Abstract
Introduction:
Major depression is associated with an increased risk for and mortality from coronary artery disease (CAD), however the mechanisms by which this occurs are not clear. Depression, which is linked to stress, is associated with changes in brain areas involved in memory and the stress response, and it is likely that these regions play an important role in this increased risk. This study assessed the effects of stress on brain and cardiac function in patients with CAD with and without depression.
Methods:
CAD patients with (N=17) and without (N=21) major depression based on the Structured Clinical Interview for DSM-IV (DSM-IV) and/or a Hamilton Depression Scale score of nine or greater underwent imaging of the brain with high resolution positron emission tomography (HR-PET) and [O-15] water and imaging of the heart with single photon emission tomography (SPECT) and [Tc-99m] sestamibi during mental stress (mental arithmetic) and control conditions.
Results:
Patients with CAD and major depression showed increased parietal cortex activation and a relative failure of medial prefrontal/anterior cingulate activation during mental stress compared to CAD patients without depression. Depressed CAD patients with stress-induced myocardial ischemia, however, when compared to depressed CAD patients without showed increased activation in rostral portions of the anterior cingulate.
Conclusions:
These findings are consistent with a role for brain areas implicated in stress and depression in the mechanism of increased risk for CAD morbidity and mortality in CAD patients with the diagnosis of major depression.
Introduction
The relationship between major depression and coronary artery disease (CAD) is well established (Carney and Freedland, 2017; Gan et al., 2014; Meijer et al., 2011; Vaccarino and Bremner, 2014; Vaccarino et al., 2009). Depression is associated with an increased risk of mortality which is primarily related to increased cardiovascular death (Anstey and Luszcz, 2002; Wulsin et al., 1999). The presence of depression also worsens outcomes in CAD patients regardless of whether the symptoms were present at the time of hospitalization for cardiac events or developed later (Mallik et al., 2006; Parashar et al., 2006). The mechanism by which depression increases CAD risk, however, is unclear.
Depression is associated with stress (Kendler et al., 2000), and patients with major depression show alterations in stress responsive neurohormonal systems, including cortisol and norepinephrine (Bremner, J.D. et al., 2003a; Carroll, 1982; Carroll et al., 1976; Charney et al., 1982; Delgado and Moreno, 2000; Lake et al., 1982; Nemeroff et al., 1984; Ordway et al., 1994; Roy et al., 1988; Traskman et al., 1980; Young et al., 1994). In some cases these changes are reversible with treatment (Charney et al., 1981; Golden et al., 1988). Increased sympathetic tone and other alterations in neurohormonal function in patients with depression (Carney et al., 1999; Veith et al., 1994) may contribute to the increase in mortality from CAD (Kannel et al., 1987; Vaccarino and Bremner, 2014; Vaccarino and Bremner, 2017). Central changes in the brain, including responses to stress, likely underlie these alterations in peripheral autonomic and neurohormonal function.
Brain imaging studies have shown alterations in several brain regions involved in stress and memory in patients with major depression, including the amygdala, hippocampus, and medial prefrontal cortex/anterior cingulate (Bremner, 2005; Cheng et al., 2016; Kumar et al., 2004; Sheline, 2003; Smith and Eyler, 2006). Structural magnetic resonance imaging (MRI) studies in patients with major depression showed smaller volumes of the hippocampus (Bremner et al., 2000; Bremner et al., 2004; Caetano et al., 2004; Campbell et al., 2004; Cole et al., 2011; Frodl et al., 2004; Hickie et al., 2005; Janssen et al., 2004; Krishnan et al., 1991; Lloyd et al., 2004; Mervaala et al., 2000; Opel et al., 2014; Posener et al., 2003; Sala et al., 2004; Sheline et al., 2003; Sheline et al., 1999; Sheline et al., 1996; Steffens et al., 2000; Vakili et al., 2000; Videbech and Ravnkilde, 2004; Vythilingam et al., 2002), orbitofrontal cortex and anterior cingulate (Ballmaier et al., 2004; Brambilla et al., 2002; Bremner, 2002; Bremner et al., 2002; Kumar et al., 2000; Lacerda et al., 2004; Lai et al., 2000; Lee et al., 2003; Smith and Eyler, 2006; Steffens and Krishnan, 1998). Patients with late-life depression showed an increase in “unidentified bright object” (UBO) lesions in the white matter and peri-ventricular areas on T2-weighted MRI (Greenwald et al., 1998; Hickie et al., 1997; Kumar et al., 1997; Kumar et al., 1998; Lenze et al., 1999; Narayan et al., 1999; Steffens and Krishnan, 1998) felt to represent small infarcts that disrupt neural circuits mediating mood (Steffens and Krishnan, 1998). Functional neuroimaging studies using functional MRI (fMRI), positron emission tomography (PET) and single photon emission tomography (SPECT) have also shown altered function in these regions as well as the amygdala (Abercrombie et al., 1998; Austin et al., 1992; Baxter et al., 1989; Bench et al., 1992; Biver et al., 1994; Bremner, 2005; Bremner et al., 1997a, b; Bremner, J.D. et al., 2003a; Bremner, J. D. et al., 2003; Bremner et al., 2007; Bremner et al., 2004; Buchsbaum et al., 1984; Cheng et al., 2016; de Asis et al., 2001; Drevets et al., 2002; Drevets et al., 1997; Ebert et al., 1991; George et al., 1997; George et al., 1994; Grant et al., 2011; Hurwitz et al., 1990; Kennedy et al., 2001; Kumar et al., 2004; Lacerda et al., 2004; Martinot et al., 1990; Mayberg, 1994; Mayberg et al., 1997; Mayberg et al., 1994; Mayberg et al., 1999; Mayberg et al., 1992; Mayberg et al., 1990; Ring et al., 1994; Smith and Eyler, 2006; Smith, G.S. et al., 2002). These alterations reversed with treatment (Baxter et al., 1989; Bremner et al., 2007; Brody et al., 2001; Goodwin et al., 1993; Kennedy et al., 2001; Martinot et al., 1990; Mayberg et al., 2000; Smith, G. et al., 2002; Smith, G.S. et al., 2002).
Studies have also shown that stress, which is linked to depression, can induce myocardial ischemia in CAD patients using a standardized mental stress protocol (Arri et al., 2016; Ramadan et al., 2013; Vaccarino et al., 2014; Wei et al., 2014a; Wei et al., 2014b){Hammadah, 2017 #9649}. Mental stress-induced myocardial ischemia (MSI) often occurs without pain, does not require diseased coronary arteries, and has been hypothesized to be related to coronary vasospasm (Deanfield et al., 1984; Lacy et al., 1995) and/or peripheral vasoconstriction during stress (Arri et al., 2016; Ramadan et al., 2013; Sullivan et al., 2018; Vaccarino et al., 2018). MSI occurs at lower heart rates than those required for physical stress-induced ischemia, and often occurs in patients without physical stress-induced myocardial ischemia (Krantz et al., 1991; LaVeau et al., 1989; Ramachandruni et al., 2006; Rozanski et al., 1988; Schang and Pepine, 1977). Similar to depression, MSI is more common than women than in men and is associated with worse outcomes .{Vaccarino, 2016 #9664}{Vaccarino, 2009 #9134}{Sullivan, 2018 #9648}
MSI may represent the mechanism by which depression increases the risk for CAD (Wei et al., 2014a). Brain areas involved in memory and the stress response that have been implicated in depression, including the medial prefrontal cortex/anterior cingulate/orbitofrontal cortex (Bremner et al., 1997b; Bremner, J. D. et al., 2003; Cheng et al., 2016; Drevets et al., 1997; Mayberg et al., 1997), likely play a role in MSI. This region has been shown to modulate peripheral cardiovascular function, including heart rate variability (Thayer et al., 2009), and peripheral cardiovascular and neurohormonal responses to stress (Campanella and Bremner, 2016; Vaccarino and Bremner, 2017). In a recent study in a general CAD population not selected for psychiatric disorders we found increased activation with public speaking and arithmetic mental stress in rostral anterior cingulate, inferior frontal gyrus, and parietal cortex, and additional insula activation with mental arithmetic, in MSI compared to non-MSI CAD patients CAD (Bremner et al., 2018). This prior study involved a general CAD population. In the current study we studied a non-overlapping sample of CAD patients who were selected based on the presence or absence of depression in order to assess brain correlates of stress in patients with CAD with and without depression. Base on prior studies of depression and our studies of MSI we hypothesized that patients with CAD and depression would show a relatively blunted response response to stress in the medial prefrontal/anterior cingulate area compared to CAD patients without depression, but that MSI would be associated with increased activation in this area.
Methods
Patient Population
Sixty patients with coronary artery disease (CAD) were recruited from the Emory University Hospital and Clinics and by advertisement. CAD diagnosis was based on a prior diagnosis of myocardial ischemia, previous cardiac catheterization showing any degree of stenosis, or coronary revascularization. Subjects with depression met DSM-IV-TR criteria for major depression as measured by the Structured Clinical Interview for DSMIV (SCID) interview (First et al., 1995) and/or had a score on the Hamilton Depression Scale (Hamilton, 1960) of nine or greater. Patients were excluded with a history of unstable angina, myocardial infarction, or decompensated heart failure in the past week. Patients were also excluded with a history of meningitis, traumatic brain injury, neurological disorder, organic mental disorder, dyslexia, history of loss of consciousness of greater than one minute, history of current alcohol abuse or substance abuse or dependence (past year) based on the SCID, history of schizophrenia, schizoaffective disorder, psychotic depression, mania/hypomania, anorexia/bulimia, based on the SCID, history of serious medical disorder other than cardiovascular disease, e.g. cancer, renal failure, or evidence of a major abnormality based on laboratory studies that contraindicated participation, active suicidal ideation, history of oral or inhaled steroid usage (past year), current use of exogenous estrogens or progesterone (“hormone replacement therapy”) in the past 3 months, or current antipsychotic medication treatment (past one month).
Patients on cardiac medications including beta blockers or statins were not excluded. Medications were held the morning of the imaging tests. Subjects treated with stable doses of antidepressants were included, but subjects undergoing active changes in antidepressant medications during the study period were excluded. This protocol was approved by the Emory University Investigation Review Board (IRB). All patients provided written informed consent for participation.
Of the 60 original CAD patients, 11 were excluded based on study criteria, and six dropped out before completing the study assessments. An additional five subjects completed the study but did not have a PET scan of the brain either for technical reasons or in one case due to claustrophobia in the scanner. A total of 38 patients completed all study procedures and had a usable PET scan, including 17 patients with depression and 21 patients without depression.
Assessments
All patients were evaluated for psychiatric diagnosis with the Structured Clinical Interview for DSMIV (SCID) interview (First et al., 1995). Depression symptoms were evaluated with the Hamilton Depression Scale, a reliable and valid measure of depressive symptoms based on clinician interview (Hamilton, 1960). The Addiction Severity Index (ASI) interview was used to assess lifetime alcohol abuse (McClellan et al., 1985). The Subjective Units of Distress Scale (SUDS) is a measure of subjective distress widely used in cognitive behavioral therapy. Subjects are asked to rate current subjective distress on a linear scale of 0 to 100 with 100 being the highest level of distress. The SUDS was used to assess the level of stress attained in the cognitive challenge to verify that the procedure was stressful for the subjects. Analogue ratings of fear, nervousness, high, and anger (scale of 0–4, with 4 being extreme) were also performed to assess emotional state at the time of each of the scans, as previously described (Bremner et al., 2009; Bremner et al., 1999).
Medication history and socio-demographic factors were assessed with a structured questionnaire. Subjects were assessed for past history of psychotropic usage and usage of antidepressant, mood stabilizer and antipsychotic medication treatment in the past. Medical information including previous CHD events and procedures, age of onset of CHD, current medications, and CHD risk factors (blood pressure, lipid and glucose levels, history of diabetes, current and past smoking, and body mass index), were also assessed.
Imaging Methods
Subjects underwent single photon emission computed tomography (SPECT) cardiac imaging on a dedicated research cardiac imaging scanner (Philips Cardio MD) and high resolution positron emission tomography (HR-PET) imaging of the brain with the High Resolution Research Tomograph (HRRT, CTI, Knoxville TN, 2 mm resolution) (Schmand et al., 1999; Weinhard et al., 2000).
For cardiac imaging each patient underwent SPECT imaging of the heart at rest on a separate day from the mental stress day. After confirmation of proper positioning, resting myocardial perfusion images were acquired for 10 minutes after the injection of 8 mCi 99mTc-sestamibi.
For the mental stress day, subjects underwent scanning of the brain with HR-PET and SPECT scanning of the heart. The mental stress day consisted of an arithmetic mental stress task and a counting control using methods we have previously described and that have been shown to increase stress as measured by subjective ratings, heart rate, blood pressure and cortisol response (Bremner et al., 2009; Bremner, J.D. et al., 2003b; Hammadah et al., 2016; Vaccarino et al., 2014). Patients underwent four HR-PET scans of the brain, two while counting out loud (“counting controls”) and two during mental arithmetic, with a SPECT scan of the heart following the second mental arithmetic task. Each task lasted for two minutes. At the beginning of the study a physician wearing a white laboratory coat entered the room. The testing physician was blind to the diagnosis and clinical care of the patient. The mental arithmetic cognitive challenge battery included arithmetic (serial subtraction, addition, multiplication and division) cognitive tasks performed under time pressure and with negative feedback regarding the performance and the time spent in the task given. The level of difficulty was increased until subjects were unable to successfully complete three consecutive tasks. The purpose of calibrating difficulty to individual performance is to have a similar level of stress for all subjects regardless of ability. Subjective ratings of distress were obtained at baseline and after the cognitive challenge as assessed with the Subjective Units of Distress Scale (SUDS) as well as analogue ratings of fear, nervousness, high, and anger.
Ten seconds after the beginning of each task patients received an intravenous injection of 20 mCi radiolabeled water followed immediately by HR-PET imaging of the brain for 80 seconds. One minute after the onset of the second mental stress condition subjects received an intravenous injected dose of 8 mCi 99mTc-sestamibi followed 45 minutes later by a SPECT scan of myocardial perfusion with mental stress. Gated images were also obtained for measurement of ejection fraction and evaluation of regional wall motion abnormalities. Myocardial images from baseline and mental stress were reconstructed in short axis, vertical long axis and horizontal long axis views.
Data Analysis
A Chi-square test was used to test the association between categorical risk factors and depression status. The distribution of continuous variables was examined for normality as a requirement for parametric testing. Data were analyzed using SAS 9.4 and statistical significance was evaluated using an alpha=0.05 cut-point.
Cardiac data were analyzed using the Emory Toolbox, a validated instrument for display and quantitation of cardiac SPECT imaging data (Garcia et al., 2007; Van Train et al., 1994), and were additionally scored by a Nuclear Medicine physician blinded to subject diagnosis using a 20-segment bull’s eye diagram of the heart. This diagram is used to rate perfusion abnormalities at rest and stress on a scale of 0 (normal) to 4 (absent perfusion) for each of 20 segments of the heart and to develop a quantitative index of perfusion with rest and stress. The myocardial perfusion score represented the stress score subtracted from the rest score. Ischemia was defined as a myocardial perfusion score of 3 or greater with at least one segment representing a moderate stress-induced perfusion defect (score of 2 in that segment).
