Summary:
Sphingolipids, including ceramides, glycosphingolipids, sphingomyelin, and sphingosine-1-phosphate, have been recognized as important molecules that regulate critical cellular functions. Although originally studied in the context of lysosomal storage diseases, the roles of these compounds in more common disorders involving metabolism, vascular disease, and aberrant growth has been the focus of recent studies, including in disorders that affect the kidneys. These efforts have led to new insights into Fabry disease, a classic disorder of lysosomal function that results in renal failure as well as in more common renal diseases including diabetic nephropathy and polycystic kidney disease. Pathways for glycosphingolipid synthesis can be targeted with orally available small-molecule inhibitors, creating new opportunities for the treatment of both rare and common kidney diseases.
Keywords: Glycosphingolipids, glucosylceramide, diabetes, Fabry disease, polycystic kidney disease, substrate reduction
Johann Ludwig Wilhelm Thudicum, a physician and chemist, is credited with the discovery of sphingolipids.1 It is believed that Thudicum was impressed by the enigmatic properties of these novel compounds and he referred to them as sphingolipids as an allusion to the riddle of the sphinx. Although much has been elucidated about this class of lipids following Thudicum’s characterization almost 130 years ago, the roles of sphingolipids in health and disease remains an active area of exploration.
An important milestone in sphingolipid biology was the discovery by Christian de Duve of the lysosome as a distinct cellular organelle.2 The work of de Duve and Wattiaux2 work was followed rapidly by the observation that the loss of activity of lysosomal hydrolases involved in sphingolipid metabolism was the causal basis of a variety of disorders that were phenotypically unique and robust. His seminal work was an important catalyst in efforts to understand the roles of these lipids in health and disease.3 In particular, the recognition that lysosomal storage disorders result in the pathologic accumulation of phospholipids including sphingomyelin, ceramides, and glycolipids led to the identification of a myriad of additional complex structures that fall within the category of sphingolipids, the distinct pathways responsible for their synthesis and catabolism, and the genes and transcription factors that regulate their metabolism.
The functions of sphingolipids are remarkably varied and include recognized roles as structural elements that define the physical properties of membranes, as membrane receptors, as receptor ligands, as intracellular second messengers, and as transcriptional regulators. Much of the work on the biochemistry and functional biology of sphingolipids has focused on the kidney owing to the diversity of sphingolipids found there and renal diseases that are sphingolipid-based. This brief review highlights historically important work and more recent efforts to understand the role and importance of sphingolipid metabolism in both rare disorders and more common clinical syndromes involving the kidney. The primary goals of this review are to introduce the reader to the basics of sphingolipid biology and to argue that insights into the functional roles of sphingolipids are providing opportunities for the development of therapeutics that target both rare and common renal disorders. Although several excellent reviews are available that detail the biochemistry and cellular biology of these compounds, the reader is referred to a particular scholarly and comprehensive treatise in this area by Merrill.4
STRUCTURES AND METABOLIC PATHWAYS OF SPHINGOLIPIDS
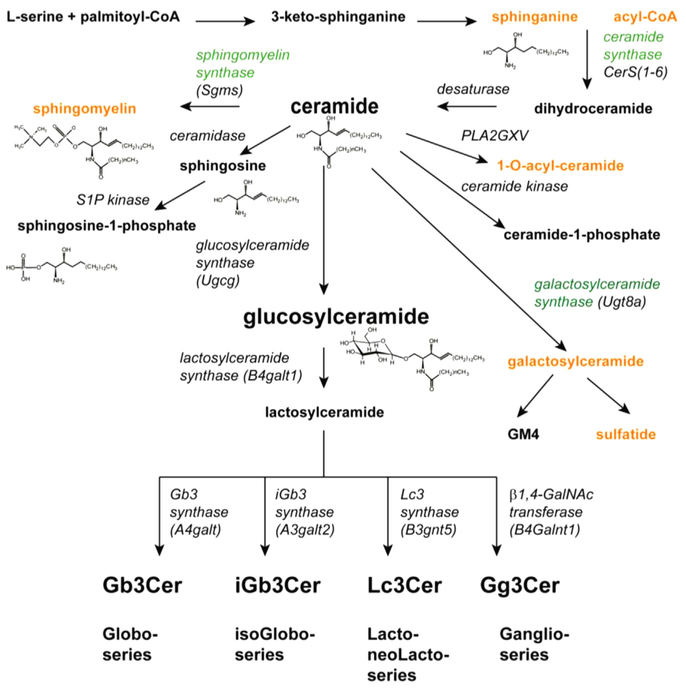
A sphingolipid is defined as a compound containing a long-chain base or sphingoid amine. Ceramides, a type of sphingolipid, are defined by the presence of an acyl group in amide linkage. Glycosphingolipids are defined by the presence of one or more sugar groups at carbon 1 of the long-chain base. If this base is unmodified by acylation or glycosylation, then one of three common sphingoid amines are commonly present, namely sphingosine, dihydrosphingosine, or phytosphingosine. These bases vary in terms of saturation, typically with a 4,5-double bond, and in terms of the presence of hydroxyl groups. Sphingoid base synthesis begins with the condensation of serine and palmitoyl-CoA to form dihydrosphingosine. Sphingosine is not formed directly from dihydrosphingosine, rather dihydrosphingosine is first acylated to form dihydroceramide. Dihydroceramide subsequently is desaturated, forming ceramide. Sphingosine can be formed via the action of a ceramidase, a deacylase, and further metabolized. However, ceramide is also a central intermediate in the formation of a variety of other sphingolipids. The most abundant of these products is sphingomyelin, formed by the exchange of phosphorylcholine from phosphatidylcholine to ceramide.5 Sphingomyelin comprises 10% to 15% of the total phospholipid in the plasma membrane. Other products of ceramide include ceramide 1-phosphate, 1-O-acylceramide, and glycosphingolipids.
