Abstract
The prognosis of patients with advanced‐stage lung squamous cell carcinoma (LUSQ) is poor, and effective treatment protocols are limited. Our continuous analyses of antitumor microRNAs (miRNAs) and their oncogenic targets have revealed novel oncogenic pathways in LUSQ. Analyses of our original miRNA expression signatures indicated that both strands of miR‐144 (miR‐144‐5p, the passenger strand; miR‐144‐3p, the guide strand) showed decreased expression in cancer tissues. Additionally, low expression of miR‐144‐5p significantly predicted a poor prognosis in patients with LUSQ by The Cancer Genome Atlas database analyses (overall survival, P = 0.026; disease‐free survival, P = 0.023). Functional assays revealed that ectopic expression of miR‐144‐5p and miR‐144‐3p significantly blocked the malignant abilities of LUSQ cells, eg, cancer cell proliferation, migration, and invasion. In LUSQ cells, 13 and 15 genes were identified as possible oncogenic targets that might be regulated by miR‐144‐5p and miR‐144‐3p, respectively. Among these targets, we identified 3 genes (SLC44A5, MARCKS, and NCS1) that might be regulated by both strands of miR‐144. Interestingly, high expression of NCS1 predicted a significantly poorer prognosis in patients with LUSQ (overall survival, P = 0.013; disease‐free survival, P = 0.048). By multivariate analysis, NCS1 expression was found to be an independent prognostic factor for patients with LUSQ patients. Overexpression of NCS1 was detected in LUSQ clinical specimens, and its aberrant expression enhanced malignant transformation of LUSQ cells. Our approach, involving identification of antitumor miRNAs and their targets, will contribute to improving our understanding of the molecular pathogenesis of LUSQ.
Keywords: lung squamous cell carcinoma, microRNA, miR‐144‐3p, miR‐144‐5p, NCS1
Abbreviations
- Ago2
Argonaute2
- D2R
dopamine D2 receptor
- DFS
disease‐free survival
- GEO
Gene Expression Omnibus
- LUAD
lung adenocarcinoma
- LUSQ
lung squamous cell carcinoma
- MARCKS
myristoylated alanine rich protein kinase C substrate
- miRNA
microRNA
- NCS1
neuronal calcium sensor 1
- NSCLC
non‐small cell lung cancer
- OS
overall survival
- qRT‐PCR
real‐time quantitative RT‐PCR
- RCC
renal cell carcinoma
- RISC
RNA‐induced silencing complex
- siRNA
small interfering RNA
- SLC44A5
solute carrier family 44 member 5
- TCGA
The Cancer Genome Atlas
1. INTRODUCTION
Lung cancer is the leading cause of cancer‐related deaths worldwide among both men and women.1 Lung cancer includes 2 major clinicopathological categories: small cell lung cancer and NSCLC. The latter accounts for approximately 85% of all cases of lung cancer, and NSCLC tumors consist mainly of 3 subtypes: LUAD, LUSQ, and large cell carcinoma.2 In patients with LUAD, currently developed treatment strategies (eg, epidermal growth factor receptor tyrosine kinase inhibitors, inhibitors of anaplastic lymphoma kinase, and immune checkpoint inhibitors) have dramatically improved OS rates in patients.3, 4, 5, 6 In contrast, in patients with LUSQ, the lack of early diagnostic tools and effective treatment protocols has resulted in a poor OS rate in patients with LUSQ.7, 8 Therefore, identification of therapeutic target molecules is essential for achieving improved outcomes in patients with LUSQ.
MicroRNAs are a class of small, noncoding RNAs (19‐22 nt in length). These molecules regulate gene expression by repressing translation or cleaving RNA transcripts in a sequence‐dependent manner.9 Interestingly, a single miRNA species could regulate a vast number of protein‐coding and noncoding RNA transcripts.10 In human disease cells, aberrantly expressed miRNAs trigger the failure of orderly and controlled RNA networks.11 Numerous studies have shown that aberrant expression of miRNAs and dysregulated RNA networks are deeply involved in human diseases, including cancer.12, 13, 14, 15, 16
Through our continuing work, we have identified antitumor miRNAs and their target oncogenic genes in LUSQ.17, 18, 19, 20, 21, 22 Moreover, detailed analyses of our original miRNA expression signatures by RNA sequencing have revealed that passenger strands of miRNAs actually act as antitumor miRNAs and are involved in cancer pathogenesis.23, 24, 25 Additionally, our recent studies showed that both strands of the miR‐144 duplex (ie, the passenger strand miR‐144‐5p and the guide strand miR‐144‐3p) are downregulated in bladder cancer and RCC tissues and act as antitumor miRNAs.26, 27 The involvement of both strands of the miRNA duplex in cancer pathogenesis is a new concept for cancer research.
Several cohort analyses by TCGA database have indicated that low expression of miR‐144‐5p predicts poor prognosis in patients with LUSQ (OS, P = 0.026; DFS, P = 0.023). Thus, both strands of the miR‐144 duplex are involved in LUSQ molecular pathogenesis. Here, we aimed to verify that miR‐144‐5p and miR‐144‐3p possess antitumor functions. We also sought to identify their molecular targets, thereby revealing new details of LUSQ pathogenesis.
2. MATERIALS AND METHODS
2.1. Clinical specimen collection, cell lines, and cell culture
The present study was approved by the Bioethics Committee of Kagoshima University Hospital (Kagoshima, Japan) (approval nos 26‐164). Written prior informed consent and approval were obtained from all patients. In total, 30 LUSQ specimens and 20 noncancerous lung specimens were collected from patients who underwent thoracic surgery at Kagoshima University Hospital from 2010 to 2013. The pathological stages of LUSQ were classified according to the International Association for the Study of Lung Cancer TNM classification, 7th edition.28 The clinicopathological features of the patients are shown in Table 1. The procedure for RNA extraction from formalin‐fixed, paraffin‐embedded specimens was described in previous studies.19
Table 1.
Characteristics of lung cancer and noncancerous cases
| A. Characteristics of lung cancer cases | |
|---|---|
| n (%) | |
| Total number | 30 |
| Median age, years (range) | 71 (50‐88) |
| Sex | |
| Male | 29 (96.7) |
| Female | 1 (3.3) |
| Pathological stage | |
| IA | 5 (16.7) |
| IB | 9 (30.0) |
| IIA | 2 (6.7) |
| IIB | 6 (20.0) |
| IIIA | 7 (23.3) |
| IIIB | 1 (3.3) |
| B. Characteristics of noncancerous cases | |
|---|---|
| n | |
| Total number | 20 |
| Median age, years (range) | 70.5 (50‐88) |
| Sex | |
| Male | 20 |
| Female | 0 |
Pathological stage of lung cancer was classified according to the International Association for the Study of Lung Cancer TNM classification, 7th edition.28
In addition, we evaluated 2 LUSQ cell lines (EBC‐1 and SK‐MES‐1), obtained from the Japanese Cancer Research Resources Bank (Osaka, Japan) and ATCC (Manassas, VA, USA), respectively. Cell culture, extraction of total RNA, and extraction of protein were carried out as described in our earlier reports.18, 19, 21
2.2. Quantitative real‐time RT‐PCR
The procedure for qRT‐PCR has been described previously.18, 22, 29 The expression levels of miRNAs were analyzed by TaqMan qRT‐PCR assays (assay IDs 002148 and 002676; Applied Biosystems, Foster City, CA, USA). Data were normalized to the expression of RNU48 (assay ID 001006; Applied Biosystems). NCS1 expression levels were determined using TaqMan probes and primers (assay ID Hs00179522_m1; Applied Biosystems), and GAPDH (assay ID Hs99999905_m1; Applied Biosystems) was used for normalization.
