Abstract
Immunotherapy may be an effective way to prevent postoperative recurrence of renal cell carcinoma. Streptavidin‐interleukin‐2 (SA‐IL‐2) surface‐modified tumor cell vaccine developed through our protein‐anchor technology could induce specific antitumor T‐cell responses, but this immunotherapy cannot completely eradicate the tumor. These effector T cells highly expressed programmed death receptor‐1 (PD‐1), and the expression of programmed death ligand‐1 (PD‐L1) in the tumor environment also was upregulated after SA‐IL‐2‐modified vaccine therapy. PD‐1/PD‐L1 interaction promotes tumor immune evasion. Adding PD‐1 blockade to SA‐IL‐2‐modified vaccine therapy increased the number of CD4+, CD8+ and CD8+interferon‐γ+ but not CD4+Foxp3+ T cells. PD‐1 blockade could rescue the activity of tumor‐specific T lymphocytes induced by the SA‐IL‐2‐modified vaccine. Combination therapy delayed tumor growth and protected mice against a second Renca cells but not melanoma cells challenge. Taken together, PD‐1 blockade could reverse immune evasion in the treatment with SA‐IL‐2‐modified vaccine, and eventually induce a stronger specific antitumor immune response against renal cell carcinoma.
Keywords: immunotherapy, interleukin 2, programmed death receptor‐1, renal cell carcinoma, vaccine
Abbreviations
- DC
dendritic cell
- hPBL
human peripheral blood lymphocyte
- IFN
interferon
- IL‐2
interleukin‐2
- PD‐1
programmed death receptor‐1
- PD‐L1
programmed death ligand‐1
- RCC
renal cell carcinoma
- SA
streptavidin
- TME
tumor microenvironment
- Tregs
regulatory T cells
1. INTRODUCTION
Renal cell carcinoma is one of the most common urological neoplasms, accounting for 2%‐3% of all adult malignancies.1 Treatment options currently applied for RCC include surgery and systemic treatment with a combination of drugs.2 Recurrent disease after radical nephrectomy or nephron‐sparing surgery is a serious clinical problem in the treatment of RCC.3 Effective therapies are needed to reduce the recurrence rate of RCC. Cytokines (i.e. IL‐2, IFN‐α) have been largely used to prevent recurrence in the past few years.4 However, decreased frequencies of circulating myeloid and dendritic cells may partly limit the therapeutic efficacy.5 Long‐term complete responders are rare, so non‐specific immunotherapy is rarely used nowadays.6
At present, therapeutic vaccines have been extensively used to generate endogenous tumor‐reactive T cells for cancer treatment.7, 8 As a result of the weak immunogenicity of tumor cells, cytokines with immunopotentiating effects are widely used for immunotherapy of tumors.9, 10 We have developed a technology that immobilizes SA‐tagged bioactive cytokines on the surface of biotinylated tumor cells to prepare tumor cell vaccines. This type of therapeutic vaccine not only maintains the biological activity of the cytokine but also contains all tumor antigens.11, 12, 13 This type of therapeutic vaccine can induce specific antitumor T‐cell responses, which could significantly delay tumor growth. However, this immunotherapy could not completely eradicate the tumor, and the therapeutic effect was usually restricted to the early stage of tumor growth. The limited therapeutic effect of this vaccination approach might be because vaccine‐generated T cells become dysfunctional when they infiltrate into the TME. Immunity decreases with tumor growth because of tumor immune evasion existing in the TME.14 Immune checkpoint blockade is a major advance in clinical immunotherapy. Blockade of PD‐1/PD‐L1 has shown therapeutic efficacy in the treatment of several advanced malignancies.15, 16
Programmed death receptor‐1/PD‐L1 signaling plays a pivotal role in tumor immune evasion.17 PD‐1 mainly expresses on activated T cells. PD‐L1 expresses on tumor cells, allophycocyanin‐streptavidin(APC), B cells, and parenchymal cells. PD‐1/PD‐L1 interaction inhibits antitumor immune response by blunting the functions of T cells or inducing T‐cell death.18 Several studies have reported a high level of PD‐L1 expression in RCC.19 PD‐L1 expression in the TME is correlated with a poor prognosis.20 Anti‐PD‐1 antibody has a specific therapeutic effect in treating RCC by rescuing dysfunctional T cells. However, anti‐PD‐1 monotherapy could not induce a specific antitumor immune response.21, 22 Cancer vaccine‐based immunotherapy is a potential strategy to activate effector T‐cell trafficking into the TME.
As a T‐cell growth factor, IL‐2 has been largely used to treat RCC in the past few years.23 This study showed that SA‐IL‐2 surface‐modified whole tumor cell vaccine could enhance tumor‐specific cytotoxic T‐lymphocyte activity and antitumor responses in vivo. However, PD‐1/PD‐L1 signaling limited the therapeutic efficacy of SA‐IL‐2‐modified vaccine. Therefore, we speculated that PD‐1 blockade combined with tumor vaccine might hold greater potential for eliciting a strong specific immune response. In the present study, we evaluated the efficacy of a combination therapy involving the SA‐IL‐2 surface‐modified tumor cells vaccine and PD‐1 blockade in a RCC mouse model. This preclinical study may provide some experimental basis for applying this type of combination therapy in the treatment of human RCC.
2. MATERIALS AND METHODS
2.1. Animals and cells
Mouse renal tumor cell line Renca and mouse melanoma cell line B16‐F10 (obtained from the American Type Culture Collection) were stored at our laboratory. Cell lines were authenticated every 6‐12 months. Renca cells were cultured in RPMI1640 (containing 10% FBS, 1% penicillin/streptomycin and 0.1% sodium pyruvate) in a 5% CO2 humidified incubator. MycoplasmaOUT (Genloci, Nanjing, China) was used to protect the cells against mycoplasma contamination. SA‐IL‐2 and SA‐GFP fusion proteins were prepared by our laboratory. BALB/C mice were purchased from the experimental animal center of Southern Medical University (Guangzhou, China). All animal experiments were carried out in accordance with relevant laws and institutional guidelines (Ethical number: L2016152).