PET brain images were realigned to the first image in the scanning session using statistical parametric mapping (spm). Images were then transformed into a common neuroanatomical space, smoothed, and subjected to statistical analysis using methods previously described by us in detail (Bremner et al., 1999; Fani et al., 2011). Analysis of variance was used to compare brain perfusion by voxel within hypothesized regions (anterior cingulate, medial prefrontal cortex) in CAD patients with and without depression during mental stress. Hypothesized regions as reviewed above were defined as the stereotactical coordinates of medial prefrontal cortex and anterior cingulate as defined by a common stereotactical atlas of the brain (Talairach and Tournoux, 1988). Significance was defined as a minimal cluster of 11 voxels with a p<0.005 in hypothesized areas (medial prefrontal cortex and anterior cingulate). This method minimizes Type I and Type II error (Lane et al., 1997; Reiman et al., 1997). Exploratory analyses of other brain areas were also performed and are displayed in the tables. with minimum voxel clusters of 11 voxels.
Results
Depressed patients (N=17) compared to non-depressed (N=21) patients with CAD had higher body mass index, fewer years of education, and more symptoms of depression (Table 1). There were no differences between groups in other factors including age, race, gender, rates of diabetes, smoking history, high cholesterol history, or hypertension. Depressed patients took more antidepressants, anxiolytics, and diuretics, but depressed and non-depressed CAD patients showed similar use of beta-blockers, angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers, statins, and vasodilators. Forty one percent of CAD patients with depression and 32% of CAD patients without depression had mental stress-induced myocardial ischemia, a difference that was not statistically significant.
Table 1.
Demographic and Risk Factors for CAD Patients with and without Depression1
| Non-Depressed (N=21) |
Depressed (N=17) |
|
|---|---|---|
| Age | 58 (10 SD) | 61 (6 SD) |
| Gender | 4 F/17 M | 5 F/12 M |
| Race | 38% AA/62% | 29% AA/65% |
| Cauc/3% Asian | Cauc/6% NA | |
| Years of Education |
16 (3 SD) | 14 (2 SD)* |
| BMI | 30 (5 SD) | 36 (8 SD)* |
| Ham-D Score | 14 (4 SD) | 2 (2 SD)* |
| Hypertension | 60% | 88% |
| Dyslipidemia | 65% | 75% |
| Diabetes | 25% | 50% |
| Smoking (current) |
10% | 13% |
| Smoking (lifetime) |
65% | 38% |
| Percentage of patients taking: | ||
| Antidepressants | 14% | 41% |
| ACE Inhibitors | 29% | 47% |
| Angiotensin Receptor Inhibitors |
10% | 18% |
| Diuretics | 5% | 41% |
| Vasodilators | 10% | 29% |
| Anxiolytics | 5% | 29% |
| Beta Blockers | 57% | 59% |
| Statins | 62% | 71% |
CAD=coronary artery disease; F=female; M=male; AA=African American; Cauc=Caucasian; NA=Native American; BMI=body mass index; Ham-D=Hamilton Depression Scale; ACE=angiotensin converting enzyme.
p<.05
In the group as a whole, stress resulted in increased activation in superior, frontal gyrus, anterior cingulate, orbital and rectal gyrus, parietal cortex (superior and inferior parietal lobules), superior temporal gyrus, amygdala / parahippocampal gyrus, and midbrain (Tables 2–7). When non-depressed and depressed patients were examined separately, only the non-depressed patients showed stress-induced activation in medial prefrontal cortex, which includes anterior cingulate, orbitofrontal cortex (orbital and rectal gyrus), and subcallosal gyrus (Table 2). Depressed patients additionally showed increased activation with stress in parietal cortex (angular gyrus) (Table 4), and direct comparison showed that depressed patients activated this area to a greater degree with stress than non-depressed patients (Table 6).
Table 2.
Areas of Increased Activation During Mental Arithmetic Stress in CAD Patients Without Depression
| Z score | Voxel Number |
Talairach Coordinates |
Brain region | BA | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 4.34 | 141 | −32 | 19 | −6 | L. Inferior Frontal Gyrus | 47 |
| 2.82 | −30 | 17 | −16 | L. Inferior Frontal Gyrus | 47 | |
| 4.85 | 102 | 32 | 13 | −16 | R. Inferior Frontal Gyrus | 47 |
| 4.80 | 1392 | 10 | 19 | 60 | R. Precentral Gyrus | 6 |
| 4.68 | −6 | 46 | 31 | L. Superior Frontal Gyrus | 9 | |
| 4.57 | 14 | 14 | 49 | R. Superior Frontal Gyrus | 6 | |
| 4.33 | 183 | 48 | 27 | 4 | R. Inferior Frontal Gyrus | 45 |
| 3.10 | 53 | 12 | 9 | R. Precentral Gyrus | 44 | |
| 4.17 | 86 | 34 | 22 | 45 | R. Middle Frontal Gyrus | 8 |
| 2.74 | 28 | 14 | 47 | R. Middle Frontal Gyrus | 6 | |
| 3.93 | 31 | 48 | 21 | 27 | R. Middle Frontal Gyrus | 46 |
| 3.59 | 80 | −18 | 60 | −10 | L. Superior Frontal Gyrus | 10 |
| 3.10 | −14 | 54 | −4 | L. Superior Frontal Gyrus | 10 | |
| 2.80 | −14 | 47 | −2 | L. Anterior Cingulate | 32 | |
| 3.70 | 78 | 22 | 44 | 29 | R. Superior Frontal Gyrus | 9 |
| 3.20 | 22 | 45 | 40 | R. Superior Frontal Gyrus | 8 | |
| 3.53 | 74 | 46 | 1 | 52 | R. Middle Frontal Gyrus | 6 |
| 3.52 | 27 | −38 | 13 | 27 | L. Middle Frontal Gyrus | 9 |
| 3.49 | 24 | 40 | 21 | −3 | R. Inferior Frontal Gyrus | 47 |
| 3.47 | 55 | 42 | 38 | −15 | R. Middle Frontal Gyrus | 11 |
| 2.72 | 40 | 35 | −8 | R. Middle Frontal Gyrus | 47 | |
| 3.19 | 32 | 4 | −26 | 71 | R. Medial Frontal Gyrus | 6 |
| 3.01 | 14 | −30 | 66 | R. Precentral Gyrus | 4 | |
| 3.19 | 20 | −34 | 30 | 26 | L. Middle Frontal Gyrus | 9 |
| 3.10 | 24 | 12 | 65 | 12 | R. Superior Frontal Gyrus | 10 |
| 3.09 | 10 | 10 | 59 | −20 | R. Superior Frontal Gyrus | 11 |
| 3.08 | 12 | −16 | 38 | −20 | L. Inferior Frontal Gyrus | 11 |
| 3.07 | 19 | 36 | 51 | 9 | R. Middle Frontal Gyrus | 10 |
| 3.18 | 22 | 12 | 42 | −17 | R. Middle Frontal Gyrus | 11 |
| 3.13 | 27 | 40 | 2 | 37 | R. Middle Frontal Gyrus | 6 |
| 2.96 | 40 | 3 | 26 | R. Precentral Gyrus | 6 | |
| 3.32 | 15 | −42 | −23 | 55 | L. Postcentral Gyrus | 3 |
| 3.26 | 11 | −14 | 41 | 7 | L. Anterior Cingulate | 32 |
| 3.20 | 14 | 0 | 37 | 9 | L. Anterior Cingulate | 24 |
| 3.18 | 19 | 4 | 38 | −25 | R. Rectal Gyrus | 11 |
| 3.88 | 22 | 10 | 26 | −20 | R. Rectal Gyrus | 11 |
| 3.45 | 52 | −12 | 25 | 41 | L. Sub-Gyral | 8 |
| 3.49 | 43 | 57 | −10 | −11 | R. Sub-Gyral | 21 |
| 3.54 | 68 | −8 | 57 | 12 | L. Medial Frontal Gyrus | 10 |
| 2.93 | 10 | −4 | −26 | 62 | L. Medial Frontal Gyrus | 6 |
| 2.83 | 14 | −12 | 49 | 16 | L. Medial Frontal Gyrus | 10 |
| 3.25 | 15 | 44 | −41 | 37 | R. Supramarginal Gyrus | 40 |
| 3.66 | 76 | 40 | −64 | 47 | R. Inferior Parietal Lobule | 7 |
| 4.01 | 49 | −38 | −63 | 53 | L. Superior Parietal Lobule | 7 |
| 4.00 | 135 | 48 | −48 | 47 | R. Inferior Parietal Lobule | 40 |
| 3.05 | 32 | −50 | 43 | R. Inferior Parietal Lobule | 40 | |
| 3.00 | 38 | −56 | 54 | R. Superior Parietal Lobule | 7 | |
| 3.97 | 26 | 55 | −43 | 30 | R. Inferior Parietal Lobule | 40 |
| 3.68 | 63 | −39 | −5 | R. Middle Temporal Gyrus | 21 | |
| 3.61 | 53 | −26 | −7 | R. Middle Temporal Gyrus | 21 | |
| 3.56 | 41 | 48 | 9 | −24 | R. Superior Temporal Gyrus | 38 |
| 3.13 | 51 | 3 | −27 | R. Middle Temporal Gyrus | 21 | |
| 3.82 | 56 | −46 | −22 | −7 | L. Superior Temporal Gyrus | 22 |
| 3.46 | 13 | 51 | −15 | −30 | R. Inferior Temporal Gyrus | 20 |
| 2.99 | 12 | 55 | −51 | −6 | R. Middle Temporal Gyrus | 37 |
| 4.39 | 268 | 59 | −21 | −1 | R. Superior Temporal Gyrus | 21 |
| 3.53 | 26 | 12 | 2 | −3 | R. Lentiform Nucleus (Globus Pallidus) | |
| 3.22 | 16 | 6 | 2 | R. Lentiform Nucleus (Putamen) | ||
| 4.21 | 40 | −14 | 8 | 3 | L. Putamen | |
| 3.41 | 11 | −18 | −9 | −2 | L. Parahippocampal Gyrus | 27 |
| 3.01 | 19 | 16 | −93 | −2 | R. Lingual Gyrus | 17 |
| 3.55 | 17 | 18 | −85 | 3 | R. Lingual Gyrus | 17 |
| 3.31 | 10 | 10 | −82 | −4 | R. Lingual Gyrus | 18 |
| 3.31 | 19 | 26 | −84 | −8 | R. Middle Occiptal Gyrus | 18 |
| 3.28 | 13 | −4 | −18 | −11 | L. Red Nucleus | |
| 5.17 | 1047 | −26 | −83 | −23 | L. Cerebellum | |
| 4.29 | −30 | −77 | −31 | L. Cerebellum | ||
| 4.18 | −18 | −80 | −36 | L. Cerebellum | ||
| 3.87 | 62 | −2 | −34 | −25 | L. Cerebellum | |
| 3.39 | 0 | −37 | −32 | L. Cerebellum | ||
| 3.94 | 31 | 12 | −52 | −21 | R. Cerebellum | |
| 3.75 | 40 | −14 | −53 | −14 | L. Cerebellum | |
| 3.68 | 136 | 10 | −77 | −21 | R. Cerebellum | |
| 3.63 | 4 | −71 | −18 | R. Cerebellum | ||
| 3.38 | 18 | −75 | −28 | R. Cerebellum | ||
| 3.57 | 109 | −6 | −52 | −39 | L. Cerebellum | |
| 3.55 | −4 | −64 | −29 | L. Cerebellum | ||
| 3.38 | 0 | −54 | −23 | L. Cerebellum | ||
| 3.43 | 60 | 30 | −75 | −27 | R. Cerebellum | |
| 3.19 | 40 | −75 | −21 | R. Cerebellum | ||
| 3.27 | 22 | −12 | −40 | −20 | L. Cerebellum | |
Table 7.
Areas of Greater Decreased Activation During Mental Arithmetic Stress in CAD Patients With Depression versus Without Depression
| Z score | Voxel Number |
Talairach Coordinates |
Brain region | BA | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 3.55 | 31 | −44 | −34 | 19 | L. Insula | 13 |
| 3.45 | 55 | 40 | −8 | −1 | R. Insula | 13 |
| 4.11 | 45 | −40 | 2 | −2 | L. Insula | 13 |
| 2.71 | −42 | −1 | −11 | L. Superior Temporal Gyrus | 13 | |
| 3.63 | 23 | −51 | −6 | −11 | L. Superior Temporal Gyrus | 21 |
| 14 | −50 | −35 | 5 | L. Middle Temporal Gyrus | 22 | |
| 3.46 | 33 | 50 | −60 | 5 | R. Middle Temporal Gyrus | 37 |
| 2.93 | 53 | −64 | 0 | R. Inferior Temporal Gyrus | 19 | |
| 3.23 | 12 | −59 | −39 | 5 | L. Middle Temporal Gyrus | 22 |
| 3.17 | 12 | 46 | 7 | −19 | R. Superior Temporal Gyrus | 38 |
| 3.63 | 45 | −46 | −20 | −6 | L. Superior Temporal Gyrus | 22 |
| 3.92 | 131 | 4 | −49 | 26 | R. Cingulate Gyrus | 31 |
| 3.46 | 8 | −35 | 34 | R. Cingulate Gyrus | 31 | |
| 3.16 | 24 | −8 | −27 | 40 | L. Cingulate Gyrus | 31 |
| 2.91 | −10 | −35 | 39 | L. Cingulate Gyrus | 31 | |
| 2.94 | 17 | 8 | 4 | 47 | R. Cingulate Gyrus | 24 |
| 3.26 | −2 | −43 | 24 | L. Posterior Cingulate | 23 | |
| 3.12 | 15 | 2 | −59 | 23 | R. Posterior Cingulate | 31 |
| 3.26 | 20 | −64 | 8 | R. Posterior Cingulate | 30 | |
| 3.06 | 13 | 10 | 44 | −15 | R. Orbital Gyrus | 11 |
| 3.92 | 59 | 12 | −82 | −5 | R. Lingual Gyrus | 18 |
| 3.28 | 18 | −83 | 2 | R. Lingual Gyrus | 17 | |
| 3.83 | 23 | −18 | −29 | −4 | L. Parahippocampal Gyrus | 27 |
| 3.43 | 16 | 6 | −80 | 33 | R. Cuneus | 19 |
| 3.58 | 20 | 12 | −83 | 19 | R. Cuneus | 18 |
| 3.06 | 13 | 18 | −52 | 45 | R. Precuneus | 7 |
| 3.00 | 12 | 0 | −71 | 27 | L. Precuneus | 31 |
| 2.81 | 4 | −67 | 22 | R. Precuneus | 31 | |
| 3.52 | 34 | −4 | −62 | 38 | L. Precuneus | 7 |
| 3.17 | 16 | 40 | −78 | −5 | R. Inferior Occipital Gyrus | 19 |
| 3.31 | 21 | −51 | −68 | 8 | L. Middle Occipital Gyrus | 19 |
| 3.48 | 19 | 42 | 21 | −5 | R. Inferior Frontal Gyrus | 47 |
| 3.19 | 15 | 28 | 7 | −13 | R. Inferior Frontal Gyrus | 47 |
| 3.71 | 28 | 0 | 34 | −18 | L. Medial Frontal Gyrus | 11 |
| 3.12 | 16 | −10 | 18 | 50 | L. Superior Frontal Gyrus | 6 |
| 3.59 | 70 | −4 | −28 | 57 | L. Paracentral Lobule | 6 |
| 3.44 | −10 | −37 | 66 | L. Paracentral Lobule | 4 | |
| 3.29 | 13 | 55 | −43 | 27 | R. Inferior Parietal Lobule | 40 |
| 3.27 | 39 | 51 | −1 | 17 | R. Precentral Gyrus | 6 |
| 3.25 | 18 | −42 | −23 | 50 | L. Postcentral Gyrus | 3 |
| 3.16 | 19 | −8 | −17 | 6 | L. Thalamus | |
| 5.06 | 183 | 42 | −61 | −15 | R. Cerebellum | |
| 3.35 | 32 | −59 | −14 | R. Cerebellum | ||
| 4.81 | 85 | −20 | −63 | −21 | L. Cerebellum | |
| 3.31 | −16 | −67 | −28 | L. Cerebellum | ||
| 4.36 | 196 | −14 | −53 | −16 | L. Cerebellum | |
| 3.47 | −12 | −40 | −20 | L. Cerebellum | ||
| 3.44 | −4 | −49 | −18 | L. Cerebellum | ||
| 3.01 | 15 | −26 | −85 | −25 | L. Cerebellum | |
| 2.97 | 28 | 34 | −58 | −41 | R. Cerebellum | |
| 3.20 | 13 | −40 | −71 | −15 | L. Cerebellum | |
| 3.41 | 10 | −20 | −88 | −18 | L. Cerebellum | |
| 3.28 | 13 | −42 | −50 | −21 | L. Cerebellum | |
| 4.02 | 123 | −10 | −62 | −39 | L. Cerebellum | |
| 3.24 | −4 | −51 | −44 | L. Cerebellum | ||
| 2.97 | −2 | −72 | −37 | L. Cerebellum | ||
| 3.92 | 93 | 24 | −52 | −34 | R. Cerebellum | |
| 3.77 | 22 | −62 | −28 | R. Cerebellum | ||
| 3.79 | 40 | 6 | −73 | −15 | R. Cerebellum | |
Table 4.
