Summary
Ichthyosis vulgaris is caused by loss-of-function mutations in the filaggrin gene (FLG) and is characterized clinically by xerosis, scaling, keratosis pilaris, palmar and plantar hyperlinearity, and a strong association with atopic disorders. According to the published studies presented in this review article, FLG mutations are observed in approximately 7.7% of Europeans and 3.0% of Asians, but appear to be infrequent in darker-skinned populations. This clinical review article provides an overview of ichthyosis vulgaris epidemiology, related disorders and pathomechanisms. Not only does ichthyosis vulgaris possess a wide clinical spectrum, recent studies suggest that carriers of FLG mutations may have a generally altered risk of developing common diseases, even beyond atopic disorders. Mechanistic studies have shown increased penetration of allergens and chemicals in filaggrin-deficient skin, and epidemiological studies have found higher levels of hand eczema, irritant contact dermatitis, nickel sensitization and serum vitamin D levels. When relevant, individuals should be informed about an increased risk of developing dermatitis when repeatedly or continuously exposed to nickel or irritants. Moreover, with our current knowledge, individuals with ichthyosis vulgaris should be protected against neonatal exposure to cats to prevent atopic dermatitis and should abstain from smoking to prevent asthma. Finally, they should be advised against excessive exposure to factors that decrease skin barrier functions and increase the risk of atopic dermatitis.
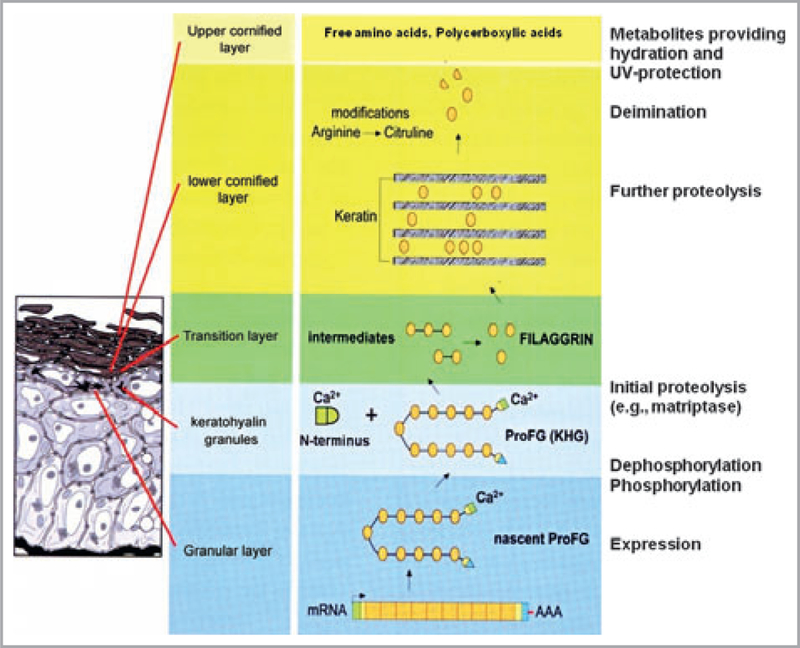
Ichthyosis vulgaris (IV; OMIM 146700) is characterized clinically by xerosis, hyperkeratosis, excess scaling, keratosis pilaris, palmar and plantar hyperlinearity, and a strong association with atopic disorders.1–3 The pathogenesis of IV was long linked to a decrease in the size and number, or even a complete absence of epidermal (F-type) keratohyaline granules.4–6 These structures contain large profilaggrin molecules that are cleaved into 10–12 filaggrin proteins during terminal epidermal differentiation, ensuring proper aggregation of keratin filaments in the cytosol as well as generation of much of the skin’s ‘natural moisturizing factor’ (NMF) (Fig. 1).2,7 Successful genotyping of the filaggrin gene (FLG)8 showed that loss-of-function mutations are surprisingly common in Europeans and Asians.9,10 Details about the genomics and the cellular features of filaggrin, as well as the wealth of implications of this discovery for atopic disorders, were recently reviewed,9 but the focus on atopic dermatitis (AD) potentially obscures the importance of filaggrin deficiency for IV. Here, we provide a clinical update of IV, as we believe that there is a need to alert practising physicians about its wide clinical spectrum. Even in the absence of AD, the skin of individuals with IV differs fundamentally from normal skin, which may alter the propensity to develop a host of disorders beyond the atopic ones.
Fig 1.
Processing of profilaggrin during terminal differentiation. Profilaggrin is synthesized and phosphorylated in the granular layer, and stored in keratohyaline granules. At the granular to cornified cell transition, profilaggrin is dephosphorylated and cleaved by proteases to filaggrin. The N-terminus is cleaved from profilaggrin and associates with other proteins in the cytoplasm and nucleus. Filaggrin aggregates keratin filaments in cornified cells (macrofibrils) that are retained in cornified cells. Filaggrin is then graded by proteases including caspase-14. The resulting free amino acids and their deinimated products carry out various functions in the cornified cells including hydration and ultraviolet (UV) photoprotection. UCA, trans-urocanic acid. Reproduced and modified with permission of the author and Elsevier.7 This article was published in Dermatologica Sinica, vol. 27, Presland RB, Function of filaggrin and caspase-14 in formation and maintenance of the epithelial barrier function, pp. 1–14. Copyright © The Taiwanese Dermatological Association, 2009.
Search strategy and selection criteria
An online literature search using PubMed ⁄Medline was performed from December 2011 to February 2012, using the following search terms: ichthyosis vulgaris, filaggrin, atopic disease, atopy, atopic dermatitis, asthma, sensitization, rhinitis, food Allergy, skin barrier, epidemiology, clinical, genetic, histology, complication, and risk factors. The prevalence of FLG mutations in the general population was reviewed in Asians, southern Europeans and dark-pigmented individuals when estimates on at least 50 individuals in a population were available. Studies on lightly pigmented, primarily northern and central European caucasian individuals, were included only when data on at least 200 individuals were found. Studies on prevalence estimates from selected atopic populations were excluded, as were studies where the combined FLG mutation carrier frequency could not be retrieved. Control populations consisting of hypernormal controls, that is individuals who were selected to rule out atopic disease, were excluded except for one African population.11 Carriers of compound heterozygous mutations were included within the homozygous carrier category throughout this article.
Ichthyosis vulgaris is defined by mutations in FLG
Genetic linkage analyses on IV families mapped FLG to the epidermal differentiation complex on chromosome 1q21.12,13 More recently, genotyping showed that loss-of-function mutations in the FLG gene are the cause of IV,8 and that the condition is inherited in a semidominant manner with 83–96% penetrance.14–16 Mutations result in a truncated profilaggrin protein, which cannot be processed into functional filaggrin subunits.17 It still remains possible that mutations in related genes could result in truncated filaggrin proteins.11,18
FLG mutations cause IV in both caucasian and Asian populations,15,16,19–25 but they tend to be population specific with different and sometimes mutually exclusive mutations between these groups.9 Even within European populations, there are regional differences. While the R501X and 2282del4 mutations account for about 80% of mutations in northern European descendants, they are much less common in southern European descendants.26–29 Heterozygous advantage is considered when carriers of one mutation possess a survival advantage over homozygous recessive and ⁄or homozygous dominant genotypes. As there seems to be a latitude-dependent prevalence gradient across Europe, FLG mutations may offer higher survival rates (see below). The Chinese-Singaporean population’s eight different mutations account for about 80%.30 Also, S2554X and 3321delA mutations are very prevalent in the Japanese, but less frequent in Koreans.31 The prevalence of IV in darkly pigmented populations appears to be low,10,11 but more studies are required to confirm these observations. The prevalence estimates shown in Tables 1–3 could potentially underestimate the true prevalence of IV in these populations, because FLG mutations specific to Europeans were initially used to identify mutation carrier frequencies in Asians, Africans and southern Europeans. However, the median prevalence of FLG mutations among Europeans and Asians was 7·7% (range 2·7–14·2) and 3·0% (range 0–7·3), respectively.
Table 1.
