Abstract
Glioblastoma multiforme (GBM) is one of the most malignant human intracranial tumors. Temozolomide (TMZ) is the primary alkylating agent for GBM patients. However, many GBM patients are resistant to TMZ. Therefore, patients with GBM urgently need more effective therapeutic options. 20(S)‐ginsenoside‐Rg3 (20(S)‐Rg3) is a natural chemical with anti‐tumor effects, but at present there is little understanding of its functional mechanism. Several research reports have demonstrated that O6‐methylguanine DNA‐methyltransferase (MGMT) repairs damaged DNA and contributes to TMZ resistance in gliomas. In addition, recent studies have shown that MGMT gene expression could be regulated by the Wnt/β‐catenin pathway. However, whether 20(S)‐Rg3 inhibits MGMT expression and augments chemosensitivity to Temozolomide (TMZ) in glioma cells remains unclear. In this study, we explored the modulating effects of 20(S)‐Rg3 on MGMT. We used glioma cell lines, primary cell strain (including T98G, U118 and GBM‐XX; all of them are MGMT‐positive glioma cell lines) and xenograft glioma models to examine whether 20(S)‐Rg3 increased the sensitivity to TMZ and to reveal the underlying mechanisms. We found that the MGMT expression was effectively downregulated by 20(S)‐Rg3 via the Wnt/β‐catenin pathway in glioma cell lines, and TMZ resistance was significantly reversed by 20(S)‐Rg3. Meanwhile, 20(S)‐Rg3 shows no obvious cytotoxicity at its effective dose and is well tolerated in vivo. In addition, we found that 20(S)‐Rg3 significantly restrains the epithelial‐mesenchymal transition (EMT) progression of glioma cells. Taken together, these results indicate that 20(S)‐Rg3 may be a novel agent to use in treatment of GBM, especially in TMZ‐resistant GBM with high MGMT expression.
Keywords: 20(S)‐ginsenoside‐Rg3, glioblastoma multiforme, O6‐methylguanine DNA‐methyltransferase, temozolomide, Wnt/β‐catenin pathway
1. INTRODUCTION
Glioblastoma multiforme (GBM) is the most common malignant primary brain tumor of the central nervous system in adults. At present, surgery with postoperative adjuvant ionizing radiation and chemotherapy (Stupp's regimen) is the primary choice for patients with gliomas,1, 2 and temozolomide (TMZ) has been the first‐line chemotherapeutic agent for newly diagnosed patients for more than a decade.3 Although current multimodal therapies have improved, the prognosis of GBM is still very poor, with a median survival of only 14.6 months.4, 5 The rapid recurrence and TMZ resistance of glioma are the main difficulties in treatment. Therefore, it is critical to find new therapies for GBM treatment and to better understand the molecular mechanism of TMZ resistance. TMZ exerts antitumor effects by methylating the O6 position of guanine (O6‐G) in DNA and causes base mispairing by continuously inducing O6‐methylguanine adduct. It then induces cell cycle arrest and, ultimately, apoptosis.6 However, the DNA repair protein MGMT (O6‐methylguanine‐DNA‐methytransferase) reverses the mutagenic and lethal effects of TMZ by removing the methyl adducts from the O6 position of guanine; therefore, MGMT mediates the resistance of glioma cells to TMZ.7, 8, 9 Furthermore, clinical studies have demonstrated that patients with low expression of MGMT are more sensitive to TMZ chemotherapy, proving that the expression of MGMT remains a main cause of GBM's chemotherapy resistance7, 10, 11 Thus, reduction of MGMT expression in glioma cells may contribute to overcoming the resistance to TMZ.
20(S)‐ginsenoside‐Rg3 is a stereoisomer of 20(R)‐ginsenoside‐Rg3; they are the 2 stereoisomeric pairs of ginsenoside‐Rg3 which is a tetracyclic triterpenoids saponins monomer, extracted and purified from Panax ginseng C. A. Meyer.12, 13 Studies report that 20(S)‐ginsenoside‐Rg3 displays more effective clinical effects than 20(R), especially in the antitumor area.14, 15, 16, 17, 18 Several studies have shown that drug 20(S)‐Rg3 is nontoxic and well‐tolerated in organisms, including in mice, rats, dogs and humans.19, 20, 21, 22 Referring to the pharmacokinetic characteristics of 20(S)‐Rg3, Zhang et al23 report that the drug concentration of 20(S)‐Rg3 in the serum of mice with 40 mg/kg 20(S)‐Rg3 administered by i.p. injection daily for 1 week was maintained at approximately 200 μM. Taken together, these reports indicate that 20(S)‐Rg3 has great potential for clinical application. In our laboratory, we initially discovered that 20(S)‐Rg3 can effectively downregulate MGMT expression in the glioma cell line T98G. Therefore, our study mainly explored whether 20(S)‐Rg3 plays a synergistic role in improving the effect of TMZ treatment in GBM. Herein, we show that 20(S)‐Rg3 inhibits MGMT expression by modulating Wnt/β‐catenin pathways and remarkably potentiates the sensitivity of glioma to TMZ chemotherapy. Meanwhile, we found that 20(S)‐Rg3 significantly inhibits the process of epithelial mesenchymal transition in glioma cells.
2. MATERIALS AND METHODS
2.1. Reagents and chemicals
20(S)‐ginsenoside‐Rg3, molecular formula C42H72O13 and molecular weight 785.01 g/mol, was purchased from Shanghai Tauto Biotech (>98% purity; Shanghai, China). TMZ, molecular formula C6H6N6O2 and molecular weight 194.15 g/mol, was purchased from Sigma‐Aldrich.
2.2. GBM cell culture
The T98G and U118 GBM cell line were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). GBM‐XX is a primary cell strain derived from the surgical specimen of a patient with World Health Organization grade IV GBM undergoing resection in accordance with a protocol approved by the Ethics Committee of our hospital and with prior informed consent from the patient. The culture medium was composed of DMEM (Life Technologies/Gibco, Carlsbad, CA, USA) and 10% FBS (Life Technologies/Gibco, Carlsbad, CA, USA), 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco, Grand Island, NY, USA). The cells were cultured at a density of 1 × 105 cells/mL. These cells were incubated at 37°C with 5% CO2 and 100% humidity.
2.3. Total RNA extraction, reverse transcription and qPCR
Total RNA was extracted from cell lines using TRIzol (TaKaRa, Dalian, China) according to the manufacturer's protocol. For mRNA analysis, the Primer‐Script one step RT‐PCR kit (TaKaRa) was used to reverse transcribe RNA into cDNA. The cDNA templates were amplified by RT‐PCR using the SYBR Premix Dimmer Eraser kit (TaKaRa). GAPDH was used as an internal control. The real‐time PCR were performed in triplicate. The primer sequences used were as follows: for GAPDH,5′‐GTCAACGGATTTGGTCTGTATT‐3′(forward) and 5′‐AGTCTTCTGGGTGGCAGTGAT‐3′(reverse); for MGMT,5′‐GTTATGAATGTAGGAGCCCTTATG‐3′(forward) and 5′‐TGACAACGGGAATGAAGTAATG‐3′(reverse). The relative mRNA expression change was calculated using the 2−ΔΔCt method.