Areas of Increased Activation During Mental Arithmetic Stress in CAD Patients With Depression
| Z score | Voxel Number |
x | Talairach Coordinates |
BA | ||
|---|---|---|---|---|---|---|
| y | z | Brain region | ||||
| 4.02 | 196 | 51 | 24 | 17 | R. Inferior Frontal Gyrus | 45 |
| 3.43 | 42 | 15 | 23 | R. Inferior Frontal Gyrus | 46 | |
| 2.87 | 59 | 24 | 10 | R. Inferior Frontal Gyrus | 45 | |
| 3.61 | 87 | 24 | 27 | 10 | L. Inferior Frontal Gyrus | 47 |
| 3.54 | 30 | 20 | 18 | L. Inferior Frontal Gyrus | 47 | |
| 3.06 | 18 | 28 | 15 | L. Inferior Frontal Gyrus | 47 | |
| 3.39 | 25 | 28 | 17 | 16 | R. Inferior Frontal Gyrus | 47 |
| 3.25 | 22 | 32 | 25 | −3 | R. Inferior Frontal Gyrus | 47 |
| 2.85 | 28 | 29 | −8 | R. Inferior Frontal Gyrus | 47 | |
| 3.10 | 15 | 53 | 19 | 1 | R. Inferior Frontal Gyrus | 47 |
| 3.06 | 19 | 40 | 33 | −5 | R. Inferior Frontal Gyrus | 47 |
| 3.89 | 23 | 51 | 36 | 15 | R. Inferior Frontal Gyrus | 47 |
| 2.77 | 46 | 42 | 12 | R. Middle Frontal Gyrus | 11 | |
| 3.74 | 30 | 32 | 23 | 36 | R. Middle Frontal Gyrus | 9 |
| 3.63 | 55 | 44 | 33 | 6 | R. Interior Frontal Gyrus | 46 |
| 2.86 | 48 | 36 | 13 | R. Middle Frontal Gyrus | 46 | |
| 3.25 | 13 | 24 | 11 | 60 | R. Middle Frontal Gyrus | 6 |
| 3.10 | 15 | 28 | 5 | 53 | R. Middle Frontal Gyrus | 6 |
| 2.92 | 14 | 42 | 32 | 19 | R. Middle Frontal Gyrus | 46 |
| 2.85 | 29 | 30 | 10 | 40 | R. Middle Frontal Gyrus | 6 |
| 2.78 | 30 | 14 | 47 | R. Middle Frontal Gyrus | 6 | |
| 3.53 | 84 | 46 | 12 | 38 | R. Middle Frontal Gyrus | 8 |
| 3.43 | 46 | 4 | 42 | R. Middle Frontal Gyrus | 6 | |
| 3.29 | 23 | 32 | 18 | 53 | R. Middle Frontal Gyrus | 6 |
| 2.80 | 34 | 11 | 55 | R. Middle Frontal Gyrus | 6 | |
| 3.64 | 34 | 8 | 65 | 17 | R. Superior Frontal Gyrus | 10 |
| 3.29 | 32 | 10 | 34 | 52 | L. Superior Frontal Gyrus | 6 |
| 3.04 | 14 | 26 | 47 | L. Superior Frontal Gyrus | 8 | |
| 4.00 | 182 | 8 | 18 | 58 | R. Superior Frontal Gyrus | 6 |
| 3.71 | 12 | 26 | 56 | R. Superior Frontal Gyrus | 6 | |
| 3.04 | 10 | 31 | 43 | R. Superior Frontal Gyrus | 8 | |
| 3.31 | 107 | 34 | 61 | 53 | R. Superior Parietal Lobule | 7 |
| 3.30 | 38 | 54 | 56 | R. Superior Parietal Lobule | 7 | |
| 2.98 | 44 | 52 | 45 | R. Inferior Parietal Lobule | 40 | |
| 3.30 | 25 | 55 | 39 | 39 | R. Interior Parietal Lobule | 40 |
| 3.31 | 14 | 46 | 62 | 49 | L. Inferior Parietal Lobule | 40 |
| 3.48 | 18 | 51 | 66 | 33 | L. Angular Gyrus | 39 |
| 3.61 | 30 | −2 | 42 | 15 | L. Medial Frontal Gyrus | 9 |
| 3.82 | 108 | −4 | 33 | 41 | L. Medial Frontal Gyrus | 8 |
| 3.19 | 12 | 34 | 26 | L. Medial Frontal Gyrus | 9 | |
| 4.19 | 318 | 16 | 39 | 11 | R. Anterior Cingulate | 32 |
| 3.97 | 12 | 45 | 5 | R. Frontal Medial Gyrus | 10 | |
| 3.93 | 14 | 47 | 12 | R. Frontal Medial Gyrus | 10 | |
| 4.13 | 28 | 6 | 9 | 14 | R. Subcallosal Gyrus | 25 |
| 4.33 | 375 | 57 | 55 | 11 | R. Inferior Temporal Gyrus | 20 |
| 4.20 | 59 | 29 | −2 | R. Middle Temporal Gyrus | 21 | |
| 3.59 | 61 | 22 | 11 | R. Middle Temporal Gyrus | 21 | |
| 4.21 | 23 | 48 | 6 | 27 | R. Middle Temporal Gyrus | 21 |
| 4.08 | 46 | 61 | 43 | 10 | L. Middle Temporal Gyrus | 21 |
| 2.78 | 55 | 31 | −2 | L. Middle Temporal Gyrus | 21 | |
| 2.98 | 14 | 34 | 22 | 21 | R. Superior Temporal Gyrus | 38 |
| 3.06 | 13 | 44 | 60 | 39 | R. Cerebellum | |
| 3.55 | 72 | 36 | 83 | 23 | L. Cerebellum | |
| 3.17 | 26 | 79 | 21 | L. Cerebellum | ||
| 2.96 | 18 | 79 | 28 | L. Cerebellum | ||
Table 6.
Areas of Greater Increased Activation During Mental Arithmetic Stress in CAD Patients With Depression compared to CAD Patients without Depression
| Z score | Voxel Number |
Talairach Coordinates |
Brain region | BA | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 3.62 | 29 | −51 | −66 | 33 | L. Angular Gyrus | 39 |
| 3.53 | 24 | 59 | −41 | 39 | R. Inferior Parietal Lobule | 40 |
| 3.41 | 16 | 8 | 9 | −12 | R. Subcallosal Gyrus | |
| 3.12 | 24 | 16 | 39 | 11 | R. Anterior Cingulate | 32 |
| 2.89 | 12 | 18 | 44 | 54 | Left Precuneus | |
Stress in the group as a whole resulted in decreased activation in insula, postcentral and precentral gyrus, subcallosal gyrus, posterior cingulate, lingual gyrus, cuneus, and uncus (Tables 3 and 5). Depressed patients alone additionally showed decreases in precuneus, fusiform gyrus, thalamus, paracentral lobule, and lateral geniculate body (Table 5). When depressed patients were compared to nondepressed patients there were significantly greater stress-induced decreases in insula, post and precentral gyrus, anterior cingulate, subcallosal gyrus, posterior cingulate, lingual gyrus, cuneus, precuneus, middle occipital gyrus, thalamus, and paracentral lobule (Table 7).
Table 3.
Areas of Decreased Activation During Mental Arithmetic Stress in CAD Patients Without Depression
| Z score | Voxel Number |
Talairach Coordinates |
Brain region | BA | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 4.51 | 927 | −42 | −6 | 4 | L. Insula | 13 |
| 4.03 | −59 | −22 | 18 | L. Postcentral Gyrus | 40 | |
| 3.89 | −51 | −20 | 19 | L. Insula | 40 | |
| 3.68 | 17 | 42 | −18 | −6 | R. Insula | 13 |
| 3.86 | 41 | 44 | −1 | −12 | R. Superior Temporal Gyrus | 38 |
| 2.68 | 44 | 7 | −7 | R. Insula | 13 | |
| 3.68 | 17 | 42 | −18 | −6 | R. Insula | 13 |
| 4.40 | 721 | 65 | −1 | 28 | R. Precentral Gyrus | 6 |
| 4.03 | 65 | −22 | 23 | R. Postcentral Gyrus | 40 | |
| 3.73 | 61 | −8 | 26 | R. Precentral Gyrus | 4 | |
| 4.09 | 22 | 10 | −74 | 2 | R. Lingual Gyrus | 18 |
| 3.87 | 17 | 12 | 13 | 29 | R. Cingulate Gyrus | 24 |
| 3.54 | 51 | −51 | −4 | 41 | L. Precentral Gyrus | 6 |
| 3.47 | 32 | −65 | −16 | 23 | L. Postcentral Gyrus | 1 |
| 3.69 | 145 | −51 | −36 | 24 | L. Inferior Parietal Lobule | 40 |
| 3.46 | −59 | −32 | 29 | L. Inferior Parietal Lobule | 40 | |
| 3.08 | −63 | −39 | 30 | L. Inferior Parietal Lobule | 40 | |
| 3.69 | 60 | −50 | −62 | −2 | L. Inferior Temporal Gyrus | 19 |
| 3.57 | −57 | −60 | 3 | L. Middle Temporal Gyrus | 21 | |
| 3.07 | 16 | −61 | −52 | 14 | L. Superior Temporal Gyrus | 22 |
| 3.07 | 11 | −46 | −19 | 5 | L. Superior Temporal Gyrus | 22 |
| 2.82 | 11 | −26 | −89 | 15 | L. Middle Occipital Gyrus | 18 |
| 3.47 | 20 | 53 | −68 | 9 | R. Middle Occipital Gyrus | 19 |
| 2.81 | 50 | −70 | 2 | R. Middle Occipital Gyrus | 37 | |
| 3.40 | 38 | −18 | 7 | −19 | L. Uncus | 34 |
| 3.39 | 15 | 28 | 8 | 1 | R. Lentiform Nucleus (Putamen) | |
| 3.37 | 24 | −14 | −17 | 56 | L. Medial Frontal Gyrus | 6 |
| 3.34 | 28 | −6 | −64 | 31 | L. Cuneus | 7 |
| 3.33 | 11 | −20 | −64 | 31 | L. Precuneus | 7 |
| 3.18 | 13 | −8 | −56 | 40 | L. Precuneus | 7 |
Table 5.