The prevalence of loss-of-function mutations in the filaggrin gene (FLG) in European populations
| Study (first author) | Country of origin | Mutations genotyped | ntotal (controls) | Mutation carrier prevalence (%) | ntotal (individuals with AD) | Mutation carrier prevalence (%) |
|---|---|---|---|---|---|---|
| Gruber111 | Austria | R501X, 2282del4 | 110 (3) | 2·7 | – | – |
| Thyssen48 | Denmark | R501X, 2282del4 | 3335 (115) | 8·1 | – | – |
| Thyssen44 | Denmark | R501X, 2282del4 | 2500 (189) | 7·6 | – | – |
| Thyssen65 | Denmark | R501X, 2282del4 | 730 (58) | 7·9 | – | – |
| Mlitz27 | France | R501X, 2282del4, R2447X | 99 (4) | 4·0 | 97 (10) | 10·3 |
| Betz96 | Germany | R501X, 2282del4 | 449 (36) | 8·0 | 145 (22) | 15·2 |
| Marenholz83 | Germany | R501X, 2282del4, R2447X | 871 (82) | 9·4 | – | – |
| Greisenegger112 | Germany⁄Austria | R501X, 2282del4, R2447X, S3247X | 402 (31) | 7·7 | 462 (106) | 22·9 |
| Stemmler75 | Germany | R501X, 2282del4 | 667 (27)a | 9·6 | 374 (59) | 15·8 |
| Weidinger82 | Germany | R501X, 2282del4, R2447X, S3247X, 3702delG, | 2864 (221) | 7·7 | – | – |
| Cramer113 | Germany | R501X, 2282del4 | 2867 (179) | 6·2 | – | – |
| Oji15 | Germany | R501X, 2282del4 | 752 (33) | 4·6 | – | – |
| Weichenthal93 | Germany | R501X, 2282del4 | 276 (21) | 7·6 | – | – |
| Hüffmeier91 | Germany | R501X, 2282del4 | 376 (33) | 3·8 | – | – |
| Stemmler75 | Germany | R501X, 2282del4 | 324 (31) | 9·6 | 401 (62) | 15·4 |
| Novak63 | Germany | R501X, 2282del4 | 1468 (114) | 7·5 | – | – |
| Zhao94 | Ireland ⁄U.K. | R501X, 2282del4 | 2117 (170) | 8·0 | – | – |
| Palmer10 | Ireland | R501X, 2282del4 | 186 (16) | 8·6 | 52 (29) | 55·8 |
| Sandilands17 | Ireland | R501X, 2282del4, R2447X, S3247X, 3702delG | 736 (56) | 7·6 | 188 (85) | 45·2 |
| Cascella29 | Italy | R3638X | 201 (9) | 4·0 | 220 (7) | 3·0 |
| De Jongh102 | Netherlands | R501X, 2282del4 | 217 (16) | 7·4 | – | – |
| Poninska85 | Poland | R501X, 2282del4 | 510 (24) | 4·8 | – | – |
| Winge95 | Sweden | R501X, S2282del4, R2447X, S3247X | 341 (18) | 5·7 | – | – |
| Palmer10 | U.K. | R501X, 2282del4 | 1008 (94) | 9·3 | – | – |
| Barker74 | U.K. | R501X, 2282del4 | 1334 (129) | 8·8 | 163 (69)b | 42·0 |
| Brown78 | U.K. | R501X, 2282del4, R2447X, S3247X, 3702delG, 3673delC | 747 (86) | 11·5 | 184 (84)c | 40·2 |
| Brown55 | U.K. | R501X, 2282del4, R2447X, S3247X, 3702delG | 789 (112) | 14·2 | 120 | 18·1 |
| Rice64 | U.K. | R501X, 2282del4 | 5289 (477) | 9·0 | – | – |
| Henderson53 | U.K. | R501X, 2282del4 | 6971 (610) | 8·8 | – | – |
| Van Limbergen114 | U.K. | R501X, 2282del4 | 944 (103) | 10·9 | – | – |
| Total | 39 480 (3097) | 7·8 |
AD, atopic dermatitis.
A subgroup of 265 had been screened not to have AD
childhood onset and persistent AD
early childhood onset of AD.
Table 3.
The prevalence of loss-of-function mutations in the filaggrin gene (FLG) in black-skinned and American populations
| Study (first author) |
Country of origin | Mutations genotyped | ntotal (controls) | Mutation carrier prevalence (%) | ntotal (individuals with AD) | Mutation carrier prevalence (%) |
|---|---|---|---|---|---|---|
| Gao98 | African American | R501X, 2282del4 | 152 (2) | 1·3 | 187 (12) | 6·4 |
| African American | R501X | 177 (1) | 0·5 | – | – | |
| European American | R501X, 2282del4 | 156 (9) | 5·8 | 276 (77) | 27·9 | |
| Palmer10 | North Africa | R501X, 2282del4 | 124 (0) | 0 | – | – |
| Brown81 | Canada | R501X, 2282del4, R2447X, S3247X | 891 (98) | 11·0 | – | – |
| Winge11 | Ethiopia | R501X, 2282del4, R2447X, S3247X | 103 (0)a | 0 | 110 (0) | 0 |
AD, atopic dermatitis.
Individuals without past or present history of AD, dry skin or atopic manifestations.
Filaggrin’s role in the skin barrier
Normal skin
The cornified envelope (CE) is generated in the outermost part of the epidermis, where it forms a rigid structure that surrounds corneocytes, providing mechanical resistance against offending physical, chemical and microbial agents.32 The formation of the CE begins in the stratum granulosum, as involucrin becomes crosslinked beneath the plasma membrane as the keratinocytes differentiate. In parallel, keratohyaline granules, which define the granular layer composite, consist primarily of 400-kDa profilaggrin polymers,4–6 which are proteolytically cleaved and dephosphorylated into 10–12 filaggrin monomers (Fig. 1).2,7 These liberated filaggrin proteins then aggregate keratin filaments into tight bundles, resulting in collapse and flattening of corneocytes. Some of the filaggrin monomers also attach to the CE, and additional, structural proteins, including small proline-rich proteins, loricrin and trichohyalin, which, like filaggrin, are synthesized late in epidermal maturation, are also crosslinked to the CE by transglutaminases, further strengthening the structure. A monolayer of -OH ceramides is then covalently bound to the external face of the CE, forming a scaffold upon which the lipids deposited in the intercellular domain by lamellar body secretion are organized from lamellar bilayers. The final result is a multilayered structure of protein-enriched corneocytes (the ‘bricks’) surrounded by hydrophobic lipids (the ‘mortar’).
Proteolysis of filaggrin proteins is a key element for skin homeostasis. Filaggrin proteins are normally fully degraded into their constituent amino acids, including glutamine, arginine and histidine and then further hydrolysed into acidic, polycarboxylic acid osmolytes that maintain stratum corneum hydration (the so-called NMFs). This sequence of proteolysis followed by deimination occurs as environmental humidity declines below 80%, and accelerates as the humidity continues to decline,33 thereby helping to maintain skin hydration even at low ambient humidity. Histidine is also a substrate for histidase, which generates trans-urocanic acid (UCA), a major ultraviolet (UV) B-absorbing epidermal chromophore. trans-UCA is photoisomerized to cis-UCA with UVB exposure; the latter may produce oxidative DNA damage and initiate translation of genes associated with apoptosis and immunosuppression.34
Consequences of filaggrin deficiency in ichthyosis vulgaris
FLG mutations cause cytoskeletal disorganization, resulting in altered cargo loading in lamellar bodies and disorganized lamellar bilayers, as well as impaired lamellar bilayer maturation.35,36 As a result, the quantities and distribution of lipids in the stratum corneum interstices are indirectly affected by FLG mutations.36,37 The inherent reduction of filaggrin metabolites seen in patients with IV reduce the levels of NMFs, causing not only a gene dose-dependent reduction in skin hydration, but also an elevated skin surface pH, and increased transepidermal water loss (TEWL), which are all features of the xerotic skin in IV. However, it should be emphasized that TEWL and skin pH were only significantly elevated in patients with IV with double allele mutations and not in patients with single allele mutations when compared with nonmutation carriers, perhaps due to power limitations.35 The loss of filaggrin also reduces the ability of the squames to remain hydrated as they move up through the stratum corneum, resulting in excessive scale.38 Abnormal barrier function in IV drives compensatory repair mechanisms that include epidermal hyperplasia, resulting in hyperkeratosis. A synergetic effect of mutations in FLG and the steroid sulfatase gene leading to more severe ichthyosis has been reported in patients with IV and X-linked ichthyosis.39 Because of the lower levels of filaggrin proteins, individuals with IV have reduced epidermal chromophore UCA levels,27,40,41 and knockdown of filaggrin increased UV sensitivity markedly in vitro.42 This could potentially explain the allegedly higher prevalence of non-melanoma skin cancer in individuals with AD.43 Moreover, five general population studies showed that FLG mutation carriers have 10% higher mean serum vitamin D levels than controls,44 a finding that also could be explained by lower UCA levels (Table 4). The suggested heterozygous advantage of FLG mutations could be the higher serum vitamin D levels resulting in enhanced survival rates due to protection against rickets and infections.