2.4. Western blot
Western blot assay was carried out as previously described.24 Cells were washed with PBS and then lysed with RIPA lysis buffer (Solarbio, China) and protease inhibitors (Roche Applied Science, Indianapolis, Switzerland). The protein concentration was measured using the bicinchonininc acid (BCA) protein assay kit (Beyotime Biotechnology, Shanghai, China). Equal amounts of protein were subjected to 10% SDS‐polyacrylamide gel electrophoresis and transferred onto PVDF membranes. The membranes were subsequently blocked with 5% non‐fat milk for 2 hours and incubated with primary antibodies overnight at 4°C. The primary antibodies used were anti‐MGMT (1:500, Abcam, Cambridge, UK) and anti‐Survivin (1:1000, Abcam), anti‐β‐catenin, anti‐CD44, anti‐C‐Jun, anti‐C‐Myc, anti‐cyclinD1, anti‐LEF1, anti‐TCF1/TCF7, anti‐MMP7, anti‐Axin2, anti‐Met, anti‐PARP, anti‐caspase‐3, anti‐BAX, anti‐Bcl2, anti‐cleaved‐caspase‐3, anti‐E‐cadherin, anti‐N‐cadherin and anti‐Vimentin (these primary antibodies are all: 1:1000, Cell Signaling Technology, Boston, MA, USA), as well as anti‐GAPDH, and secondary antibodies were HRP‐conjugated goat anti‐mouse or goat anti‐rabbit IgG antibody (1:1000, Beyotime). All experiments were performed in triplicate.
2.5. Cell viability assay
Cell viability and proliferation was, respectively, evaluated using the cell counting kit‐8 solution (CCK‐8) assay (Dojindo, Japan). Cells were collected and seeded at a density of 5 × 103 cells/well in 96‐well plates for CCK‐8 assay. After various treatments, cells were incubated with 10 μL CCK‐8 solution for 1 hour following the manufacturer's specifications. The data was assessed by measuring the optical density (OD) at 450 nm using a microplate reader (BioTek, Winooski, VT, USA).
2.6. Wound healing assay
Approximately 1 × 106 cells were seeded into 6‐well plates and incubated at 37°C until cells reached a confluence of at least 90%. Wounds were created by scratching cell monolayers with a 200‐μL plastic pipette tip and then incubated in fresh medium containing 1% FBS and different concentrations of 20(S)‐Rg3 for 24 hours. Photographs were taken to estimate the mean number of migrating cells per field.
2.7. Transwell invasion assay
Cell invasion assays were performed using 24‐well Transwell plates (Corning, NY, USA) pre‐coated with Matrigel (BD, USA). Approximately 1 × 105 cells were seeded in the upper chamber with serum‐free medium in triplicate. Medium containing 10% FBS (300 μL) and different concentrations of 20(S)‐Rg3 was added to the lower chamber as a chemo‐attractant. After incubation for 24 hours, the cells above the Matrigel layer were removed by cotton swab, and the cells below the membrane were fixed by methanol, stained with .1% crystal violet for 10 minutes, and counted from 5 randomly chosen fields for each well.
2.8. Immunofluorescence analysis
Cells were cultured on glass coverslips in 6‐well plates, and 20(S)‐Rg3 was added to the culture media for 72 hours and then fixed in a solution of 4% paraformaldehyde in PBS for 30 minutes. The cells were permeabilized with .1% Triton X‐100 in PBS for 10 minutes, and blocked in 5% goat serum in PBS for 1 hour. Coverslips were then incubated in primary antibodies overnight at 4°C in the dark with the following dilutions: E‐cadherin, N‐cadherin and Vimentin (1:400, Cell Signaling Technology). Cells were washed and incubated in secondary antibody (Abcam) for 1.5 hour at 37°C. The slips were immediately examined using fluorescence microscopy (Olympus BX51).
2.9. Annexin V‐FITC/PI apoptosis assay
Cell apoptosis was measured using an annexin V‐FACS apoptosis detection kit (Becton Dickinson, Lake Franklin, NJ, USA) according to the manufacturer's instructions. To be brief, after treatments (20(S)‐Rg3 alone, TMZ alone and 20(S)‐Rg3 combined with TMZ for 72 hours), cells were washed twice with PBS and incubated in 300 μL 1× binding buffer containing 5‐μL annexin V‐FITC for 10 minutes and then resuspended with propidium iodide in the dark for 5 minutes at 25°C. The stained cells (containing 200 000 cells/sample) were analyzed using an FC 500 flow cytometer (Beckman Coulter, Brea, CA, USA) within 1 hour according to the manufacturer's protocol.
2.10. Tumor xenograft experiment and tumor peritoneal metastasis experiment
For the tumor xenograft experiment, each 4‐week‐old male nude mouse (6 mice per group) was subcutaneously injected with U118 cells (100 μL, 2 × 106). Tumor volumes were calculated as .5 × length × width2 on a weekly basis. Therapeutic experiments were started when the tumors reached approximately 100 mm3 after 7 days and mice were randomly divided into 2 groups (6 mice/group). Because the drug concentration in the serum of mice with 40 mg/kg 20(S)‐Rg3 administered i.p. daily for 1 week reached a peak at approximately 200 μmol/L,23 and 30 mg/kg TMZ alone was unable to significantly inhibit glioma growth in vivo xenograft models,25 20 mg/kg of 20(S)‐Rg3 and 20 mg/kg TMZ were selected for in vivo experiments. One of the groups received an i.p. injection of TMZ 20 mg/kg alone every day for a 28‐day continuum, and the other group received TMZ 20 mg/kg and 20(S)‐Rg3 20 mg/kg in combination every day for a 28‐day continuum. After 4 weeks, mice were killed and tumors were excised and weighed; total tissue protein was then extracted from tumor tissue for western blot of MGMT expression level.
For the tumor peritoneal metastasis experiment, GBM‐XX cells (100 μL, 2 × 106) were i.p. injected into each 4‐week‐old male nude mouse, and then mice were randomly divided into 2 groups (4 mice for each group); 2 groups received PBS or 20 mg/kg of 20(S)‐Rg3 every day, respectively. Mice were killed to count the peritoneal metastatic nodules after 20 days. All animal experiments were performed in the animal laboratory center of Xinhua Hospital (Shanghai JiaoTong University School of Medicine, Shanghai, China). The study protocol was approved by the Animal Care and Use committee of Xinhua Hospital.
2.11. Statistical analysis
All statistical analyses were performed using SPSS 20.0 (SPSS, Chicago, IL, USA). Data were expressed as mean values ± SD. The differences between groups were calculated using Student's t test. All the P‐values were 2‐sided and P < .05 was considered to be statistically significant.
3. RESULTS
3.1. 20(S)‐Rg3 significantly downregulated the expression of O6‐methylguanine DNA‐methyltransferase in glioma cell lines
To evaluate the toxic effect of 20(S)‐Rg3 and TMZ on GBM, three human GBM cell lines, T98G, U118 and GBM‐XX, were treated with 20(S)‐Rg3 at different concentrations (0, 60, 120, 240, 360 and 480 μmol/L) and TMZ at different concentrations (0, 75, 150, 300, 450 and 600 μmol/L) for 72 hours, respectively. MTT assay showed that 20(S)‐Rg3 and TMZ exhibited a concentration‐dependent killing of diverse GBM cell lines, with an IC50 (half maximal inhibitory concentration) value of around 200 and 250 μmol/L, respectively (Figure 1A,B). To avoid cytotoxicity using a single reagent, we used a 100 μmol/L concentration of 20(S)‐Rg3 and a 100 μmol/L concentration of TMZ for the following study.
Figure 1.
