral level, the capsaicin test was employed. In this assay, Berk SS and B6;129 control mice received an intraplantar injection of capsaicin or 10% DMSO vehicle. Spontaneous nocifensive behaviors were timed following injection of two different doses of capsaicin: 160 ng/paw or 16 ng/paw. Berk SS mice displayed spontaneous pain-like behaviors for significantly more time than B6;129 mice following 160 ng/paw treatment (Figure 6A). Following 16 ng/paw treatment, mice from both genotypes exhibited behavioral responses that were statistically similar to vehicle treatment.
Figure 6: Behavioral TRPV1 sensitization is alleviated by acute CCR2 blockade.
To assess TRPV1 sensitization and its relationship with CCR2 signaling, the capsaicin test was employed. (A.) Like calcium imaging experiments, Berk SS animals exhibited significantly larger responses to intraplantar capsaicin than B6;129 mice (two-way ANOVA significant main effect of genotype F1,51=11.64, p=0.0013, significant main effect of treatment F2,51=36.3, p<0.0001; Bonferroni multiple comparison test B6;129 160 ng vs. Berk SS 160 ng #p=0.0140, B6;129 160 ng vs. B6;129 16 ng &&p=0.0050, B6;129 160 ng vs. B6;129 vehicle &&p=0.0038, Berk SS 160 ng vs. Berk SS 16 ng ****p<0.0001, Berk SS 160 ng vs. Berk SS vehicle ****p<0.0001; n=7–12). (B.) Subcutaneous pre-treatment with RS 504393 (3 mg/kg) reduced capsaicin(160 ng)-evoked nocifensive responses in Berk SS mice, but not B6;129 controls (three-way ANOVA significant main effect of capsaicin F1,1=106.3, p<0.0001, significant main effect of genotype F1,1=7.521, p=0.0077; Bonferroni multiple comparison test Berk SS vehicle/capsaicin vs. Berk SS RS 504393/capsaicin #p=0.0230, B6;129 vehicle/vehicle vs. B6;129 vehicle/capsaicin ***p=0.0002, B6;129 RS 504393/vehicle vs. B6;129 RS 504393/capsaicin &&p=0.0013, Berk SS vehicle/vehicle vs. Berk SS vehicle/capsaicin ****p<0.0001, Berk SS RS 504393/vehicle vs. Berk SS RS 504393/capsaicin &&&p=0.0008, B6;129 vehicle/capsaicin vs. Berk SS vehicle/capsaicin ++p=0.0057; B6;129 vehicle/capsaicin vs. Berk SS RS 504393/capsaicin p>0.05; n=10).
A separate cohort of mice were pretreated with RS 504393 or 5% DMSO vehicle prior to intraplantar injection of 160 ng capsaicin to determine if CCR2 signaling was critical for the behavioral capsaicin sensitivity observed in Berk SS animals. Vehicle pre-treated Berk SS and B6;129 animals exhibited significant increases in nocifensive behavior duration following capsaicin injection (Figure 6B); capsaicin responsiveness of Berk SS mice was significantly higher than B6;129 mice. Responses to intraplantar vehicle injection were statistically similar between groups. Notably, subcutaneous injection of RS 504393 (3 mg/kg) significantly decreased nocifensive responses in Berk SS mice following intraplantar capsaicin injection, but did not statistically decrease capsaicin responses in B6;129 controls. Taken together, these results suggest that ongoing CCR2 activity in Berk SS mice is contributing to the responsiveness of peripheral TRPV1 signaling pathways.
Discussion
Pain is often the primary symptom of SCD and the leading cause for patients to seek medical care [1]. Despite high morbidity rates, surprisingly little is known about the mechanisms underlying the generation and maintenance of chronic pain in this disease. It is known, however, that both SCD patients and transgenic animal models exist in a persistent pro-inflammatory state; chemokines including CXCL1 [43], CXCL8 (interleukin 8, IL8) [38], CXCL9, CXCL10 [56], CXCL12 [43], and CX3CL1 (fractalkine) [54] are significantly elevated in the serum of SCD patients or mouse models. The focus of these studies was CCL2, a chemokine that has previously been shown to sensitize primary sensory afferents in neuropathic pain models [30]. Independent studies have reported both neuropathic pain-like symptoms [8] and increased levels of circulating CCL2 [47] in patients with SCD; future experiments should determine if reported pain measures and CCL2 serum levels in human patients are correlative. In this report, we further investigate the nociceptive role of this chemokine in transgenic mouse models of SCD. We first demonstrate that Berk SS mice have increased levels of plasma CCL2. Species-specific differences in circulating CCL2 should not be overlooked; SCD mice have ~30% increase in circulating CCL2 as compared to control animals while patients with SCD have ~600% increase in circulating CCL2 as compared to race-matched controls [47]. Despite the smaller disease-related increases of CCL2 in rodent, manipulation of this signaling pathway was still effective in reducing mechanical and cold hypersensitivity in the Berkeley and Townes transgenic animal models of SCD, providing further support that CCR2 therapy would be effective in human SCD patients.