Glycosphingolipids are the result of the addition of between 1 and 20 sugars to ceramide. When a single sugar is present, the glycosphingolipid is termed a cerebroside. Glucosylceramide and galactosylceramide formed by glucosylceramide synthase (Ugcg)6 and galactosylceramide synthase (Ugt8a),7 respectively, are the most abundant cerebrosides. Glycosphingolipids may be neutral or negatively charged. Negatively charged carbohydrate head groups are associated with sulfate or sialic acids, resulting in the formation of sulfatides or gangliosides. Both of these anionic species are abundant in the kidney.8 Approximately 85% of mammalian glycolipids have glucose as their first sugar. Most glycosphingolipids with more than one sugar are built upon the addition of galactose, resulting in the formation of lactosylceramide through the action of lactosylceramide synthase (B4galt1). Glycosphingolipids, comprising one of four series, are formed based on the next sugar to be added to the lactosylceramide. These series and the responsible enzymes include the globo series (Gb3 synthase, A4galt); the isoGlobo series (iGb3 synthase, A3galt3); the lacto-/neoLacto series (Lc synthase, B3gnt5); and ganglio series (β1,4-N-acetylgalactosamine (GalNAc) transferase, B4Galnt1) (Fig. 1).9
Figure 1.
Pathways for sphingolipid synthesis. The substrates, pathways, enzymes, and structures for the formation of the major species of sphingolipids are shown. Ceramide, formed by the desaturation of dihydroceramide, is a central metabolite and precursor for several species of sphingolipids including sphingomyelin, sphingosine and sphingosine-1-phosphate, ceramide-1-phosphate, and glucosylceramide- and galactosylceramide-based glycosphingolipids.
Abbreviations: CoA, coenzyme A; GalNAc, N-acetylgalactosamine.
Glycosphingolipids are both organ- and cell-type–specific. Although glycosphingolipids circulate in the plasma and are re-incorporated into cells, the cell specificity is owing primarily to differences in the expression and activity of specific enzymes and glycosidases that favor the synthesis and accumulation of individual species. Within cells, glycosphingolipids tend to localize to the outer leaflet of the plasma membrane. They cycle within the cell through endocytic pathways that involve the lysosome, but are extralysosomal as well.
Sphingolipids, most notably sphingomyelin and glycosphingolipids, form aggregates with cholesterol in distinct domains, commonly termed lipid rafts.10 These rafts are hypothesized to be sites for many signaling complexes that include receptors, phospholipases, tyrosine kinases, and structural proteins such as caveolins. Changes in the molar ratios and composition of these raft lipids have been reported to result in aberrant changes in ligand signaling and may provide a potential mechanism linking abnormal sphingolipid metabolism and disease.
There are more than 30 distinct glycosphingolipids (GSLs) present in mammalian kidney as defined only by their carbohydrate composition and linkages.8 However, based on the diversity of the associated sphingoid bases and fatty acyl groups that can comprise the ceramide portion of these glycosphingolipids, there are hundreds of distinct species found in the kidney. One GSL in particular has been established as the basis for renal disease, namely globotriaosylcer-amide (Gb3). Gb3, present on renal endothelial cells, is the receptor for shiga toxin and mediates injury associated with infectious forms of hemolytic uremic syndrome.11 Gb3 is also the primary substrate for the lysosomal enzyme α-galactosidase A (GLA). Loss of GLA activity is the basis for Fabry disease, which is discussed in greater detail later.12
SPHINGOLIPIDS AS CELLULAR MEDIATORS OF SIGNALING AND METABOLISM
Although sphingolipids comprise in aggregate a relatively small mass of total cell lipids, they play a disproportionately significant role in the regulation of cell metabolism and differentiation. Cell growth and death appear regulated by sphingolipids. Sphingolipids also are modulators of growth factors, are ligands in and of themselves, and also may be cellular second messengers. The potential second messenger functions of sphingolipids have been the focus of considerable effort over the past 25 years, with most attention directed toward ceramide and sphingosine-1-phosphate (S1P). Ceramide, liberated by the hydrolysis of sphingomyelin or formed via de novo synthesis, has been implicated in growth arrest and apoptosis.13 An early model for the mechanism of action of ceramide was akin to that of diacylglycerol and protein kinase C. A number of ceramide-specific targets have been identified over the years, including protein kinase C ζ and protein phosphatase 2A. However, in contrast to the well-characterized interaction of diacylglycerol and protein kinase C, it has been more difficult to show a direct binding interaction between ceramide and any of these targets at a structural level, although this has not been for lack of effort. Alternative findings that may explain the role of exogenously added ceramides in the induction of apoptotic death include the formation of membrane pores.
S1P is synthesized in the endoplasmic reticulum through the action of one of two sphingosine kinases and is metabolized via either a phosphatase or lyase (SGPL1).14 S1P has been identified as both an intra-cellular second messenger and extracellular ligand with five distinct G-protein–coupled receptors identified.15 These receptors comprise a subgroup of the larger family of lysophospholipid receptors. S1P has been implicated in a number of important physiological pathways including vascular development and lymphocyte trafficking. S1P through binding to the S1P1 receptor mediates egress of lymphocytes from lymph nodes. S1P mimetics (FTY720, fingolimod) is an S1P1-receptor agonist clinically approved for multiple sclerosis. Anti-S1P monoclonal antibodies have been developed for clinical use and are the basis for clinical trials in renal cell carcinoma and macular degeneration.
Recently, seven families with steroid-resistant nephrotic syndrome were identified to have mutations in sphingosine-1-phosphate lyase 1 (SGPL1). Additional phenotypic findings among these kindreds were ichthyo-sis, adrenal insufficiency, immunodeficiency, and neuro-logic defects.16 Nine distinct different recessive mutations in SGPL1 were identified, all of which resulted in reduced lyase activity. Although this represents a rare form of nephrotic syndrome, these findings ultimately may show a larger role for S1P in glomerular function.