2.3. Transfection of LUSQ cells with mature miRNA and siRNA
The following mature miRNA species were used in this study: mirVana miRNA mimic, hsa‐miR‑144‑5p (product ID MC12631; Applied Biosystems), hsa‐miR‑144‑3p (product ID MC11051; Applied Biosystems), and negative control miRNA, anti‐miR negative control #1 (catalog no. AM17010; Applied Biosystems). The following siRNAs were used: Stealth Select RNAi siRNA, si‐NCS1 (P/N HSS118732 and HSS118734; Invitrogen, Carlsbad, CA, USA), and negative control miRNA/siRNA, anti‐miR negative control #1 (catalog no. AM17010; Applied Biosystems). The transfection procedures were described previously.18, 20, 22
2.4. Incorporation of miR‐144‐5p and miR‐144‐3p into RISC: Assessment by Ago2 immunoprecipitation
MicroRNAs were transfected into EBC‐1 cells by reverse transfection. After a 48‐hour incubation period, miRNAs were isolated by immunoprecipitation using a microRNA Isolation Kit for Human Ago2 (Wako, Osaka, Japan). We then assessed the expression of Ago2‐conjugated miRNAs by qRT‐PCR, as described in previous studies.30, 31
2.5. Cell proliferation, migration, and invasion assays
Protocols for determining cell proliferation, migration, and invasion were described previously.17, 22
2.6. Identification of putative target genes regulated by miR‐144‐5p and miR‐144‐3p in LUSQ cells
Gene expression analyses by oligo microarray and in silico analyses were used to identify putative target genes regulated by miR‐144‐5p and miR‐144‐3p. The microarray data were deposited in the GEO repository under accession number GSE115801. Putative target genes having binding sites for miR‐144‐5p and miR‐144‐3p were detected by TargetScanHuman version 7.2 (http://www.targetscan.org/vert_72/). The GEO database (GSE19188) was used for assessment of the association between target genes and expression of NSCLC clinical specimens. Identification of miR‐144‐5p and miR‐144‐3p target genes was carried out as described in Figure S1.
2.7. Clinical database analysis
The clinical significance of miRNAs and their target genes in LUSQ was investigated with TCGA database (https://tcga-data.nci.nih.gov/tcga/). The gene expression and clinical data were retrieved from cBioPortal (http://www.cbioportal.org/) and OncoLnc (http://www.oncolnc.org) (data downloaded on April 28, 2018).31, 32, 33
2.8. Plasmid construction and dual‐luciferase reporter assay
Wild‐type or deletion‐type sequences targeted by miR‐144‐5p and miR‐144‐3p were inserted into the psiCHECK‐2 vector (C8021; Promega, Madison, WI, USA).
After cotransfecting miRNA and the constructed vector into EBC‐1 and SK‐MES‐1 cells, firefly and Renilla luciferase activities were measured using a dual Luciferase assay kit (Promega). The procedure was described previously.18, 20, 22
2.9. Western blot analysis and immunohistochemistry
The procedures for western blotting and immunohistochemistry were described previously.21, 22 Membranes were immunoblotted with monoclonal anti‐NCS1 Abs (1:1000 dilution; ab129166; Abcam, Cambridge, UK) and monoclonal anti‐GAPDH Abs (1:20 000 dilution; MAB374; EMD Millipore, Billerica, MA, USA).
Immunohistochemistry was carried out with a VECTASTAIN Universal Elite ABC Kit (catalog no. PK‐6200; Vector Laboratories, Burlingame, CA, USA) according to the manufacturer's protocol. Primary rabbit mAbs against NCS1 (ab129166; Abcam) were used at a 1:100 dilution at 4°C overnight. The characteristics of patients included in the tissue microarray are described in Table 2. The procedure for immunohistochemistry was described previously.18, 22, 34
Table 2.
Characteristics and immunohistochemical status of patients included in tissue microarray analysis
| A. Immunohistochemical status and characteristics of cases with lung squamous cell carcinoma | ||||||
|---|---|---|---|---|---|---|
| Patient no. | Grade | T | N | M | Pathological stage | Immunohistochemical staining intensity |
| 23 | 2 | 2 | 1 | 0 | IIB | (+) |
| 24 | 2 | 2 | 0 | 0 | IB | (+++) |
| 25 | 2 | 1 | 0 | 0 | IA | (+++) |
| 26 | 1 | 2 | 1 | 0 | IIB | (+) |
| 27 | 2 | 1 | 0 | 0 | IA | (++) |
| 28 | 1 | 3 | 0 | 0 | IIB | (++) |
| 29 | 1 | 2 | 0 | 0 | IB | (+++) |
| 30 | 2 | 2 | 0 | 0 | IB | (+++) |
| 31 | 3 | 2 | 0 | 0 | IB | (+++) |
| 32 | 3 | 2 | 1 | 0 | IIB | (+) |
| 33 | 3 | 2 | 0 | 0 | IB | (+) |
| 34 | 3 | 2 | 1 | 0 | IIB | (+++) |
| 35 | 2 | 3 | 1 | 0 | IIIA | (++) |
| 36 | 3 | 2 | 1 | 0 | IIA | (+++) |
| 37 | 3 | 3 | 0 | 0 | IIB | (++) |
| 38 | 3 | 2 | 0 | 0 | IB | (++) |
| 39 | 3 | 2 | 1 | 0 | IIB | (+++) |
| 40 | 3 | 2 | 0 | 0 | IB | (++) |
| 41 | 2‐3 | 3 | 0 | 0 | IIB | (+++) |
| 42 | 3 | 1 | 2 | 0 | IIIA | (+) |
| 43 | 3 | 2 | 0 | 0 | IB | (+) |
| 44 | 3 | 2 | 0 | 0 | IB | (+) |
| B. Immunohistochemical status of noncancerous cases | |
|---|---|
| Patient no. | Immunohistochemical staining intensity |
| 69 | (+) |
| 70 | (−) |
| 71 | (−) |
| 72 | (+) |
| 73 | (+) |
| 74 | (+) |
| 75 | (+) |
| 76 | (++) |
| 77 | (+) |
| 78 | (+) |
| 79 | (+) |
| 80 | (+) |
Pathological stages of lung cancer were classified according to the International Association for the Study of Lung Cancer TNM classification.28
2.10. Statistical analysis
All data were analyzed using SPSS version 23 software (IBM SPSS, Chicago, IL, USA). Differences between 2 groups were analyzed by Mann‐Whitney U tests, and those between multiple groups were examined by one‐way ANOVA and Tukey tests for post‐hoc analysis. Differences between survival rates were analyzed by Kaplan‐Meier survival curves and log‐rank statistics. Univariate and multivariate analyses for OS using TCGA database were carried out by Cox proportional hazards regression analyses.
3. RESULTS
3.1. Downregulation of miR‐144‐5p and miR‐144‐3p in LUSQ clinical specimens and their clinical significance
The expression levels of miR‐144‐5p and miR‐144‐3p were markedly decreased in LUSQ tissues in comparison with those in noncancerous tissues (P < 0.001; Figure 1A,B). Positive correlations between the expression of miR‐144‐5p and miR‐144‐3p were confirmed by Spearman's rank tests (r = 0.912 and P < 0.001; Figure 1C). In 2 cancer cell lines, EBC‐1 and SK‐MES‐1, the expression levels of miR‐144‐5p and miR‐144‐3p were extremely low (Figure 1A,B).