Here, we report that inhibition of CCR2, one of the main receptors of CCL2, leads to alleviation of both mechanical and cold behavioral hypersensitivity in mouse models of sickle cell disease. Inhibition of CCR4, another CCL2 receptor that is expressed in DRG and that has known nociceptive functions, [42] did not alleviate SCD-related hypersensitivities. These data suggest that enhanced tonic activation of CCR2, via CCL2 or additional endogenous ligands, may be necessary to maintain the nociceptor sensitization observed in SCD both in vitro and in vivo. There are multiple ways through which the CCL2-CCR2 signaling axis may contribute to neuronal sensitization. An important consideration is whether the impact of CCR2 inhibition is mediated through sensory neurons themselves, or whether intermediate cell types are necessary to confer the alleviation of pain. CCR2 is expressed by DRG neurons; expression is enhanced in neuropathic pain models, many of which are phenotypically similar to transgenic SCD mouse models [6,29,53,58,61]. In neuropathic models, CCL2 can act upon peripheral sensory neurons to enhance excitability and responsiveness, as well as on other cell types including microglia or CNS neurons [46]. To begin to address this question in SCD, we asked whether CCR2 signaling contributes to sensitization of DRG neurons in culture, as this effectively eliminates the direct contributions of the CNS and non-neuronal cell types.
CCR2 mediates cold hypersensitivity but not sensory neuron sensitization to cold stimuli
Subcutaneous administration of the CCR2 antagonist significantly decreased the behavioral cold sensitivity. Notably, inhibition of the putative cold-sensitive channel TRPA1 had no effect on this behavioral measure. In addition to behavioral cold sensitization, we also report cold sensitization in isolated small-diameter sensory neuron somata in culture. These data are consistent with our previous findings that nociceptive peripheral sensory afferents from SCD animals are sensitized to cold stimulation [63]. In afferent skin-nerve recordings, cold sensitization manifested as warmer activation thresholds; in the present calcium imaging studies, activation temperatures did not differ between genotypes. Instead, the proportion of neurons activated by the cooling stimulus was increased in DRG cultures from sickle mice. The fact that subcutaneous CCR2 inhibition alleviated cold sensitization in two behavioral assays without altering the cold sensitivity of isolated sensory neurons is noteworthy. Several possibilities may underlie these distinctions. Firstly, non-neuronal cells in the skin may contribute to cold sensitivity in situ. In fact, keratinocytes of the epidermis are known to respond directly to heat stimulation [18], and parallel mechanisms may underlie cold sensitivity as well. The hypothesis that non-neuronal skin cells may be involved is consistent with our data in which intraplantar administration of RS 504393 significantly decreased cold behavioral hypersensitivity, and with previously published experiments in which levels of secreted CCL2 from Berk SS skin were significantly decreased following treatment with a mast cell inhibitor [55]. Secondly, calcium imaging experiments were performed at a baseline starting temperature of ~23°C compared to the 32°C starting temperature in skin-nerve experiments. This difference in starting temperature and the extent of temperature deviation during the experiment are known to affect TRP channel activation [22,35]. Thirdly, in vitro calcium imaging experiments by definition involve significant axonal injury near the soma, and the culturing process and subsequent recording delay may obscure transient differences in sensitization that existed in vivo [3].