Areas of Decreased Activation During Mental Arithmetic Stress in CAD Patients With Depression
| Z score | Voxel Number |
x | Talairach Coordinates |
BA | ||
|---|---|---|---|---|---|---|
| y | z | Brain region | ||||
| 5.69 | 3555 | −40 | 2 | −3 | L. Insula | 13 |
| 5.60 | −44 | −32 | 18 | L. Superior Temporal Gyrus | 41 | |
| 5.28 | −61 | −22 | 18 | L. Postcentral Gyrus | 40 | |
| 5.51 | 2202 | 61 | −3 | 15 | R. Middle Temporal Gyrus | 21 |
| 4.94 | 40 | −8 | −1 | R. Insula | ||
| 4.87 | 61 | 2 | 7 | R. Precentral Gyrus | 6 | |
| 3.22 | 13 | −28 | −14 | 67 | L. Precentral Gyrus | 6 |
| 3.02 | 11 | 22 | −12 | 67 | R. Precentral Gyrus | 6 |
| 3.01 | 17 | −10 | −31 | 70 | L. Postcentral Gyrus | 3 |
| 3.40 | 57 | 55 | −34 | 22 | R. Insula | 13 |
| 3.09 | 53 | −26 | 18 | R. Postcentral Gyrus | 40 | |
| 2.64 | 59 | −26 | 25 | R. Inferior Parietal Lobule | 40 | |
| 3.01 | 15 | −40 | −25 | 36 | L. Postcentral Gyrus | 2 |
| 4.36 | 69 | −32 | −84 | 24 | L. Superior Occipital Gyrus | 19 |
| 3.38 | 44 | −40 | −79 | 11 | L. Middle Occipital Gyrus | 19 |
| 3.23 | 23 | 42 | −79 | 11 | R. Middle Occipital Gyrus | 19 |
| 3.21 | 15 | −26 | −87 | 6 | L. Middle Occipital Gyrus | 19 |
| 4.18 | 96 | 4 | −78 | 28 | R. Cuneus | 18 |
| 3.92 | 6 | −80 | 35 | R. Cuneus | 19 | |
| 3.69 | −2 | −86 | 26 | L. Cuneus | 19 | |
| 3.31 | 26 | −16 | −79 | 22 | L. Cuneus | 18 |
| 3.21 | 43 | −12 | −82 | 34 | L. Cuneus | 19 |
| 3.40 | 26 | 20 | −93 | 14 | R. Cuneus | 18 |
| 3.01 | 11 | 12 | −81 | 21 | R. Cuneus | 18 |
| 3.20 | 48 | 10 | −68 | 38 | R. Precuneus | 7 |
| 2.93 | 12 | −67 | 27 | R. Precuneus | 31 | |
| 3.20 | 14 | 18 | −52 | 50 | R. Precuneus | 7 |
| 4.34 | 306 | −6 | −64 | 42 | L. Precuneus | 7 |
| 3.88 | −2 | −68 | 29 | L. Precuneus | 31 | |
| 3.42 | 4 | −57 | 21 | R. Posterior Cingulate | 23 | |
| 4.06 | 177 | −2 | −43 | 26 | L. Cingulate Gyrus | 31 |
| 3.58 | 4 | −39 | 37 | R. Cingulate Gyrus | 31 | |
| 3.24 | 4 | −47 | 30 | R. Precuneus | 31 | |
| 3.37 | 11 | 4 | 26 | 17 | R. Anterior Cingulate | 24 |
| 3.95 | 87 | 0 | 13 | 29 | L. Cingulate Gyrus | 24 |
| 3.16 | −4 | 8 | 36 | L. Cingulate Gyrus | 24 | |
| 3.08 | 2 | 4 | 31 | R. Cingulate Gyrus | 24 | |
| 3.91 | 61 | −8 | −15 | 3 | L. Thalamus | |
| 3.85 | 44 | −8 | −15 | 41 | L. Cingulate Gyrus | 24 |
| 2.88 | −4 | −12 | 36 | L. Cingulate Gyrus | 24 | |
| 3.63 | 68 | −8 | −29 | 44 | L. Paracentral Lobule | 31 |
| 3.32 | −14 | −37 | 41 | L. Cingulate Gyrus | 31 | |
| 3.43 | 16 | 10 | 8 | 40 | R. Cingulate Gyrus | 32 |
| 2.95 | 12 | 8 | −25 | 34 | R. Cingulate Gyrus | 31 |
| 2.94 | 16 | −6 | 43 | R. Cingulate Gyrus | 24 | |
| 3.15 | 92 | −10 | −63 | 14 | L. Posterior Cingulate | 30 |
| 3.15 | −4 | −54 | 12 | L. Posterior Cingulate | 29 | |
| 3.50 | 18 | −16 | −58 | 7 | L. Posterior Cingulate | 30 |
| 3.71 | 38 | −6 | 1 | 59 | L. Medial Frontal Gyrus | 6 |
| 3.70 | 54 | 4 | 27 | −13 | R. Medial Frontal Gyrus | 11 |
| 3.07 | 0 | 34 | −13 | L. Medial Frontal Gyrus | 11 | |
| 3.41 | 37 | 8 | −5 | 50 | R. Medial Frontal Gyrus | 6 |
| 3.52 | 42 | 26 | 3 | −10 | R. Subcallosal Gyrus | 34 |
| 2.99 | 22 | 24 | −21 | −1 | R. Lateral Geniculum Body | |
| 2.98 | 13 | 18 | −40 | 48 | R. Paracentral Lobule | 5 |
| 3.05 | 24 | 10 | −11 | 43 | R. Paracentral Lobule | 31 |
| 3.50 | 44 | 50 | −58 | 8 | R. Middle Temporal Gyrus | 39 |
| 3.13 | 55 | −64 | 3 | R. Middle Temporal Gyrus | 37 | |
| 4.02 | 148 | −44 | −62 | 7 | L. Middle Temporal Gyrus | 37 |
| 3.39 | −50 | −68 | 9 | L. Middle Occipital Gyrus | 19 | |
| 2.93 | −50 | −58 | 14 | L. Superior Temporal Gyrus | 22 | |
| 2.91 | 21 | 50 | −67 | 16 | R. Middle Temporal Gyrus | 39 |
| 5.47 | 759 | 42 | −61 | −14 | R. Fusiform Gyrus | 37 |
| 3.35 | 24 | −40 | −71 | −12 | L. Fusiform Gyrus | 19 |
| 3.45 | 29 | −18 | 5 | −20 | L. Uncus | 34 |
| 4.12 | 113 | 16 | −68 | 2 | R. Lingual Gyrus | 18 |
| 2.84 | 22 | −74 | 0 | R. Lingual Gyrus | 18 | |
| 3.29 | 22 | −12 | −74 | −3 | R. Lingual Gyrus | 18 |
| 3.16 | 13 | 12 | −80 | −6 | R. Lingual Gyrus | 18 |
| 2.89 | 13 | −2 | −80 | 2 | L. Lingual Gyrus | 18 |
| 5.41 | 265 | −20 | −63 | −17 | L. Cerebellum | |
| 3.83 | −10 | −55 | −17 | L. Cerebellum | ||
| 3.52 | −26 | −70 | −8 | L. Lingual Gyrus | 18 | |
| 4.04 | 64 | −22 | −45 | −16 | L. Cerebellum | |
| 3.11 | 14 | −18 | −54 | −36 | L. Cerebellum | |
| 3.73 | 98 | 24 | −42 | −21 | R. Cerebellum | |
| 2.83 | 34 | −46 | −30 | R. Cerebellum | ||
| 3.63 | 22 | −42 | −49 | −18 | L. Cerebellum | |
| 3.69 | 37 | −10 | −62 | −36 | L. Cerebellum | |
| 3.65 | 26 | 26 | −52 | −31 | R. Cerebellum | |
| 4.90 | 22 | −61 | −20 | R. Cerebellum | ||
| 4.49 | 30 | −57 | −12 | R. Cerebellum | ||
Depressed patients without mental stress-induced myocardial ischemia (MSI) showed increased activation with stress in the parietal cortex (Table 8) and decreased activation in cingulate, lingual gyrus, cuneus, inferior temporal gyrus, middle and inferior occipital gyrus, thalamus and paracentral lobule (Table 9)
Table 8.
Areas of Increased Activation During Mental Arithmetic Stress in CAD Patients With Depression Without Stress-Induced Myocardial Ischemia
| Z score | Voxel Number |
Talairach Coordinates |
Brain region | BA | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 4.39 | 203 | 18 | 41 | 13 | R. Medial Frontal Gyrus | 10 |
| 3.67 | 18 | 49 | 9 | R. Medial Frontal Gyrus | 10 | |
| 3.30 | 14 | 8 | 29 | 34 | R. Medial Frontal Gyrus | 6 |
| 3.78 | 80 | 10 | 44 | 29 | R. Medial Frontal Gyrus | 9 |
| 3.37 | 10 | 43 | 40 | R. Superior Frontal Gyrus | 8 | |
| 3.11 | 15 | 8 | 63 | 17 | R. Medial Frontal Gyrus | 10 |
| 4.05 | 208 | 46 | 33 | 2 | R. Inferior Frontal Gyrus | 46 |
| 3.83 | 51 | 24 | 19 | R. Inferior Frontal Gyrus | 45 | |
| 3.45 | 44 | 36 | 20 | R. Middle Frontal Gyrus | 46 | |
| 3.84 | 52 | 51 | 36 | −15 | R. Inferior Frontal Gyrus | 47 |
| 2.97 | 55 | 36 | −9 | R. Inferior Frontal Gyrus | 47 | |
| 3.95 | 94 | 59 | −22 | −11 | R. Middle Temporal Gyrus | 21 |
| 3.87 | 63 | −12 | −4 | R. Middle Temporal Gyrus | 21 | |
| 3.88 | 21 | −42 | 4 | −42 | L. Middle Temporal Gyrus | 38 |
| 3.18 | 18 | 50 | 4 | −27 | R. Middle Temporal Gyrus | 21 |
| 3.69 | 15 | −55 | −16 | −9 | L. Middle Temporal Gyrus | 21 |
| 3.25 | 13 | 10 | 18 | 58 | R. Superior Frontal Gyrus | 6 |
| 3.20 | 16 | −2 | 31 | 44 | L. Superior Frontal Gyrus | 8 |
| 3.65 | 32 | 20 | 50 | −11 | R. Middle Frontal Gyrus | 11 |
| 3.53 | 66 | 59 | −31 | −7 | R. Middle Temporal Gyrus | 21 |
| 3.09 | 59 | −29 | 3 | R. Superior Frontal Gyrus | 22 | |
| 3.47 | 72 | 48 | 10 | 47 | R. Middle Frontal Gyrus | 6 |
| 2.90 | 46 | 4 | 40 | R. Middle Frontal Gyrus | 6 | |
| 2.77 | 46 | 12 | 38 | R. Middle Frontal Gyrus | 8 | |
| 3.44 | 34 | 55 | 29 | −5 | R. Inferior Frontal Gyrus | 47 |
| 3.20 | 17 | −30 | 19 | −18 | L. Inferior Frontal Gyrus | 47 |
| 3.12 | 16 | −50 | 34 | −15 | L. Inferior Frontal Gyrus | 47 |
| 3.40 | 11 | 18 | 17 | 36 | R. Cingulate Gyrus | 32 |
| 3.00 | 12 | 46 | −70 | 37 | R. Precuneus | 39 |
| 2.94 | 16 | −28 | −75 | 44 | L. Superior Parietal Lobule | 7 |
| 2.86 | 24 | 18 | 24 | 49 | R. Superior Frontal Gyrus | 8 |
| 3.38 | 53 | 34 | −63 | 53 | R. Superior Parietal Lobule | 7 |
| 3.16 | 13 | 44 | −52 | 45 | R. Inferior Parietal Lobule | 40 |
| 4.25 | 94 | −12 | −79 | −35 | L. Cerebellum | |
| 2.77 | −26 | −79 | −35 | L. Cerebellum | ||
| 3.07 | 20 | 12 | −81 | −20 | R. Cerebellum | |
| 2.69 | 10 | −83 | −26 | R. Cerebellum | ||
| 3.90 | 43 | −26 | −79 | −21 | L. Cerebellum | |
| 3.98 | 66 | 20 | −77 | −30 | R. Cerebellum | |
Table 9.
Areas of Decreased Activation During Mental Arithmetic Stress in CAD Patients With Depression Without Stress-Induced Myocardial Ischemia
| Z score | Voxel Number |
Talairach Coordinates |
Brain region | BA | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 4.83 | 2360 | −40 | 2 | −2 | L. Insula | 13 |
| 4.81 | −48 | −12 | 28 | L. Precentral Gyrus | 6 | |
| 4.71 | −40 | 2 | 7 | L. Insula | 13 | |
| 4.80 | 1266 | 40 | −12 | 2 | R. Insula | 13 |
| 4.20 | 42 | −15 | 15 | R. Insula | 13 | |
| 4.18 | 59 | −1 | 22 | R. Precentral Gyrus | 6 | |
| 3.04 | 18 | −34 | −17 | 12 | L Insula | 13 |
| 2.83 | 53 | −26 | 16 | R Postcentral Gyrus | 43,40 | |
| 3.25 | 15 | 67 | −32 | 16 | R Postcentral Gyrus | 43,40 |
| 3.23 | 46 | −4 | −56 | 12 | L Posterior Cingulate | 30,23 |
| 2.95 | 2 | −55 | 19 | Posterior Cingulate | 23,30 | |
| 3.39 | 28 | 65 | −18 | 19 | R Postcentral Gyrus | 43,40 |
| 2.90 | 16 | 24 | −14 | 67 | R Precentral Gyrus | 6 |
| 2.98 | 18 | −14 | −32 | 68 | L Paracentral Lobule | 4 |
| 3.28 | 23 | 20 | −40 | 46 | R Cingulate Gyrus | 31 |
| 4.01 | 107 | −2 | −45 | 26 | L. Cingulate Gyrus | 31 |
| 3.76 | 131 | 12 | −10 | 43 | R. Cingulate Gyrus | 31 |
| 3.61 | −8 | −15 | 41 | L. Cingulate Gyrus | 24 | |
| 3.28 | 10 | −5 | 50 | R. Cingulate Gyrus | 24 | |
| 3.42 | 46 | 10 | −28 | 33 | R. Cingulate Gyrus | 23 |
| 2.59 | 8 | −37 | 31 | R. Cingulate Gyrus | 31 | |
| 3.51 | 43 | 0 | 28 | −13 | L. Medial Frontal Gyrus | 11 |
| 3.63 | 29 | −6 | −25 | 53 | L. Medial Frontal Gyrus | 6 |
| 2.73 | −8 | −27 | 44 | L. Cingulate Gyrus | 31 | |
| 3.08 | 27 | −2 | 11 | 29 | L Anterior Cingulate | 24 |
| 3.25 | 33 | −6 | 2 | 42 | R Anterior Cingulate | 32,24 |
| 3.83 | 32 | 26 | 3 | −10 | R. Subcallosal Gyrus | 34 |
| 3.28 | 14 | −16 | −37 | 41 | L Posterior Cingulate | 31 |
| 3.16 | 20 | 6 | −37 | 39 | L Posterior Cingulate | 31 |
| 3.77 | 26 | −40 | 39 | −4 | L. Middle Frontal Gyrus | 47 |
| 3.31 | 42 | −40 | −3 | 55 | L Middle Frontal Gyrus | 6 |
| 2.94 | 20 | 34 | −74 | −11 | R Fusiform Gyrus | 19 |
| 4.00 | 13 | −20 | −88 | −14 | L. Fusiform Gyrus | 18 |
| 3.36 | 13 | −28 | −85 | 6 | L Middle Occipital Gyrus | 19 |
| 3.27 | 13 | 24 | −92 | −11 | R Inf. Occipital Gyrus | 18 |
| 3.75 | 29 | 40 | −80 | −3 | R. Inf. Occipital Gyrus | 19 |
| 3.66 | 36 | −30 | −84 | 26 | L. Sup. Occipital Gyrus | 19 |
| 3.21 | 12 | −50 | −46 | 8 | L Sup. Temporal Gyrus | 22 |
| 3.19 | 22 | 36 | −81 | 10 | R Middle Occipital Gyrus | 19 |
| 3.15 | 12 | −28 | −71 | −12 | L Middle Occipital Gyrus | 19 |
| 2.90 | 23 | −36 | −79 | 13 | L Middle Occipital Gyrus | 18 |
| 2.74 | −40 | −79 | 6 | L Middle Occipital Gyrus | 18 | |
| 2.98 | 11 | −2 | −78 | 4 | Lingual Gyrus | 18 |
| 3.63 | 57 | 14 | −68 | 5 | L. Lingual Gyrus | 18 |
| 2.70 | 20 | −74 | 2 | R. Lingual Gyrus | 18 | |
| 3.60 | 31 | 22 | −72 | 28 | R. Precuneus | 31 |
| 3.06 | 18 | 14 | −68 | 33 | R. Precuneus | 7 |
| 3.30 | 21 | −16 | −79 | 22 | L Cuneus | 18 |
| 3.46 | 29 | −2 | −68 | 33 | L. Cuneus | 7 |
| 3.56 | 42 | −10 | −80 | 35 | L. Cuneus | 19 |
| 4.42 | 52 | −6 | −16 | 1 | L. Thalamus | |
| 4.24 | 200 | −46 | −60 | 10 | L. Mid. Temporal Gyrus | 39 |
| 3.94 | −51 | −64 | −2 | L. Inf. Temporal Gyrus | 19 | |
| 3.35 | 56 | 50 | −25 | 9 | R Sup. Temporal Gyrus | 22 |
| 4.16 | 480 | 20 | −59 | −22 | R. Cerebellum | |
| 4.12 | 22 | −42 | −21 | R. Cerebellum | ||
| 3.93 | 24 | −57 | −14 | R. Cerebellum | ||
| 3.56 | 153 | −20 | −61 | −19 | L. Cerebellum | |
| 3.27 | −10 | −51 | −16 | L. Cerebellum | ||
| 3.18 | −10 | −61 | −14 | L. Cerebellum | ||
Depressed patients with MSI had increased activation in middle frontal gyrus and rostral anterior cingulate with stress (Table 10) and decreased function in caudal anterior cingulate, putamen, and superior occipital gyrus (Table 11).