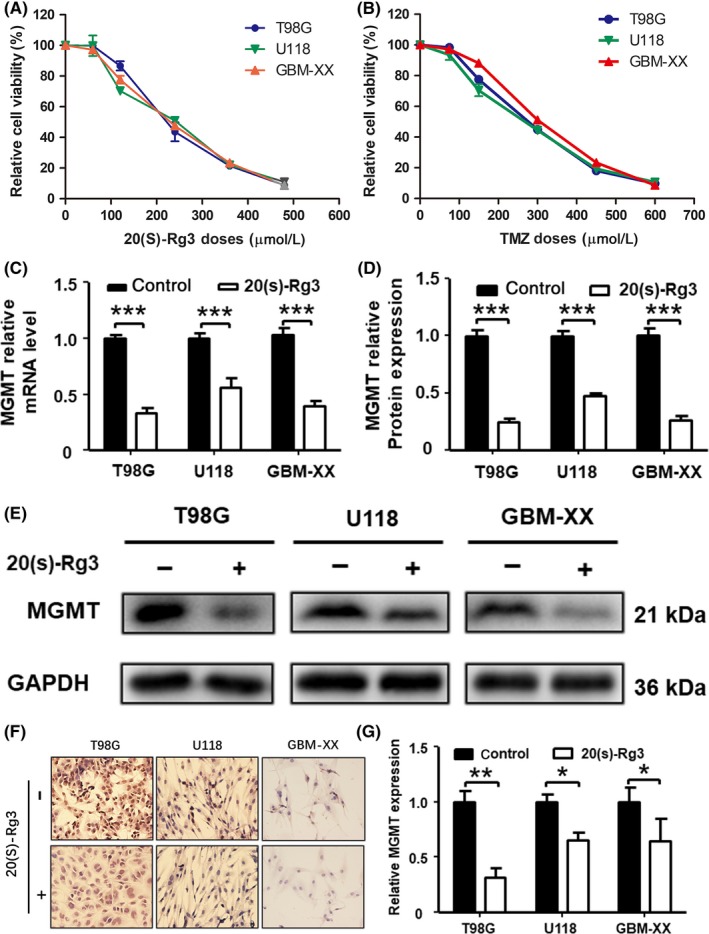
20(S)‐Rg3 inhibits the expression of O6‐methylguanine DNA‐methyltransferase (MGMT) in glioma cell lines. T98G, U118 and GBM‐XX cells were seeded in 96‐well flat‐bottom plates at 5000 cells/well, cultured in DMEM supplemented with 10% FBS, and then treated with increasing concentrations of 20(S)‐Rg3 or temozolomide (TMZ), or DMSO as a control; 72 h later, 10 μL of cell counting kit‐8 mix reagent was added to 100 μL of media per well, and the cells were incubated at 37°C for 2 h. The optical density (OD) was measured at 450 nm with a spectrophotometer. A,B, The half maximal inhibitory concentration of 20(S)‐Rg3 and TMZ on glioma cells is approximately 200 and 250 μmol/L. C, T98G, U118 and GBM‐XX were treated with 20(S)‐Rg3 (100 μmol/L) for 72 h, and the total RNA was extracted with TRIzol; then the expression of MGMT mRNA level was determined by quantitative real‐time PCR (n = 4). ***P < .001 compared with the control group. D,E, T98G, U118 and GBM‐XX were treated with 20(S)‐Rg3 (100 μmol/L); 72 h later, expression of MGMT was determined by western blot (n = 4). ***P < .001 compared with control group. F,G, T98G, U118 and GBM‐XX cells were cultured on coverslips. After the cells attached to the slips over 24 h, 20(S)‐Rg3 (100 μmol/L) was added to the culture media for 72 h. Then expression of MGMT in glioma cells was determined by immunocytochemistry assay (n = 4). *P < .05, **P < .01 compared with control group
To explore whether 20(S)‐Rg3 will exert an effect on the expression of MGMT, we first detected MGMT expression levels before and after treatment with 20(S)‐Rg3 (100 μmol/L for 72 hours) in glioma cell lines T98G, U118 and GBM‐XX by qRT‐PCR. As show in Figure 1C, MGMT levels in GBM cell lines were significantly depressed by 20(S)‐Rg3. Western blotting showed that all cells, U118 and GBM‐XX were MGMT‐positive, while with the treatment of 20(S)‐Rg3 (100 μmol/L for 72 hours), MGMT expression was significantly suppressed in all glioma cell lines (Figure 1D,E). In addition, the immunocytochemistry results are similar to those of quantitative RT‐PCR and western blot (Figure 1F,G).
3.2. GinsenosideRg3 inhibits O6‐methylguanine DNA‐methyltransferase expression in glioblastoma by modulating Wnt/beta‐catenin pathways
To determine whether the 20(S)‐Rg3 inhibits MGMT expression is related to Wnt/β‐catenin signaling, a series of western blot experiments were carried out. These results suggested that expression of the key downstream effector β‐catenin of the Wnt signaling pathway is obviously suppressed by 20(S)‐Rg3 (100 μmol/L for 3 days). As shown in Figure 2, the related nuclear transcription factors LEF1 and TCF1/TCF7 were distinctly decreased. In addition, the target genes CD44, C‐Jun, C‐Myc, cyclinD1, Survivin and MMP7 were changed similarly to those of MGMT as well, but there was no obvious effect on MET and Axin‐2.
Figure 2.
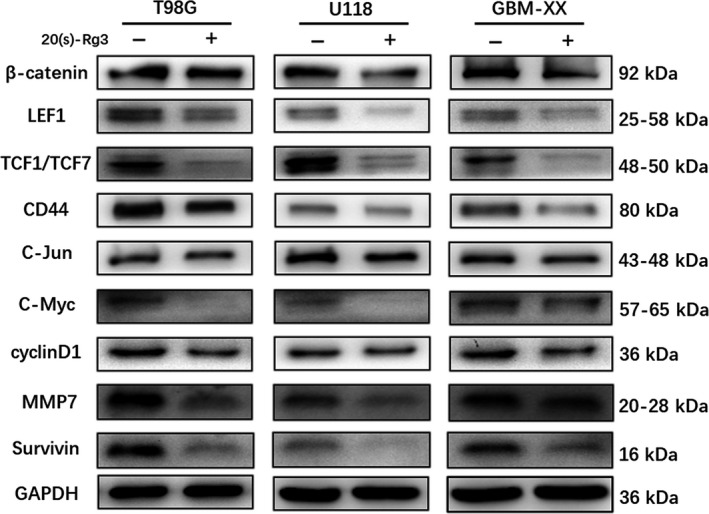
20(S)‐Rg3 inhibits Wnt/β‐catenin pathways activity. After treatment of 20(S)‐Rg3 (100 μmol/L), Wnt/β‐catenin pathway‐related proteins were determined by western blot. The expression of β‐catenin was repressed in all of T98G, U118 and GBM‐XX cell lines, 2 important transcriptional factors of Wnt/β‐catenin signaling, LEF1 and TCF1/TCF7, were both depressed by 20(S)‐Rg3. Meanwhile, the target genes CD44, C‐Jun, C‐Myc, cyclinD1, MMP7 and survivin were decreased, and the changes were similar to those of MGMT
3.3. 20(S)‐Rg3 augments temozolomide‐mediated chemotherapy
To explore whether the 20(S)‐Rg3 could augment TMZ‐mediated chemotherapy by downregulating the expression of MGMT, we performed a cell viability assay. The assay demonstrated that 20(S)‐Rg3 (100 μmol/L for 3 days) had no obvious cytotoxicity by itself, and TMZ (100 μmol/L for 3 days) alone did not cause significant cytotoxicity in cell lines T98G, U118 and GBM‐XX. However, as shown in Figure 3A,B,C, 20(S)‐Rg3 significantly enhanced the cytotoxicity of TMZ (100 μmol/L for 3 days) in all three glioma cell lines. In addition, to investigate whether compared with the treatment of 20(S)‐Rg3+ TMZ on glioblastoma cells simultaneously, pretreatment of 20(S)‐Rg3 on glioblastoma cells may be more effective, we pretreated cells with 20(S)‐Rg3 (100 μmol/L) and then performed CCK8 assays. As shown in Figure S1, the results showed that pretreatment of 20(S)‐Rg3 was more effective in the first 24 hours on T98G and U118 cells, but for 72 hours, there was no significant difference in the results on all three glioblastoma cell lines. Taken together, the results suggested that MGMT expression after 20(S)‐Rg3 pretreatment for 72 hours was lower than 20(S)‐Rg3 treatment for 24 hours, and sensitivity of TMZ to glioblastoma cells is negatively correlated with the expression of MGMT in glioblastoma cells.