CCR2 mediates TRPV1 sensitivity in SCD
SCD mice exhibit TRPV1 sensitization on both the behavioral and neuronal levels. Behaviorally, TPRV1 sensitization manifested as mechanical hypersensitivity. It should be noted that unlike in the plantar cold testing, the less severe model of SCD (i.e., Townes AS) exhibited mechanical withdrawal thresholds that were statistically similar to those observed in the severe Berk SS model of SCD. Previously published data from our lab demonstrate an intermediate phenotype for AS mice when compared to SS mice of the same genetic background (Townes SS) [64]. The highly variable nature of von Frey testing, sample size, and number of post hoc analyses, may contribute to the observed similarities between genotypes (i.e., Townes AS and Berk SS) in these experiments. Regardless, acute inhibition of TRPV1 channels significantly increased mechanical paw withdrawal thresholds in Berk SS mice. Acute CCR2 inhibition also decreased mechanical hypersensitivity in Berk SS mice. Furthermore, RS 504393 treatment decreased both the amount of time Berk SS mice exhibited nocifensive responses to intraplantar capsaicin injections, and the proportion of isolated neurons from these animals that were responsive to low concentrations of capsaicin. Although we tested three concentrations of RS 504393 (0.1–10 μM), only 10 μM fully inhibited the enhanced number of isolated SCD neurons that responded to 5 nM capsaicin. We predict that concentrations between 1 and 10 μM would partially inhibit the capsaicin-responsive SCD neurons. Collectively, this evidence supports the hypothesis that CCR2 mediates mechanical hypersensitivity through sensitization of TRPV1.
Several lines of evidence suggest that TRPV1 sensitization is not likely mediated by an increase in TRPV1 expression. First, Trpv1 expression is not enhanced in whole DRG of sickle mice, at least at the level of mRNA [63]. Secondly, we observed that acute inhibition of CCR2 was able to mitigate the mechanical sensitization on a much faster time scale than would be required if this effect were mediated by an alteration of gene transcription, translation, and transport to the plasma membrane. Together, these data suggest that a similar proportion of sensory neurons express TRPV1, and likely at similar levels, but that existing TRPV1 channels may be sensitized to activation. Since the TRPV1 antagonist was administered intraperitoneally for all behavioral experiments, central sensitization of TRPV1, a phenomenon previously reported by others [51], cannot be ruled out. However, it has previously been shown that sickle sensory neurons retain sensitization to TRPV1 even once isolated from the central nervous system, both in the form of teased fiber recordings and patch clamp recordings of isolated DRG neurons [26]. Furthermore, low blood brain barrier permeability of A-425619 make peripheral sensitization a more likely contributor to the observed behavioral manifestations [27].
Potential mechanisms underlying CCR2-mediated sensitization
Overall, our evidence suggests that CCR2 activation leads to TRPV1 sensitization, and thereby contributes to mechanical hypersensitivity. Of note, a brief three minutes of CCR2 inhibition was sufficient to mitigate the sensitization of TRPV1 in calcium imaging experiments. This rapid effectiveness strongly suggests the effects of RS 504393 are mediated by sensory neurons themselves. CCR2 is a Gαi-linked G protein-coupled receptor; its activation leads to inhibition of adenylyl cyclase and mobilization of calcium stores [59]. While the mechanisms underlying CCR2 activation and resulting calcium flux and chemotaxis in immune cells has been extensively studied, much less is known about how chemokine receptors induce neuronal sensitization. One possibility is that Gβγ-mediated activation of PLC leading to release of calcium from intracellular stores [40] might directly contribute to TRPV1 sensitization, given the intrinsic connection between calcium signaling and neuronal activation. However, such a mechanism may require persistent tonic activation of CCR2 to maintain neuronal sensitization, and ligand-mediated activation of CCR2 results in receptor internalization from the plasma membrane [59]. Measuring CCL2 release in DRG cultures and testing capsaicin sensitization via calcium imaging in the presence of an effective CCL2 neutralizing antibody will be helpful in confirming the viability of this possibility. Alternatively, neuronal CCR2 activation might lead to direct Gβγ-mediated sensitization of TRPV1 channels. A similar mechanism of Gβγ subunits coupling with ion channels has been proposed for other GPCRs, such as MrgprA3 activation leading to the activation of TRPA1 [60].
These results provide insight into immune-mediated regulation of hypersensitivity in SCD and could inform future development of treatments for chronic SCD pain. The lack of adequate pain management therapies available for these patients results in an enormous health care burden for both the individual and society [52,57]. The inadequate pain control is further complicated by discrimination at the level of access to care [25], largely due to a pervasive stigmatization that patients with SCD are frequently abusing opioid medications, despite the clear physiological basis for the pain these patients report [21]. Of note, several clinical trials are addressing the utility and feasibility of CCR2 antagonists, and antagonists of other chemokine receptors for indications ranging from arthritis to pain [28]. Therefore, there is a distinct therapeutic potential that CCR2 inhibition may be of significant clinical benefit to individuals with SCD.