Table 10.
Areas of Increased Activation During Mental Arithmetic Stress in CAD Patients With Depression and Stress-Induced Myocardial Ischemia
| Z score | Voxel Number |
Talairach Coordinates |
Brain region | BA | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 4.43 | 109 | 57 | −53 | −11 | R. Middle Temporal Gyrus | 37 |
| 3.91 | 25 | 40 | 6 | −37 | R. Middle Temporal Gyrus | 38 |
| 3.36 | 38 | 14 | −38 | R. Middle Temporal Gyrus | 38 | |
| 3.40 | 12 | 48 | −2 | −40 | R. Middle Temporal Gyrus | 38 |
| 3.24 | 18 | −61 | −45 | −10 | L. Middle Temporal Gyrus | 21 |
| 2.92 | 11 | 20 | −78 | −8 | R. Cuneus | 17 |
| 3.57 | 12 | 36 | −80 | 41 | R. Precuneus | 19 |
| 3.97 | 64 | 10 | 22 | 47 | R. Medial Frontal Gyrus | 8 |
| 3.13 | 14 | −2 | 35 | 41 | L. Medial Frontal Gyrus | 8 |
| 3.58 | 104 | 16 | 46 | 23 | R. Superior Frontal Gyrus | 9 |
| 3.51 | 12 | 40 | 16 | R. Medial Frontal Gyrus | 9 | |
| 2.94 | 13 | 20 | 66 | 8 | R. Superior Frontal Gyrus | 10 |
| 3.35 | 24 | 38 | 37 | −7 | R. Middle Frontal Gyrus | 47 |
| 3.52 | 32 | 36 | −44 | 43 | R. Inferior Parietal Lobule | 40 |
| 3.29 | 69 | 57 | 33 | 6 | R. Inferior Parietal Lobule | 46 |
| 3.11 | 51 | 36 | 13 | R. Middle Frontal Gyrus | 46 | |
| 3.01 | 44 | 33 | 4 | R. Inferior Frontal Gyrus | 46 | |
| 2.97 | 15 | −48 | 25 | 26 | L. Middle Frontal Gyrus | 46 |
| 2.98 | 14 | 12 | 34 | −10 | R. Anterior Cingulate | 10 |
| 3.26 | 20 | 8 | 45 | −2 | R. Anterior Cingulate | 32 |
| 3.29 | 23 | −28 | 20 | 3 | L. Claustrum | |
| 3.11 | 12 | 14 | 47 | −23 | R. Orbital Gyrus | 11 |
| 3.08 | 16 | 4 | −54 | −28 | R. Cerebellum | |
| 2.90 | 24 | −28 | −62 | −36 | L. Cerebellum | |
| 2.84 | −28 | −60 | −27 | L. Cerebellum | ||
| 2.68 | −34 | −64 | −30 | L. Cerebellum | ||
Table 11.
Areas of Decreased Activation During Mental Arithmetic Stress in CAD Patients With Depression and Stress-Induced Myocardial Ischemia
| Z score | Voxel Number |
Talairach Coordinates |
Brain region | BA | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 5.34 | 451 | −10 | −64 | 36 | L. Precuneus | 7 |
| 3.82 | 2 | −63 | 23 | R. Precuneus | 31 | |
| 3.33 | −16 | −55 | 30 | L. Cingulate Gyrus | 31 | |
| 3.71 | 128 | 16 | −53 | 32 | R. Precuneus | 31 |
| 3.52 | 14 | −65 | 27 | R. Precuneus | 31 | |
| 2.94 | 18 | −63 | 20 | R. Precuneus | 31 | |
| 3.21 | 28 | 14 | −54 | 54 | R. Precuneus | 7 |
| 2.74 | 8 | −48 | 48 | R. Precuneus | 7 | |
| 3.18 | 23 | −18 | −86 | 37 | L. Cuneus | 19 |
| 2.93 | 18 | 10 | −84 | 34 | R. Cuneus | 19 |
| 2.63 | 2 | −82 | 39 | R. Cuneus | 19 | |
| 3.67 | 24 | 24 | −86 | 28 | R. Cuneus | 19 |
| 4.31 | 209 | −42 | −23 | 12 | L. Transv. Temporal Gyrus | 41 |
| 4.09 | −46 | −32 | 18 | L. Insula | 13 | |
| 3.53 | 61 | 34 | 4 | −5 | R. Claustrum | |
| 3.28 | 36 | −8 | −6 | R. Claustrum | ||
| 2.85 | 42 | −6 | −1 | R. Insula | 13 | |
| 3.09 | 19 | −34 | −86 | 25 | L. Superior Occipital Gyrus | 19 |
| 4.04 | 51 | −50 | −35 | 29 | L. Inferior Parietal Lobule | 40 |
| 4.04 | 89 | −53 | −61 | 16 | L. Middle Temporal Gyrus | 19 |
| 4.00 | −59 | −55 | 21 | L. Superior Temporal Gyrus | 22 | |
| 3.96 | 20 | 50 | −54 | 8 | R. Superior Temporal Gyrus | 39 |
| 3.15 | 18 | 44 | −16 | −8 | L. Superior Temporal Gyrus | 22 |
| 3.92 | 59 | 51 | −67 | 16 | R. Middle Temporal Gyrus | 39 |
| 3.53 | 35 | −30 | −7 | 6 | L. Putamen | |
| 3.71 | 61 | 0 | −41 | 26 | L. Cingulate Gyrus | 31 |
| 2.65 | −4 | −45 | 34 | L. Cingulate Gyrus | 31 | |
| 3.41 | 18 | −6 | 8 | 38 | L. Cingulate Gyrus | 32 |
| 3.53 | 89 | −6 | −41 | 44 | L. Cingulate Gyrus | 31 |
| 3.26 | −6 | −27 | 38 | L. Cingulate Gyrus | 31 | |
| 2.77 | −14 | −31 | 42 | L. Cingulate Gyrus | 31 | |
| 3.52 | 23 | −14 | −63 | 12 | L. Posterior Cingulate | 30 |
| 3.45 | 17 | 18 | 50 | −6 | R. Medial Frontal Gyrus | 10 |
| 3.44 | 37 | −40 | −10 | 11 | L. Sub Gyral | 21 |
| 3.30 | 25 | 26 | 40 | 18 | R. Middle Frontal Gyrus | 10 |
| 3.24 | 24 | 16 | −33 | 3 | R. Thalamus | |
| 3.05 | 19 | 36 | −36 | 55 | R. Postcentral Gyrus | 40 |
| 3.73 | 27 | −63 | −22 | 18 | L. Postcentral Gyrus | 40 |
| 3.71 | 99 | −63 | −9 | 19 | L. Postcentral Gyrus | 43 |
| 3.55 | −57 | 3 | 13 | L. Precentral Gyrus | 6 | |
| 3.56 | 31 | 55 | −6 | 37 | R. Precentral Gyrus | 6 |
| 3.30 | 21 | 61 | −14 | 32 | R. Precentral Gyrus | 4 |
| 3.19 | 14 | 51 | −1 | 26 | R. Precentral Gyrus | 6 |
| 3.19 | 14 | 42 | −7 | 45 | R. Precentral Gyrus | 6 |
| 2.69 | 42 | −10 | 36 | R. Precentral Gyrus | 6 | |
| 3.11 | 28 | 61 | −5 | 13 | R. Precentral Gyrus | 43 |
| 3.17 | 12 | −40 | −6 | 41 | L. Precentral Gyrus | 6 |
| 2.99 | 22 | 34 | −80 | 11 | R. Fusiform Gyrus | 19 |
| 3.88 | 22 | 22 | −77 | 28 | R. Cerebellum | |
| 3.14 | 18 | 26 | −46 | 21 | R. Cerebellum | |
| 3.30 | 19 | −24 | −61 | 15 | L. Cerebellum | |
Comparison of ischemic to non-ischemic showed they had greater activations with stress in the rostral anterior cingulate (Table 12, Figure 2) and greater decreases with stress in dorsal anterior cingulate (Table 13).
Table 12.
Areas of Greater Increases in Activation During Mental Arithmetic Stress in CAD Patients With Depression With Compared to Those Without Stress-Induced Myocardial Ischemia
| Z score | Voxel Number |
Talairach Coordinates |
Brain region | BA | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 4.18 | 52 | −57 | −33 | 44 | L. Inferior Parietal Lobule | 40 |
| 2.82 | 12 | 63 | −31 | 42 | R. Inferior Parietal Lobule | 40 |
| 3.00 | 12 | 61 | −49 | 26 | R. Supramarginal Gyrus | 40 |
| 3.84 | 72 | 8 | 45 | −2 | R. Anterior Cingulate | 32 |
| 3.13 | 10 | 36 | 10 | R. Medial Frontal Gyrus | 10 | |
| 3.31 | 16 | 10 | 22 | 47 | R. Medial Frontal Gyrus | 8 |
| 3.45 | 25 | −8 | 47 | 16 | L. Medial Frontal Gyrus | 9 |
| 2.89 | 11 | −2 | 42 | 29 | L. Medial Frontal Gyrus | 9 |
| 3.03 | 29 | 2 | 34 | 24 | R. Rectal Gyrus | 11 |
| 3.63 | 19 | −32 | 19 | 40 | L. Middle Frontal Gyrus | 8 |
| 3.51 | 38 | 20 | −90 | −11 | R. Fusiform Gyrus | 18 |
| 3.42 | 13 | 32 | −12 | 13 | R. Hippocampus | |
| 3.32 | 16 | 20 | −74 | −8 | R. Lingual Gyrus | 18 |
| 3.14 | 29 | 34 | 2 | 9 | R. Claustrum | |
| 3.10 | 19 | −40 | 6 | 13 | L. Insula | 13 |
| 2.99 | 18 | 51 | −8 | 2 | R. Superior Temporal Gyrus | 22 |
| 2.93 | 11 | −48 | −57 | 30 | L. Superior Temporal Gyrus | 39 |
| 3.40 | 13 | 38 | 14 | 38 | R. Middle Temporal Gyrus | 38 |
| 3.38 | 16 | −50 | −49 | −4 | R. Middle Temporal Gyrus | 37 |
| 2.85 | 11 | 59 | −53 | −11 | R. Inferior Temporal Gyrus | 20 |
| 3.35 | 28 | −6 | −69 | 25 | L. Cerebellum | |
Figure 2.
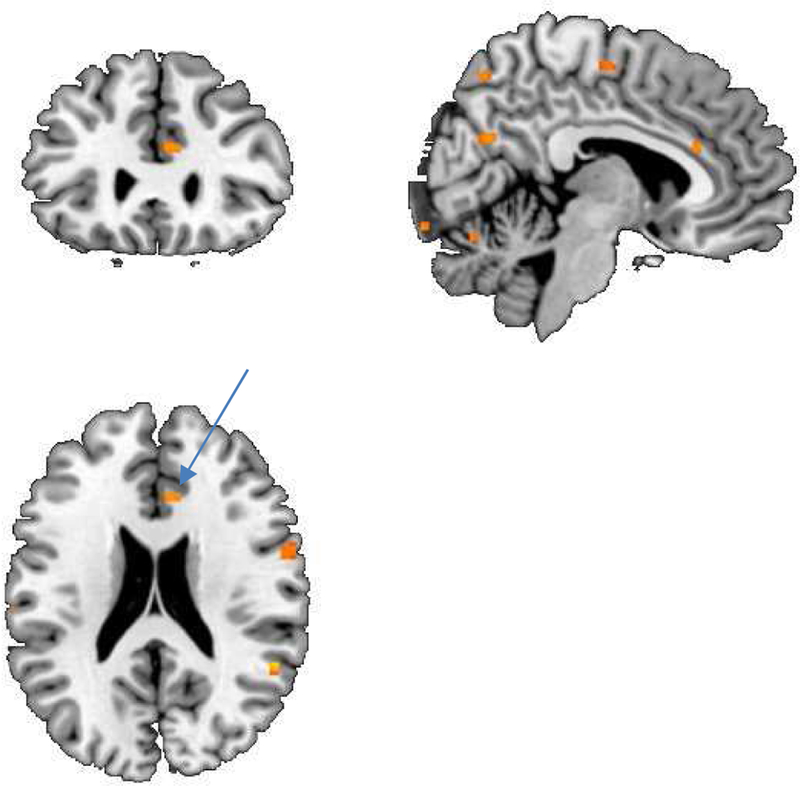
SPM maps of brain function measured with PET in CHD patients with depression and stress-induced myocardial ischemia compared to those without stress-induced myocardial ischemia showing increased activation with stress in the anterior cingulate (yellow area, arrow).
Table 13.
Areas of Greater Decreases in Activation During Mental Arithmetic Stress in CAD Patients With Depression With v Those Without Stress-Induced Myocardial Ischemia
| Z score | Voxel Number |
Talairach Coordinates |
Brain region | BA | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 4.42 | 139 | −10 | −64 | 36 | L. Precuneus | 7 |
| 2.97 | −8 | −67 | 29 | L. Cuneus | 7 | |
| 4.04 | 36 | 16 | −53 | 32 | R. Precuneus | 31 |
| 3.58 | 31 | −53 | −63 | 14 | L. Middle Temporal Gyrus | 19 |
| 3.35 | 24 | −42 | −23 | 14 | L. Transverse Temp. Gyrus | 41 |
| 3.52 | 30 | 20 | 50 | −8 | R. Medial Frontal Gyrus | 10 |
| 3.23 | 46 | 24 | 42 | 18 | R. Medial Frontal Gyrus | 9 |
| 2.72 | 22 | 47 | 11 | R. Medial Frontal Gyrus | 10 | |
| 3.44 | 32 | −6 | −39 | 44 | L. Cingulate Gyrus | 31 |
| 3.22 | 12 | 36 | 58 | 1 | R. Middle Frontal Gyrus | 10 |
| 3.41 | 27 | 53 | 29 | −5 | R. Inferior Frontal Gyrus | 47 |
| 3.21 | 19 | 16 | −35 | 4 | R. Thalamus | |
| 3.13 | 14 | 14 | −12 | −16 | R. Parahippocampal Gyrus | 34 |
| 2.98 | 14 | 32 | −73 | −26 | R. Cerebellum | |
| 4.99 | 67 | 22 | −77 | −28 | R. Cerebellum | |
Discussion
This study showed that CAD patients with depression demonstrated altered functional responses to stress compared to CAD patients without depression in brain regions involved in memory, emotion, and the fear response. Specifically, depressed patients compared to non-depressed showed increased activation with stress in parietal cortex (angular gyrus) and a failure of activation in medial prefrontal cortex (anterior cingulate, orbitofrontal cortex, and subcallosal gyrus). Depressed patients with stress-induced myocardial ischemia showed greater activation in rostral anterior cingulate during stress than depressed non-ischemic patients, with deactivations in more posterior portions of the cingulate. These brain areas are all involved in stress, memory, and regulation of peripheral cardiovascular function (Campanella and Bremner, 2016).