2.2. Vaccine preparation
We used 30% ethanol (v/v) to inactivate Renca cells, and this method could maintain cell shape and size.24 Ethanol‐fixed Renca cells were incubated with EZ‐Link Sulfo‐NHS‐LC‐Biotin (Pierce, Rockford, IL, USA) for 1 hour at room temperature. The biotinylated Renca cells were then incubated with SA‐IL‐2 fusion protein (100 ng/106 cells) for 1 hour. The Renca cell anchored SA‐IL‐2 protein is a whole tumor cell vaccine.
To evaluate the anchoring efficiency of SA‐IL‐2 protein, the Renca cell anchored SA‐IL‐2 protein was stained with phycoerythrin (PE)‐labeled anti‐IL‐2 (eBioscience, San Diego, CA, USA) for 30 minutes. Flow cytometry was used to detect the SA‐IL‐2 protein anchored on the surface of Renca cells.
2.3. Biological activity of SA‐IL‐2 anchored on the surface of Renca cells
The SA‐IL‐2‐modified Renca cell vaccine was collected and lysed by three cycles of freezing and thawing. Membrane fractions were harvested and resuspended in complete medium. Bioactivity of SA‐IL‐2 protein anchored on the surface of Renca cells was assessed by hPBL proliferation. SA‐GFP was set as the negative control and recombinant IL‐2 was set as the positive control. Different concentrations of cell membrane fractions were added to 1 × 104 hPBL in each well, and then incubated in a 5% CO2 humidified incubator for 3 days. Then, 20 μL MTT (5 mg/mL) was added to each well for 4 hours and 200 μL DMSO was used to terminate the reaction. Optical density (OD) at 570 nm was read on a microplate reader.
2.4. Immunotherapy with SA‐IL‐2‐modified Renca cell vaccine
A total of 2 × 106 Renca cells suspended in 100 μL PBS were injected s.c. into the hind leg of BALB/C mice (6 weeks) to established a subcutaneous model. Mice were then randomly assigned to four groups (anchored IL‐2 vaccine group; soluble SA‐IL‐2 group; ethanol‐fixed Renca cell group; and PBS group). For the anchored IL‐2 vaccine group, mice were injected s.c. with SA‐IL‐2 surface‐modified vaccine (1 × 106 cells/100 μL) on days 0, 4, 8 and 12 after tumor injection. For the other three groups, the mice were injected s.c. with soluble SA‐IL‐2 (1 ng/100 μL), ethanol‐fixed Renca cells (1 × 106 cells/100 μL), and PBS (100 μL) on days 0, 4, 8 and 12 after tumor injection, respectively.
2.5. Programmed death ligand‐1 expression and PD‐1+CD8+ T‐cell infiltration after SA‐IL‐2‐modified vaccine therapy
On day 19 after tumor injection, tumor tissues were collected from each group. The frozen sections were fixed in cold acetone for 15 minutes and incubated with rabbit anti‐mouse PD‐L1 (eBioscience) or rabbit anti‐mouse PD‐1 (eBioscience) + rat anti‐mouse CD8 (eBioscience) for 1 hour. Slides were washed with TBST for 5 minutes and secondary Abs (Alexa Fluor 647‐labeled goat anti‐rabbit Abs and/or Alexa Fluor 488‐labeled goat anti‐rat Abs; eBioscience) were added and incubated for 30 minutes. Slides were washed with TBST for 10 minutes and mounted with a Vectashield DAPI containing kit (Sigma, Saint Louis, MO, USA). PD‐1+CD8+ T cells and expression of PD‐L1 in the TME were then observed through a fluorescence microscope (Eclipse E800; Nikon, Tokyo, Japan).
2.6. Combination therapy with SA‐IL‐2‐modified vaccine and PD‐1 blockade
A subcutaneous model was established by injecting 2 × 106 Renca cells suspended in 100 μL PBS into the hind leg of BALB/C mice (6 weeks). Mice were then randomly assigned to four groups (IgG group; anti‐PD‐1 group; anchored IL‐2 + IgG group; and anchored IL‐2 + anti‐PD‐1 group [combined group]). Mice in the combined group were injected s.c. with SA‐IL‐2‐modified vaccine (1 × 106 cells/100 μL) and injected i.p. with anti‐PD‐1 antibody (100 μg) on days 0, 4, 8 and 12 after tumor injection. For the other three control groups, mice were injected s.c. or injected i.p. with IgG (100 μg), anti‐PD‐1 (100 μg) or SA‐IL‐2 surface‐modified Renca cell vaccine (1 × 106 cells/100 μL) + IgG (100 μg), respectively. All mice were maintained on a 12‐hour light/dark cycle and provided with free access to feed and water. During the observation, tumor volume was recorded. Tumor specimens were collected and tumor weight was evaluated.
2.7. Specific immune response induced by combination therapy
Splenocytes were collected from the mice of each group on day 19 after tumor challenge. The splenocytes were cultured with inactivated Renca cells plus 20 U/mL hIL‐2 (R&D Systems, Minneapolis, MN, USA) for 5 days. The effector cells (splenocytes) and target cells (Renca cells) were cocultured in a 96−well plate at the desired E:T ratios (number of effector cells : number of target cells). The supernatant was collected after 4 hours of incubation. CytoTox 96 non‐radioactive cytotoxicity assay (Promega, Madison, WI, USA) was used to measure lactate dehydrogenase (LDH) content to assess the cytotoxicity of tumor‐specific CTL. CTL was calculated as follows:
To determine the specific immunity against Renca cells in vivo on day 60, the surviving mice in the combined group were injected s.c. with 2 × 106 Renca cells (in the left hind leg) and melanoma cells (in the right hind leg), and tumor volume was recorded.