Table 4.
The association between loss-of-function mutations in the filaggrin gene (FLG) and serum vitamin D concentrations in five cohorts from the general population in Denmark and Germany was strong and positive, probably due to lower levels of the ultraviolet B photoreceptor urocanic acid in mutation carriers, a metabolite of the filaggrin molecules44
| Cohort | Age of participants (years) | n | Proportion with FLG mutations (%) | Effect of FLG mutations on serum vitamin D levels (%)a |
|---|---|---|---|---|
| COPSAC | 4 | 277 | 11·9 | 2·21 |
| GINI ⁄LISA | 10 | 1238 | 6·6 | 13·20 |
| Health2006 | 18–69 | 3112 | 8·1 | 12·16 |
| Monica | 40–70 | 2500 | 7·6 | 7·20 |
| KORA F4 | 32–81 | 2823 | 7·9 | 11·37 |
| Pooled difference | 10 10 (95% CI 6 70–13 60) |
CI, confidence interval.
Adjusted for gender, age, season of vitamin D testing, atopic dermatitis, body mass index and supplementary vitamin D intake.
Increased penetration of chemicals and allergens
Increased permeation of chemicals and allergens occurs across filaggrin-deficient skin35,45,46 explaining the increased risk of sensitization to aeroallergens and haptens in IV.47,48 Phthalates are immunomodulatory chemicals that are used in personal care products, perfumes and plastics.49 Up to 40% higher urinary excretion of phthalates has been observed in FLG mutation carriers (manuscript in preparation). Yet it is unknown, whether this may affect endocrine reproductive functions.
Gene–environment interactions for the development of atopic disorders
Certain environmental risk factors should be avoided in individuals with IV to prevent the development of atopic disorders. There is convincing evidence that cat, but not dog, ownership strongly increases the risk of AD in newborns and children with FLG mutations.50,51 Moreover, a significant interaction between FLG mutations and tobacco smoking has been shown to favour the development of asthma, including decreased respiratory function.52 However, no association was found between passive smoking and the development of AD in British children.53 While there are indications that FLG mutation carriers might have become more susceptible to environmental changes, accounting for the rise in AD prevalence,54 it is important to emphasize that even homozygous FLG mutation carriers, with a much higher risk of AD than heterozygous carriers, may still not develop dermatitis and that long-term remissions of AD are indeed possible.55–57 Also, only a subset of patients with AD has mutations in FLG. No clinical studies have yet investigated the burden of known environmental skin exposures, including exposure to dust mites, low humidity, excessive use of soaps, or other factors known to exacerbate barrier dysfunction thereby potentially increasing the risk of inflammation.
Clinical features of ichthyosis vulgaris
Xerosis, scaling and skin fissures (chapping)
The clinical onset of IV typically occurs within the first years of life.58–60 Individuals intermittently or persistently suffer from xerosis, which manifests itself as fine (powdery) and sometimes even coarse (polygonal) scaling of the extensor surfaces of the extremities, the scalp, central part of the face and the trunk (Fig. 2).61 The extensor surfaces of the lower limbs are more often affected in adults than in children,58 while the more hydrated axillae, antecubital and popliteal fossae are rarely involved.59 Scales are typically centrally adherent with loose edges and are smaller in children than in adults.58 When overlying the shins, they can be darker and thicker and may have a mosaic pattern. Finer scales can be greyish, silvery and glossy.59 Scales in the scalp may resemble dandruff clinically in adult patients, but IV is not known to predispose to fungal infections. It comes as no surprise that patients with IV report xerosis and apply body moisturizers significantly more often than nonmutation carriers.62–64 Chapping, defined as painful fissures of the hands, fingers and heels, a feature that is strongly influenced by environmental humidity, was found in 76% of British school children with IV,59 and was associated with FLG mutations in patients with AD, and in adults from the general population.65,66 Finally, it has been claimed that hypohidrosis and heat intolerance, as observed in lamellar ichthyosis, is an often neglected feature of moderate-to-severe IV.15 It is not clear whether this is related to AD.
Fig 2.
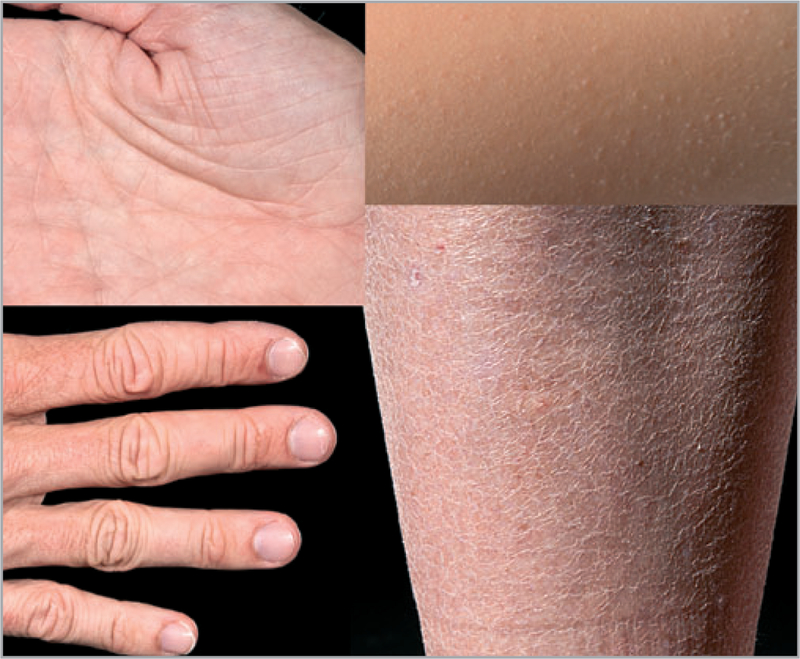
Ichthyosis vulgaris clinical characteristics. Upper left, palmar hyperlinearity; upper right, keratosis pilaris; lower left, hyperkeratosis on the dorsal aspects of the fingers; lower right, xerosis and scaling.
Palmar hyperlinearity and keratosis pilaris
Palmar and plantar hyperlinearity, defined as exaggerated skin markings (dermatoglyphics), and keratosis pilaris, defined by keratotic elevations around hair follicle orifices, are frequently observed in individuals with IV (Fig. 2). Keratosis pilaris was noted in 100%, 66% and 30%, respectively, of homozygous, heterozygous and wild-type juvenile FLG mutation carriers.55 The positive and negative predictive values (PPV and NPV) for keratosis pilaris were 53% and 90%, respectively, whereas the PPV and NPV for palmar hyperlinearity were 71% and 90%, respectively.55
Phenotypic variability in ichthyosis vulgaris
In interpreting these data, recall that latitude-dependent changes in environmental humidity will alter skin hydration, potentially affecting phenotype appearance and prevalence. Hence, low humidity is expected to amplify disease, presumably due to accelerated proteolysis of residual filaggrin, resulting in an increased prevalence of certain clinical features, such as keratosis pilaris and palmar hyperlinearity, whereas high humidity will lower IV prevalence, as seen in Singaporean Chinese patients.24 Although both of these phenotypic characteristics are strongly associated with IV, these features can also occur in individuals with normal skin as they are driven by ambient low humidity (Tables 5 and 6).67 It is acknowledged that many factors other than ambient humidity may influence the phenotypic variability.
Table 5.
Post-genetic era studies that have investigated the prevalence of keratosis pilaris and palmar hyperlinearity. The list may not be exhaustive
| Study (first author) | Population | Individuals in the study who were FLG mutation carriers vs. wild-type carriers | Keratosis pilaris, n (%) |
Palmar hyperlinearity, n (%) |
||
|---|---|---|---|---|---|---|
| FLG carrier | Wild type | FLG carrier | Wild type | |||
| Cai97 | Patients with AD | 56 vs. 172 | 5 (8·9) | 11 (6·4) | 42 (75·0) | 60 (34·9) |
| Sergeant62 | Patients referred with ‘discrete skin lesions’ | 32 vs. 252 | 8 (25·0) | 34 (14·0) | 8 (25·0) | 25 (11·0) |
| Brown55 | School children | 107 vs. 671 | 60 (56·0) | 201 (30·0) | 82 (76·6) | 84 (12·5) |
AD, atopic dermatitis.
Table 6.