The medial prefrontal cortex, including anterior cingulate, orbitofrontal cortex, and subcallosal gyrus, plays an important role in emotional regulation (Devinsky et al., 1995; Vogt et al., 1992). Multiple studies have implicated this region in the symptomatology of depression (Bremner et al., 1997b; Bremner et al., 2005; Bremner, J. D. et al., 2003; Drevets et al., 1997; George et al., 1994; Mayberg, 1994). The anterior cingulate also regulates the peripheral neurohormonal and autonomic systems involved in cardiovascular responses to stress (Diorio et al., 1993; Nagai et al., 2010; Napadow et al., 2008). It sends inhibitory inputs to the amygdala, which mediates fear memories (Morgan and LeDoux, 1995; Quirk et al., 2006). Lesions of the medial prefrontal cortex result in a failure to extinguish fear reactions, as well as failure to mount peripheral cortisol and sympathetic response to stress (Devinsky et al., 1995; Vogt et al., 1992). Decreased medial prefrontal activation with stress in depressed CAD patients is consistent with prior studies of depression. Altered function of this area likely plays a role in the increase in cardiovascular events in patients with depression (Vaccarino and Bremner, 2014; Vaccarino and Bremner, 2017; Vaccarino et al., 2016).
CAD patients with stress-induced myocardial ischemia demonstrated altered brain responses to stress compared to those without, including increased activation in rostral anterior cingulate. We recently published a study of brain correlates of MSI in a larger sample of CAD patients. This sample, unlike the current study, was not not selected for psychiatric disorders, and there was no overlap between subjects in the two studies. In that study we found increased activation with public speaking and arithmetic mental stress in rostral anterior cingulate, inferior frontal gyrus, and parietal cortex, and additional insula activation with mental arithmetic, in MSI compared to non-MSI CAD patients (Bremner et al., 2018). The current study replicated the finding of increased rostral anterior cingulate activation with stress in MSI. The smaller sample size in the current study may explain the lack of activation in areas seen in the prior study, including parietal cortex and inferior frontal gyrus. As noted elsewhere, increased anterior cingulate activation with stress may drive increased peripheral cardiovascular and neurohormonal responses that lead to myocardial ischemia. Since this was only seen in MSI patients, however, it suggests that there is a subsample of CAD patients with depression who are susceptible to MSI through this brain region. It does not provide an explanation, however, for the link between depression and CAD, since in the CAD depressed patients as a whole there was blunted rostral anterior cingulate activation compared to non-depressed CAD patients. Evidence from other studies, including the lack of an association between early trauma and MSI, suggests that MSI may not be completely attributable to psychiatric disorders. The relationship between depression and CAD is likely more complex, and may involve common genetic or other factors {Vaccarino, 2008 #8315}.
This prior study involved a general CAD population. In the current study we studied a non-overlapping sample of CAD patients who were selected based on the presence or absence of depression in order to assess brain correlates of stress in patients with CAD with and without depression. Base on prior studies of depression and our studies of MSI we hypothesized that patients with CAD and depression would show a relatively blunted response response to stress in the medial prefrontal/anterior cingulate area compared to CAD patients without depression, but that MSI would be associated with increased activation in this area.
This is consistent with a prior study of MSI in patients with CAD not selected for history of depression (Bremner et al., 2018), and suggests a mechanism by which the brain mediates MSI. This is logical since appraisal of threat by brain regions involved in fear, memory, and emotion, is an important part of the stress response (LeDoux, 1996; Phillips and LeDoux, 1992). Patients with depression and stress-induced myocardial ischemia showed increased activation in more anterior parts of cingulate involved in emotion and decreases in more posterior portions involved in assessment of threat in context, time and space (Devinsky et al., 1995; Vogt et al., 1992). One possibility is that increased anterior cingulate function leads to exaggerated peripheral sympathetic and neurohormonal responses to stress in some vulnerable patients, leading to increased stress-induced myocardial ischemia.
Stress was associated with an increase in parietal function in the depressed compared to the nondepressed CAD patients, specifically angular gyrus. The parietal lobe plays an important role in perception of the self in space and time and contextual cues as well as visuospatial memory (Bremner et al., 1995; Jonides et al., 1993; Pardo et al., 1991; Petersen et al., 1988; Zandbelt et al., 2013). It has been hypothesized that increased parietal lobe function with stress could be a neural correlate of heightened awareness or a hypervigilant response to stress (Bremner, 2003; Bremner et al., 1995). Studies have implicated this region in stress-related psychiatric disorders (van Rooij et al., 2015). Individuals at increased risk for cardiovascular disease in prior studies were found to have an increased task-related activation in the parietal cortex (Chuang et al., 2014). This area has also been shown to modulate peripheral cardiovascular responses to stress (de Morree et al., 2013). These findings suggest that increased parietal cortical response to stress could play a role in increased cardiovascular events in patients with depression.
This research is subject to several limitations. Findings from the current study are limited to patients with CAD and are not generalizable to e.g. patients with depression without CAD. Laboratory-induced stress may not be relevant to the types of stressors CAD patients with depression encounter in daily life. Nevertheless, we have used this paradigm in a number of studies and have found that it has a certain amount of ecological validity. The sample size of the current study was limited, and this was particularly relevant to comparison of depressed CAD patients with and without myocardial ischemia. The results should be therefore be considered preliminary, and future studies should focus on CAD patients with depression comparing those with and without stress-induced myocardial ischemia.
In summary, the current study found that patients with CAD and depression showed altered brain responses to mental stress compared to CAD patients without depression in brain areas involved in fear, memory, and peripheral modulation of neurophysiological responses to stress. Specific findings included an increase in parietal cortex activation and decreased anterior cingulate/medial prefrontal cortex response to stress in the CAD patients with depression relative to CAD patients without depression. CAD patients with depression who also developed stress-induced myocardial ischemia, however, had increased rostral anterior cingulate activation with stress compared to CAD patients with depression who did not have stress-induced myocardial ischemia. These findings suggest a pathway by which the brain may mediate the increased cardiovascular morbidity and mortality in some patients with CAD and depression implicating brain areas involved in emotion and autonomic regulation by which stress in certain vulnerable individuals could mediate this effect. Although research in this area is limited, the findings suggest that interventions like mindfulness training or biofeedback that have been shown to affect brain function and reduce stress and anxiety may be useful in the prevention of morbidity and mortality in some vulnerable patients with CAD. This is particularly true for those vulnerable to stress-induced myocardial ischemia, and is not necessarily limited to CAD patients with depression.
Figure 1.
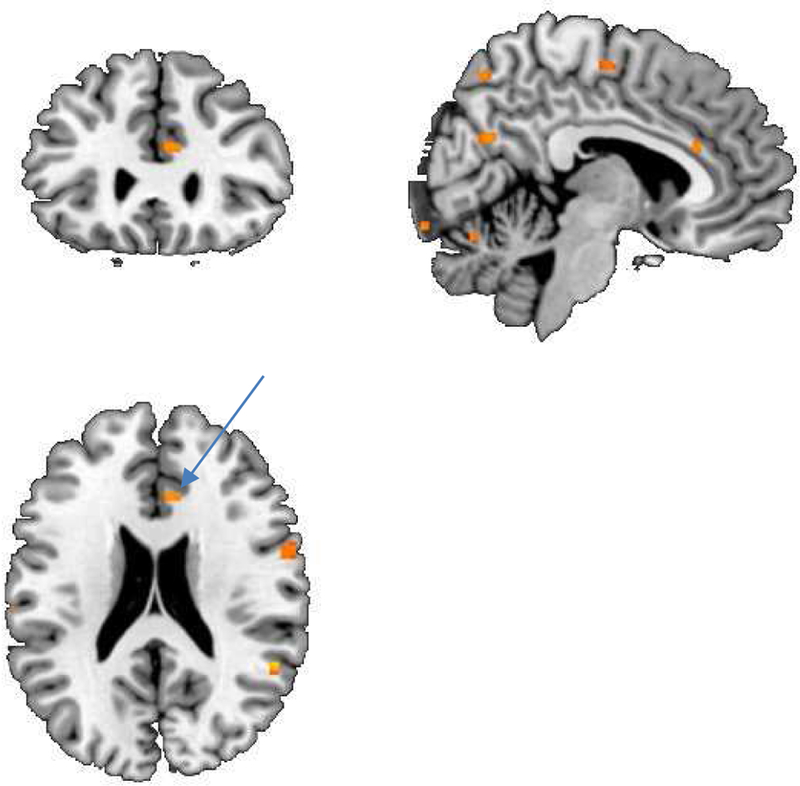
SPM maps of brain function measured with PET in CHD patients with and without depression. There was a greater anterior cingulate (arrow) response to mental stress in the CHD patients without depression compared to CHD patients with depression during mental stress (i.e. failure of anterior cingulate/medial prefrontal activation with mental stress in heart disease and depression, yellow area).
ACKNOWLEDGMENTS:
This study was supported by NIH research grants to JDB R01 HL088726, K24 MH076955, T32 MH067547–01, R01 MH56120, S10 RR016917 and NIH grants to LVV K24 HL077506, R01 AG026255, R01 HL068630 and R21 HL093665. We wish to acknowledge Lai Reed, M.B.A., Carolina LeCours, Kelly Tracey, and Karen Sykes for assistant with clinical assessments, and Delicia Votaw, C.N.M.T., and Margie Jones, C.N.M.T., for their assistance with imaging and analysis procedures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Abercrombie HD, Schaefer SM, Larson CL, Oakes TR, Lindgren KA, Holden JE, Perlman SB, Turski PA, Krahn DD, Benca RM, Davidson RJ, 1998. Metabolic rate in the right amygdala predicts negative affect in depressed patients. Neuroreport 9, 3301–3307. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Luszcz MA, 2002. Mortality risk varies according to gender and change in depressive status in very old adults. Psychosom. Med 64, 880–888. [DOI] [PubMed] [Google Scholar]
- Arri SS, Ryan M, Redwood SR, Marber MS, 2016. Mental stress-induced myocardial ischaemia. Heart 102, 472–480. [DOI] [PubMed] [Google Scholar]
- Austin MP, Dougall N, Ross M, Murray C, O’Carroll RE, Moffoot A, Ebmeier KP, Goodwin GM, 1992. Single photon emission tomography with 99mTc-exametazime in major depression and the pattern of brain activity underlying the psychotic/neurotic continuum. J. Affect. Disord 26, 31–43. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Toga AW, Blanton RE, Sowell ER, Lavretsky H, Peterson J, Pham D, Kumar A, 2004. Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: an MRI-based parcellation of the prefrontal cortex. Am. J. Psychiatry 161(1), 99–108. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, Gerner RH, Smida RM, 1989. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch. Gen. Psychiatry 46, 243–249. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Friston KJ, Brown RG, Scott LC, Frackowiak RSJ, Dolan RJ, 1992. The anatomy of melancholia: focal abnormalities of cerebral blood flow in major depression. Psychol. Med 22, 607–615. [DOI] [PubMed] [Google Scholar]
- Biver F, Goldman S, Delvenne V, Luxen A, De Maertelaer V, Hubain P, Mendlewicz J, Lotstra F, 1994. Frontal and parietal metabolic disturbances in unipolar depression. Biol. Psychiatry 36, 381–388. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Nicoletti MA, Harenski K, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC, 2002. Anatomical MRI study of subgenual prefrontal cortex in bipolar and unipolar subjects. Neuropsychopharmacology 27, 792–799. [DOI] [PubMed] [Google Scholar]
- Bremner JD, 2002. Structural changes in the brain in depression and relationship to symptom recurrence. CNS Spectrums 7, 129–139. [DOI] [PubMed] [Google Scholar]
- Bremner JD, 2003. Functional neuroanatomical correlates of traumatic stress revisited 7 years later, this time with data. Psychopharmacol. Bull 37(2), 6–25. [PubMed] [Google Scholar]
- Bremner JD, 2005. Changes in brain volume in major depression. Depression: Mind and Brain 2(2), 38–46. [Google Scholar]
- Bremner JD, Campanella C, Khan Z, Shah M, Hammadah M, Wilmot K, Al Mheid I, Lima BB, Garcia EV, Nye J, Ward L, Kutner MH, Raggi P, Pearce BD, Shah AJ, Quyyumi AA, Vaccarino V, 2018. Brain correlates of mental stress-induced myocardial ischemia. Psychosom. Med [DOI] [PMC free article] [PubMed]
- Bremner JD, Cheema FA, Ashraf A, Afzal N, Fani N, Reed J, Musselman DL, Ritchie JC, Faber T, Votaw JR, Nemeroff CB, Vaccarino V, 2009. Effects of a cognitive stress challenge on myocardial perfusion and plasma cortisol in coronary heart disease patients with depression. Stress Health 25, 267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Innis RB, Salomon RM, Staib L, Ng CK, Miller HL, Bronen RA, Duncan J, Krystal JH, Rich D, Malison R, Price LH, Dey H, Soufer R, Charney DS, 1997a. PET measurement of cerebral metabolic correlates of tryptophan depletion-induced depressive relapse. Arch. Gen. Psychiatry 54, 364–374. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Innis RB, Salomon RM, Staib L, Ng CK, Miller HL, Bronen RA, Duncan J, Krystal JH, Rich D, Malison R, Price LH, Dey H, Soufer R, Charney DS, 1997b. Positron emission tomography measurement of cerebral metabolic correlates of tryptophan depletion-induced depressive relapse. Arch. Gen. Psychiatry 54, 364–374. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS, 1995. Functional neuroanatomical correlates of the effects of stress on memory. J. Trauma. Stress 8, 527–554. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller H, Charney DS, 2000. Hippocampal volume reduction in major depression. American Journal of Psychiatry 157, 115–117. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Staib L, Kaloupek D, Southwick SM, Soufer R, Charney DS, 1999. Positron emission tomographic (PET)-based measurement of cerebral blood flow correlates of traumatic reminders in Vietnam combat veterans with and without posttraumatic stress disorder (PTSD). Biol. Psychiatry 45, 806–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, Grillon C, Charney DS, 2005. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual abuse-related posttraumatic stress disorder. Psychol. Med 35(6), 791–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Ng CK, Vermetten E, Nazeer A, Oren D, Berman RM, Charney DS, 2003a. Regional brain metabolic correlates of positron emission tomographic measurement of alpha-methylparatyrosine-induced depressive symptoms: Implications for the neural circuitry of depression. J. Am. Med. Assoc 289, 3125–3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Ng CK, Vermetten E, Nazeer A, Oren DA, Berman RM, Charney DS, 2003. Regional brain metabolic correlates of alpha-methylparatyrosine-induced depressive symptoms: implications for the neural circuitry of depression. JAMA 289(23), 3125–3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Adil J, Khan S, Nazeer A, Afzal N, McGlashan T, Anderson G, Heninger GR, Southwick SM, Charney DS, 2003b. Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology 28, 733–750. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Charney DS, 2007. Effects of antidepressant treatment on neural correlates of emotional and neutral declarative verbal memory in depression. J. Affect. Disord 101(1–3), 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, Staib LH, Charney DS, 2002. Reduced volume of orbitofrontal cortex in major depression. Biol. Psychiatry 51, 273–279. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Vaccarino LV, Charney DS, 2004. Deficits in hippocampal and anterior cingulate function during verbal declarative memory encoding in mid-life depression. Am. J. Psychiatry 161(4), 637–645. [DOI] [PubMed] [Google Scholar]
