Abstract
Background:
Hematopoietic stem cell transplantation (HCT) conditioning regimens that can reduce relapse risk without increasing non-relapse mortality (NRM) are needed. We tested the safety of timed-sequential delivery of myeloablative dose of busulfan in older patients and younger patients with comorbidities.
Methods:
In this open label, non-stratified, randomized phase II clinical trial, patients with hematological malignancies up to 75 years of age were randomized 1:1 by a computer generated program in a block size of 4 to receive a total intravenous busulfan dose to achieve an area under the curve (AUC) of 16,000 μmol.min (16K) or 20,000 μmol.min (20K) based on pharmacokinetic analysis, with fludarabine 40 mg/m2 intravenously for four days (Bu-Flu). The investigators and the research nurses were blinded to the block size to conceal allocation. The primary endpoint was day 100 NRM analyzed by intention-to-treat. No interim analyses were planned and the accrual is complete.
Findings:
Forty-nine patients were randomized to the 16K arm and 48 to the 20K arm. Median age was 60 (range, 18-75) years. Over 50% had an HCT-Comorbidity Index (HCT-CI) ≥ 3, and 42% had a high or very high Disease Risk Index (DRI). Day 100 NRM was 4.1% (95% confidence interval [CI], 0%-9.7%) in the 16K arm and 6.3% (95% CI, 0%-13.2%) in the 20K arm (P= 0.65). In multivariate analysis, HCT-CI was an independent predictor of NRM (hazard ratio [HR], 2.12; 95% CI, 1.37-3.29; P=0.0008). Infection was the most common grade 3 to 5 toxicity that occurred equally in the 20K arm (n=25) and the 16K arm (n=24). Mucositis (n=10 versus n=3), idiopathic pneumonia syndrome (n=9 versus n=2), and culture-negative neutropenic fever (n=16 versus n=8) were more common in the 20K arm than in the 16K arm, respectively.
Interpretation:
Myeloablative doses of busulfan administered in a timed-sequential manner with fludarabine results in low NRM in older patients. Whether this approach can further reduce the risk of relapse requires additional studies.
Introduction
Reduced-intensity conditioning (RIC) widens the prospect of hematopoietic stem cell transplantation (HCT) for older patients and those with comorbidities. Compared with myeloablative conditioning, RIC reduces morbidity and non-relapse mortality (NRM), but at the expense of a higher relapse rate, which translates to a similar overall survival (OS) rate.(1-5) Therefore, a less toxic myeloablative regimen that can be tolerated by older and frail patients could improve the results of HCT.
Current pre-HCT conditioning regimens consist of 4 to 8 days of chemotherapy with or without radiation. Longer regimens or regimens using a sequence of chemotherapy agents have not been studied. Timed-sequential therapy—delivery of a second course of chemotherapy 8 to 10 days after the first one—was developed to enhance antitumor effects. Clinical studies of timed-sequential therapy outside of the HCT setting, in patients with acute myeloid leukemia, showed promising efficacy.(6-8) In the HCT setting, giving a course of induction chemotherapy 4 to 14 days before the start of RIC showed encouraging results, suggesting that sequential administration of chemotherapy agents may kill more leukemia cells without causing a major increase in toxicity.(9, 10) Yet, the concept of timed-sequential therapy has not been applied to HCT conditioning regimens.
To adapt these principles in the HCT setting, we sought to modify the sequence of busulfan administration in our myeloablative busulfan plus fludarabine (Bu-Flu) conditioning regimen.(11) We hypothesized that timed-sequential administration of busulfan over two weeks would reduce toxicity and allow myeloablative doses of busulfan to be safely delivered to older patients and patients with comorbidities. The purpose of this randomized phase II study was to compare the safety of two myeloablative timed-sequential Bu-Flu conditioning regimens: one with a lower dose of busulfan (area under the curve [AUC] of 16 000 μmol.min; 16K arm) and one with a higher dose (AUC of 20 000 μmol.min; 20K arm).
PATIENTS AND METHODS
Study design and participants
This was an equally randomized, non- stratified, open label, phase II clinical trial comparing two timed-sequential Bu-Flu conditioning regimens (16K versus 20K arms). This clinical study (The University of Texas MD Anderson Cancer Center protocol 2011-0958; ClinicalTrials.gov identifier NCT01572662) was approved by the Institutional Review Board at MD Anderson Cancer Center. The research was conducted in accordance with the Helsinki Declaration, and all participants provided written informed consent. The study was conducted and the data were collected at The University of Texas MD Anderson Cancer Center. The initial eligibility criteria included patients with any hematological malignancy up to the age of 70 years; however, after safety was determined, the age limit was expanded to 75 years. Further inclusion criteria were the availability of an 8/8 human leukocyte antigen-matched related or unrelated donor, determined by high-resolution typing; adequate pulmonary, cardiac, renal and hepatic functions; and Karnofsky performance score of ≥ 70 or Eastern Cooperative Oncology Group performance score of 0 to 1, and any number of comorbidities [Supplement: protocol]. Patients with prior HCT, HIV or uncontrolled infections were excluded.
Randomization and masking
Block randomization with a block size of 4 was used to assign patients to the treatment arm. The randomization was conducted by the Clinical Oncology Research System (CORe), which is the institutional patient registration system for clinical trials. The allocation sequence was generated via a computer program maintained by the CORe. The investigators and the research nurses who enrolled the participants were blinded to the block size. The study team and the participants were aware of the treatment arm once it was assigned.
