Abstract
Background/Objectives
Sensory function has been associated with neurological disease, but there are few prospective studies. We investigated the relationship between olfactory dysfunction and a subsequent diagnosis of dementia.
Design
Longitudinal study of a population representative of older adults in the United States.
Setting
Home interviews (National Social Life, Health, and Aging Project).
Participants
Men and women ages 57–85 years (n=2,906).
Measurements
Objective odor identification ability was measured at baseline using a validated 5-item test (Sniffin’ Sticks). Five years later, physician diagnosis of dementia was reported by the respondent or a proxy if they were too sick to interview or deceased. The association between baseline olfactory dysfunction and an interval dementia diagnosis was tested using multivariate logistic regression, controlling for age, gender, race/ethnicity, education, comorbidities (modified Charlson Index), and cognition at baseline (Short Portable Mental Status Questionnaire).
Results
Older adults with olfactory dysfunction had more than twice the odds of subsequently developing dementia five years later (OR 2.13, 95% CI: 1.32–3.43), controlling for the above covariates. Increasing number of odor identification errors was associated with increased probability of an interval dementia diagnosis (p=0.044, 1-df linear-trend test).
Conclusion
We show for the first time in a nationally-representative sample that home-dwelling older adults with normal cognition yet more difficulty identifying odors face higher odds of being diagnosed with dementia five years later, independent of other significant risk factors. This validated five-item odor identification test is an efficient, low-cost component of the physical examination that can provide useful information while assessing patients at risk for dementia. Use of such testing may provide an opportunity for early interventions to reduce the attendant morbidity and public health burden of dementia.
Keywords: Aged, Dementia, Longitudinal Studies, Olfaction Disorders, Smell
Introduction
Older adults with dementia have a higher prevalence of olfactory dysfunction in cross-sectional studies1. However, in the few longitudinal studies that assessed whether olfactory dysfunction precedes the development of Alzheimer’s Disease (AD), the most common type of dementia2–7, not all found a significant predictive relationship. Moreover, subjects in these longitudinal studies were from at-risk groups, relatively small samples or homogenous populations. In some studies, it was also possible that the apparent predictive power of olfaction was an artifact of its association with cognitive abilities at baseline and did not have predictive power above and beyond the predictive effects of baseline cognition itself. Thus, it remains uncertain if olfactory dysfunction is a predictive marker of subsequent dementia in the diverse general population of older US adults. We investigated the relationship between olfactory dysfunction and a subsequent diagnosis of dementia within five years in the National Social Life, Health, and Aging Project (NSHAP), a nationally representative, probability sample of home-dwelling Americans aged 57–85.
Methods
Study Population
We studied 2,906 NSHAP respondents who were interviewed at baseline (2005–6) and re-interviewed at five-year follow-up (2010–11) in their homes by professional interviewers (from NORC at the University of Chicago); this was done after excluding respondents who reported a pre-existing physician diagnosis of dementia (0.7%), those who did not complete olfactory testing (2.2%), and those who did not provide complete demographic information (0.4%). Both interviews included assessments of demographic, social, psychological, and biological measures, including olfactory ability. To select its sample, NSHAP followed a standard multistage area probability sample design, covering all geographic areas of the US. This sample is representative of the US home-dwelling population aged 57–85 (born 1920–1947)8. Further details regarding the study design and data collection are published elsewhere9. By design, the population was cognitively intact at baseline (mean Short Portable Mental Status Questionnaire, SPMSQ: 9.2 ± 1.0 out of 10). Additional baseline characteristics of the population are presented in Table 1. This study was approved by the Institutional Review Boards at NORC and The University of Chicago.
Table 1.