- Brody AL, Saxena S, Stoessel P, Gillies LA, Fairbanks LA, Alborzian S, Phelps ME, Huang SC, Wu HM, Ho ML, Ho MK, Au SC, Maidment K, Baxter LR, 2001. Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy: preliminary findings. Arch. Gen. Psychiatry 58, 631–640. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, DeLisi LE, Holcomb H, Cappelletti J, King AC, Johnson J, Hazlett E, Dowling-Zimmerman S, Post RM, Morihisa J, Carpenter W, Cohen R, Pickar D, Weinberger DR, Margolin R, Kessler RM, 1984. Anteroposterior gradients in cerebral glucose use in schizophrenia and affective disorders. Arch. Gen. Psychiatry 41, 1159–1166. [DOI] [PubMed] [Google Scholar]
- Caetano SC, Hatch JP, Brambilla P, Sassi RB, Nicoletti M, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC, 2004. Anatomical MRI study of hippocampus and amygdala in patients with current and remitted major depression. Psychiatry Res 132(2), 141–147. [DOI] [PubMed] [Google Scholar]
- Campanella C, Bremner JD, 2016. Neuroimaging of PTSD., in: Bremner JD (Ed.) Posttraumatic Stress Disorder: From Neurobiology to Treatment Wiley-Blackwell, Hoboken, New Jersey, pp. 291–320. [Google Scholar]
- Campbell S, Marriott M, Nahmias C, MacQueen GM, 2004. Lower hippocampal volume in patients suffering from depression: A meta-analysis. Am. J. Psychiatry 161, 598–607. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, 2017. Depression and coronary heart disease. Nature reviews. Cardiology 14, 145–155. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Veith RC, 1999. Major depression, heart rate, and plasma norepinephrine in patients with coronary heart disease. Biol. Psychiatry 45, 458–463. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, 1982. The dexamethasone suppression test for melancholia. Br. J. Psychiatry 140, 292–304. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Curtis GC, Davies BM, Mendels J, Sugarman AA, 1976. Urinary free cortisol excretion in depression. Journal of Psychology and Medicine 6, 43–50. [DOI] [PubMed] [Google Scholar]
- Charney DS, Heninger GR, Sternberg F, Hafsted KM, Giddings S, Landis DH, 1982. Adrenergic receptor sensitivity in depression. Arch. Gen. Psychiatry 39, 290–294. [DOI] [PubMed] [Google Scholar]
- Charney DS, Menkes DB, Heninger GR, 1981. Receptor sensitivity and the mechanism of action of antidepressants. Arch. Gen. Psychiatry 38, 1160–1180. [DOI] [PubMed] [Google Scholar]
- Cheng W, Rolls ET, Qiu J, Liu W, Tang Y, Huang CC, Wang X, Zhang J, Lin W, Zheng L, Pu J, Tsai SJ, Yang AC, Lin CP, Wang F, Xie P, Feng J, 2016. Medial reward and lateral non-reward orbitofrontal cortex circuits change in opposite directions in depression. Brain 139(Pt 12), 3296–3309. [DOI] [PubMed] [Google Scholar]
- Chuang Y-F, Eldreth D, Erickson KI, Varma V, Harris G, Fried LP, REbok GW, Tanner EK, Carlson MC, 2014. Cardiovascular risks and brain function: a functional magnetic resonance imaging study of executive function in older adults. Neurobiol. Aging 35(6), 1396–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J, Costafreda SG, McGuffin P, Fu CH, 2011. Hippocampal atrophy in first episode depression: a meta-analysis of magnetic resonance imaging studies. . J. Affect. Disord 134(1–3), 483–487. [DOI] [PubMed] [Google Scholar]
- de Asis JM, Stern E, Alexopoulos GS, Pan H, Van Gorp W, Blumberg H, Kalayam B, Eidelberg D, Kiosses D, Silbersweig DA, 2001. Hippocampal and anterior cingulate activation deficits in patients with geriatric depression. American Journal of Psychiatry 158, 1321–1323. [DOI] [PubMed] [Google Scholar]
- de Morree HM, Szabo BM, Rutten G-J, Kop WJ, 2013. Central nervous system involvement in the autonomic responses to psychological distress. Neth. Heart J 21, 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deanfield JD, Shea M, Kensett M, Horlock P, Wilson RA, deLandsheere CM, Selwyn AP, 1984. Silent myocardial ischaemia due to mental stress. Lancet 2(8410), 1001–1005. [DOI] [PubMed] [Google Scholar]
- Delgado PL, Moreno FA, 2000. Role of norepinephrine in depression. J. Clin. Psychiatry 61(Supp 1), S5–12. [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA, 1995. Contributions of anterior cingulate to behavior. Brain 118, 279–306. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ, 1993. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J. Neurosci 13(9), 3839–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME, 2002. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacology, Biochemistry, and Behavior 71(3), 431–447. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JRJ, Todd RD, Reich T, Vannier M, Raichle ME, 1997. Subgenual prefrontal cortex abnormalities in mood disorders. Nature 386, 824–827. [DOI] [PubMed] [Google Scholar]
- Ebert D, Feistel H, Barocka A, 1991. Effects of sleep deprivation on the limbic system and the frontal lobes in affective disorders: A study with Tc-99m-HMPAO SPECT. Psychiatry Research: Neuroimaging 40, 247–251. [DOI] [PubMed] [Google Scholar]
- Fani N, Ashraf A, Afzal N, Jawed F, Kitayama N, Reed L, Bremner JD, 2011. Increased neural response to trauma scripts in posttraumatic stress disorder following paroxetine treatment: A pilot study. Neurosci. Lett 491(3), 196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Williams JBW, Gibbon M, 1995. Structured Clinical Interview for DSMIV-Patient Edition (SCID-P) American Psychiatric Press, Washington, D.C. [Google Scholar]
- Frodl T, Meisenzahl EM, Zill P, Baghai T, Rujescu D, Leinsinger G, Bottlender R, Schule C, Zwanzger P, Engel RR, Rupprecht R, Bondy B, Reiser M, Moller H-J, 2004. Reduced hippocampal volumes associated with the long variant of the serotonin transporter polymorphism in major depression. Arch. Gen. Psychiatry 61, 177–183. [DOI] [PubMed] [Google Scholar]
- Gan Y, Gong Y, Tong X, Sun H, Cong Y, Dong X, Wang Y, Xu X, Yin X, Deng J, Li L, Cao S, Lu Z, 2014. Depression and the risk of coronary heart disease: a meta-analysis of prospective cohort studies. BMC Psychiatry 14, 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia EV, Faber TL, Cooke CD, Folks RD, Chen J, Santana C, 2007. The increasing role of quantification in clinical nuclear cardiology: the Emory approach. . J. Nucl. Cardiol 14, 420–432. [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, Parekh PI, Rosinsky N, Ring HA, Pazzaglia PJ, Marangell LB, Callahan AM, Post RM, 1997. Blunted left cingulate activation in mood disorder subjects during a response interference task (the Stroop). J. Neuropsychiatry Clin. Neurosci 9(1), 55–63. [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, Post RM, 1994. Prefrontal cortex dysfunction in clinical depression. Depression 2, 59–72. [Google Scholar]
- Golden RN, Markey SP, Risby ED, Rudorfer MV, Cowdry RW, Potter WZ, 1988. Antidepressants reduce whole-body norepinephrine turnover while enhancing 6-hydroxymelatonin output. Arch. Gen. Psychiatry 45, 150–154. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, Austin MP, Dougall N, Ross M, Murray C, O’Carroll RE, Moffoot A, Prentice N, Ebmeier KP, 1993. State changes in brain activity shown by the uptake of 99mTc- exametazine with single photon emission tomography in major depression before and after treatment. J. Affect. Disord 29, 245–255. [DOI] [PubMed] [Google Scholar]
- Grant MM, Cannistraci C, Hollon SD, Gore J, Shelton R, 2011. Childhood trauma history differentiates amygdala response to sad faces within MDD. J. Psychiatr. Res 45(7), 886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald BS, Kramer-Ginsberg E, Krishnan KRR, Ashtari M, Auerbach C, Patel M, 1998. Neuroanatomical localization of magnetic resonance imaging signal hyperintensities in geriatric depression. Stroke 29, 613–617. [DOI] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. Journal of Neurology Neurosurgery and Psychiatry 12, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Shah AJ, Sun Y, Pearce B, Garcia EV, Kutner M, Bremner JD, Esteves F, Raggi P, Sheps DS, Vaccarino V, Quyyumi AA, 2016. The Mental Stress Ischemia Prognosis Study (MIPS): Objectives, study design, and prevalence of inducible ischemia. Psychosom. Med [DOI] [PMC free article] [PubMed]
- Hickie I, Naismith S, Ward PB, Turner K, Scott E, Mitchell P, Wilhelm K, Parker G, 2005. Reduced hippocampal volumes and memory loss in patients with early- and late-onset depression. Br. J. Psychiatry 186, 197–202. [DOI] [PubMed] [Google Scholar]
- Hickie I, Scott E, Wilhelm K, Brodaty H, 1997. Subcortical hyperintensities on magnetic resonance imaging in patients with severe depression-a longitudinal evaluation. Biol. Psychiatry 42, 367–374. [DOI] [PubMed] [Google Scholar]
- Hurwitz TA, Clark C, Murphy E, Klonoff H, Martin WRW, Pate BD, 1990. Regional cerebral glucose metabolism in major depressive disorder. Can. J. Psychiatry 35, 684–688. [DOI] [PubMed] [Google Scholar]
- Janssen J, Hulshoff Pol HE, Lampe IK, Schnack HG, de Leeus FE, Kahn RS, Heeren TJ, 2004. Hippocampal changes and white matter lesions in early-onset depression. Biol. Psychiatry 56(11), 825–831. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Koeppe RA, Awh E, Minoshima S, Mintun MA, 1993. Spatial working memory in humans as revealed by PET. Nature 363, 623–625. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Kannel C, Paffenbarger RSJ, Cupples PH, Cupples LA, 1987. Heart rate and cardiovascular mortality: The Framingham Study. Am. Heart J 113, 1489–1494. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Thornton LM, Gardner CO, 2000. Stressful life events and previous episodes in the etiology of major depression in women: an evaluation of the “kindling” hypothesis. American Journal of Psychiatry 157, 1243–1251. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Evans KR, Kruger S, Mayberg HS, Meyer JH, McCann S, Arifuzzman AI, Houle S, Vaccarino FJ, 2001. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. American Journal of Psychiatry 158(6), 899–905. [DOI] [PubMed] [Google Scholar]
- Krantz DS, Helmers KF, Bairey CN, Nebel LE, Hedges SM, Rozanski A, 1991. Cardiovascular reactivity and mental stress-induced myocardial ischemia in patients with coronary artery disease. Psychosom. Med 53, 1–12. [DOI] [PubMed] [Google Scholar]
- Krishnan KRR, Doraiswamy PM, Figiel GS, Husain MM, Shaw SA, Na C, Boyko OB, McDonald WM, Nemeroff CB, Ellinwood EH Jr., 1991. Hippocampal abnormalities in depression. Journal of Neuropsychiatry & Clinical Neuroscience 3, 387–391. [DOI] [PubMed] [Google Scholar]
- Kumar A, Bilker W, Lavretsky H, Gottlieb G, 2000. Volumetric asymmetries in late-onset mood disorders: an attenuation of frontal asymmetry with depression severity. Psychiatry Res 100(1), 41–47. [DOI] [PubMed] [Google Scholar]
- Kumar A, Gupta RC, Albert Thomas M, Alger J, Wyckoff N, Hwang S, 2004. Biophysical changes in normal-appearing white matter and subcortical nuclei in late-life major depression detected using magnetization transfer. Psychiatry Res 130(2), 131–140. [DOI] [PubMed] [Google Scholar]
- Kumar A, Schweizer E, Jin Z, Miller D, Bilker W, Swan LL, Gottlieb G, 1997. Neuroanatomical substrates of late-life minor depression. A quantitative magnetic resonance imaging study. Arch. Neurol 54, 613–617. [DOI] [PubMed] [Google Scholar]
- Kumar A, Zin Z, Bilker W, Udupa J, Gottlieb G, 1998. Late onset minor and major depression: early evidence for common neuroanatomical substrates detected by using MRI. Proceedings of the National Academy of Sciences USA 95(13), 7654–7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacerda ALT, Keshavan MS, Hardan AY, Yorbik O, Brambilla P, Sassi RB, Nicoletti M, Mallinger AG, Frank E, Kupfer DJ, Soares JC, 2004. Anatomic evaluation of the orbitofrontal cortex in major depressive disorder. Biol. Psychiatry 55, 353–358. [DOI] [PubMed] [Google Scholar]
- Lacy CR, Contrada RJ, Robbins ML, Tannenbaum AK, Moreyra AE, Chelton S, Kostis JB, 1995. Coronary vasoconstriction induced by mental stress (simulated public speaking). Am. J. Cardiol 75, 503–505. [DOI] [PubMed] [Google Scholar]
- Lai TJ, Payne ME, Byrum CE, Steffens D, Krishnan KR, 2000. Reduction of orbital frontal cortex volume in geriatric depression. Biol. Psychiatry 48, 971–975. [DOI] [PubMed] [Google Scholar]
- Lake CR, Pickar D, Ziegler MG, Lipper S, Slater S, Murphy DL, 1982. High plasma NE levels in patients with major affective disorder. Am. J. Psychiatry 139, 1315–1318. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Ahern CE, Schwartz GE, Davidson RJ, 1997. Neuroanatomical correlates of happiness, sadness, and disgust. Am. J. Psychiatry 154, 926–933. [DOI] [PubMed] [Google Scholar]
- LaVeau PJ, Rozanski A, Krantz DS, Cornell CE, Cattanach L, Zaret BL, 1989. Transient left ventricular dysfunction during provocative mental stress in patients with coronary artery disease. Am. Heart J 118(1), 1–8. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, 1996. The Emotional Brain: The Mysterious Underpinnings of Emotional Life Simon & Schuster, New York, N.Y. [Google Scholar]
- Lee SH, Payne ME, Steffens DC, McQuoid DR, Lai TJ, Provenzale JM, Krishnan KR, 2003. Subcortical lesion severity and orbitofrontal cortex volume in geriatric depression. Biol. Psychiatry 54(5), 529–533. [DOI] [PubMed] [Google Scholar]
- Lenze E, Cross D, McKeel D, Neuman RJ, Sheline YI, 1999. White matter hyperintensities and gray matter lesions in physically healthy depressed subjects. Am. J. Psychiatry 156, 1602–1607. [DOI] [PubMed] [Google Scholar]
- Lloyd AJ, Ferrier IN, Barber R, Gholkar A, Young AH, O’Brien JT, 2004. Hippocampal volume change in depression: late and early-onset illness compared. Br. J. Psychiatry 184, 488–495. [DOI] [PubMed] [Google Scholar]
- Mallik S, Spertus JA, Reid KJ, Krumholz HM, Rumsfeld JS, Weintraub WS, Agarwal P, Santra M, Bidyasar S, Lichtman JH, Wenger NK, Vaccarino V, 2006. Depressive symptoms after acute myocardial infarction: evidence for highest rates in younger women. Arch. Intern. Med 166(8), 876–883. [DOI] [PubMed] [Google Scholar]