Procedures - Conditioning Regimen and Supportive Care
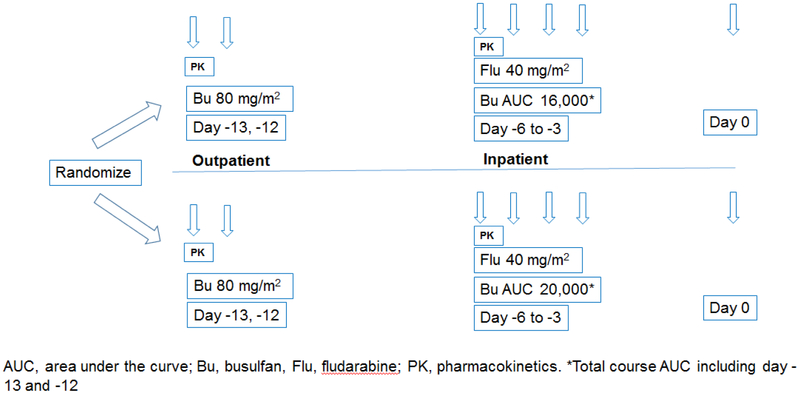
The conditioning regimen consisted of busulfan (distributed by Otsuka American Pharmaceuticals, Rockville, MD; manufactured by Patheon Mfg Service LLC, Greenville, NC or Baxter Oncology, Westfalen, Germany) and fludarabine (distributed and manufactured by Leucadia Pharmaceuticals, Carlsbad, CA, USA) [Figure 1], Dosing of busulfan was determined on the basis of pharmacokinetic (PK) analyses conducted after the first (day −13) and the third (day −6) busulfan doses, as described previously. (12) Patients were randomized to receive a total busulfan dose that would achieve a target AUC of either 16 000 ± 12% μmol.min (16Karm) or 20 000 ± 12% μmol.min (20Karm). On days −13 and −12 before HOT, patients received 80 mg/m2 busulfan intravenously (IV) daily in an outpatient clinic. Busulfan PK analysis was done after the first dose on day −13, based on which all patients needed adjustments for day−6 and day−5 doses of busulfan. Additional chemotherapy was administered during inpatient treatment from day −6 through day −3, including fludarabine 40 mg/m2 IV once daily and once daily busulfan IV given over 3 hours by controlled rate infusion pump starting immediately after the completion of fludarabine. Second PK analysis was performed after day −6 dose of busulfan to adjust the final two doses on day −4 and day −3 to meet the target total AUC. Three patients (2 in the 16K arm and 1 in the 20K arm) did not have day −6 PK analysis performed. Of the patients who had day −6 PK testing done, 92 needed subsequent dose adjustments to achieve target AUC.
Figure 1: Study Schema.
Patients were randomized to either 16K or 20K arm. All patients received out-patient dose of busulfan 80 mg/m2 on day −13 and day -12. Busulfan PK analysis was done after the first dose on day −13. Patients were then admitted on day −6 and received additional once daily IV doses of busulfan based on the PK analysis immediately after the completion of fludarabine 40 mg/m2 IV given once daily through day −3. HCT was performed on day 0.
Graft-versus-host disease (GVHD) prophylaxis consisted of tacrolimus starting on day −2, aiming for a level of 8 to 11 ng/mL, and methotrexate 5 mg/m2 IV given on days 1, 3, 6, and 11. Phenytoin was given for anti-seizure prophylaxis. Antimicrobial prophylaxis was given according to institutional standards. Granulocyte-colony stimulating factor (G-CSF) at a dose of 5 mcg/kg/day subcutaneously was started on day 7 and continued until neutrophil engraftment, defined as an absolute neutrophil count of > 500 × 106/L for 3 consecutive days. Donor bone marrow (BM) or G-CSF-primed peripheral blood progenitor cells (PBPC) were procured using standard mobilization protocols and apheresis techniques. All donors provided written informed consent. Unrelated donor BM was obtained through the National Marrow Donor Program according to applicable guidelines.
Outcomes
The primary objective was to compare the rates of NRM at day 100 in the 2 treatment arms. Bayesian sequential monitoring rules using the methods of Thall et al.(13) were used to monitor the day 100 NRM rate and to stop the study early if there was a high chance that the NRM rate was higher in one arm than in the other or higher than 20% in either arm. Specifically, if the posterior probability of NRM being higher in one arm than in the other or being higher than 20% was greater than 99%, the study would be stopped. Beta (0.40, 1.60) priors were used to estimate the probability of NRM in each arm. The trial design was simulated 2000 times for several scenarios, with the operating characteristics as summarized in the table S1. If the true NRM rates were 5% and 20% in the 16K and the 20K arms, respectively, then the probability that 16K arm would be selected was 87.4%. A sample size of 100 patients ensured more than 80% probability of selecting 16K arm when the NRM rates were 5% and 20%, respectively. No interim analyses were planned. After 98 patients were enrolled, the MD Anderson Data and Safety Monitoring Board approved the study team’s request to stop the randomization and to continue the study as a single-arm study with increased accrual onto the higher dose arm. The total accrual was almost complete at that time, and there were no significant differences in NRM between the arms and virtually no chance of seeing a difference at the end of the trial (predictive probability =0.0003). Our secondary objectives were to obtain preliminary estimates of efficacy and differences between the 2 arms by analyzing OS, progression-free survival (PFS), neutrophil and platelet engraftment, and rates of acute and chronic GVHD. Platelet engraftment was defined as a platelet count greater than 20 × 109/L for 7 days without transfusion. The Common Terminology Criteria forAdverse Events (CTCAE) v5.0 was used to grade toxicities, which were monitored daily until engraftment or until the day of discharge, whichever was later, and at least weekly thereafter until day 100. The MD Anderson Data and Safety Monitoring Board monitored the trial on a yearly basis.
Statistical Analysis
Categorical variables were compared between treatment arms using Fisher’s exact test. Continuous variables were compared using the Wilcoxon rank sum test. The rate of day 100 NRM was estimated in a competing risks framework with relapse as the competing risk using the cumulative incidence method of Gooley et al.(14) The association between NRM and variables of interest was assessed using the method of Fine and Gray.(15) Among patients in whom engraftment occurred, the median time to engraftment was compared using the Wilcoxon rank sum test. Acute and chronic GVHD were assessed with competing risks of relapse and death, again using the methods of Gooley et al. and Fine and Gray.(14, 15) Kaplan-Meier curves were used to estimate OS and PFS, and the log-rank test was used to test differences by treatment arm. Cox proportional hazards regression models were fit to model the association between OS and PFS and covariates of interest. All analyses were performed on intention-to-treat basis.