Characteristics of the sample at baseline, survey-weighted to represent the US population of home-dwelling older adults (n=2,906).
| Characteristic | % |
|---|---|
|
| |
| Odors correctly identified | |
| Olfactory Dysfunction (0–3 correct) | 22.0 |
| 0 | 1.0 |
| 1 | 2.2 |
| 2 | 4.9 |
| 3 | 13.8 |
| Normosmic (4–5 correct) | 78.0 |
| 4 | 29.4 |
| 5 | 48.7 |
|
| |
| Age (years, mean ± SD) | 68.0 ± 7.6 |
| 57–64 years | 41.6 |
| 65–74 years | 34.9 |
| 75–85 years | 23.4 |
|
| |
| Gender | |
| Men | 48.9 |
| Women | 51.1 |
|
| |
| Race/ethnicity | |
| White | 80.8 |
| Black | 9.9 |
| Hispanic, non-Black | 6.9 |
| Other | 2.5 |
|
| |
| Education | |
| No college | 45.1 |
| Some college or higher | 54.9 |
|
| |
| Comorbidities (modified Charlson Index, mean ± SD) | 1.8 ± 1.7 |
|
| |
| Cognition (SPMSQ Score, mean ± SD) | 9.2 ± 1.0 |
| Among respondents with olfactory dysfunction | 8.8 ± 1.5 |
| Among normosmic respondents | 9.3 ± 0.9 |
SD=Standard deviation, SPMSQ = Short Portable Mental Status Questionnaire
Olfactory Assessment at Baseline
Objective odor identification ability was evaluated at baseline using a validated 5-item test10, 11. Odors were presented via Sniffin’ Sticks odor pens (Burghart Messtechnik; Wedel, Germany) and respondents were asked to identify each odor by choosing from a set of four picture/word prompts. Refusals were coded as incorrect. Using previously validated cutpoints10, 11, respondents who identified 4–5 odors correctly were classified as normosmic, whereas respondents who identified only 3 or fewer odors correctly were classified as having some form of olfactory dysfunction. Using these criteria, 22.0% of older US adults had objective olfactory dysfunction, a percentage that aligns with other estimates of olfactory dysfunction in older adults12, 13.
Interval Dementia Diagnosis at Five-year Follow-up
At five-year follow-up, we determined physician diagnosis of dementia during home interviews with the respondent or a proxy if respondents were too sick to interview or deceased. Prior studies have found that proxy reports of date and cause of death match or exceed the accuracy of information from death certificates and that proxy reports of dementia are reasonably accurate when compared to psychometric testing14–16.
Proxy interviews were unattainable for 296 respondents (10.2%). We classified these respondents conservatively as normal (presented results). We found similar effect sizes and significance levels either when we classified all of them as having dementia or excluded them altogether.
Potential Confounding Variables
Our analyses accounted for numerous potential confounders, including age, gender, race/ethnicity, education, comorbidity, and baseline cognition (Supplementary Table S1). Age and gender have long been known to be associated with olfactory function12. Race, an established olfactory risk factor17, was measured via self-report according to standard NIH questions. Respondents were classified as White, Black, or Hispanic (those who reported their race as “Black/African American” and answered “Yes” to Hispanic ethnicity were classified as Black). Small sample sizes necessitated combining into a single “Other” category those reporting their race as “American Indian or Alaskan Native,” “Asian,” or “Other”. Education was measured by highest degree or certification earned. Comorbid diseases were measured with the Charlson Index modified for the NSHAP survey.
It is likely that respondents diagnosed with dementia might exhibit cognitive decline at baseline. Further, we have previously reported in a cross-sectional analysis that olfaction is associated with cognition within the normal cognitive range18. Therefore, cognitive function at baseline was included as a covariate in order to test the hypothesis that olfactory dysfunction would predict the development of dementia above and beyond baseline cognition itself. Cognitive function at baseline was measured with a modified version of the Short Portable Mental Status Questionnaire (SPMSQ, scores from 0–10).
Statistical Analysis
NSHAP used a national probability sample of home-dwelling older US adults (born 1920–1947). The participation rates were considered excellent for surveys of this type: 75.5% at baseline (2005–2006) and 74% at follow-up (2010–2011; the conditional response rate among returning respondents was 89%). In addition, NSHAP oversampled African Americans and Hispanics, as well as males and older individuals, so as to obtain roughly equal numbers of sampled individuals in each of six gender by age categories8. Respondent-level weights were calculated to adjust for differential non-response to survey participation as well as the planned oversampling based on race/ethnicity, gender, and age. This permits estimation of parameters for the US population of older, home-dwelling adults10, 11. All values presented use these respondent-level weights. Design-based standard errors were calculated using the linearization method together with the strata and Primary Sampling Unit indicators provided with the dataset. Sensitivity, specificity, positive predictive value, and negative predictive value were calculated using standard formulas. All statistical analyses were conducted using Stata Version 14.0.