Figure 3.
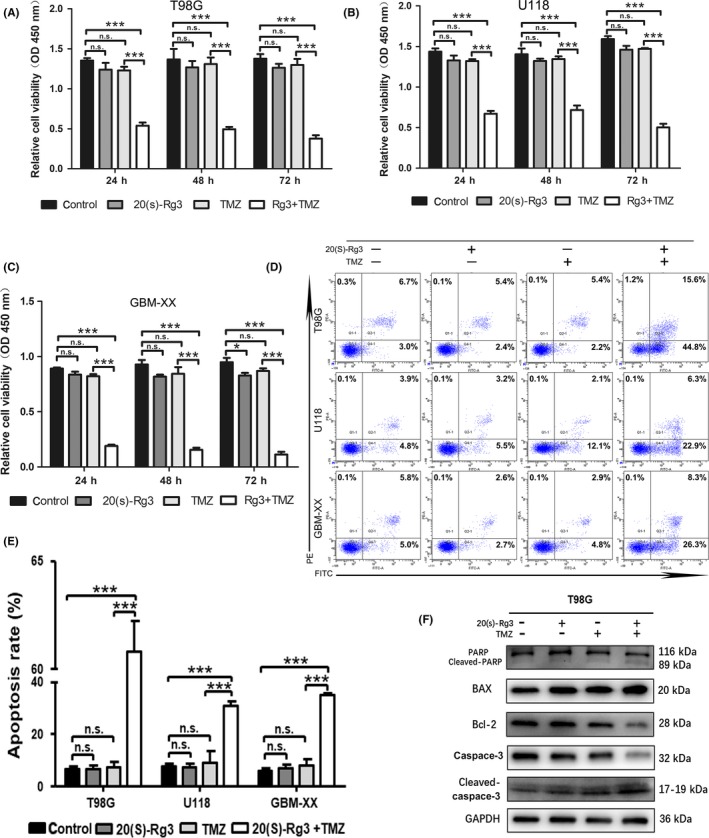
20(S)‐Rg3 augments temozolomide (TMZ)‐mediated chemotherapy. A,B,C, T98G, U118 and GBM‐XX cells were seeded in 96‐well plates at a density of 5 × 103 cells/well. After 12 h of cell attachment, we treated them with TMZ (100 μmol/L) and/or 20(S)‐Rg3 (100 μmol/L), or DMSO as a control for 72 h. Then cell viability was determined by cell counting kit‐8 mix reagent at indicated time points (24, 48, 72 h). As shown in cell viability assays, 20(S)‐Rg3 (100 μmol/L) had no cytotoxicity by itself. However, 20(S)‐Rg3 significantly enhanced the cytotoxicity of TMZ (n = 6), *P < .05, ***P < .001 compared with control group, n.s., not statistically significant. D,E, The combination of 20(S)‐Rg3 and TMZ significantly increase the apoptosis of glioma cells. T98G, U118 and GBM‐XX cells were treated with 20(S)‐Rg3 (100 μmol/L) or/and TMZ (100 μmol/L) for 72 h, and then the apoptosis rates of glioma cells were determined by flow cytometry (n = 3). ***P < .001 compared with control group; n.s., not statistically significant. F, After treatment of 20(S)‐Rg3 (100 μmol/L) and/or TMZ (100 μmol/L) on T98G for 48 h, the expression of apoptosis‐related proteins including BAX, Bcl‐2, caspase‐3, cleaved‐caspase‐3, PARP and cleaved‐PARP, were determined by western blot. As shown in the figure, 20(S)‐Rg3+ TMZ increased the expression of BAX, cleaved‐caspase‐3 and cleaved‐PARP, and conversely decreased Bcl‐2, caspase‐3 and PARP expression in glioma cell line T98G
Similar results were shown in the analysis of flow cytometry. From Figure 3D,E, compared with the control, when 20(S)‐Rg3 (100 μmol/L for 3 days) or TMZ (100 μmol/L for 3 days) alone was used on glioma cell lines, neither of them could induce an increase in cell apoptosis obviously; however, the combination of 20(S)‐Rg3 and TMZ can significantly increase the apoptotic effect of glioma cells. For T98G cells, the early and the late apoptosis percentage was 5.4% and 2.2% in the control group, 5.4% and 2.2% in the 20(S)‐Rg3 group, and 6.7% and 3.0% in the TMZ group, while in the 20(S)‐Rg3+ TMZ group the early apoptosis percentage was up to 15.6% and the late apoptosis percentage was up to 44.8% (P < .05). The results in U118 and GBM‐XX cell lines were consistent with T98G.
We carried out western blotting experiments to detect changes in the expression of apoptosis‐related proteins in T98G cells, including BAX, Bcl‐2, caspase‐3, cleaved‐caspase‐3, PARP and cleaved‐PARP. As shown in Figure 3F, 20(S)‐Rg3+ TMZ increased the expression of BAX, cleaved‐caspase‐3, cleaved‐PARP and conversely decreased Bcl‐2, caspase‐3 and PARP expression in glioma cell line T98G. These results illustrated that 20(S)‐Rg3(100 μmol/L for 3 days) + TMZ (100 μmol/L for 3 days) could induce apoptosis in glioma cells through mitochondrial signaling pathways.
3.4. 20(S)‐Rg3 displays synergistic activity with temozolomide in in vivo xenograft models
To further examine the effect of 20(S)‐Rg3 synergistic activity with TMZ in vivo, U118 cells were injected into nude mice subcutaneously. According to some references, 23 , 25 we selected 20 mg/kg 20(S)‐Rg3 and 20 mg/kg TMZ for the in vivo experiments. When the subcutaneous tumors reached a mean group size of around 100 mm3, mice were treated every day for 4 weeks with 20 mg/kg TMZ alone or 20 mg/kg 20(S)‐Rg3+ 20 mg/kg TMZ. 20(S)‐Rg3 showed superior synergistic activity with TMZ. Compared to TMZ alone, 20(S)‐Rg3 plus TMZ treatment showed dramatically suppressed subcutaneous tumor growth (Figure 4A,B). Western blot for the subcutaneous tumors indicated that the MGMT level in tumor tissue was significantly suppressed (Figure 4C). In addition, the combination of 20(S)‐Rg3 and TMZ did not display adverse consequences or obvious toxicity; it was well‐tolerated in nude mice.