Pregenetic studies that have investigated the prevalence of keratosis pilaris and palmar hyperlinearity. The list may not be exhaustive
| Study (first author) | Population | Individuals in the study with IV vs. controls | Keratosis pilaris, n (%) |
Palmar hyperlinearity, n (%) |
||
|---|---|---|---|---|---|---|
| Patients with IV | Controls | Patients with IV | Controls | |||
| Mevorah121 | Patients with IV and controls without concomitant AD | 35 ⁄247 | 26 (74·3) | 103 (42) | 28 (80) | 20 (8) |
| Wells59 | School children | 169 ⁄0 | 57 (34) | – | 121 (72) | – |
| Chen24 | Patients | 8 ⁄– | – | – | 8 (100) | – |
| Oji15 | Patients | 26 ⁄– | – | – | 25 (96·2) | – |
| Winge11 | Patients | 7 ⁄103 | 7 (100) | 0 | 7 (100) | 0 |
| Uehara122 | Patients with AD | 66 ⁄112 | – | – | 45 (68) | 4 (3·5) |
| Kuokkanen60 | Patients with IV | 21 ⁄– | 12 (57) | – | 12 (57) | – |
AD, atopic dermatitis; IV, ichthyosis vulgaris.
As homozygous FLG mutation carriers have complete absence of filaggrin expression, they usually display a stable skin phenotype with chronic presence of the features of IV. Heterozygous carriers display a milder phenotype, which can be masked by the effects of high humidity or application of skin-care products that restore and maintain skin hydration. It is estimated that approximately 30% of patients with IV who consult a physician for their skin condition are heterozygous carriers and 70% homozygous.8,68 According to Wells,1 ‘some female patients take care of themselves so well that it may be impossible to see that they are affected’. Skin symptoms improve as environmental humidity increases; in fact, 80% of patients with IV report improvement during the summer.58 Accordingly, IV is typically present, and more severe, during the winter in temperate climates when a drop in humidity may result in further hydrolysis of residual filaggrin (in heterozygous carriers) into its constituent amino acids and their deiminated carboxylic acid derivatives. Overall, very few individuals with IV consult a physician for their skin condition, but the phenotype is not only highly prevalent, but also dynamic, and aggravated by a decline in environmental humidity.
Diagnosis and micropathology
Detection of IV, for the purpose of preventing complications of filaggrin deficiency, including atopic diseases, should be considered as a part of the routine medical work-up of paediatric patients and also in older patients who present with dermatitis. A family history should then be obtained together with inspection of the skin, including the palms and soles. IV can be diagnosed provisionally based on clinical presentation and family history, and confirmed if appropriate by genetic testing or histological examination. Light microscopy, immunofluorescence and electron microscopy (EM) are all useful, and EM may be used to distinguish between heterozygous and homozygous carriers.15,69 Light microscopy typically shows a stratum corneum that is moderately orthohyperkeratotic, but it can also display a basket-weave pattern, or it can be compact or laminated.58 The stratum corneum is thicker than normal, but thin in comparison with other hyperkeratotic disorders, such as psoriasis or lamellar ichthyosis, and epidermal proliferation rates are significantly lower.70 The relatively low mitotic count compared with other ichthyoses, together with relatively modest scaling, may contribute to the thicker stratum corneum observed in individuals with IV. The granular cell layer is either completely absent or strongly reduced. Under EM, keratohyaline granules may be ‘crumbly’ or absent and upon close examination, perinuclear keratin retractions can be seen in granular cells, probably because FLG mutations confer alterations in keratin intermediate filament organization that result in a distinctive cytoskeletal abnormality.35
Progression of ichthyosis vulgaris to atopic disorders
A recent study showed that about half of Irish patients with AD were FLG mutation carriers.10 These findings were subsequently confirmed in numerous studies and in large meta-analyses.71,72 Double-allele FLG mutations in AD are characterized by: (i) early onset;73–77 (ii) disease persistence;64,74,78 (iii) a severe course;73 and (iv) concomitant aeroallergen sensitization.47,73,79 Filaggrin-deficient mice, with completely absent filaggrin synthesis corresponding to homozygous carrier status, show increased penetration of allergens, which is followed by the development of antigen-specific antibodies.46 Moreover, they develop AD after exposure to lower doses of hapten than wild-type mice.45 FLG mutations do not appear to be associated with atopic features such as Dennie-Morgan lines (infraorbital fold), Herthoge’s sign, orbital darkening or white dermographism.63,80 Finally, there is a wide array of epidemiological data supporting both an increased risk and severity of asthma, rhinitis and food allergies with FLG mutations.10,53,71,77,81–86 Probably, the inherent barrier abnormality caused by FLG mutations is the main factor that allows continuous penetration of allergens, resulting in a gradual conversion to a T helper 2 immunophenotype.
Ichthyosis vulgaris and other disorders
Although AD and psoriasis susceptibility loci are located on chromosome 1q21,87,88 no association has been found between FLG mutation and psoriasis, at least in caucasians89–95 (Table 7). Moreover, no association was shown with acne vulgaris.62 FLG mutations in AD have been positively associated with other skin diseases, including alopecia areata,96 recurrent skin infections,97 eczema herpeticum,98 early onset and persistence of hand eczema, and contact Allergy to topical products.99 A decreased threshold for the development of allergic and irritant contact dermatitis was observed in mice,45 and FLG mutations are associated with acute and chronic irritant contact dermatitis in patients from tertiary clinics,100,101 but not with allergic contact dermatitis (except nickel).102,103 However, there are indications that FLG mutations increase the risk of contact sensitization as much as fivefold in individuals with dermatitis emphasizing that recruitment of immune cells is crucial for sensitization.104 Notably, the barrier abnormality due to filaggrin deficiency is probably a key predisposing factor for all these disorders. Yet, these epidemiological studies need to be carefully interpreted, as FLG mutation carriers may display avoidance behaviour that could introduce bias into the analyses. For example, it is likely that FLG mutation carriers routinely avoid wet work because of their impaired skin barrier. Hand eczema in FLG mutation carriers displays a distinct phenotype characterized by its dorsal localization, palmar hyperlinearity and skin fissures.105,106 FLG mutations have been associated with nickel sensitization, although the correlation was restricted to individuals without ear piercings in one of the studies.48,63 The onset of allergic nickel dermatitis was significantly associated with early age in FLG mutation carriers.107 Finally, two independent cohorts suggest that FLG mutation carriers display a higher risk of developing type 2 diabetes, perhaps due to frequent application of topical corticosteroids, resulting in secondary diabetes.108
Table 7.
Disorders that have, and have not, been associated with ichthyosis vulgaris defined by filaggrin gene (FLG) loss-of-function mutations
| Disorders that have been associated with FLG mutations |
| Atopic disorders |
| Atopic dermatitis (and an elevated risk following neonate cat exposure)10,50,51,71 |
| Asthma (and elevated risk following tobacco smoking)52,71,72,84,85 |
| Allergic rhinitis51,71,82 |
| Food allergies81 |
| Sensitization to aeroallergens82 |
| Skin disorders |
| Staphylococcus aureus infection in atopic dermatitis97 |
| Herpes simplex infection in atopic dermatitis (eczema herpeticum)98 |
| Severe course of alopecia areata in individuals with atopic disease96 |
| Nickel sensitization and allergic nickel dermatitis48,63,99,107 Hand eczema99,100,102,105 |
| Other |
| Diabetes type 2108 |
| Disorders that have not been associated with FLG mutations |
| Skin disorders |
| Acne vulgaris62,118 |
| Psoriasis vulgaris28,89–95 |
| Other |
| Psoriatic arthritis91 |
| Rheumatoid arthritis91 |
| Inflammatory bowel disease114,123 |
| Keratoconus26 |
| Sarcoidosis123 |
| Impaired hearing in childhood124 |
Prevention and rational therapy
Therapeutic considerations for patients with IV have been reviewed recently.68,109 A primary objective is to remove excess scales and to treat xerosis without causing irritation. Repeated daily application of emollients containing either lactic or glycolic acid (5–15%), propylene glycol (10–25%) and ⁄or urea (2–10%) have been recommended.109 However, for the majority of individuals with IV, application of emollients with a high-lipid content, but without skin sensitizers should suffice. Emollient therapy showed a promising effect in reducing AD in predisposed individuals,110 but these studies need to be confirmed by further controlled studies. Occupational advice about avoidance of professions involving wet work or excessive metal and contact irritant exposure should be considered. Cats in the household, as well as tobacco smoking should be discouraged.50,52 Currently, attempts are being made to identify compounds that can upregulate filaggrin expression in FLG heterozygous individuals and to modify protein translation so that nonsense mutations can be bypassed.9
Table 2.