We tested the association between baseline olfactory dysfunction and an interval dementia diagnosis using multivariate logistic regression, controlling for age, gender, race/ethnicity, education, comorbidities, and baseline cognitive function. We tested the incremental risk attributed to each additional odor identification error using a one degree of freedom linear trend test. Results are presented as odds ratios and 95% confidence intervals (CI). Statistical significance was set at p<0.05.
Results
At five-year follow-up, 4.1% of older adults reported a new physician diagnosis of dementia (Supplementary Table S2). The sensitivity of olfactory dysfunction was 47%; nearly one-half of older adults reporting a new physician diagnosis of dementia had olfactory dysfunction on testing five years prior. The specificity was 79%; over three-quarters of older adults without a new dementia diagnosis had normal olfactory testing five years prior. The positive predictive value of olfactory dysfunction in predicting a dementia diagnosis five years later was 9%, while the negative predictive value was 97%.
Thus, older adults with olfactory dysfunction had more than twice the odds of developing dementia five years later (OR 2.13, 95% CI: 1.32–3.43), controlling for age, gender, race/ethnicity, education, comorbidities, and baseline cognitive function (Table 2).
Table 2.
Effects of baseline olfactory dysfunction on an interval dementia diagnosis at five-year follow-up (logistic regression, n=2,906).
| Covariates | Odds Ratio (95% Confidence Interval) | p-value |
|---|---|---|
|
| ||
| Olfactory Dysfunction (vs. Normosmic) | 2.13 (1.32–3.43) | 0.002 |
|
| ||
| Age (per year) | 1.06 (1.01–1.11) | 0.02 |
|
| ||
| Gender (Women vs. Men) | 1.02 (0.56–1.86) | 0.95 |
|
| ||
| Race/ethnicity | ||
| White (ref) | -- | -- |
| Black | 0.84 (0.42–1.68) | 0.62 |
| Hispanic, non-Black | 0.73 (0.34–1.58) | 0.42 |
| Other | 0.32 (0.04–2.59) | 0.28 |
|
| ||
| Education (Some College or Higher vs. No College) | 0.87 (0.47–1.60) | 0.64 |
|
| ||
| Comorbidities (per 1 point on modified Charlson Index) | 1.02 (0.91–1.15) | 0.68 |
|
| ||
| Cognition (per 1 point on SPMSQ Score) | 0.73 (0.61–0.87) | 0.001 |
SPMSQ = Short Portable Mental Status Questionnaire
As expected, higher cognitive function at baseline was associated with a lower likelihood of interval dementia diagnosis (OR 0.73, 95% CI: 0.61–0.87). In addition, dementia was more likely to develop among older adults (OR 1.06, 95% CI: 1.01–1.11), further supporting the validity of our diagnostic measure. Nonetheless, olfactory dysfunction had a predictive value over and above baseline cognition, equivalent to aging 13 years. Indeed, we found that each additional odor identification error increased the odds of dementia independent of all of the covariates (p=0.044, Figure 1).
Figure 1.