Sphingolipid synthesis begins in the endoplasmic reticulum with the synthesis of dihydrosphingosine via the activity of serine palmitoyl-CoA. Further synthesis leading to ceramide and sphingomyelin synthesis also are endoplasmic reticulum–associated. Ceramide subsequently is transported to the Golgi apparatus where glucosylceramide is formed at the cytoplasmic side of the Golgi. The newly synthesized cerebroside flips to the intraluminal side of the Golgi where further glycosylation steps occur.17
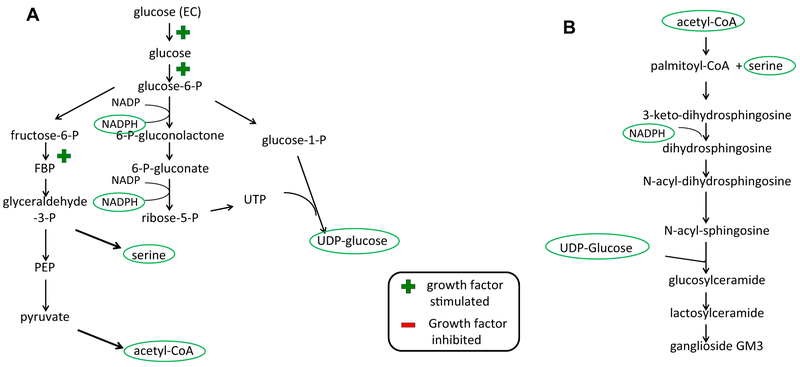
Sphingolipid synthesis is tightly coupled to cell growth and proliferation. Recent insights into the manner in which glucose is used under conditions in which tissues are fully differentiated and quiescent versus conditions associated with proliferation provide clues as to why this may be the case.18 In the presence of oxygen, most cells in the renal cortex metabolize glucose to carbon dioxide by the metabolism of pyruvate through the tricarboxylic acid cycle. This fuels oxidative phosphorylation to maximally produce ATP, an important energy source for cellular functions including transport across the tubular epithelia. Aerobic glycolysis, on the other hand, although less efficient in terms of ATP formation, is favorable with regard to the formation of metabolites and cofactors required for cell growth and proliferation. Under stimuli that favor cell proliferation or hypertrophy, such as growth factor stimulation, aerobic glycolysis is favored. In other words, although glucose can be fully metabolized through oxidative phosphorylation to produce 38 molecules of ATP, this will not produce the other critical substrates for cell growth. For example, to synthesize a single molecule of palmitate a cell requires only 7 molecules of ATP, but also 8 molecules of acetyl-CoA (16 carbons), and 14 molecules of nicotinamide adenine dinucleotide phosphate (NADPH) (28 electrons). One molecule of glucose can produce two NADPH molecules and one uridine triphosphate (UTP), one acetyl-CoA, or one serine. To form a single molecule of UDP-glucose or UDP-galactose, two glucose molecules must be metabolized (Fig. 2A). Consistent with the importance of metabolism of glucose through nonoxidative pathways is the observation that growth factors such as epidermal growth factor favor glucose metabolism through aerobic glycolysis. This occurs in part through stimulated uptake of glucose across the plasma membrane, its conversion to glucose-6-phosphate, and inhibition of pyruvate flux through oxidative phosphorylation pathways.
Figure 2.
Glycolytic pathways for the generation of critical substrates in glycosphingolipid synthesis. (A) Major intermediates and products of aerobic glycolysis. In the presence of growth factors such as epidermal growth factor, glucose metabolism generates acetyl-CoA, serine, UDP glucose, and NADPH. (B) Denotes the points at which the products of aerobic glycolysis are used for the synthesis of glucosylceramide. Abbreviations: CoA, coenzyme A; EC, extra-cellular; FBP, fructose 1,6-bisphosphate; PEP, phosphoenolpyruvate; UDP, uridine diphosphate; UTP, uridine triphosphate.
It is noteworthy that many of the products of aerobic glycolysis are substrates or co-factors for glycosphingolipid synthesis (Fig. 2B). These include serine, acetyl-CoA, UDP glucose, and UDP galactose, as well as reducing equivalents in the form of NADPH. It is not surprising therefore that disorders of aberrant renal growth are associated with increased expression of glycosphingolipids. These disorders include renal cell carcinoma, diabetic nephropathy,19 and polycystic kidney disease.20 These findings have led investigators to consider whether these pathways may serve not only as cellular markers for these disorders, but also whether they may play a role in the pathogenesis of these disorders.
In recent years, investigators have focused on the lysosome as a critical site at which cells sense their metabolic status. Central to this regulation is the transcription factor EB (TFEB), a member of the micropthalmia associated (MiT) family of transcription factors. TFEB binds to a 10-base E-box–like palindromic sequence termed the coordinated lysosomal expression and regulation motif that is shared among many lysosomal genes. Activation of TFEB (and other MiT family members) results in lysosomal biogenesis and lysosomal enzyme activity, leading to an enhancement of catabolic activity within cells. However, TFEB coordinates the transcription of many other genes, notably those associated with lysosomal exocytosis and autophagy.21 TFEB is post-translationally regulated by phosphorylation at Ser142 and Ser211. Importantly, two kinases that regulate cellular growth, mammalian target of rapamycin complex 1 (mTORC1) and mitogen-activated protein kinase, phosphorylate TFEB under nutrient-rich conditions, leading to TFEB sequestration in the cytosol and prevention of nuclear translocation. mTORC1 is localized to the lysosomal membrane in its active state where it is believed to be activated by amino acids, thus serving as a nutrient sensor. During starvation, mTORC1 is released from the lysosomal membrane where it becomes inactive. Based on these findings, a model has emerged that links nutrient sensing at the lysosome with mTORC1, a master regulator of cellular growth, and a master transcription factor TFEB that regulates autophagy and lysosomal function.22
INHIBITORS OF GLUCOSYLCERAMIDE SYNTHASE
The identification of glucosylceramide as a target for small molecule inhibitors was first proposed by Radin23 as the basis for the treatment of Gaucher disease. In contrast to strategies designed to increase or replace the deficient hydrolase, substrate reduction was proposed as a means for reducing the substrate that accumulated within lysosomes. In Gaucher disease, in which the loss of β-glucocerebrosidase activity resulted in glucosylceramide accumulation, or in Fabry disease, in which loss of α-galactosidase A activity resulted in globotriaosylceramide accumulation, inhibition of glucosylceramide was proposed to be beneficial.
D-threo-1-phenyl-2-decanoylamino-3-morpholinopropanol (PDMP) was the first reversible inhibitor of glucosylceramide synthase and was modeled after chloramphenicol.24 Initial work defined a critical scaffold or pharmacophore required for activity. A modest increase in activity was achieved after a series of fatty acyl substitutions for the 2-acyl group25 and cyclic amine for the morpholino function.26 A significant increase in activity was achieved by application of Hansch analysis to aromatic substitutions.27 Specifically, substitution of an ethylenedioxyphenyl group for the phenyl group led to the identification of a series of compounds with low nanomolar activity.