Figure 1.
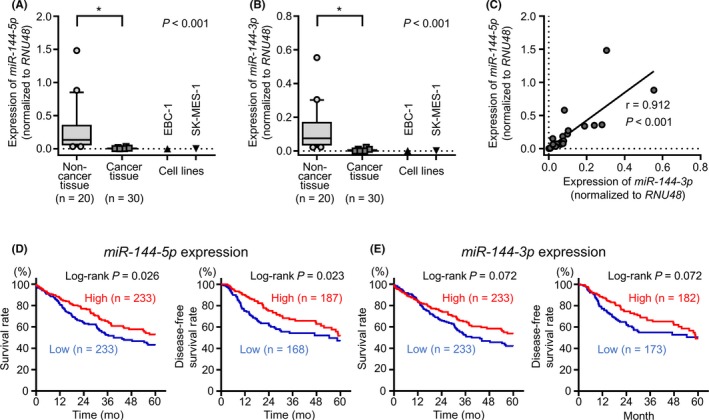
Expression levels of miR‐144‐5p and miR‐144‐3p in clinical lung squamous cell carcinoma (LUSQ) specimens and cell lines, and analysis of the expression levels of miR‐144‐5p and miR‐144‐3p in patients with LUSQ using The Cancer Genome Atlas (TCGA) database. A, Expression levels of miR‐144‐5p in clinical specimens and cell lines (EBC‐1 and SK‐MES‐1). B, Expression levels of miR‐144‐3p in clinical specimens and cell lines (EBC‐1 and SK‐MES‐1). Data were normalized to RNU48. *P < 0.001. C, Correlation between the expression levels of miR‐144‐5p and miR‐144‐3p in clinical specimens. Expression of miR‐144‐5p and miR‐144‐3p in clinical specimens showed a positive correlation (r = 0.912, P < 0.001). D, Kaplan‐Meier curve of 5‐year overall survival and 5‐year disease‐free survival according to miR‐144‐5p expression among patients with LUSQ in TCGA database (P = 0.026 and P = 0.023, respectively). E, Kaplan‐Meier curve of 5‐year overall survival and 5‐y disease‐free survival according to miR‐144‐3p expression in patients with LUSQ in TCGA database (P = 0.072 and P = 0.072, respectively)
By analyses using TCGA database, low expression of miR‐144‐5p significantly predicted poor prognosis compared with high expression of miR‐144‐5p (5‐year OS, P = 0.026; 5‐year DFS, P = 0.023; Figure 1D). Low expression of miR‐144‐3p also tended to predict poor prognosis compared with high expression of miR‐144‐3p in LUSQ patients (5‐year OS, P = 0.072; 5‐year DFS, P = 0.072; Figure 1E).
3.2. Ectopic expression of miR‐144‐5p and miR‐144‐3p suppressed cancer cell proliferation, migration, and invasion in LUSQ cells
To verify the functional roles of miR‐144‐5p and miR‐144‐3p in LUSQ, EBC‐1 and SK‐MES‐1 cells were transfected with mature miR‐144‐5p and miR‐144‐3p sequences. Cell proliferation assays showed significant inhibition of cell growth in miR‐144‐5p and miR‐144‐3p transfectants (Figure 2A). Moreover, cell migration and Matrigel invasion were significantly inhibited by miR‐144‐5p or miR‐144‐3p transfection (Figures 2B,C). In migration and invasion analyses, miR‐144‐5p had stronger antitumor effects than miR‐144‐3p.
Figure 2.
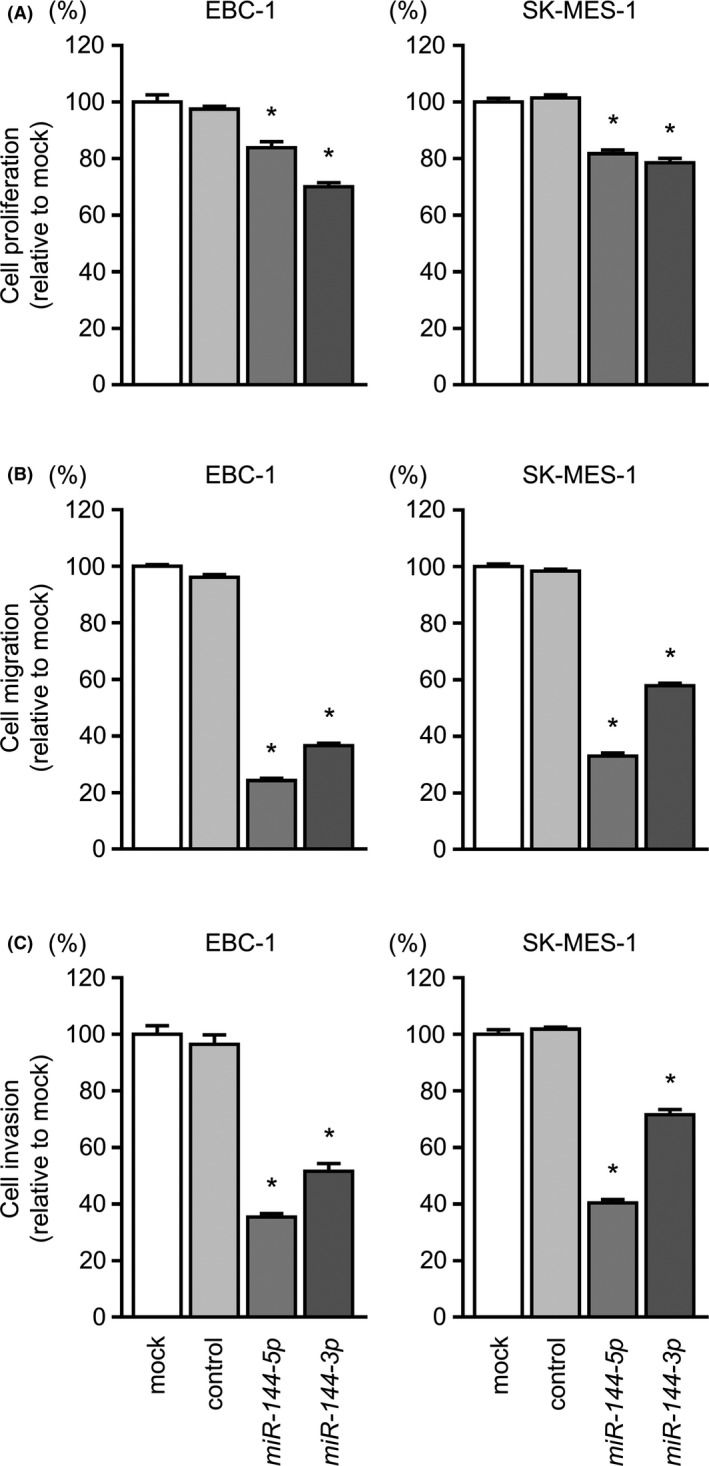
Functional assays of miR‐144‐5p and miR‐144‐3p in lung squamous cell carcinoma cell lines. A, Cell proliferation was determined by XTT assays 72 hours after transfection with miR‐144‐5p and miR‐144‐3p. B, Cell migration was measured by wound healing assays. C, Cell invasion was determined by Matrigel invasion assays. Cell proliferation, migration, and invasion were significantly suppressed in miR‐144‐5p and miR‐144‐3p transfectants compared with those in mock and control transfectants. *P < 0.001
3.3. Incorporation of miR‐144‐5p and miR‐144‐3p into RISC in LUSQ cells
To verify whether the miR‐144‐5p passenger strand could be incorporated into the RISC in LUSQ cells, we undertook immunoprecipitation for Ago2, which is essential for formation of the RISC, in cells transfected with either miR‐144‐5p or miR‐144‐3p. Isolated Ago2‐bound miRNAs were analyzed by qRT‐PCR to examine the interactions between miR‐144‐5p or miR‐144‐3p and Ago2. A conceptual diagram of this analysis is shown in Figure 3A.
