Abstract
The traditional Inuit diet includes wild birds, fish and marine mammals, which can contain high concentrations of the neurotoxicant methylmercury (MeHg). Hydroelectric development may increase MeHg concentrations in traditional foods. Consumption advisories are often used to mitigate such risks and can result in reduced intake of traditional foods. Data from a dietary survey, MeHg exposure assessment and risk analysis for individuals in three Inuit communities in Labrador, Canada (n = 1,145) in 2014 indicate reducing traditional food intake is likely to exacerbate deficiencies in n-3 polyunsaturated fatty acids and vitamins B12 and B2. Traditional foods accounted for < 5% of per-capita calories but up to 70% of nutrients consumed. Although consumption advisories could lower neurodevelopmental risks associated with an increase in MeHg exposure (90th-percentile ΔIQ = −0.12 vs. −0.34), they may lead to greater risks of cardiovascular mortality (90th-percentile increase: +58% to +116% vs. +25%) and cancer mortality (90th-percentile increase +2% to +4% vs. no increase). Conversely, greater consumption of locally caught salmon mostly unaffected by hydroelectric flooding would lower all these risks (90th-percentile ΔIQ = +0.4; cardiovascular risk: −45%; cancer risk: −1.4%). We thus conclude that continued consumption of traditional foods is essential for Inuit health in these communities.
Keywords: fish advisory, methylmercury, indigenous health, dietary transition, nutrition
1. Introduction
Traditional foods consumed by northern Inuit populations include locally caught fish, birds and marine mammals. These foods are critical sources of micronutrients and high-quality protein in regions that have limited access to other fresh foods. However, some traditional foods contain elevated concentrations of contaminants such as methylmercury (MeHg) that biomagnify in aquatic environments.1 MeHg exposures among indigenous populations across Canada tend to be higher than the national average due to relatively greater consumption of fish and marine mammals.2, 3 Fish consumption advisories are a common policy response to elevated environmental levels of MeHg.4–7 However, even targeted food advisories can lead to decreased overall consumption of seafood.8, 9 In indigenous populations, some food consumption advisories related to elevated MeHg levels have led to decreased overall consumption of seafood.10, 11
MeHg has a half-life of 50–70 days in the human body and thus dietary changes can alter exposures over shorter timescales than many other hydrophobic organic pollutants such as polychlorinated biphenyls (PCBs) that persist in the body for decades.12, 13 Negative effects of MeHg exposure on the brain of the developing fetus and young children have been shown to persist into adulthood and this is the endpoint used by most regulatory agencies to establish risk threholds.14–16 An association between elevated prenatal MeHg exposures and neurodevelopmental impairment has similarly been shown among Inuit children.17, 18 MeHg exposure has also been associated with cardiovascular health risks for adults.19–22
Dietary advice to indigenous populations must balance the competing goals of minimizing contaminant exposures with ensuring nutritional sufficiency.10, 11, 23 Thus, quantitative studies quantifying the likely health risks or benefits from different dietary interventions among indigenous populations are needed and are the focus of this work. Past studies have examined risk tradeoffs between increased MeHg exposure and n-3 polyunsaturated fatty acids (n-3 PUFAs) in seafood.24–27 Cardiovascular and neurodevelopmental benefits of n-3 PUFAs can offset negative impacts of MeHg exposure.27 The World Health Organization (WHO) and the Food and Agriculture Organization (FAO) have however recommended that risk-benefit evaluations for seafood consumption consider a broader suite of nutrients, including iron, zinc, and vitamins A, D, B2 and B12.28
Dietary changes exert changes on health endpoints through diverse, competing mechanisms. For example, red and processed meats increase cardiovascular risks by triggering the formation of proartherosclerotic trimethylamines and raise colorectal cancer risks through production of carcinogenic N-nitroso compounds in the gastrointestinal tract.29–31 Conversely, fruits, vegetables and nuts are rich in compounds that reduce cardiovascular risks by inhibiting platelet aggregation and oxidation of arterial cholesterol (antioxidants and polyphenols), mediating glucose homeostasis (fiber) and regulating blood pressure (potassium and magnesium).32–36 There is strong evidence that vitamin D exerts a generalized cancer-protective effect through the apoptotic and antiangiogenic properties of its metabolite 1,25(OH) D.37–39 The Global Burden of Disease study synthesizes the most well-established causal relationships between intake of various foods and nutrients and different cancers and cardiovascular diseases.40, 41
Here, we use results from a dietary survey and food subsidy data to better understand consumption preferences and the nutritional importance of locally harvested foods for three Inuit communities in the Lake Melville region of Labrador, Canada. In this region, local Inuit are concerned about potential increases in MeHg exposure from traditional foods following completion of a hydroelectric power facility upstream of their traditional hunting and fishing territory. Flooding of hydroelectric reservoirs leads to a pulse in MeHg production in the saturated soils, which can enter the overlying water and accumulate in fish and wildlife. In previous work, we estimated increases in MeHg concentrations of traditional foods at their peak due to the local hydroelectric development using a biogeochemical model.42 Modeled peak increases in ranged from nine times 2014 levels for some freshwater species most affected by the hydroelectric development to zero for offshore marine species.42
In this study, we conduct a screening level analysis of potential risks associated with higher MeHg concentrations in traditional foods. We compare the estimated magnitudes of projected MeHg exposure risks to those attributable to changes in nutrient intake following a dietary transition away from traditional foods using established dose-response relationships. We conduct this analysis to better understand potential health implications of dietary consumption advisories for indigenous populations more generally.
2. Methods
2.1. Food frequency questionnaire and hair Hg analysis
We developed a food frequency questionnaire (FFQ) to measure intake of traditional foods and store-bought seafood among Labrador Inuit settled in three communities downstream from the Churchill River: 1) Happy Valley – Goose Bay, 2) North West River, and 3) Rigolet (SI Figure S1). We enrolled a total of 1,145 individuals, representing roughly 40% of the Inuit population in these communities (SI Table S1). Responses were weighted by demographic categories according to the 2011 Census to provide statistically relevant estimates for the whole population (SI Table S2).43 A team of 26 trained research assistants recruited from the Inuit community administered the survey instrument.
The FFQ asked respondents to recall their intake of 64 traditional foods (locally caught seafood, land mammals, birds, plants and berries) and 24 store-bought seafood types over 24-hour, one-month and three-month recall periods. Foods measured by the FFQ are listed in SI Tables S3 (locally caught seafood), S4 (other locally caught foods) and S5 (store bought seafood). The FFQ was administered across three seasons to assess seasonal variability in consumption pWinter (March–April, n = 231), Spring (June–July, n = 294), and Summer (August–September, n = 1,054). Enrollment was maximized for the Summer survey period, which we use for risk calculations unless otherwise noted. Self-reported age, sex, height and weight was included in the survey. All FFQ respondents in the Spring and Summer periods were asked to provide hair samples. In total, 656 hair samples corresponding to 571 unique individuals were collected (157 in Spring and 499 in Summer).
All work involving human subjects (recruitment, survey design, data analysis and reporting) was reviewed and approved by the Office of Human Research Administration at the Harvard T.H. Chan School of Public Health, the Newfoundland and Labrador Health Research Ethics Authority and the Nunatsiavut Government Inuit Research Advisor. The Nunatsiavut Government provided input on all research plans and has assumed responsibility for disseminating research findings to community members and provincial and federal policymakers.44, 45 Additional information on the FFQ design and implementation, participant recruitment and hair Hg analysis is provided in the SI.
2.2. Modeled MeHg exposures
We modeled MeHg exposures for all Inuit individuals in 2014 using dietary recall data for the 88 traditional and store-bought foods and associated MeHg concentrations (modeled as lognormal distributions). For locally caught foods, we directly measured MeHg concentrations in 22 species representing 81% of per-capita MeHg exposures from this category in 2014. All data sources for MeHg concentrations were originally reported by Li et al.46et al. and Calder et al.42 and are provided in SI Tables S3 (locally caught seafood), S4 (other locally caught foods) and S5 (store-bought seafood).
To correct for overreporting bias associated with species-specific recall, we scaled reported species-specific intakes to match reported total consumption of three food categories (local seafood, store-bought seafood and other locally caught foods) following Lincoln et al.47 We probabilistically simulated hair mercury concentrations (10,000 Monte Carlo trials) for each individual using the one-compartment model developed by the U.S. Environmental Protection Agency’s (U.S. EPA), lognormal distributions for MeHg concentrations in food items (Tables S3–S5), and probabilistically distributed toxicokinetic parameters48, following Li et al.12
The median ratio between measured hair Hg and simulated hair Hg was 0.96 in the larger-scale Summer survey period, suggesting that there is very little bias in the dietary model we developed (measured-to-modeled ratio close to 1). To ensure that population-wide MeHg exposures are not overestimated by the dietary model, modeled seafood intake was scaled such that simulated hair Hg matched measured Hg for all individuals with available hair Hg data. For others, seafood intake was scaled by the median bias (0.96).
2.3. Dietary intake of other foods
We estimated consumption of market foods other than seafood that have high nutritional content using sales data for the community of Rigolet for the same years as our dietary survey (2014–2015).49 Data on the edible supply of foods have been successfully used to estimate dietary composition and caloric sufficiency in other populations.50–52 Data were obtained from a Canadian federal subsidy program (Nutrition North), which subsidizes 42 nutrient-dense and perishable store-bought foods or food categories in remote communities.53 We estimated population-wide intake of nutrient-dense store-bought foods based on the magnitude of food subsidies and by subtracting retail and consumer waste fractions54 (SI Table S6). We assumed the composition of market foods sold in Rigolet was similar for the other two communities (Happy Valley-Goose Bay, Northwest River). Since traditional food intake was smaller in these communities in 2014, we allowed for proportionally greater consumption of store-bought nutrient-dense foods.