RESULTS
Patient Characteristics
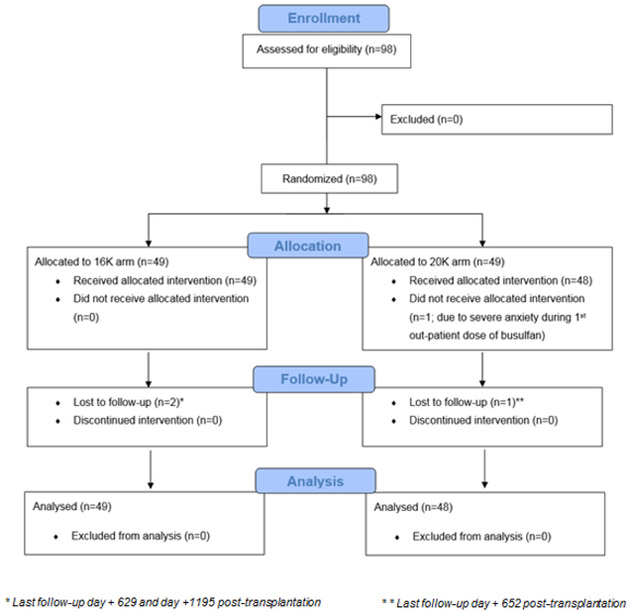
Between April 18, 2012 and December 9, 2015, 98 patients were assessed for eligibility and equally randomized between the arms. One patient in the 20K arm did not receive the allocated intervention. Of the 97 patients who received timed-sequential busulfan conditioning, 49 (50.5%) were randomized to the 16K arm and 48 (49.5%) to the 20K arm [Figure 2: Consort diagram]. The average total IV busulfan dose given per patient was 9.7 mg/kg (range, 5.4-14.6) in the 16K arm and 12.5 mg/kg (range, 8.5-16.7) in the 20K arm. Overall, the median age at HCT was 60 years (range, 18 to 75). Patients in the 20K arm were older (median, 62 years) than in the 16K arm (median, 57 years; P = 0.05). Male patients made up 66% (n = 64) of the study cohort; 56% (n = 54) had an unrelated donor, and a majority (n = 72, 74%) received a PBPC graft. The most common diagnoses were acute myeloid leukemia and myelodysplastic syndrome (n = 53, 55%). More patients in the 20K arm than in the 16K arm had acute myeloid leukemia or myelodysplastic syndrome (60% versus 49%) and multiple myeloma (23% versus 10%); P = 0.02. We found no other significant differences between the groups. Overall, more than 70% of patients with acute myeloid leukemia or myelodysplastic syndrome had relapsed or primary refractory disease with induction failure prior to HCT. More than half of the patients (n = 50, 52%) had an HCT-Specific Comorbidity Index (HCT-CI) of 3 or higher, and 42% (n = 41) had a high or very high Disease Risk Index (DRI).(16) The median follow-up duration was 16.7 ( interquartile range, 11.7-21.4) months among survivors [Table 1].
Figure 2: CONSORT diagram illustrating patient flow.
A total of 98 patients were randomized equally to 16K and 20K arms including 49 patients each. One patient in the 20K arm withdrew from the study and did not receive the allocated intervention. Analysis included 49 patients enrolled in the 16K arm and 48 patients in the 20K arm. Two patients were lost to follow-up (day + 629 and day +1195 post HCT) in the 16K arm and one patient was lost to follow-up (day +652 post HCT).
Table 1.
Baseline Patient Characteristics
| Characteristic | Arm 1 AUC = 16K No. (%) |
Arm 2 AUC = 20K No. (%) |
P value |
|---|---|---|---|
| No. of patients | 49 | 48 | |
| Total busulfan dose (mg/kg), average (range) | 9.7 (5.4-14.6) | 12.5 (8.5-16.7) | |
| Age at HCT in years, median (range) | 57(18-75) | 62 (41-75) | 0.05 |
| Age group | |||
| < 50 years | 9(18) | 6(13) | |
| 50-59 years | 19 (39) | 14 (29) | |
| 60-69 years | 17 (35) | 22 (46) | |
| 70-75 years | 4(8) | 6(13) | |
| Sex | |||
| Male | 36 (73) | 28 (58) | 0.14 |
| Female | 13 (27) | 20 (42) | |
| Race | |||
| White | 36 (73) | 41 (85) | 0.21 |
| Other | 13 (27) | 7 (15) | |
| Diagnosis | |||
| Acute myeloid leukemia/Myelodysplastic syndrome | 24 (49) | 29 (60) | 0.02 |
| Complete remission | 6 | 8 | |
| Relapsed (untreated/ hypoplastic marrow) | 3 | 3 | |
| Primary induction failure | 15 | 18 | |
| Chronic myeloid leukemia/ Myeloproliferative disorder | 17 (35) | 7 (15) | |
| Chronic phase | 3 | 1 | |
| Accelerated phase | 1 | 1 | |
| Primary induction failure | 10 | 3 | |
| Untreated/clonal evolution | 3 | 2 | |
| Myeloma | 5(10) | 11(23) | |
| Prior autologous HCT | 4 | 8 | |
| Prior allogeneic HCT | - | 1 | |
| Lymphoma | 3(6) | 0 | |
| Acute lymphoblastic leukemia | 0 | 1(2) | |
| No. of previous lines of chemotherapy, median (range) | 1(0-10) | 2(0-9) | 0.36 |
| Donor | |||
| HLA-matched unrelated | 27 (55) | 27 (56) | 1.00 |
| HLA-matched sibling | 22 (45) | 21 (44) | |
| Graft source | |||
| PBPC | 37 (76) | 35 (73) | 0.82 |
| BM | 12 (24) | 13 (27) | |
| Refined DRI | 0.79 | ||
| Low | 2 (4) | 1 (2) | |
| Intermediate | 28 (57) | 25 (52) | |
| High | 17 (35) | 18 (38) | |
| Very high | 2 (4) | 4 (8) | |
| HCT-CI | |||
| 0-2 | 28 (57) | 19 (40) | 0.11 |
| ≥3 | 21 (43) | 29 (60) |
Abbreviations: AUC, area under the curve; BM, bone marrow; DRI, Disease Risk Index; HCT, hematopoietic cell transplantation; HCT-CI, Hematopoietic Cell Transplantation-Specific Comorbidity Index; HLA, human leukocyte antigen; PBPC, peripheral blood progenitor cells.
Engraftment and Chimerism
One patient in the 16K arm failed to engraft. Among patients in whom engraftment was successful, the median time to neutrophil engraftment was 12 days (range, 10-21 days) in the 16K arm and 12 days (range, 10-23 days) in the 20K arm (P = 0.22). Six (12%) patients in the 16K arm and 2 (4%) patients in the 20K arm did not have platelet engraftment, defined as the attainment of platelet count of 20 × 109/L or more without platelet transfusion for seven consecutive days. Among those who had platelet engraftment, the median time to engraftment was 13 days (range, 7-111 days) in the 16K arm and 13 days (range, 7-70 days) in the 20K arm (P = 0.52) [Table 2].
Table 2.