Figure 3.
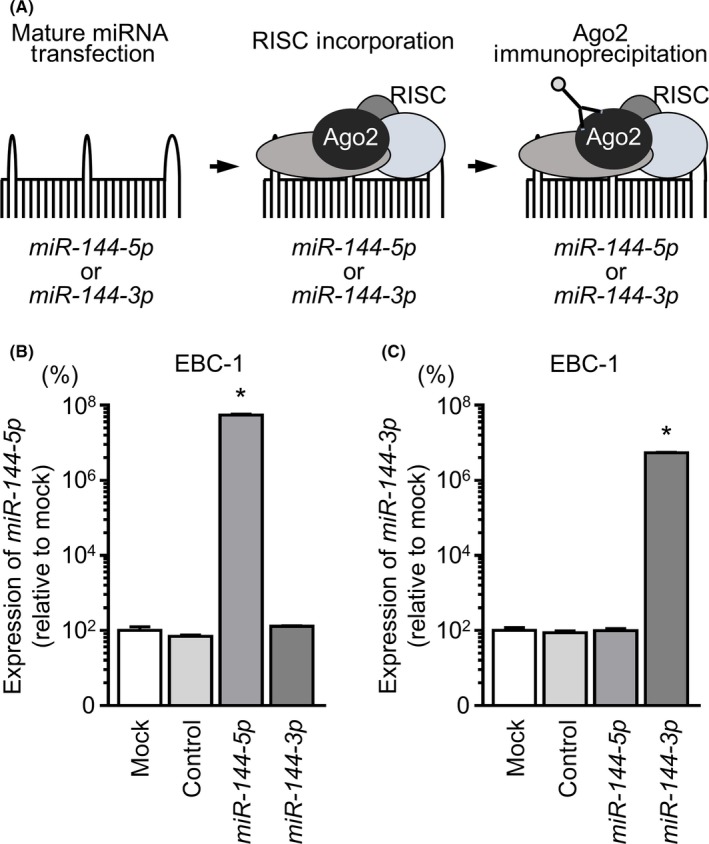
Incorporation of miR‐144‐5p and miR‐144‐3p into the RNA‐induced silencing complex (RISC) in lung squamous cell carcinoma cell lines. A, Schematic diagram of isolation of RISC‐incorporated microRNAs by Argonaute2 (Ago2) immunoprecipitation. In LUSQ cells, if miR‐144‐5p or miR‐144‐3p regulated gene activities, miR‐144‐5p or miR‐144‐3p was incorporated into the RISC. By carrying out immunoprecipitation with Ago2, which has a central role in the RISC, miR‐144‐5p or miR‐144‐3p incorporated into the RISC was detected. B, Expression levels of miR‐144‐5p following immunoprecipitation by Ago2. C, Expression levels of miR‐144‐3p following immunoprecipitation by Ago2. *P < 0.001
In EBC‐1 cells, miR‐144‐5p transfectants showed higher expression levels of miR‐144‐5p than did mock transfectants or miR‐control or miR‐144‐3p (P < 0.001) transfectants (Figure 3B). Similarly, after miR‐144‐3p transfection, we detected miR‐144‐3p using Ago2 immunoprecipitation (P < 0.001; Figure 3C).
3.4. Identification of putative target genes regulated by miR‐144‐5p and miR‐144‐3p in LUSQ cells
Next, we aimed to identify miR‐144‐5p and miR‐144‐3p target genes. To this end, we undertook a combination of in silico and genome‐wide gene expression analyses, as shown in Figure S1. Using TargetScanHuman database analysis, 1785 and 3776 putative target genes were found to have binding sites for miR‐144‐5p and miR‐144‐3p, respectively. Among these genes, we selected genes that were upregulated in NSCLC clinical expression profiles from the GEO database (accession no. GSE19188). Next, we merged gene expression analysis data using miR‐144‐5p‐ or miR‐144‐3p‐transfected SK‐MES‐1 cells (GEO accession no. GSE115801). This analysis yielded miR‐144‐5p‐ and miR‐144‐3p‐controlled genes (n = 13 and 15, respectively) in LUSQ cells (Table S1 and S2).
Overall, 3 putative target genes (NCS1, SLC44A5, and MARCKS) were coordinately regulated by both miR‐144‐5p and miR‐144‐3p (Table 3). The TCGA database analysis showed that high NCS1 expression significantly predicted a poor prognosis in patients with LUSQ (5‐year OS, P = 0.013; 5‐year DFS, P = 0.048; Figure 4A). Subsequently, we focused on NCS1 and validated the functional significance of LUSQ cells.
Table 3.
Common putative target genes regulated by miR‐144‐5p and miR‐144‐3p in lung squamous cell carcinoma cells
| Entrez gene ID | Gene symbol | Description | SK‐MES‐1 miR‐144‐5p transfectant Log2 ratio | SK‐MES‐1 miR‐144‐3p transfectant Log2 ratio | GSE19188 Log fold‐change | TCGA database 5‐y OS P value |
|---|---|---|---|---|---|---|
| 23413 | NCS1 | Neuronal calcium sensor 1 | −1.04 | −1.04 | 1.31 | 0.013 |
| 204962 | SLC44A5 | Solute carrier family 44 member 5 | −1.28 | −1.96 | 3.07 | 0.325 |
| 4082 | MARCKS | Myristoylated alanine rich protein kinase C substrate | −1.53 | −1.04 | 1.44 | 0.872 |
Lower and upper percentiles of The Cancer Genome Atlas (TCGA) database were both 50.
GSE, Gene Expression Omnibus dataset results; OS, overall survival.
Figure 4.