We used the relationship developed by Mifflin et al.55 that has applied among indigenous populations56 to estimate total energy expenditure (E) of each Inuit individual based on self-reported body mass, height, and age from survey data (eq. 1):
| (1) |
where W is body mass (kg), H is height (cm), A is age (years) and α = 1 and β = 1.7 for men and α = 0 and β = 1.6 for women. For each individual, the difference between estimated energy expenditure and the caloric intake accounted for by locally caught traditional foods (data from the FFQ) and nutrient-dense market foods (data from Nutrition North) was assumed to correspond to comparatively nutrient-sparse foods such as potato chips and sweetened beverages. There are very few foods that do not qualify for Nutrition North subsidies that have nutritional value and, in other Inuit populations, they are not widely consumed.57
2.4. Nutritional content of store bought foods
We used the Canadian Nutrient File58 to estimate nutrient intake from store-bought nutrient-dense foods and traditional foods. Government nutritional databases include traditional and market foods consumed by Inuit populations and have been successfully used to estimate population-wide nutrient intake for other indigenous groups (e.g., Quebec Inuit).59 Five foods with no data were matched to foods in the United States Department of Agriculture (USDA) Nutrient Database60. Nutrient contents were available for 90% of foods by calories. For other foods, the average nutrient content of similar food categories was used. For this purpose, we group all foods (traditional and store-bought together) according to the following food categories: dairy, egg, fish liver, fish muscle, fish roe, fowl/poultry, fruit, grain, marine mammal, processed meat, red meat, shellfish, terrestrial mammal and vegetables. The compiled database of nutritional information for all foods studied here is included in the SI (Excel file: Appendix A, SI Table S6). We consider that dietary supplementation has a negligible impact on population-wide nutrient intake because dietary supplement tends to be rare among indigenous populations.61–63
2.5. Traditional food substitution scenarios
We developed five traditional food substitution scenarios to bound the possible range of dietary changes that might occur in the future. These are: [1] Traditional foods are replaced by nutrient-dense store-bought foods subsidized by the Nutrition North program (SI Figure S2). [2] Traditional foods are replaced by processed meat as an alternative protein source. [3] Traditional foods are replaced by vegetables. This scenario was used to provide a lower envelope of nutritional risks. However, the Nunatsiavut Government identified this scenario as highly unlikely given current consumption preferences. We nevertheless include it as a best-case scenario for market-based food substitution. [4] Traditional foods are replaced by nutrient-sparse foods such as snack foods. [5] Traditional foods high in MeHg are substituted with Atlantic salmon. The Nunatsiavut Government identified Atlantic salmon as a preferred food item. We use this scenario to investigate the health impacts of traditional diet adaptation instead of replacement with store-bought foods. For all scenarios, caloric consumption is assumed to be constant at the estimated 2014 values based on Eq. 1 above.
2.6. Screening-level risk assessment
We quantified the magnitudes of neurodevelopmental, cardiovascular and cancer risks associated with the five dietary scenarios described above and compared them to those associated with projected future MeHg exposures at 2014 diet. Projected future MeHg exposures result from increased MeHg content in traditional foods as a result of upstream hydroelectric development calculated by Calder et al.42. The probabilistic projections of future MeHg levels in local traditional foods are included in SI Table S7. We do not consider cancer risks associated with MeHg in traditional foods because the U.S. EPA classifies MeHg as a possible human carcinogen, noting there is ‘no persuasive evidence’ for human carcinogenicity.16
Neurodevelopmental risks to children are expressed in terms of change in IQ and are modeled by considering diets of women of childbearing age (16–49 following McDowell et al.64). We retain the confounder-adjusted dose-response functions summarized in Table 1. We express cardiovascular and cancer risks as the relative risk (RR) of mortality compared to 2014, calculated from changes in intake of various foods and nutrients. A RR of greater than 1.0 represents an increase in risk, and a RR of less than 1.0 represents a decrease in risk.
Table 1:
Summary of dose-response relationships used for screening-level risk assessment of neurodevelopmental risk
| Predictor | Outcome | Dose-response functiona | Reference |
|---|---|---|---|
| MeHg | IQ points | 1.07 per 0.5 g Hg g−1 hair (95% CI: 1.03–1.11) | Virtanen et al.19 |
| n-3 PUFAs | IQ points | mode = +1.3; min = 0.8; max = 1.8 per g DHA day−1 | Cohen et al.70 |
MeHg dose-response function is normal distribution and n-3 PUFA dose-response function is triangular distribution following the authors. Risks accrue to children born to mothers (females aged 16–49) with modeled intakes.
Cardiovascular and cancer risks are quantified as the product of individual confounder-adjusted relationships following Fleming et al.65. RRs are presented in the literature corresponding to certain incremental doses. We assume these are proportional over the range of incremental changes explored here and scale RRs presented in the literature to changes in food substitution scenarios. We consider relative risks for cardiovascular and cancer deaths based on diet for all individuals over 25 following Forouzanfar et al.66. Dose-response functions for cardiovascular and cancer mortality are expressed in terms of risks of more specific causes of death (e.g., risk of cancer at certain sites). We consider the share of overall cardiovascular (Table 2) and cancer (Table 3) mortality represented by the outcome in each dose-response relationship in order to calculate net impacts on the risk of total cardiovascular and cancer mortality. A mathematical derivation (equations S1–S3) is presented in the SI.
Table 2:
Summary of lognormally distributed dose-response relationships used for screening-level risk assessment risk of cardiovascular mortality
| Predictor | Outcome | Median (95% CI) relative risk per change (+) in intake | Reference |
|---|---|---|---|
| MeHg | SCDb | 1.07 (1.03–1.11) per 0.5 g Hg g−1 hair | Virtanen et al.19 |
| n-3 PUFAsa | IHDc | 0.866 (0.792–0.943) per 0.1 g day−1 | Forouzanfar et al.40, Chowdhury et al.71 |
| Fiber | IHDc | 0.754 (0.678–0.831) per 20 g day−1 | Threapleton et al.29, Forouzanfar et al.40 |
| Fruit | IHDc | 0.867 (0.829–0.962) per 100 g day−1 | Wang et al.32, Forouzanfar et al.40 |
| Fruit | ISd | 0.719 (0.604–0.8401) per 100 g day−1 (b) | Forouzanfar et al.40, Hu et al.72 |
| Fruit | HSe | 0.868 (0.661–0.762) per 100 g day−1 | Forouzanfar et al.40, Hu et al.72 |
| Nuts | IHDc | 0.944 (0.845–0.914) per 4.05 g day−1 (b) | Forouzanfar et al.40, Afshin et al.73 |
| Processed meat | IHDc | 1.603 (1.022–2.271) per 50 g day−1 | Forouzanfar et al.40, Micha et al.74 |
| Trans fatty acids | IHDc | 1.414 (1.281–1.567) per 2% energy intakef | Forouzanfar et al.40, Mozaffarian and Clarke75 |
| Vegetables | IHDc | 0.96 (0.93–0.99) per 106 g day−1 (g) | Wang et al.32 |
| Vegetables | ISd and HSe | 0.89 (0.81–0.98) per 200 g day−1 (h) | Hu et al.72 |
As DHA + EPA.
Sudden cardiac death represents 27.4% of total cardiovascular mortality CDC76.
Ischemic heart disease represents 70% of total cardiovascular mortality CDC76.
Ischemic stroke represents 13% of total cardiovascular mortality CDC76.
Hemorrhagic stroke represents 6% of total cardiovascular mortality CDC76.
Table 3:
Summary of lognormally distributed dose-response relationships used for screening-level risk assessment of risk of cancer mortality
| Predictor | Cancer site (mortality) | Median (95% CI) relative risk per change (+) in intake | Reference |
|---|---|---|---|
| Calcium | Colon/rectuma | 0.729 (0.831–0.963) per 1 g day−1 | Forouzanfar et al.40, WCRF and AICR78 |
| Fiber | Colon/rectuma | 0.809 (0.741–0.882) per 20 g day−1 | Forouzanfar et al.40, WCRF and AICR78 |
| Fruit | Esophagusb | 0.867 (0.776–0.968) per 100 g day−1 | Forouzanfar et al.40, Liu et al.79 |
| Fruit | Trachea/bronchus/lungc | 0.929 (0.890–0.970) per 100 g day−1 | Forouzanfar et al.40, Vieira et al.80 |
| Milk | Colon/rectuma | 0.898 (0.831–0.963) per 226.8 g day−1 | Forouzanfar et al.40, WCRF and AICR78 |
| Processed meat | Colon/rectuma | 1.179 (1.092–1.267) per 50 g day−1 | Forouzanfar et al.40, WCRF and AICR78 |
| Red meat | Colon/rectuma | 1.167 (1.033–1.309) per 100 g day−1 | Forouzanfar et al.40, WCRF and AICR78 |
| Sodium | Stomachd | 1.18 (1.02–1.38) per 1 g day−1 (e) | Forouzanfar et al.40, WCRF and AICR78 |
| Vegetables | Esophagusb | 0.840 (0.780–0.920) per 100 g day−1 (f) | Forouzanfar et al.40, Liu et al.79 |
| Vitamin D | General | 0.69 (0.55–0.86) per 1,429 IU day−1 | Garland et al.81 |
Dietary dose-response functions for risk of cardiovascular and cancer death are based on the Global Burden of Disease study.40 We excluded benefits related to increased whole grain consumption because we could not quantify the ratio of whole to processed grains in the baseline diet and intake of whole grains is small in similar northern indigenous populations.61, 67 We account for cardiovascular risks associated with consumption of red and processed meats and benefits of fruits, nuts and vegetables as a function of intake of the whole food and thus do not separately consider constituent nutrients in these foods (e.g., sodium in red meat). There is strong evidence that vitamin D exerts a generalized cancer-protective effect, especially in northern populations.37–39 We thus also consider a negative association between vitamin D and risk of cancer mortality68 following earlier work by Grant et al.69 who calculated cancer mortality in Canada attributable to vitamin D deficiency. This analysis is carried out using probabilistically distributed parameters for relative risk of cancer and cardiovascular effects and for IQ gains and decrements, allowing for explicit calculation of the uncertainties inherent to this analysis.