2.8. Flow cytometry analysis of T‐cell subsets after combination therapy
To determine the therapeutic effect of the SA‐IL‐2‐modified vaccine together with PD‐1 blockade on T‐cell subsets, 100 μL blood was collected from each group on day 19 after tumor injection. To detect CD4+ and CD8+ T‐cell subsets, cells were stained with FITC‐labeled anti‐mCD4 and APC‐labeled anti‐mCD8 (eBioscience). To detect IFN‐γ+CD8+, PD‐1+CD8+ and CD4+Foxp3+ T‐cell subsets, cells were first stained with APC‐labeled anti‐mCD8 or FITC‐labeled anti‐mCD4 (Biolegend, San Diego, CA, USA), and then stained with PE‐labeled anti‐IFN‐γ, PE‐labeled anti‐PD‐1 or PE‐labeled anti‐FoxP3 antibody (Biolegend). T‐cell subsets were detected by flow cytometry.
2.9. Immunohistochemical analyses for tumor‐infiltrating T lymphocytes
Tumor specimens were collected from each group on day 19 after tumor injection. All specimens were embedded in freezing medium. The frozen tissue sections were fixed in cold acetone for 15 minutes, and then incubated with primary antibody (anti‐mCD4 [Bioworld, New Brunswick, NJ, USA] or anti‐mCD8 [Epitomics, Burlingame, CA, USA]) for 1 hour. CD4+ or CD8+ T lymphocytes were visualized using the anti‐rat Ig SABC kit (Spring Bioscience, Pleasanton, CA, USA). The number of CD4+ and CD8+ T lymphocytes were counted through a microscope (Eclipse E800; Nikon).
2.10. Concentrations of IFN‐γ, IL‐4, IL‐10 and IL‐12 after combination therapy
Peripheral blood was collected from each group on day 19 after tumor injection. The blood was congealed at room temperature for 20 minutes. The supernatant was collected by centrifugation at 1006.2 g for 5 minutes. ELISA (R&D Systems) was used to measure the concentrations of IFN‐γ, IL‐4, IL‐10 or IL‐12.
2.11. Upregulation of PD‐L1 expression induced by IFN‐γ
To determine the role of IFN‐γ in upregulating PD‐L1 expression, Renca cells were cultured in the presence of IFN‐γ (500 IU/mL) for 72 hours. Cells were then stained with PE‐labeled anti‐mPD‐L1, and mean fluorescence intensity was measured by flow cytometry.
2.12. Effect of PD‐1 blockade on tumor‐specific cytotoxic activity
Splenocytes were collected from mice in the SA‐IL‐2‐modified vaccine group on day 19 after tumor injection. PD‐1+CD8+ T‐cell subset was sorted by magnetic beads. The PD‐1+CD8+ T cells served as effector cells (1 × 105 cells/mL in RPMI‐1640 medium). Renca cells cultured with IFN‐γ for 72 hours served as target cells (1 × 104/mL in RPMI‐1640 medium). Anti‐mPD‐1 antibody (eBscience) was used to block PD‐1, with isotype IgG antibody as a control. CTL was assessed by the CytoTox 96 non‐radioactive cytotoxicity assay (Promega).
2.13. Statistics
FACS, ELISA, immunohistochemistry, and immunofluorescence data were analyzed using one‐way ANOVA. Repeated measures analysis was used to compare the tumor volume among groups. All analyses were carried out using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA), with P < .05 considered indicative of statistical significance.
3. RESULTS
3.1. Anchoring efficiency and bioactivity of SA‐IL‐2 protein
Results of flow cytometry assay showed that 99.2% ethanol‐fixed Renca cells were anchored with SA‐IL‐2 fusion protein (Figure 1A). Moreover, SA‐IL‐2 fusion protein anchored on the surface of Renca cells still maintained its biological activity in a dosage‐dependent way (Figure 1B).
Figure 1.
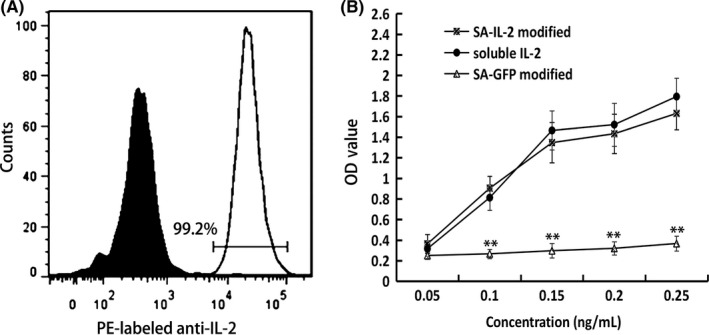
Analysis of SA‐IL‐2 protein anchored on the surface of Renca cells. A, Flow cytometry was used to assay the presence of SA‐IL‐2 anchored on the surface of Renca cells, with biotinylated Renca cells as a negative control. B, Biological activity of SA‐IL‐2 immobilized on the surface of Renca cells was assessed. SA‐GFP was the negative control and soluble IL‐2 was the positive control. All the experiments were replicated at least three times (**P < .01). IL, interleukin; OD, optical density; PE, phycoerythrin
3.2. Programmed death ligand‐1 expression and PD‐1+CD8+ T‐cell infiltration in TME after SA‐IL‐2‐modified vaccine therapy
As shown in Figure 2, expression of PD‐L1 (red fluorescence) in the TME was upregulated after SA‐IL‐2‐modified vaccine therapy. Although more CD8+ T cells (green fluorescence) infiltrated into the TME in the SA‐IL‐2‐modified vaccine group than that in the other groups (P < .05), the number of PD‐1+CD8+ T cells (yellow fluorescence) also increased (P < .05), which eventually resulted in immune evasion.
Figure 2.