Outcomes
| Outcome | Arm 1 AUC = 16K |
Arm 2 AUC = 20K |
P value |
|---|---|---|---|
| Graft failure, n | 1 | 0 | |
| Time to engraftment, median (range), days* | |||
| Neutrophil | 12 (10-21) | 12 (10-23) | 0.22 |
| Platelet | 13 (7-111) | 13 (7-70) | 0.52 |
| GVHD, cumulative incidence (95% CI) | |||
| Acute, grade II-IV | 37% (23%-50%) | 27% (14%-40%) | 0.3 |
| Acute, grade III-IV | 2% (0-6%) | 13% (3%-22%) | 0.05 |
| Chronic | 43% (26%-61%) | 55% (39%-70%) | 0.16 |
| Extensive chronic | 31% (17%-44%) | 36% (21%-50%) | 0.61 |
| 1-year relapse, cumulative incidence (95% CI) | 34% (20%-49%) | 33% (19%-47%) | 0.83 |
| Non-relapse mortality, cumulative incidence (95% CI) | |||
| Day 100 | 4% (0-10%) | 6.3% (0-13%) | 0.65 |
| 1 year | 20.6% (9%-32%) | 22% (10%-34%) | 0.69 |
| 1-year PFS (95% CI) | 48% (36%-65%) | 49% (36%-66%) | 0.59 |
| 1-year OS (95% CI) | 59% (46%-74%) | 59% (46%-75%) | 0.47 |
Among patients with successful engraftment
Abbreviations: AUC, area under the curve; CI, confidence interval; GVHD, graft-versus-host disease; PFS, progression-free survival; OS, overall survival
Microsatellite polymorphism analysis at day 30 showed a median of 100% (range, 71%-100%) myeloid cells of donor origin in the 16K arm and a median of 100% (range, 87%-100%) in the 20K arm. At day 100, the corresponding numbers were 100% (range, 0%- 100%) and 100% (range, 92%-100%) in the 16K and 20K arms, respectively. In the T-cell compartment, a median of 81% (range, 5%-100%) of cells were of donor origin in the 16K arm and 83% (range, 6%-100%) in the 20K arm at day 30, increasing to a median of 99% (range, 18%-100%) in the 16K arm and 93% (range, 8%-100%) in the 20K arm at day 100. At 6 months, 100% of cells in myeloid as well as T cell compartments were of donor origin in both arms.
GVHD
The cumulative incidence of grade II-V acute GVHD at day 100 was 37% (95% confidence interval [CI], 23%-50%) in the 16K arm and 27% (95% CI, 14%-40%) in the 20K arm (P = 0.3). Seven patients developed grade III-IV acute GVHD - one in the 16K arm and six in the 20K arm, [Table S2] with a cumulative incidence of 2% (95% CI, 0%- 6%) and 13% (95% CI, 3%-22%), respectively, (P = 0.05). The cumulative incidence of chronic GVHD was 43% (95% CI, 26%-61%) and 55% (95% CI, 39%-70%) in the 16K and 20K arms, respectively (P = 0.16). The incidence of extensive-stage chronic GVHD was 31% (95% CI, 17%-44%) and 36% (95% CI, 21%-50%), respectively, in the 16K and 20K arms (P = 0.61) [Table 2].
Survival Data and Prognostic factors
NRM
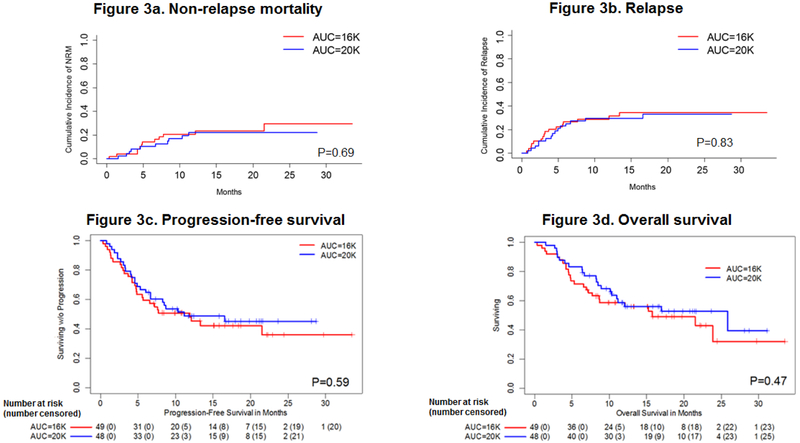
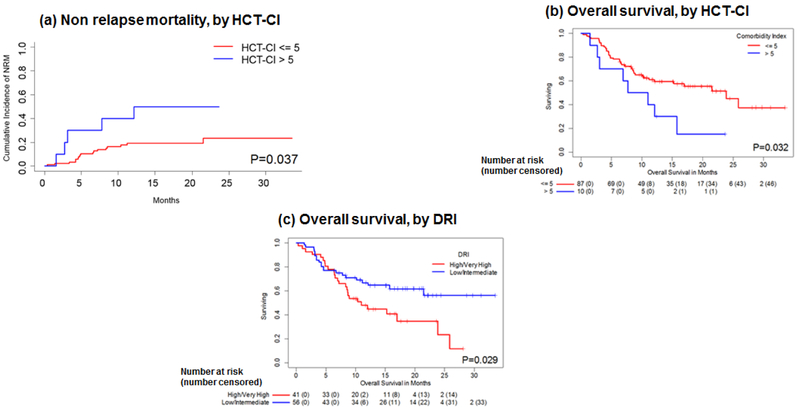
There were 2 deaths out of 49 patients in the 16K arm (both from bacterial infection) and 3 deaths out of 48 patients in the 20K arm (1 from GVHD and 2 from bacterial infection) before day 100.The estimated day 100 NRM rates were 4.1% (95% CI, 0%-9.7%) in the 16K arm and 6.3% (95% CI, 0%-13.2%) in the 20K arm (P = 0.65). Between day 101 and 1 year, there were a total of 14 additional non-relapse related deaths (acute GVHD (n=8), chronic GVHD (n=1), bacterial infection (n=4) and pneumonia (n=1)) [Table S3]. The 1-year NRM rates were 20.6% (95% CI, 9.0%-32.1%) in the 16K arm and 22.0% (95% CI, 9.7%-34.4%) in the 20K arm (P = 0.69) [Table 2 and Figure 3a]. In the multivariate model adjusted for age, donor type, graft source, and treatment arm, HCT-CI was an independent significant predictor of NRM (hazard ratio [HR], 2.12; 95% CI, 1.37-3.29; P = 0.0008) [Figure 4a]. The higher dose busulfan had no significant impact on NRM as compared to the lower dose (HR=0.48, 95% CI, 0.09-2.43, P=0.37).