The prevalence of loss-of-function mutations in the filaggrin gene (FLG) in Asian populations
| Study (first author) | Country of origin | Mutations genotyped | ntotal (controls) | Mutation carrier prevalence (%) | ntotal (individuals with AD) | Mutation carrier prevalence (%) |
|---|---|---|---|---|---|---|
| Li80 | China | 478insA, Q1070X, 4026delT, Q1712X, Q2397X, 7145del4 and 8001del4, 3222del4, 3321delA, 4271delAA, S1515X, Q1790X, 5757del4, 6834del5, Q2417X, E2422X, 7945delA and K4671X | 301 (12) | 4·0 | 339 (88) | 26·0 |
| Ma115 | China | E2422X, Q2417X, S2554X, S2889X, S3296X, R4307X, 3321delA, 7945delA | 169 (11) | 6·5 | 160 (24) | 15·0 |
| Chen24 | China | 441delA, 1249insG, 7945delA, Q2417X, R4307X, E2422X | 160 (1) | 1·0 | – | – |
| Zhang77 | China (Han) | R826X, 3222del4, R1140X, 4271delAA, Q1790X, 5757del4, 6834del5, 6950del8, S2706X, K4671X, 441delA, R501X, 3321delA, R1474X, Q2417X, E2422X, 7945delA, R4306X | 92 (0) | 0 | 261 (82) | 31·4 |
| Li84 | China | 2231delA, 3222del4, S1302X, Q2397X, K4671X | 301 (12) | 4·0 | – | – |
| Nomura23 | Japan | R501X, 2282del4, 3702delG, S2554X, 3321delA | 156 (0) | 0 | 143 (8) | 5·6 |
| Nomura116 | Japan | S2554X, 3321delA, S2889X, S3296X | 133 (4) | 1·5 | 102 (21) | 11·1 |
| Nomura,20 | Japan | R501X, S2554X, 2889X, S3296X, 3321delA, 1695X, Q1701X, Lys4021X | 134 (5) | 3·8 | 137 (37) | 27·0 |
| Nemoto-Hasebe117 | ||||||
| Imoto79 | Japan | 3321delA, S2554X, S2889X, S3296X | 1499 (98) | 6·5 | – | – |
| Lee31 | Korea | S2554X, 3321delA | 133 (2) | 1·5 | 42 (1) | 2·4 |
| Chen24 | Singapore | 441delA, 1249insG, 7945delA, Q2417X, R4307X, E2422X | 100 (0) | 0 | – | – |
| Common118 | Singapore | p.S406X, c.1249insG, c.2284del4, c.3321delA, p.S1302X, p.S15 15X, c.6950del8, p.Q2417X, p.E2422X, c.7945delA, p.S2706X, p.R4307X, c.6834del5 c.8157delC | 434 (32) | 7·3 | – | – |
| Zhang22 | China | 3321delAa | 100 (3) | 3·0 | – | – |
| Ching119 | China | R501X, 2282del4, R2447X, S2554X, S2889X 3321delA | 191 (0) | 0 | 174 (4) | 2·3 |
| Chen57 | Singapore | 441delA, G323X, Q368X, S406X, 1249insG, R501X, 3321delA, S1302X, 4275del2, S1515X, Q1745X, 6950_6957del8, Q2417X, E2422X, 7945delA, S2706X, R4307XS1302X, 4275del2, S1515X, Q1745X, 6950_6957del8, Q2417X, E2422X, 7945delA, S2706X, R4307X | 433 (30) | 6·9 | 390 (83) | 21·3 |
| Wang120 | Taiwan | T454A, P478S, E498D, H519N, R3270C, Q3322Q, R501X, 2282del4, S3247X, S3296X, S2554X, S2889X, 3321delA, E1795X, E2422X, Q2417X, P478S | 212 (8) | 3·8 | 212 (17) | 14·7 |
| Total | 4336 (218) | 5·0 |
AD, atopic dermatitis.
3321del A was the only FLG mutations that was found among 27 mutations used for screening. Please refer to text for complete list.
What’s already know about this topic?
Ichthyosis vulgaris is a common disorder characterized clinically by xerosis, excess scaling, hyperkeratosis, keratosis pilaris, and palmar and plantar hyperlinearity, as well as a strong association with atopic disorders.
What does this study add?
This review updates the reader on the broader perspective of ichthyosis vulgaris based on recent findings and suggests that clinicians, when appropriate, should warn individuals with ichthyosis vulgaris against environmental exposures, for example nickel, irritants, cats in the household and smoking, as these may cause secondary disease.
Acknowledgments
Funding sources
J.P.T. was funded by an unrestricted grant from the LEO Pharma Research Foundation as part of an award for past research activities. E.G.-G. was funded by an unrestricted research grant from ISDIN as a part of a national Spanish award.
Footnotes
Conflicts of interest
None declared.
References
- 1.Wells RS. Ichthyosis. Br Med J 1966; 2:1504–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elias PM, Williams ML, Crumrine D, Schmuth M. Inherited disorders of corneocyte proteins. In: Ichthyoses: Clinical, Biochemical, Pathogenic and Diagnostic Assessment (Elias PM, Williams ML, Crumrine D, Schmuth M, eds). Basel: Karger, 2010; 98–131. [DOI] [PubMed] [Google Scholar]
- 3.Oji V, Tadini G, Akiyama M et al. Revised nomenclature and classification of inherited ichthyoses: results of the First Ichthyosis Consensus Conference in Soreze 2009. J Am Acad Dermatol 2010; 63:607–41. [DOI] [PubMed] [Google Scholar]
- 4.Fleckman P, Holbrook KA, Dale BA et al. Keratinocytes cultured from subjects with ichthyosis vulgaris are phenotypically abnormal. J Invest Dermatol 1987; 88:640–5. [DOI] [PubMed] [Google Scholar]
- 5.Sybert VP, Dale BA, Holbrook KA. Ichthyosis vulgaris: identification of a defect in synthesis of filaggrin correlated with an absence of keratohyaline granules. J Invest Dermatol 1985; 84:191–4. [DOI] [PubMed] [Google Scholar]
- 6.Feinstein A, Ackerman AB, Ziprkowski L. Histology of autosomal dominant ichthyosis vulgaris and X-linked ichthyosis. Arch Dermatol 1970; 101:524–7. [PubMed] [Google Scholar]
- 7.Presland RB. Function of filaggrin and caspase-14 in formation and maintenance of the epithelial barrier function. Dermatol Sin 2009; 27:1–14. [Google Scholar]
- 8.Smith FJ, Irvine AD, Terron-Kwiatkowski A et al. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat Genet 2006; 38:337–42. [DOI] [PubMed] [Google Scholar]
- 9.Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med 2011; 365:1315–27. [DOI] [PubMed] [Google Scholar]
- 10.Palmer CN, Irvine AD, Terron-Kwiatkowski A et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet 2006; 38:441–6. [DOI] [PubMed] [Google Scholar]
- 11.Winge MC, Bilcha KD, Lieden A et al. Novel filaggrin mutation but no other loss-of-function variants found in Ethiopian patients with atopic dermatitis. Br J Dermatol 2011; 165:1074–80. [DOI] [PubMed] [Google Scholar]
- 12.Compton JG, DiGiovanna JJ, Johnston KA et al. Mapping of the associated phenotype of an absent granular layer in ichthyosis vulgaris to the epidermal differentiation complex on chromosome 1. Exp Dermatol 2002; 11:518–26. [DOI] [PubMed] [Google Scholar]
- 13.Zhong W, Cui B, Zhang Y et al. Linkage analysis suggests a locus of ichthyosis vulgaris on 1q22. J Hum Genet 2003; 48:390–2. [DOI] [PubMed] [Google Scholar]
- 14.Sandilands A, O’Regan GM, Liao H et al. Prevalent and rare mutations in the gene encoding filaggrin cause ichthyosis vulgaris and predispose individuals to atopic dermatitis. J Invest Dermatol 2006; 126:1770–5. [DOI] [PubMed] [Google Scholar]
- 15.Oji V, Seller N, Sandilands A et al. Ichthyosis vulgaris: novel FLG mutations in the German population and high presence of CD1a+ cells in the epidermis of the atopic subgroup. Br J Dermatol 2009; 160:771–81. [DOI] [PubMed] [Google Scholar]
- 16.Gruber R, Janecke AR, Fauth C et al. Filaggrin mutations p.R501X and c.2282del4 in ichthyosis vulgaris. Eur J Hum Genet 2007; 15:179–84. [DOI] [PubMed] [Google Scholar]
- 17.Sandilands A, Terron-Kwiatkowski A, Hull PR et al. Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat Genet 2007; 39:650–4. [DOI] [PubMed] [Google Scholar]
- 18.Liu P, Yang Q, Wang X et al. Identification of a genetic locus for ichthyosis vulgaris on chromosome 10q22.3-q24.2. J Invest Dermatol 2008; 128:1418–22. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Guo Y, Wang W et al. Associations of FLG mutations between ichthyosis vulgaris and atopic dermatitis in Han Chinese. Allergy 2011; 66:1253–4. [DOI] [PubMed] [Google Scholar]
- 20.Nomura T, Akiyama M, Sandilands A et al. Prevalent and rare mutations in the gene encoding filaggrin in Japanese patients with ichthyosis vulgaris and atopic dermatitis. J Invest Dermatol 2009; 129:1302–5. [DOI] [PubMed] [Google Scholar]