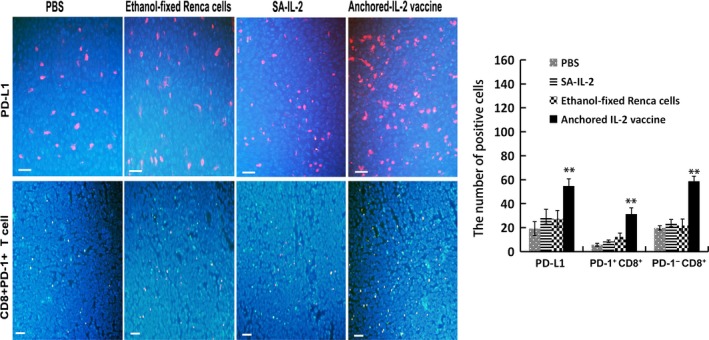
Programmed death receptor‐1 (PD‐1)/programmed death ligand‐1 (PD‐L1) signaling exists in the tumor microenvironment after interleukin (IL)‐2‐modified vaccine therapy. Immunofluorescence assays were carried out to evaluate PD‐L1 expression and infiltration of PD‐1+ CD8+ T cells in the tumor tissues. DAPI (blue fluorescence) stains the cell nucleus, and tumor cells are presented as blue fluorescence. Red fluorescence represents PD‐L1 positive cells, green fluorescence represents CD8+ T cells, and yellow fluorescence represents PD‐1+ CD8+ T cells (Bar, 200 μm). Results of statistical analyses showed significant differences in the number of positive cells (n = 15). All the experiments were replicated at least three times (**P < .01)
3.3. Programmed death receptor‐1 blockade enhanced therapeutic efficacy of SA‐IL‐2‐modified vaccine
We examined whether blocking PD‐1 could augment the antitumor activity of the SA‐IL‐2‐modified vaccine. According to the results, combination therapy with the SA‐IL‐2‐modified vaccine and PD‐1 blockade induced a stronger antitumor response, which significantly delayed tumor growth (P < .05; Figure 3A) and reduced wet tumor weight (P < .05; Figure 3B).
Figure 3.
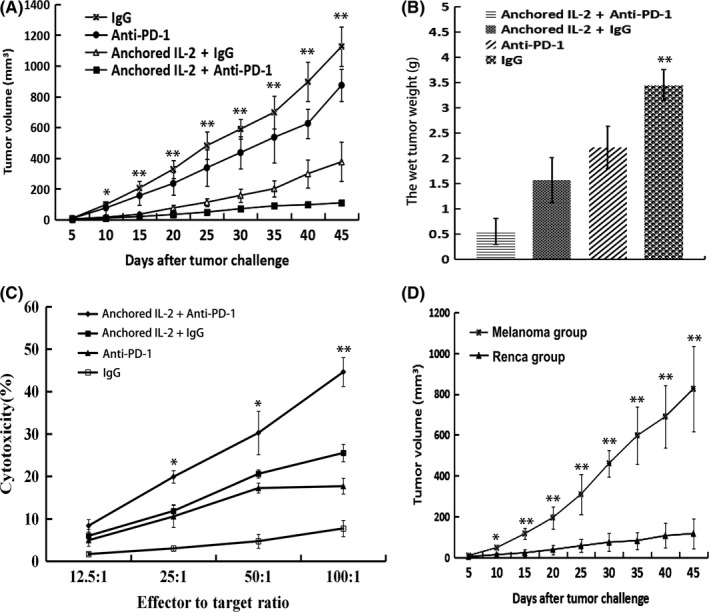
Therapeutic efficacy of combination therapy involving SA‐IL‐2‐modified vaccine and programmed death receptor‐1 (PD‐1) blockade. A, Combination therapy effectively delayed tumor growth compared with the SA‐IL‐2‐modified vaccine or anti‐PD‐1 alone (n = 15). B, Wet tumor weight of each treated group. C, Combination therapy induced the strongest tumor‐specific cytotoxic activities against Renca cells. D, On day 60 after tumor injection, surviving mice in the combined group were injected s.c. with 2 × 106 Renca cells (in the left hind leg) and melanoma cells (in the right hind leg), and tumor volume was recorded (n = 7). All experiments were replicated at least three times (*P < .05, **P < .01). IL, interleukin
In the tumor‐specific lymphocyte cytotoxicity assays, PD‐1 blockade could enhance the tumor‐specific cytotoxic activity induced by the SA‐IL‐2‐modified vaccine (Figure 3C). Moreover, the SA‐IL‐2‐modified vaccine combined with PD‐1 blockade could effectively protect mice against a second set of Renca cells but not a melanoma cells challenge (Figure 3D), which also showed that PD‐1 blockade combined with the SA‐IL‐2‐modified vaccine could effectively enhance tumor‐specific immune response.
3.4. T‐cell subsets after combination therapy with SA‐IL‐2‐modified vaccine and PD‐1 blockade
As shown in Figure 4A,B, the proportions of different T‐cell subsets were measured by flow cytometery. Both the proportions of CD4+ and CD8+ T cells in the combined group were the highest among the four groups. To further evaluate the effectiveness of combination therapy in enhancing immune responses, the proportions of IFN‐γ+CD8+, PD‐1+CD8+ and CD4+Foxp3+ T‐cell subsets of each group were also detected. The results showed that the proportion of IFN‐γ+CD8+ T cells in the combined group was the highest among the four groups, suggesting that the SA‐IL‐2‐modified vaccine combined with PD‐1 blockade promoted the production of IFN‐γ in CD8+ T cells. SA‐IL‐2‐modified vaccine increased the percentage of CD4+Foxp3+ T cells (Tregs), but adding PD‐1 blockade to the SA‐IL‐2‐modified vaccine decreased the percentage of Tregs relative to that of SA‐IL‐2‐modified vaccine monotherapy. Although the combined group had more CD8+ T cells than other groups, the proportions of PD‐1+CD8+ T cells also increased. Therefore, the tumor‐specific CD8+ T‐cell response generated by the SA‐IL‐2‐modified vaccine requires enhancement by blocking PD‐1/PD‐L1.
Figure 4.