Figure 3: Outcomes by treatment arm, univariate estimates:
(a) non-relapse mortality, (b) relapse, (c) progression free survival, and (d) overall survival. The numbers below figures 3c and 3d denote total number of patients at risk (number censored)
Figure 4: Outcomes by HCT-CI and DRI, univariate estimates:
(a) non-relapse mortality by HCT-CI, (b) overall survival by HCT-CI, and (c) overall survival by DRI. The numbers below figures 4b and 4c denote total number of patients at risk (number censored)
Relapse
Overall, 16 of 49 patients (33%) relapsed in the 16K arm and 15 of 48 (31%) patients in the 20K arm. The cumulative incidence of relapse was 34% (95% CI, 20%-49%) in the 16K arm and 33% (95% CI, 19%-47%) in the 20K arm (P = 0.69) [Table 2 and Figure 3b]. In the multivariate analysis adjusted for age and other covariates, DRI was an independent significant predictor of relapse, while the treatment arm had no impact (HR=0.99; 95% CI, 0.47- 2.07, P=0.98). Patients with a high or very high DRI had about 2.5 times greater risk of relapse than those with a low or intermediate DRI (HR, 2.50; 95% CI, 1.21-5.16; P = 0.01). Also, the risk of relapse was about 2.2 times higher in patients who had a sibling donor than in those who had an unrelated donor, although the difference did not reach statistical significance (HR, 2.21; 95% CI, 0.95-5.19; P = 0.07).
PFS and OS
Twenty-one of 49 patients (43%) were alive without relapse or progression by the end of the study period in the 16K arm and 23 of 48 patients (48%) in the 20K arm. The median PFS was 11.9 months (95% CI, 5.6-nonestimable [N.E.]) in the 16K arm and 11.2 months (95% CI, 6.7-N.E.) in the 20K arm. The PFS rate at 1 year was 48% (95% CI, 36%-65%) in the 16K arm and 49% (95% CI, 36%-66%) in the 20K arm (P = 0.59) [Table 2 and Figure 3c]. In the multivariate analysis adjusted for age, treatment arm and other covariates, no factor emerged as a significant predictor of PFS, although there was a trend towards inferior PFS with increasing HCT-CI (HR, 1.12; 95% CI, 0.99-1.27; P = 0.07) and in patients with a high or very high DRI (HR, 1.71; 95% CI, 0.96-3.06; P = 0.07).
Overall, 24 of 49 patients (49%) in the 16K arm and 26 of 48 patients (54%) in the 20K arm were alive by the end of the study period. The median OS was 15.8 months (95% CI, 8.6-N.E.) in the 16K arm and 25.9 months (95% CI, 11.0-N.E.) in the 20K arm. The estimated 1-year OS rate was 59% (95% CI, 46%-74%) in the 16K arm and 59% (95% CI, 46%-75%) in the 20K arm (P = 0.47) [Table 2 and Figure 3d], As with PFS, there were no significant independent predictors of OS in the multivariate analysis adjusted for age, treatment arm and other covariates, although there was a trend towards inferior OS with increasing HCT-CI (HR, 1.12; 95% CI, 0.99-1.28; P= 0.07) [Figure 4b] and in patients with a high or very high DRI (HR, 1.78; 95% CI, 0.97-3.28; P= 0.06) [Figure 4c], Disease relapse was the most common cause of death (55%), followed by acute GVHD (19%) and bacterial infections (17%) [Tables S3 and S4].
Toxicity
A total of 53 grade 3 to 5 adverse events (AEs) were noted in 37 patients in the 16K arm and 71 AEs were noted in 41 patients in the 20K arm [Table 3]. About half of the AEs were grade 3. There was no significant difference in the proportion of patients experiencing grade 3 to 5 toxicities between the 2 arms (P= 0.31). However, several AEs were more common in the 20K arm than in the 16K arm, including mucositis/neutropenic colitis (21% versus 6%; P= 0.04); pulmonary complications, including idiopathic pneumonia syndrome (IPS, 23% versus 4%; P= 0.03); and culture-negative neutropenic fever (33% versus 16%; P = 0.06). The incidence of documented infections (52% versus 49%) and other toxicities exhibited a similar pattern. We observed no cases of hepatic veno-occlusive disease (VOD), but asymptomatic hyperbilirubinemia was seen in 3 patients in the 16K arm and 5 patients in the 20K arm.
Table 3.
Grade 3 to 5 Adverse Events
| Adverse Event | Arm 1 AUC = 16K No. (%) |
Arm 2 AUC = 20K No. (%) |
P value |
|---|---|---|---|
| No of patients | 49 | 48 | |
| Primary graft failure (Grade 4) | 1 (2) | 0 | 1.00 |
| HEMATOLOGIC | 3 (6) | 2 (4) | 1.00 |
| Grade 3 Red cell aplasia # | 2 (4) | 2 (4) | |
| Grade 3 Autoimmune hemolytic anemia | 1 (2) | 0 | |
| CARDIAC | 1 (2) | 0 | 1.00 |
| Grade 3 Congestive heart failure | 1 (2) | 0 | |
| HEPATIC | 5 (10) | 6 (13) | 0.76 |
| Grade 3 Hyperbilirubinemia* | 3 (6) | 3 (6) | |
| Grade 4 Hyperbilirubinemia* | 0 | 2 (4) | |
| Grade 3 Elevation of alkaline phosphatase | 2 (4) | 0 | |
| Grade 3 Transaminitis | 0 | 1 (2) | |
| INFECTIONS** | 24 (49) | 25 (52) | 0.84 |
| Grade 3 Viral infection | 5 (10) | 5 (10) | |
| Grade 3 Fungal infection | 1 (2) | 1 (2) | |
| Grade 3 Bacterial infection | 12 (24) | 14 (29) | |
| Grade 4 Bacterial infection | 2 (4) | 2 (4) | |
| Grade 5 Bacterial infection | 4 (8) | 3 (6) | |
| Grade 3 Presumed viral encephalopathy | 2 (4) | 0 | 0.49 |
| Grade 3 Neutropenic fever*** | 8 (16) | 16 (33) | 0.06 |
| Grade 3 Cellulitis | 1 (2) | 0 | 1.00 |
| PULMONARY | 3 (6) | 11 (23) | 0.02 |
| Grade 3 Engraftment syndrome | 0 | 2 (4) | |
| Grade 3 IPS | 2 (4) | 8 (17) | |
| Grade 4 IPS | 0 | 1 (2) | |
| Grade 5 Pneumonia | 1 (2) | 0 | |
| GASTROINTESTINAL | 3 (6) | 10 (21) | 0.04 |
| Grade 3 Mucositis | 3 (6) | 9 (19) | |
| Grade 3 Neutropenic colitis | 0 | 1 (2) | |
| RENAL | 1 (2) | 1 (2) | 1.00 |
| Grade 3 Renal insufficiency | 1 (2) | 1 (2) |
due to major ABO incompatibility
Asymptomatic hyperbilirubinemia; resolved spontaneously. No cases of venoocclusive disease.