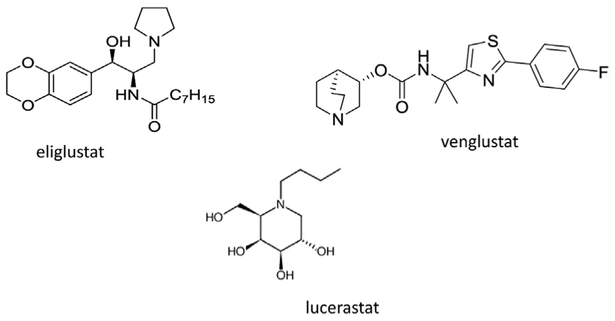
When these second-generation analogues were studied in mouse models of Gaucher and Fabry disease, significant reductions in glucosylceramide and globotriaosylceramide, respectively, were observed.28,29 This led to the clinical development of the carbon-8 substituted ethylenedioxyphenyl analogue as a treatment for Gaucher disease type 1.30 After a series of clinical trials, including two pivotal phase 3 studies, eliglustat tartrate was approved in 2014 as the first stand alone oral therapy for Gaucher disease type 1.31,32
Other chemotypes have been developed as glucosylceramide synthase inhibitors (Fig. 3). Imino sugars, including N-butyldeoxynojirimycin, originally were developed as antiviral agents owing to their α-glucosidase inhibitory activity.33 N-butyldeoxynojirimycin (miglustat) inhibits glucosylceramide synthase in the micromolar range, but also inhibits lysosomal and plasma membrane β-glucocerebrosidase with higher affinities, raising the likely possibility that the therapeutic benefits of this compound in Gaucher disease is secondary to its chaperone and not substrate reduction activity.34 The off-target effects on intestinal glucosidases result in significant gastrointestinal toxicities and have precluded its use as a first-line agent for Gaucher disease.
Figure 3.
Examples of chemotypes that are glucosylceramide synthase inhibitors.
SPHINGOLIPIDS IN RARE KIDNEY DISEASE: FABRY DISEASE
Fabry disease is an inherited lysosomal storage disease arising from the partial or complete loss of GLA activity. GLA hydrolyzes terminal 1-α−4 glycosidic linkages on neutral glycosphingolipids including globotriaosylceramide, globotriaosylsphingosine, and galabiosylceramide. This X-linked disorder is associated with progressive renal insufficiency often resulting in renal failure, cardiac disease, and a vasculopathy resulting in stroke.35 The disease is pathologically manifest as the lysosomal accumulation of GSLs throughout the kidney, including podocytes, tubular epithelium, interstitial cells, and endothelial cells. In addition, high levels of Gb3 and lyso-Gb3 circulate in the plasma of affected patients. Although a great deal of insight has been elucidated regarding the genetic basis and structural abnormities of GLA, the precise mechanisms responsible for the development of renal insufficiency and the vasculopathy remain unclear.
Much attention has focused on abnormalities in the endothelial cell biology associated with GLA deficiency. A knockout mouse lacking Gla activity has been informative in this regard. Although the mice lack detectable Gla activity, they fail to show a spontaneous vascular phenotype or loss of renal function. However, the mice are susceptible to inducible models of vasculopathy including oxidant-induced arterial thrombosis,36 accelerated atherogenesis,37 and impaired relaxation in precontracted aortic rings and mesenteric arteries.38
Several experimental findings in this model were consistent with the defect being localized to the vasculature in general and the endothelial cell in particular. First, bone marrow transplants between the knockout (KO) mice and wild-type mice showed that the thrombotic defect was localized to the vasculature and not in circulating blood cells such as platelets, which also have Gb3. Second, the vasorelaxation defect was eliminated by removal of the endothelial cells from the aortic rings. Third, the vasorelaxation defect could be bypassed with restricted exposure of calcium ionophore to the endothelial cells. This form of calcium-induced vasorelaxation was consistent with the defect residing not only within the endothelial cell layer, but at the level of the plasma membrane.
These findings raised the possibility that changes in the sphingolipid content of endothelial cells were not restricted to the lysosome, but rather secondarily might affect cellular domains that included the plasma membrane. A model of Fabry disease using primary cultures of mouse aortic endothelial cells was established.39When these cells were compared with those from wild-type mice, Gb3 accumulation was observed in plasma membrane fractions and in lipid raft fractions as well.40The increase in Gb3 was associated with a decrease in the molar ratio of cholesterol, suggesting that Gla deficiency fundamentally could alter the membrane composition and structure within the lipid raft domains. In endothelial cells these domains are associated with caveolae, small invaginations within the endothelial cells characterized by the presence of high-molecular-weight oligomers of the protein caveolin-1. Indeed, the Gla null mouse endothelial cells were characterized by a loss of these high-molecular-weight oligomers.41
An important signaling molecule that is localized to caveolae is endothelial nitric oxide synthase (eNOS). Decreased NO bioavailability has been linked to loss of vasorelaxation response, and eNOS uncoupling results in a pro-oxidant state leading to both thrombosis and atherosclerosis secondary to the formation of peroxynitrite. The hypothesis arose from these observations that the vasculopathy observed with loss of GLA activity was the result of abnormal eNOS function. In support of this hypothesis, eNOS expression and activities were decreased in both the endothelial cell model and Gla null aortas.
An important consequence of eNOS uncoupling is the production of nitrogen-containing oxidants such as peroxynitrite. This pathway can be distinguished from other oxidant-associated pathways by assaying tyrosine adducts42 (Table 1). A 50-fold increase in 3-nitrotyrosine content was measured by mass spectrometry in an endothelial cell line in which Gla levels were decreased by small interfering RNA. Other adducts including chlorotyrosine, dityrosine, and ortho- and meta-tyrosine were no different from control cells. Similar changes were observed in the plasma and aortas of KO mice when compared with wild-type controls. Finally, bio-banked plasma from classic Fabry disease patients showed significant increases in 3-nitrotyrosine levels as well.43 Future work will need to confirm whether 3-nitrotyrosine is a valid biomarker of Fabry disease and whether reductions in plasma or tissue-associated 3-nitrotyrosine levels track with various treatment modalities such as enzyme replacement therapy.
Table 1.