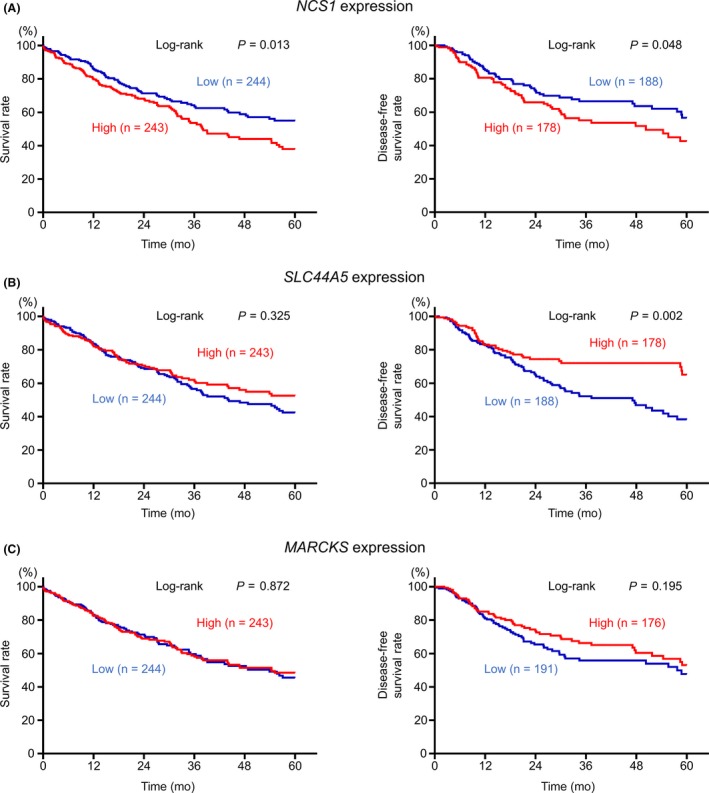
A, Kaplan‐Meier curve of 5‐year overall survival (OS) and 5‐year disease‐free survival (DFS) according to NCS1 expression among patients with lung squamous cell carcinoma (LUSQ) in The Cancer Genome Atlas (TCGA) database. The prognosis and DFS of patients with LUSQ with high expression of NCS1 was significantly worse than those with low expression (P = 0.013 and P = 0.048, respectively). B, Kaplan‐Meier curve of 5‐year OS and 5‐year DFS according to SLC44A5 expression among patients with LUSQ in TCGA database. There were no significant differences between the prognosis of patients with LUSQ according to SLC44A5 expression (P = 0.325). Conversely, DFS in patients with LUSQ was significantly higher in the high expression group than in the low expression group (P = 0.002). C, Kaplan‐Meier curve of 5‐year OS and 5‐year DFS according to MARCKS expression among patients with LUSQ in TCGA database. There were no significant differences in prognosis or DFS in patients with LUSQ according to MARCKS expression (P = 0.872 and P = 0.195, respectively)
3.5. Direct regulation of NCS1 by miR‐144‐5p and miR‐144‐3p in LUSQ cells
We then examined whether NCS1 mRNA and NCS1 protein expression were suppressed by restoration of miR‐144‐5p and miR‐144‐3p in LUSQ cells. NCS1 mRNA and NCS1 protein levels were significantly decreased by miR‐144‐5p or miR‐144‐3p transfection compared with those in mock‐ or miR‐control‐transfected cells (Figure 5A,B).
Figure 5.
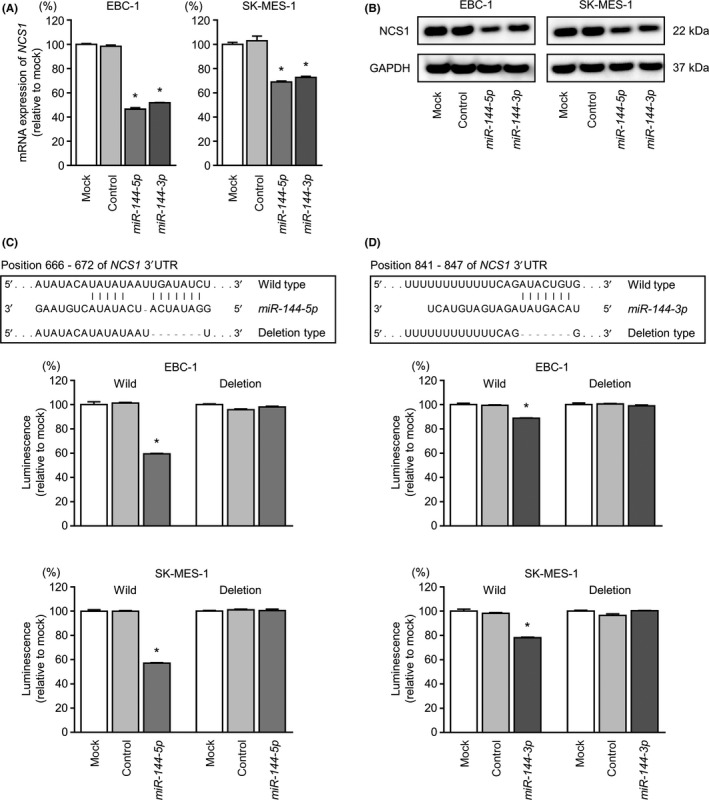
Direct regulation of NCS1 by miR‐144‐5p and miR‐144‐3p in lung squamous cell carcinoma cell lines. A, Expression levels of NCS1 mRNA 72 hours after transfection with miR‐144‐5p and miR‐144‐3p using GAPDH as an internal control. B, NCS1 protein expression by western blot analysis 72 hours after transfection with miR‐144‐5p and miR‐144‐3p in EBC‐1 and SK‐MES‐1 cell lines. GAPDH was used as a loading control. C, Putative miR‐144‐5p binding sites in the 3′‐UTR of NCS1 mRNA. Dual luciferase reporter assays using vectors encoding putative miR‐144‐5p target sites in the NCS1 3′‐UTR (position 666‐672) for both wild‐type and deleted regions. Normalized data were calculated as Renilla/firefly luciferase activity ratios. D, Putative miR‐144‐3p binding sites in the 3′‐UTR of NCS1 mRNA. Dual luciferase reporter assays using vectors encoding putative miR‐144‐3p target sites in the NCS1 3′‐UTR (position 841‐847) for both wild‐type and deleted regions. Normalized data were calculated as Renilla/firefly luciferase activity ratios. *P < 0.001
Next, we carried out luciferase reporter assays in EBC‐1 and SK‐MES‐1 cells to validate the direct binding of miR‐144‐5p and miR‐144‐3p to NCS1 mRNA. The TargetScanHuman database projected the existence of binding sites in the 3′‐ UTR of NCS1 (miR‐144‐5p, position 666‐672; miR‐144‐3p, position 841‐847; Figure 5C,D). Accordingly, we undertook luciferase reporter assays with vectors that contained either the wild‐type or deletion‐type 3′‐UTR of NCS1. Our results showed that the luminescence intensities were markedly reduced by transfection with miR‐144‐5p or miR‐144‐3p and the vector harboring the wild‐type 3′‐UTR of NCS1. In contrast, transfection with the deletion‐type vector did not reduce the luminescence intensities in EBC‐1 or SK‐MES‐1 cells (Figure 5C,D). These findings indicated that both strands of the miR‐144 duplex bound directly to the 3′‐UTR of NCS1.
3.6. NCS1 knockdown inhibited cell proliferation, migration, and invasion in LUSQ cells
We examined the effects of NCS1 knockdown in EBC‐1 and SK‐MES‐1 cells using 2 types of si‐NCS1 oligos: si‐NCS1‐1 and si‐NCS1‐2. Both siRNAs effectively downregulated NCS1 mRNA and NCS1 protein expression (Figure 6A,B).
Figure 6.
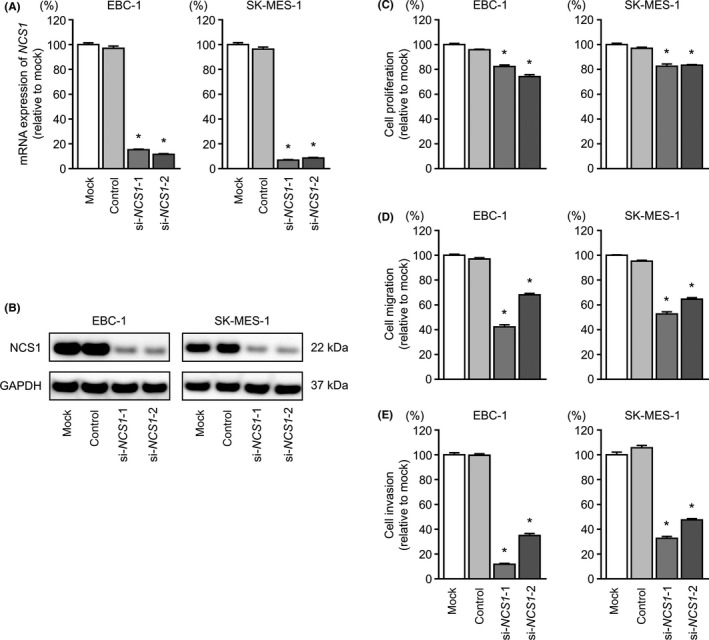
Effects of NCS1 silencing in lung squamous cell carcinoma cell lines. A, mRNA expression of NCS1 72 hours after transfection with si‐NCS1 using GAPDH as an internal control. B, NCS1 protein expression by western blot analysis 72 hours after transfection with si‐NCS1‐1 and si‐NCS1‐2 in EBC‐1 and SK‐MES‐1 cell lines. GAPDH was used as a loading control. C, Cell proliferation was identified by XTT assays 72 hours after transfection with si‐NCS1‐1 and si‐NCS1‐2. D, Cell migration was measured by wound healing assays. E, Cell invasion was determined by Matrigel invasion assays. *P < 0.001
Cancer cell proliferation, migration, and invasive abilities were markedly inhibited by si‐NCS1 transfection compared with those in mock or control EBC‐1 and SK‐MES‐1 cells (Figure 6C‐E).
