LDL Receptor-related Protein-1 (LRP1) was cloned and identified as a probable apolipoprotein-E receptor by Herz et al.1 Strickland et al2 then reported that LRP1 functions as the receptor for the activated form of the proteinase inhibitor, α2-macroglobulin. Subsequent work by numerous laboratories defined the first novel paradigm regarding LRP1, which is its ability to function as an endocytic receptor for as many as one hundred diverse ligands, in each case, satisfying the criteria of receptor specificity and saturability. Rapid endocytosis and efficient LRP1 recycling allow transport of associated cargo to lysosomes for degradation; clearance of proteins from the cellular microenvironment was the first recognized pathway by which LRP1 may regulate cell biology and tissue physiology.3 Because LRP1 internalizes multiprotein complexes, which may include other membrane proteins active in cell-signaling, LRP1 regulates cell-signaling indirectly, by altering the composition of the plasma membrane proteome.4 LRP1 also regulates cell-signaling directly, by binding cell-signaling adapter and scaffold proteins after it is phosphorylated at specific residues in its cytoplasmic tail and by interaction with receptors such as the N-methyl-D-aspartate receptor, Trk receptors, and p75NTR.5–7 A second novel paradigm regarding the function of LRP1 is its ability to activate distinct and sometimes opposing signaling responses upon binding different ligands.8 This unique LRP1 activity may allow LRP1 to function as a radar system for the cell, sensing changes in the cellular microenvironment and directing context-appropriate cellular responses. LRP1 has been implicated in phagocytosis, efferocytosis, and antigen presentation.9–11 Finally, membrane-anchored LRP1 is a substrate for transmembrane proteinases that release a soluble form of the receptor, which may be biologically activity in immunity.12,13 These various activities of LRP1 are summarized in the accompanying Figure The challenge now is to understand how the activities of LRP1 are integrated at the cellular and tissue levels to regulate physiology and various forms of pathology.
Figure.
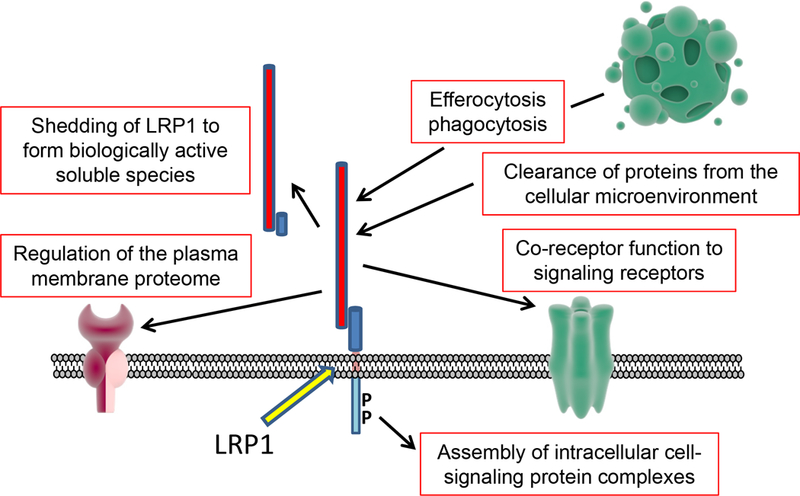
LRP1 regulates cell physiology by diverse mechanisms shown here. In vascular smooth muscle cells, LRP1 deficiency regulates expression of numerous proteins and causes changes in cell physiology that impact contractility.
In this issue of ATVB, Au et al.14 describe novel mechanisms by which LRP1 may regulate vascular smooth muscle contractility and the phenotype of vascular smooth muscle cells (VSMCs). The focus of this study is highly justified because of genome-wide association studies, which have identified single nucleotide polymorphisms in the LRP1 gene that are associated with abdominal aortic aneurysm development in humans.15,16 Mice in which LRP1 is deleted conditionally in VSMCs also develop aneurysms in the context of a diverse array of structural abnormalities in arteries.17,18
During development and in response to various cues in the cellular microenvironment, VSMCs undergo substantial changes in differentiation, gene expression, and phenotype.19 To measure contractility of LRP1-deficient and control VSMCs under isometric conditions, Au et al.14 performed aortic ring contraction assays using segments of descending thoracic aorta. Responses to a number of agonists were either attenuated or completely absent; however, calyculin A triggered increased responses in aortic rings from mice with LRP1-deficient VSMCs, arguing against a generalized loss of contractile machinery as the responsible mechanism. To explore this finding, the investigators undertook a discovery approach, using proteomics to identify over 200 proteins that are regulated when LRP1 is deficient in VSMCs. Many of the identified proteins express activities related to VSMC contractility and the function of the cytoskeleton. α2δ−1, a chaperone for voltage-gated Ca2+ channels, bound directly to LRP1, suggesting a novel mechanism by which LRP1 may regulate these channels. LRP1 deficiency in VSMCs also resulted in abnormal function of Ryanodine Receptors, which are instrumental in controlling calcium release from the sarcoplasmic reticulum.
A strength of this paper is the utilization of two distinct mouse model systems for deleting LRP1 in VSMCs. In the first model system, Cre recombinase is active during development whereas in the second, Cre recombinase is expressed as a fusion protein, which may be activated in adult mice treated with Tamoxifen. The combination of systems allowed the investigators to probe for genetic compensation following LRP1 deletion, which is particularly important given that there are receptors in the LDL Receptor gene family with overlapping activities.5 A second strength of this paper is replication of key results using aortic rings and primary cultures of VSMCs.