Figure 4.
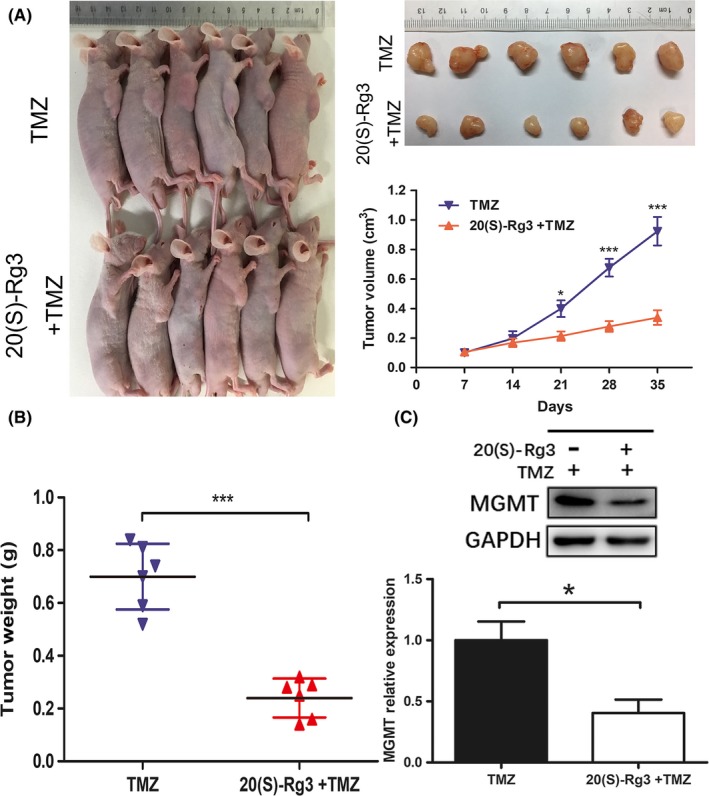
20(S)‐Rg3 displays synergistic activity with temozolomide (TMZ) in vivo. A,B, Tumor xenografts were established by subcutaneous inoculation of U118 cells into the armpit of the right upper limb of nude mice. Seven days later, tumors reached approximately 100 mm3; mice were treated every day for 4 wks with 20 mg/kg TMZ alone or 20 mg/kg 20(S)‐Rg3+ 20 mg/kg TMZ. The size of tumors in nude mice was monitored weekly with a vernier caliper; 4 wks later, mice were killed and tumors were excised and weighed (n = 6). *P < .05, ***P < .001. C, O6‐methylguanine DNA‐methyltransferase (MGMT) expression level of tumor tissue was determined by western blot (n = 3). *P < .05 compared with control group
3.5. 20(S)‐Rg3 restrains the epithelial‐mesenchymal transition progression of glioma cells in vitro and in vivo
In addition, we found that 20(S)‐Rg3 significantly inhibited the migration and invasion of glioma cells. As shown in Figure 5, the results of wound healing (Fig. 5a, b) and transwell invasion assays (Figure 5C,D) indicated that 20(S)‐Rg3 dramatically inhibited cell migration and invasion in cell lines T98G, U118 and GBM‐XX in a dose‐dependent manner.
Figure 5.
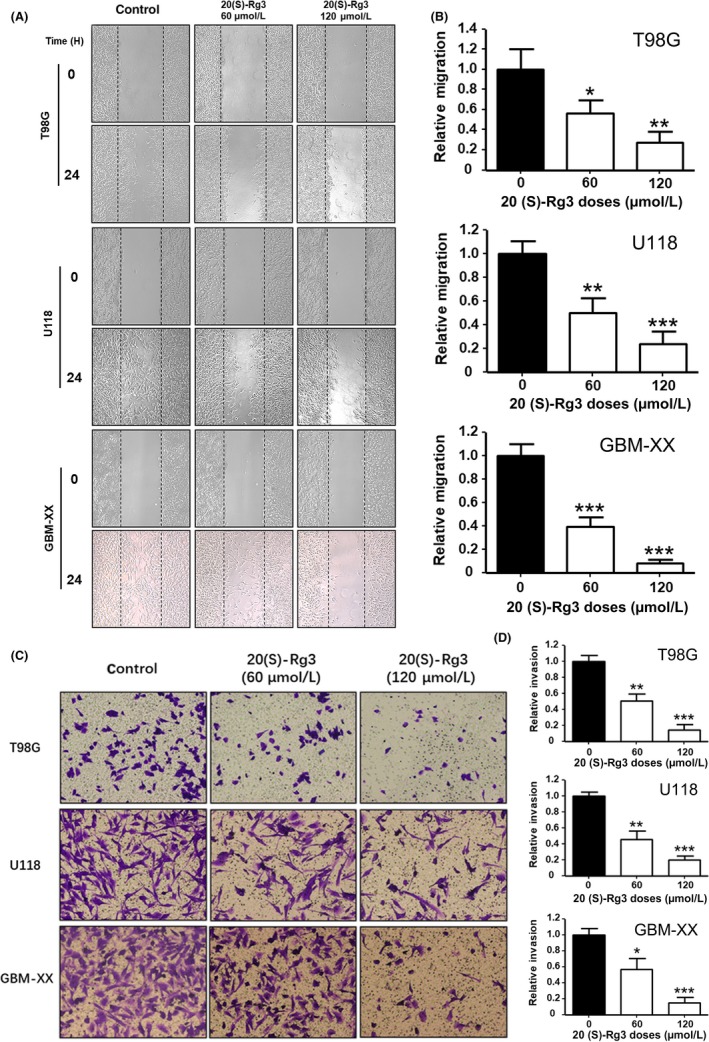
20(S)‐Rg3 inhibits migration and invasion of glioma cells. A, T98G, U118 and GBM‐XX cells were seeded into 6‐well plates and incubated at 37°C until cells reached a confluence of at least 90%, then scratched and stimulated with 20(S)‐Rg3 (60, 120 μmol/L) or without 20(S)‐Rg3, and migration was monitored by cells within the wound area at 0 and 24 h. B, Migrated cells were quantitated using the ImageJ software program (n = 4). *P < .05, **P < .01, ***P < .001. C, 1 × 105 T98G, U118 and GBM‐XX cells were seeded in the upper chamber with serum‐free medium in 24‐well Transwell plates that were pre‐coated with Matrigel; 300 μL complete culture medium containing 10% FBS and 20(S)‐Rg3 (0, 60, 120 μmol/L) was added to the lower chamber as a chemo‐attractant. We dyed them with crystal violet 24 h later. D, Invasive cells were quantitated using the ImageJ software program (n = 3). *P < .05, **P < .01, ***P < .001 compared with control group
To further explore the mechanism of inhibition of glioma cell migration and invasion by 20(S)‐Rg3, we performed western blot assays for epithelial‐mesenchymal transformation‐related proteins, including E‐cadherin, N‐cadherin and vimentin. Western blot analyses showed that the expression levels of an epithelial marker, E‐cadherin, were increased by 20(S)‐Rg3(120 μmol/L for 3 days) in T98G, U118 and GBM‐XX cell lines, and of mesenchymal markers, N‐cadherin and Vimentin, were the opposite to E‐cadherin in all three glioma cell lines (Figure 6A). In addition, the results of immunofluorescence analyses in T98G and GBM‐XX were similar to those of western blot assays (Figure 6B). These results suggested that 20(S)‐Rg3 inhibits the epithelial cells to transdifferentiate into mesenchymal cells; in other words, 20(S)‐Rg3 could restrain the EMT progression of glioma cells.