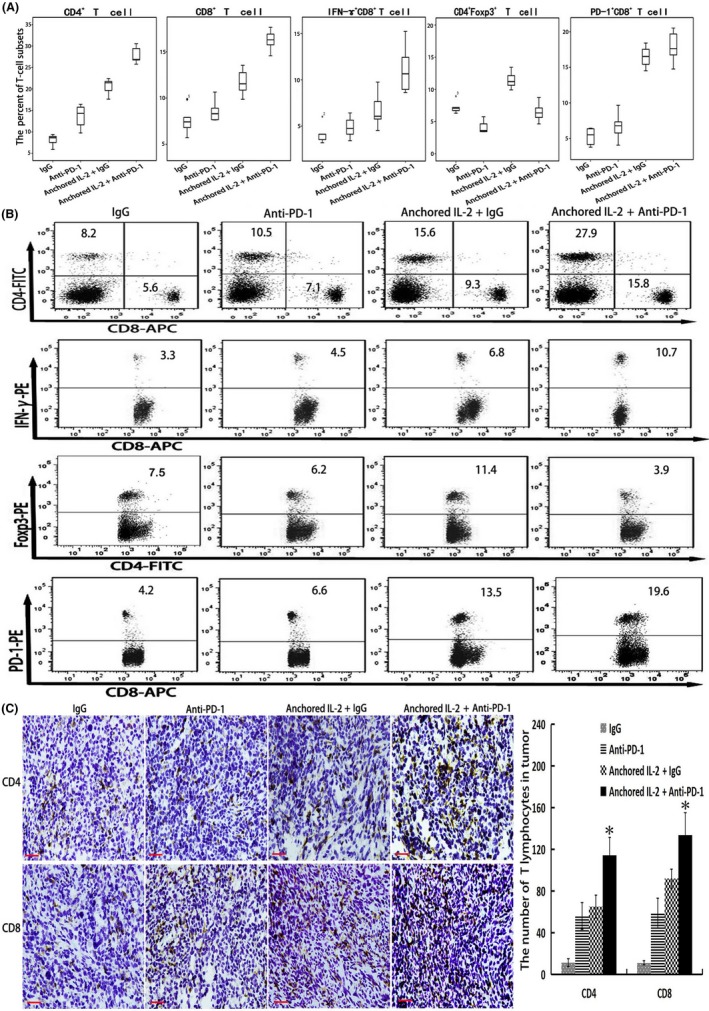
Analysis of T‐cell subsets after combination therapy involving SA‐IL‐2‐modified vaccine and programmed death receptor‐1 (PD‐1) blockade. Flow cytometry or immunohistochemistry was used to detect T‐cell subsets. A, Statistical results of proportions of T‐cell subsets (CD4+ T cells, CD8+ T cells, CD8+ IFN‐γ+ T cells, CD8+ PD‐1+ T cells and CD4+Foxp3+ T cells) in the blood (n = 15). B, Representative results for evaluating differences in the proportions of T‐cell subsets of each group (n = 15). C, Immunohistochemistry was carried out to evaluate tumor‐infiltrating CD4+ and CD8+ T cells in the tumor microenvironment of each group (n = 15; bar, 200 μm). All the experiments were replicated at least three times (*P < .05). IL, interleukin
In addition, the results of immunohistochemistry showed that the number of tumor‐infiltrating CD4+ or CD8+ T cells in the combined group was the highest among the four groups (Figure 4C), suggesting that PD‐1 blockade could rescue the tumor‐infiltrating T lymphocytes generated by SA‐IL‐2‐modified vaccine.
3.5. Concentrations of IFN‐γ, IL‐4, IL‐10 and IL‐12 after combination therapy
Certain cytokines in vivo may reflect immune status. As shown in Figure 5, the combined group had a higher concentration of IFN‐γ than either the SA‐IL‐2‐modified vaccine or PD‐1 blockade alone. Moreover, the combined group displayed the highest concentration of IL‐12 (P < .05) but the lowest concentration of IL‐10 among the four groups (P < .05). No significant difference in the concentration of IL‐4 was found among the four groups (P > .05).
Figure 5.
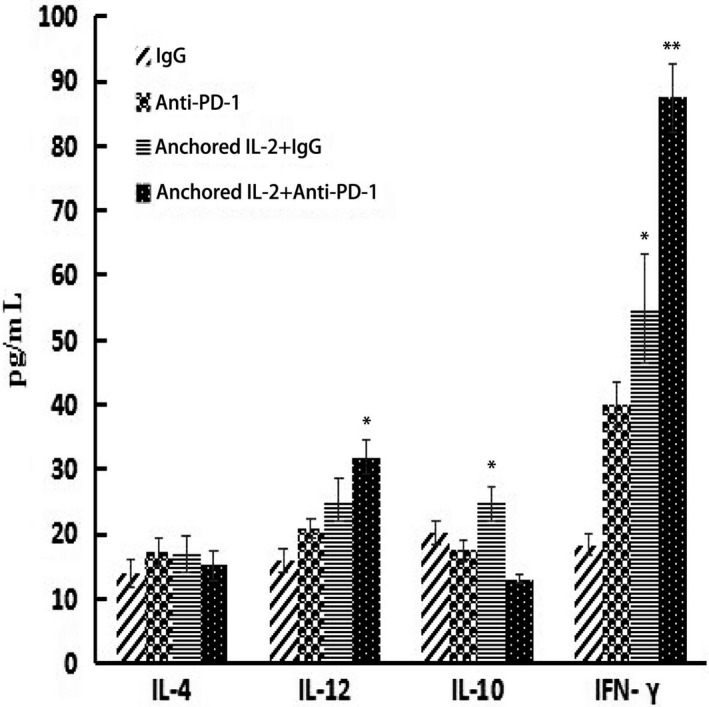
Concentrations of interferon (IFN)‐γ, interleukin (IL)‐4, IL‐10 and IL‐12 after combination therapy. Sera were collected from the mice of each group on day 19 after tumor injection. ELISA was used to measure IFN‐γ, IL‐4, IL‐10 and IL‐12 (n = 15). All the experiments were replicated at least three times (*P < .05, **P < .01)
3.6. Interferon‐γ upregulated PD‐L1 expression of tumor cells
To better study the effect of IFN‐γ on the PD‐L1 expression of tumor cells, we examined the PD‐L1 expression of tumor cells after culturing with IFN‐γ for 72 hours. Results showed that the PD‐L1 expression of tumor cells was significantly upregulated (P < .05; Figure 6A), supporting the important role of IFN‐γ in promoting PD‐L1 expression.