Looking forward, the study by Au et al,14 defining changes in VSMCs using LRP1 “loss of function” model systems, sets the stage for future work examining how various LRP1 ligands may regulate VSMC physiology. Is there specificity in the response of VSMCs to different LRP1 ligands, as has been defined in cells such as PC12 cells?8 How are the results of Au et al.14 relevant to the role of VSMCs in atherogenesis? Finally, and perhaps most importantly, is LRP1 a therapeutic target for vascular disease?
Acknowledgments
Source of Funding
This work was supported in part by grants R01 HL136395 and R01 NS097590 from the National Institutes of Health.
Footnotes
Disclosures
None
References
- 1.Herz J, Hamann U, Rogne S, Myklebost O, Gausepohl H, Stanley KK. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J. 1988;7:4119–4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strickland DK, Ashcom JD, Williams S, Burgess WH, Migliorini M, Argraves WS. Sequence identity between the alpha 2-macroglobulin receptor and low density lipoprotein receptor-related protein suggests that this molecule is a multifunctional receptor. J Biol Chem. 1990;265:17401–17404 [PubMed] [Google Scholar]
- 3.Pizzo SV. Serpin receptor 1: a hepatic receptor that mediates the clearance of antithrombin III-proteinase complexes. The American Journal of Medicine. 1989;87:10S–14S [DOI] [PubMed] [Google Scholar]
- 4.Gonias SL, Wu L, Salicioni AM. Low density lipoprotein receptor-related protein: Regulation of the plasma membrane proteome. Thromb Haemostas. 2004;91:1056–1064 [DOI] [PubMed] [Google Scholar]
- 5.Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108:779–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Geer P Phosphorylation of LRP1: regulation of transport and signal transduction. Trends in Cardiovascular Medicine. 2002;12:160–165 [DOI] [PubMed] [Google Scholar]
- 7.Gonias SL, Campana WM. LDL receptor-related protein-1: A regulator of inflammation in atherosclerosis, cancer, and injury to the nervous system. Amer J Pathol 2014;184:18–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantuano E, Lam MS, Gonias SL. LRP1 assembles unique co-receptor systems to initiate cell signaling in response to tissue-type plasminogen activator and myelin-associated glycoprotein. J Biol Chem. 2013;288:34009–34018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaultier A, Wu X, Le Moan N, Takimoto S, Mukandala G, Akassoglou K, Campana WM, Gonias SL. Low-density lipoprotein receptor-related protein 1 is an essential receptor for myelin phagocytosis. J Cell Sci. 2009;122:1155–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subramanian M, Hayes CD, Thome JJ, Thorp E, Matsushima GK, Herz J, Farber DL, Liu K, Lakshmana M, Tabas I. An AXL/LRP-1/RANBP9 complex mediates DC efferocytosis and antigen cross-presentation in vivo. J Clin Invest. 2014;124:1296–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Binder RJ, Srivastava PK. Essential role of CD91 in re-presentation of gp96-chaperoned peptides. Proc Natl Acad Sci U S A. 2004;101:6128–6133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quinn KA, Grimsley PG, Dai YP, Tapner M, Chesterman CN, Owensby DA. Soluble low density lipoprotein receptor-related protein (LRP) circulates in human plasma. J Biol Chem. 1997;272:23946–23951 [DOI] [PubMed] [Google Scholar]
- 13.Gorovoy M, Gaultier A, Campana WM, Firestein GS, Gonias SL. Inflammatory mediators promote production of shed LRP1/CD91, which regulates cell signaling and cytokine expression by macrophages. J Leukocyte Biol. 2010;88:769–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Au D, Hernadez-Ochoa O, Fondrie, Hampton B, Migliorini M, Galisteo R, Schneider M, Daugherty A, Rateri D, Strickland D, Muratoglu S. LRP1 regulates smooth muscle contractility by modulating Ca2+signaling and expression of cytoskeletal related proteins. in press (this issue of ATVB) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bown MJ, Jones GT, Harrison SC, Wright BJ, Bumpstead S, Baas AF, Gretarsdottir S, Badger SA, Bradley DT, Burnand K, Child AH, Clough RE, Cockerill G, Hafez H, Scott DJ, Futers S, Johnson A, Sohrabi S, Smith A, Thompson MM, van Bockxmeer FM, Waltham M, Matthiasson SE, Thorleifsson G, Thorsteinsdottir U, Blankensteijn JD, Teijink JA, Wijmenga C, de Graaf J, Kiemeney LA, Assimes TL, McPherson R, Consortium CA, Global BC, Consortium D, Consortium V, Folkersen L, Franco-Cereceda A, Palmen J, Smith AJ, Sylvius N, Wild JB, Refstrup M, Edkins S, Gwilliam R, Hunt SE, Potter S, Lindholt JS, Frikke-Schmidt R, Tybjaerg-Hansen A, Hughes AE, Golledge J, Norman PE, van Rij A, Powell JT, Eriksson P, Stefansson K, Thompson JR, Humphries SE, Sayers RD, Deloukas P, Samani NJ. Abdominal aortic aneurysm is associated with a variant in low-density lipoprotein receptor-related protein 1. American Journal of Human Genetics. 2011;89:619–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradley DT, Badger SA, McFarland M, Hughes AE. Abdominal aortic aneurysm genetic associations: Mostly false? A systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2016;51:64–75 [DOI] [PubMed] [Google Scholar]
- 17.Boucher P, Gotthardt M, Li WP, Anderson RG, Herz J. LRP: Role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–332 [DOI] [PubMed] [Google Scholar]
- 18.Muratoglu SC, Belgrave S, Hampton B, Migliorini M, Coksaygan T, Chen L, Mikhailenko I, Strickland DK. LRP1 protects the vasculature by regulating levels of connective tissue growth factor and HtrA1. Arteriosclerosis, Thrombosis, and Vascular Biology. 2013;33:2137–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801 [DOI] [PubMed] [Google Scholar]