Supplementary Material
Acknowledgements
Katelyn E. Sadler and Katherine J. Zappia contributed equally to this work; K.J.Z. helped to formulate the initial hypothesis and experimental approach and acquired funding; performed experiments, data analysis and assisted with writing the manuscript; K.E.S. contributed to experimental design, performance of experiments, data analysis and writing and editing of the manuscript throughout the review process. The authors would like to thank Dr. Aniko Szabo for expert statistical consulting, Anthony Menzel for technical assistance, and Francie Moehring for her thoughtful comments and review of this manuscript. This research was supported by NIH grants NS040538 (to C.L.S.), NS070711 (to C.L.S. and C.A.H.), HL128371 (to C.A.H.), and NS087716 (to K.J.Z.). The Research and Education Component of the Advancing a Healthier Wisconsin Endowment at the Medical College of Wisconsin provided partial support. K.J.Z. is a member of the Medical Scientist Training Program at MCW, which is partially supported by a training grant from NIGMS, T32-GM080202. The authors have no conflicts of interest to declare.
References
- [1].Ballas SK. Pain management of sickle cell disease. Hematol Oncol Clin North Am 2005;19:785–802. [DOI] [PubMed] [Google Scholar]
- [2].Ballas SK, Gupta K, Adams-Graves P. Sickle cell pain : a critical reappraisal. Blood 2013;120:3647–3656. [DOI] [PubMed] [Google Scholar]
- [3].Barabas ME, Kossyreva EA, Stucky CL. TRPA1 Is Functionally Expressed Primarily by IB4-Binding, Non-Peptidergic Mouse and Rat Sensory Neurons. PLoS One 2012;7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt S-E, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007;448:204–8. [DOI] [PubMed] [Google Scholar]
- [5].Belcher JD, Bryant CJ, Nguyen J, Bowlin PR, Kielbik MC, Bischof JC, Hebbel RP, Vercellotti GM. Transgenic sickle mice have vascular inflammation. Blood 2003;101:3953–3959. [DOI] [PubMed] [Google Scholar]
- [6].Bhangoo S, Ren D, Miller RJ, Henry KJ, Lineswala J, Hamdouchi C, Li B, Monahan PE, Chan DM, Ripsch MS, White FA. Delayed functional expression of neuronal chemokine receptors following focal nerve demyelination in the rat: A mechanism for the development of chronic sensitization of peripheral nociceptors. Mol Pain 2007;3:1744-8069-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bogen O, Dina OA, Gear RW, Levine JD, Bogen. Dependence of MCP-1 hyperlgesia on the IB4-binding protein versican. Neuroscience 2009;159:780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brandow AM, Farley RA, Panepinto JA. Neuropathic pain in patients with sickle cell disease. Pediatr Blood Cancer 2014;61:512–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Brandow AM, Stucky CL, Hillery CA, Hoffmann RG, Panepinto JA. Patients with sickle cell disease have increased sensitivity to cold and heat. Am J Hematol 2013;88:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Brenner DS, Golden JP, Gereau IV RW. A novel behavioral assay for measuring cold sensation in mice. PLoS One 2012;7:e39765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brousseau DC, Panepinto JA, Nimmer M, Hoffmann RG. The number of people with sickle-cell disease in the United States: National and state estimates. Am J Hematol 2010;85:77–78. [DOI] [PubMed] [Google Scholar]
- [12].Cain DM, Vang D, Simone DA, Hebbel RP, Gupta K. Mouse models for studying pain in sickle cell disease: effects of strain, age, and acuteness. Br J Haematol 2012;156:535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Carmo AAF, Costa BRC, Vago JP, de Oliveira LC, Tavares LP, Nogueira CRC, Ribeiro ALC, Garcia CC, Barbosa AS, Brasil BSAF, Dusse LM, Barcelos LS, Bonjardim CA, Teixeira MM, Sousa LP. Plasmin Induces In Vivo Monocyte Recruitment through Protease-Activated Receptor-1–, MEK/ERK-, and CCR2-Mediated Signaling. J Immunol 2014;193:3654–3663. [DOI] [PubMed] [Google Scholar]