- Martinot JL, Hardy P, Feline A, Huret J-D, Mazoyer B, Attar-Levy D, Pappata S, Syrota A, 1990. Left prefrontal glucose hypometabolism in the depressed state: A confirmation. Am. J. Psychiatry 147, 1313–1317. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, 1994. Frontal lobe dysfunction in secondary depression. Journal of Neuropsychiatry & Clinical Neuroscience 6, 428–442. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, McGinnis S, Glass TG, Martin CC, Fox PT, 1997. Cingulate function in depression: a potential predictor of treatment response. Neuroreport 8, 1057–1061. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA, 2000. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol. Psychiatry 48(8), 830–843. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lewis PJ, Regenold W, Wagner HN, 1994. Paralimbic hypoperfusion in unipolar depression. J. Nucl. Med 35, 929–934. [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT, 1999. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. American Journal of PsychiatryThe 156(5), 675–682. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Starkstein SE, Peyser CE, Brandt J, Dannals RF, Folstein SE, 1992. Paralimbic frontal lobe hypometabolism in depression associated with Huntington’s disease. Neurology 42, 1791. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Starkstein SE, Sadzot B, Preziosi T, Andrezejewski PL, Dannals RF, Wagner HN, Robinson RG, 1990. Selective hypometabolism in the inferior frontal lobe in depressed patients with Parkinson’s Disease. Ann. Neurol 28, 57–64. [DOI] [PubMed] [Google Scholar]
- McClellan AT, Luborsky A, Cacciola J, Griffith J, Evans F, Bar HL, O’Brien CP, 1985. New data from the addiction severity index: reliability and validity in three centers. J. Nerv. Ment. Dis 73, 412–423. [DOI] [PubMed] [Google Scholar]
- Meijer A, Conradi HJ, Bos EH, Thombs BD, van Melle JP, de Jonge P, 2011. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis of 25 years of research. Gen. Hosp. Psychiatry 33(3), 203–216. [DOI] [PubMed] [Google Scholar]
- Mervaala E, Fohr J, Konogen M, Valkonen-Korhonen M, Vainio P, Partanen J, Tiihonen J, Vinamaki H, Karjalainen AK, Lehtonen J, 2000. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol. Med 30, 117–125. [DOI] [PubMed] [Google Scholar]
- Morgan CA, LeDoux JE, 1995. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav. Neurosci 109(4), 681–688. [DOI] [PubMed] [Google Scholar]
- Nagai M, Hoshide S, Kario K, 2010. The insular cortex and cardiovascular system: a new insight into the brain-heart axis. J. Am. Soc. Hypertens 4(4), 174–182. [DOI] [PubMed] [Google Scholar]
- Napadow V, Dhond R, Conti G, Makris N, Brown EN, Barbieri R, 2008. Brain correlates of autonomic modulation: combining heart rate variability with fMRI. Neuroimage 42(1), 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan M, Bremner JD, Kumar A, 1999. Neuroanatomical substrates of late-life mental disorders. J. Geriatr. Psychiatry Neurol 12, 95–106. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W, 1984. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science 226, 1342–1344. [DOI] [PubMed] [Google Scholar]
- Opel N, Redlich R, Zwanzger P, Grotegerd1 D, Arolt V, Heindel W, Konrad C, Kugel H, Dannlowski U, 2014. Hippocampal atrophy in major depression: a function of childhood maltreatment rather than diagnosis? Neuropsychopharmacology 39(12), 2723–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordway G, Smith I, Haycock J, 1994. Elevated tyrosine hydroxylase in the locus coeruleus of suicide victims. J. Neurochem 62, 680–685. [DOI] [PubMed] [Google Scholar]
- Parashar S, Rumsfeld JS, Spertus JA, Reid KJ, Wenger NK, Krumholz HM, Amin A, Weintraub WS, Lichtman J, Dawood N, Vaccarino V, 2006. Time course of depression and outcome of myocardial infarction. Arch. Intern. Med 166(18), 2035–2043. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, Raichle ME, 1991. Localization of a human system for sustained attention by positron emission tomography. Nature 349, 61–64. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun MA, Raichle ME, 1988. Positron emission tomographic studies of the cortical anatomy of single word processing. Nature 331, 585–589. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE, 1992. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci 106, 274–285. [DOI] [PubMed] [Google Scholar]
- Posener JA, Wang L, Price JL, Gado MH, Province MA, Miller MI, Babb CM, Csernansky JG, 2003. High-dimensional mapping of the hippocampus in depression. Am. J. Psychiatry 160, 83–89. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F, 2006. Prefrontal mechanisms in extinction of conditioned fear. Biol. Psychiatry 60(4), 337–343. [DOI] [PubMed] [Google Scholar]
- Ramachandruni S, Fillingim RB, McGorray SP, Schmalfuss CM, Cooper GR, Schofield RS, Sheps DS, 2006. Mental stress provokes ischemia in coronary artery disease subjects without exercise- or adenosine-induced ischemia. J. Am. Coll. Cardiol 47(5), 987–991. [DOI] [PubMed] [Google Scholar]
- Ramadan R, Sheps D, Esteves F, Zafari AM, Bremner JD, Vaccarino V, Quyyumi AA, 2013. Myocardial ischemia during mental stress: role of coronary artery disease burden and vasomotion. JAHA 2, e000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Lane RD, Ahern GL, Schwartz GE, Davidson RJ, Friston KJ, Yun L-S, Chen K, 1997. Neuroanatomical correlates of externally and internally generated human emotion. Am. J. Psychiatry 154(7), 918–925. [DOI] [PubMed] [Google Scholar]
- Ring RA, Bench CJ, Trimble MR, Brooks DJ, Frackowiak RSJ, Dolan RJ, 1994. Depression in Parkinson’s Disease: A positron emission study. Br. J. Psychiatry 165, 333–339. [DOI] [PubMed] [Google Scholar]
- Roy A, Pickar D, DeJong J, Karoum F, Linnoila M, 1988. Norepinephrine and its metabolites in cerebrospinal fluid, plasma, and urine: Relationship to hypothalamic-pituitary-adrenal axis function in depression. Arch. Gen. Psychiatry 45, 849–857. [DOI] [PubMed] [Google Scholar]
- Rozanski A, Bairey CN, Krantz DS, Friedman J, Resser KJ, Morell M, Hilton-Chalfen S, Hestrin L, Bietendorf J, Berman DS, 1988. Mental stress and the induction of silent myocardial ischemia in patients with coronary artery disease. N. Engl. J. Med 318(16), 1005–1012. [DOI] [PubMed] [Google Scholar]
- Sala M, Perez J, Soloff P, Ucelli di Nemi S, Caverzasi E, Soares JC, Brambilla P, 2004. Stress and hippocampal abnormalities in psychiatric disorders. Eur. Neuropsychopharmacol 14(5), 393–405. [DOI] [PubMed] [Google Scholar]
- Schang SJ, Pepine CJ, 1977. Transient asymptomatic S-T segment depression during daily activity. Am. J. Cardiol 39, 396–402. [DOI] [PubMed] [Google Scholar]
- Schmand M, Weinhard K, Casey ME, Eriksson L, Jones WF, Reed JH, Treffert J, Lenox M, Luk P, Bao J, Young JW, Baker K, Miller SD, Knoess C, Vollmar S, Richerzhagen N, Flugge G, Heiss WD, Nutt R, 1999. Performance evaluation of a new LSO High Resolution Research Tomograph-HRRT. IEEE Trans. Med. Imaging 2(October Nuclear Medicine Science), 1067–1071. [Google Scholar]
- Sheline YI, 2003. Neuroimaging studies of mood disorder effects on the brain. Biol. Psychiatry 54(3), 338–352. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC, 2003. Untreated depression and hippocampal volume loss. American Journal of Psychiatry 160, 1516–1518. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Sanhavi M, Mintun MA, Gado MU, 1999. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J. Neurosci 19, 5034–5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Wang P, Gado M, Csernansky J, Vannier M, 1996. Hippocampal atrophy in recurrent major depression. Proceedings of the National Academy of Sciences USA 93(9), 3908–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G, Reynolds C, Houck P, Dew M, Ma Y, Mulsant B, Pollock B, 2002. The glucose metabolic response to total sleep deprivation, recovery sleep and acute antidepressant treatment as functional neuroanatomic correlates of treatment outcome in geriatric depression. Am. J. Geriatr. Psychiatry 10, 561–567. [PubMed] [Google Scholar]
- Smith GS, Eyler LT, 2006. Structural neuroimaging in geriatric psychiatry. Am. J. Geriatr. Psychiatry 14(10), 809–811. [DOI] [PubMed] [Google Scholar]
- Smith GS, Kramer E, Hermann CR, Goldberg S, Ma Y, Dhawan V, Barnes A, Chaly T, Belakhleff A, Laghrissi-Thode F, Greenwald B, Eidelberg D, Pollock BG, 2002. Acute and chronic effects of citalopram on cerebral glucose metabolism in geriatric depression. Am. J. Geriatr. Psychiatry 10(6), 715–723. [PubMed] [Google Scholar]
- Steffens DC, Byrum CE, McQuoid DR, Greenburg DL, Payne ME, Blichington TT, McFall JR, Krishnan KRR, 2000. Hippocampal volume in geriatric depression. Biol. Psychiatry 48, 301–309. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Krishnan KRR, 1998. Structural neuroimaging and mood disorders: Recent findings, implications for classification, and future directions. Biol. Psychiatry 43, 705–712. [DOI] [PubMed] [Google Scholar]
- Sullivan S, Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Alkhoder A, Isakadze N, Shah A, Levantsevych O, Pimple PM, Kutner M, Ward L, Garcia EV, Nye J, Mehta PK, Lewis TT, Bremner JD, Raggi P, Quyyumi AA, Vaccarino V, 2018. Sex differences in hemodynamic and microvascular mechanisms of myocardial ischemia induced by mental stress. Arterioscler. Thromb. Vasc. Biol 38(2), 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P, 1988. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System, An Approach to Cerebral Imaging Georg Thieme, Stuttgart, Germany. [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH, 2009. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann. Behav. Med 37(2), 141–153. [DOI] [PubMed] [Google Scholar]
- Traskman L, Tybring G, Asberg M, Bertilsson L, Lantto O, Schalling D, 1980. Cortisol in the CSF of depressed and suicidal patients. Arch. Gen. Psychiatry 37, 761–767. [DOI] [PubMed] [Google Scholar]
- Vaccarino V, Bremner JD, 2014. Psychiatric and behavioral aspects of cardiovascular disease., in: Bonow RO, Mann DL, Zipes OP, Libby P (Eds.), Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine Saunders, Philadelphia, PA. [Google Scholar]
- Vaccarino V, Bremner JD, 2017. Behavioral, emotional and neurobiological determinants of coronary heart disease risk in women. Neurosci. Biobehav. Rev 74((Pt B)), 297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Mayer E, Bremner JD, 2016. Stress and Health., in: Bremner JD (Ed.) Posttraumatic Stress Disorder: From Neurobiology to Treatment Wiley-Blackwell Press, Hoboken, N.J. [Google Scholar]
- Vaccarino V, Shah AJ, Rooks C, Ibeanu I, Nye JA, Pimple P, Salerno A, D’Marco L, Karohl C, Bremner JD, Raggi P, 2014. Sex differences in mental stress-induced myocardial ischemia in young survivors of an acute myocardial infarction. Psychosom. Med 76(3), 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Sullivan S, Hammadah M, Wilmot K, Al Mheid I, Ramadan R, Elon L, Pimple PM, Garcia EV, Nye J, Shah AJ, Alkhoder A, Levantsevych O, Gay H, Obideen M, Huang M, Lewis TT, Bremner JD, Quyyumi AA, Raggi P, 2018. Mental stress-induced-myocardial ischemia in young patients with recent myocardial infarction: Sex differences and mechanisms. Circulation 137, 794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Votaw J, Faber T, Veledar E, Murrah NV, Jones LR, Zhao J, Su S, Goldberg J, Raggi JP, Quyyumi AA, Sheps DS, Bremner JD, 2009. Major depression and coronary flow reserve detected by positron emission tomography. Arch. Intern. Med 169, 1668–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakili K, Pillay SS, Lafer B, Fava M, Renshaw PF, Bonello-Cintron CM, Yurgelun-Todd DA, 2000. Hippocampal volume in primary unipolar major depression: A magnetic resonance imaging study. Biol. Psychiatry 47, 1087–1090. [DOI] [PubMed] [Google Scholar]
- van Rooij SJH, Geuze E, Kennis M, Rademaker AR, Vink M, 2015. Neural correlates of inhibition and contextual cue processing related to treatment response in PTSD. Neuropsychopharmacology 40, 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Train KF, Garcia EV, Maddahi J, Areeda J, Cooke CD, Kiat H, Silagan G, Folks R, Reidman J, Matzer L, 1994. Multicenter trial validation for quantitative analysis of same-day rest-stress technetium-99m-sestamibi myocardial tomograms. J. Nucl. Med 35, 609–618. [PubMed] [Google Scholar]
- Veith RC, Lewis L, Linares OA, 1994. Sympathetic nervous system activity in major depression: basal and desipramine-induced alterations in plasma norepinephrine kinetics. Arch. Gen. Psychiatry 51(411–422). [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B, 2004. Hippocampal volume and depression: a meta-analysis of MRI studies. American Journal of Psychiatry 161(11), 1957–1966. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR, 1992. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb. Cortex 2, 435–443. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Heim C, Newport CD, Miller AH, Vermetten E, Anderson E, Bronen R, Staib L, Charney DS, Nemeroff CB, Bremner JD, 2002. Childhood trauma associated with smaller hippocampal volume in women with major depression. American Journal of Psychiatry 159, 2072–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Pimple P, Shah AJ, Rooks C, Bremner JD, Nye JA, Ibeanu I, Murrah N, Shallenberger L, Raggi P, Vaccarino V, 2014a. Depressive symptoms are associated with mental stress-induced myocardial ischemia after acute myocardial infarction. . PLoS One 9, e102986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Rooks C, Ramadan R, Shah AJ, Bremner JD, Quyyumi AA, Kutner M, Vaccarino V, 2014b. Meta-analysis of mental sress-induced myocardial ischemia and subsequent cardiac events in patients with coronary artery disease. Am. J. Cardiol 114(2), 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhard K, Schmand M, Casey ME, Baker K, Bao J, Eriksson L, Jones WF, Knoess C, Lenox M, Lercher M, Luk P, Michel C, Reed JH, Richerzhagen N, Treffert J, Vollmar S, Young JW, Heiss WD, Nutt R, 2000. The ECAT HRRT: Performance and first clinical application of the new high resolution research tomograph. IEEE Trans. Med. Imaging 3(Nuclear Science Symposium Conference Record), 17/12–17/16. [Google Scholar]
- Wulsin LR, Vaillant GE, Wells VE, 1999. A systematic review of the mortality of depression. Psychosom. Med 61, 6–17. [DOI] [PubMed] [Google Scholar]
- Young EA, Haskett RF, Grunhaus L, 1994. Increased circadian activation of the hypothalamic pituitary adrenal axis in depressed patients in the evening. Arch. Gen. Psychiatry 51, 701–707. [DOI] [PubMed] [Google Scholar]
- Zandbelt BB, Bloemendaal M, Neggers SFW, Kahn RS, Vink M, 2013. Expectations and violations: delineating the neural network of proactive inhibitory control. Hum. Brain Mapp 34, 2015–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]