Figure 6.
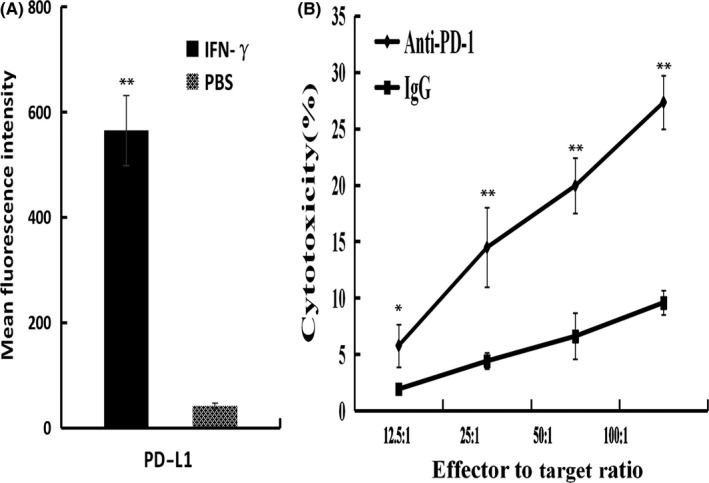
Possible mechanism by which programmed death receptor‐1 (PD‐1) blockade rescues tumor‐specific T lymphocytes induced by SA‐IL‐2‐modified vaccine. A, Renca cells were cultured with or without interferon (IFN)‐γ for 72 h, and programmed death ligand‐1 (PD‐L1) expression of tumor cells was measured by flow cytometry. B, PD‐1+ CD8+ T cells isolated from mice treated with the SA‐IL‐2‐modified vaccine served as effector cells, and the Renca cells expressing PD‐L1 served as target cells. CTL was carried out with or without anti‐PD‐1 antibody. All the experiments were replicated at least three times (*P < .05, **P < .01). IL, interleukin
3.7. Programmed death receptor‐1 blockade enhanced tumor‐specific cytotoxic activity
To test whether PD‐1 blockade can augment specific antitumor activity, PD‐1+CD8+ T cells isolated from mice in the SA‐IL‐2‐modified vaccine group served as effector cells, and the Renca cells that expressed PD‐L1 served as target cells. Both effector cells and target cells were cultured with or without anti‐PD‐1 antibody. Results showed that the tumor‐specific cytotoxic activity induced by SA‐IL‐2‐modified vaccine therapy was enhanced by PD‐1 blockade (Figure 6B).
4. DISCUSSION
There is currently no effective treatment for recurrence of renal cell carcinoma. In the present study, we report a combination immunotherapy for the treatment of RCC. This type of whole‐tumor‐cell vaccine not only contains all of the tumor‐associated antigens but also maintains the biological activity of cytokine.11, 12, 13 The SA‐IL‐2‐modified vaccine could induce tumor‐specific antitumor response. However, monotherapy is difficult to completely eradicate tumors. Although this vaccination approach can induce unprecedentedly high T‐cell response, the therapeutic effect is usually restricted to the early stage of tumor growth. The tumor microenvironment contains a strong immunosuppressive network.25 Currently, immune escape and immune checkpoint are under intense investigation. Antibodies blocking PD‐1 or PD‐L1 could enhance the antitumor effect by reversing immune evasion.26 In this study, we explored whether blocking the PD‐1/PD‐L1 axis can optimize our strongly immunogenic but moderately efficacious vaccination platform.
Programmed death receptor‐1/PD‐L1 interaction inhibits the function and activity of effector T lymphocytes.27 Upregulation of PD‐L1 in the TME predicts poor prognosis in patients with cancer.28 Blocking the PD‐1/PD‐L1 pathway has shown promising results in several carcinomas.29 In this study, we showed that the TME highly expressed PD‐L1 after tumor vaccine therapy. Treatment with repeated doses of vaccine still could not eradicate the tumor, because T cells become dysfunctional through PD‐1/PD‐L1 interaction. These PD‐1+ T cells produce fewer cytokines and show less proliferation.30 Some published studies have reported the therapeutic efficacy of anti‐PD‐1 monotherapy in RCC.26, 31 Anti‐PD‐1 monotherapy induces limited antitumor efficacy, likely because PD‐1 blockade cannot induce tumor‐specific immune response.32 Although anti‐PD‐1 monotherapy increased the number of CD8+ T cells infiltrating TME, the antitumor effect of anti‐PD‐1 monotherapy was inferior to that of the SA‐IL‐2‐modified vaccine group or combined group. This finding shows that PD‐1/PD‐L1 blockade should be carried out on the basis of the vaccine‐induced effector T‐cell response.
SA‐IL‐2‐modified vaccine treatment increased the number of CD8+ T cells infiltrating into tumor tissues, but these CD8+ T cells also expressed PD‐1. Moreover, CD8+ T cells have the capacity to produce IFN‐γ, which is a potent stimulator of PD‐L1 expression in tumor cells.33 PD‐1/PD‐L1 interaction inhibits the function of CD8+ T cells, which eventually resulted in tumor progression. It is intriguing to explore whether blocking PD‐1/PD‐L1 can overcome this immune evasion. IFN‐γ is a known mediator of PD‐L1 expression in cancer cell lines.34 In the in vitro study, we showed that IFN‐γ could upregulate the PD‐L1 expression in tumor cells. These findings suggest that upregulation of PD‐L1 expression in the TME subsequently results in immune evasion. Therefore, an effective immunotherapy should combine a driver of tumor‐specific immunity with checkpoint blockade. A promising approach to accomplish this would be combining PD‐1/PD‐L1 blockade with a tumor vaccine that can induce tumor‐specific immunity.