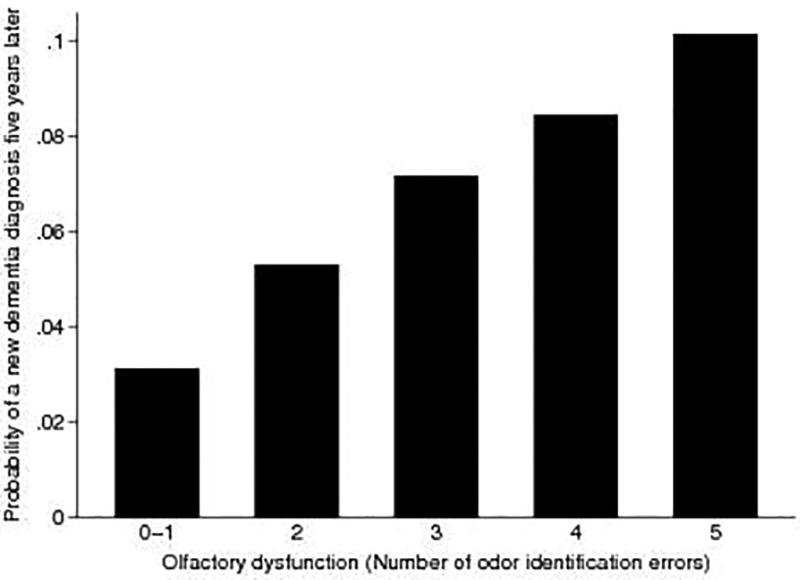
Olfactory dysfunction (number of odor identification errors) predicts increased probability of physician dementia diagnosis five years later.
As a stronger test of the predictive effects of olfaction independent of baseline cognition, we restricted our analysis to older adults who, at baseline, tested within the normal cognitive range. In case the initial findings were driven by those with impaired cognition at baseline, we excluded respondents whose baseline SPMSQ scores were below the traditional cutoff for likely dementia (<8). The sensitivity of olfactory dysfunction was similar: 43% of older adults reporting a new dementia diagnosis had olfactory dysfunction on testing five years prior. Furthermore, among older adults who tested within the normal cognitive range, poor olfaction continued to strongly predict an interval dementia diagnosis (OR 2.21, 95% CI: 1.33–3.69, n=2,677).
Discussion
We show for the first time that home-dwelling older US adults with more difficulty identifying odors have greater odds of receiving a new physician diagnosis of dementia five years later (based on respondent or proxy reports). The strengths of this study include a design that represents the general US population, demographic diversity, the largest sample size to date, and control of key covariates. Specifically, we demonstrate the predictive power of olfaction above and beyond cognition by including baseline cognitive function as a covariate in the analyses and by confirming results after excluding those with SPMSQ scores indicating high dementia risk. An additional strength that gives this study its broad implications is that olfaction is predictive of dementia even in cognitively intact older adults, extending prior work with high risk populations and smaller, more homogenous samples1–7.
Why would olfactory dysfunction precede the development of dementia? One potential explanation is that the neuropathology underlying dementia, such as AD, (amyloid-β plaques and paired helical filament tau tangles) may begin earlier in the olfactory system than the cortex19–21. If so, this type of neuropathology in the olfactory system would impair its function prior to the detrimental effects in other parts of the central nervous system, including cognition. Indeed, a post-mortem study of brains obtained at autopsy of older adults who had completed olfactory testing prior to death (mean ± standard deviation of 2.2 ± 1.2 years pre-mortem) found that neuropathological findings associated with AD accounted for 12% of the variation in odor identification ability22. The association between olfactory dysfunction and dementia could also arise from other shared pathological mechanisms to which the olfactory system is more vulnerable, such as decreased age-related regenerative capacity, reduced physiologic repair, or worsening immunopathology. Additional studies of this area will be needed to test and fine-tune these hypotheses.
On a practical basis for clinicians, a validated five-item odor identification test10, 11 can potentially serve as an efficient, low-cost component of the physical examination that provides useful information in assessing older patients at risk for dementia. This tool is a non-invasive biomarker that provides additional information alongside other relevant clinical information, established risk factors, and neuropsychological testing to aid in an earlier diagnosis of dementia. The ability to identify individuals at risk for dementia prior to overt cognitive impairment would allow for earlier intervention, with the potential to improve patient outcomes, and would offer patients and their families more time to plan ahead.