Figure 6.
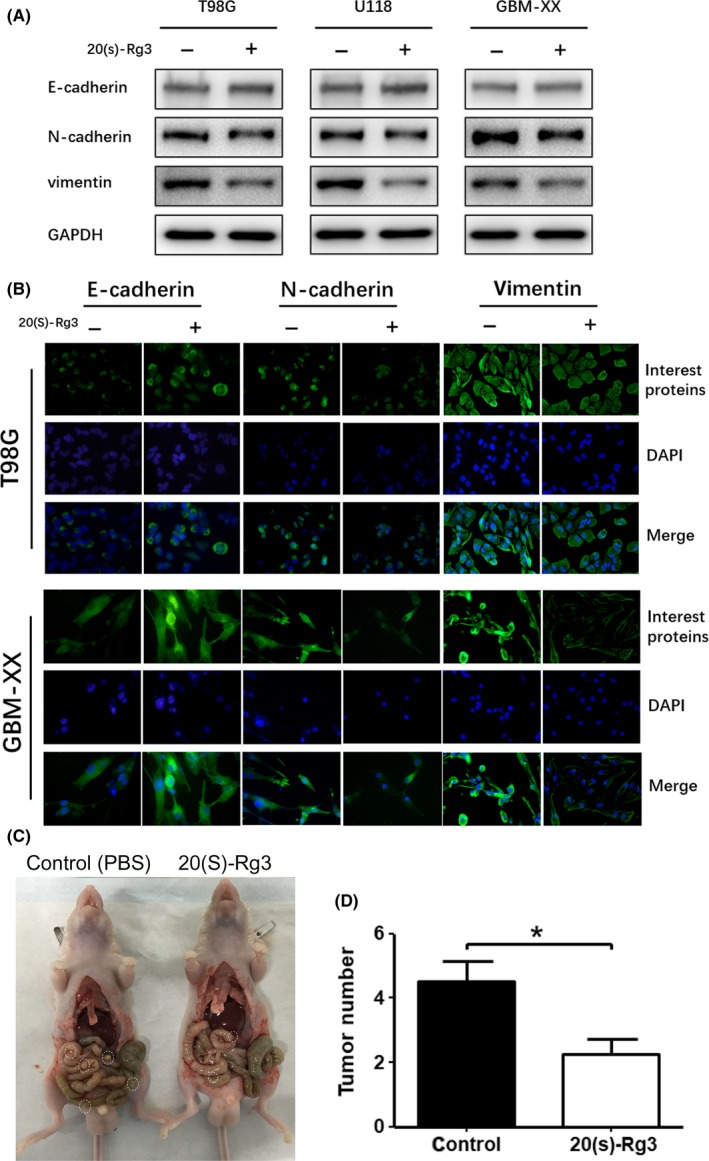
20(S)‐Rg3 increased the expression level of E‐cadherin and decreased the expression levels of N‐cadherin and vimentin. A, After treatment of 20(S)‐Rg3(120 μmol/L for 48 h), epithelial‐mesenchymal transformation‐related proteins, including E‐cadherin, N‐cadherin and vimentin, were determined by western blot. The expression of E‐cadherin in T98G, U118 and GBM‐XX increased; meanwhile, N‐cadherin and vimentin decreased uniformly. B, Immunofluorescence and assay showed the same result as with western blots in T98G and GBM‐XX cells. C,D Effect of 20(S)‐Rg3 on peritoneal spreading and metastasis in vivo. GBM‐XX cells (100 μL, 2 × 106) were injected i.p. into each 4‐wk‐old male nude mouse. Seven days later, mice received PBS or 20 mg/kg of 20(S)‐Rg3 every day by i.p. injection for 20 d. After 20 d, mice were killed and peritoneal metastatic nodules were calculated. n = 4, *P < .05 compared with the control group
Finally, to investigate the effect of 20(S)‐Rg3 on glioma cell peritoneal spreading in vivo, we injected GBM‐XX cells into nude mice intraperitoneally. Seven days later, 20 mg/kg of 20(S)‐Rg3 was injected in one group of mice every day by i.p. injection for 20 days in a row, while the control group mice were injected with equal amount of PBS. Twenty days later, the number of intraperitoneal metastatic nodules in the 20(S)‐Rg3 (20 mg/kg/day) treatment group was significantly less than in the control group (Figure 6C,D). Thus, 20(S)‐Rg3 can prevent glioma cell peritoneal spreading and metastasis in vivo.
4. DISCUSSION
As the most common malignant intracranial tumor, glioblastoma has a very fast course and extremely poor prognosis. At present, the treatment options for glioma patients are still very limited. The Stupp's regimen has been widely used in clinical practice for over a decade, and increases the life expectancy of GBM patients. Postoperative adjuvant chemotherapy plays an important role in Stupp's regimen, and TMZ is the first choice for chemotherapeutic agents. The addition of TMZ can bring additional benefits to patients with gliomas for an additional average 2.5 months to overall survival (OS).2, 24, 26, 27 However, the emergence of TMZ resistance in the majority of GBM patients brings another great challenge to the treatment of patients.28
The alkylating agent TMZ can spontaneously dissolve into the reactive intermediate 5‐(3‐methyl‐1‐triazeno)imidazole‐4‐carboxamide in the human body, and then it methylates the N7/O6 positions of guanine and the N3 position of adenine.24 The most important mismatch in these 3 methylation positions is the O6 position of guanine (O6‐G), which causes DNA base mispairing by continuously inducing O6‐methylguanine adduct, finally leading to cell cycle arrest and cellular apoptosis. This is how TMZ exerts its chemotherapeutic effects.29
Unfortunately, MGMT is frequently expressed in tumor cells of glioma patients. MGMT mediates the chemoresistance of glioma cells to TMZ by directly removing the methyl group from O6‐meG.30 After each MGMT molecule transfers the methyl group from O6‐meG, it will deactivate itself. Therefore, the cytotoxicity of TMZ is theoretically determined by the expression level of MGMT.31, 32 Because of these factors, much effort has been directed towards increasing the TMZ chemosensitivity by downregulating the expression of MGMT of glioma cells. Shirai et al33 reported that TMZ itself was shown to partially deplete MGMT protein in tumors, and MGMT would be depleted by a high dose of TMZ by suicide inhibition. O6‐BG, sulforaphane and STAT3 inhibitor have been shown to reverse resistance to TMZ by targeting downregulation of MGMT expression.34, 35, 36
20(S)‐Rg3 is a small molecule agent with a molecular weight of 785.01 g/mol, and it is reported that 20(S)‐Rg3 can be maintained in plasma with a high drug concentration, and is nontoxic and well‐tolerated.23 20(S)‐Rg3 has been reported to have remarkable anti‐tumor effects; however, its mechanism remains poorly understood. In this study, we used 2 MGMT‐positive glioma cell lines and an MGMT‐positive primary cell strain and xenograft glioma models to examine whether 20(S)‐Rg3 potentiates the sensitivity to TMZ and to reveal the underlying mechanisms.