- [14].Cataldo G, Rajput S, Gupta K, Simone DA. Sensitization of nociceptive spinal neurons contributes to pain in a transgenic model of sickle cell disease. Pain 2015;156:722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chantrathammachart P, Mackman N, Sparkenbaugh E, Wang J-G, Parise LV, Kirchhofer D, Key NS, Pawlinski R. Tissue factor promotes activation of coagulation and inflammation in a mouse model of sickle cell disease. Blood 2012;120:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53:55–63. [DOI] [PubMed] [Google Scholar]
- [17].Chirico EN, Pialoux V. Role of oxidative stress in the pathogenesis of sickle cell disease. IUBMB Life 2012;64:72–80. [DOI] [PubMed] [Google Scholar]
- [18].Chung M-K, Lee H, Mizuno A, Suzuki M, Caterina MJ. TRPV3 and TRPV4 mediate warmth-evoked currents in primary mouse keratinocytes. J Biol Chem 2004;279:21569–75. [DOI] [PubMed] [Google Scholar]
- [19].Craig MJ, Loberg RD. CCL2 (Monocyte Chemoattractant Protein-1) in cancer bone metastases. Cancer Metastasis Rev 2007;25:611–619. [DOI] [PubMed] [Google Scholar]
- [20].Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 1980;20:441–62. [DOI] [PubMed] [Google Scholar]
- [21].Feliu MH, Wellington C, Crawford RD, Wood M, Edwards L, Byrd G, Edwards CL. Opioid management and dependency among adult patients with sickle cell disease. Hemoglobin 2011;35:485–94. [DOI] [PubMed] [Google Scholar]
- [22].Fujita F, Uchida K, Takaishi M, Sokabe T, Tominaga M. Ambient temperature affects the temperature threshold for TRPM8 activation through interaction of phosphatidylinositol 4,5-bisphosphate. J Neurosci 2013;33:6154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Garrison SR, Kramer AA, Gerges NZ, Hillery CA, Stucky CL. Sickle cell mice exhibit mechanical allodynia and enhanced responsiveness in light touch cutaneous mechanoreceptors. Mol Pain 2012;8:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hassell KL. Population Estimates of Sickle Cell Disease in the U.S. Am J Prev Med 2010;38:S512–S521. [DOI] [PubMed] [Google Scholar]
- [25].Haywood CJ, Tanabe P, Naik R, Beach MC, Lanzkron S. The impact of race and disease on sickle cell patient wait times in the emergency department. Am J Emerg Med 2013;31:651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hillery CA, Kerstein PC, Vilceanu D, Barabas ME, Retherford D, Brandow AM, Wandersee NJ, Stucky CL. Transient receptor potential vanilloid 1 mediates pain in mice with severe sickle cell disease. Blood 2011;118:3376–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Honore P, Wismer CT, Mikusa J, Zhu CZ, Zhong C, Gauvin DM, Gomtsyan A, El Kouhen R, Lee C-H, Marsh K, Sullivan JP, Faltynek CR, Jarvis MF. A-425619 [1-Isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea], a Novel Transient Receptor Potential Type V1 Receptor Antagonist, Relieves Pathophysiological Pain Associated with Inflammation and Tissue Injury in Rats. J Pharmacol Exp Ther 2005;314. [DOI] [PubMed] [Google Scholar]
- [28].Horuk R Chemokine receptor antagonists: overcoming developmental hurdles. Nat Rev Drug Discov 2009;8:23–33. [DOI] [PubMed] [Google Scholar]
- [29].Jung H, Bhangoo S, Banisadr G, Freitag C, Ren D, White FA, Miller RJ. Visualization of Chemokine Receptor Activation in Transgenic Mice Reveals Peripheral Activation of CCR2 Receptors in States of Neuropathic Pain. J Neurosci 2009;29:8051–8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jung H, Toth PT, White FA, Miller RJ. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J Neurochem 2008;104:254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kohli DR, Li Y, Khasabov SG, Gupta P, Kehl LJ, Ericson ME, Nguyen J, Gupta V, Hebbel RP, Simone DA, Gupta K. Pain-related behaviors and neurochemical alterations in mice expressing sickle hemoglobin: modulation by cannabinoids. Blood 2010;116:456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lennertz RC, Kossyreva EA, Smith AK, Stucky CL. TRPA1 mediates mechanical sensitization in nociceptors during inflammation. PLoS One 2012;7:e43597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Llorián-Salvador M, Pevida M, González-Rodríguez S, Lastra A, Fernández-García M-T, Hidalgo A, Baamonde A, Menéndez L. Analgesic effects evoked by a CCR2 antagonist or an anti-CCL2 antibody in inflamed mice. Fundam Clin Pharmacol 2016;30:235–247. [DOI] [PubMed] [Google Scholar]