The antitumor effect depends mainly on cytotoxic effect by Th1 immune response. The immune system is a very complex network, including Th1 and Th2. Therefore, to evaluate the effect of combination therapy on the immune system, both Th1 and Th2 were evaluated in this experiment. Several cytokines in vivo may reflect immune status, such as IFN‐γ, IL‐12, IL‐10 and IL‐4.23, 34 Combination therapy involving SA‐IL‐2‐modified vaccine and PD‐1 blockade could induce stronger tumor‐specific CD8+ T‐cell and CD4+ T‐cell responses. SA‐IL‐2‐modified vaccine monotherapy increased the proportion of CD4+Foxp3+ T cells. The high frequency of Tregs could decrease the number of mature DC and disrupt the interactions between DC and T cells by direct contact or by changing the immune microenvironment.29 Interestingly, adding PD‐1 blockade to SA‐IL‐2‐modified vaccine therapy could reduce the proportion of CD4+Foxp3+ T cells. Although the combination therapy could increase the number of IFN‐γ‐producing CD8+ T cells, the number of PD‐1+CD8+ T cell also increased at the same time. The tumor‐specific CD8+ T cell response generated by the SA‐IL‐2‐modified vaccine can be enhanced by blocking PD‐1, which could explain why the combination therapy could further inhibit tumor growth. PD‐1 blockade could overcome immune evasion in the treatment with SA‐IL‐2‐modified vaccine.
A number of factors potentially affect the therapeutic effect of vaccines.35 The present study has not evaluated the role of myeloid cells or stromal tissue in the combination therapy with the SA‐IL‐2‐modified vaccine and PD‐1 blockade. Further studies will be needed to evaluate the effect of tumor vaccine combined with blocking other immune checkpoints (anti‐CTLA‐4, anti‐LAG3, anti‐TIM3). These studies can optimize the treatment of RCC in the future.
In conclusion, the SA‐IL‐2‐modified vaccine can induce a tumor‐specific immune response. It is suitable to combine with immune checkpoint blockade. The present study supports the notion that PD‐1 blockade combined with the SA‐IL‐2‐modified vaccine could induce a stronger antitumor immunity than the vaccine or PD‐1 blockade alone. Our future studies will continue to explore related immune mechanisms and the effect of combination therapy with other immune checkpoint blockade. These findings may provide an experimental basis for applying this type of combination therapy in the treatment of human renal cell carcinoma.
CONFLICTS OF INTEREST
Authors declare no conflicts of interest for this article.
ACKNOWLEDGMENT
This study was supported by National Natural Science Foundation of China (No. 81272844).
Zhang X, Shi X, Li J, et al. Combination immunotherapy with interleukin‐2 surface‐modified tumor cell vaccine and programmed death receptor‐1 blockade against renal cell carcinoma. Cancer Sci. 2019;110:31–39. 10.1111/cas.13842
REFERENCES
- 1. Maher ER. Genomics and epigenomics of renal cell carcinoma. Semin Cancer Biol. 2013;23:10‐17. [DOI] [PubMed] [Google Scholar]
- 2. Lane BR, Kattan MW. Predicting outcomes in renal cell carcinoma. Curr Opin Urol. 2005;15:289‐297. [DOI] [PubMed] [Google Scholar]
- 3. Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal cell carcinoma. N Engl J Med. 2013;369:722‐731. [DOI] [PubMed] [Google Scholar]
- 4. Passalacqua R, Caminiti C, Buti S, et al. Adjuvant low‐dose interleukin‐2 (IL‐2) plus interferon‐α (IFN‐α) in operable renal cell carcinoma (RCC): a phase III, randomized, multicentre trial of the Italian Oncology Group for Clinical Research (GOIRC). J Immunother. 2014;37:440‐447. [DOI] [PubMed] [Google Scholar]
- 5. Kruck S, Bedke J, Kuczyk MA, et al. Second‐line systemic therapy for the treatment of metastatic renal cell cancer. Expert Rev Anticancer Ther. 2012;12:777‐785. [DOI] [PubMed] [Google Scholar]
- 6. Atkins M, Regan M, McDermott D, et al. Carbonic anhydrase IX expression predicts outcome of interleukin 2 therapy for renal cancer. Clin Cancer Res. 2005;11:3714‐3721. [DOI] [PubMed] [Google Scholar]
- 7. Moschini M, Sharma V, Delloglio P, et al. Comparing long‐term outcomes of primary and progressive carcinoma invading bladder muscle after radical cystectomy. BJU Int. 2016;117:604‐610. [DOI] [PubMed] [Google Scholar]
- 8. Kawashima H, Obayashi A, Kawamura M, et al. Galectin 9 and PINCH, novel immunotherapy targets of renal cell carcinoma: a rationale to find potential tumour antigens and the resulting cytotoxic T lymphocytes induced by the derived peptides. BJU Int. 2014;113:320‐332. [DOI] [PubMed] [Google Scholar]
- 9. Fisher SA, Aston WJ, Chee J, et al. Transient Treg depletion enhances therapeutic anti‐cancer vaccination. Immun Inflamm Dis. 2016;5:16‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Singh NP, Yolcu ES, Taylor DD, et al. A novel approach to cancer immunotherapy: tumor cells decorated with CD80 generate effective antitumor immunity. Cancer Res. 2003;63:4067‐4073. [PubMed] [Google Scholar]
- 11. Galanis E, Vile R, Russell SJ. Delivery systems intended for in vivo gene therapy of cancer: targeting and replication competent viral vectors. Crit Rev Oncol Hematol. 2001;38:177‐192. [DOI] [PubMed] [Google Scholar]
- 12. Shi X, Zhang X, Li J, et al. Sequential administration of GM‐CSF and IL‐2 surface‐modified MB49 cells vaccines against the metastatic bladder cancer. Urol Oncol. 2013;31:883‐893. [DOI] [PubMed] [Google Scholar]