3. Results and Discussion
3.1. Methylmercury exposures in 2014 among Lake Melville Inuit
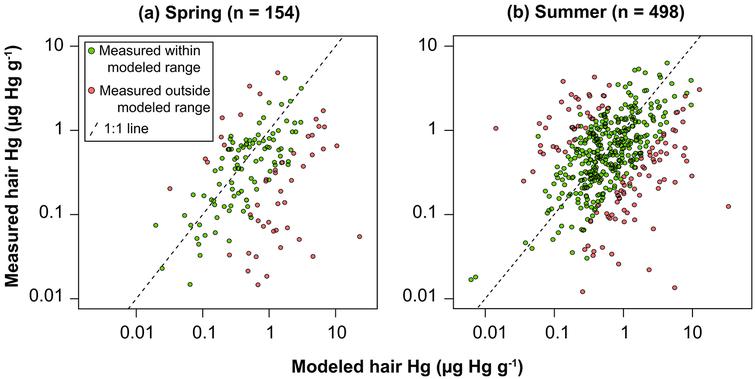
Measured Hg concentrations in the hair of Inuit individuals participating in our survey ranged from 6.8 ng g−1 to 6,200 ng g−1. We find that between 67% (spring) and 71% (summer) of all measured hair Hg samples fall within the modeled ranges of exposure (Figure 1, green circles). This is better than many recent surveys47, 83–85 and may reflect lower inter-individual variability in pharmacokinetics among a relatively homogeneous survey population and the relatively smaller range of available fish with more consistent bioaccessibility.12, 86 The Inuit Health Study (IHS) previously characterized MeHg exposures among Inuit in the community of Rigolet and other communities on the Labrador Coast but excluding Happy Valley – Goose Bay and North West River. The geometric mean blood Hg (3.2 μg L−1) reported in the IHS is equivalent to approximately 0.8 μg g−1 hair87, 88 and compares well to Spring and Summer mean hair levels (0.77 μg g−1) measured in this study in Rigolet. MeHg exposures measured in 2014 are generally lower than other Inuit populations. For instance, the IHS reported geometric mean blood Hg equal to 9.0 μg L−1 for Inuit in Nunavut89, while Dewailly et al.90 reported a geometric mean of 10.8 μg L−1 for the Inuit of Nunavik (northern Quebec).
Figure 1.
Comparison of measured and probabilistically modeled hair Hg concentrations for Labrador Inuit in the Lower Lake Melville Region during Spring and Summer survey periods. Green circles indicate measured values that fall within the modeled range. Red circles indicate measured values that fall outside the probabilistically modeled range of hair Hg concentrations. Individuals who did not report consuming seafood, birds or marine mammals (8 in Spring, median hair Hg = 0.036 μg g−1 and 26 in Summer, median hair Hg = 0.049 μg g−1) are excluded. R2 = 0.13 (Spring), 0.11 (Summer).
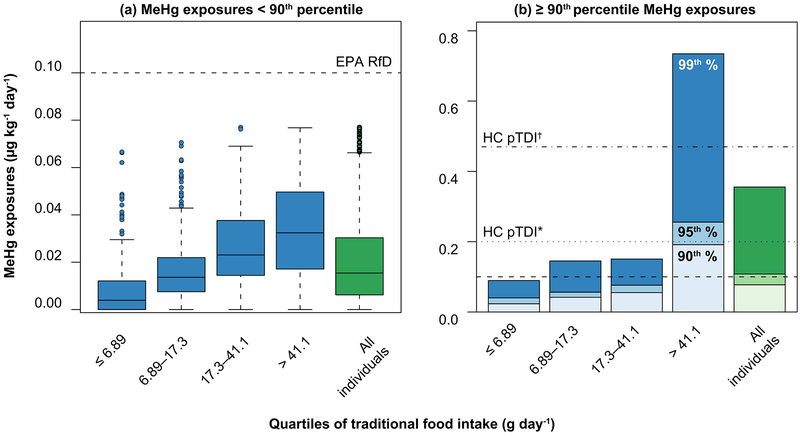
Exposures to MeHg of during the Summer survey period in 2014 were approximately double the median exposure of the general Canadian population.2, 48 However, the majority of individuals in the survey population fall below the reference dose (RfD) for exposure established by the U.S. EPA of 0.1 μg kg−1 day−1 and Health Canada’s provisional tolerable daily intake (pTDI) of 0.2–0.47 μg kg−1 day−1 (Figure 2, SI Table S8).91 Across the three Inuit communities, only individuals above the 90th percentile of MeHg exposures had daily intake levels that exceeded the U.S. EPA’s RfD (Figure 2). For example, the 95th percentile of MeHg exposures ranged from 0.10 μg kg−1 day−1 in Happy Valley–Goose Bay to 0.27 μg kg−1 day−1 in Rigolet, and 0.12 μg kg−1 day−1 when averaged across the three communities. In Summer 2014, the fractions of Lake Melville Inuit exceeding the Health Canada pTDI and the US EPA RfD were approximately 1% and 7% respectively (SI Table S8). For all survey periods, older individuals, men, and individuals residing in the community of Rigolet have higher MeHg exposures (SI Table S8). Therefore, in 2014, risks associated with MeHg exposures were generally low and concentrated among individuals at higher exposure percentiles.
Figure 2.
Distributions of MeHg exposures for Inuit in the Lower Lake Melville Region of Labrador by quartile of traditional food consumption (Summer 2014). Panel (a) shows values below the 90th percentile of MeHg exposures and Panel (b) shows the distribution for highly exposed individuals in the population (at or above 90th percentile of MeHg exposures). EPA RfD denotes the U.S. EPA reference dose for methylmercury and HC pTDI indicates the Health Canada provisional tolerable daily intakes for women of childbearing age and children (*) and for everyone else (†).
Individuals with the highest MeHg exposures in 2014 had the highest intake of traditional foods. Per-capita, roughly 70% of all MeHg intake came from traditional foods. Among individuals with MeHg exposures ≥ 90th percentile, 90% of all MeHg intake came from traditional foods. In Summer 2014, individuals in the lowest quartile of traditional food intake (≤ 6.86 g day−1) received 24% of their MeHg exposure from traditional foods compared to 80% among the highest quartile (>41.1 g day−1). Median MeHg exposure was 0.043 μg kg−1 day−1 for individuals in the highest quartile of traditional food consumption compared to 0.003 μg kg−1 day−1 among individuals in the lowest intake quartile. Mean MeHg per-capita exposures in the Summer survey period (0.035 μg kg−1 day−1) were significantly different from the Spring period (0.024 μg kg−1 day−1, p < 0.001, Wilcox rank-sum test) but not the Winter (0.046 μg kg−1 day−1p >0.05). This mirrors trends in traditional food intake. Mean traditional food consumption was significantly lower in the Spring period (28.5 g day−1) compared to the Summer period (36.52 g day−1, p < 0.001, Wilcox rank-sum test). Mean traditional food consumption in Winter was 38.7 g day−1 but was not statistically different from either Spring or Summer. Therefore, while population-wide MeHg exposure risks are generally low, these risks are sensitive to the MeHg content of local foods.
Although locally caught Atlantic salmon is relatively low in MeHg (Table S3), it was the single greatest contributor to overall MeHg intakes in all three communities including among individuals with MeHg exposures ≥ 90th percentile (24–29% of overall per-capita intake across communities in Summer 2014). Other foods contributing more than 5% to overall MeHg intakes in Summer 2014 were brook trout, Atlantic cod, tern eggs, duck and seal muscle (locally caught) and fresh cod, canned tuna and fresh tuna (store-bought). Individuals with MeHg exposures ≥90th percentile had similar sources of MeHg intake as the study population as a whole. SI Figure S2 presents the breakdown of per-capita MeHg sources in Summer 2014 for all individuals in the three communities studied and for individuals in all communities with MeHg exposures ≥90th percentile.
3.2. 2014 diet composition
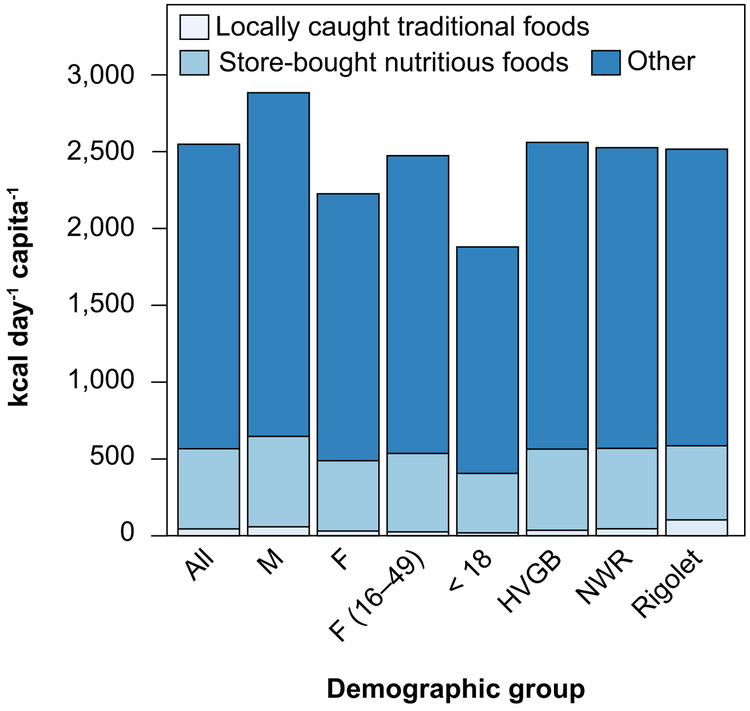
Survey data from the summer of 2014 suggest 91% of the population consumes traditional foods. However, these foods are only account for approximately 2% of mean caloric intake and 11% for the 95th percentile consumer (Figure 3). Across the three communities, consumption of traditional foods is highest in Rigolet (mean = 4% of total calories) and lowest in Happy Valley–Goose Bay (mean = <1% of total calories) (Figure 3). Prior work has reported similar findings for other Inuit communities, with higher rates of country food consumption in more northern communities that have less access to market alternatives.88
Figure 3.