- [34].Manci EA, Hillery CA, Bodian CA, Zhang ZG, Lutty GA, Coller BS. Pathology of Berkeley sickle cell mice : similarities and differences with human sickle cell disease. Blood 2006;107:1651–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].McKemy DD. How cold is it? TRPM8 and TRPA1 in the molecular logic of cold sensation. Mol Pain 2005;1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].McMillin M, Frampton G, Thompson M, Galindo C, Standeford H, Whittington E, Alpini G, DeMorrow S. Neuronal CCL2 is upregulated during hepatic encephalopathy and contributes to microglia activation and neurological decline. J Neuroinflammation 2014;11:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Menetski J, Mistry S, Lu M, Mudgett JS, Ransohoff RM, Demartino JA, Macintyre DE, Abbadie C. Mice overexpressing chemokine ligand 2 (CCL2) in astrocytes display enhanced nociceptive responses. Neuroscience 2007;149:706–14. [DOI] [PubMed] [Google Scholar]
- [38].Michaels L, Ohene-Frempong K, Zhao H, Douglas SD. Serum levels of substance P are elevated in patients with sickle cell disease and increase further during vaso-occlusive crisis. Blood 1998;92:3148–3151. [PubMed] [Google Scholar]
- [39].Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GM, Patapoutian A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science (80- ) 2005;307:1468–1472. [DOI] [PubMed] [Google Scholar]
- [40].Myers S, Wong L, Charo I. Signal transduction and ligand specificity of the human monocyte chemoattractant protein-1 receptor in transfected embryonic kidney cells. J Biol Chem 1995;270:5786–5792. [DOI] [PubMed] [Google Scholar]
- [41].Nolan VG, Zhang Y, Lash T, Sebastiani P, Steinberg MH. Association between wind speed and the occurrence of sickle cell acute painful episodes: results of a case-crossover study. Br J Haematol 2008;143:433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Oh S, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ. Chemokines and glycoprotein 120 produce pain hypersensitivity directly exciting primary nociceptive neurons. J Neurosci 2001;21:5027–5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ostadebrahimi H, Jamali Z, Nazari M, Bahri M, Farahmandnia Z, Khandany BK, Taheri M, Khorramdelazad H, Hakimizadeh E, Zaker F, Rezaeian M, Hassanshahi G. CXC chemokines CXCL1, CXCL9, CXCL10 and CXCL12 are variably expressed in patients with sickle cell disease and carriers: Are they predictive tools for disease complications? Clin Lab 2014;60:99–104. [DOI] [PubMed] [Google Scholar]
- [44].Paszty C Transgenic Knockout Mice with Exclusively Human Sickle Hemoglobin and Sickle Cell Disease. Science (80- ) 1997;278:876–878. [DOI] [PubMed] [Google Scholar]
- [45].Pevida M, González-Rodríguez S, Lastra A, García-Suárez O, Hidalgo A, Menéndez L, Baamonde A. Involvement of Spinal Chemokine CCL2 in the Hyperalgesia Evoked by Bone Cancer in Mice: A Role for Astroglia and Microglia. Cell Mol Neurobiol 2014;34:143–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pevida M, Lastra A, Hidalgo A, Baamonde A, Menéndez L. Spinal CCL2 and microglial activation are involved in paclitaxel-evoked cold hyperalgesia. Brain Res Bull 2013;95:21–7. [DOI] [PubMed] [Google Scholar]
- [47].Qari MH, Dier U, Mousa S a. Biomarkers of inflammation, growth factor, and coagulation activation in patients with sickle cell disease. Clin Appl Thromb Hemost 2012;18:195–200. [DOI] [PubMed] [Google Scholar]
- [48].Ryan TM, Ciavatta DJ, Townes TM. Knockout-Transgenic Mouse Model of Sickle Cell Disease. Science (80- ) 1997;278:873–876. [DOI] [PubMed] [Google Scholar]