Organism documented.
Cultures negative; no obvious focus of infection.
Abbreviations: AUC, area under the curve; IPS, idiopathic pneumonia syndrome
Discussion
In this randomized phase II trial, we tested the safety of two myeloablative Bu-Flu conditioning regimens, one with a lower dose of busulfan (AUC 16 000 μmol.min) and one with a higher dose (AUC 20 000 μmol.min), using a timed-sequential approach in older and medically infirm patients. In our study population, in which more than half of patients were aged 60 years or older, 52% had an HCT-CI score of 3 or higher, and 42% had a high or very high DRI, we demonstrated that high myeloablative doses of busulfan can be safely administered to patients up to 75 years old. The rates of NRM at day 100 and at 1 year were 4% and 20%, respectively in the 16K arm and 6% and 22%, respectively in the 20K arm, which compare favorably to the NRM reported with RIC HCT in older patients (2, 17-20) and myeloablative HCT in younger patients with lower comorbidities.(1, 21, 22)
The remarkable safety of this approach was made possible by delivering myeloablative chemotherapy in a timed-sequential manner, although the precise mechanism by which the regimen’s antitumor efficacy is preserved while its toxicity is reduced is not clearly understood. This effect could simply be attributable to the lengthening of the treatment course, which allows recovery of healthy cells and thereby reduces overall toxicity. Early in vitro studies indicated that timed-sequential chemotherapy may recruit surviving leukemic cells into a proliferative state, thus making them more susceptible to subsequent cytotoxic chemotherapy. (23) Studies also suggest that the benefits of this approach may be attributable to soluble factors or humoral substances in plasma that may be released in response to a first course of chemotherapy and synergize with the second course of chemotherapy delivered 8 to 10 days later.(24, 25)
Additionally, PK monitoring allows precise dosing of busulfan, which is safer and leads to better outcomes than fixed-dose busulfan.(12) Nevertheless, administration of myeloablative conditioning is generally restricted to patients younger than 60 to 65 years of age,(17, 26-28) and patients over the age of 65 and those who have comorbidities are less likely to undergo HCT in general.(29) Although the use of RIC dramatically lowers the risk of NRM, this decrease is offset by a higher risk of relapse thereby leading to similar long-term outcomes among patients who receive myeloablative or RIC transplantation.(1-3, 5, 27) However, in a direct randomized comparison of myeloablative and RIC HCT by the Blood and Marrow Transplant Clinical Trials Network, myeloablative conditioning had a reduced risk of relapse and a strong trend towards improved OS despite its greater risk of NRM, suggesting that higher doses of chemotherapy should be used in patients who can tolerate them.(17)
In our study, the average busulfan dose administered was 9.7 mg/kg in the 16K arm and 12.5 mg/kg in the 20K arm. These are considerably higher than the busulfan dose used in RIC regimens (6.4 mg/kg iv) and are comparable to the dose used in myeloablative regimens containing busulfan (12.8 mg/kg iv).(11, 17, 18) The higher myeloablative dose of busulfan administered in the 20K arm did not compromise the time to neutrophil (median, 12 days in each arm) or platelet (median, 13 days in each arm) engraftment. Also, it did not add to the risk of grade II-IV acute GVHD (27% in the 20K arm versus 37% in the 16K arm; P = 0.3), chronic GVHD (55% versus 43%; P = 0.16) or extensive chronic GVHD (36% versus 31%; P = 0.61). Moreover, no patient developed VOD, especially as our regimen avoided the use of other agents known to increase the risk of VOD, such as cyclophosphamide-based conditioning (30) or sirolimus for GVHD prophylaxis (31). In a subgroup analysis comparing the 2 treatment arms in patients younger and older than 65 years, we found no differences in NRM, PFS, or OS in the 16K and 20K arms [Figure S1], indicating that the higher dose of busulfan was also safe in patients 65 to 75 years of age.
We acknowledge some limitations of our study. First, the two arms were not completely balanced; patients in the 20K arm were somewhat older and tended to have higher comorbidities than those in the 16K arm. Therefore, not surprisingly, patients in the higher-dose arm were more likely to experience grade 3 to 5 toxicities such as mucositis, IPS and neutropenic fever than were those in the lower-dose arm. The higher incidence of IPS in the 20K arm is in keeping with prior studies that showed increased risk with myeloablative doses of busulfan and in older patients. (32, 33) Nevertheless, only one patient in the 16K arm and none in the 20K arm had IPS-related mortality. Similarly, other toxicities were managed successfully by aggressive supportive care and did not contribute to excess NRM. Next, although NRM was remarkably low at day 100, it increased to 20-22% by 1 year in both arms. Further exploration of this revealed that late acute GVHD was the major contributor (57%) to the deaths beyond day 100, which occurred while the immunosuppression was tapered. Moreover, we observed a trend towards a higher risk of grade III-IV acute GVHD in the 20K arm (12.5% versus 2.1%; P = 0.05), and more patients in the 20K arm died of acute GVHD (n = 6) than in the 16K arm (n = 3). Furthermore, acute GVHD is known risk factor for IPS, which was more common in the 20K arm. Given GVHD related morbidity and mortality, these findings collectively call for the testing of novel GVHD prophylaxis regimens such as post-transplantation cyclophosphamide, which may reduce late NRM. Next, our study included a heterogeneous population with various diseases, which may have diluted the expected responses.
With the establishment of the safety of the timed-sequential myeloablative Bu-Flu regimen, its efficacy needs to be further evaluated. In our study where more than 70% of the patients with acute myeloid leukemia or myelodysplastic syndrome had relapsed or primary refractory disease with induction failure pre HCT and which was statistically powered to compare the primary outcome (NRM), we found no differences in the rates of relapse (33% versus 34%; P = 0.83), PFS (49% versus 48%; P = 0.59), or OS (59% each) between arms, despite the fact that the 20K arm included older population with higher comorbidity scores than the 16K arm. Larger trials with balanced patient characteristics will shed more light on the efficacy of this regimen. Prospective studies should also evaluate the impact of adding novel GVHD prophylaxis regimens, such as post-transplantation cyclophosphamide. Another subject for future research is whether further extension of the time between the two courses of busulfan, which has several potential advantages, can modulate NRM and relapse risk. Such a regimen could be implemented at centers that lack on-site capability for PK testing. Additionally, longer-duration conditioning chemotherapy would allow the inclusion of novel targeted agents in the regimen, which may further enhance antitumor efficacy. Myeloablative conditioning used in our study did not result in added risk of NRM, which was made possible simply by prolonging the duration of chemotherapy over 2 weeks rather than the conventional short course of high dose chemotherapy given over 4-5 consecutive days. Although our study harnessed the concept of timed sequential therapy using busulfan-based conditioning, it may be studied with different regimens.