Estimated contributions of different food types to total calories consumed by Inuit in the Lower Lake Melville region of Labrador, Canada. M = male; F = female; HVGB = Happy Valley – Goose Bay; NWR = North West River.
Using food subsidy data, we estimate that store-bought nutrient-dense foods account for 25% of total per-capita caloric intake. SI Figure S3 presents the composition of this nutrient-dense store-bought food component of the diet. For store-bought seafood, dietary survey data agree to within 12% of estimates based on the food subsidy program data, providing partial validation of this method. The remaining fractions of all caloric consumption estimated from individual body weight must come from store bought food such as nutrient-sparse snack foods and sweetened beverages. We estimate that these other foods account for approximately 70% of all calories consumed across demographic groups. These findings agree with prior research that has reported Inuit populations consume traditional foods, fruits and vegetables only one third as frequently as foods with low nutrient content.92
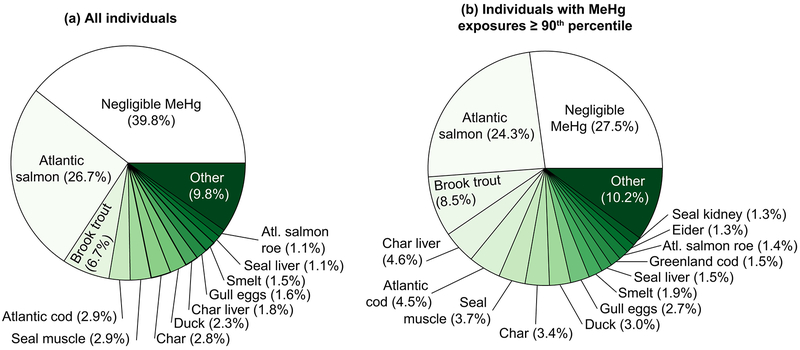
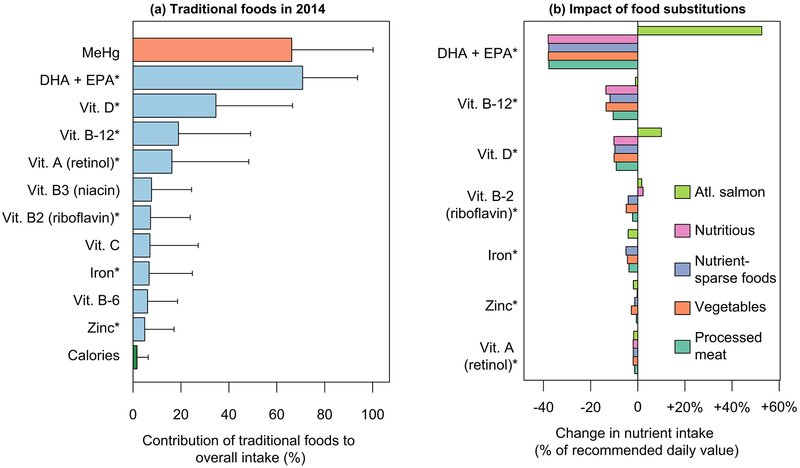
Types of traditional foods consumed by individuals vary widely (Figure 4). On a per-capita basis across the three communities, 90% of the calories from traditional foods are derived from 24 foods. Some traditional foods such as berries and seal blubber contain negligible quantities of MeHg (Figure 4). Foods with negligible Hg account for 40% of the total calories from traditional foods for all individuals surveyed (Figure 5). On a per-capita basis, 11 of the 24 major traditional foods consumed contain negligible amounts of MeHg. Survey data indicate that the greatest diversity in traditional food consumption occurs among individuals with the highest MeHg exposures (Figure 2b). Among the most highly exposed individuals to MeHg (90th percentile), traditional foods with negligible MeHg content account for only 28% of total calories (nine foods). Among all individuals, including among individuals with MeHg exposures greater than the 90th percentile, the most widely consumed traditional foods with negligible Hg were berries, goose, partridge, moose, caribou, seal blubber and rabbit.
Figure 4.
Fraction of caloric intake from traditional foods for top 90% of foods among Lake Melville Inuit for (a) all individuals and (b) individuals with MeHg exposures above the 90th percentile in summer 2014 (0.08 μg kg−1 day−1). Dietary data from full-scale summer survey. Widely consumed foods (in the top 90% of contributors to overall calories from traditional foods) include berries, goose, partridge, moose, caribou, seal blubber and rabbit.
Figure 5.
Role of traditional foods for nutrient and MeHg intake and impacts of traditional food substitution. Panel (a) shows the estimated proportion of MeHg and several key nutrients from traditional foods based on survey data for 2014. Panel (b) shows the modeled impact of traditional food substitution on intakes relative to recommended daily values assuming several hypothetical traditional food replacement scenarios. Substitution scenarios for traditional foods include: (1) locally caught Atlantic salmon, (2) nutrient-dense store-bought foods (“Nutritious”), (3) nutrient-sparse junk foods, (4) vegetables and (5) processed meat. Shaded bars represent per-capita averages, and lines represent the 5th–95th percentile individuals. * denotes per-capita intake below recommended daily values based on US FDA recommendations94 and 500 mg day−1 for DHA + EPA93.
Consumption of traditional foods increases linearly with age, with each year of age associated with a 0.8 g day−1 increase in traditional food intake (R2 = 0.10, p < 0.001). Traditional foods supply a significantly higher fraction of dietary calories for men (mean = 1.9%) compared to women (mean = 1.4%, p < 0.001, SI Table S9). Per-capita caloric significance is reported in the SI for locally caught traditional seafood (Table S3), other locally caught traditional food (Table S4), store-bought seafood (Table S5) and other store-bought nutritious foods (Table S6).
3.3. Importance of traditional foods for intake of nutrients
Despite their low contribution to total caloric intake, traditional foods are the predominant source of several key nutrients (Figure 5). Traditional foods supply approximately 70% of the n-3 PUFAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) across the population (Figure 5). They are an important source of vitamins D (35%), B12 (19%), B6 (6%), A (16%), B2, B3 and C and iron (7%), and zinc (5%). Baseline dietary analysis suggests that intake on average across the population of iron, n-3 fatty acids, vitamins A, B2 and B12 and zinc are currently below dietary reference values.93, 94 Other studies have similarly found traditional foods are richer in these nutrients than the market-based components of indigenous diets.95–98
3.4. Nutritional impact of traditional food substitution scenarios
While 2014 MeHg exposures were generally low, these exposures are likely to increase as a result of upstream hydroelectric development.42 Food consumption advisories are commonly used to control these risks but have unpredictable effects.4, 10, 11 Here, we describe the nutritional impacts of several hypothetical responses to food consumption advisories.
Modeled dietary transitions to market foods, following the scenarios outlined above, generally exacerbate deficiencies in n-3 PUFA and vitamins B12, D, B2, A, iron and zinc intake among indigenous Inuit (Figure 5b). We find a small net gain in vitamin B-2 intake in the nutrient-dense foods replacement scenario. Modeled reductions as a fraction of daily recommended intake range from 1–2% for vitamin A to 37% for n-3 PUFAs. Replacement of traditional foods with an equivalent amount of locally caught Atlantic salmon has a mixed impact on nutritional sufficiency. Under the Atlantic salmon replacement scenario, average intake of n-3 PUFAs and vitamin D increases by 53% and 10% of recommended daily values respectively and leads to modest declines in intake of vitamins A and B-12, iron and zinc that are less 5% of daily values (Figure 5b).
3.5. Screening-level analysis of risks and benefits
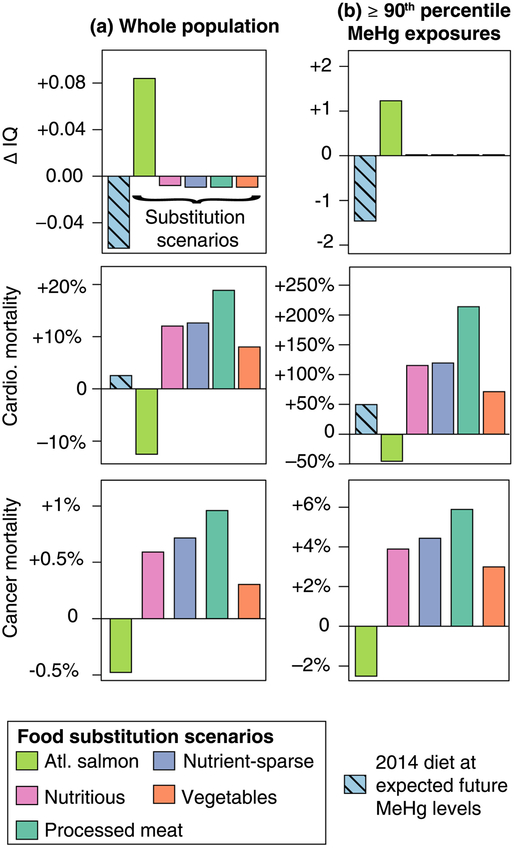
In 2014, intake of traditional foods among Inuit women of childbearing age generally had a small net positive impact on child neurodevelopment due to relatively low MeHg levels in traditional foods and benefits from n-3 PUFA intake. The median IQ decrement attributable to present-day MeHg exposures from traditional foods is 0.02 points (95th population percentile: 0.14 points). After accounting for benefits from n-3 PUFAs in traditional foods, median (5th–95th population percentiles) net impact on IQ is a gain of 0.01 points (decrement of 0.014 to gain of 0.19) IQ points. Increased consumption of greater quantities of low MeHg traditional foods would further increase this net benefit.