In addition to olfactory testing, several biomarkers for dementia have been proposed, including cerebrospinal fluid biomarkers (e.g. concentrations of amyloid-β and tau), structural brain imaging (hippocampal volume23), and inflammatory molecules24. We found that our five-item odor identification test has a sensitivity of 47% and a specificity 79% five years in advance of any evidence of cognitive impairment. These characteristics compare reasonably to other proposed dementia biomarkers when cognitive decline is already in process: for example, a recent Cochrane Review found that in individuals with mild cognitive impairment amyloid-β in cerebrospinal fluid has a sensitivity of 81% at the median specificity of 64% for predicting conversion to AD25, a condition considered a precursor to AD. However, despite the efforts to identify biomarkers for dementia, none of the candidate biomarkers thus far have achieved the appropriate screening characteristics to recommend their utilization for the general population26. Indeed, the United States Preventive Services Task Force gives screening for cognitive impairment among older adults an “I” grade, concluding that the current evidence is insufficient to assess the balance of benefits and harms. More detailed study of the additional value of olfactory testing in this clinical context is warranted.
We found that the positive predictive value of olfactory dysfunction in predicting a subsequent dementia diagnosis within five years was 9% in home-dwelling older Americans, meaning that 91% of older adults who failed our olfactory test did not develop dementia within the five year follow-up period. We note that the positive predictive value of a test depends on the prevalence of disease in the population studied, implying that the positive predictive value of olfactory dysfunction will increase in at-risk populations. Further, the positive predictive value will likely improve as the follow-up period increases, given that risk for dementia increases with age.
Further research is needed to determine the test characteristics of olfactory dysfunction in different populations and follow-up periods. Regardless of test characteristics, primary care physicians will need to thoughtfully evaluate when olfactory testing is appropriate for their individual patients, taking into consideration the benefits of early dementia risk stratification given the lack of available therapies and the emotional and financial harms that can result from misdiagnosis.
Beyond aiding in an earlier diagnosis of dementia by alerting physicians to consider other factors that may affect disease development, we speculate that olfactory testing may help to distinguish clinically between different dementia subtypes. Cross-sectional studies have shown associations between olfactory dysfunction and both vascular dementia and frontotemporal dementia27, 28. Indeed, prior studies suggest that different types of olfactory disability (e.g., odor identification vs. odor discrimination vs. odor threshold) may be associated with different forms of dementia28. Finally, we speculate that olfactory testing may be useful in predicting progression of disease and response to treatment29. This remains to be tested in future work.
Our study has several limitations. As our primary outcome, we rely on interview reports of physician-diagnosed dementia, and we are therefore most likely underestimating the incidence of new dementia cases, although proxy reports of dementia are reasonably accurate when compared to psychometric testing14–16. A higher incidence of dementia would likely be revealed by psychometric testing were it not infeasible in a time-limited, many-item, and omnibus nationally-representative survey.23Additionally, while we control for many known confounders, as in any cohort analysis, there may be additional confounders associated with both olfactory function and dementia that are unaccounted for in our analyses, such as smoking and depression.
In summary, older adults with olfactory dysfunction are twice as likely to develop dementia five years later as those with normal olfaction. Use of simple olfactory testing in the primary care setting may provide an opportunity for targeted, early interventions to reduce the attendant morbidity and public health burden of dementia.
Supplementary Material
Supplementary Table S1. Cross-sectional association between selected covariates and olfactory dysfunction (univariate logistic regressions, n=2,906).
Supplementary Table S2. New dementia diagnosis by a physician at five-year follow-up, reported by respondent or proxy; survey weighted value (raw value).
Acknowledgments
This work was supported by the National Institute on Aging (AG030481; AG036762; AG029795; AG048511; AG033903), the National Institute of Allergy and Infectious Disease (Chronic Rhinosinusitis Integrative Studies Program [CRISP], AI106683), the Institute for Translational Medicine at The University of Chicago (KL2RR025000), and the McHugh Otolaryngology Research Fund. DRA was supported by a Pritzker Fellowship from the Pritzker School of Medicine at The University of Chicago. The funders had no role in the design, methods, subject recruitment, data collection, analysis or preparation of this paper.
Susie Kim and Katherine McKeough (both Section of Otolaryngology-Head and Neck Surgery) provided logistical support. Timothy M. Mulcahy (NORC) provided technical support. Written permission has been obtained from all persons named in the acknowledgement.
Footnotes
The authors have no conflicts of interest.