Conclusion
In this randomized trial, we demonstrated the safety of administering myeloablative busulfan using the timed-sequential approach in older individuals. Patients up to the age of 75 years tolerated the timed-sequential myeloablative Bu-Flu conditioning regimen well with an extremely low NRM. Despite the presence of more patients with higher comorbidities and older age in the 20K arm, toxicities and NRM were not increased than in the 16K arm. This regimen holds considerable promise and merits further testing.
Supplementary Material
Figure S1: Outcomes by age and treatment arm, univariate estimates: Sub group analysis of patients younger or older than 65 years of age showed no difference in non-relapse mortality (a, b), progression free survival (c, d) or overall survival (e, f) in the 16K versus 20K arms.
Research in context.
Evidence before this study
We did not do a formal systemic review, but we searched PubMed for published studies of allogeneic hematopoietic stem cell transplantation (HCT) conditioning regimen intensity in patients with hematological malignancies, with no specific date restrictions. We used the search terms “allogeneic hematopoietic stem cell transplantation” AND “conditioning” OR “myeloablative” OR “reduced-intensity conditioning (RIC).” Although multiple studies showed higher risk of relapse and lower non-relapse mortality (NRM) with RIC leading to similar survival as with myeloablative conditioning, a recent randomized phase III clinical trial showed superior outcomes with myeloablative conditioning than with RIC in patients younger than age 65. However, limited prospective data are available to suggest the safety and feasibility of using myeloablative conditioning in patients older than 60-65 years who usually receive RIC.
Added value of this study
Herein, we demonstrate that myeloablative doses of busulfan, guided by pharmacokinetic monitoring and administered in a timed-sequential manner, along with fludarabine (Bu-Flu) can be given safely to patients up to age 75. Our regimen resulted in an extremely low NRM of up to 6% at day 100 post HCT, in a group where a majority had a high HCT co-morbidity index (HCT-CI) of 3 or more, and almost half had a high or very high revised disease risk index (DRI).
Implications of all the available evidence
The concept of delivering myeloablative conditioning chemotherapy over weeks in a timed-sequential manner with our Bu-Flu regimen or other regimens with or without novel targeted agents warrants further investigation to test its efficacy in older patients and those with comorbidities. This approach may allow better disease control without increasing toxicity.
Data sharing statement:
Will individual participant data be available (including data dictionaries)? Yes, de-identified data can be shared, if approved by our institutional review board.
What data in particular will be shared? Individual participant data that underlie the results reported in this article, after de-identification (text, tables, figures, and appendices).
What other documents will be available? Study protocol.
When will data be available (start and end dates)? Beginning 9 months and ending 36 months following article publication.
With whom? Investigators whose proposed use of the data has been approved by an independent review committee (“learned intermediary”) identified for this purpose and if approved by our institutional review board.
For what types of analyses? For individual participant data meta-analysis.
By what mechanism will data be made available? Proposals may be submitted up to 36 months following article publication. After 36 months the data will not be available.
Acknowledgments
Acknowledgments: We thank Amy Ninetto of the Department of Scientific Publications at the MD Anderson Cancer Center for editing the manuscript.
Role of the funding source
The biostatistician (R.B.) received funding from the Cancer Center Support Grant (NCI Grant P30 CA016672). The grant provider had no role in the study design, data collection, data analysis, interpretation of the results, or writing of the report. The first four authors had full access to the raw data (U.R.P., R.S.M., R.B., and J.C.). All authors approved the manuscript. The corresponding author had the final responsibility to submit for publication.
Research support: Cancer Center Support Grant (NCI Grant P30 CA016672)
Footnotes
ClinicalTrials.gov Identifier: NCT01572662
Trial registration: ClinicalTrials NCT01572662
Disclosure of Conflicts of Interest: The authors declared no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bornhauser M, Kienast J, Trenschel R, Burchert A, Hegenbart U, Stadler M, et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomised phase 3 trial. Lancet Oncol. 2012;13(10):1035–44. [DOI] [PubMed] [Google Scholar]
- 2.Lioure B, Bene MC, Pigneux A, Huynh A, Chevallier P, Fegueux N, et al. Early matched sibling hematopoietic cell transplantation for adult AML in first remission using an age-adapted strategy: long-term results of a prospective GOELAMS study. Blood. 2012;119(12):2943–8. [DOI] [PubMed] [Google Scholar]
- 3.Ringden O, Labopin M, Ehninger G, Niederwieser D, Olsson R, Basara N, et al. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol. 2009;27(27):4570–7. [DOI] [PubMed] [Google Scholar]
- 4.Mohty M, Labopin M, Volin L, Gratwohl A, Socie G, Esteve J, et al. Reduced-intensity versus conventional myeloablative conditioning allogeneic stem cell transplantation for patients with acute lymphoblastic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood. 2010;116(22):4439–43. [DOI] [PubMed] [Google Scholar]
- 5.Luger SM, Ringden O, Zhang MJ, Perez WS, Bishop MR, Bornhauser M, et al. Similar outcomes using myeloablative vs reduced-intensity allogeneic transplant preparative regimens for AML or MDS. Bone Marrow Transplant. 2012;47(2):203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castaigne S, Chevret S, Archimbaud E, Fenaux P, Bordessoule D, Tilly H, et al. Randomized comparison of double induction and timed-sequential induction to a “3 + 7” induction in adults with AML: long-term analysis of the Acute Leukemia French Association (ALFA) 9000 study. Blood. 2004;104(8):2467–74. [DOI] [PubMed] [Google Scholar]