Patterns of Tyrosine Oxidation Based on Oxidant Source
| Ortho-tyrosine | Meta-tyrosine | Dityrosine | Nitrotyrosine | Chlorotyrosine | |
|---|---|---|---|---|---|
| Glycoxidation | ↑ | ↑ | ↑ | — | — |
| MPO | — | — | ↑ | ↑ | ↑ |
| RNS by MPO | ↑ | ↑ | ↑ | ||
| RNS by peroxynitrite | ↑ | ↑ | ↑ | ↑↑ | — |
| Superoxide (mitochondria, NAD(P)H oxidase) | ↑ | ↑ | ↑ | ↑* | ↑† |
| Glucose-PUFA | ↑ | ↑ | — | — | — |
Abbreviations: MPO, myeloperoxidase; NAD(P)H, nicotinamide adenine dinucleotide phosphate; PUFA, polyunsaturated fatty acid; RNS, reactive nitrogen species; ↑, increased; ↑↑, markedly increased; —, no change.
Adapted with permission from Pennathur et al.61
In the presence of nitric oxide.
In the presence of MPO.
Some experimental evidence supports a causal role in vasculopathy for globo series glycolipids that is present in nonendothelial cell compartments of blood vessels. Lyso-Gb3 has been identified as an important biomarker that tracks with the presence and severity of Fabry disease.44Lyso-Gb3 is reported to stimulate smooth muscle cell proliferation in in vitro models of the disease.
Substrate reduction therapy presently is being investigated as a potential treatment for Fabry disease. Two glucosylceramide synthase inhibitors are the subject of active trials. A phase 1b safety study using the imino sugar analogue, lucerastat, has been completed.45Another chemotype, venglustat (GZ-402671), with significantly higher potency, is in phase 2 clinical development for Fabry disease.46
SPHINGOLIPIDS IN ABERRANT RENAL GROWTH: DIABETIC NEPHROPATHY
A growing body of work has emerged exploring the role of sphingolipids in metabolic disease including insulin resistance and fatty liver. Several studies have documented changes in sphingolipids in the setting of diabetes or inflammatory disorders that result in metabolic disease. Gaucher disease also has been explored for clues in the mechanisms linking insulin resistance and fatty liver to alterations in glucosylceramide levels. Although the limitations of this review preclude a detailed discussion of this work, in general investigators have explored two models for understanding the potential importance of sphingolipids in the pathogenesis of these syndromes.
The first set of models has explored the secondary changes in sphingolipid metabolism that are present in the setting of hyperglycemia and that may modulate secondary pathways associated with insulin resistance and fatty liver disease. The second set of models has focused on the potential roles of sphingolipids, including ceramides, glycosphingolipids, and sphingosine-1-phosphate as signaling molecules that mediate changes in signaling through the insulin receptor or inflammatory responses typical of steatohepatitis and steatosis. These models, of course, are not mutually exclusive because changes in cell signaling such as insulin resistance may result in secondary changes in hyperglycemia, hyperlipidemia, or inflammatory responses that may feedback to amplify some of these abnormalities. Two pathways are offered that are illustrative of how altered sphingolipid metabolism can result in aberrant signaling in the setting of diabetes.
Metabolic profiling as measured by mass spectrometry and genetic analyses have shown increased ceramides and associated sphingolipids in the setting of obesity and nonalcoholic steatohepatitis.47 This may be secondary to inflammatory pathways that are intimately tied to the up-regulation of sphingolipid synthesis. This may be the result of increased formation of saturated fatty acids, which activate Toll-like receptor-4 signaling with downstream activation of nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor (IkB) kinase and de novo ceramide synthesis. Ceramide synthesis in and of itself can further result in insulin resistance, oxidative stress, and inflammation.48
Glycosphingolipids have been shown to interact directly with the insulin receptor itself to regulate insulin signaling. The insulin receptor and its associated signaling molecules co-localize to lipid rafts in the plasma membrane. These rafts are enriched for cholesterol and sphingolipids, most notably sphingomyelin, and are de-enriched for glycerophospholipids. Glycosphingolipids also localize to these rafts with the exception of ganglioside GM3, which resides outside of the raft domain. Under conditions of inflammation-associated insulin resistance, such as exposure to tumor necrosis factor-α, ganglioside GM3 synthesis is up-regulated and the insulin receptor is sequestered outside of the cholesterol-enriched raft, resulting in insulin resistance as measured by a decrease in protein kinase B (AKT) and insulin-receptor phosphorylation.49 This effect may be explained by a direct binding interaction that was shown between the membrane-spanning domain of the insulin receptor and GM3.50 These observations suggest that ganglioside GM3 may sequester the insulin receptor outside of its usual raft domain and uncouple the receptor from other domain-associated signaling molecules. Supporting this model is the observation that KO mice lacking GM3 synthase are insulin sensitive51 and that inhibitors of glucosylceramide synthase restore insulin sensitivity in both in vitro and in vivo models of type 2 diabetes.52,53
An early clue that there might be a specific role for GSLs in regulating renal hypertrophy was suggested by work correlating testosterone exposure to increased glucosylceramide synthesis and kidney growth. Subsequently, an association with diabetes was suggested by early work on glucosylceramide synthase inhibitors. In these studies, streptozotocin-treated rats were noted to not develop renal hypertrophic responses when treated with PDMP, an early inhibitor with micromolar activity.19 In addition to decreasing glucosylceramide based glycosphingolipid levels, PDMP also increased ceramide levels through inhibition of 1-O-acylceramide synthase.54 Thus, these effects may have been owing to glycosphingolipid depletion or ceramide accumulation.
This observation has been supported further in in vitro55 and in vivo mouse models of type 2 diabetes in which at least two separate classes and more potent classes of glycolipid synthase inhibitors have been used. In these models, including the ob/ob mouse, hepatic levels of glucosylceramide and ganglioside GM3 are increased. These changes are consistent with the predicted increase in glycosphingolipids associated with aerobic glycolysis. These inhibitors also are effective in reversing the hepatic steatosis observed in mice on a high-fat diet. More recently, mesangial cell hypertrophy studied in vitro has been shown to be reversed with a highly specific glucosylceramide synthase inhibitor.52
The mechanism whereby inhibition of glucosylceramide synthase prevents renal hypertrophy secondary to hyperglycemia is not clear. On the one hand, glucosylceramide synthase inhibition results in the reversal of insulin resistance and improvement in hyperglycemia as measured by lower blood glucose and hemoglobin A1c levels. This change may be both sufficient and necessary to prevent the renal growth response. On the other hand, there may be a glucose-independent effect of glycolipid synthesis inhibition on the renal growth response. In support of this latter possibility, these inhibitors have been studied in a second model of aberrant renal growth: polycystic kidney disease.