Author Contributions
Study concept and design: Adams, Pinto, McClintock, Dale
Acquisition, analysis, or interpretation of data: Adams, Kern, Wroblewski, McClintock, Dale, Pinto
Drafting of the manuscript: Adams
Critical revision of the manuscript for important intellectual content: Adams, Kern, Wroblewski, McClintock, Dale, Pinto
Statistical analysis: Adams, Wroblewski
References
- 1.Sun GH, Raji CA, Maceachern MP, Burke JF. Olfactory identification testing as a predictor of the development of Alzheimer's dementia: a systematic review. Laryngoscope. 2012;122:1455–1462. doi: 10.1002/lary.23365. [DOI] [PubMed] [Google Scholar]
- 2.Devanand DP, Lee S, Manly J, et al. Olfactory deficits predict cognitive decline and Alzheimer dementia in an urban community. Neurology. 2015;84:182–189. doi: 10.1212/WNL.0000000000001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schubert CR, Carmichael LL, Murphy C, Klein BE, Klein R, Cruickshanks KJ. Olfaction and the 5-year incidence of cognitive impairment in an epidemiological study of older adults. J Am Geriatr Soc. 2008;56:1517–1521. doi: 10.1111/j.1532-5415.2008.01826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahar-Fuchs A, Moss S, Rowe C, Savage G. Awareness of olfactory deficits in healthy aging, amnestic mild cognitive impairment and Alzheimer's disease. International psychogeriatrics / IPA. 2011;23:1097–1106. doi: 10.1017/S1041610210002371. [DOI] [PubMed] [Google Scholar]
- 5.Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett DA. Olfactory identification and incidence of mild cognitive impairment in older age. Archives of general psychiatry. 2007;64:802–808. doi: 10.1001/archpsyc.64.7.802. [DOI] [PubMed] [Google Scholar]
- 6.Yaffe K, Freimer D, Chen H, et al. Olfaction and risk of dementia in a biracial cohort of older adults. Neurology. 2017;88:456–462. doi: 10.1212/WNL.0000000000003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts RO, Christianson TJ, Kremers WK, et al. Association Between Olfactory Dysfunction and Amnestic Mild Cognitive Impairment and Alzheimer Disease Dementia. JAMA Neurol. 2016;73:93–101. doi: 10.1001/jamaneurol.2015.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Muircheartaigh C, Eckman S, Smith S. Statistical design and estimation for the National Social Life, Health, and Aging Project. Journal of Gerontology: Social Sciences. 2009;64B:i12–19. doi: 10.1093/geronb/gbp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzman R. The National Social Life, Health, and Aging Project: An Introduction. Journal of Gerontology: Social Sciences. 2009:64–65. doi: 10.1093/geronb/gbp078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kern DW, Wroblewski KE, Schumm LP, Pinto JM, McClintock MK. Field Survey Measures of Olfaction: The Olfactory Function Field Exam (OFFE) Field Methods. 2014 doi: 10.1177/1525822X14547499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller C, Renner B. A new procedure for the short screening of olfactory function using five items from the "Sniffin' Sticks" identification test kit. American journal of rhinology. 2006;20:113–116. [PubMed] [Google Scholar]
- 12.Murphy C, Schubert CR, Cruickshanks KJ, Klein BEK, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. JAMA : the journal of the American Medical Association. 2002;288:2307–2312. doi: 10.1001/jama.288.18.2307. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman HJ, Rawal S, Li CM, Duffy VB. New chemosensory component in the U.S. National Health and Nutrition Examination Survey (NHANES): first-year results for measured olfactory dysfunction. Reviews in endocrine & metabolic disorders. 2016 doi: 10.1007/s11154-016-9364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McClintock MK, Dale W, Laumann EO, Waite L. Empirical redefinition of comprehensive health and well-being in the older adults of the United States. Proceedings of the National Academy of Sciences of the United States of America. 2016 doi: 10.1073/pnas.1514968113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kemp NM, Brodaty H, Pond D, Luscombe G. Diagnosing dementia in primary care: the accuracy of informant reports. Alzheimer Dis Assoc Disord. 