3.7. Expression of NCS1 in LUSQ clinical specimens and its clinical significance
Immunohistochemical analyses of LUSQ clinical specimens indicated that NCS1 protein was strongly expressed in LUSQ cells, but showed infrequent and weak expression in normal lung cells (Table 2 and Figure 7).
Figure 7.
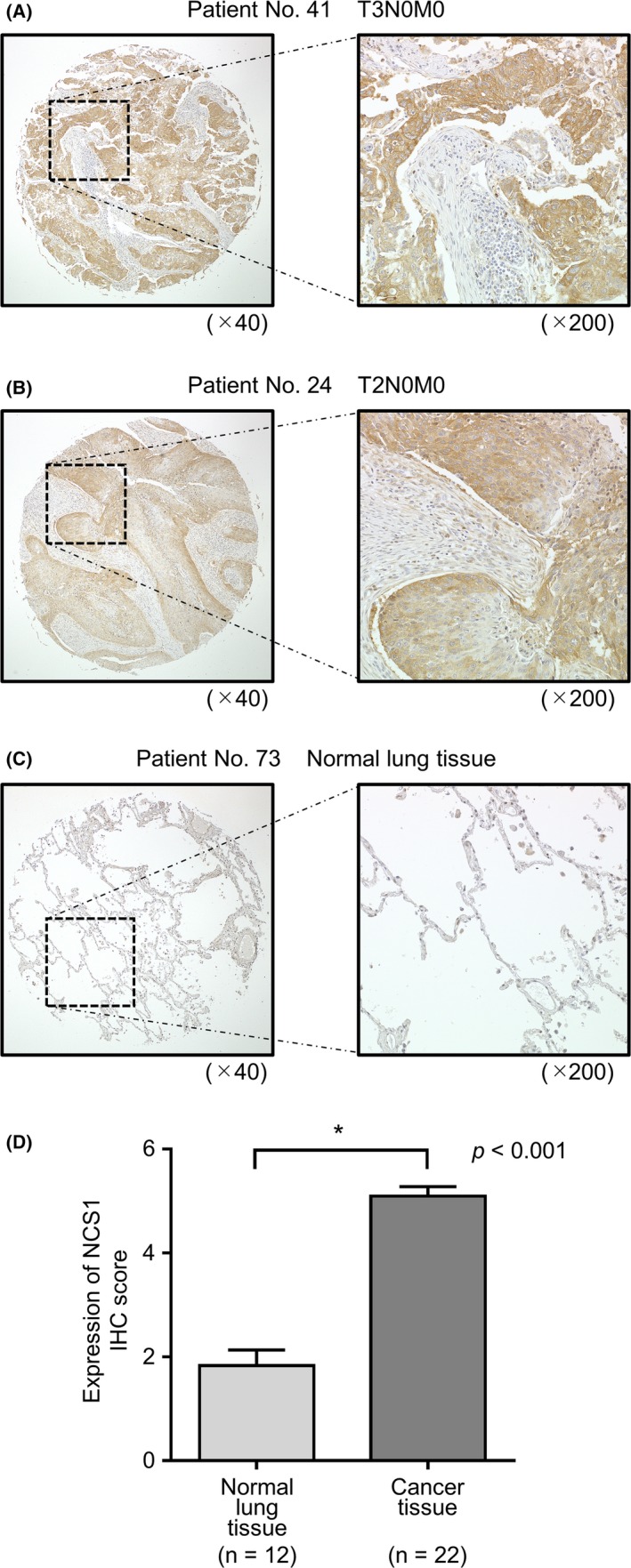
Expression of neuronal calcium sensor 1 (NCS1) in clinical lung squamous cell carcinoma (LUSQ) tissue. A‐C, Immunohistochemistry staining of NCS1 in LUSQ specimens and normal lung tissue using a tissue microarray. Overexpression of NCS1 was observed in the cytoplasm of cancer cells, whereas negative or low expression of NCS1 was observed in normal cells. D, Comparison of the scoring of NCS1 expression in clinical lung specimens. NCS1 expression in LUSQ tissue was significantly higher than in normal lung tissue. *P < 0.001
Finally, univariate and multivariate Cox hazard regression analyses were used to evaluate the clinical significance of NCS1 expression for OS in patients with LUSQ. Multivariate analysis showed that NCS1 expression was an independent predictive factor for OS (hazard ratio = 1.508, P = 0.007; Figure 8).
Figure 8.
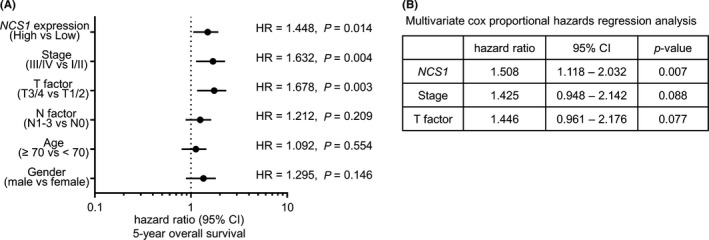
Analysis of the expression levels of NCS1 in patients with lung squamous cell carcinoma using The Cancer Genome Atlas (TCGA) database. A, Forest plot of univariate Cox proportional hazards regression analysis of 5‐year overall survival using TCGA database. B, Multivariate Cox proportional hazards regression analysis of 5‐year overall survival using TCGA database. CI, confidence interval; HR, hazard ratio
4. DISCUSSION
In our previous studies, we showed that both strands of the miR‐145 duplex (the guide strand miR‐145‐5p and the passenger strand miR‐145‐3p) were significantly downregulated in cancer tissues. These miRNAs were also found to have antitumor functions, and their targets were involved in NSCLC pathogenesis.20, 35 Thus, research focusing on both strands of the miRNA duplex is important for improving outcomes in cancer.