Wnt/β‐catenin signaling plays a pivotal role in the occurrence and development of many types of malignant tumors, including GBM.37, 38, 39, 40 Inhibition of the β‐catenin expression level and the transcription factor TCF/LEF1 expression level for Wnt/β‐catenin signaling lead to downregulation of Wnt/β‐catenin signaling activity. Targeting Wnt/β‐catenin pathways provide a new therapeutic strategy for GBM.41, 42 Recent studies have shown that the Wnt/β‐catenin pathway could be involved in regulating the expression level of MGMT in cancers.43, 44 In the present study, we revealed that 20(S)‐Rg3(100 μmol/L for 3 days) significantly inhibits the expression level of MGMT protein in glioma cells by modulating Wnt/β‐catenin signaling in vitro. The key downstream effector β‐catenin and 2 important nuclear transcription factors for Wnt/β‐catenin pathways were all depressed by 20(S)‐Rg3, leading to several target genes being decreased. He et al45 reported that ginsenoside Rg3 inhibits colorectal tumor growth by downregulating the Wnt/β‐catenin pathway, which is partly consist with our result.
Meanwhile, 20(S)‐Rg3 by itself showed no significant cytotoxicity at its effective dose (100 μmol/L for 3 days), but when 20(S)‐Rg3 was given concomitantly with TMZ, it strongly enhanced the pro‐apoptotic effect of TMZ. 20(S)‐Rg3 inhibited the viability of glioma cells and induced apoptosis by decreasing the expression of caspase‐3, PARP and anti‐apoptosis protein Bcl‐2, and increasing the expression of the pro‐apoptosis proteins Bax, cleaved‐PARP and cleaved caspase‐3. Accumulating studies have reported that increased MGMT gene expression is closely related to the occurrence of TMZ resistance.46, 47 To our knowledge, we have revealed for the first time that 20(S)‐Rg3 inhibits MGMT gene expression by modulating Wnt/β‐catenin/MGMT pathways and potentiates chemosensitivity to TMZ in glioma cells in vitro and in vivo.
Moreover, it is generally recognized that epithelial‐mesenchymal transition (EMT) causes dissemination of tumor cells from primary sites by inducing polarized epithelial tumor cells to acquire mesenchymal cell phenotype. Thus, it is considered as an important factor in promoting the cell migration and invasion ability of tumor cells.48, 49 Therefore, we examined the effect of 20(S)‐Rg3 on migration and invasiveness of glioma cells, and checked the expression of critical EMT markers, E‐cadherin, N‐cadherin and vimentin, and found that 20(S)‐Rg3 significantly restrains the epithelial‐mesenchymal transition progression of glioma cells in vitro and in vivo. However, the underlying mechanism of 20(S)‐Rg3 that inhibits the EMT process on glioma cells is uncertain at present, and in future we will carry out further related exploration.
In sum, we revealed that 20(S)‐Rg3 demonstrates good reversal performance of TMZ resistance in MGMT‐positive glioma treatment; meanwhile, 20(S)‐Rg3 effectively restrains the epithelial‐mesenchymal transition progression of glioma cells. Our study suggests that 20(S)‐Rg3 could potentially be used to counteract TMZ resistance in MGMT‐positive glioblastomas.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Supporting information
Chen Z, Wei X, Shen L, Zhu H, Zheng X. 20(S)‐ginsenoside‐Rg3 reverses temozolomide resistance and restrains epithelial‐mesenchymal transition progression in glioblastoma. Cancer Sci. 2019;110:389–400. 10.1111/cas.13881
Funding information
The Foundation for Interdisciplinary Research of Shanghai JiaoTong University, (Grant/Award Number: YG2015MS65).
REFERENCES
- 1. Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet (London, England). 2018;392(10145):432‐446. [DOI] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987‐996. [DOI] [PubMed] [Google Scholar]
- 3. Stupp R, Brada M, van den Bent MJ, Tonn JC, Pentheroudakis G. High‐grade glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2014;25(Suppl 3):iii93‐iii101. [DOI] [PubMed] [Google Scholar]
- 4. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5‐year analysis of the EORTC‐NCIC trial. Lancet Oncol. 2009;10(5):459‐466. [DOI] [PubMed] [Google Scholar]
- 5. Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492‐507. [DOI] [PubMed] [Google Scholar]
- 6. Christmann M, Verbeek B, Roos WP, Kaina B. O(6)‐Methylguanine‐DNA methyltransferase (MGMT) in normal tissues and tumors: enzyme activity, promoter methylation and immunohistochemistry. Biochem Biophys Acta. 2011;1816(2):179‐190. [DOI] [PubMed] [Google Scholar]
- 7. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997‐1003. [DOI] [PubMed] [Google Scholar]
- 8. Esteller M, Garcia‐Foncillas J, Andion E, et al. Inactivation of the DNA‐repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343(19):1350‐1354. [DOI] [PubMed] [Google Scholar]
- 9. Wick W, Weller M, van den Bent M, et al. MGMT testing–the challenges for biomarker‐based glioma treatment. Nat Rev Neurol. 2014;10(7):372‐385. [DOI] [PubMed] [Google Scholar]
- 10. Kitange GJ, Carlson BL, Schroeder MA, et al. Induction of MGMT expression is associated with temozolomide resistance in glioblastoma xenografts. Neuro Oncol. 2009;11(3):281‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dahlrot RH, Dowsett J, Fosmark S, et al. Prognostic value of O‐6‐methylguanine‐DNA methyltransferase (MGMT) protein expression in glioblastoma excluding nontumour cells from the analysis. Neuropathol Appl Neurobiol. 2018;44(2):172‐184. [DOI] [PubMed] [Google Scholar]
- 12. Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58(11):1685‐1693. [DOI] [PubMed] [Google Scholar]
- 13. Jia L, Zhao Y, Liang XJ. Current evaluation of the millennium phytomedicine‐ ginseng (II): collected chemical entities, modern pharmacology, and clinical applications emanated from traditional Chinese medicine. Curr Med Chem. 2009;16(22):2924‐2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lim CJ, Choi WY, Jung HJ. Stereoselective skin anti‐photoaging properties of ginsenoside Rg3 in UV‐B‐irradiated keratinocytes. Biol Pharm Bull. 2014;37(10):1583‐1590. [DOI] [PubMed] [Google Scholar]
- 15. Park EH, Kim YJ, Yamabe N, et al. Stereospecific anticancer effects of ginsenoside Rg3 epimers isolated from heat‐processed American ginseng on human gastric cancer cell. J Ginseng Res. 2014;38(1):22‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kwok HH, Guo GL, Lau JK, et al. Stereoisomers ginsenosides‐20(S)‐Rg(3) and ‐20(R)‐Rg(3) differentially induce angiogenesis through peroxisome proliferator‐activated receptor‐gamma. Biochem Pharmacol. 2012;83(7):893‐902. [DOI] [PubMed] [Google Scholar]
- 17. Park MW, Ha J, Chung SH. 20(S)‐ginsenoside Rg3 enhances glucose‐stimulated insulin secretion and activates AMPK. Biol Pharm Bull. 2008;31(4):748‐751. [DOI] [PubMed] [Google Scholar]