2002;16:171–176. doi: 10.1097/00002093-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Halanych JH, Shuaib F, Parmar G, et al. Agreement on cause of death between proxies, death certificates, and clinician adjudicators in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Am J Epidemiol. 2011;173:1319–1326. doi: 10.1093/aje/kwr033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinto JM, Schumm LP, Wroblewski KE, Kern DW, McClintock MK. Racial Disparities in Olfactory Loss Among Older Adults in the United States. Journals of Gerontology: MEDICAL SCIENCES Cite journal as J Gerontol A Biol Sci Med Sci. 2014;69:323–329. doi: 10.1093/gerona/glt063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schumm LP, Kern DW, Wroblewski KE, et al. Association for Chemoreception Sciences. Bonita Springs, FL: Apr 19, 2013. Odor Identification and Cognition in a Nationally Representative Sample of Older Adults. [Google Scholar]
- 19.Arnold SE, Lee EB, Moberg PJ, et al. Olfactory epithelium amyloid-beta and paired helical filament-tau pathology in Alzheimer disease. Ann Neurol. 2010;67:462–469. doi: 10.1002/ana.21910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kovacs T, Cairns NJ, Lantos PL. Olfactory centres in Alzheimer's disease: olfactory bulb is involved in early Braak's stages. Neuroreport. 2001;12:285–288. doi: 10.1097/00001756-200102120-00021. [DOI] [PubMed] [Google Scholar]
- 21.Attems J, Lintner F, Jellinger KA. Olfactory involvement in aging and Alzheimer's disease: an autopsy study. Journal of Alzheimer's disease : JAD. 2005;7:149–157. doi: 10.3233/jad-2005-7208. discussion 173–180. [DOI] [PubMed] [Google Scholar]
- 22.Wilson RS, Arnold SE, Schneider JA, Tang Y, Bennett DA. The relationship between cerebral Alzheimer's disease pathology and odour identification in old age. Journal of neurology, neurosurgery, and psychiatry. 2007;78:30–35. doi: 10.1136/jnnp.2006.099721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell MB, Shaughnessy LW, Shirk SD, Yang FM, Atri A. Neuropsychological test performance and cognitive reserve in healthy aging and the Alzheimer's disease spectrum: a theoretically driven factor analysis. Journal of the International Neuropsychological Society : JINS. 2012;18:1071–1080. doi: 10.1017/S1355617712000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craig-Schapiro R, Fagan AM, Holtzman DM. Biomarkers of Alzheimer's disease. Neurobiology of disease. 2009;35:128–140. doi: 10.1016/j.nbd.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ritchie C, Smailagic N, Noel-Storr AH, et al. Plasma and cerebrospinal fluid amyloid beta for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI) The Cochrane database of systematic reviews. 2014:Cd008782. doi: 10.1002/14651858.CD008782.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams JW, Plassman BL, Burke J, Benjamin S. Preventing Alzheimer's disease and cognitive decline. Evidence report/technology assessment. 2010:1–727. [PMC free article] [PubMed] [Google Scholar]
- 27.Alves J, Petrosyan A, Magalhaes R. Olfactory dysfunction in dementia. World journal of clinical cases. 2014;2:661–667. doi: 10.12998/wjcc.v2.i11.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luzzi S, Snowden JS, Neary D, Coccia M, Provinciali L, Lambon Ralph MA. Distinct patterns of olfactory impairment in Alzheimer's disease, semantic dementia, frontotemporal dementia, and corticobasal degeneration. Neuropsychologia. 2007;45:1823–1831. doi: 10.1016/j.neuropsychologia.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Velayudhan L, Lovestone S. Smell identification test as a treatment response marker in patients with Alzheimer disease receiving donepezil. Journal of clinical psychopharmacology. 2009;29:387–390. doi: 10.1097/JCP.0b013e3181aba5a5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Cross-sectional association between selected covariates and olfactory dysfunction (univariate logistic regressions, n=2,906).
Supplementary Table S2. New dementia diagnosis by a physician at five-year follow-up, reported by respondent or proxy; survey weighted value (raw value).