In this study, we found that both miR‐144‐5p and miR‐144‐3p had tumor‐suppressing effects in LUSQ cells and controlled several oncogenes. In an analysis of previous studies of the functional significance of the miR‐144 duplex, the antitumor function of miR‐144‐3p was reported in several type of cancers.36, 37, 38, 39, 40, 41 For example, expression of miR‐144‐3p suppressed cancer cell proliferation in glioblastoma and hepatocellular carcinoma through targeting c‐MET and SGK3, respectively.36, 37 In laryngeal squamous cell carcinoma, miR‐144‐3p inhibited the cancer cell epithelial‐mesenchymal transition phenotype through targeting ETS‐1.38 These data indicated that miR‐144‐3p was a pivotal tumor suppressor, and that downregulation of miR‐144‐3p enhanced cancer cell aggressiveness. Recent studies reported that some long noncoding RNAs (metastasis‐associated lung adenocarcinoma transcript 1 [MALAT1] and taurine upregulated 1 [TUG1]) acted as competing endogenous RNAs and that their overexpression attenuated the expression of miR‐144‐3p in cancer cells.39, 40 Another study showed that interleukin‐1β affected miR‐144‐3p at the transcriptional level and that interleukin‐1β levels were significantly higher in patients with LUAD and LUSQ.41
In contrast to miR‐144‐3p analyses, few reports have examined the functional significance of miR‐144‐5p in cancer cells. Analyses of miRNA signatures by RNA sequencing showed that both strands of miR‐144‐5p and miR‐144‐3p were frequently downregulated in several cancers.26, 27 In our studies, we focused on both strands of the miR‐144 duplex and investigated the antitumor functions and targets of the duplex in cancer cells.26, 27 Our previous studies showed that both strands of the miR‐144 duplex had antitumor functions in bladder cancer and RCC and that both miRNAs coordinately targeted CCNE1/CCNE2 and SDC3, respectively.26, 27 This study is the third paper reporting the antitumor functions of both strands of the miR‐144 duplex in cancer cells. Importantly, low expression of miR‐144‐5p and miR‐144‐3p significantly predicted short survival in patients with RCC.27 Moreover, high expression of the oncogenic genes controlled by these miRNAs also predicted short survival in patients with RCC, suggesting that the miR‐144 duplex and its targets were deeply involved in RCC pathogenesis.27
In this study, 3 genes (NCS1, SLC44A5, and MARCKS) were found to be coordinately controlled by both miR‐144‐5p and miR‐144‐3p in LUSQ cells. MARCKS is a major substrate of protein kinase C.42 Recent studies have shown that MARCKS plays a pivotal role in cancer development and progression.42 Moreover, in lung cancer, aberrant MARCKS expression was detected in clinical specimens, and MARCKS expression was implicated in this disease.43, 44
Among the 3 targets, we focused on NCS1 because its aberrant expression significantly predicted poor prognosis in patients. The NCS1 protein is a member of the NCS family, which harbors a functional Ca2+ binding domain and N‐terminally myristoylated site.45, 46 Previous studies have reported that NCS1 is a multifunctional protein involved in exocytosis, neurite outgrowth, neuroprotection, axonal regeneration, and nuclear Ca2+ regulation.45, 46 Furthermore, NCS1 interacts with various proteins to control their functions.45, 46 For example, NCS1 interacts with D2R and inhibits D2R‐mediated signaling pathways.47 Interestingly, activation of D2R‐mediated signaling suppresses lung cancer aggressiveness.48 Moreover, aberrant expression of NCS1 could interfere with D2R‐mediated signaling and might be involved in LUSQ cell progression.
Our functional assays showed that inhibition of NCS1 by siRNA suppressed cancer cell migration and invasion in LUSQ cells. Moreover, in multivariate Cox proportional hazards regression analysis, expression of NCS1 predicted poor prognosis in patients with LUSQ. Another study showed that overexpression of NCS1 promoted cell invasion and migration in breast cancer cells and that NCS1 overexpression was associated with poor prognosis in these patients.49 These findings indicate that aberrantly expressed NCS1 is involved in cancer cell aggressiveness. Thus, NCS1 could be a novel diagnostic and therapeutic target for patients with LUSQ.
In conclusion, genes coordinately controlled by the miR‐144 duplex (miR‐144‐5p and miR‐144‐3p) were found to be related to LUSQ pathogenesis. Our findings describing the involvement of the passenger strand miR‐144‐5p are the first report of this phenomenon in LUSQ pathogenesis. NCS1 expression was directly regulated by the miR‐144 duplex in LUSQ cells. Aberrantly expressed NCS1 enhanced LUSQ cell aggressiveness. Thus, elucidation of antitumor miRNAs controlling RNA networks could provide novel prognostic markers and therapeutic targets for this disease.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
ACKNOWLEDGMENTS
This study was supported by KAKENHI grants 18K09338, 17K09660, and 16K11224.
Uchida A, Seki N, Mizuno K, et al. Involvement of dual‐strand of the miR‐144 duplex and their targets in the pathogenesis of lung squamous cell carcinoma. Cancer Sci. 2019;110:420–432. 10.1111/cas.13853
Funding information
KAKENHI grants 18K09338, 17K09660, and 16K11224.
REFERENCES
- 1. Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability‐adjusted life‐years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Travis WD. Pathology of lung cancer. Clin Chest Med. 2011;32:669‐692. [DOI] [PubMed] [Google Scholar]
- 3. Zhou C, Wu YL, Chen G, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first‐line treatment of EGFR mutation‐positive advanced non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802). Ann Oncol. 2015;26:1877‐1883. [DOI] [PubMed] [Google Scholar]
- 4. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR‐mutated advanced non‐small‐cell lung cancer. N Engl J Med. 2018;378:113‐125. [DOI] [PubMed] [Google Scholar]
- 5. Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in untreated ALK‐positive non‐small‐cell lung cancer. N Engl J Med. 2017;377:829‐838. [DOI] [PubMed] [Google Scholar]
- 6. Reck M, Rodriguez‐Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med. 2016;375:1823‐1833. [DOI] [PubMed] [Google Scholar]
- 7. Sandler AB, Schiller JH, Gray R, et al. Retrospective evaluation of the clinical and radiographic risk factors associated with severe pulmonary hemorrhage in first‐line advanced, unresectable non‐small‐cell lung cancer treated with Carboplatin and Paclitaxel plus bevacizumab. J Clin Oncol. 2009;27:1405‐1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Paz‐Ares L, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation‐positive advanced non‐small‐cell lung cancer: overall survival data from the phase IIb LUX‐Lung 7 trial. Ann Oncol. 2017;28:270‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281‐297. [DOI] [PubMed] [Google Scholar]
- 10. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gulyaeva LF, Kushlinskiy NE. Regulatory mechanisms of microRNA expression. J Transl Med. 2016;14:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer. 2015;15:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baer C, Claus R, Plass C. Genome‐wide epigenetic regulation of miRNAs in cancer. Cancer Res. 2013;73:473‐477. [DOI] [PubMed] [Google Scholar]
- 14. Koshizuka K, Hanazawa T, Arai T, Okato A, Kikkawa N, Seki N. Involvement of aberrantly expressed microRNAs in the pathogenesis of head and neck squamous cell carcinoma. Cancer Metastasis Rev. 2017;36:525‐545. [DOI] [PubMed] [Google Scholar]
- 15. Mizuno K, Mataki H, Seki N, Kumamoto T, Kamikawaji K, Inoue H. MicroRNAs in non‐small cell lung cancer and idiopathic pulmonary fibrosis. J Hum Genet. 2017;62:57‐65. [DOI] [PubMed] [Google Scholar]