- [49].Sakurada T, Katsumata K, Tan-No K, Sakurada S, Kisara K. The capsaicin test in mice for evaluating tachykinin antagonists in the spinal cord. Neuropharmacology 1992;31:1279–1285. [DOI] [PubMed] [Google Scholar]
- [50].Smith WR, Penberthy LT, Bovbjerg VE, McClish DK, Roberts JD, Dahman B, Aisiku IP, Levenson JL, Roseff SD. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med 2008;148:94–101. [DOI] [PubMed] [Google Scholar]
- [51].Spicarova D, Adamek P, Kalynovska N, Mrozkova P, Palecek J. TRPV1 receptor inhibition decreases CCL2-induced hyperalgesia. Neuropharmacology 2014;81:75–84. [DOI] [PubMed] [Google Scholar]
- [52].Steiner CA, Miller JL. Sickle Cell Disease Patients in U.S. Hospitals, 2004. Statistical Brief # 21. Int Classif 2006;332:1–9. [PubMed] [Google Scholar]
- [53].Sun JH, Yang B, Donnelly DF, Ma C, LaMotte RH. MCP-1 enhances excitability of nociceptive neurons in chronically compressed dorsal root ganglia. J Neurophysiol 2006;96:2189–99. [DOI] [PubMed] [Google Scholar]
- [54].Unal S, Ozdemir O, Ozcimen AA, Oztas Y. Increase of serum fractalkine and fractalkine gene expression levels in sickle cell disease patients. Int J Hematol 2015;101:114–118. [DOI] [PubMed] [Google Scholar]
- [55].Vincent L, Vang D, Nguyen J, Gupta M, Luk K, Ericson ME, Simone DA, Gupta K. Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood 2013;122:1853–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wallace KL, Marshall MA, Ramos SI, Lannigan JA, Field JJ, Strieter RM, Linden J. NKT cells mediate pulmonary inflammation and dysfunction in murine sickle cell disease through production of IFN-γ and CXCR3 chemokines. Blood 2009;114:667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Weisberg D, Balf-Soran G, Becker W, Brown S-E, Sledge W. “I’m Talking About Pain”: Sickle cell disease patients with extremely high hospital use. J Hosp Med 2013;8:42–46. [DOI] [PubMed] [Google Scholar]
- [58].White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, Steflik J, Cortright DN, LaMotte RH, Miller RJ. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci 2005;102:14092–14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].White GE, Iqbal AJ, Greaves DR. CC chemokine receptors and chronic inflammation--therapeutic opportunities and pharmacological challenges. Pharmacol Rev 2013;65:47–89. [DOI] [PubMed] [Google Scholar]
- [60].Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, Mas-related G protein–coupled receptor–mediated itch. Nat Neurosci 2011;14:595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wu H, Bogdanov M, Zhang Y, Sun K, Zhao S, Song A, Luo R, Parchim NF, Liu H, Huang A, Adebiyi MG, Jin J, Alexander DC, Milburn MV, Idowu M, Juneja HS, Kellems RE, Dowhan W, Xia Y. Hypoxia-mediated impaired erythrocyte Lands’ Cycle is pathogenic for sickle cell disease. Sci Rep 2016;6:29637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Yusuf HR, Atrash HK, Grosse SD, Parker CS, Grant AM. Emergency Department Visits Made by Patients with Sickle Cell Disease. A Descriptive Study, 1999–2007. Am J Prev Med 2010;38:S536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zappia KJ, Garrison SR, Hillery CA, Stucky CL. Cold hypersensitivity increases with age in mice with sickle cell disease. Pain 2014;155:2476–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zappia KJ, Guo Y, Retherford D, Wandersee NJ, Stucky CL, Hillery CA. Characterization of a mouse model of sickle cell trait: parallels to human trait and a novel finding of cutaneous sensitization. Br J Haematol 2017;179:657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Zhang Z-J, Dong Y-L, Lu Y, Cao S, Zhao Z-Q, Gao Y-J. Chemokine CCL2 and its receptor CCR2 in the medullary dorsal horn are involved in trigeminal neuropathic pain. J Neuroinflammation 2012;9:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zhou Y, Tang H, Liu J, Dong J, Xiong H. Chemokine CCL2 modulation of neuronal excitability and synaptic transmission in rat hippocampal slices. J Neurochem 2011;116:406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.