Increased MeHg concentrations in local foods may pose neurodevelopmental and cardiovascular risks. As described above, increased exposures are likely to disproportionately impact individuals who already have high MeHg exposures and exceed regulatory reference doses. However, mitigating these risks with reduced consumption of local foods may also pose risks. Here, we present the results of our analysis comparing risks from increased MeHg exposures to risks from potential dietary transitions. We conducted a screening-level estimate of the risks (Figure 6) associated with peak forecasted increases in MeHg concentrations in traditional foods due to hydroelectric flooding by Calder et al.42. Even at peak MeHg concentrations, assuming the same magnitudes and species consumed as reported in the 2014 dietary survey, the population median IQ decrement for women of childbearing age is relatively low (0.06 points). However, among individuals with MeHg exposures greater than the 90th percentile, median impacts are much larger (loss of 1.4 IQ points). These estimates must be viewed as uncertain due to inter-individual differences in sensitivity to MeHg exposure and the toxicokinetics of MeHg absorption in the human body.86, 99
Figure 6.
Comparison of neurodevelopmental, cardiovascular and cancer risks for traditional food substitution scenarios compared to risks associated with projected future MeHg levels in traditional foods from Calder et al.42 Panel (a) shows median risks for the whole population. Panel (b) shows individuals at or above the 90th percentile of MeHg exposures in summer 2014 (0.08 μg kg−1 day−1). Traditional food substitution scenarios include: (1) locally caught Atlantic salmon, (2) the representative basket of subsidized nutrient-dense store-bought foods (“Nutritious”), (3) nutrient-sparse junk foods, (4) vegetables and (5) processed meat. Excess cardiovascular and cancer mortality risks are presented as fractions of present-day risks (+0% corresponds to a relative risk of 1, meaning no change relative to 2014).
Substitution of traditional foods with store-bought alternatives represents a large reduction (70% per-capita) of n-3 PUFA intake (Figure 6). We estimate that this may also lead to small neurodevelopmental impacts for most individuals (median IQ decrement of 0.01 across substitution scenarios). However, replacement of traditional foods with locally caught Atlantic salmon results in estimated gains of 0.08 IQ points (population-wide median) and 1.2 points (>90th percentile MeHg exposures). This reflects a large increase in n-3 PUFA intake associated with additional Atlantic Salmon consumption that greatly outweighs minor increases in MeHg exposure (SI Table S9).
We estimate that cardiovascular risks associated with peak MeHg exposures in traditional foods are smaller than under any store-bought food replacement scenario (Figure 6). Across the population, median risk of cardiovascular mortality associated with projected increases in MeHg in traditional foods increases by 3% relative to present-day (RR = 1.03). For individuals with MeHg exposures at the 90th percentile or greater, estimated risk of cardiovascular mortality increases dramatically (RR = 1.5). While the magnitude of these impacts is highly uncertain due to variability in susceptibility to MeHg exposure across populations, this analysis provides a quantitative estimate for the potential difference in magnitude of risks from MeHg in comparison to dietary changes. If traditional foods are replaced by processed meat, median RR of cardiovascular mortality is 1.19 across the population and 3.14 for individuals with MeHg exposures >90th percentile. Median RR of cardiovascular mortality is 1.08 at the across the population when the dietary replacement is fruits and vegetables and 1.73 for individuals with MeHg exposures >90th percentile. The replacement scenarios for nutrient-dense market foods and junk foods fall within this envelope (Figure 6).
Replacement of high MeHg traditional foods by locally caught Atlantic salmon has the opposite impact of market foods on RR of cardiovascular mortality. This replacement scenario leads to greater net benefits for cardiovascular health than all store-bought alternative scenarios. Median RR of cardiovascular mortality under this scenario is 0.88 across the population and 0.55 for individuals with MeHg exposures >90th percentile (Figure 6, SI Table S10).
We estimate that replacing traditional foods with store-bought foods under all scenarios will increase the RR of cancer mortality. Greater than 95% of this effect for the nutrient-dense foods scenario is attributable to reduced intake of vitamin D. Median RR of colorectal cancer is 1.01 due to reduced fiber intake (75% of individuals) and increased consumption of red and processed meat. Gains in calcium intake for 95% of individuals and increased milk consumption do not offset these risks. Increased sodium intake (97% of individuals) results in a small increase in RR of gastric cancer (RR = 0.01 at the 95th percentile). Colorectal cancers account for more than three times as many deaths as gastric cancers in Newfoundland and Labrador100, and so the increased risk of colorectal cancer is a relatively stronger driver of overall cancer risks. Replacement of the representative basket of traditional foods with locally caught Atlantic salmon provides small net reductions in overall cancer risks relative to present-day (median RR = 0.995; >90th percentile individuals: RR = 0.97) (Figure 6, SI Table S11).
3.6. Study strengths and limitations
To our knowledge, this study is the most comprehensive survey of Inuit diet and MeHg exposures (n = 1,145). We provide a detailed characterization of diet variability among Lake Melville Inuit evaluated with direct hair Hg measurements. Our dietary MeHg exposure model performed better than several other recent studies, likely reflecting the relatively homogeneous sources of MeHg across the population and the use of extensive local data. This study provides an assessment of the magnitude of projected risks associated with elevated MeHg exposures in comparison with the neurodevelopmental, cardiovascular, cancer and nutritional risks posed by possible dietary changes.
We were limited by the availability of intake data for store-bought foods (other than seafood, which we measured on the FFQ), which are available only on a per-capita basis for the community of Rigolet. Therefore, our characterization of present-day nutritional sufficiency and composition of store-bought foods could not describe interindividual variability. Dietary recall data is often biased, and although we designed the study so as to evaluate (with hair Hg measurements) and control for some biases (e.g., correcting for species-specific recall biases by asking redundant “total” recall questions) as described above, we are limited by the accuracy of the reports of survey respondents. Our evaluation of dietary model performance with respect to hair Hg and calculation of MeHg exposures from hair Hg measurements depends on self-reported measures of height and weight, which may be estimates. Although there was little evident bias in the larger-scale Summer survey round (Figure 1), these factors likely contributed to the random error observed.
Our analysis does not account for other possible second-order effects of traditional food substitutions. For instance, isocaloric dietary substitution is associated with a mean reduction in protein intake of 2–11% across dietary scenarios. While per-capita intake of protein continues to exceed the recommended daily allowance, increasing the proportion of calories from carbohydrates and fats may lead to overall greater caloric intake via reduced satiety and higher insulin production, thus increasing weight gain and obesity-related risks.101, 102 Consumption rates of locally caught traditional foods in the Inuit communities studied here are lower than those for other indigenous communities across Canada. For example, British Columbia First Nations consume roughly three times the per-capita amounts reported here.103 This implies health and nutritional impacts associated with dietary transitions may be greater in other populations.
We have not addressed the physical or psychosocial dimensions of hunting and fishing and the preparation and consumption of traditional foods. Hunting traditional foods represents vigorous physical activity, and loss of access to traditional foods has been linked to adverse mental and social outcomes, implying substitution of traditional foods may present additional risks to those quantified here.104–106 Fruits and vegetables are not a significant part of the traditional Inuit diet107, and our analysis suggests they account for roughly 2% of caloric intake at present day. Replacement of traditional foods with fruits and vegetables is acknowledged to be less likely than by other foods such as red meat or other snack foods. This scenario is included as a better-case scenario for store-bought alternatives.
3.7. Implications for risk mitigation strategies
Food consumption advisories are routinely used to mitigate potential risks from elevated contaminant exposures. However, these advisories have unpredictable effects and can lead to reduced overall intake of traditional foods among indigenous populations. This study is the first to calculate the plausible range of health impacts from elevated MeHg exposures as compared to potential outcomes of risk-mitigation strategies. Our analysis suggests that replacing traditional foods with store-bought alternatives may lead to increases in cardiovascular and cancer risks among Lake Melville Inuit. Conversely, we estimate that replacement with locally caught Atlantic salmon will lead to net benefits for neurodevelopmental and cardiovascular health and reduce cancer risks relative to the present-day diet. Atlantic salmon is already a large component of traditional diet of our survey respondents, accounting for approximately 25% of calories from traditional foods. These results reinforce the potential benefits of dietary advice that promotes nutrient-dense, low-MeHg traditional foods. We have shown that in the local diet, there are many commonly consumed (and therefore familiar) foods with negligible levels of Hg, intake of which could be promoted in order to maximize net health benefits of the traditional diet.
Reducing the diversity of traditional foods consumed has mixed impacts on nutritional sufficiency, which must be considered when making recommendations about dietary choices among indigenous populations. Nutrient shortfalls are common in indigenous populations, and our findings suggest that this is the case among Lake Melville Inuit. Therefore, independent of contaminant levels, there may be a role for dietary interventions that promote increased intake of nutritious foods and possibly dietary supplements. Taken together, findings presented here underline the importance of protecting northern food webs from environmental contamination and of promoting traditional foods among indigenous populations.
Supplementary Material
Research ethics approval.
This work was carried out following the approval of the following research ethics authorities:
Office of Human Research Administration, Harvard T.H. Chan School of Public Health (case IRB13–1483)
Newfoundland and Labrador Research Ethics Board (case 14.004)
Nunatsiavut Government Health Research Ethics Authority
Highlights.
Traditional foods account for up to 70% of essential nutrients
They account for the majority of bioaccumulative contaminant exposures
Food advisories may increase median risk of cardiovascular mortality
Advisories may also increase cancer risks associated with red meat consumption
Acknowledgments
We thank Marina Biasutti-Brown, Tom Sheldon, Rodd Laing and Trevor Bell, as well as the research assistants hired by the NG, for their help in sample collection and development and administration of the dietary survey.