- 7.Zeidner JF, Foster MC, Blackford AL, Litzow MR, Morris LE, Strickland SA, et al. Randomized multicenter phase II study of flavopiridol (alvocidib), cytarabine, and mitoxantrone (FLAM) versus cytarabine/daunorubicin (7+3) in newly diagnosed acute myeloid leukemia. Haematologica. 2015;100(9):1172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karp JE, Blackford A, Smith BD, Alino K, Seung AH, Bolanos-Meade J, et al. Clinical activity of sequential flavopiridol, cytosine arabinoside, and mitoxantrone for adults with newly diagnosed, poor-risk acute myelogenous leukemia. Leuk Res. 2010;34(7):877–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woods WG, Kobrinsky N, Buckley JD, Lee JW, Sanders J, Neudorf S, et al. Timed-sequential induction therapy improves postremission outcome in acute myeloid leukemia: a report from the Children's Cancer Group. Blood. 1996;87(12):4979–89. [PubMed] [Google Scholar]
- 10.Schmid C, Schleuning M, Schwerdtfeger R, Hertenstein B, Mischak-Weissinger E, Bunjes D, et al. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood. 2006;108(3):1092–9. [DOI] [PubMed] [Google Scholar]
- 11.Andersson BS, de Lima M, Thall PF, Wang X, Couriel D, Korbling M, et al. Once daily i.v. busulfan and fludarabine (i.v. Bu-Flu) compares favorably with i.v. busulfan and cyclophosphamide (i.v. BuCy2) as pretransplant conditioning therapy in AML/MDS. Biol Blood Marrow Transplant. 2008;14(6):672–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersson BS, Thall PF, Valdez BC, Milton DR, Al-Atrash G, Chen J, et al. Fludarabine with pharmacokinetically guided IV busulfan is superior to fixed-dose delivery in pretransplant conditioning of AML/MDS patients. Bone Marrow Transplant. 2017;52(4):580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thall PF, Simon RM, Estey EH. New statistical strategy for monitoring safety and efficacy in single-arm clinical trials. J Clin Oncol. 1996;14(1):296–303. [DOI] [PubMed] [Google Scholar]
- 14.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999; 18(6):695–706. [DOI] [PubMed] [Google Scholar]
- 15.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 16.Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative Versus Reduced-Intensity Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndromes. J Clin Oncol. 2017;35(11):1154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devine SM, Owzar K, Blum W, Mulkey F, Stone RM, Hsu JW, et al. Phase II Study of Allogeneic Transplantation for Older Patients With Acute Myeloid Leukemia in First Complete Remission Using a Reduced-Intensity Conditioning Regimen: Results From Cancer and Leukemia Group B 100103 (Alliance for Clinical Trials in Oncology)/Blood and Marrow Transplant Clinical Trial Network 0502. J Clin Oncol. 2015;33(35):4167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martino R, Perez-Simon JA, Moreno E, Queralto JM, Caballero D, Mateos M, et al. Reduced-intensity conditioning allogeneic blood stem cell transplantation with fludarabine and oral busulfan with or without pharmacokinetically targeted busulfan dosing in patients with myeloid leukemia ineligible for conventional conditioning. Biol Blood Marrow Transplant. 2005;11(6):437–47. [DOI] [PubMed] [Google Scholar]
- 20.Schetelig J, Bornhauser M, Kiehl M, Schwerdtfeger R, Kroger N, Runde V, et al. Reduced-intensity conditioning with busulfan and fludarabine with or without antithymocyte globulin in HLA-identical sibling transplantation--a retrospective analysis. Bone Marrow Transplant. 2004;33(5):483–90. [DOI] [PubMed] [Google Scholar]
- 21.Sebert M, Porcher R, Robin M, Ades L, Boissel N, Raffoux E, et al. Equivalent outcomes using reduced intensity or conventional myeloablative conditioning transplantation for patients aged 35 years and over with AML. Bone Marrow Transplant. 2015;50(1):74–81. [DOI] [PubMed] [Google Scholar]
- 22.Marks DI, Wang T, Perez WS, Antin JH, Copelan E, Gale RP, et al. The outcome of full-intensity and reduced-intensity conditioning matched sibling or unrelated donor transplantation in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first and second complete remission. Blood. 2010;116(3):366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karp JE, Ross DD, Yang W, Tidwell ML, Wei Y, Greer J, et al. Timed sequential therapy of acute leukemia with flavopiridol: in vitro model for a phase I clinical trial. Clin Cancer Res. 2003;9(1):307–15. [PubMed] [Google Scholar]
- 24.Karp JE, Donehower RC, Dole GB, Burke PJ. Correlation of drug-perturbed marrow cell growth kinetics and intracellular 1-B-D-arabinofuranosylcytosine metabolism with clinical response in adult acute myelogenous leukemia. Blood. 1987;69(4):1134–40. [PubMed] [Google Scholar]
- 25.Karp JE, Burke PJ. Enhancement of drug cytotoxicity by recruitment of leukemic myeloblasts with humoral stimulation. Cancer Res. 1976;36(10):3600–3. [PubMed] [Google Scholar]
- 26.Alyea EP, Kim HT, Ho V, Cutler C, DeAngelo DJ, Stone R, et al. Impact of conditioning regimen intensity on outcome of allogeneic hematopoietic cell transplantation for advanced acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2006;12(10):1047–55. [DOI] [PubMed] [Google Scholar]
- 27.Aoudjhane M, Labopin M, Gorin NC, Shimoni A, Ruutu T, Kolb HJ, et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT). Leukemia. 2005;19(12):2304–12. [DOI] [PubMed] [Google Scholar]
- 28.Alyea EP, Kim HT, Ho V, Cutler C, Gribben J, DeAngelo DJ, et al. Comparative outcome of nonmyeloablative and myeloablative allogeneic hematopoietic cell transplantation for patients older than 50 years of age. Blood. 2005;105(4):1810–4. [DOI] [PubMed] [Google Scholar]
- 29.Getta BM, Kishtagari A, Hilden P, Tallman MS, Maloy M, Gonzales P, et al. Allogeneic Hematopoietic Stem Cell Transplantation Is Underutilized in Older Patients with Myelodysplastic Syndromes. Biol Blood Marrow Transplant. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44(6):778–83. [DOI] [PubMed] [Google Scholar]
- 31.Cutler C, Stevenson K, Kim HT, Richardson P, Ho VT, Linden E, et al. Sirolimus is associated with veno-occlusive disease of the liver after myeloablative allogeneic stem cell transplantation. Blood. 2008;112(12):4425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukuda T, Hackman RC, Guthrie KA, Sandmaier BM, Boeckh M, Maris MB, et al. Risks and outcomes of idiopathic pneumonia syndrome after nonmyeloablative and conventional conditioning regimens for allogeneic hematopoietic stem cell transplantation. Blood. 2003; 102(8):2777–85. [DOI] [PubMed] [Google Scholar]
- 33.Bilgrami SF, Metersky ML, McNally D, Naqvi BH, Kapur D, Raible D, et al. Idiopathic pneumonia syndrome following myeloablative chemotherapy and autologous transplantation. Ann Pharmacother. 2001;35(2):196–201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Outcomes by age and treatment arm, univariate estimates: Sub group analysis of patients younger or older than 65 years of age showed no difference in non-relapse mortality (a, b), progression free survival (c, d) or overall survival (e, f) in the 16K versus 20K arms.