SPHINGOLIPIDS IN ABERRANT RENAL GROWTH: POLYCYSTIC KIDNEY DISEASE
Polycystic kidney disease is a clinical syndrome that encompasses autosomal-dominant and autosomal-recessive forms of the disorder. Multiple phenotypes of polycystic kidney disease have been described that encompass both adult and pediatric forms of the disorder, as well as varying degrees of extrarenal manifestations. Common to all forms of polycystic kidney disease is cystogenesis and progressive renal failure. Multiple mechanisms for cytogenesis have been described, although most of the products of polycystic kidney disease–associated genes are expressed in either primary cilia or centrosomes. Several pathways have been identified that result in cyst formation and growth. They include adenlyl-associated proliferation, wingless-related integration site (wnt) pathways, calcium dysregulation, and AKT-mTOR signaling.56–58
As detailed earlier, aerobic glycolysis favors the generation of glucose metabolites that are important in glucosylceramide-based glycolipid formation. Earlier studies have reported increased levels of both glucosylceramide and lactosylceramide in polycystic kidneys.20 Subsequently, sphingolipid analyses were performed in human polycystic kidney disease samples and in juvenile cystic kidney (jck), hypomorphic Nphp3 (pcy), and conditional polycystin-1 (PKD1) mouse models. As previously reported for the pck mice, glucosylceramide and ganglioside GM3 levels were increased with minimal, if any, change in ceramide content.59
Treatment of each knockout mouse model with a potent inhibitor of glucosylceramide synthase, Genz-123346, resulted in inhibition of cystogenesis. An analysis of molecular pathways in the jck mouse kidneys was consistent with a G1/S cell-cycle arrest as well as inhibition of the mTOR pathway. In a follow-up study, jck mice were crossed with those knocked out for the GM3 synthase (St3gal5) gene. Cystogenesis was inhibited and similar decrements in cell cycle and mTOR-associated protein phosphorylation were observed.60
TREATMENT OPPORTUNITIES AND FUTURE DIRECTIONS
The successful development of eliglustat tartrate as the first stand-alone substrate reduction therapy for a glycosphingolipidosis has accelerated work in the development of alternative compounds for targeting other glycosphingolipidoses. Proof-of-principle studies for eliglustat originally were conducted in models of Fabry disease. Because the clinical manifestations are primarily peripheral and do not involve central nervous system (CNS) deposition of Gb3, this disorder represents the most promising target for substrate reduction therapy. Two candidate drugs are currently in clinical development for Fabry disease and include venglustat and lucerastat. Enzyme replacement therapy presently is available for Fabry disease and so treatment alternatives will remain available. However, other sphingolipidoses represent disorders for which there is truly unmet medical need. These include Gaucher disease types 2 and 3 as well as Tay-Sachs, Sandhoff disease, and GM1 gangliosidosis. Although the successful use of glucosylceramide synthase inhibitors for these diseases is not a foregone conclusion, a necessary precondition for this approach is the identification of a potent and specific CNS permeant inhibitor. Miglustat, an imino sugar, distributes into the CNS but lacks the potency, specificity, and safety profile to be such a candidate. Small clinical trials in Tay-Sachs and GM1 gangliosidosis have not been promising in this regard. Venglustat represents a more promising candidate. A major challenge to the successful development of substrate reduction agents for these latter diseases will be the limited number of subjects, a less well-defined natural history, variable phenotypes, the lack of good biomarkers that track with the progression of disease, and the long time to outcome.
Targeting GSL synthesis for disorders in which glycosphingolipidoses contribute to the cause or progression of the disease represent additional clinical targets for glycolipid synthesis inhibition. Aside from disorders such as insulin resistance and metabolic syndrome discussed earlier, other examples include multiple myeloma, Parkinson’s disease, and infectious diseases involving bacteria, viruses, and fungal organisms. For many of these disorders the role of glycosphingolipids is well established, such as the finding of the B subunit of verotoxin to Gb3. In other cases, the precise role of glycosphingolipids in the pathogenesis of the disorder and benefit for substrate reduction is less well established. Nevertheless, clinical trials still are planned.
Finally, the GSL synthesis inhibitors that block glucose flux through aerobic glycolysis presents a novel therapeutic opportunity to inhibit or reverse aberrant growth or metabolic disease. Included is cancer, diabetic nephropathy, and polycystic kidney disease. Although the potential impact of developing a successful GSL synthase inhibitor for these diseases is high, the mechanistic justification for treatment with a GSL synthesis inhibitor remains to be elucidated. Finding the holy grail, a unifying hypothesis that explains the nexus between glycosphingolipid synthesis inhibition and growth arrest, will be a critical step in accomplishing this goal.
Acknowledgments
Financial support: Supported in part by National Institutes of Health grants UH2NS092981and 1RO1HL22416.
Footnotes
Conflict of interest statement: The author is an inventor on patents covering the composition of matter, use, and synthesis of eliglustat tartrate and related analogues. He is an employee of the University of Michigan, which receives licensing fees and royalties for the use of eliglustat tartrate for the treatment of Gaucher disease type 1.