Funding sources
This work was supported by the Canadian Northern Contaminants Program, ArcticNet Inc., Tides Canada Oak Arctic Marine Fund Program, and the Nunatsiavut Government (NG). RSDC acknowledges support from a graduate fellowship from the Natural Sciences and Engineering Research Council of Canada (grant 575792). SB acknowledges support from National Institutes of Health (grant 5T32ES007069).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arctic Monitoring and Assessment Program (AMAP), AMAP Assessment 2015: Human Health in the Arctic Oslo, Norway, 2015. [Google Scholar]
- 2.Lye E; Legrand M; Clarke J; Probert A, Blood total mercury concentrations in the Canadian population: Canadian health measures survey cycle 1, 2007–2009. Can J Public Health 2013, 104, (3), e246–e251. DOI: https://www.jstor.org/stable/canajpublheal.104.3.e246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Oostdam J; Donaldson SG; Feeley M; Arnold D; Ayotte P; Bondy G, … Kalhok S, Human health implications of environmental contaminants in Arctic Canada: A review. Sci Total Environ 2005, 351–352, 165–246. DOI: 10.1016/j.scitotenv.2005.03.034 [DOI] [PubMed] [Google Scholar]
- 4.Passos CJ; Mergler D, Human mercury exposure and adverse health effects in the Amazon: a review. CadSaude Publica 2008, 24, S503–S520. DOI: 10.1590/S0102-311X2008001600004 [DOI] [PubMed] [Google Scholar]
- 5.Hydro-Québec; Conseil cri de la santé et des services sociaux de la Baie James, Le guide alimentaire despoissons nordiques. Région de la Baie-James 2013.
- 6.Hydro-Quebec Production, Guide de consommation des poissons pour les plans d’eau de la region de la riviere Saint-Maurice en Haute-Mauricie 2014.
- 7.Agence de la santé et des services sociaux de la Côte-Nord; Hydro-Quebec Production; CHU de Quebec; Institute national de sante publique, Le guide alimentaire des poissons et fruits de mer de la Côte-Nord 2013.
- 8.Shimshack JP; Ward MB, Mercury advisories and household health trade-offs. J Health Econ 2010, 29, (5), 674–85. DOI: 10.1016/j.jhealeco.2010.05.001 [DOI] [PubMed] [Google Scholar]
- 9.Teisl MF; Fromberg E; Smith AE; Boyle KJ; Engelberth HM, Awake at the switch: improving fish consumption advisories for at-risk women. Sci Total Environ 2011, 409, (18), 3257–66. DOI: 10.1016/j.scitotenv.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 10.Furgal C; Powell S; Myers H, Digesting the message about contaminants and country foods in the Canadian North: a review and recommendations for future research and action. Arctic 2005, 55, (2), 103–114. DOI: 10.14430/arctic404 [DOI] [Google Scholar]
- 11.Wheatley B; Paradis S, Balancing human exposure, risk and reality: questions raised by the Canadian aboriginal methylmercury program. Neurotoxicology 1996, 17, (1), 241–249. [PubMed] [Google Scholar]
- 12.Li M; von Stackelberg K; Rheinberger CM; Hammitt JK; Krabbenhoft DP;Yin R; Sunderland EM, Insights from mercury stable isotopes into factors affecting the internal body burden of methylmercury in frequent fish consumers. Elementa 2016, 4, 000103 DOI: 10.12952/journal.elementa.000103 [DOI] [Google Scholar]
- 13.Binnington MJ; Quinn CL; McLachlan MS; Wania F, Evaluating the effectiveness of fish consumption advisories: modeling prenatal, postnatal, and childhood exposures to persistent organic pollutants. Environ Health Perspect 2014, 122, (2), 178–86. DOI: 10.1289/ehp.1206380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karagas M; Choi AL; Oken E; Horvat M; Schoeny R; Kamai E, … Korrick S, Evidence on the human health effects of low level methylmercury exposure. Environ Health Perspect 2012, 120, (6), 799–806. DOI: 10.1289/ehp.1104494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Debes F; Weihe P; Grandjean P, Cognitive deficits at age 22 years associated with prenatal exposure to methylmercury. Cortex 2016, 74, 358–69. DOI: 10.1016/j.cortex.2015.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.United States Environmental Protection Agency (US EPA), Methylmercury (MeHg); CASRN22967-92-6 Integrated Risk Information System (IRIS) Washington, DC, 2002. [Google Scholar]
- 17.Boucher O; Jacobson S; Plusquellec P; Dewailly E; Ayotte P; Forget-Dubois N, … Muckle G, Prenatal methylmercury, postnatal lead exposure, and evidence of attention deficit/hyperactivity disorder among Inuit children in Arctic Quebec. Environ Health Perspect 2012, 120, (10), 1456–1461. DOI: 10.1289/ehp.1204976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weihe P; Hansen JC; Katsuyuki M; Debes F; Jorgensen PJ; Steuerwald U, … Grandjean P, Neurobehavioral performance of Inuit children with increased prenatal exposure to methylmercury. Int J CircumpolHeal 2016, 61, 41–49. DOI: 10.3402/ijch.v61i0.17404 [DOI] [PubMed] [Google Scholar]
- 19.Virtanen JK; Rissanen TH; Voutilainen S; Tuomainen TP, Mercury as a risk factor for cardiovascular diseases. JNutr Biochem 2007, 18, (2), 75–85. DOI: 10.1016/j.jnutbio.2006.05.001 [DOI] [PubMed] [Google Scholar]
- 20.Farina M; Rocha JB; Aschner M, Mechanisms of methylmercury-induced neurotoxicity: evidence from experimental studies. Life Sci 2011, 89, (15–16), 555–63. DOI: 10.1016/j.lfs.2011.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salonen JT; Seppanen K; Nyyssonen K; Korpela H; Kauhanen J; Kantola M,… Salonen R, Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in Eastern Finnish men. Circulation 1995, 91, (3), 645–655. DOI: 10.1161/01.cir.91.3.645 [DOI] [PubMed] [Google Scholar]
- 22.Roman HA; Walsh TL; Coull BA; Dewailly E; Guallar E; Hattis D,… Rice G, Evaluation of the cardiovascular effects of methylmercury exposures: current evidence supports development of a dose-response function for regulatory benefits analysis. Environ Health Perspect 2011, 119, (5), 607–14. DOI: 10.1289/ehp.1003012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laird BD; Goncharov AB; Egeland GM; Chan HM, Dietary advice on Inuit traditional food use needs to balance benefits and risks of mercury, selenium, and n3 fatty acids. J Nutr 2013, 143, (6), 923–30. DOI: 10.3945/jn.112.173351 [DOI] [PubMed] [Google Scholar]
- 24.European Food Safety Authority (EFSA), Scientific opinion on health benefits of seafood (fish and shellfish) consumption in relation to health risks associated with exposure to methylmercury. EFSA Journal 2014, 12, (7). DOI: 10.2903/j.efsa.2014.3761 [DOI] [Google Scholar]
- 25.Ginsberg GL; Toal BF, Quantitative approach for incorporating methylmercury risks and omega-3 fatty acid benefits in developing species-specific fish consumption advice. Environ Health Perspect 2009, 117, (2), 267–75. DOI: 10.1289/ehp.11368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stern AH; Korn LR, An approach for quantitatively balancing methylmercury risk and omega-3 benefit in fish consumption advisories. Environ Health Perspect 2011, 119, (8), 1043–1046. DOI: 10.1289/ehp1002824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahaffey KR; Sunderland EM; Chan HM; Choi AL; Grandjean P; Marien K, … Yasutake A, Balancing the benefits of n-3 polyunsaturated fatty acids and the risks of methylmercury exposure from fish consumption. Nutr Rev 2011, 69, (9), 493–508. DOI: 10.1111/j.1753-4887.2011.00415.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Food and Agricultural Organization of the United Nations (FAO); World Health Organization (WHO), Joint FAO/WHO Expert Consultation on the Risks and Benefits of Fish Consumption, 25–29 January 2010, Rome, Italy World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- 29.Threapleton DE; Greenwood DC; Evans CE; Cleghorn CL; Nykjaer C; Woodhead C, … Burley VJ, Dietary fibre intake and risk of cardiovascular disease: systematic review and meta-analysis. Brit Med J 2013, 347, f6879 DOI: 10.1136/bmj.f6879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whelton PK; Appel LJ; Sacco RL; Anderson CA; Antman EM; Campbell N, … Van Horn LV, Sodium, blood pressure, and cardiovascular disease: further evidence supporting the American Heart Association sodium reduction recommendations. Circulation 2012, 126, (24), 2880–9. DOI: 10.1161/CIR.0b013e318279acbf [DOI] [PubMed] [Google Scholar]
- 31.Koeth RA; Wang Z; Levison BS; Buffa JA; Org E; Sheehy BT, … Hazen SL, Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013, 19, (5), 576–85. DOI: 10.1038/nm.3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X; Ouyang Y; Liu J; Zhu M; Zhao G; Bao W; Hu FB, Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. Brit Med J 2014, 349, g4490 DOI: 10.1136/bmj.g4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blomhoff R; Carlsen MH; Andersen LF; Jacobs DR, Health benefits of nuts: potential role of antioxidants. Brit JNutr 2006, 96, (S2), S52–S60. DOI: 10.1017/BJN20061864 [DOI] [PubMed] [Google Scholar]
- 34.Ludwig DS; Pereira MA; Kroenke CH; Hilner JE; Van Horn L; Slattery ML; Jacobs DR Jr, Dietary fiber, weight gain, and cardiovascular disease risk factors in young adults. J Amer Med Assoc 1999, 282, (16), 1539–1546. DOI: 10.1001/jama.282.16.1539 [DOI] [PubMed] [Google Scholar]
- 35.Kelly JH; Sabate J, Nuts and coronary heart disease: an epidemiological perspective. Brit J Nutr 2006, 96, (S2), S61–S67. DOI: 10.1017/BJN20061865 [DOI] [PubMed] [Google Scholar]
- 36.Rissanen TH; Voutilainen S; Virtanen JK; Venho B; Vanharanta M; Mursu J; Salonen JT, Low intake of fruits, berries and vegetables is associated with excess mortality in men: the Kuopio Ischaemic Heart Disease Risk Factor (KIHD) Study. J Nutr 2003, 133, (1), 199–204. DOI: 10.1093/jn/133.1.199 [DOI] [PubMed] [Google Scholar]
- 37.Giovannucci E, The epidemiology of vitamin D and cancer incidence and mortality: a review (United States). Caner Cause Control 2005, 16, (2), 83–95. DOI: 10.1007/s10552-004-1661-4 [DOI] [PubMed] [Google Scholar]
- 38.Giovannucci E; Liu Y; Rimm EB; Hollis BW; Fuchs CS; Stampfer MJ; Willett WC, Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer I 2006, 98, (7), 451–459. DOI: 10.1093/jnci/djj101 [DOI] [PubMed] [Google Scholar]
- 39.Grant WB, Relation between prediagnostic serum 25-hydroxyvitamin D level and incidence of breast, colorectal, and other cancers. JPhotoch Photobio B 2010, 101, (2), 130–6. DOI: 10.1016/j.jphotobiol.2010.04.008 [DOI] [PubMed] [Google Scholar]
- 40.Forouzanfar MH; Afshin A; Alexander LT; Anderson HR; Bhutta ZA; Biryukov S, … Murray CJL, Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, (10053), 1659–1724. DOI: 10.1016/S0140-6736(16)31679-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H; Naghavi M; Allen C; Barber RM; Bhutta ZA; Carter A, … Coates MM, Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, (10053), 1459–1544. DOI: 10.1016/S0140-6736(16)31012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calder RSD; Schartup AT; Li M; Valberg AP; Balcom PH; Sunderland EM, Future impacts of hydroelectric power development on methylmercury exposures of Canadian indigenous communities. Environ Sci Technol 2016, 50, (23), 13115–13122. DOI: 10.1021/acs.est.6b04447 [DOI] [PubMed] [Google Scholar]
- 43.Statistics Canada, Census Profile. 2011. Census Accessed 2018-03-18 from http://www12.statcan.gc.ca/census-recensement/2011/dp-pd/prof/index.cfm?Lang=E.