- 13. Zhu YT, Pang SY, Lei CY, et al. Development of a therapy against metastatic bladder cancer using an interleukin‐2 surface‐modified MB49 bladder cancer stem cells vaccine. Stem Cell Res Ther. 2015;6:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang X, Shi X, Li J, et al. Novel immunotherapy for metastatic bladder cancer using vaccine of human interleukin‐2 surface‐modified MB49 cells. Urology. 2011;78:722.e1‐722.e6. [DOI] [PubMed] [Google Scholar]
- 15. Kolacinska A, Cebula‐Obrzut B, Pakula L, et al. Immune checkpoints: cytotoxic T‐lymphocyte antigen 4 and programmed cell death protein‐1 in breast cancer surgery. Oncol Lett. 2015;10:1079‐1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sonpavde G, Pond GR, Mullane S, et al. Outcomes in patients with advanced urothelial carcinoma after discontinuation of programmed death (PD)‐1 or PD ligand‐1 inhibitor therapy. BJU Int. 2017;119:579‐584. [DOI] [PubMed] [Google Scholar]
- 17. Hendriks D, He Y, Koopmans I, et al. Programmed Death Ligand 1 (PD‐L1)‐targeted TRAIL combines PD‐L1‐mediated checkpoint inhibition with TRAIL‐mediated apoptosis induction. Oncoimmunology. 2016;5:e1202390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Q, Wu X. Primary and acquired resistance to PD‐1/PD‐L1 blockade in cancer treatment. Int Immunopharmacol. 2017;46:210‐219. [DOI] [PubMed] [Google Scholar]
- 19. Zhu J, Armstrong AJ, Friedlander TW, et al. Biomarkers of immunotherapy in urothelial and renal cell carcinoma: PD‐L1, tumor mutational burden, and beyond. J Immunother Cancer. 2018;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim KS, Sekar RR, Patil D, et al. Evaluation of programmed cell death protein 1 (PD‐1) expression as a prognostic biomarker in patients with clear cell renal cell carcinoma. Oncoimmunology. 2018;7:e1413519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Botta GP, Granowicz E, Costantini C. Advances on immunotherapy in genitourinary and renal cell carcinoma. Transl Cancer Res. 2017;6:17‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kawakami F, Sircar K, Rodriguez‐Canales J, et al. Programmed cell death ligand 1 and tumor‐infiltrating lymphocyte status in patients with renal cell carcinoma and sarcomatoid dedifferentiation. Cancer. 2017;123:4823‐4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pili R, Quinn DI, Hammers HJ, et al. Immunomodulation by entinostat in renal cell carcinoma patients receiving high‐dose interleukin 2: a multicenter, single‐arm, phase I/II trial (NCI‐CTEP#7870). Clin Cancer Res. 2017;23:7199‐7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malek TR, Yu A, Zhu L, et al. IL‐2 family of cytokines in T regulatory cell development and homeostasis. J Clin Immunol. 2008;28:635‐639. [DOI] [PubMed] [Google Scholar]
- 25. Yin W, He Q, Hu Z, et al. A novel therapeutic vaccine of GM‐CSF/TNF‐alpha surface‐modified RM‐1 cells against the orthotopic prostatic cancer. Vaccine. 2010;28:4937‐4944. [DOI] [PubMed] [Google Scholar]
- 26. Massari F, Santoni M, Ciccarese C, et al. PD‐1 blockade therapy in renal cell carcinoma: current studies and future promises. Cancer Treat Rev. 2015;41:114‐121. [DOI] [PubMed] [Google Scholar]
- 27. Fu J, Malm IJ, Kadayakkara DK, et al. Preclinical evidence that PD1 blockade cooperates with cancer vaccine TEGVAX to elicit regression of established tumors. Cancer Res. 2014;74:4042‐4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hamanishi J, Mandai M, Matsumura N, et al. PD‐1/PD‐L1 blockade in cancer treatment: perspectives and issues. Int J Clin Oncol. 2016;21:462‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hirai M, Kitahara H, Kobayashi Y, et al. Regulation of PD‐L1 expression in a high‐grade invasive human oral squamous cell carcinoma microenvironment. Int J Oncol. 2017;50:41‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rebelatto MC, Midha A, Mistry A, et al. Development of a programmed cell death ligand‐1 immunohistochemical assay validated for analysis of non‐small cell lung cancer and head and neck squamous cell carcinoma. Diagn Pathol. 2016;11:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Albiges L, Fay AP, Xie W, et al. Efficacy of targeted therapies after PD‐1/PD‐L1 blockade in metastatic renal cell carcinoma. Eur J Cancer. 2015;51:2580‐2586. [DOI] [PubMed] [Google Scholar]
- 32. Soares KC, Rucki AA, Wu AA, et al. PD‐1/PD‐L1 blockade together with vaccine therapy facilitates effector T‐cell infiltration into pancreatic tumors. J Immunother. 2015;38:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bellmunt J, Powles T, Vogelzang NJ. A review on the evolution of PD‐1/PD‐L1 immunotherapy for bladder cancer: the future is now. Cancer Treat Rev. 2017;54:58‐67. [DOI] [PubMed] [Google Scholar]
- 34. de Alwis R, Bangs DJ, Angelo MA, et al. Immunodominant Dengue virus‐specific CD8+ T cell responses are associated with a memory PD‐1+ phenotype. J Virol. 2016;90:4771‐4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gannavaram S, Bhattacharya P, Ismail N, et al. Modulation of innate immune mechanisms to enhance Leishmania vaccine‐induced immunity: role of coinhibitory molecules. Front Immunol. 2016;7:187. [DOI] [PMC free article] [PubMed] [Google Scholar]