REFERENCES
- 1.Thudichum JLW. A Treatise on the Chemical Constitution of Brain. London: Bailliere, Tindall, and Cox, 1884 [Google Scholar]
- 2.De Duve C, Wattiaux R. Functions of lysosomes. Annu Rev Physiol 1966;28:435–92. [DOI] [PubMed] [Google Scholar]
- 3.Futerman AH, van Meer G. The cell biology of lysosomal storage disorders. Nat Rev Mol Cell Biol 2004;5:554–65. [DOI] [PubMed] [Google Scholar]
- 4.Merrill AH Jr. Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem Rev. 2011;111:6387–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ullman MD, Radin NS. The enzymatic formation of sphingomyelin from ceramide and lecithin in mouse liver. J Biol Chem 1974;249:1506–12. [PubMed] [Google Scholar]
- 6.Basu S, Kaufman B, Roseman S. Enzymatic synthesis of glucocerebroside by a glucosyltransferase from embryonic chicken brain. J Biol Chem 1973;248:1388–94. [PubMed] [Google Scholar]
- 7.Morell P, Radin NS. Synthesis of cerebroside by brain from uridine diphosphate galactose and ceramide containing hydroxy fatty acid. Biochemistry. 19698:506–512. [DOI] [PubMed] [Google Scholar]
- 8.Shayman JA, Radin NS. Structure and function of renal glycosphingolipids. Am J Physiol 1991;260:F291–302. [DOI] [PubMed] [Google Scholar]
- 9.Kolter T, Proia RL, Sandhoff K. Combinatorial ganglioside biosynthesis. J Biol Chem 2002;277:25859–62. [DOI] [PubMed] [Google Scholar]
- 10.van Meer G Invisible rafts at work. Traffic. 2004;5:211–2. [DOI] [PubMed] [Google Scholar]
- 11.Nutikka A, Binnington-Boyd B, Lingwood CA. Methods for the identification of host receptors for Shiga toxin. Methods Mol Med 2003;73:197–208. [DOI] [PubMed] [Google Scholar]
- 12.Brady RO, Gal AE, Bradley RM, Martensson E, Warshaw AL, Laster L. Enzymatic defect in Fabry’s disease. Ceramidetrihexosidase deficiency. N Engl J Med 1967;276:1163–7. [DOI] [PubMed] [Google Scholar]
- 13.Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. J Lipid Res 2009;50 (Suppl:):S91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fyrst H, Saba JD. An update on sphingosine-1-phosphate and other sphingolipid mediators. Nat Chem Biol 2010;6:489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blaho VA, Hla T. Regulation of mammalian physiology, development, and disease by the sphingosine 1-phosphate and lysophosphatidic acid receptors. Chem Rev 2011;111: 6299–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lovric S, Goncalves S, Gee HY, Oskouian B, Srinivas H, Choi WI, et al. Mutations in sphingosine-1-phosphate lyase cause nephrosis with ichthyosis and adrenal insufficiency. J Clin Invest 2017;127:912–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeckel D, Karrenbauer A, Burger KN, van Meer G, Wieland F. Glucosylceramide is synthesized at the cytosolic surface of various Golgi subfractions. J Cell Biol 1992;117:259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zador IZ, Deshmukh GD, Kunkel R, Johnson K, Radin NS, Shayman JA. A role for glycosphingolipid accumulation in the renal hypertrophy of streptozotocin-induced diabetes-mellitus. J Clin Invest 1993;91:797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deshmukh GD, Radin NS, Gattone VH, Shayman JA. Abnormalities of glycosphingolipid, sulfatide, and ceramide in the polycystic (Cpk/Cpk) mouse. J Lipid Res 1994;35:1611–8. [PubMed] [Google Scholar]
- 21.Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, et al. A gene network regulating lysosomal biogenesis and function. Science. 2009;325:473–7. [DOI] [PubMed] [Google Scholar]
- 22.Napolitano G, Ballabio A. TFEB at a glance. J Cell Sci 2016;129:2475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radin NS. Treatment of Gaucher disease with an enzyme inhibitor. Glycoconj J 1996;13:153–7. [DOI] [PubMed] [Google Scholar]
- 24.Shayman JA, Lee L, Abe A, Shu LM. Inhibitors of glucosylceramide synthase 2000. Sphingolipid Metab Cell Signal Pt A 2000;311:373–87. [DOI] [PubMed] [Google Scholar]
- 25.Abe A, Inokuchi J, Jimbo M, Shimeno H, Nagamatsu A, Shayman JA, et al. Improved inhibitors of glucosylceramide synthase. J Biochem 1992;111:191–6. [DOI] [PubMed] [Google Scholar]
- 26.Carson KG, Ganem B, Radin NS, Abe A, Shayman JA. Studies on morpholinosphingolipids - potent inhibitors of glucosylcer-amide synthase. Tetrahedron Lett 1994;35:2659–62. [Google Scholar]
- 27.Lee L, Abe A, Shayman JA. Improved inhibitors of glucosylceramide synthase. J Biol Chem 1999;274:14662–9. [DOI] [PubMed] [Google Scholar]
- 28.Abe A, Gregory S, Lee L, Killen PD, Brady RO, Kulkarni A, et al. Reduction of globotriaosylceramide in Fabry disease mice by substrate deprivation. J Clin Invest 2000;105:1563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marshall J, McEachern KA, Chuang WL, Hutto E, Siegel CS, Shayman JA, et al. Improved management of lysosomal glucosylceramide levels in a mouse model of type 1 Gaucher disease using enzyme and substrate reduction therapy. J Inherit Metab Dis 2010;33:281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shayman JA. Eliglustat Tartrate: Glucosylceramide synthase inhibitor treatment of type 1 Gaucher disease. Drugs Future 2010;35:613–20. [PMC free article] [PubMed] [Google Scholar]
- 31.Cox TM, Drelichman G, Cravo R, Balwani M, Burrow TA, Martins AM, et al. Eliglustat compared with imiglucerase in patients with Gaucher’s disease type 1 stabilised on enzyme replacement therapy: a phase 3, randomised, open-label, non-inferiority trial. Lancet. 2015;385:2355–62. [DOI] [PubMed] [Google Scholar]
- 32.Mistry PK, Lukina E, Ben Turkia H, Amato D, Baris H, Dasouki M, et al. Effect of oral eliglustat on splenomegaly in patients with Gaucher disease type 1: the ENGAGE randomized clinical trial. JAMA. 2015;313:695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ficicioglu C Review of miglustat for clinical management in Gaucher disease type 1. Ther Clin Risk Manag. 2008;4: 425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abian O, Alfonso P, Velazquez-Campoy A, Giraldo P, Pocovi M, Sancho J. Therapeutic strategies for Gaucher disease: miglustat (NB-DNJ) as a pharmacological chaperone for glucocerebrosidase and the different thermostability of velaglucerase alfa and imiglucerase. Mol Pharm. 2011;8:2390–7. [DOI] [PubMed] [Google Scholar]