- 44.Durkalec A; Sheldon T; Bell T, Eds., Scientific Report. Lake Melville: Avativut, Kanuittailinnivut Nunatsiavut Government: Nain, NL, 2016. [Google Scholar]
- 45.Durkalec A; Sheldon T; Bell T, Eds., Summary for Policymakers. Lake Melville: Avativut, Kanuittailinnivut Nunatsiavut Government: Nain, NL, 2016. [Google Scholar]
- 46.Li M; Schartup AT; Valberg AP; Ewald JD; Krabbenhoft DP; Yin R, … Sunderland EM, Environmental origins of methylmercury accumulated in subarctic estuarine fish indicated by mercury stable isotopes. Environ Sci Technol 2016, 50, (21), 11559–11568. DOI: 10.1021/acs.est.6b03206 [DOI] [PubMed] [Google Scholar]
- 47.Lincoln RA; Shine JP; Chesney EJ; Vorhees DJ; Grandjean P; Senn DB, Fish consumption and mercury exposure among Louisiana recreational anglers. Environ Health Perspect 2011, 119, (2), 245–51. DOI: 10.1289/ehp.1002609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stern AH, Estimation of the interindividual variability in the one-compartment pharmacokinetic model for methylmercury: implications for the derivation of a reference dose. Regul ToxicolPharm 1997, 25, (3), 277–288. DOI: 10.1006/rtph.1997.1105 [DOI] [PubMed] [Google Scholar]
- 49.Aboriginal Affairs and Northern Development Canada (AANDC), Access to Information Act: request A-2016–00810/VN 2016.
- 50.Douglass JS; Fleming KH; Barraj LM; Heimbach JT, Using Food Consumption Data to Determine Exposure to Toxins in Handbook of Human Toxicology, Massaro EJ, Ed. CRC Press: Boca Raton, FL, 1997; pp 305–326. [Google Scholar]
- 51.Sunderland EM, Mercury exposure from domestic and imported estuarine and marine fish in the U.S. seafood market. Environ Health Perspect 2007, 115, (2), 235–42. DOI: 10.1289/ehp.9377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sunderland EM; Li M; Bullard K, Decadal changes in the edible supply of seafood and methylmercury exposure in the United States. Environ Health Perspect 2018, 126, (1), 017006 DOI: 10.1289/EHP2644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Government of Canada, Nutrition North Accessed 2017-06-23 from www.nutritionnorthcanada.gc.ca/eng/1415385762263/1415385790537.
- 54.Gustavsson J; Cederberg C; Sonersson U; van Otterdijk R; Meybeck A, Global Food Losses and Food Waste: Extent, Causes and Prevention United Nations Food and Agriculture Organization (UN FAO): Rome, Italy, 2011. [Google Scholar]
- 55.Mifflin MD; St Jeor ST; Hill LA; Scott BJ; Daugherty SA; Koh Y, A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 1990, 51, (2), 241–247. DOI: 10.1093/ajcn/51.2.241 [DOI] [PubMed] [Google Scholar]
- 56.Kattelmann KK; Conti K; Ren C, The Medicine Wheel nutrition intervention: a diabetes education study with the Cheyenne River Sioux Tribe. J Am Diet Assoc 2010, 110, (5 Suppl), S44–51. DOI: 10.1016/j.jada.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 57.Sheehy T; Kolahdooz F; Roache C; Sharma S, Changing dietary patterns in the Canadian Arctic: Frequency of consumption of foods and beverages by. Food Nutr Bull 2014, 35, (2), 244–252. DOI: 10.1177/156482651403500211 [DOI] [PubMed] [Google Scholar]
- 58.Health Canada, Canadian Nutrient File Accessed 2018-01-15 from https://food-nutrition.canada.ca/cnf-fce/index-eng.jsp.
- 59.Rochette L; Blanchet C, Methodological Report. Qanuippitaa? (How Are We?) Institut national de sante publique du Quebec: Quebec, QC, 2004. [Google Scholar]
- 60.United States Department of Agriculture (USDA), Food Composition Databases Accessed 2017-06-16 from https://ndb.nal.usda.gov/ndb/.
- 61.Kuhnlein HV; Receveur O; Soueida R; Berti PR, Unique patterns of dietary adequacy in three cultures of Canadian Arctic indigenous peoples. Public Health Nutr 2008, 11, (4), 349–60. DOI: 10.1017/S1368980007000353 [DOI] [PubMed] [Google Scholar]
- 62.Schaefer SE; Erber E; Trzaskos JP; Roache C; Osborne G; Sharma S, Sources of Food Affect Dietary Adequacy of Inuit Women of Childbearing Age in Arctic Canada. J Health PopulNutr 2011, 29, (5). DOI: 10.3329/jhpn.v29i5.8899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lepage C; Carignan G; Patry P; Saucier A; Jette M, Use of Services and Consumption of Medications in A Health Profile of the Inuit, Jette M, Ed. Sante Quebec: Montreal, QC. [Google Scholar]
- 64.McDowell MA; Dillon CF; Osterloh J; Bolger PM; Pellizzari E; Fernando R, … Mahaffey KR, Hair Mercury Levels in U.S. Children and Women of Childbearing Age: Reference Range Data from NHANES 1999–2000. Environ Health Perspect 2004, 112, (11), 1165–1171. DOI: 10.1289/ehp.7046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fleming ST; Rastogi A; Dmitrienko A; Johnson KD, A comprehensive prognostic index to predict survival based on multiple comorbidities: a focus on breast cancer. Med Care 1999, 601–614. DOI: https://www.jstor.org/stable/3767021 [DOI] [PubMed] [Google Scholar]
- 66.Forouzanfar MH; Alexander L; Anderson HR; Bachman VF; Biryukov S; Brauer M, … Murray CJ, Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, (10010), 2287–2323. DOI: 10.1016/S0140-6736(15)00128-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gates A; Skinner K; Gates M, The diets of school-aged Aboriginal youths in Canada: a systematic review of the literature. J Hum Nutr Diet 2015, 28, (3), 246–61. DOI: 10.1111/jhn.12246 [DOI] [PubMed] [Google Scholar]
- 68.Kuhnlein HV; Chan HM, Environment and contaminants in traditional food systems of northern indigenous peoples. Ann Rev Nutr 2000, 20, (1), 595–626. DOI: 10.1146/annurev.nutr.20.1.595 [DOI] [PubMed] [Google Scholar]
- 69.Grant WB; Schwalfenberg GK; Genuis SJ; Whiting SJ, An estimate of the economic burden and premature deaths due to vitamin D deficiency in Canada. Mol Nutr Food Res 2010, 54, (8), 1172–81. DOI: 10.1002/mnfr.200900420 [DOI] [PubMed] [Google Scholar]
- 70.Cohen JT; Bellinger DC; Connor WE; Shaywitz BA, A quantitative analysis of prenatal intake of n-3 polyunsaturated fatty acids and cognitive development. Am JPrev Med 2005, 29, (4), 366–74. DOI: 10.1016/j.amepre.2005.06.008 [DOI] [PubMed] [Google Scholar]
- 71.Chowdhury R; Stevens S; Gorman D; Pan A; Warnakula S; Chowdhury S, … Hu FB, Association between fish consumption, long chain omega 3 fatty acids, and risk of cerebrovascular disease: systematic review and meta-analysis. Brit Med J 2012, 345, e6698 DOI: 10.1136/bmj.e6698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu D; Huang J; Wang Y; Zhang D; Qu Y, Fruits and vegetables consumption and risk of stroke: a meta-analysis of prospective cohort studies. Stroke 2014, 45, (6), 1613–9. DOI: 10.1161/STROKEAHA.114.004836 [DOI] [PubMed] [Google Scholar]
- 73.Afshin A; Micha R; Khatibzadeh S; Mozaffarian D, Consumption of nuts and legumes and risk of incident ischemic heart disease, stroke, and diabetes: a systematic review and meta-analysis. Am J Clin Nutr 2014, 100, (1), 278–288. DOI: 10.3945/ajcn.113.076901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Micha R; Wallace SK; Mozaffarian D, Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation 2010, 121, (21), 2271–83. DOI: 10.1161/CIRCULATIONAHA.109.924977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mozaffarian D; Clarke R, Quantitative effects on cardiovascular risk factors and coronary heart disease risk of replacing partially hydrogenated vegetable oils with other fats and oils. Eur JClinNutr 2009, 63, (S2), S22 DOI: 10.1038/sj.ejcn.1602976 [DOI] [PubMed] [Google Scholar]