- 21.Sinclair C, O’Toole EA, Paige D et al. Filaggrin mutations are associated with ichthyosis vulgaris in the Bangladeshi population. Br J Dermatol 2009; 160:1113–15. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Liu S, Chen X et al. Novel and recurrent mutations in the filaggrin gene in Chinese patients with ichthyosis vulgaris. Br J Dermatol 2010; 163:63–9. [DOI] [PubMed] [Google Scholar]
- 23.Nomura T, Sandilands A, Akiyama M et al. Unique mutations in the filaggrin gene in Japanese patients with ichthyosis vulgaris and atopic dermatitis. J Allergy Clin Immunol 2007; 119:434–40. [DOI] [PubMed] [Google Scholar]
- 24.CheH, HC, SandilandA et al. Unique and recurrent mutations in the filaggrin gene in Singaporean Chinese patients with ichthyosis vulgaris. J Invest Dermatol 2008; 128:1669–75. [DOI] [PubMed] [Google Scholar]
- 25.Hsu CK, Akiyama M, Nemoto-Hasebe I et al. Analysis of Taiwanese ichthyosis vulgaris families further demonstrates differences in FLG mutations between European and Asian populations. Br J Dermatol 2009; 161:448–51. [DOI] [PubMed] [Google Scholar]
- 26.Droitcourt C, Touboul D, Ged C et al. A prospective study of filaggrin null mutations in keratoconus patients with or without atopic disorders. Dermatology 2011; 222:336–41. [DOI] [PubMed] [Google Scholar]
- 27.Mlitz V, Latreille J, Gardinier S et al. Impact of filaggrin mutations on Raman spectra and biophysical properties of the stratum corneum in mild to moderate atopic dermatitis. J Eur Acad Dermatol Venereol 2012; 26:983–90. [DOI] [PubMed] [Google Scholar]
- 28.Giardina E, Paolillo N, Sinibaldi C et al. R501X and 2282del4 filaggrin mutations do not confer susceptibility to psoriasis and atopic dermatitis in Italian patients. Dermatology 2008; 216:83–4. [DOI] [PubMed] [Google Scholar]
- 29.Cascella R, Foti Cuzzola V, Lepre T et al. Full sequencing of the FLG gene in Italian patients with atopic eczema: evidence of new mutations, but lack of an association. J Invest Dermatol 2011; 131:982–4. [DOI] [PubMed] [Google Scholar]
- 30.Brown SJ, McLean WH. One remarkable molecule: filaggrin. J Invest Dermatol 2012; 132:751–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee DE, Park SY, Han JY et al. Association between filaggrin mutations and atopic dermatitis in Korean pregnant women. Int J Dermatol 2011. doi: 10.1111/j.1365-4632.2011.05062.x. (Epub ahead of print). [DOI] [PubMed]
- 32.Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol 2005; 6:328–40. [DOI] [PubMed] [Google Scholar]
- 33.Scott IR, Harding CR. Filaggrin breakdown to water binding compounds during development of the rat stratum corneum is controlled by the water activity of the environment. Dev Biol 1986; 115:84–92. [DOI] [PubMed] [Google Scholar]
- 34.Gibbs NK, Norval M. Urocanic acid in the skin: a mixed blessing? J Invest Dermatol 2011; 131:14–17. [DOI] [PubMed] [Google Scholar]
- 35.Gruber R, Elias PM, Crumrine D et al. Filaggrin genotype in ichthyosis vulgaris predicts abnormalities in epidermal structure and function. Am J Pathol 2011; 178:2252–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Angelova-Fischer I, Mannheimer AC, Hinder A et al. Distinct barrier integrity phenotypes in filaggrin-related atopic eczema following sequential tape stripping and lipid profiling. Exp Dermatol 2011; 20:351–6. [DOI] [PubMed] [Google Scholar]
- 37.Jungersted JM, Scheer H, Mempel M et al. Stratum corneum lipids, skin barrier function and filaggrin mutations in patients with atopic eczema. Allergy 2010; 65:911–18. [DOI] [PubMed] [Google Scholar]
- 38.Scott IR. Alterations in the metabolism of filaggrin in the skin after chemical and ultraviolet-induced erythema. J Invest Dermatol 1986; 87:460–5. [DOI] [PubMed] [Google Scholar]
- 39.Ramesh R, Chen H, Kukula A et al. Exacerbation of X-linked ichthyosis phenotype in a female by inheritance of filaggrin and steroid sulfatase mutations. J Dermatol Sci 2011; 64:159–62. [DOI] [PubMed] [Google Scholar]
- 40.Kezic S, Kemperman PM, Koster ES et al. Loss-of-function mutations in the filaggrin gene lead to reduced level of natural moisturizing factor in the stratum corneum. J Invest Dermatol 2008; 128:2117–19. [DOI] [PubMed] [Google Scholar]
- 41.Kezic S, Kammeyer A, Calkoen F et al. Natural moisturizing factor components in the stratum corneum as biomarkers of filaggrin genotype: evaluation of minimally invasive methods. Br J Dermatol 2009; 161:1098–104. [DOI] [PubMed] [Google Scholar]
- 42.Mildner M, Jin J, Eckhart L et al. Knockdown of filaggrin impairs diffusion barrier function and increases UV sensitivity in a human skin model. J Invest Dermatol 2010; 130:2286–94. [DOI] [PubMed] [Google Scholar]
- 43.Jensen AO, Svaerke C, Kormendine FD et al. Atopic dermatitis and risk of skin cancer: a Danish nationwide cohort study (1977– 2006). Am J Clin Dermatol 2012; 13:29–36. [DOI] [PubMed] [Google Scholar]
- 44.Thyssen JP, Thuesen BH, Huth C et al. Skin barrier abnormality due to FLG mutations is associated with increased serum vitamin D concentrations. J Allergy Clin Immunol 2012; 130:1204–7. [DOI] [PubMed] [Google Scholar]
- 45.Scharschmidt TC, Man MQ, Hatano Y et al. Filaggrin deficiency confers a paracellular barrier abnormality that reduces inflammatory thresholds to irritants and haptens. J Allergy Clin Immunol 2009; 124:496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fallon PG, Sasaki T, Sandilands A et al. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat Genet 2009; 41:602–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weidinger S, Illig T, Baurecht H et al. Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. J Allergy Clin Immunol 2006; 118:214–19. [DOI] [PubMed] [Google Scholar]
- 48.Thyssen JP, Johansen JD, Linneberg A et al. The association between null mutations in the filaggrin gene and contact sensitization to nickel and other chemicals in the general population. Br J Dermatol 2010; 162:1278–85. [DOI] [PubMed] [Google Scholar]
- 49.Kimber I, Dearman RJ. An assessment of the ability of phthalates to influence immune and allergic responses. Toxicology 2010; 271:73–82. [DOI] [PubMed] [Google Scholar]
- 50.Bisgaard H, Simpson A, Palmer CN et al. Gene–environment interaction in the onset of eczema in infancy: filaggrin loss-of-function mutations enhanced by neonatal cat exposure. PLoS Med 2008; 5:e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schuttelaar ML, Kerkhof M, Jonkman MF et al. Filaggrin mutations in the onset of eczema, sensitization, asthma, hay fever and the interaction with cat exposure. Allergy 2009; 64:1758–65. [DOI] [PubMed] [Google Scholar]
- 52.Berg ND, Husemoen LL, Thuesen BH et al. Interaction between filaggrin null mutations and tobacco smoking in relation to asthma. J Allergy Clin Immunol 2012; 129:374–80. [DOI] [PubMed] [Google Scholar]
- 53.Henderson J, Northstone K, Lee SP et al. The burden of disease associated with filaggrin mutations: a population-based, longitudinal birth cohort study. J Allergy Clin Immunol 2008; 121:872–7. [DOI] [PubMed] [Google Scholar]
- 54.Thyssen JP, Linneberg A, Johansen JD et al. Atopic diseases by filaggrin mutations and birth year. Allergy 2012; 67:705–8. [DOI] [PubMed] [Google Scholar]
- 55.Brown SJ, Relton CL, Liao H et al. Filaggrin null mutations and childhood atopic eczema: a population-based case–control study. J Allergy Clin Immunol 2008; 121:940–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thyssen JP, Carlsen BC, Bisgaard H et al. Individuals who are homozygous for the 2282del4 and R501X filaggrin null mutations do not always develop dermatitis and complete long-term remission is possible. J Eur Acad Dermatol Venereol 2012; 26:386–9. [DOI] [PubMed] [Google Scholar]