- 16. Koshizuka K, Hanazawa T, Fukumoto I, Kikkawa N, Okamoto Y, Seki N. The microRNA signatures: aberrantly expressed microRNAs in head and neck squamous cell carcinoma. J Hum Genet. 2017;62:3‐13. [DOI] [PubMed] [Google Scholar]
- 17. Mataki H, Enokida H, Chiyomaru T, et al. Downregulation of the microRNA‐1/133a cluster enhances cancer cell migration and invasion in lung‐squamous cell carcinoma via regulation of Coronin1C. J Hum Genet. 2015;60:53‐61. [DOI] [PubMed] [Google Scholar]
- 18. Mataki H, Seki N, Chiyomaru T, et al. Tumor‐suppressive microRNA‐206 as a dual inhibitor of MET and EGFR oncogenic signaling in lung squamous cell carcinoma. Int J Oncol. 2015;46:1039‐1050. [DOI] [PubMed] [Google Scholar]
- 19. Mizuno K, Seki N, Mataki H, et al. Tumor‐suppressive microRNA‐29 family inhibits cancer cell migration and invasion directly targeting LOXL2 in lung squamous cell carcinoma. Int J Oncol. 2016;48:450‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mataki H, Seki N, Mizuno K, et al. Dual‐strand tumor‐suppressor microRNA‐145 (miR‐145‐5p and miR‐145‐3p) coordinately targeted MTDH in lung squamous cell carcinoma. Oncotarget. 2016;7:72084‐72098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kumamoto T, Seki N, Mataki H, et al. Regulation of TPD52 by antitumor microRNA‐218 suppresses cancer cell migration and invasion in lung squamous cell carcinoma. Int J Oncol. 2016;49:1870‐1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suetsugu T, Koshizuka K, Seki N, et al. Downregulation of matrix metalloproteinase 14 by the antitumor miRNA, miR‐150‐5p, inhibits the aggressiveness of lung squamous cell carcinoma cells. Int J Oncol. 2018;52:913‐924. [DOI] [PubMed] [Google Scholar]
- 23. Yonemori K, Seki N, Idichi T, et al. The microRNA expression signature of pancreatic ductal adenocarcinoma by RNA sequencing: anti‐tumour functions of the microRNA‐216 cluster. Oncotarget. 2017;8:70097‐70115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koshizuka K, Nohata N, Hanazawa T, et al. Deep sequencing‐based microRNA expression signatures in head and neck squamous cell carcinoma: dual strands of pre‐miR‐150 as antitumor miRNAs. Oncotarget. 2017;8:30288‐30304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goto Y, Kurozumi A, Arai T, et al. Impact of novel miR‐145‐3p regulatory networks on survival in patients with castration‐resistant prostate cancer. Br J Cancer. 2017;117:409‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matsushita R, Seki N, Chiyomaru T, et al. Tumour‐suppressive microRNA‐144‐5p directly targets CCNE1/2 as potential prognostic markers in bladder cancer. Br J Cancer. 2015;113:282‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamada Y, Arai T, Kojima S, et al. Regulation of antitumor miR‐144‐5p targets oncogenes: direct regulation of syndecan‐3 and its clinical significance. Cancer Sci. 2018;109:2919‐2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mirsadraee S, Oswal D, Alizadeh Y, Caulo A, van Beek E Jr. The 7th lung cancer TNM classification and staging system: review of the changes and implications. World J Radiol. 2012; 4: 128‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mizuno K, Mataki H, Arai T, et al. The microRNA expression signature of small cell lung cancer: tumor suppressors of miR‐27a‐5p and miR‐34b‐3p and their targeted oncogenes. J Hum Genet. 2017;62:671‐678. [DOI] [PubMed] [Google Scholar]
- 30. Yamada Y, Koshizuka K, Hanazawa T, et al. Passenger strand of miR‐145‐3p acts as a tumor‐suppressor by targeting MYO1B in head and neck squamous cell carcinoma. Int J Oncol. 2018;52:166‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koshizuka K, Hanazawa T, Kikkawa N, et al. Regulation of ITGA3 by the anti‐tumor miR‐199 family inhibits cancer cell migration and invasion in head and neck cancer. Cancer Sci. 2017;108:1681‐1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Science Signal. 2013; 6: pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arai T, Okato A, Kojima S, et al. Regulation of spindle and kinetochore‐associated protein 1 by antitumor miR‐10a‐5p in renal cell carcinoma. Cancer Sci. 2017;108:2088‐2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kojima S, Chiyomaru T, Kawakami K, et al. Tumour suppressors miR‐1 and miR‐133a target the oncogenic function of purine nucleoside phosphorylase (PNP) in prostate cancer. Br J Cancer. 2012;106:405‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Misono S, Seki N, Mizuno K, et al. Dual strands of the miR‐145 duplex (miR‐145‐5p and miR‐145‐3p) regulate oncogenes in lung adenocarcinoma pathogenesis. J Hum Genet. 2018;63:1015‐1028. [DOI] [PubMed] [Google Scholar]
- 36. Lan F, Yu H, Hu M, Xia T, Yue X. miR‐144‐3p exerts anti‐tumor effects in glioblastoma by targeting c‐Met. J Neurochem. 2015;135:274‐286. [DOI] [PubMed] [Google Scholar]
- 37. Wu M, Huang C, Huang X, Liang R, Feng Y, Luo X. MicroRNA‐144‐3p suppresses tumor growth and angiogenesis by targeting SGK3 in hepatocellular carcinoma. Oncol Rep. 2017;38:2173‐2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang SY, Lu ZM, Lin YF, et al. miR‐144‐3p, a tumor suppressive microRNA targeting ETS‐1 in laryngeal squamous cell carcinoma. Oncotarget. 2016;7:11637‐11650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang Y, Zhang Y, Yang T, et al. Long non‐coding RNA MALAT1 for promoting metastasis and proliferation by acting as a ceRNA of miR‐144‐3p in osteosarcoma cells. Oncotarget. 2017;8:59417‐59434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cao J, Han X, Qi X, Jin X, Li X. TUG1 promotes osteosarcoma tumorigenesis by upregulating EZH2 expression via miR‐144‐3p. Int J Oncol. 2017;51:1115‐1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu C, Xu B, Zhou Y, et al. Correlation between serum IL‐1beta and miR‐144‐3p as well as their prognostic values in LUAD and LUSC patients. Oncotarget. 2016;7:85876‐85887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fong LWR, Yang DC, Chen CH. Myristoylated alanine‐rich C kinase substrate (MARCKS): a multirole signaling protein in cancers. Cancer Metastasis Rev. 2017;36:737‐747. [DOI] [PubMed] [Google Scholar]
- 43. Reddy SP, Natarajan V, Dudek AZ. MARCKS is marked in combating lung cancer growth and acquired resistance. Am J Respir Crit Care Med. 2014;190:1084‐1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen CH, Statt S, Chiu CL, et al. Targeting myristoylated alanine‐rich C kinase substrate phosphorylation site domain in lung cancer. Mechanisms and therapeutic implications. Am J Respir Crit Care Med. 2014; 190: 1127‐1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Burgoyne RD, Haynes LP. Sense and specificity in neuronal calcium signalling. Biochim Biophys Acta. 2015;1853:1921‐1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Boeckel GR, Ehrlich BE. NCS‐1 is a regulator of calcium signaling in health and disease. Biochim Biophys Acta. 2018;1865:1660‐1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kabbani N, Negyessy L, Lin R, Goldman‐Rakic P, Levenson R. Interaction with neuronal calcium sensor NCS‐1 mediates desensitization of the D2 dopamine receptor. J Neurosci. 2002;22:8476‐8486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hoeppner LH, Wang Y, Sharma A, et al. Dopamine D2 receptor agonists inhibit lung cancer progression by reducing angiogenesis and tumor infiltrating myeloid derived suppressor cells. Mol Oncol. 2015;9:270‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moore LM, England A, Ehrlich BE, Rimm DL. Calcium sensor, NCS‐1, promotes tumor aggressiveness and predicts patient survival. Mol Cancer Res. 2017;15:942‐952. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