- 76.Centers for Disease Control and Prevention (CDC), Death rates for 358 selected causes, by 10-year age groups, race, and sex: United States, 1999–2007 National Vital Statistics System; 2010. [Google Scholar]
- 77.Drewnowski A, Sensory properties of fats and fat replacements. Nutr Rev 1992, 50, (4), 17–20. DOI: 10.1111/j.1753-4887.1992.tb01285.x [DOI] [PubMed] [Google Scholar]
- 78.World Cancer Research Fund (WCRF); American Institute for Cancer Research (AICR), Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective AICR: Washington, DC, 2007. [Google Scholar]
- 79.Liu J; Wang J; Leng Y; Lv C, Intake of fruit and vegetables and risk of esophageal squamous cell carcinoma: a meta-analysis of observational studies. Int J Cancer 2013, 133, (2), 473–85. DOI: 10.1002/ijc.28024 [DOI] [PubMed] [Google Scholar]
- 80.Vieira AR; Abar L; Vingeliene S; Chan D; Aune D; Navarro-Rosenblatt D, … Norat T, Fruits, vegetables and lung cancer risk: a systematic review and meta-analysis. Ann Oncol 2015, 27, (1), 81–96. DOI: 10.1093/annonc/mdv381 [DOI] [PubMed] [Google Scholar]
- 81.Garland CF; Grant WB; Mohr SB; Gorham ED; Garland FC, What is the Dose-Response Relationship between Vitamin D and Cancer Risk? Nutr Rev 2007, 65, (8), 91–95. DOI: 10.1301/nr.2007.aug.S91-S95 [DOI] [PubMed] [Google Scholar]
- 82.Howlader N; Noone A; Krapcho M; Miller D; Bishop K; Altekruse S, … Cronin K, Eds., SEER Cancer Statistics Review, 1975–2013 Bethesda, MD, 2016. [Google Scholar]
- 83.Canuel R; de Grosbois SB; Atikessé L; Lucotte M; Arp P; Ritchie C, … Anderson R, New evidence on variations of human body burden of methylmercury from fish consumption. Environ Health Perspect 2006, 114, (2), 302–306. DOI: 10.1289/ehp.7857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gosselin NH; Brunet RC; Carrier G; Bouchard M; Feeley M, Reconstruction of methylmercury intakes in indigenous populations from biomarker data. J Expo Sci Env Epid 2006, 16, (1), 19–29. DOI: 10.1038/sj.jea.7500433 [DOI] [PubMed] [Google Scholar]
- 85.Sirot V; Guerin T; Mauras Y; Garraud H; Volatier JL; Leblanc JC, Methylmercury exposure assessment using dietary and biomarker data among frequent seafood consumers in France CALIPSO study. Environ Res 2008, 107, (1), 30–8. DOI: 10.1016/j.envres.2007.12.005 [DOI] [PubMed] [Google Scholar]
- 86.Basu N; Goodrich JM; Head J, Ecogenetics of mercury: from genetic polymorphisms and epigenetics to risk assessment and decision-making. Environ Toxicol Chem 2014, 33, (6), 1248–58. DOI: 10.1002/etc.2375 [DOI] [PubMed] [Google Scholar]
- 87.World Health Organization (WHO), Methylmercury Environmental Health Criteria; Geneva, Switzerland, 1990. [Google Scholar]
- 88.Chan HM, Contaminant Assessment in Nunatsiavut. Inuit Health Survey 2007–2008 2011. [Google Scholar]
- 89.Chan HM, Contaminant Assessment in Nunavut. Inuit Health Survey 2007–2008 2011. [Google Scholar]
- 90.Dewailly E; Ayotte P; Pereg D; Dery S; Dallaire R; Fontaine J; Cote S, Exposure to environmental contaminants in Nunavik: metals. Nunavik Inuit Health Survey 2004 2004. [Google Scholar]
- 91.Health Canada, Mercury: Your Health and the Environment: A Resource Tool Accessed 2017-11-21 from https://www.canada.ca/en/health-canada/services/environmental-workplace-health/reports-publications/environmental-contaminants/mercury-your-health-environment-resource-tool.html.
- 92.Hopping BN; Erber E; Mead E; Sheehy T; Roache C; Sharma S, Socioeconomic indicators and frequency of traditional food, junk food, and fruit and vegetable consumption amongst Inuit adults in the Canadian Arctic. J Hum Nutr Diet 2010, 23 Suppl 1, 51–8. DOI: 10.1111/j.1365-277X.2010.01100.x [DOI] [PubMed] [Google Scholar]
- 93.Kris-Etherton PM; Grieger JA; Etherton TD, Dietary reference intakes for DHA and EPA. ProstagLeukotr Ess 2009, 81, (2), 99–104. DOI: 10.1016/j.plefa.2009.05.011 [DOI] [PubMed] [Google Scholar]
- 94.United States Food and Drug Administration (US FDA), A Food Labeling Guide: Guidance for Industry College Park, MD, 2013. [Google Scholar]
- 95.Gagne D; Blanchet R; Lauziere J; Vaissiere E; Vezina C; Ayotte P, … Turgeon O’Brien H, Traditional food consumption is associated with higher nutrient intakes in Inuit children attending childcare centres in Nunavik. Int J Circumpol Heal 2012, 71, 18401 DOI: 10.3402/ijch.v71i0.18401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kuhnlein H; Receveur O, Local cultural animal food contributes high levels of nutrients for arctic Canadian indigenous adults and children. J Nutr 2007, 137, (4), 1110–1114. DOI: 10.1093/jn/137.4.1110 [DOI] [PubMed] [Google Scholar]
- 97.Nakano T; Fediuk K; Kassi N; Kuhnlein HV, Food use of Dene/Metis and Yukon children. Int J Circumpol Heal 2005, 64, (2). DOI: 10.3402/ijch.v64i2.17966 [DOI] [PubMed] [Google Scholar]
- 98.Sheehy T; Kolahdooz F; Roache C; Sharma S, Traditional food consumption is associated with better diet quality and adequacy among Inuit adults in Nunavut, Canada. Int J FoodSci Nutr 2015, 66, (4), 445–51. DOI: 10.3109/09637486.2015.1035232 [DOI] [PubMed] [Google Scholar]
- 99.Grandjean P; Budtz-Jorgensen E, An ignored risk factor in toxicology: The total imprecision of exposure assessment. Pure Appl Chem 2010, 82, (2), 383–391. DOI: 10.1351/PAC-CON-09-05-04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Statistics Canada, Age-standardized five-year survival estimates for primary sites of cancer, ICD-O-3 (October 2011 CCR file), by sex, 3 years of cases, Canada and selected provinces. CANSIM Accessed 2018-03-19.
- 101.Simpson SJ; Raubenheimer D, The Nature of Nutrition: A Unifying Framework from Animal Adaptation to Human Obesity Princeton University Press: Princeton, NJ, 2012. [Google Scholar]
- 102.Mozaffarian D; Hao T; Rimm EB; Willett WC; Hu FB, Changes in diet and lifestyle and long-term weight gain in women and men. New Engl J Med 2011, 364, (25), 2392–404. DOI: 10.1056/NEJMoa1014296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chan HM; Receveur O; Sharp D; Schwartz H; Ing A; Tikhonov C, First Nations Food, Nutrition and Environment Study: Results from British Columbia (2008/2009) University of Northern British Columbia: Prince George, BC, 2011. [Google Scholar]
- 104.Kirmayer LJ; Fletcher C; Watt R, Locating the Ecocentric Self: Inuit Concepts of Mental Health and Illness in Healing Traditions: The Mental Health of Aboriginal Peoples in Canada, Kirmayer LJ, Ed. UBC Press: Vancouver, BC, 2009. [Google Scholar]
- 105.King M; Smith A; Gracey M, Indigenous health part 2: the underlying causes of the health gap. Lancet 2009, 374, (9683), 76–85. DOI: 10.1016/S0140-6736(09)60827-8 [DOI] [PubMed] [Google Scholar]
- 106.Sharma S, Assessing diet and lifestyle in the Canadian Arctic Inuit and Inuvialuit to inform a nutrition and physical activity intervention programme. J Hum Nutr Diet 2010, 23 Suppl 1, 5–17. DOI: 10.1111/j.1365-277X.2010.01093.x [DOI] [PubMed] [Google Scholar]
- 107.Cordain L; Eaton SB; Miller JB; Mann N; Hill K, The paradoxical nature of hunter-gatherer diets: meat-based, yet non-atherogenic. Eur J Clin Nutr 2002, 56, (S1), S42 DOI: 10.1038/sj.ejcn.1601353 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.