- 57.Chen H, Common JE, Haines RL et al. Wide spectrum of filaggrin-null mutations in atopic dermatitis highlights differences between Singaporean Chinese and European populations. Br J Dermatol 2011; 165:106–14. [DOI] [PubMed] [Google Scholar]
- 58.Ziprkowski L, Feinstein A. A survey of ichthyosis vulgaris in Israel. Br J Dermatol 1972; 86:1–8. [DOI] [PubMed] [Google Scholar]
- 59.Wells RS, Kerr CB. Clinical features of autosomal dominant and sex-linked ichthyosis in an English population. Br Med J 1966; 1:947–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuokkanen K Ichthyosis vulgaris. A clinical and histopathological study of patients and their close relatives in the autosomal dominant and sex-linked forms of the disease. Acta Derm Venereol Suppl (Stockh) 1969; 62:1–72. [PubMed] [Google Scholar]
- 61.Wells RS, Kerr CB. Genetic classification of ichthyosis. Arch Dermatol 1965; 92:1–6. [PubMed] [Google Scholar]
- 62.Sergeant A, Campbell LE, Hull PR et al. Heterozygous null alleles in filaggrin contribute to clinical dry skin in young adults and the elderly. J Invest Dermatol 2009; 129:1042–5. [DOI] [PubMed] [Google Scholar]
- 63.Novak N, Baurecht H, Schafer T et al. Loss-of-function mutations in the filaggrin gene and allergic contact sensitization to nickel. J Invest Dermatol 2008; 128:1430–5. [DOI] [PubMed] [Google Scholar]
- 64.Rice NE, Patel BD, Lang IA et al. Filaggrin gene mutations are associated with asthma and eczema in later life. J Allergy Clin Immunol 2008; 122:834–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thyssen JP, Ross-Hansen K, Johansen JD et al. Filaggrin loss-of-function mutation R501X and 2282del4 carrier status is associated with fissured skin on the hands: results from a cross-sectional population study. Br J Dermatol 2011; 166:46–53. [DOI] [PubMed] [Google Scholar]
- 66.Thyssen JP, Menne T, Zachariae C. [Minerva – no title]. BMJ 2012; 344:e2441. [Google Scholar]
- 67.Brown SJ, Relton CL, Liao H et al. Filaggrin haploinsufficiency is highly penetrant and is associated with increased severity of eczema: further delineation of the skin phenotype in a prospective epidemiological study of 792 school children. Br J Dermatol 2009; 161:884–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oji V, Traupe H. Ichthyosis: clinical manifestations and practical treatment options. Am J Clin Dermatol 2009; 10:351–64. [DOI] [PubMed] [Google Scholar]
- 69.Fleckman P, Brumbaugh S. Absence of the granular layer and keratohyalin define a morphologically distinct subset of individuals with ichthyosis vulgaris. Exp Dermatol 2002; 11:327–36. [DOI] [PubMed] [Google Scholar]
- 70.Frost P, Van Scott EJ. Ichthyosiform dermatoses. Classification based on anatomic and biometric observations. Arch Dermatol 1966; 94:113–26. [DOI] [PubMed] [Google Scholar]
- 71.van den Oord RA, Sheikh A. Filaggrin gene defects and risk of developing allergic sensitisation and allergic disorders: systematic review and meta-analysis. BMJ 2009; 339:b2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rodriguez E, Baurecht H, Herberich E et al. Meta-analysis of filaggrin polymorphisms in eczema and asthma: robust risk factors in atopic disease. J Allergy Clin Immunol 2009; 123:1361–70. [DOI] [PubMed] [Google Scholar]
- 73.Weidinger S, Rodriguez E, Stahl C et al. Filaggrin mutations strongly predispose to early-onset and extrinsic atopic dermatitis. J Invest Dermatol 2007; 127:724–6. [DOI] [PubMed] [Google Scholar]
- 74.Barker JN, Palmer CN, Zhao Y et al. Null mutations in the filaggrin gene (FLG) determine major susceptibility to early-onset atopic dermatitis that persists into adulthood. J Invest Dermatol 2007; 127:564–7. [DOI] [PubMed] [Google Scholar]
- 75.Stemmler S, Parwez Q, Petrasch-Parwez E et al. Two common loss-of-function mutations within the filaggrin gene predispose for early onset of atopic dermatitis. J Invest Dermatol 2007; 127:722–4. [DOI] [PubMed] [Google Scholar]
- 76.Flohr C, England K, Radulovic S et al. Filaggrin loss-of-function mutations are associated with early-onset eczema, eczema severity and transepidermal water loss at 3 months of age. Br J Dermatol 2010; 163:1333–6. [DOI] [PubMed] [Google Scholar]
- 77.Zhang H, Guo Y, Wang W et al. Mutations in the filaggrin gene in Han Chinese patients with atopic dermatitis. Allergy 2011; 66:420–7. [DOI] [PubMed] [Google Scholar]
- 78.Brown SJ, Sandilands A, Zhao Y et al. Prevalent and low-frequency null mutations in the filaggrin gene are associated with early-onset and persistent atopic eczema. J Invest Dermatol 2008; 128:1591–4. [DOI] [PubMed] [Google Scholar]
- 79.Imoto Y, Enomoto H, Fujieda S et al. S2554X mutation in the filaggrin gene is associated with allergen sensitization in the Japanese population. J Allergy Clin Immunol 2010; 125:498–500. [DOI] [PubMed] [Google Scholar]
- 80.Li M, Liu Q, Liu J et al. Mutations analysis in filaggrin gene in northern China patients with atopic dermatitis. J Eur Acad Dermatol Venereol 2013; 27:169–74. [DOI] [PubMed] [Google Scholar]
- 81.Brown SJ, Asai Y, Cordell HJ et al. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut Allergy. J Allergy Clin Immunol 2011; 127:661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weidinger S, O’Sullivan M, Illig T et al. Filaggrin mutations, atopic eczema, hay fever, and asthma in children. J Allergy Clin Immunol 2008; 121:1203–9. [DOI] [PubMed] [Google Scholar]
- 83.Marenholz I, Kerscher T, Bauerfeind A et al. An interaction between filaggrin mutations and early food sensitization improves the prediction of childhood asthma. J Allergy Clin Immunol 2009; 123:911–16. [DOI] [PubMed] [Google Scholar]
- 84.Li M, Chen X, Chen R et al. Filaggrin gene mutations are associated with independent atopic asthma in Chinese patients. Allergy 2011; 66:1616–17. [DOI] [PubMed] [Google Scholar]
- 85.Poninska J, Samolinski B, Tomaszewska A et al. Filaggrin gene defects are independent risk factors for atopic asthma in a Polish population: a study in ECAP cohort. PLoS ONE 2011; 6:e16933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Muller S, Marenholz I, Lee YA et al. Association of filaggrin loss-of-function-mutations with atopic dermatitis and asthma in the Early Treatment of the Atopic Child (ETAC) population. Pediatr Allergy Immunol 2009; 20:358–61. [DOI] [PubMed] [Google Scholar]
- 87.Cookson WO, Ubhi B, Lawrence R et al. Genetic linkage of childhood atopic dermatitis to psoriasis susceptibility loci. Nat Genet 2001; 27:372–3. [DOI] [PubMed] [Google Scholar]
- 88.Capon F, Novelli G, Semprini S et al. Searching for psoriasis susceptibility genes in Italy: genome scan and evidence for a new locus on chromosome 1. J Invest Dermatol 1999; 112:32–5. [DOI] [PubMed] [Google Scholar]
- 89.Hu Z, Xiong Z, Xu X et al. Loss-of-function mutations in filaggrin gene associate with psoriasis vulgaris in Chinese population. Hum Genet 2012; 131:1269–74. [DOI] [PubMed] [Google Scholar]
- 90.Thyssen J, Johansen J, Carlsen B et al. The filaggrin null genotypes R501X and 2282del4 seem not to be associated with psoriasis: results from general population study and meta-analysis. J Eur Acad Dermatol Venereol 2012; 26:782–4. [DOI] [PubMed] [Google Scholar]
- 91.Hu¨ ffmeier U, Traupe H, Oji V et al. Loss-of-function variants of the filaggrin gene are not major susceptibility factors for psoriasis vulgaris or psoriatic arthritis in German patients. J Invest Dermatol 2007; 127:1367–70. [DOI] [PubMed] [Google Scholar]