- 35.Eng CM, Fletcher J, Wilcox WR, Waldek S, Scott CR, Sillence DO, et al. Fabry disease: baseline medical characteristics of a cohort of 1765 males and females in the Fabry Registry. J Inherit Metab Dis. 2007;30:184–92. [DOI] [PubMed] [Google Scholar]
- 36.Eitzman DT, Bodary PF, Shen YC, Khairallah CG, Wild SR, Abe A, et al. Fabry disease in mice is associated with age-dependent susceptibility to vascular thrombosis. J Am Soc Nephrol. 2003;14:298–302. [DOI] [PubMed] [Google Scholar]
- 37.Bodary PF, Shen YS, Vargas FB, Bi XM, Ostenso KA, Gu SF, et al. alpha-Galactosidase A deficiency accelerates atherosclerosis in mice with apolipoprotein E deficiency. Circulation. 2005;111:629–32. [DOI] [PubMed] [Google Scholar]
- 38.Park JL, Whitesall SE, D’Alecy LG, Shu LM, Shayman JA. Vascular dysfunction in the alpha-galactosidase A-knockout mouse is an endothelial cell-, plasma membrane-based defect. Clin Exp Pharmacol Physiol. 2008;35:1156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shu LM, Murphy HS, Cooling L, Shayman JA. An in vitro model of Fabry disease. J Am Soc Nephrol. 2005;16:2636–45. [DOI] [PubMed] [Google Scholar]
- 40.Shu L, Shayman JA. Caveolin-associated accumulation of globotriaosylceramide in the vascular endothelium of alpha-galactosidase A null mice. J Biol Chem 2007;282:20960–7. [DOI] [PubMed] [Google Scholar]
- 41.Shu L, Shayman JA. Glycosphingolipid mediated caveolin-1 oligomerization. J Glycomics Lipidomics. 2012;S2:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heinecke JW. Oxidized amino acids: culprits in human atherosclerosis and indicators of oxidative stress. Free Radic Biol Med 2002;32:1090–101. [DOI] [PubMed] [Google Scholar]
- 43.Shu L, Vivekanandan-Giri A, Pennathur S, Smid BE, Aerts JM, Hollak CE, et al. Establishing 3-nitrotyrosine as a biomarker for the vasculopathy of Fabry disease. Kidney Int. 2014;86:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aerts JM, Groener JE, Kuiper S, Donker-Koopman WE, Strijland A, Ottenhoff R, et al. Elevated globotriaosylsphingo-sine is a hallmark of Fabry disease. Proc Natl Acad Sci U S A 2008;105:2812–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guerard N, Zwingelstein C, Dingemanse J. Lucerastat, an iminosugar for substrate reduction therapy: pharmacokinetics, tolerability, and safety in subjects with mild, moderate, and severe renal function impairment. J Clin Pharmacol. 2017;57: 1425–31. [DOI] [PubMed] [Google Scholar]
- 46.Marshall J, Sun Y, Bangari DS, Budman E, Park H, Nietupski JB, et al. CNS-accessible inhibitor of glucosylceramide synthase for substrate reduction therapy of neuronopathic Gaucher disease. Mol Ther 2016;24:1019–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bijl N, Sokolovic M, Vrins C, Langeveld M, Moerland PD, Ottenhoff R, et al. Modulation of glycosphingolipid metabolism significantly improves hepatic insulin sensitivity and reverses hepatic steatosis in mice. Hepatology. 2009;50:1431–41. [DOI] [PubMed] [Google Scholar]
- 48.Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab. 2012;15:585–94. [DOI] [PubMed] [Google Scholar]
- 49.Tagami S, Inokuchi Ji J, Kabayama K, Yoshimura H, Kitamura F, Uemura S, et al. Ganglioside GM3 participates in the pathological conditions of insulin resistance. J Biol Chem 2002;277:3085–92. [DOI] [PubMed] [Google Scholar]
- 50.Kabayama K, Sato T, Saito K, Loberto N, Prinetti A, Sonnino S, et al. Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. Proc Natl Acad Sci U S A 2007;104:13678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamashita T, Hashiramoto A, Haluzik M, Mizukami H, Beck S, Norton A, et al. Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc Natl Acad Sci U S A 2003;100:3445–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao HM, Przybylska M, Wu IH, Zhang JH, Maniatis P, Pacheco J, et al. Inhibiting glycosphingolipid synthesis ameliorates hepatic steatosis in obese mice. Hepatology. 2009;50: 85–93. [DOI] [PubMed] [Google Scholar]
- 53.Aerts JM, Ottenhoff R, Powlson AS, Grefhorst A, van Eijk M, Dubbelhuis PF, et al. Pharmacological inhibition of glucosylceramide synthase enhances insulin sensitivity. Diabetes. 2007;56:1341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shayman JA, Kelly R, Kollmeyer J, He Y, Abe A. Group XV phospholipase A, a lysosomal phospholipase A. Prog Lipid Res 2011;50:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Subathra M, Korrapati M, Howell LA, Arthur JM, Shayman JA, Schnellmann RG, et al. Kidney glycosphingolipids are elevated early in diabetic nephropathy and mediate hypertrophy of mesangial cells. Am J Physiol Renal Physiol 2015;309: F204–F215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamaguchi T, Wallace DP, Magenheimer BS, Hempson SJ, Grantham JJ, Calvet JP. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem 2004;279:40419–30. [DOI] [PubMed] [Google Scholar]
- 57.Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet 2005;37:537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quarmby LM, Parker JD. Cilia and the cell cycle?. J Cell Biol 2005;169:707–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Natoli TA, Smith LA, Rogers KA, Wang B, Komarnitsky S, Budman Y, et al. Inhibition of glucosylceramide accumulation results in effective blockade of polycystic kidney disease in mouse models. Nat Med 2010;16:788–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Natoli TA, Husson H, Rogers KA, Smith LA, Wang B, Budman Y, et al. Loss of GM3 synthase gene, but not sphingosine kinase 1, is protective against murine nephronophthisis-related polycystic kidney disease. Hum Mol Genet 2012;21:3397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pennathur S, Wagner JD, Leeuwenburgh C, Litwak KN, Heinecke JW. A hydroxyl radical-like species oxidizes cynomolgus monkey artery wall proteins in early diabetic vascular disease. J Clin Invest 2001;107:853–60. [DOI] [PMC free article] [PubMed] [Google Scholar]