- 18. Lee DC, Lau AS. Effects of Panax ginseng on tumor necrosis factor‐alpha‐mediated inflammation: a mini‐review. Molecules (Basel, Switzerland). 2011;16(4):2802‐2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu JP, Lu D, Nicholson RC, Li PY, Wang F. Toxicity of a novel anti‐tumor agent 20(S)‐ginsenoside Rg3: a 26‐week intramuscular repeated administration study in Beagle dogs. Food Chem Toxicol. 2011;49(8):1718‐1727. [DOI] [PubMed] [Google Scholar]
- 20. Liu JP, Lu D, Nicholson RC, Zhao WJ, Li PY, Wang F. Toxicity of a novel anti‐tumor agent 20(S)‐ginsenoside Rg3: a 26‐week intramuscular repeated administration study in rats. Food Chem Toxicol. 2012;50(10):3388‐3396. [DOI] [PubMed] [Google Scholar]
- 21. Lee JY, Jung KH, Morgan MJ, et al. Sensitization of TRAIL‐induced cell death by 20(S)‐ginsenoside Rg3 via CHOP‐mediated DR5 upregulation in human hepatocellular carcinoma cells. Mol Cancer Ther. 2013;12(3):274‐285. [DOI] [PubMed] [Google Scholar]
- 22. Zhao Q, Li P, Jiang J, Hu P. Pharmacokinetics of single ascending doses and multiple doses of 20(S)‐ginsenoside Rg3 in Chinese healthy volunteers. Eur J Drug Metab Pharmacokinet. 2016;41(6):845‐853. [DOI] [PubMed] [Google Scholar]
- 23. Zhang F, Li M, Wu X, et al. 20(S)‐ginsenoside Rg3 promotes senescence and apoptosis in gallbladder cancer cells via the p53 pathway. Drug Des Devel Ther. 2015;9:3969‐3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. You CG, Sheng HS, Xie CR, Zhang N, Zheng XS. FM19G11 inhibits O(6) ‐methylguanine DNA‐methyltransferase expression under both hypoxic and normoxic conditions. Cancer Med. 2018;7(7):3292‐3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yu Z, Chen Y, Wang S, Li P, Zhou G, Yuan Y. Inhibition of NF‐kappaB results in anti‐glioma activity and reduces temozolomide‐induced chemoresistance by down‐regulating MGMT gene expression. Cancer Lett. 2018;428:77‐89. [DOI] [PubMed] [Google Scholar]
- 26. Ng K, Kim R, Kesari S, Carter B, Chen CC. Genomic profiling of glioblastoma: convergence of fundamental biologic tenets and novel insights. J Neurooncol. 2012;107(1):1‐12. [DOI] [PubMed] [Google Scholar]
- 27. Weller M, Stupp R, Reifenberger G, et al. MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat Rev Neurol. 2010;6(1):39‐51. [DOI] [PubMed] [Google Scholar]
- 28. Grossman SA, Ye X, Piantadosi S, et al. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res. 2010;16(8):2443‐2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fu D, Calvo JA, Samson LD. Balancing repair and tolerance of DNA damage caused by alkylating agents. Nat Rev Cancer. 2012;12(2):104‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Nifterik KA, van den Berg J, van der Meide WF, et al. Absence of the MGMT protein as well as methylation of the MGMT promoter predict the sensitivity for temozolomide. Br J Cancer. 2010;103(1):29‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Belanich M, Randall T, Pastor MA, et al. Intracellular Localization and intercellular heterogeneity of the human DNA repair protein O(6)‐methylguanine‐DNA methyltransferase. Cancer Chemother Pharmacol. 1996;37(6):547‐555. [DOI] [PubMed] [Google Scholar]
- 32. Wang C, Abegg D, Hoch DG, Adibekian A. Discovery of a potent and selective inhibitor of the DNA repair protein MGMT. Angew Chem Int Ed Engl. 2016;55(8):2911‐2915. [DOI] [PubMed] [Google Scholar]
- 33. Shirai K, Chakravarti A. Towards personalized therapy for patients with glioblastoma. Expert Rev Anticancer Ther. 2011;11(12):1935‐1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kreklau EL, Liu N, Li Z, Cornetta K, Erickson LC. Comparison of single‐ versus double‐bolus treatments of O(6)‐benzylguanine for depletion of O(6)‐methylguanine DNA methyltransferase (MGMT) activity in vivo: development of a novel fluorometric oligonucleotide assay for measurement of MGMT activity. J Pharmacol Exp Ther. 2001;297(2):524‐530. [PubMed] [Google Scholar]
- 35. Lan F, Yang Y, Han J, Wu Q, Yu H, Yue X. Sulforaphane reverses chemo‐resistance to temozolomide in glioblastoma cells by NF‐kappaB‐dependent pathway downregulating MGMT expression. Int J Oncol. 2016;48(2):559‐568. [DOI] [PubMed] [Google Scholar]
- 36. Kohsaka S, Wang L, Yachi K, et al. STAT3 inhibition overcomes temozolomide resistance in glioblastoma by downregulating MGMT expression. Mol Cancer Ther. 2012;11(6):1289‐1299. [DOI] [PubMed] [Google Scholar]
- 37. Flahaut M, Meier R, Coulon A, et al. The Wnt receptor FZD1 mediates chemoresistance in neuroblastoma through activation of the Wnt/beta‐catenin pathway. Oncogene. 2009;28(23):2245‐2256. [DOI] [PubMed] [Google Scholar]
- 38. Zhang ZM, Wu JF, Luo QC, et al. Pygo2 activates MDR1 expression and mediates chemoresistance in breast cancer via the Wnt/beta‐catenin pathway. Oncogene. 2016;35(36):4787‐4797. [DOI] [PubMed] [Google Scholar]
- 39. Han P, Li JW, Zhang BM, et al. The lncRNA CRNDE promotes colorectal cancer cell proliferation and chemoresistance via miR‐181a‐5p‐mediated regulation of Wnt/beta‐catenin signaling. Mol Cancer. 2017;16(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liebelt BD, Shingu T, Zhou X, Ren J, Shin SA, Hu J. Glioma stem cells: signaling, microenvironment, and therapy. Stem Cells Int. 2016;2016:7849890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de Sousa EMF, Vermeulen L. Wnt signaling in cancer stem cell biology. Cancers. 2016;8(7):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Song Y, Lee S, Kim JR, Jho EH. Pja2 inhibits Wnt/beta‐catenin signaling by reducing the level of TCF/LEF1. Int J Stem Cells. 2018;11(2):242‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li ZY, Huang GD, Chen L, et al. Tanshinone IIA induces apoptosis via inhibition of Wnt/betacatenin/MGMT signaling in AtT20 cells. Mol Med Rep. 2017;16(5):5908‐5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wickstrom M, Dyberg C, Milosevic J, et al. Wnt/beta‐catenin pathway regulates MGMT gene expression in cancer and inhibition of Wnt signalling prevents chemoresistance. Nat Commun. 2015;6:8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. He BC, Gao JL, Luo X, et al. Ginsenoside Rg3 inhibits colorectal tumor growth through the down‐regulation of Wnt/ss‐catenin signaling. Int J Oncol. 2011;38(2):437‐445. [DOI] [PubMed] [Google Scholar]
- 46. Blough MD, Westgate MR, Beauchamp D, et al. Sensitivity to temozolomide in brain tumor initiating cells. Neuro Oncol. 2010;12(7):756‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Spiegl‐Kreinecker S, Pirker C, Filipits M, et al. O6‐methylguanine DNA methyltransferase protein expression in tumor cells predicts outcome of temozolomide therapy in glioblastoma patients. Neuro Oncol. 2010;12(1):28‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thiery JP. Epithelial‐mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442‐454. [DOI] [PubMed] [Google Scholar]
- 49. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial‐mesenchymal transitions in development and disease. Cell. 2009;139(5):871‐890. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