- 92.Chang YC, Wu WM, Chen CH et al. Association between P478S polymorphism of the filaggrin gene and risk of psoriasis in a Chinese population in Taiwan. Arch Dermatol Res 2008; 300:133–7. [DOI] [PubMed] [Google Scholar]
- 93.Weichenthal M, Ruether A, Schreiber S et al. Filaggrin R501X and 2282del4 mutations are not associated with chronic plaque-type psoriasis in a German cohort. J Invest Dermatol 2007; 127:1535–7. [DOI] [PubMed] [Google Scholar]
- 94.Zhao Y, Terron-Kwiatkowski A, Liao H et al. Filaggrin null alleles are not associated with psoriasis. J Invest Dermatol 2007; 127:1878–82. [DOI] [PubMed] [Google Scholar]
- 95.Winge MC, Suneson J, Lysell J et al. Lack of association between filaggrin gene mutations and onset of psoriasis in childhood. J Eur Acad Dermatol Venereol 2013; 27:e124–7. [DOI] [PubMed] [Google Scholar]
- 96.Betz RC, Pforr J, Flaquer A et al. Loss-of-function mutations in the filaggrin gene and alopecia areata: strong risk factor for a severe course of disease in patients comorbid for atopic disease. J Invest Dermatol 2007; 127:2539–43. [DOI] [PubMed] [Google Scholar]
- 97.Cai SC, Chen H, Koh WP et al. Filaggrin mutations are associated with recurrent skin infection in Singaporean Chinese patients with atopic dermatitis. Br J Dermatol 2012; 166:200–3. [DOI] [PubMed] [Google Scholar]
- 98.Gao PS, Rafaels NM, Hand T et al. Filaggrin mutations that confer risk of atopic dermatitis confer greater risk for eczema herpeticum. J Allergy Clin Immunol 2009; 124:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thyssen JP, Carlsen BC, Menne T et al. Filaggrin null mutations increase the risk and persistence of hand eczema in subjects with atopic dermatitis: results from a general population study. Br J Dermatol 2010; 163:115–20. [DOI] [PubMed] [Google Scholar]
- 100.Molin S, Vollmer S, Weiss EH et al. Filaggrin mutations may confer susceptibility to chronic hand eczema characterized by combined allergic and irritant contact dermatitis. Br J Dermatol 2009; 161:801–7. [DOI] [PubMed] [Google Scholar]
- 101.Visser MJ, Landeck L, Campbell LE et al. Impact of loss-of-function mutations in the filaggrin gene and atopic dermatitis on the development of occupational irritant contact dermatitis. Br J Dermatol 2013; 168:326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.de Jongh CM, Khrenova L, Verberk MM et al. Loss-of-function polymorphisms in the filaggrin gene are associated with an increased susceptibility to chronic irritant contact dermatitis: a case–control study. Br J Dermatol 2008; 159:621–7. [DOI] [PubMed] [Google Scholar]
- 103.Carlsen BC, Johansen JD, Menne´ T et al. Filaggrin null mutations and association with contact Allergy and allergic contact dermatitis: results from a tertiary Dermatology clinic. Contact Dermatitis 2010; 63:89–95. [DOI] [PubMed] [Google Scholar]
- 104.Thyssen JP, Linneberg A, Ross-Hansen K et al. Filaggrin mutations are strongly associated with contact sensitization in individuals with dermatitis. Contact Dermatitis 2013. doi: 10.1111/cod.12021. [DOI] [PubMed]
- 105.Thyssen JP, Carlsen BC, Johansen JD et al. Filaggrin null-mutations may be associated with a distinct subtype of atopic hand eczema. Acta Derm Venereol 2010; 90:528. [DOI] [PubMed] [Google Scholar]
- 106.Carson CG, Rasmussen MA, Thyssen JP et al. Clinical presentation of atopic dermatitis by filaggrin gene mutation status during the first 7 years of life in a prospective cohort study. PLoS ONE 2012; 7:e48678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ross-Hansen K, Menne´ T, Johansen JD et al. Nickel reactivity and filaggrin null mutations – evaluation of the filaggrin bypass theory in a general population. Contact Dermatitis 2011; 64:24–31. [DOI] [PubMed] [Google Scholar]
- 108.Thyssen JP, Linneberg A, Carlsen BC et al. A possible association between a dysfunctional skin barrier (filaggrin null-mutation status) and diabetes: a cross-sectional study. BMJ Open 2011; 1:e000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vahlquist A, Ganemo A, Virtanen M. Congenital ichthyosis: an overview of current and emerging therapies. Acta Derm Venereol 2008; 88:4–14. [DOI] [PubMed] [Google Scholar]
- 110.Simpson EL, Berry TM, Brown PA et al. A pilot study of emollient therapy for the primary prevention of atopic dermatitis. J Am Acad Dermatol 2010; 63:587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gruber R, Janecke AR, Grabher D et al. Lower prevalence of common filaggrin mutations in a community sample of atopic eczema: is disease severity important? Wien Klin Wochenschr 2010; 122:551–7. [DOI] [PubMed] [Google Scholar]
- 112.Greisenegger E, Novak N, Maintz L et al. Analysis of four prevalent filaggrin mutations (R501X, 2282del4, R2447X and S3247X) in Austrian and German patients with atopic dermatitis. J Eur Acad Dermatol Venereol 2010; 24:607–10. [DOI] [PubMed] [Google Scholar]
- 113.Cramer C, Link E, Horster M et al. Elder siblings enhance the effect of filaggrin mutations on childhood eczema: results from the 2 birth cohort studies LISAplus and GINIplus. J Allergy Clin Immunol 2010; 125:1254–60. [DOI] [PubMed] [Google Scholar]
- 114.Van Limbergen J, Russell RK, Nimmo ER et al. Filaggrin loss-of-function variants are associated with atopic comorbidity in pediatric inflammatory bowel disease. Inflamm Bowel Dis 2009; 15:1492–8. [DOI] [PubMed] [Google Scholar]
- 115.Ma L, Zhang L, Di ZH et al. Association analysis of filaggrin gene mutations and atopic dermatitis in Northern China. Br J Dermatol 2010; 162:225–7. [DOI] [PubMed] [Google Scholar]
- 116.Nomura T, Akiyama M, Sandilands A et al. Specific filaggrin mutations cause ichthyosis vulgaris and are significantly associated with atopic dermatitis in Japan. J Invest Dermatol 2008; 128:1436–41. [DOI] [PubMed] [Google Scholar]
- 117.Nemoto-Hasebe I, Akiyama M, Nomura T et al. FLG mutation p.Lys4021X in the C-terminal imperfect filaggrin repeat in Japanese patients with atopic eczema. Br J Dermatol 2009; 161:1387–90. [DOI] [PubMed] [Google Scholar]
- 118.Common JE, Brown SJ, Haines RL et al. Filaggrin null mutations are not a protective factor for acne vulgaris. J Invest Dermatol 2011; 131:1378–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ching GK, Hon KL, Ng PC et al. Filaggrin null mutations in childhood atopic dermatitis among the Chinese. Int J Immunogenet 2009; 36:251–4. [DOI] [PubMed] [Google Scholar]
- 120.Wang IJ, Lin TJ, Kuo CF et al. Filaggrin polymorphism P478S, IgE level and atopic phenotypes. Br J Dermatol 2011; 164:791–6. [DOI] [PubMed] [Google Scholar]
- 121.Mevorah B, Marazzi A, Frenk E. The prevalence of accentuated palmoplantar markings and keratosis pilaris in atopic dermatitis, autosomal dominant ichthyosis and control dermatological patients. Br J Dermatol 1985; 112:679–85. [DOI] [PubMed] [Google Scholar]
- 122.Uehara M, Hayashi S. Hyperlinear palms: association with ichthyosis and atopic dermatitis. Arch Dermatol 1981; 117:490–1. [DOI] [PubMed] [Google Scholar]
- 123.Ruether A, Stoll M, Schwarz T et al. Filaggrin loss-of-function variant contributes to atopic dermatitis risk in the population of Northern Germany. Br J Dermatol 2006; 155:1093–4. [DOI] [PubMed] [Google Scholar]
- 124.Rodriguez S, Hall AJ, Granell R et al. Carrier status for the common R501X and 2282del4 filaggrin mutations is not associated with hearing phenotypes in 5,377 children from the ALSPAC cohort. PLoS ONE 2009; 4:e5784. [DOI] [PMC free article] [PubMed] [Google Scholar]